Abstract
Kaposi's sarcoma-associated herpesvirus (KSHV) encodes a cellular dihydrofolate reductase (DHFR) homologue. Methotrexate (MTX), a potent anti-inflammatory agent, inhibits cellular DHFR activity. We investigated the effect of noncytotoxic doses of MTX on latency and lytic KSHV replication in two KSHV-infected primary effusion lymphoma cell lines (BC-3 and BC-1) and in MTX-resistant BC-3 cells (MTX-R-BC-3 cells). Treatment with MTX completely prevented tetradecanoyl phorbol acetate-induced viral DNA replication and strongly decreased viral lytic transcript levels, even in MTX-resistant cells. However, the same treatment had no effect on transcription of cellular genes and KSHV latent genes. One of the lytic transcripts inhibited by MTX, ORF50/Rta (open reading frame), is an immediate-early gene encoding a replication-transcription activator required for expression of other viral lytic genes. Therefore, transcription of genes downstream of ORF50/Rta was inhibited, including those encoding the viral G-protein-coupled receptor (GPCR), viral interleukin-6, and K12/kaposin, which have been shown to be transforming in vitro and oncogenic in mice. Resistance to MTX has been documented in cultured cells and also in patients treated with this drug. However, MTX showed an inhibitory activity even in MTX-R-BC-3 cells. Two currently available antiherpesvirus drugs, cidofovir and foscarnet, had no effect on the transcription of these viral oncogenes and ORF50/Rta. MTX is the first example of a compound shown to downregulate the expression of ORF50/Rta and therefore prevent viral transforming gene transcription. Given that the expression of these genes may be important for tumor development, MTX could play a role in the future management of KSHV-associated malignancies.
Kaposi's sarcoma-associated herpesvirus (KSHV; also called human herpesvirus 8) is a recently discovered gammaherpesvirus linked to Kaposi's sarcoma (13). KSHV has been found to be present in all epidemiologic forms of Kaposi's sarcoma, including classical, endemic African, and AIDS types (29) as well as an uncommon but recognized complication in patients with organ transplants (55). KSHV is also associated with lymphoproliferative disorders, including primary effusion lymphoma and multicentric Castleman's disease (4, 10). In Kaposi's sarcoma and primary effusion lymphoma, the majority of the tumor cells display a latent infection with KSHV, while lytic replication is limited to a small subset of cells, presumably reflecting spontaneous reactivation from the latent state that can also be induced in vitro by treatment with12-O-tetradecanoyl phorbol 13-acetate (TPA) or sodium butyrate (SB) (47, 48).
A regulated cascade of viral gene expression characterizes lytic herpesvirus replication. Immediate-early genes, which mainly encode activators of gene transcription, are expressed first and lead to upregulation of early genes whose proteins are involved in viral DNA replication. Late genes, which encode mainly structural proteins, are expressed after newly replicated viral DNA. The KSHV lytic cycle is driven to completion by an immediate-early gene, ORF50/Rta (replication and transcription activator of viral lytic cycle genes), which is a homologue of the BRLF1/Rta gene encoded by Epstein-Barr virus (EBV), a herpesvirus that is closely related to KSHV (57). The ORF50/Rta product is necessary and sufficient to activate expression of viral lytic cycle genes (25).
KSHV encodes a large number of proteins that have homology to cellular proteins that regulate cell proliferation (14), cell death (15), and signal transduction (3, 24). These proteins include chemokine and cytokine homologues (36): viral G-protein-coupled receptor (11), viral IL-6 (41), viral FLIP, a viral Fas-associated death domain-like interleukin-1β-converting enzyme (FLICE)-inhibitory protein (59), viral cyclin, a homologue of D-type cyclin (ORF72) (11, 14), and three chemokines, viral macrophages inflammatory proteins MIP-I, -II, and -III (36). Additionally, KSHV is the only human virus encoding a dihydrofolate reductase (DHFR) (ORF2) (42, 54) homologue of cellular DHFR, which reduces folic acid to tetrahydrofolic acid and is required for purine and thymydilate biosynthesis. Methotrexate (MTX), a folic acid antagonist and a potent anti-inflammatory agent, inhibits the cellular DHFR. The affinity of DHFR for MTX is far greater then its affinity for folic acid or dihydrofolic acid, and therefore very large doses of folic acid are necessary to reverse the effect of MTX. This drug was first developed for treatment of malignancies such as leukemia and lymphoma and is now used at low doses mainly as therapy for rheumatoid arthritis, other inflammatory diseases (23, 60), and psoriasis (8).
In recent studies, MTX has been reported to have antiviral action against human cytomegalovirus and murine cytomegalovirus. Murine cytomegalovirus and human cytomegalovirus infection of quiescent NIH 3T3 cells stimulates transcription, expression, and cellular activity of DHFR (30, 31). In quiescent NIH 3T3 cells, MTX suppresses human cytomegalovirus and murine cytomegalovirus replication at the level of DNA synthesis. However, in MTX-resistant NIH 3T3 sublines, it has no activity due to cellular DHFR overexpression (31).
In vitro and in vivo studies have shown that, in addition to its anti-cellular DHFR activity, MTX has an anti-inflammatory action due to the capacity of MTX to promote adenosine release from fibroblasts and endothelial cells (5, 17, 18). Adenosine, acting at one or more of its receptors on monocytes/macrophages, inhibits tumor necrosis factor alpha (TNF-α), interleukin-6 (IL-6), and IL-8 production (7, 44, 49) and on endothelial cells, inhibits the secretion of IL-6 and IL-8. KSHV encodes homologues to human IL-6 and IL-8 receptors that are important for viral replication and pathogenesis.
Viral IL-6 is a multifunctional cytokine that promotes hematopoiesis, plasmacytosis, and angiogenesis (2), and viral G-protein-coupled receptor (GPCR) is a constitutive signaling receptor homologous to human IL-8 receptors type A (CXCR 1) and B (CXCR 2) (3, 11). Signaling by viral GPCR eventually leads to vascular endothelial growth factor (VEGF) expression, thereby inducing angiogenesis via paracrine mechanisms (6). These studies suggest that it is possible that MTX might affect these viral gene products as it does cellular homologs. Thus, we investigated whether methotrexate has any activity on KSHV replication and transcription in two KSHV-infected primary effusion lymphoma (PEL) cell lines (BC-3 and BC-1) and in MTX-resistant BC-3 cells (MTX-R-BC-3 cells).
KSHV gene transcripts have been classified into three classes based on their presence and upregulation in uninduced and TPA-induced BC-1 cells: class I (constitutive, strictly latent), class II (present in very low levels in uninduced cells but upregulated with TPA treatment), and class III (present only after TPA induction) (54). In this report we show that, in latently KSHV-infected PEL cell lines, the inhibition of ORF50/Rta transcription with MTX abolishes the induction of the entire viral lytic cycle with either TPA or sodium butyrate. Therefore, transcription of class II and III genes (54), which are downstream of ORF50/Rta, cannot be activated. The same treatment with MTX had no effect on transcription of latent genes of class I, cellular DHFR, β-actin, and c-Fos.
Resistance to methotrexate may develop in cell lines as well as in blast cells from patients with leukemia during treatment with MTX, and resistance has been associated with decreased uptake of the drug and overexpression of cellular DHFR. To address this issue, we established two BC-3 cell lines resistant to 0.5 and 1 μM MTX. We show by Northern blot, immunofluorescence, and Western blot that, although the cells had developed a resistance to the drug, TPA induction of KSHV class II and III gene transcription and translation is markedly reduced either in the presence or in the absence of MTX.
Currently available antiherpesvirus drugs such as acyclovir, adefovir, foscarnet, and cidofovir have been shown to reduce the number of cells expressing late KSHV lytic gene products but not the number of cells expressing the nonstructural immediate-early lytic gene products (62) such as ORF50/Rta, viral IL-6, K12/kaposin, and viral GPCR. Thus, we compared the effect of MTX with two of these antiviral drugs, cidofovir, a nucleotide analog, and foscarnet, a pyrophosphate analog. Both target the herpesvirus DNA polymerase during lytic replication. In contrast to MTX, we show that these two inhibitors have no effect on ORF50/Rta, viral IL-6, K12/kaposin, and viral GPCR transcription in both uninduced and TPA-induced BC-1 and BC-3 cells.
MATERIALS AND METHODS
Cell cultures.
BC-3 (KSHV-positive and EBV-negative cell line) (4) and BC-1 (KSHV- and EBV-positive cell line) (10) are B-cell lines derived from a body cavity-based B lymphoma; BJAB is a KSHV- and EBV-negative lymphoid cell line (35). Cells were grown in RPMI 1640 medium (Gibco-BRL) supplemented with 20% fetal calf serum, 2 mM glutamine, penicillin, and streptomycin (100 U/μl each). Cell viability was assessed by counting trypan blue-excluding cells on a hemacytometer. All cells counts were performed in triplicate.
