Abstract
Patients infected with hepatitis C virus (HCV) have an impaired response against HCV antigens while keeping immune competence for other antigens. We hypothesized that expression of HCV proteins in infected dendritic cells (DC) might impair their antigen-presenting function, leading to a defective anti-HCV T-cell immunity. To test this hypothesis, DC from normal donors were transduced with an adenovirus coding for HCV core and E1 proteins and these cells (DC-CE1) were used to stimulate T lymphocytes. DC-CE1 were poor stimulators of allogeneic reactions and of autologous primary and secondary proliferative responses. Autologous T cells stimulated with DC-CE1 exhibited a pattern of incomplete activation characterized by enhanced CD25 expression but reduced interleukin 2 production. The same pattern of incomplete lymphocyte activation was observed in CD4+ T cells responding to HCV core in patients with chronic HCV infection. However, CD4+ response to HCV core was normal in patients who cleared HCV after alpha interferon therapy. Moreover, a normal CD4+ response to tetanus toxoid was found in both chronic HCV carriers and patients who had eliminated the infection. Our results suggest that expression of HCV structural antigens in infected DC disturbs their antigen-presenting function, leading to incomplete activation of anti-HCV-specific T cells and chronicity of infection. However, presentation of unrelated antigens by noninfected DC would allow normal T-cell immunity to other pathogens.
Hepatitis C virus (HCV) is a single-stranded RNA virus belonging to the Flaviviridae family (25). Although some patients exhibit acute self-limited infection, a characteristic feature of HCV is the high incidence of persistent infection and chronic hepatitis, with a strong risk for the development of hepatocellular carcinoma (10). This high incidence of chronicity suggests that the virus has developed efficient mechanisms to escape host immune responses. Indeed, although most patients have anti-HCV antibodies (38), cellular immune responses are weak in chronically infected patients. It has been reported that CD4+ T-cell responses against viral antigens are vigorous in individuals who have cleared HCV after acute infection or after treatment with alpha interferon (IFN-α) (11, 21, 26, 35). By contrast, patients who fail to respond to therapy exhibit poor T-cell reactivity against viral proteins. Regarding CD8+ lymphocytes, low responses have been detected in infected patients (7, 32, 37), and recent reports show that these cells play a critical role during the acute phase of the disease (8). Thus, chronicity of HCV infection is related to the inability of HCV-specific CD4+ and CD8+ T cells to accomplish efficient effector functions. These defects, however, are not the result of general immunosuppression since T-cell immunity to other pathogens is well preserved in patients with chronic HCV infection.
HCV may evade immune surveillance by different mechanisms including mutations in regions that are targets for the immune system (13, 40, 41) and infection of the cells involved in the induction of the immune response (27, 39). Among antigen-presenting cells (APC), dendritic cells (DC) are characterized by potent immunostimulatory properties for both primary and secondary T-cell responses (4). These cells capture and process antigens, migrate to lymphoid tissues, express costimulatory molecules, and produce cytokines and chemokines which activate and attract T cells. It has been reported that APC (B cells, monocytes) are sites of active HCV replication (23, 28). Moreover, it has been shown that HCV can infect monocyte-derived DC (3). Some viruses have been shown to infect DC and interfere with their immunostimulatory functions as a result of interaction of viral proteins with molecules implicated in intracellular processes (12, 15, 34). Due to the ability of HCV to infect DC, it is conceivable that HCV antigens are presented to CD4+ lymphocytes by HCV-infected DC. If HCV proteins affect DC function, this would result in abnormal priming of anti-HCV-specific T cells and defective antiviral immunity favoring chronicity of the infection.
It has been recently reported that HCV structural proteins were able to reduce the stimulatory activity of murine DC in mixed leukocyte reactions (MLR) (17) and that DC from HCV-infected patients exhibit impaired allostimulatory ability (3, 18). It is not known, however, whether HCV structural proteins might affect the ability of DC to induce primary and secondary autologous T-cell responses and whether abnormal priming of HCV-specific T lymphocytes occurs in patients with chronic HCV infection. In the present work we show that DC transduced with an adenovirus coding for HCV core and E1 proteins (DC-CE1) exhibit altered antigen-presenting function for all class II-presented antigens, leading to abnormal priming and incomplete activation of CD4+ T cells. The pattern of incomplete activation of CD4+ T cells stimulated by DC-CE1 is identical to that seen in anti-HCV core-specific CD4+ T cells from patients with chronic hepatitis C. DC from HCV chronic carriers are, however, capable of inducing normal CD4+ T-cell responses against recall antigens such as tetanus toxoid. Our findings indicate that expression of viral proteins inside HCV-infected DC makes these cells unable to stimulate anti-HCV T-cell responses, thus facilitating chronicity of the infection.
MATERIALS AND METHODS
Antigens.
Peptides PADRE (1) and p45 (F. Borrás-Cuesta, unpublished data) are able to bind to several HLA-DR molecules. They were synthesized manually in a multiple-peptide synthesizer using 9-fluorenylmethoxy carbonyl (Fmoc) chemistry. The ninhydrin test of Kaiser was used to monitor every step. At the end of the synthesis they were cleaved and deprotected with trifluoroacetic acid and washed with diethyl ether. Purity of the peptides was always above 90%. HCV core protein (genotype 1b) expressed in baculovirus was a kindly gift of B. Rodgers (Murex, Dartford, England). Tetanus toxoid (TT) was a kindly gift of B. Gander (ETH; Zurich, Switzerland).
Subjects. (i) Healthy donors.
Buffy coats from healthy individuals were obtained from Blood Bank of Navarra.
(ii) Patients.
Twenty-six patients with chronic HCV infection were selected for this study. All of them were anti-HCV positive and had had raised transaminase levels for more than 6 months. They received IFN-α treatment (3 megaunits daily for 2 months and 3 megaunits three times a week for 8 to 10 months) and were classified into the following groups: (a) 10 patients (6 males and 4 females; mean age and standard deviation, 39.0 ± 6.4 years) experienced biochemical and virological responses (normal serum transaminases and negative serum HCV RNA) lasting for at least 6 months after stopping IFN-α therapy (designated hereafter as sustained response patients [SR]), and (b) 16 patients (11 males and 5 females; mean age and standard deviation, 52.5 ± 12.3 years) failed to normalize serum transaminases and remained viremic after IFN-α treatment (designated hereafter as nonresponders [NR]). All patients were monitored for at least 6 months after stopping IFN-α therapy. All NR had chronic hepatitis without cirrhosis. This study was approved by our Institutional Review Board.
Recombinant adenoviruses.
Recombinant adenoviruses RAdCMVCE1, coding for HCV core and E1 proteins, and RAdCMVLacZ, expressing β-galactosidase, have been previously described (5). Viruses were propagated on 293 cells, purified in a CsCl isopicnic banding step, and kept in aliquots at −80°C.
Generation of DC.