Bone marrow endothelial cells immortalized with simian virus 40 (SV40) (BMEC-SV40) were grown in Dulbecco's modified Eagle's medium (DMEM) (Gibco-BRL) supplemented with 10% fetal calf serum, 2 mM glutamine, penicillin, and streptomycin (100 U/μl).
Induction of KSHV lytic replication and drug treatment.
Viral lytic replication was induced by treating 4 × 105 cells/ml with 20 ng of TPA/ml or 0.3 M sodium butyrate (Sigma Chemical Co., St. Louis, Mo.) for 48 h, and eight concentrations of MTX (Sigma Chemical Co., St. Louis, Mo.), ranging from 1 nM to 10 μM, were added simultaneously with TPA or sodium butyrate. Kinetics experiment was performed by adding 1 μM MTX 0, 4, 8, 12, and 24 h after chemical induction. To establish the MTX-resistant cultures, BC-3 cells were incubated in medium supplemented with increasing concentrations of MTX up to 1 μM and then maintained in 1 μM MTX. After 2 months in culture, the cells were able to grow in the presence of 1 μM MTX at the same proliferation rate as the controls grown in the absence of MTX.
Antiviral drug treatment was performed on uninduced and TPA-induced BC-3 cells in the presence of 0.5 μM cidofovir [1-(S)-3-hydroxy-2-(phosphonomethoxypropylcytosine] (Gilead Sciences Inc., Foster City, Calif.) or 0.5 mM foscarnet (trisodium phosphonoformate hexahydrate) (Sigma Chemical Co., St. Louis, Mo.) for 48 h.
RNA extraction and Northern blot analysis.
Total RNA was extracted from 2.5 × 107 cells per sample (4 × 105 cells/ml) of BC-3 and BC-1 with Tri reagent (Molecular Research Center, Inc., Cincinnati, Ohio), and mRNA was selected with a Poly (A) Tract mRNA isolation kit (Promega, Madison, Wis.) following the manufacturer's instructions. To eliminate any contaminating DNA, the RNA samples were treated with 2 U of RNase-free DNase (Promega). Northern blots were performed by loading 1 μg of mRNA onto a 1.5% formaldehyde-agarose gel and transferred onto a Gene Screen nylon membrane (DuPont, Boston, Mass.).
Filters were hybridized at 42°C with 32P-labeled probes obtained by amplification of the viral DNA with the following primers: Kaposin /K12, 5′-GGATAGAGGCTTAACGGTGTTTGTG-3′ and 5′-TGCAACTCGTGTCCTGAATGC-3′; viral MIP II (ORF K4), 5′-GGTACCAGCTGGACAGAAGC-3′ and 5′-TGACTGCCTTGCTTTGTTTG-3′; viral IL-6 (ORF K2), 5′-ATGTGCTGGTTCAAGTTGTGG-3′ and 5′-GATGGCTGGTAGTTTCAGATG-3′; viral FLIP, 5′-AAATAACTCATTGTGCCCGC-3′ and 5′-TTAAAGGAGGAGGGCAGGTT-3′. The MCP (major capsid protein) (21), viral GPCR (ORF 74) (11), viral DHFR (16), and human DHFR (16) primers have already been described. Human β-actin and c-fos (Stratagene, La Jolla, Calif.) probes were used as a quantitative control.
Detection of viral genome replication.
Genomic DNA was isolated from 2 × 105 cells per sample (4 × 105 cells/ml) of BC-3 and BC-1 cells by the phenol-chloroform method (53), and 2 μg was quantitatively slot blotted in triplicate. Hybridization was performed with a KSHV-GPCR probe (11) 32P labeled by random priming (RediPrime DNA labeling system; Amersham Life Science). Relative hybridization intensity was standardized to the total DNA hybridization with a human β-globin probe, and the effect of MTX was expressed as a percentage of the hybridization in the untreated control DNA. Specific hybridization was quantitated on a Molecular Dynamics 425E PhosphorImager. Results are the averages of three experiments with three repeats per sample with standard deviations.
Viral infection.
SV40-BMEC were plated in six-well dishes (three plates/sample), at the density of 2 × 105, 24 h before viral inoculum. Medium was replaced with 1 ml of supernatant obtained from uninduced, TPA-induced, and TPA-induced in the presence of 1 μM MTX BC-3 cells, and Polybrene (4 μg/ml) (Sigma) was added to each sample. Plates were centrifuged at 3,500 × g for 1 h at 25°C, then the cells were washed with phosphate-buffered saline (PBS), and new medium was added. Four days after infection, total RNA was extracted and treated with RNase-free DNase (Promega, Madison, Wis.) to eliminate any contaminant DNA. Reverse transcription system (Promega, Madison, Wis.) was used for cDNA synthesis, and a fragment of 192 bp in the ORF73 gene was amplified by PCR with the primers 5′-CGGAGCTAAAGAGTCTGGTG-3′ and 5′-GCAGTCTCCAGAGTCTTCTC-3′.
The amplified products were transferred onto nylon membranes, and cDNA was hybridized with an internal probe.
Immunoblotting.
Cells were lysed in mammalian protein extraction reagent (Pierce). Equal amounts of total proteins (100 μg) were separated by electrophoresis in 4 to 20% Tris-glycine gels (Novex) and transferred to Immobilon-P membranes (Millipore). K12/kaposin proteins were detected using a 1:750 dilution of a mouse anti-kaposin antibody (39), followed by a horseradish peroxidase-conjugated anti-mouse immunoglobulin (Ig) secondary antibody. The specific K12/kaposin signal was visualized by using enhanced chemiluminescence.
Immunofluorescence assay.
Immunofluorescence assay was performed using a polyclonal antibody against viral IL-6. Cells were washed once with PBS, counted, spotted on slides (105/slide), air-dried, and fixed in acetone for 10 min. The slides were blocked with 20% normal goat serum in PBS for 30 min and then incubated overnight at 4°C with the primary antibody followed by fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit Ig (28). Staining controls included omission of primary antibody. Cell nuclei were counterstained with DAPI (4′,6′-diamidino-2-phenylindole dihydrochloride; Boehringer Mannheim).
Cytokine assay.
Assays were done by enzyme-linked immunosorbent assay in a commercial laboratory (Cytokine Core Laboratory, Baltimore, Md.).
RESULTS
MTX dose-response curve on ORF50/Rta mRNA levels.
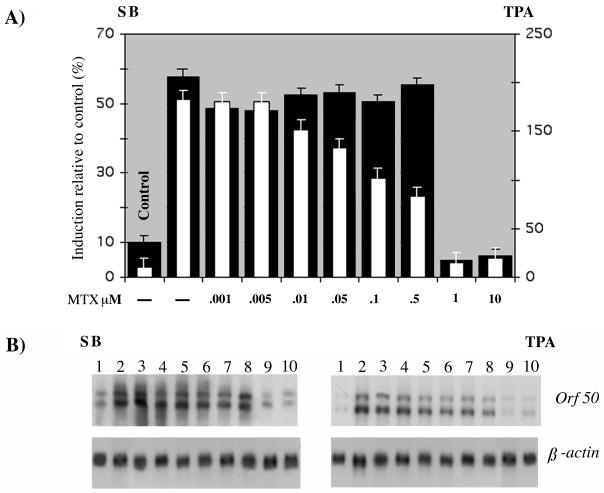
To study the effect of MTX on viral gene transcription, we began the analysis by determining the minimal effective concentration of MTX. mRNA was extracted from BC-3 cells uninduced and induced with TPA or sodium butyrate for 48 h and treated with eight concentrations of MTX (1 nM, 5 nM, 10 nM, 50 nM, 0.1 μM, 0.5 μM, 1 μM, and 10 μM) added simultaneously with TPA. The results are shown as percentage of induction relative to uninduced cells (Fig. 1A); 1 μM MTX proved to be the minimal concentration that prevents the induction of ORF50 transcripts with both TPA and sodium butyrate. This result suggests that MTX has a general effect that does not depend on the signal transduction pathway of the inducing compound, since TPA and sodium butyrate use different mechanism of induction. Of note, in TPA-induced cells, the inhibition was dose dependent, while in sodium butyrate-induced cells, the inhibition was not linear, although the minimal effective concentration was equivalent for both TPA and sodium butyrate.
FIG. 1.
(A) MTX dose-response curve on ORF50/Rta mRNA levels in BC-3 cells induced with TPA and sodium butyrate (SB). Hybridization was performed with an ORF50/Rta probe. Bars indicate the values for BC-3 cells treated with MTX and induced with sodium butyrate (solid bars) or TPA (open bars). Control indicates the value for uninduced and untreated BC-3 cells. (B) Northern blots.
MTX inhibits KSHV DNA replication.
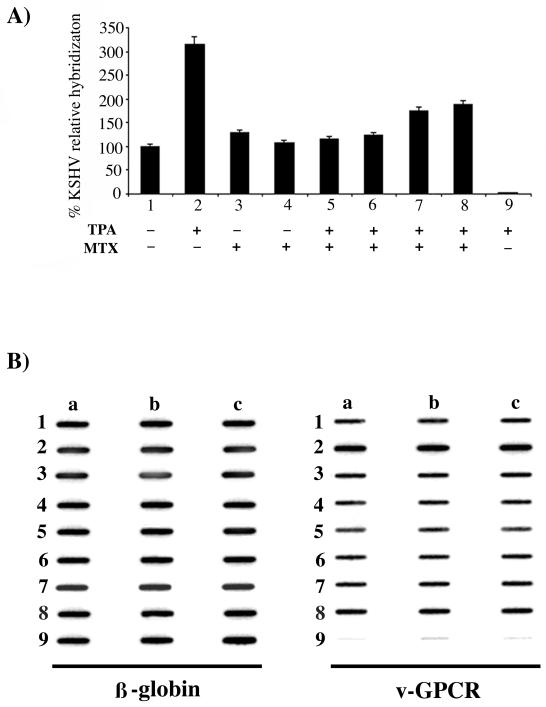
To determine the effect of MTX on KSHV DNA replication, BC-3 cells were treated with TPA to induce the viral lytic cycle, and 1 μM or 10 μM MTX was added simultaneously with TPA or 24 h after induction (Fig. 2A). As shown previously, viral DNA replication in BC-3 cells increased threefold after TPA treatment (21), but induction was completely abolished in samples treated simultaneously with MTX and TPA. The effect was less dramatic when MTX was added 24 h after TPA induction. The experiments were performed three times, and in all experiments, there was a 15 to 30% decrease in the percentage of viable cells (most likely this reflects the toxic effects of TPA), but no difference was detected between the MTX-treated and untreated TPA-induced cells. Similar results were obtained in BC-1 cells (data not shown).
FIG. 2.
(A) Effect of MTX on KSHV DNA replication in uninduced and TPA-induced BC-3 cells. Hybridization was performed with a KSHV GPCR probe. Relative hybridization intensity was standardized to the total DNA hybridization with a human β-globin probe, and the effect of MTX was expressed as a percentage of the untreated control DNA hybridization. Samples: 1, uninduced; 2, TPA induced; 3, uninduced and treated with 1 μM MTX; 4, uninduced and treated with 10 μM MTX; 5, TPA induced for 48 h in the presence of 1 μM MTX; 6, TPA induced for 48 h in the presence of 10 μM MTX; 7, TPA induced and after 24 h treated with 1 μM MTX for an additional 24 h; 8, TPA induced and after 24 h treated with 10 μM MTX for an additional 24 h; 9, TPA induced BJAB. (B) Data representative of three slot blot experiments with three repeats per sample (a, b, and c) for β-globin and viral GPCR (v-GPCR).
Effect of MTX on viral and cellular transcript levels.
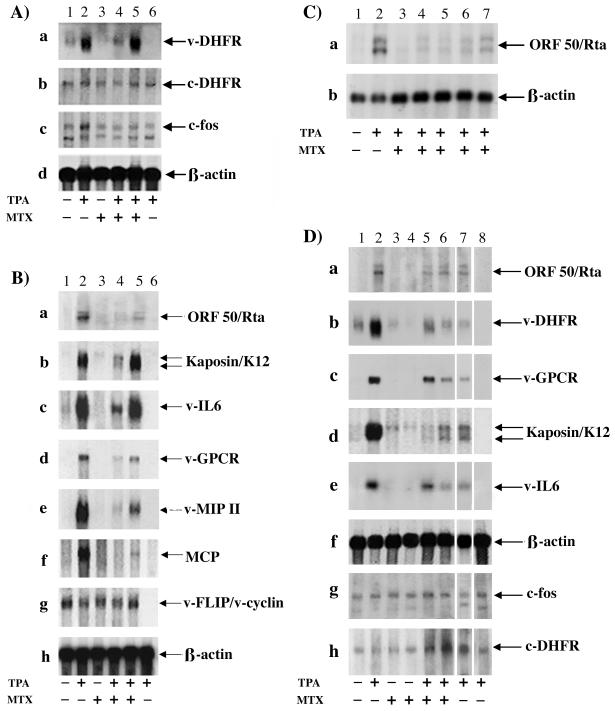
To determine the effect of MTX on viral gene transcription, Northern blots were performed. mRNA was extracted from uninduced and TPA-induced BC-3 cells for 48 h, and 1 μM MTX was added simultaneously with TPA or 24 h after TPA induction. As shown in Fig. 3A, viral DHFR transcript levels (lane 1) were strongly induced by TPA (lane 2); however, TPA induction of viral DHFR transcripts was abolished when MTX was added at the same time as TPA (lane 4). In contrast, when MTX was added 24 h after TPA induction (lane 5), transcript levels were similar to those in TPA-induced cells without MTX (lane 2). Cellular mRNA levels of cellular DHFR, c-Fos, and human β-actin were not affected by TPA and MTX treatment, showing no evidence of toxicity.
FIG. 3.
Northern blot analysis of mRNA extracted from BC-3 cells uninduced or TPA induced for 48 h in the presence of MTX. (A) RNA was hybridized with viral DHFR, cellular DHFR, c-fos, and human β-actin probes. Lane 1, uninduced; lane 2, TPA induced; lane 3, uninduced and treated with 1 μM MTX; lane 4, TPA induced in the presence of 1 μM MTX; lane 5, TPA induced and 24 h after induction treated with 1 μM MTX for an additional 24 h; lane 6, TPA induced BJAB. (B) mRNA was hybridized with ORF50/Rta, kaposin/K12, viral IL-6, viral GPCR, viral MIP II, MCP, viral FLIP/cyclin, and human β-actin probes. Lane 1, uninduced; lane 2, TPA induced; lane 3, uninduced and treated with 1 μM MTX for 48 h; lane 4, TPA induced for 48 h in the presence of 1 μM MTX; lane 5, TPA induced and 24 h after induction treated with 1 μM MTX for an additional 24 h; lane 6, TPA induced BJAB. (C) Kinetics of ORF50/Rta. BC-3 cells were induced with TPA for 48 h, and 1 μM MTX was added 0, 4, 8, 12, and 24 h after induction. Lane 1, uninduced; lane 2, TPA induced; lane 3, TPA induced and MTX added 0 h after induction; lane 4, TPA induced and MTX added 4 h after induction; lane 5, TPA induced and MTX added 8 h after induction; lane 6, TPA induced and MTX added 12 h after induction; lane 7, TPA induced and MTX added 24 h after induction. (D) Northern blot analysis of mRNA extracted from BC-3 and MTX-R-BC-3 cells. RNA was hybridized with ORF50/Rta, viral DHFR, viral GPCR, K12/kaposin, viral IL-6, human β-actin, c-fos, and cellular DHFR probes. Lane 1, BC-3; lane 2, TPA induced BC-3; lane 3, 0.5 μM MTX-R-BC-3 cells maintained in 0.5 μM MTX; lane 4, 1 μM MTX-R-BC-3 cells maintained in 1 μM MTX; lane 5, 0.5 μM MTX-R-BC-3 cells, TPA induced in the presence 0.5 μM MTX; lane 6, 1 μM MTX-R-BC-3 cells, TPA induced in the presence 1 μM MTX; lane 7, 1 μM MTX-R-BC-3 cells, TPA induced in the absence of 1 μM MTX; lane 8, TPA induced BJAB.
To evaluate the effect of MTX on transcript induction of other viral genes, we examined the transcripts produced by uninduced and TPA-induced BC-3 cells for 48 h in the presence of 1 μM MTX (Fig. 3B). Probes specific for ORF50/Rta (immediate-early gene, class II), K12/kaposin (early gene, class II), viral IL-6 (early gene, class II), viral GPCR (early gene, class II), viral MIP II (early gene, class II), MCP (late gene, class III), and viral FLIP/viral cyclin (strictly latent genes, class I) were used to analyze mRNA. As shown in Fig. 3B from a to f, TPA treatment induced class II and III viral gene transcription (Fig. 3B, lane 2), which was strongly reduced by adding 1 μM MTX at the same time as TPA (Fig. 3B, lane 4). In contrast, transcription of class I latent genes viral FLIP/viral cyclin, which are constitutively expressed and not inducible by TPA, was totally unaffected by MTX treatment. Only partial inhibition of transcript induction was observed when MTX was added 24 h after TPA induction (Fig. 3B, lane 5). A human β-actin probe was used as a quantitative control.
These results indicate that MTX, when added simultaneously with TPA, inhibits the induction of immediate-early, early, and late lytic viral gene transcripts. RNA was also hybridized with a probe complementary to ORF73, the gene encoding latent nuclear antigen (LANA, class I), and no inhibition was detected (data not shown). Therefore, MTX interferes with the KSHV lytic cycle activation but has no effect on viral latency, since transcription of strictly latent genes (class I) was not affected. The same experiments were performed with BC-1 cells, and identical results were obtained (data not shown).