DC were generated from buffy coats from normal donors or from fresh heparinized blood from HCV patients. Peripheral blood mononuclear cells (PBMC) were separated by using a Ficoll gradient, and CD14+ cells were enriched by using CD14 Microbeads (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. CD14− cells were frozen to be used as a source of CD4+ T lymphocytes in stimulation experiments. The purity of CD14+ cells was always above 95%, as confirmed by flow cytometry. These cells were plated in six-well plates at 1 × 106 to 2 × 106 cells/ml in RPMI 1640 medium containing l-glutamine (2 mM), gentamicin (10 μg/ml), penicillin (50 U/ml), streptomycin (50 μg/ml), and HEPES (5 mM) supplemented with 10% heat-inactivated human AB serum (complete medium [CM]) plus 1,000 U of granulocyte-macrophage colony-stimulating factor per ml and 1,000 U of interleukin 4 (IL-4) per ml and cultured for 7 days at 37°C and 5% CO2. Cells were refed with fresh medium containing cytokines on days 3 to 4 of culture.
Infection with adenovirus.
DC harvested at day 7 of culture with cytokines were infected with recombinant defective adenoviruses expressing HCV core and E1 genes or control LacZ gene. Cells were resuspended at 107 cells/ml and incubated with adenoviruses at a multiplicity of infection (MOI) of 500. After 1 h, CM was added to dilute the DC to a final concentration of 1 × 106 to 2 × 106 cells/ml. Cells were harvested 24 h later, washed twice, and used for flow cytometry analysis and stimulation of T lymphocytes.
Flow-cytometric analysis of DC.
Analysis of the expression of DC surface molecules was done 24 h after infection with adenovirus. Cells (105/well) were stained at 4°C in phosphate-buffered saline containing 2% fetal calf serum with the following antibodies: anti-CD11c (Ancell, Bayport, Minn.), anti-CD54 (Southern Biotechnology, Birmingham, Ala.), and isotype control. After 30 min, cells were washed and incubated with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse immunoglobulin G (Sigma, Madrid, Spain). FITC-labeled antibodies were used for CD40 (BD-Pharmingen, San Diego, Calif.) and HLA-DR (BD-Pharmingen), and phycoerythrin (PE)-labeled antibodies were used for CD14 (BD-Pharmingen), CD80 (BD-Pharmingen), CD83 (Immunotech, Marseille, France), and CD86 (Cytognos, Salamanca, Spain), together with their corresponding isotype-matching controls. After a washing, surface expression of the different molecules was analyzed by using a FACSCalibur flow cytometer (Becton Dickinson).
Cell viability 4 and 7 days after infection was analyzed by staining DC with annexin V-FITC and propidium iodide in binding buffer (Annexin Apoptosis Detection kit; BD-Pharmingen) at 4°C. Fifteen minutes later, cells were resuspended in binding buffer and analyzed.
MLR.
MLR were carried out with nonadherent cells (2 × 105/well) obtained from normal donors, stimulated in 96-well plates in CM for 5 days with graded numbers of allogeneic DC transduced with different adenoviruses. Cells were pulsed with 0.5 μCi/well of [3H]thymidine for the last 18 h. They were harvested, and radioactivity was measured by a scintillation counter (Topcount; Packard, Meriden, Conn.).
Induction of antigen-specific CD4+ T-cell responses with adenovirus-transduced DC.
CD4+ cells were purified from the CD14− fraction by positive selection using CD4-Dynabeads (Dynal, Oslo, Norway). The purity levels of the cell preparations were always above 90 to 95%, as analyzed by flow cytometry. Triplicate cultures of CD4+ cells (105/well) were stimulated with autologous DC (104/well) transduced with different adenoviruses in 96-well U-bottomed plates in CM in the absence or in the presence of different antigens. Peptides were used at a concentration of 50 μM, and TT was used at 5 limit of flocculation (Lf)/ml. For T-cell proliferation assays, cells were pulsed with 0.5 μCi/well of [3H]thymidine on day 6 and harvested 18 h later. Incorporated radioactivity was measured as described above. In experiments for measurement of IL-2 production, cells were incubated with 10 μg of anti-IL-2 receptor antibody (BT563; Biotest Pharma, Dreieich, Germany) per ml and supernatants were harvested on day 7. IL-2 production was assessed by examining the ability to support the growth of an IL-2-dependent CTLL mouse cell line as previously described (21). Data are reported as stimulation index (SI), which is the ratio of mean response to antigen over mean response to a negative control.
The immune response was also analyzed by determining CD25 or CD69 expression by flow cytometry. Three days after stimulation of CD4+ T cells with DC, cells were double stained with FITC-antiCD4 and PE-antiCD25 or Cy-Chrome-antiCD69 (BD-Pharmingen) antibodies. Lymphocytes were gated, and 104 gated cells were analyzed for the expression of both molecules.
Cellular immune response in patients with chronic HCV infection. (i) IL-2 production.
PBMC were isolated from fresh heparinized blood by Ficoll-Hypaque centrifugation and washed three times with saline. PBMC were resuspended in CM, plated at 3 × 105 cells/well, and cultured in triplicate in 96-well flat-bottomed plates with 10 μg of anti-IL-2 receptor antibody per ml in the presence or absence of HCV core protein (1 μg/ml) or TT (5 Lf/ml). After 7 days of culture, supernatants were harvested and stored at −20°C. IL-2 content was assessed as described above. Data are reported as stimulation index (SI), which is the ratio of mean response to antigen over mean response to a negative control in the absence of antigen.
(ii) Flow cytometric analysis of CD4+ T-cell stimulation.
When CD25 expression was used as a marker of T-cell activation, PBMC (3 × 105 cells/well) were incubated in the presence or absence of HCV core protein (1 μg/ml) or TT (5 Lf/ml) in CM at 37°C and 5% CO2 during 3 days. Then, cells were washed and double stained with FITC-antiCD4 (Sigma) and PE-antiCD25 (Pharmingen) antibodies. After washing, surface expressions of the different molecules were measured. Lymphocytes were gated, and 104 gated cells were analyzed for the expressions of both molecules. The percentage of double-positive cells was determined for each patient by comparison to isotype-matched fluorochrome-tagged negative controls. Results were expressed as the difference between the percentage of CD4+ CD25+ cells in the presence of antigen minus the percentage of CD4+ CD25+ cells in the absence of antigen. The percentage of CD4+ CD25+ cells in the absence of antigen was always below 0.5%.
Isolation of CD4+ CD25+ cells.
PBMC (2 × 107 cells/well) were incubated at 107 cells/ml in six-well plates in the presence of HCV core protein (1 μg/ml) or TT (5 Lf/ml) for 3 days. Then, cells were washed and CD4+ cells were positively selected by using anti-CD4 Dynabeads according to manufacturer instructions. CD4+ cells were incubated with anti-CD25 monoclonal antibodies (Pharmingen) during 30 min at 4°C. After being washed with phosphate-buffered saline, CD25+ cells were positively selected by using anti-immunoglobulin G-Dynabeads (Dynal). CD4+ CD25+ purified cells were lysed by incubation with 100 μl of lysis buffer prior to mRNA isolation.