Kinetics of ORF50/Rta transcript inhibition.
It has been demonstrated that ORF50/Rta transcription is induced within 4 h of the addition of TPA, while early lytic gene transcripts appeared 8 to 13 h and late transcripts between 20 and 30 h after chemical induction (58). As our previous experiments showed that the inhibitory effect of MTX was much less evident when it was added 24 h after TPA induction, we performed a kinetics experiment to identify whether ORF50/Rta was the initial target. BC-3 cells were induced with TPA, and MTX was added at 0, 4, 8, 12, and 24 h after induction. All samples were collected 48 h after TPA treatment. mRNA was extracted from each sample, and ORF50/Rta transcript levels were monitored by Northern blot analyses. Figure 3C shows that the induction of ORF50/Rta transcripts observed with TPA treatment (lane 2) was blocked when TPA and MTX were added simultaneously (Fig. 3C, lane 3). However, transcript levels gradually increased when MTX was added 4, 8, 12, and 24 h after TPA induction (Fig. 3C, lanes 4, 5, 6, and 7, respectively). β-Actin levels were monitored as an internal control for the Northern blots.
MTX inhibits KSHV gene transcription in MTX-R-BC-3 cells.
Resistance mechanisms to MTX have been studied extensively in animal models and in patients treated for long periods with MTX. To determine whether KSHV could develop resistance as well, two sublines of BC-3 cells were selected to grow in the presence of 0.5 μM and 1 μM MTX. After 2 months in culture with MTX, we analyzed the levels of viral DHFR and other viral transcripts. Northern blot analyses were performed with mRNA extracted from MTX-R-BC-3 cells TPA induced for 48 h in the presence or in the absence of MTX. mRNA from uninduced BC-3 and MTX-R-BC-3 cells was used as a control (Fig. 3D from a to h; BC-3, lane 1; 0.5 μM and 1 μM MTX-R-BC-3 cells, lanes 3 and 4, respectively).
Figure 3D shows that in TPA-induced MTX-R-BC-3 cells (0.5 μM and 1 μM MTX; Fig. 3D, lanes 5 and 6), transcript levels of ORF50/Rta, viral DHFR, viral GPCR, K12/kaposin, and viral IL-6 genes were considerably decreased compared to the levels detected in TPA-induced BC-3 cells (Fig. 3D, from a to e, lane 2). In contrast, transcript levels of β-actin, c-Fos, and cellular DHFR were identical in uninduced BC-3 cells (Fig. 3D, lane 1) and MTX-R-BC-3 cells (Fig. 3D, lanes 3 and 4) or TPA-induced BC-3 (Fig. 3D, lane 2) and 0.5 μM and 1 μM MTX-R-BC-3 cells (Fig. 3D, lanes 5 and 6). In the absence of MTX, TPA induction of KSHV class II gene transcripts (lane 7) was similar to that detected in MTX-R-BC-3 cells TPA induced in the presence of MTX.
These results indicate that (i) KSHV did not develop a total resistance to MTX, given that in MTX-R cells the TPA induction of ORF50/Rta and early lytic transcripts was still inhibited compared to the sensitive BC-3 cells and (ii) the ability of the selected resistant cells to express lytic transcripts after TPA induction in the absence of MTX is not the same as the parental cell population. In fact, a moderate increase in viral transcript levels was detected in MTX-R-BC-3 cells TPA induced in the absence of MTX (Fig. 3D, lane 7) in comparison with the parental sensitive uninduced BC-3 cells (Fig. 3D, lane 1). In contrast, the transcript levels in MTX-R-BC-3 cells TPA induced in the absence of MTX (Fig. 3D, lane 7) are much lower then those detected in the parental sensitive TPA-induced BC-3 cells (Fig. 3D, lane 2), but similar to those detected in MTX-R-BC-3 cells TPA induced in the presence of MTX (Fig. 3D, lane 6). Of note in TPA-induced MTX-R-BC-3 cells (Fig. 3D, lanes 5, 6, and 7), the level of cellular DHFR transcripts is higher then in MTX-sensitive BC-3 cells (Fig. 3D, lanes 1 and 2).
MTX inhibits the synthesis of viral IL-6 and K12/kaposin proteins.
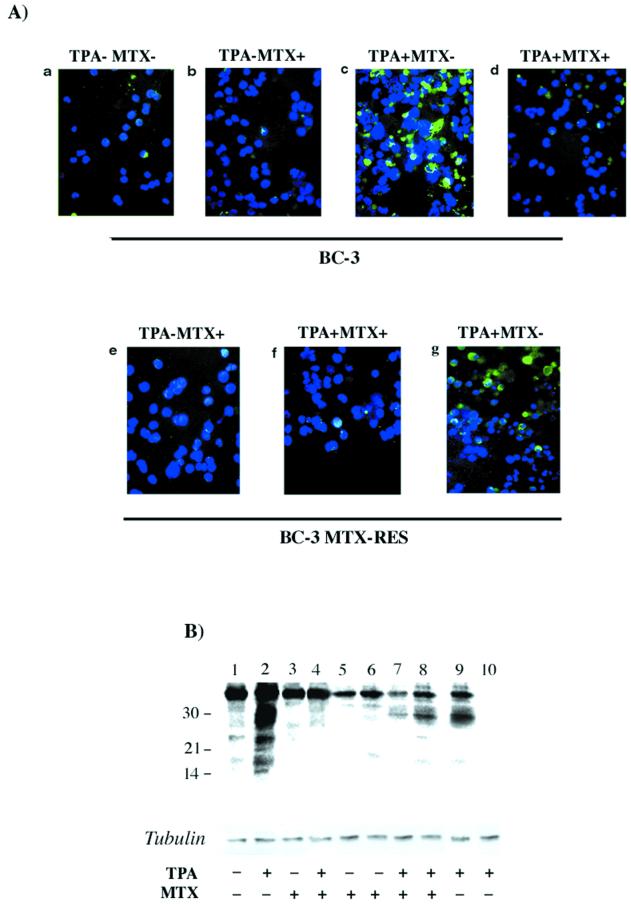
KSHV IL-6 gene expression in uninduced and TPA-induced BC-3 cells and uninduced and TPA-induced 1 μM MTX-R-BC-3 cells maintained in the presence of MTX was analyzed by immunofluorescence assay (Fig. 4A). All samples were collected 48 h after induction. A polyclonal antibody against viral IL-6 was used as the primary antibody (cytoplasmic green signal), and DAPI was used to counterstain the nuclei (blue signal). Induction of viral IL-6 expression was abolished in almost 90% of TPA-induced BC-3 cells in the presence of 1 μM MTX (Fig. 4A, panel d) compared to the TPA-induced BC-3 in the absence of MTX (Fig. 4A, panel c). In uninduced MTX-R-BC-3 cells (Fig. 4A, panel e), there was no change in the level of viral IL-6 expression compared to the control nonresistant uninduced BC-3 cells (Fig. 4A, panel a). However, in MTX-R-BC-3 cells induced with TPA in the presence of MTX, viral IL-6 expression was dramatically reduced (Fig. 4A, panel f) compared to the control TPA-induced BC-3 cells (Fig. 4A, panel c). This effect was less impressive when the MTX-R-BC-3 cells were induced in the absence of MTX (Fig. 4A, panel g).
FIG. 4.
(A) Immunofluorescence assay. Merged images of viral IL-6 polyclonal antibody cytoplasmic signal (green) and nuclear DAPI signal (blue). KSHV IL-6 expression in uninduced and TPA-induced BC-3 cells for 48 h in the presence of MTX, and 1 μM MTX-R-BC-3 cells uninduced and TPA induced. BC-3 cells (a), BC-3 cells with 1 μM MTX (b), BC-3 cells TPA induced (c), BC-3 cells TPA induced in the presence of MTX (d), 1 μM MTX-R-BC-3 cells (e), 1 μM MTX-R-BC-3 cells TPA induced in the presence of MTX (f), and 1 μM MTX-R-BC-3 cells TPA induced in the absence of MTX (g). (B) Western blot. Cellular extracts of BC-3 and 1 μM MTX-R-BC-3 cells, uninduced and TPA induced and treated with 1 μM MTX for 48 h were analyzed. Lane 1, uninduced BC-3; lane 2, TPA induced BC-3; lane 3, BC-3 uninduced with 1 μM MTX; lane 4, BC-3 TPA induced in the presence of 1 μM MTX; lane 5, 0.5 μM MTX-R-BC-3 cells; lane 6, 1 μM MTX-R-BC-3 cells; lane 7, TPA induced 0.5 μM MTX-R-BC-3 in the presence of MTX; lane 8, TPA induced 1 μM MTX-R-BC-3 in the presence of MTX; lane 9, TPA induced 1 μM MTX-R-BC-3 cells in the absence of MTX; lane 10, TPA induced BJAB cells.
Expression of a constitutive latent gene (LANA) was also assayed by immunofluorescence assay using a monoclonal antibody against LANA/ORF73 (ABI, Columbia, Md.), and no difference between untreated and MTX-treated cells was detected (data not shown). Similar results have been obtained with BC-1 cells (data not shown).
To determine the effects of MTX on the synthesis of kaposin proteins, cell extracts of uninduced as well as TPA-induced PEL cell lines, BC-3 treated with 1 μM MTX for 48 h, and 1 μM MTX-R-BC-3 cells were analyzed by Western blot. Cellular extracts from untreated samples were used as controls. Results are shown in Fig. 4B. Multiple forms of kaposin proteins ranging in size from approximately 16 to 44 kDa were detected in untreated control BC-3 cells (Fig. 4B, lane 1). Higher levels of proteins were observed in the TPA-induced cells (Fig. 4B, lane 2). In contrast to this, no kaposin proteins were detected in cell extracts from MTX-treated BC-3 cells, whether or not TPA was added (Fig. 4B, lanes 3 and 4), or in MTX-R-BC-3 cells (Fig. 4B, lanes 5 and 6 [uninduced] and lanes 7 and 8) TPA induced in the presence of MTX.
Similar results were obtained when the MTX-R-BC-3 cells were induced in the absence of MTX (Fig. 4B, lane 9). However, higher-molecular-mass bands (30 and 44 kDa) were observed in MTX-treated sensitive BC-3 uninduced or TPA induced (Fig. 4B, lane 3 and 4, respectively) and MTX-R-BC-3 cells uninduced (Fig. 4B, lanes 5 and 6) or TPA induced (Fig. 4B, lanes 7, 8, and 9). This can be attributed to the K12/kaposin expression in low abundance during the latency. No signal was detected in cell extracts from a control, KSHV-negative B-cell line, BJAB (Fig. 4B, lane 10). These data indicate that 1 μM MTX specifically inhibited the TPA induction of viral IL-6 and kaposin protein synthesis in sensitive TPA-induced BC-3, BC-1, and MTX-R-BC-3 cells, although in the last cells a slight increase in kaposin protein was detectable. These results are consistent with those obtained with Northern blot analyses.
Viral particles produced in the presence of MTX are not infectious.
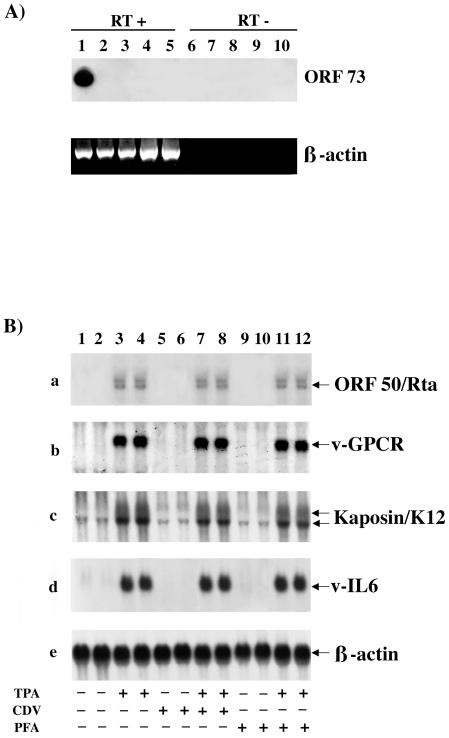
In our previous experiments, although clearly reduced, some of the viral gene transcripts were still detectable after TPA induction with MTX treatment. Therefore it was essential to determine whether BC-3 cells TPA induced in the presence of MTX could produce infectious virions. To address this issue, SV40-BMEC were infected with supernatants obtained from uninduced, TPA-induced, and TPA-induced in the presence of 1 μM MTX BC-3 cells. SV40-BMEC overlaid with supernatant from BJAB cells was used as a negative control; 4 μg of Polybrene (Sigma) was added to each sample to enhance the infection (19). After 4 days, RNA was extracted, and reverse transcription (RT)-PCR was performed with ORF73-specific primers. Figure 5A shows that viral transcripts could be amplified only in cells infected with supernatant obtained from BC-3 cells TPA induced without MTX (lane 1). No viral transcripts were detected in cells infected with supernatant obtained from BC-3 cells uninduced or TPA induced in the presence of MTX (lanes 2 and 3).
FIG. 5.
(A) RT-PCR of RNA from SV40-BMEC infected with supernatants of BC-3 cells, uninduced, TPA induced, and TPA induced in the presence of 1 μM MTX. Polybrene was used in all samples. Primers specific for ORF73 were used, and amplified fragments were hybridized with an internal probe. Lane 1, SV40-BMEC infected with supernatant of TPA induced BC-3; lane 2, SV40-BMEC infected with supernatant of uninduced BC-3; lane 3, SV40-BMEC infected with supernatant of TPA induced BC-3 in the presence of MTX; lane 4, SV40-BMEC infected with supernatant of BJAB; lane 5, SV40-BMEC without Polybrene. Lanes 6 to 10, same samples as in lanes 1 to 5, respectively, without RT. β-Actin was used as an internal control for cDNA synthesis. (B) Northern blot analysis of RNA extracted from uninduced and TPA induced BC-3 cells after 48 h of treatment with cidofovir (CDV) and foscarnet (PFA) and hybridized with ORF50/Rta, viral GPCR, K12/kaposin, viral IL-6, and human β-actin probes. Samples were loaded in duplicate. Lanes 1 and 2, uninduced; lanes 3 and 4, TPA induced; lanes 5 and 6, BC-3 uninduced and treated with 0.5 μM cidofovir; lane 7 and 8, TPA induced in the presence of 0.5 μM cidofovir; lanes 9 and 10, uninduced and treated with 0.5 mM foscarnet; lanes 11 and 12, TPA induced in the presence of 0.5 mM foscarnet.
Cidofovir and foscarnet have no effect on ORF50/Rta, viral IL-6, viral GPCR, and K12/kaposin gene transcription.
None of the currently available antiherpesvirus drugs has been reported to inhibit KSHV immediate-early lytic and transforming genes. Therefore we examined their effect on ORF50/Rta, viral GPCR, K12/kaposin, and viral IL-6 gene transcription in uninduced and TPA-induced BC-3 cells treated for 48 h with cidofovir or foscarnet, two well-known antiviral drugs which have already been shown to inhibit viral genomic replication and expression of KSHV late lytic genes (21, 27, 34, 40). Northern blots were performed to analyze the levels of ORF50/Rta, viral GPCR, K12/kaposin, and viral IL-6 transcripts (Fig. 5B). Since ORF50/Rta, viral GPCR, and viral IL-6 transcripts are not detectable in the absence of TPA, the activity of cidofovir and foscarnet could not be assessed in uninduced cells (Fig. 5B, a, b, and d, BC-3, lanes 1 and 2; BC-3 treated with cidofovir, lanes 5 and 6; BC-3 treated with foscarnet, lanes 9 and 10).
Although K12/kaposin is TPA inducible, low levels of transcripts are visible without induction. However, no difference in transcript levels of K12/kaposin (Fig. 5B, c) was detected among BC-3 cells (Fig. 5B, lanes 1 and 2) and BC-3 cells treated with cidofovir (Fig. 5B, lanes 5 and 6) or foscarnet (Fig. 5B, lanes 9 and 10). High levels of ORF50/Rta, viral GPCR, K12/kaposin, and viral IL-6 transcripts were observed after TPA induction, yet no difference was detected among TPA-induced BC-3 cells (Fig. 5B, all panels, lanes 3 and 4) and TPA-induced cells treated with cidofovir (Fig. 5B, all panels, lanes 7 and 8) or foscarnet (Fig. 5B, all panels, lanes 11 and 12).
Human cytokine production.
It has been demonstrated that inflammatory cytokines can modulate KSHV replication (12). Since MTX promotes adenosine release in the inflamed sites (5) and adenosine diminishes TNF-α and other inflammatory cytokine production (7, 44, 49), we analyzed the cytokine expression pattern of TPA-induced BC-3 cells treated with different doses of MTX. The cells treated with MTX showed a dose-dependent decrease in TNF-α production, from 2-fold with 1 nM MTX to 14-fold with 1 μM MTX compared to the untreated controls (Table 1). In contrast, the levels of IL-10, IL-1β, and IL-6 were not significantly different in untreated and treated cells. The observed MTX-induced reduction in levels of TNF-α supports the hypothesis that adenosine could mediate this effect. Interestingly, in TPA-induced BC-3 cells, either treated or untreated with MTX, IL-8 production was markedly increased, presumably due to the TPA treatment.
TABLE 1.