Differential display analysis of mRNA from CD4+ CD25+ cells.
mRNA from CD4+ CD25+ cells was isolated by using poly(T)-coated magnetic beads (Dynabeads mRNA Direct; Dynal) according to manufacturer instructions. Quantitation of mRNA was carried out by using Ribogreen RNA quantitation kit (Molecular Probes, Eugene, Oreg.). Differential display gene analysis was performed with purified mRNA, using oligo(dT)-anchored primer T7dT12-AP6 and arbitrary primer M13r-ARP4 (Hieroglyph mRNA Profile Kit; Genomix, Beckman Instruments, Fullerton, Calif.) as previously described (24). A nucleotide sequence homology search analysis of the GenBank database was performed with the FASTA program.
PCR for β-glucuronidase expression in CD4+ CD25+ lymphocytes.
CD4+ CD25+ cells obtained after in vitro stimulation of PBMC from different patients in the presence of 1 μg of HCV core protein per ml were lysed for mRNA isolation as described before. mRNA was reverse transcribed as previously described (6). PCR amplifications were carried out using specific primers for β-glucuronidase (upper primer, CTCCGTATGTGGATGTGATC; lower primer, ATCCAGACCCAGATGGTACT, to amplify a 244-bp fragment located between nucleotides 1504 and 1748) or for β-actin (upper primer, TCTACAATGAGCTGCGTGTG; lower primer, GGTGAGGATCTTCATGAGGT, to amplify a 314-bp fragment located between nucleotides 1319 and 2079) as described previously (6). PCR-derived fragments were amplified by 30 cycles (20 s at 94°C, 15 s at 56°C, and 30 s at 72°C) for β-glucuronidase detection and by 25 cycles (20 s at 94°C, 15 s at 55°C, and 30 s at 72°C) for β-actin detection. Amplified cDNA was electrophoresed through 1% agarose, visualized by UV after ethidium bromide staining, and quantified by image analysis using a commercial software (Molecular Analyst/PC; Bio-Rad, Hercules, Calif.). mRNA for β-actin had been previously shown to be constantly expressed in PBMC from chronic HCV patients (6). Results of β-glucuronidase expression for each patient were normalized with those of β-actin and were expressed as the ratio of β-glucuronidase expression to β-actin expression.
RESULTS
Characterization of DC transduced with an adenovirus expressing HCV proteins.
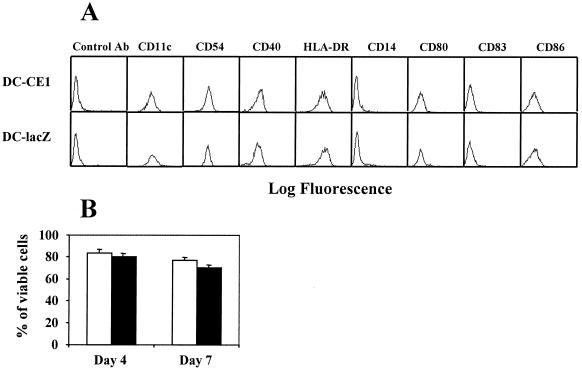
In this work we have tested the hypothesis that infection of DC by HCV might impair their antigen-presenting function, resulting in defective activation of antigen-specific T cells. Since the proportion of infected DC present in PBMC from patients with chronic hepatitis C is thought to be low, we infected monocyte-derived DC from normal donors with defective recombinant adenovirus RAdCMVCE1 (coding for HCV core and E1 proteins) or with control adenovirus RAdCMVLacZ (carrying reporter gene lacZ), to study the effect of HCV proteins on DC antigen-presenting function. With a MOI of 500, more than 70% of DC were infected (as estimated by staining with anti-HCV core antibodies or β-galactosidase; data not shown). Phenotypic analysis of DC 24 h after infection showed that they were negative for CD14, expressed CD11c, CD40, CD54, and HLA-DR, and had low or no expression of CD80, CD83, and CD86 (Fig 1). No differences in the expression of these markers were observed between DC infected with RAdCMVCE1 and those infected with RAdCMVLacZ. The endocytic activities of the two groups of DC were assessed by incubation with FITC-dextran and flow cytometry analysis. These experiments did not reveal differences between DC expressing core and E1 and DC expressing LacZ (data not shown). In order to discard the possibility of a toxic effect in our cultures due to expression of HCV proteins, DC viability was analyzed 4 and 7 days after infection with RAdCMVLacZ or with RAdCMVCE1. Thus, after adenoviral infection, we incubated DC with or without purified T cells and measured DC viability through annexin V and propidium iodide staining. As shown in Fig. 1B, DC viability after infection with RAdCMVLacZ was similar to that observed after infection with RAdCMVCE1. Equivalent results were obtained with DC incubated in the absence of T cells (data not shown).
FIG. 1.
Phenotypic analysis and cell viability of DC infected with recombinant adenovirus expressing HCV core and E1 genes or with control adenovirus. Monocyte-derived DC obtained from normal donors were cultured with cytokines for 7 days and infected with recombinant adenovirus (RAdCMVCE1 [DC-CE1] or RAdCMVLacZ [DC-lacZ]). (A) Twenty-four hours after infection cells were harvested and labeled with antibodies, and surface expressions of the different molecules were analyzed by flow cytometry. (B) RAdCMVCE1-infected DC (open bars) and RAdCMVLacZ-infected DC (filled bars) were cultured in the presence of purified T cells and stained with annexin V and propidium iodide 4 and 7 days after adenoviral infection. Results are expressed as the percentages of cells negative for both markers.
Stimulation of T-cell responses using DC expressing HCV genes: T-cell proliferation and IL-2 production assays.
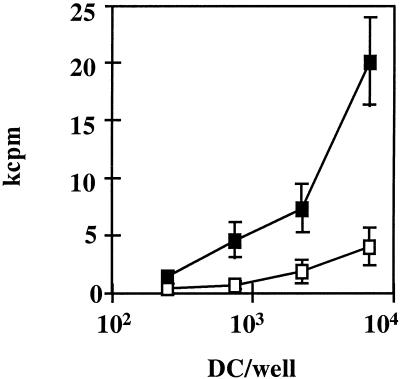
To analyze the stimulatory capacity of DC expressing HCV genes or LacZ, these cells were first used in MLR assays. Thus, lymphocytes from normal donors were stimulated with allogeneic DC infected with RAdCMVCE1 or RAdCMVLacZ, and proliferation was measured 5 days later. As shown in Fig. 2 in a representative case, DC expressing HCV proteins had a lower stimulatory ability than that of DC infected with control adenovirus. Although DC were extensively washed after adenoviral infection, we wished to discard the possibility that an infection of T cells by RAdCMV-CE1, due to some carry over of viral particles, might account for defective T-cell activation. To this aim, purified T cells were incubated with a MOI of 500 of RAdCMVLacZ (the same MOI used for DC infection). Twenty-four hours later, 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) staining revealed that less than 1% of the T cells were infected (data not shown). These data indicate that adenoviral infection of T cells is not the cause of impaired T-cell function after interaction of these cells with DC transduced with RAdCMV-CE1.