Production of human cytokines by TPA-induced BC-3 cells treated with different doses of MTX
| MTX dose (nM) | TPA | Cytokine concn (pg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| IL-10 | IL-12 | IL-1β | IL-6 | IL-8 | TNF-α | ||
| 0 | − | 210 | 3.1 | 0 | 238 | 2.1 | 43.6 |
| 0 | + | 207 | 0 | 0 | 247 | 27.1 | 42.8 |
| 1 | + | 206 | 0 | 0 | 247 | 26.1 | 19.4 |
| 5 | + | 207 | 0 | 0 | 244 | 32.8 | 13.2 |
| 10 | + | 207 | 0 | 0 | 247 | 25.6 | 10.2 |
| 50 | + | 207 | 0 | 0 | 245 | 37.4 | 14.7 |
| 100 | + | 205 | 0 | 0 | 249 | 31.1 | 9.20 |
| 500 | + | 205 | 0 | 0 | 252 | 28.8 | 13.4 |
| 1,000 | + | 203 | 0.95 | 0 | 247 | 22.7 | 3.10 |
DISCUSSION
In this study, we report a novel effect of MTX on KSHV replication, although exactly how MTX mediates its effect at the cellular level is not fully understood. We found that 1 μM MTX totally prevents TPA induction of viral DNA replication and inhibits TPA induction of transcription and expression of lytic genes, while it does not interfere with cellular gene transcription. In fact, transcript levels of c-fos, β-actin, and cellular DHFR were identical in both MTX-treated and untreated cells. In contrast, transcription of strictly latent viral genes is not affected by the treatment.
KSHV encodes an immediate-early gene, ORF50/Rta, and its expression is required to reactivate and drive the viral lytic cycle to completion (25, 32, 33, 57). Moreover, it has been demonstrated that viral GPCR promoter activity is dependent on the immediate-early transactivator ORF50/Rta (26). We show that in BC-3 cells treated with TPA in the presence of MTX, MCP (late lytic gene, class III) transcripts were not TPA inducible, since their levels were similar to those of the untreated control cells, while viral DHFR, ORF50/Rta, K12/kaposin, viral IL-6, viral GPCR, and viral MIP II (immediate-early and early lytic genes, class II) transcripts were expressed at lower levels compared to the TPA-induced cells in the absence of MTX.
KSHV early lytic genes (class II) are present at very low levels in uninduced cells; they can also autoregulate their own expression (20, 50) or be regulated by latent genes (i.e., LANA upregulates viral IL-6 [1]); most likely our data could reflect these events. Although some early lytic transcripts are detectable after MTX treatment, under the same conditions late structural transcripts are not present, and the virions produced in the presence of MTX are not infectious (Fig. 5A). Furthermore, in cells treated simultaneously with TPA and MTX, no induction of viral IL-6 and K12/kaposin expression was detected by immunofluorescence assay and Western blot (Fig. 4A and B). Similar results have been obtained in MTX-R-BC-3 cells, although the degree of inhibition was less impressive then in the sensitive parental BC-3 cells. We could not evaluate the expression of viral GPCR after MTX treatment because no satisfactory antibodies are available.
In view of the kinetics of transcription of these genes, ORF50/Rta is induced within 4 h of the chemical induction, its message is the first to appear, and it is resistant to cycloheximide, an inhibitor of protein synthesis, and to phosphonoacetic acid, an inhibitor of herpesvirus DNA polymerase. Viral MIP II and viral IL-6 transcripts appear 8 to 13 h after induction, and viral DHFR, K12/kaposin, and viral GPCR are slightly delayed, and the expression of all of them is inhibited by cycloheximide but resistant to phosphonoacetic acid (58). ORF50/Rta is a transcriptional activator of viral lytic gene expression, and its message is the first to appear after TPA induction. Thus, if expression of ORF50/Rta is blocked, transcription of all other lytic genes downstream of ORF50/Rta is not activated, and the entire viral lytic cascade cannot initiate. In fact, we show that as transcription of ORF50/Rta takes place within 24 h of chemical induction, MTX was much less effective when added 24 after TPA induction. These results suggest that the mechanism of MTX against KSHV differs from that well defined as competitive inhibitor of DHFR.
Cinquina et al. (16) have shown that among several DHFR inhibitors, including MTX, KSHV DHFR was less sensitive then cellular DHFR, and neither of them proved to be a stoichiometric inhibitor with selective activity against KSHV DHFR. They showed that the same amount of inhibitor (aminopterin) that blocked the enzymatic activity in vitro and cell proliferation did not block KSHV DNA replication and late gene expression, suggesting that viral DHFR activity is not essential for viral replication (16). Moreover, it has been shown by other studies that expression of early lytic genes does not depend on viral DNA replication (58). In fact, in TPA-induced cells, ORF50/Rta transcription occurs before viral DHFR transcription; therefore, the inhibition of ORF50/Rta by MTX cannot be secondary to the inhibition of viral DHFR. Additionally, MTX inhibits the switch from latency to TPA-induced lytic cycle in cells resistant to MTX, confirming that the block of lytic cycle induction is not related to inhibition of DNA replication.
Although cultured cells become resistant to MTX by at least four biochemical mechanisms, overproduction of cellular DHFR is the most common. In contrast to this, our results show that in MTX-R-BC-3 cells viral DHFR is not overexpressed even though a modest TPA induction of some viral early lytic genes is detectable after long-term treatment with MTX. This supports our hypothesis that ORF50/Rta transcription and consequent induction of the viral lytic cycle are repressed by MTX with a mechanism that is likely to differ from its previous well defined inhibition of cellular DHFR activity.
Several pathways that transmit signals from the surface of the cell to the transcriptional machinery control cellular transcriptional regulators. We could speculate that MTX might inactivate one of these pathways by inhibiting one or more components of the basal transcriptional machinery; thus, ORF50/Rta transcription cannot be activated and as a consequence, the signal transduction cascade that induces entry of KSHV into lytic cycle is blocked.
Another mechanism of action for MTX could be its capacity to promote an increase in adenosine release (5, 17, 18). Adenosine inhibits the generation of chemoattractants and inflammatory cytokines such as IL-6, TNF-α, IL-8, and IL-1 (7, 44, 49). Their presence stimulates the production of basic fibroblast growth factor and VEGF, which cooperate to induce angiogenesis and Kaposi's sarcoma lesion formation (51, 52). Therefore, suppression of cytokine signaling might interfere with the switch mechanism from latency to lytic cycle, since the disease caused by KSHV may be associated with the reactivation of latent virus. However, various doses of adenosine, ranging from 100 nM to 100 μM, and TNF-α, ranging from 15 to 50 ng/ml, did not have a direct effect on viral reactivation (data not shown).
Previous studies indicate that, although most spindle cells in Kaposi's sarcoma tumors are latently infected, a small subset of cells support lytic viral replication (46, 56). Latent genes, particularly LANA, which interacts with both p53 and Rb pathways (22, 45), most likely are the major factors in cellular transformation. However, increasing evidence suggests that lytic gene expression may also play a role in tumorigenesis. Thus, the switch of the virus from latency to lytic replication appears to be important not only for viral propagation but also for viral pathogenesis.
The target of all antiherpesvirus drugs is the lytic rather than the latent cycle, and among them, foscarnet and cidofovir have the best activity against KSHV (27, 37). However, we show that foscarnet and cidofovir do not have any effect on ORF50/Rta and other early lytic gene transcription. This result is not unexpected since foscarnet and cidofovir act as inhibitors of the viral DNA polymerase, and in the cascade of viral gene expression, early lytic genes are transcribed before viral DNA replication. Among these early lytic genes, viral IL-6, viral GPCR, and K12/kaposin may be particularly significant for cellular transformation and viral pathogenesis, since in previous reports they have been demonstrated to be transforming in mouse fibroblast assays and oncogenic in mice (2, 3, 6, 38). Furthermore, recently it has been shown that viral GPCR induces angioproliferative lesions in animals (61) and viral GPCR transgenic mice develop Kaposi's sarcoma-like lesions (9) and that viral IL-6 can stimulate the human IL-6-induced signaling pathways, activates secretion of VEGF, and mimics many of the activities of human IL-6 (2, 43).
To date, MTX is the only drug that can downregulate KSHV oncogenes and all other early lytic gene expression by downregulating the transcription of ORF50/Rta. Given that the expression of these early genes may be necessary for viral propagation and tumor development, MTX could play a role in the future management of KSHV-associated malignancies.
Experiments designed to further assess the mechanism of MTX in downregulation of ORF50/Rta transcription and to determine whether the residual viral products after MTX treatment could still sustain angiogenesis are under way.
Acknowledgments
We thank Claudio Basilico and Jan T. Vilcek for helpful discussions and Michael Bouchard for critical reading of the manuscript. We are grateful to Bala Chandran for the generous gift of the antibody against K12/kaposin.
This work was supported by the Howard Gilman Foundation, by the NYU Center for AIDS Research (CFAR), and by a postdoctoral fellowship from FIRC (Federazione Italiana per la Ricerca sul Cancro) to Francesca Curreli.
REFERENCES
- 1.An, J., A. K. Lichtenstein, G. Brent, and M. B. Rettig. 2002. The Kaposi sarcoma-associated herpesvirus (KSHV) induces cellular interleukin 6 expression: role of the KSHV latency-associated nuclear antigen and the AP1 response element. Blood 99:649-654. [DOI] [PubMed] [Google Scholar]
- 2.Aoki, Y., E. S. Jaffe, Y. Chang, K. Jones, J. Teruya-Feldstein, P. S. Moore, and G. Tosato. 1999. Angiogenesis and hematopoiesis induced by Kaposi's sarcoma-associated herpesvirus-encoded interleukin-6. Blood 93:4034-4043. [PubMed] [Google Scholar]
- 3.Arvanitakis, L., E. Geras-Raaka, A. Varma, M. C. Gershengorn, and E. Cesarman. 1997. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385:347-350. [DOI] [PubMed] [Google Scholar]
- 4.Arvanitakis, L., E. A. Mesri, R. G. Nador, J. W. Said, A. S. Asch, D. M. Knowles, and E. Cesarman. 1996. Establishment and characterization of a primary effusion (body cavity-based) lymphoma cell line (BC-3) harboring Kaposi's sarcoma-associated herpesvirus (KSHV/HHV-8) in the absence of Epstein-Barr virus. Blood 88:2648-2654. [PubMed] [Google Scholar]
- 5.Asako, H., R. E. Wolf, and D. N. Granger. 1993. Leukocyte adherence in rat mesenteric venules: effects of adenosine and methotrexate. Gastroenterology 104:31-37. [DOI] [PubMed] [Google Scholar]
- 6.Bais, C., B. Santomasso, O. Coso, L. Arvanitakis, E. G. Raaka, J. S. Gutkind, A. S. Asch, E. Cesarman, M. C. Gerhengorn, and E. A. Mesri. 1998. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature 391:86-89. [DOI] [PubMed] [Google Scholar]
- 7.Bouma, M. G., R. K. Stad, F. A. van den Wildenberg, and W. A. Buurman. 1994. Differential regulatory effects of adenosine on cytokine release by activated human monocytes. J. Immunol. 153:4159-4168. [PubMed] [Google Scholar]
- 8.Bright, R. D. 1999. Methotrexate in the treatment of psoriasis. Cutis 64:332-334. [PubMed] [Google Scholar]
- 9.Cesarman, E., E. A. Mesri, and M. C. Gershengorn. 2000. Viral G protein-coupled receptor and Kaposi's sarcoma: a model of paracrine neoplasia? J. Exp. Med. 191:417-422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cesarman, E., P. S. Moore, P. H. Rao, G. Inghirami, D. M. Knowles, and Y. Chang. 1995. In vitro establishment and characterization of two acquired immunodeficiency syndrome-related lymphoma cell lines (BC-1 and BC-2) containing Kaposi's sarcoma-associated herpesvirus-like (KSHV) DNA sequences. Blood 86:2708-2714. [PubMed] [Google Scholar]
- 11.Cesarman, E., R. G. Nador, F. Bai, R. A. Bohenzky, J. J. Russo, P. S. Moore, Y. Chang, and D. M. Knowles. 1996. Kaposi's sarcoma associated herpesvirus (KSHV/HHV-8) contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J. Virol. 70:8218-8223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chang, J., R. Renne, D. Dittmer, and D. Ganem. 2000. Inflammatory cytokines and the reactivation of Kaposi's sarcoma-associated herpesvirus lytic replication. Virology 266:17-25. [DOI] [PubMed] [Google Scholar]
- 13.Chang, Y., E. Cesarman, M. S. Pessin, F. Lee, J. Culpepper, D. M. Knowles, and P. S. Moore. 1994. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science 266:1865-1869. [DOI] [PubMed] [Google Scholar]
- 14.Chang, Y., P. S. Moore, S. J. Talbot, C. H. Boshoff, T. Zarkowska, D. Godden-Kent, H. Paterson, R. A. Weiss, and S. Mittnacht. 1996. Cyclin encoded by KS herpesvirus. Nature 382:410. [DOI] [PubMed] [Google Scholar]
- 15.Cheng, E. H. Y., J. Nicholas, D. S. Bellows, G. S. Hayward, H. G. Guo, M. S. Reitz, and J. M. Hardwick. 1997. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc. Natl. Acad. Sci. USA 94:690-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cinquina, C. C., E. Grogan, R. Sun, S. F. Lin, G. P. Beardsley, and G. Miller. 2000. Dihydrofolate reductase from Kaposi's sarcoma-associated herpesvirus. Virology 268:201-217. [DOI] [PubMed] [Google Scholar]
- 17.Cronstein, B. N., M. A. Eberle, H. E. Gruber, and R. I. Levin. 1991. Methotrexate inhibits neutrophil function by stimulating adenosine release from connective tissue cells. Proc. Natl. Acad. Sci. USA 88:2441-2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cronstein, B. N., D. Naime, and E. Ostad. 1993. The antiinflammatory mechanism of methotrexate. Increased adenosine release at inflamed sites diminishes leukocyte accumulation in an in vivo model of inflammation. J. Clin. Investig. 92:2675-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Damico, R., and P. Bates. 2000. Soluble receptor-induced retroviral infection of receptor-deficient cells. J. Virol. 74:6469-6475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Deng, H., A. Young, and R. Sun. 2000. Auto-activation of the rta gene of human herpesvirus-8/Kaposi's sarcoma-associated herpesvirus. J. Gen Virol. 81:3043-3048. [DOI] [PubMed] [Google Scholar]
- 21.Flore, O., and S. J. Gao. 1997. Effect of DNA synthesis inhibitors on Kaposi's sarcoma-associated herpesvirus cyclin and major capsid protein gene expression. AIDS Res. Hum. Retroviruses 13:1229-1233. [DOI] [PubMed] [Google Scholar]
- 22.Friborg, J., Jr., W. Kong, M. O. Hottiger, and G. J. Nabel. 1999. p53 inhibition by the LANA protein of KSHV protects against cell death. Nature 402:889-894. [DOI] [PubMed] [Google Scholar]
- 23.Furst, D. E., and J. M. Kremer. 1988. Methotrexate in rheumatoid arthritis. Arthritis Rheum. 31:305-314. [DOI] [PubMed] [Google Scholar]
- 24.Gao, S. J., C. Boshoff, S. Jayachandra, R. A. Weiss, Y. Chang, and P. S. Moore. 1997. KSHV ORF K9 (vIRF) is an oncogene which inhibits the interferon signaling pathway. Oncogene 15:1979-1985. [DOI] [PubMed] [Google Scholar]
- 25.Gradoville, L., J. Gerlach, E. Grogan, D. Shedd, S. Nikiforow, C. Metroka, and G. Miller. 2000. Kaposi's sarcoma-associated herpesvirus open reading frame 50/Rta protein activates the entire viral lytic cycle in the HH-B2 primary effusion lymphoma cell line. J. Virol. 74:6207-6212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeong, J., J. Papin, and D. Dittmer. 2001. Differential regulation of the overlapping Kaposi's sarcoma-associated herpesvirus vGCR (orf74) and LANA (orf73) promoters. J. Virol. 75:1798-1807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kedes, D. H., and D. Ganem. 1997. Sensitivity of Kaposi's sarcoma-associated herpesvirus replication to antiviral drugs—implications for potential therapy. J. Clin. Investig. 99:2082-2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kedes, D. H., E. Operskalski, M. Busch, R. Kohn, J. Flood, and D. Ganem. 1996. The seroepidemiology of human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus): distribution of infection in KS risk groups and evidence for sexual transmission. Nat. Med. 2:918-924. [DOI] [PubMed] [Google Scholar]
- 29.Knowles, D. M., and E. Cesarman. 1997. The Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) in Kaposi's sarcoma, malignant lymphoma, and other diseases. Ann. Oncol. 8:123-129. [PubMed] [Google Scholar]
- 30.Lembo, D., A. Angeretti, M. Gariglio, and S. Landolfo. 1998. Murine cytomegalovirus induces expression and enzyme activity of cellular dihydrofolate reductase in quiescent cells. J. Gen. Virol. 79:2803-2807. [DOI] [PubMed] [Google Scholar]
- 31.Lembo, D., R. Cavallo, M. Cornaglia, A. Mondo, L. Hertel, A. Angeretti, and S. Landolfo. 1999. Overexpression of cellular dihydrofolate reductase abolishes the anticytomegaloviral activity of methotrexate. Arch. Virol. 144:1397-1403. [DOI] [PubMed] [Google Scholar]