FIG. 2.
DC expressing HCV core and E1 genes have a lower allogeneic stimulatory capacity. Monocyte-derived DC obtained from normal donors were infected with RAdCMVCE1 (open squares) or with RAdCMVLacZ (filled squares). Twenty-four hours after infection, graded numbers of DC were incubated in flat-bottomed 96-well plates for 5 days with 105 nonadherent allogeneic cells obtained from a normal donor. [3H]thymidine was added for the last 18 h, and after harvesting, cell proliferation was measured in kilocounts per minute (kcpm). A representative case from four donors tested is shown.
To investigate the effect of HCV proteins on the antigen-presenting function of DC in autologous primary and secondary T-cell responses, purified CD4+ T lymphocytes were stimulated with antigens presented by autologous DC infected with RAdCMVCE1 or RAdCMVLacZ. Two chimeric synthetic peptides (PADRE and p45) known to bind to several HLA-DR molecules were chosen as antigens. Because of their nature, these peptides presumably would activate only naïve CD4+ T-cells. As shown in Fig. 3A and B (showing results from two representative donors), stimulation of CD4+ T cells with RAdCMVLacZ-infected DC presenting the synthetic peptides induces proliferation that is peptide specific. However, when DC expressing HCV core and E1 proteins were used as stimulators, no response against the synthetic peptides was observed.
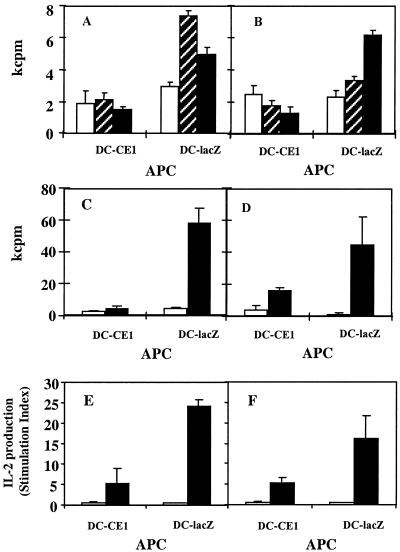
FIG. 3.
DC expressing HCV core and E1 genes have poor stimulatory capacity for autologous primary and recall CD4+ T-cell responses. Monocyte-derived DC obtained from normal donors were infected with RAdCMVCE1 (DC-CE1) or with RAdCMVLacZ (DC-lacZ) and used as APC to stimulate CD4+ cells. DC (104) were incubated for 7 days with purified autologous CD4+ T cells (105) in the absence or in the presence of different antigens; proliferation was measured in kilocounts per minute (kcpm). (A and B) Induction of primary responses: cells were incubated without antigen (open bars) or in the presence of a 50 μM concentration of peptides p45 (hatched bars) or PADRE (filled bars). (C and D) Induction of recall responses: cells were incubated without antigen (open bars) or in the presence of 5 Lf of TT per ml (filled bars). Cells were pulsed with [3H]thymidine for the last 18 h of culture, and then they were harvested and proliferation was measured. (E and F) IL-2 production by CD4+ cells stimulated with DC incubated without antigen (open bars) or in the presence of 5 Lf of TT per ml (filled bars). Panels A to F show results from representative experiments obtained with six different donors.
DC have also been implicated in the maintenance of secondary responses. In order to characterize the effect of HCV genes on the ability of DC to induce recall responses, CD4+ T cells were stimulated with autologous DC infected with RAdCMVCE1 or RAdCMVLacZ plus the recall antigen TT. A potent proliferative reaction against TT was induced with RAdCMVLacZ-infected DC, whereas very poor responses to this antigen were observed when DC infected with RAdCMVCE1 as APC were used (Fig. 3C and D).
We also analyzed the stimulatory potential of DC infected with RAdCMVCE1 or RAdCMVLacZ in assays estimating IL-2 production. In the case of primary immune responses, very low or no production of IL-2 was detected (data not shown). However, in recall responses (in parallel with data obtained in proliferation assays) DC infected with the control adenovirus were able to induce production of high levels of IL-2 against TT while DC infected with RAdCMVCE1 had much lower stimulatory capacity (Fig. 3E and F).
Expression of CD25 by CD4+ T cells stimulated by DC expressing HCV genes.
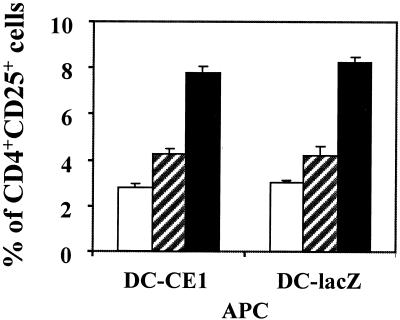
Among the three components of IL-2 receptor, the IL-2Rα chain (CD25) demonstrates the most tightly regulated expression and is a marker of T-cell activation. CD25 is not expressed by resting T cells but upon TCR stimulation CD25 transcription is induced, leading to formation of high-affinity IL-2R (trimeric α:β:γc complex) and acquisition of IL-2 responsiveness (29). Thus, we decided to study the stimulatory ability of DC expressing HCV genes measured as CD25 expression on CD4+ T cells in autologous primary and secondary responses. To this aim, purified CD4+ T cells obtained from normal donors were stimulated with autologous DC transduced with RAdCMVCE1 or RAdCMVLacZ, and 3 days later CD25 expression was analyzed by flow cytometry. Figure 4 shows that DC expressing HCV genes were able to stimulate CD25 expression in CD4+ T lymphocytes with the same efficiency (differences not significant) as DC transduced with the control adenovirus after incubation with peptide PADRE or with TT. Similar results were obtained with a particular donor when we measured CD69 expression as another activation marker (data not shown). Thus, these results show that although DC expressing HCV core and E1 genes are not able to activate CD4+ T cells to proliferate or to produce IL-2, they can stimulate the expression of activation markers in this cell population. Therefore CD4+ T cells acquire a state of incomplete activation after recognition of antigens on DC expressing HCV structural proteins.
FIG. 4.
CD25 expression of CD4+ T cells stimulated by DC. Monocyte-derived DC obtained from normal donors were infected with RAdCMVCE1 (DC-CE1) or with RAdCMVLacZ (DC-lacZ) and used to stimulate CD4+ T cells. DC were incubated with purified autologous CD4+ T cells in the absence (open bars) or in the presence of peptide PADRE (50 μM) (hatched bars) or TT (5 Lf/ml) (filled bars). Three days later, cells were double stained with phycoerythrin-labeled anti-CD25 and FITC-labeled anti-CD4 antibodies. Lymphocytes were gated, and 104 cells were analyzed for the expression of CD25 and CD4. Results represent the percentages of lymphocytes expressing both molecules. Results are representative of five different experiments.
Cellular immune response against HCV core protein in HCV-infected patients: IL-2 production versus CD25 expression.
As mentioned, presentation of antigens by DC transduced with HCV core and E1 genes fails to induce proliferation and IL-2 production by CD4+ T cells but stimulates CD25 expression in this cell population. In patients with chronic hepatitis C, HCV-specific T cells are conceivably exposed to HCV antigens on the membranes of HCV-infected APC. To see whether HCV core-specific T cells from the patients show the same pattern of defective activation as that found in T cells which recognize the antigen on DC expressing core and E1, we incubated PBMC from NR (patients with persistent HCV infection) and SR (patients who had cleared the virus after therapy) in the presence of HCV core and determined IL-2 production and CD25 expression by CD4+ T cells. Similar experiments were performed using the recall antigen TT.
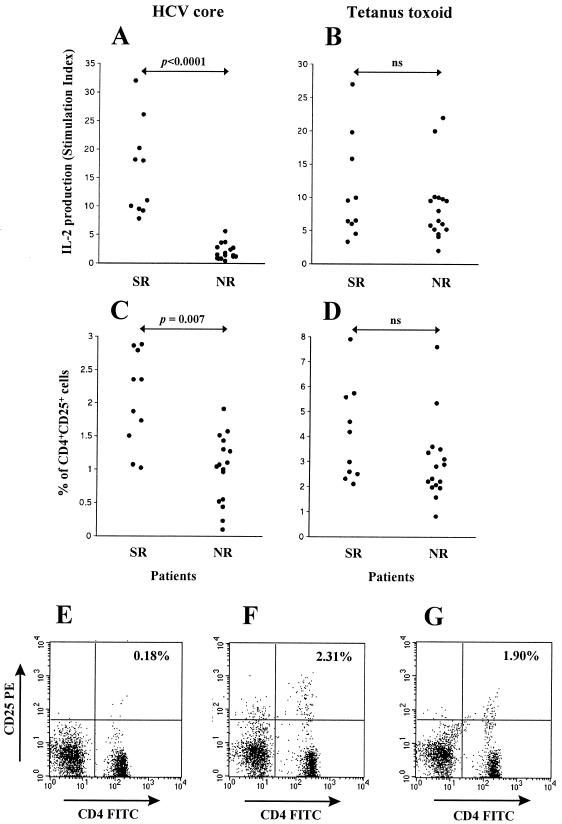
We found that IL-2 production in response to HCV core was markedly decreased in NR compared to SR (mean values, 2.01 ± 1.38 SI in NR versus 16.2 ± 8.16 SI in SR; P < 0.0001) (Fig. 5A). In contrast, the IL-2 response against the recall antigen TT showed no differences between SR and NR (mean values, 8.64 ± 5.42 SI in NR versus 10.83 ± 7.66 SI in SR) (Fig. 5B). We then determined by flow cytometry the percentages of CD4+ T cells expressing CD25 after incubation with HCV core protein or TT in SR and NR patients. We found that SR and NR exhibited similar percentages of CD4+ CD25+ cells after incubation with TT (Fig. 5D) while the percentage of CD4+ CD25+ cells in response to HCV core was reduced in NR compared to SR (2.05 ± 0.71% in SR versus 0.88 ± 0.55% in NR; P = 0.007) (Fig. 5C). However, while IL-2 production was depressed in all NR individuals, a substantial proportion of these patients exhibited percentages of CD25+ cells in the range found in SR (Fig. 5A and C). It should be noted that while the proportion of CD4+ CD25+ cells in response to HCV core was appreciable in HCV-infected individuals, the percentage of CD4+ CD25+ cells observed with healthy subjects after incubation of PBMC with HCV core protein was negligible (always below 0.2%). No correlation was found between expression of CD25 and transaminase levels, viral genotype, or viremia in NR patients (not shown). Representative examples of a healthy subject, an SR patient, and an NR patient are shown in Fig. 5E, F, and G, respectively. Thus, our findings indicate that many NR patients have circulating CD4+ T cells with the ability to recognize HCV core, but these cells respond to the viral antigen with a pattern of incomplete activation characterized by expression of CD25 but absent production of IL-2, similar to that found in CD4+ T cells stimulated by DC expressing HCV antigens.
FIG. 5.
IL-2 production and percentages of CD4+ CD25+ cells in response to HCV core or TT Antigens in HCV patients. PBMC from 10 SR patients and 16 NR patients were stimulated in vitro in the presence of 1 μg of HCV core antigen per ml (A and C) or 5 Lf of TT per ml (B and D). IL-2 production (expressed as SI) (A and B) and the percentages of CD4+ CD25+ cells as estimated by flow cytometry (C and D) were measured at days 7 and 3, respectively. Panels E to G are representative examples of the percentages of CD4+ CD25+ cells in a healthy seronegative control (E), an SR patient (F), and an NR patient (G) after stimulation with HCV core.
Differential mRNA expression in CD4+ CD25+ cells activated with TT or with HCV core protein.
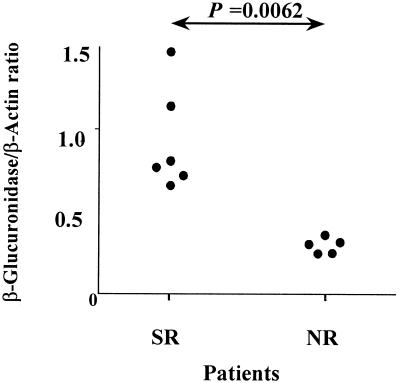
To study differences in gene expression between CD25+ cells synthesizing IL-2 and CD25+ cells failing to produce IL-2, we performed mRNA differential display analysis in purified CD4+ CD25+ cells from an NR patient after incubation with TT or HCV core. We found that β-glucuronidase mRNA was overexpressed in CD25+ T cells obtained in the presence of TT in comparison to those obtained in the presence of HCV core. After identification of this differentially expressed gene, selected experiments were carried out with a subgroup of the above-studied patients. PBMC from six SR and five NR patients were incubated in the presence of HCV core protein, and 3 days later, CD4+ CD25+ cells were purified and mRNA was extracted. We performed reverse transcription-PCRs using specific primers for β-glucuronidase and β-actin (the latter was used as a control gene). Figure 6 shows that SR express higher levels of β-glucuronidase mRNA than do NR (ratio of β-glucuronidase over β-actin, 0.94 ± 0.31 for SR patients versus 0.31 ± 0.04 for NR patients; P = 0.0062). β-Glucuronidase has been reported as a marker of T-cell activation and maturation (14, 30). Thus, these findings further indicate that in NR patients circulating CD4+ T cells which recognize HCV core fail to undergo complete activation after interaction with this viral antigen.