- 32.Lukac, D. M., J. R. Kirshner, and D. Ganem. 1999. Transcriptional activation by the product of open reading frame 50 of Kaposi's sarcoma-associated herpesvirus is required for lytic viral reactivation in B cells. J. Virol. 73:9348-9361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lukac, D. M., R. Renne, J. R. Kirshner, and D. Ganem. 1998. Reactivation of Kaposi's sarcoma-associated herpesvirus infection from latency by expression of the ORF50 transactivator, a homolog of the EBV R protein. Virology 252:304-312. [DOI] [PubMed] [Google Scholar]
- 34.Medveczky, M. M., E. Horvath, T. Lund, and P. G. Medveczky. 1997. In vitro antiviral drug sensitivity of the Kaposi's sarcoma-associated herpesvirus. AIDS 11:1327-1332. [DOI] [PubMed] [Google Scholar]
- 35.Menezes, J., W. Leibold, G. Klein, and G. Clements. 1975. Establishment and characterization of an Epstein-Barr virus (EBV)-negative lymphoblastoid B cell line (BJAB) from an exceptional EBV-genome negative African Burkitt's lymphoma. Biomedicine 22:276-280. [PubMed] [Google Scholar]
- 36.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 37.Morfeldt, L., and J. Torssander. 1994. Long-term remission of Kaposi's sarcoma following foscarnet treatment in HIV-infected patients. Scand. J. Infect. Dis. 26:749-752. [DOI] [PubMed] [Google Scholar]
- 38.Muralidhar, S., A. M. Pumfery, M. Hassani, M. R. Sadaie, N. Azumi, M. Kishishita, J. N. Brady, J. Doniger, P. Medveczky, and L. J. Rosenthal. 1998. Identification of kaposin (open reading frame k12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene. J. Virol. 72:4980-4988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muralidhar, S., G. Veytsmann, B. Chandran, D. Ablashi, J. Doniger, and L. J. Rosenthal. 2000. Characterization of the human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) oncogene, kaposin (ORF K12). J. Clin. Virol. 16:203-213. [DOI] [PubMed] [Google Scholar]
- 40.Neyts, J., and E. De Clercq. 1997. Antiviral drug susceptibility of human herpesvirus 8. Antimicrob. Agents Chemother. 41:2754-2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nicholas, J., V. R. Ruvolo, W. H. Burns, G. Sandford, X. Y. Wan, D. Ciufo, S. B. Hendrickson, H. G. Guo, G. S. Hayward, and M. S. Reitz. 1997. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat. Med. 3:287-292. [DOI] [PubMed] [Google Scholar]
- 42.Nicholas, J., J. C. Zong, D. J. Alcendor, D. M. Ciufo, L. J. Poole, R. T. Sarisky, C. J. Chiou, X. Zhang, X. Wan, H. G. Guo, M. S. Reitz, and G. S. Hayward. 1998. Novel organizational features, captured cellular genes, and strain variability within the genome of KSHV. J. Natl. Cancer Inst. Monogr. 23:79-88. [DOI] [PubMed] [Google Scholar]
- 43.Osborne, J., P. S. Moore, and Y. Chang. 1999. KSHV-encoded viral IL-6 activates multiple human IL-6 signaling pathways. Hum. Immunol. 60:921-927. [DOI] [PubMed] [Google Scholar]
- 44.Parmely, M. J., W. W. Zhou, C. K. Edwards, D. R. Borcherding, R. Silverstein, and D. C. Morrison. 1993. Adenosine and a related carbocyclic nucleoside analogue selectively inhibit tumor necrosis factor-alpha production and protect mice against endotoxin challenge. J. Immunol. 151:389-396. [PubMed] [Google Scholar]
- 45.Radkov, S. A., P. Kellam, and C. Boshoff. 2000. The latent nuclear antigen of Kaposi sarcoma-associated herpesvirus targets the retinoblastoma-E2F pathway and with the oncogene Hras transforms primary rat cells. Nat. Med. 6:1121-1127. [DOI] [PubMed] [Google Scholar]
- 46.Renne, R., D. Blackbourn, D. Whitby, J. Levy, and D. Ganem. 1998. Limited transmission of Kaposi's sarcoma-associated herpesvirus in cultured cells. J. Virol. 72:5182-5188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Renne, R., W. Zhong, B. Herndier, M. McGrath, N. Abbey, D. Kedes, and D. Ganem. 1996. Lytic growth of Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in culture. Nat. Med. 2:342-346. [DOI] [PubMed] [Google Scholar]
- 48.Sadler, R., L. Wu, B. Forghani, R. Renne, W. Zhong, B. Herndier, and D. Ganem. 1999. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J. Virol. 73:5722-5730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sajjadi, F. G., K. Takabayashi, A. C. Foster, R. C. Domingo, and G. S. Firestein. 1996. Inhibition of TNF-alpha expression by adenosine: role of A3 adenosine receptors. J. Immunol. 156:3435-3442. [PubMed] [Google Scholar]
- 50.Sakakibara, S., K. Ueda, J. Chen, T. Okuno, and K. Yamanishi. 2001. Octamer-binding sequence is a key element for the autoregulation of Kaposi's sarcoma-associated herpesvirus ORF50/Lyta gene expression. J. Virol. 75:6894-6900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Samaniego, F., P. D. Markham, R. Gendelman, R. C. Gallo, and B. Ensoli. 1997. Inflammatory cytokines induce endothelial cells to produce and release basic fibroblast growth factor and to promote Kaposi's sarcoma-like lesions in nude mice. J. Immunol. 158:1887-1894. [PubMed] [Google Scholar]
- 52.Samaniego, F., P. D. Markham, R. Gendelman, Y. Watanabe, V. Kao, K. Kowalski, J. A. Sonnabend, A. Pintus, R. C. Gallo, and B. Ensoli. 1998. Vascular endothelial growth factor and basic fibroblast growth factor present in Kaposi's sarcoma (KS) are induced by inflammatory cytokines and synergize to promote vascular permeability and KS lesion development. Am. J. Pathol. 152:1433-1443. [PMC free article] [PubMed] [Google Scholar]
- 53.Sambrook, J., E. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 54.Sarid, R., O. Flore, R. A. Bohenzky, Y. Chang, and P. S. Moore. 1998. Transcription mapping of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) genome in a body cavity-based lymphoma cell line (BC-1). J. Virol. 72:1005-1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Shepherd, F. A., E. Maher, C. Cardella, E. Cole, P. Greig, J. A. Wade, and G. Levy. 1997. Treatment of Kaposi's sarcoma after solid organ transplantation. J. Clin. Oncol. 15:2371-2377. [DOI] [PubMed] [Google Scholar]
- 56.Staskus, K. A., W. D. Zhong, K. Gebhard, B. Herndier, H. Wang, R. Renne, J. Beneke, J. Pudney, D. J. Anderson, D. Ganem, and A. T. Hasse. 1997. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J. Virol. 71:715-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sun, R., S. F. Lin, L. Gradoville, Y. Yuan, F. Zhu, and G. Miller. 1998. A viral gene that activates lytic cycle expression of Kaposi's sarcoma-associated herpesvirus. Proc. Natl. Acad. Sci. USA 95:10866-10871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun, R., S. F. Lin, K. Staskus, L. Gradoville, E. Grogan, A. Haase, and G. Miller. 1999. Kinetics of Kaposi's sarcoma-associated herpesvirus gene expression. J. Virol. 73:2232-2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Thome, M., P. Schneider, K. Hofmann, H. Fickenscher, E. Meinl, F. Neipel, C. Mattmann, K. Burns, J. L. Bodmer, M. Schroter, C. Scaffidi, P. H. Krammer, M. E. Peter, and J. Tschopp. 1997. Viral FLICE-inhibitory proteins (FLIPs) prevent apoptosis induced by death receptors. Nature 386:517-521. [DOI] [PubMed] [Google Scholar]
- 60.Weinblatt, M. E. 1995. Methotrexate for chronic diseases in adults. N. Engl. J. Med. 332:330-331. [DOI] [PubMed] [Google Scholar]
- 61.Yang, T. Y., S. C. Chen, M. W. Leach, D. Manfra, B. Homey, M. Wiekowski, L. Sullivan, C. H. Jenh, S. K. Narula, S. W. Chensue, and S. A. Lira. 2000. Transgenic expression of the chemokine receptor encoded by human herpesvirus 8 induces an angioproliferative disease resembling Kaposi's sarcoma. J. Exp. Med. 191:445-454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zoeteweij, J. P., S. T. Eyes, J. M. Orenstein, T. Kawamura, L. Wu, B. Chandran, B. Forghani, and A. Blauvelt. 1999. Identification and rapid quantification of early- and late-lytic human herpesvirus 8 infection in single cells by flow cytometric analysis: characterization of antiherpesvirus agents. J. Virol. 73:5894-5902. [DOI] [PMC free article] [PubMed] [Google Scholar]