FIG. 6.
β-Glucuronidase mRNA expression in CD4+ CD25+ cells stimulated by HCV core in different groups of patients. CD4+ CD25+ cells were purified from six SR patients and five NR patients after 3 days of in vitro stimulation of PBMC in the presence of HCV core. mRNA was isolated, and reverse transcription-PCR for β-glucuronidase and β-actin was carried out. Resulting PCR bands were quantified, and the ratio of β-glucuronidase to β-actin for each patient is represented.
Ability of DC from HCV patients to stimulate T-cell responses.
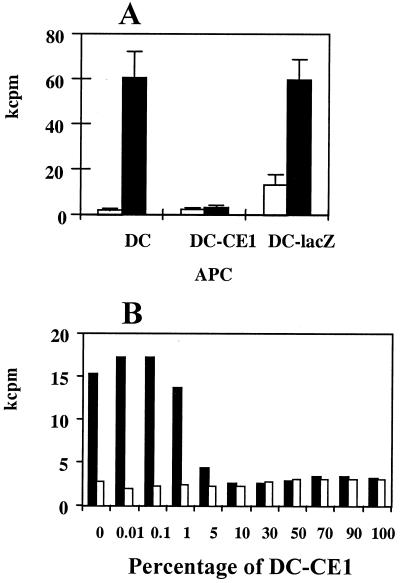
The observation that DC expressing HCV proteins induce incomplete T-cell activation while HCV-infected individuals are immunocompetent with regard to HCV-unrelated antigens suggests that only a minority of DC from HCV carriers are infected, making possible the presentation of non-HCV antigens by uninfected DC. In order to analyze the antigen-presenting function of DC from HCV-infected patients, purified CD4+ T cells from four NR patients with chronic hepatitis C were cultured with autologous DC incubated with TT, and cell proliferation was measured. As shown in Fig. 7A, in a representative case, CD4+ T cells exhibited strong proliferation in response to antigen presented by autologous DC. This is in agreement with results shown in Fig. 5B, where responses against TT are well preserved in HCV patients, showing that most DC from infected patients retain their immunostimulatory ability. However, when DC from HCV patients were transduced with RAdCMVCE1, no responses to recall antigens were found, while DC transduced with RAdCMVLacZ showed the same stimulatory activity as untransduced DC (Fig. 7A). These data confirm the inhibitory effect of HCV proteins on DC function.
FIG. 7.
DC obtained from HCV patients maintain a good capacity to stimulate recall CD4+ T-cell responses. (A) Monocyte-derived DC obtained from patients with chronic hepatitis C were used to stimulate autologous CD4+ T cells incubated in the absence (open bars) or in the presence of 5 Lf of TT per ml (filled bars). Together with adenovirus-uninfected DC, DC infected with RAdCMVCE1 (DC-CE1) and with RAdCMVLacZ (DC-lacZ) were included in the same experiment. Results show T-cell proliferation obtained after 7 days in a representative case from a group of four patients tested. (B) Normal donor-derived DC were infected with RAdCMVCE1 or with RAdCMVLacZ, pooled at different proportions (keeping a total number of 104 DC) and used to stimulate autologous CD4+ T cells. They were incubated in the absence (open bars) or in the presence of 5 Lf of TT per ml (filled bars) for 7 days, and cell proliferation was measured in kilocounts per minute (kcpm).
Taken together, our data suggest that there is a low number of HCV-infected DC in patients with chronic hepatitis C, making possible the induction of a normal T-cell reaction against the generality of antigens by noninfected DC. In order to find the minimal number of DC expressing HCV proteins required to impair T-cell responses, normal donor-derived DC were infected with RAdCMVCE1 or RAdCMVLacZ, pooled at different proportions, and used to present TT to autologous CD4+ T cells. Results from Fig. 7B show that 5% of DC expressing HCV proteins is enough to impair antigen presentation to CD4+ T cells, whereas lower percentages do not affect antigen presentation. Very consistently with our previous findings, activation of CD25 expression in CD4+ T cells reaches similar levels (around 3% of CD4+ T cells become CD25+) irrespective of the proportion of DC transduced with RAdCMVCE1 used for stimulation (data not shown).
DISCUSSION
HCV infection has a strong tendency to progress to chronicity, presumably through its ability to escape both immune surveillance and the IFN system. This virus evades immune responses without causing generalized immunosuppression. The quasispecies nature of HCV and the presence of hypervariable regions in envelope proteins prevents viral neutralization by humoral immunity despite the fact that most patients with chronic hepatitis C have high titers of antibodies against viral antigens (38). T-cell immunity appears to be the type of immune reaction which is crucial in the control of the infection. It has been reported that CD4+ T-cell response against diverse structural and nonstructural HCV proteins is vigorous in individuals who clear the virus after natural infection or after IFN treatment. In contrast, CD4+ T-cell responses are weak in individuals who progress to chronicity after acute infection and in patients with chronic hepatitis who fail to respond to therapy (11, 21, 26, 35). Thus, the last group of patients is characterized by a specific abolishment of immune reactivity against viral products, which might contribute to viral persistence and resistance to antiviral treatment.
Since APC have been shown to be sites of active HCV replication (23, 28), it seems possible that viral antigens would be presented to CD4+ T cells by HCV-infected APC. In this work we have analyzed the antigen-presenting function of monocyte-derived DC under conditions under which these cells are expressing structural HCV antigens intracellularly. To this aim, DC obtained from normal donors were transduced with adenovirus coding for core and E1 (RAdCMVCE1) or for the reporter gene lacZ (RAdCMVLacZ). A previous study reported high levels of expression of HCV structural proteins in the cells transduced with RAdCMVCE1 (5). In the present study we found that when RAdCMVCE1 is used to infect DC ex vivo, these cells showed a phenotype similar to that found in DC transduced with RAdCMVLacZ but had much lower stimulatory abilities, with impaired capacity to stimulate T cells in MLR and in autologous primary and recall responses. Interestingly, when CD4+ T-cell activation was studied by measuring CD25 expression, no differences were found between lymphocytes stimulated by DC expressing HCV proteins and those stimulated by DC expressing LacZ. It has been shown that TCR signaling induces both the expression of CD25 (favoring the formation of a high-affinity IL-2R) and the activation of IL-2 synthesis and T-cell proliferation. However, the intracellular signaling pathways stimulating IL-2 production and IL-2Rα expression diverge downstream of the TCR/CD3 complex (9, 33) and each pathway may be influenced by separate sets of conditions within the cell. Thus, TCR stimulation is sufficient to induce IL-2Rα in the absence of costimulation provided by accessory cells, whereas IL-2 secretion requires an additional signal, such as that supplied by costimulatory molecules (9, 36). Our finding that CD4+ CD25+ cells stimulated by DC expressing HCV proteins are unable to produce detectable amounts of IL-2 may suggest that these cells have been abnormally primed by APC, leading to an incomplete activation state.
Although both core and E1 are expressed in RAdCMVCE1-transduced cells (5), the disturbance in antigen-presenting function of these cells is most probably due to the effect of HCV core protein, since it has been reported to be the main HCV protein interacting with intracellular signaling molecules (19). Moreover, in vivo experiments have shown that HCV core alone was responsible for inhibiting immune responses to recombinant vaccinia virus (20). The molecular mechanisms responsible for the derangement of the functionality of APC by HCV core protein remain to be clarified. In agreement with published results (18, 22), we found that IL-12 production by DC expressing HCV proteins is decreased compared to DC transduced with RAdCMVLacZ (data not shown). However, addition of IL-12 to DC/CD4 T-cell cultures did not restore T-cell proliferation (data not shown), suggesting that reduced IL-12 production is not the main cause for abnormal T-cell priming. It has been recently reported (2) that tumor necrosis factor alpha-dependent DC maturation is impaired in patients with chronic hepatitis C, but not in patients who cleared HCV. Maturation experiments using lipopolysaccharides or tumor necrosis factor alpha have not revealed differences between DC transduced with RAdCMVCE1 and DC transduced with RAdCMVLacZ (data not shown). This may suggest that viral proteins other than core or E1 are responsible for the effects observed by these authors. Alternatively, infection by RAdCMVCE1 might interfere with other DC maturation pathways.
Analysis of antiviral immune response in HCV patients confirms that while PBMC from SR show increased IL-2 synthesis when exposed to HCV core, lymphocytes from NR fail to produce IL-2 after incubation with this antigen. Interestingly, the lack of response of NR to HCV core is not due to deletion of the cells recognizing this protein since there is an upregulation of CD25 expression by CD4+ T cells in the presence of core in a high percentage of NR. The defective activation of CD4+ T cells in NR occurs in the response to core but not to the recall antigen TT, which elicits increased IL-2 production and enhanced expression of CD25 in both NR and SR patients. The inability of HCV core-specific CD4+ cells from NR to acquire a mature activation phenotype after exposure to antigen is also demonstrated by experiments showing that CD4+ CD25+ cells from NR patients express lower levels of β-glucuronidase mRNA than cells from SR patients. Expression of β-glucuronidase has been shown to be associated with a correct maturation of T lymphocytes after antigen exposure (14, 30). β-Glucuronidase (heparanase) is a degrading enzyme which cleaves heparan sulfate proteoglycans (16), facilitating the penetration of lymphocytes through the endothelium to the site of inflammation.
Thus, chronic HCV carriers exhibit a selective deterioration of HCV-specific T-cell immunity while maintaining normal T-cell responses to other antigens. It seems possible that dysfunction of HCV-infected DC impairs T-cell response to HCV proteins while presentation of other antigens by noninfected DC allows normal T-cell reactivity to a wide range of antigenic stimuli. When a high proportion of monocyte-derived DC from the patients were transduced with RAdCMVCE1, they lost the ability to stimulate recall T-cell responses. DC titration experiments revealed that 5% of DC expressing HCV proteins is enough to impair T-cell proliferation. Since bulk monocyte-derived DC from HCV patients retain their stimulatory activity, our results suggest that only a small percentage of DC from HCV patients might be infected.
Our data, in conjunction with reports on the effects of HCV core on several cell functions (19, 20), indicate that immunization protocols aimed at inducing T-cell responses against core and E1 should not be done by using DC transduced with core and E1 genes, since expression of these genes leads to an impairment of stimulatory functions of DC. Instead, immunization against core and E1 employing DC should be done using vectors coding for fragments of these antigens, proteins added exogenously (31), or peptides encompassing epitopes recognized by CD4+ or CD8+ T cells.
In summary, our data indicate that the expression of HCV structural proteins in HCV-infected APC impairs their immunostimulatory function, causing abnormal priming of CD4+ lymphocytes. Recognition of HCV antigens on infected DC would lead to defective activation of CD4+ T cells with specificity for HCV antigens, favoring chronicity of infection.
Acknowledgments
P. Sarobe and J. J. Lasarte contributed equally to this work
We thank B. Rodgers and B. Gander for their kind gifts of HCV core protein and tetanus toxoid, respectively. We also thank M. A. Ávila, E. Fernández, U. Latasa, and E. Larrea for their help with differential display analysis and J. A. Berzofsky and I. Melero for helpful discussions and critical reading of the manuscript.
This work was supported by grants from Ministerio de Ciencia y Tecnología (SAF2001-1119) to P. Sarobe, FIS (00/0536) and FIS (01/0733) to J. J. Lasarte, CICYT (SAF2000-0059) to F. Borrás-Cuesta, and CICYT (SAF99-0084) and Fundación Ramón Areces to J. Prieto. A. López-Díaz de Cerio is a recipient of a scholarship from the Ministerio de Educación y Ciencia (AP 9672673897). N. Casares is a recipient of a scholarship from Gobierno Vasco.
REFERENCES
- 1.Alexander, J., J. Sidney, S. Southwood, J. Ruppert, C. Oseroff, A. Maewal, K. Snoke, H. M. Serra, R. T. Kubo, A. Sette, and H. M. Grey. 1994. Development of high potency universal DR-restricted helper epitopes by modification of high affinity DR-blocking peptides. Immunity 1:751-761. [DOI] [PubMed] [Google Scholar]
- 2.Auffermann-Gretzinger, S., E. B. Keeffe, and S. Levy. 2001. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood 97:3171-3176. [DOI] [PubMed] [Google Scholar]
- 3.Bain, C., A. Fatmi, F. Zoulim, J. P. Zarski, C. Trepo, and G. Inchauspe. 2001. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology 120:512-524. [DOI] [PubMed] [Google Scholar]
- 4.Banchereau, J., and R. M. Steinman. 1998. Dendritic cells and the control of immunity. Nature 392:245-252. [DOI] [PubMed] [Google Scholar]
- 5.Bruna-Romero, O., J. J. Lasarte, G. Wilkinson, K. Grace, B. Clarke, F. Borras-Cuesta, and J. Prieto. 1997. Induction of cytotoxic T-cell response against hepatitis C virus structural antigens using a defective recombinant adenovirus. Hepatology 25:470-477. [DOI] [PubMed] [Google Scholar]
- 6.Castelruiz, Y., E. Larrea, P. Boya, M. P. Civeira, and J. Prieto. 1999. Interferon alfa subtypes and levels of type I interferons in the liver and peripheral mononuclear cells in patients with chronic hepatitis C and controls. Hepatology 29:1900-1904. [DOI] [PubMed] [Google Scholar]
- 7.Cerny, A., J. G. McHutchison, C. Pasquinelli, M. E. Brown, M. A. Brothers, B. Grabscheid, P. Fowler, M. Houghton, and F. V. Chisari. 1995. Cytotoxic T lymphocyte response to hepatitis C virus-derived peptides containing the HLA A2.1 binding motif. J. Clin. Investig. 95:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooper, S., A. L. Erickson, E. J. Adams, J. Kansopon, A. J. Weiner, D. Y. Chien, M. Houghton, P. Parham, and C. M. Walker. 1999. Analysis of a successful immune response against hepatitis C virus. Immunity 10:439-449. [DOI] [PubMed] [Google Scholar]
- 9.Crabtree, G. R. 1989. Contingent genetic regulatory events in T lymphocyte activation. Science 243:355-361. [DOI] [PubMed] [Google Scholar]
- 10.Dienstag, J. L. 1983. Non-A, non-B hepatitis. I. Recognition, epidemiology, and clinical features. Gastroenterology 85:439-462. [PubMed] [Google Scholar]
- 11.Diepolder, H. M., R. Zachoval, R. M. Hoffmann, E. A. Wierenga, T. Santantonio, M. C. Jung, D. Eichenlaub, and G. R. Pape. 1995. Possible mechanism involving T-lymphocyte response to non-structural protein 3 in viral clearance in acute hepatitis C virus infection. Lancet 346:1006-1007. [DOI] [PubMed] [Google Scholar]
- 12.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 13.Frasca, L., P. Del Porto, L. Tuosto, B. Marinari, C. Scotta, M. Carbonari, A. Nicosia, and E. Piccolella. 1999. Hypervariable region 1 variants act as TCR antagonists for hepatitis C virus-specific CD4+ T cells. J. Immunol. 163:650-658. [PubMed] [Google Scholar]
- 14.Fridman, R., O. Lider, Y. Naparstek, Z. Fuks, I. Vlodavsky, and I. R. Cohen. 1987. Soluble antigen induces T lymphocytes to secrete an endoglycosidase that degrades the heparan sulfate moiety of subendothelial extracellular matrix. J. Cell. Physiol. 130:85-92. [DOI] [PubMed] [Google Scholar]
- 15.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. C. Rissoan, Y. J. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gilat, D., R. Hershkoviz, I. Goldkorn, L. Cahalon, G. Korner, I. Vlodavsky, and O. Lider. 1995. Molecular behavior adapts to context: heparanase functions as an extracellular matrix-degrading enzyme or as a T cell adhesion molecule, depending on the local pH. J. Exp. Med. 181:1929-1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hiasa, Y., N. Horiike, S. M. Akbar, I. Saito, T. Miyamura, Y. Matsuura, and M. Onji. 1998. Low stimulatory capacity of lymphoid dendritic cells expressing hepatitis C virus genes. Biochem. Biophys. Res. Commun. 249:90-95. [DOI] [PubMed] [Google Scholar]
- 18.Kanto, T., N. Hayashi, T. Takehara, T. Tatsumi, N. Kuzushita, A. Ito, Y. Sasaki, A. Kasahara, and M. Hori. 1999. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J. Immunol. 162:5584-5591. [PubMed] [Google Scholar]
- 19.Kato, N., H. Yoshida, S. Kioko Ono-Nita, J. Kato, T. Goto, M. Otsuka, K. Lan, K. Matsushima, Y. Shiratori, and M. Omata. 2000. Activation of intracellular signaling by hepatitis B and C viruses: C-viral core is the most potent signal inducer. Hepatology 32:405-412. [DOI] [PubMed] [Google Scholar]
- 20.Large, M. K., D. J. Kittlesen, and Y. S. Hahn. 1999. Suppression of host immune response by the core protein of hepatitis C virus: possible implications for hepatitis C virus persistence. J. Immunol. 162:931-938. [PubMed] [Google Scholar]
- 21.Lasarte, J. J., M. Garcia Granero, A. Lopez, N. Casares, N. Garcia, M. P. Civeira, F. Borras Cuesta, and J. Prieto. 1998. Cellular immunity to hepatitis C virus core protein and the response to interferon in patients with chronic hepatitis C. Hepatology 28:815-822. [DOI] [PubMed] [Google Scholar]
- 22.Lee, C. H., Y. H. Choi, S. H. Yang, C. W. Lee, S. J. Ha, and Y. C. Sung. 2001. Hepatitis C virus core protein inhibits interleukin 12 and nitric oxide production from activated macrophages. Virology 279:271-279. [DOI] [PubMed] [Google Scholar]
- 23.Lerat, H., S. Rumin, F. Habersetzer, F. Berby, M. A. Trabaud, C. Trepo, and G. Inchauspe. 1998. In vivo tropism of hepatitis C virus genomic sequences in hematopoietic cells: influence of viral load, viral genotype, and cell phenotype. Blood 91:3841-3849. [PubMed] [Google Scholar]
- 24.Liang, P., and A. B. Pardee. 1992. Differential display of eukaryotic messenger RNA by means of the polymerase chain reaction. Science 257:967-971. [DOI] [PubMed] [Google Scholar]
- 25.Miller, R. H., and R. H. Purcell. 1990. Hepatitis C virus shares amino acid sequence similarity with pestiviruses and flaviviruses as well as members of two plant virus supergroups. Proc. Natl. Acad. Sci. USA 87:2057-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Missale, G., R. Bertoni, V. Lamonaca, A. Valli, M. Massari, C. Mori, M. G. Rumi, M. Houghton, F. Fiaccadori, and C. Ferrari. 1996. Different clinical behaviors of acute hepatitis C virus infection are associated with different vigor of the anti-viral cell-mediated immune response. J. Clin. Investig. 98:706-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moldvay, J., P. Deny, S. Pol, C. Brechot, and E. Lamas. 1994. Detection of hepatitis C virus RNA in peripheral blood mononuclear cells of infected patients by in situ hybridization. Blood 83:269-273. [PubMed] [Google Scholar]
- 28.Muller, H. M., B. Kallinowski, C. Solbach, L. Theilmann, T. Goeser, and E. Pfaff. 1994. B-lymphocytes are predominantly involved in viral propagation of hepatitis C virus (HCV). Arch. Virol. Suppl. 9:307-316. [DOI] [PubMed] [Google Scholar]
- 29.Nelson, B. H., and D. M. Willerford. 1998. Biology of the interleukin-2 receptor. Adv. Immunol. 70:1-81. [DOI] [PubMed] [Google Scholar]
- 30.Olsen, I., G. Bou-Gharios, and D. Abraham. 1990. The activation of resting lymphocytes is accompanied by the biogenesis of lysosomal organelles. Eur. J. Immunol. 20:2161-2170. [DOI] [PubMed] [Google Scholar]
- 31.Polakos, N. K., D. Drane, J. Cox, P. Ng, M. J. Selby, D. Chien, D. T. O'Hagan, M. Houghton, and X. Paliard. 2001. Characterization of hepatitis C virus core-specific immune responses primed in rhesus macaques by a nonclassical ISCOM vaccine. J. Immunol. 166:3589-3598. [DOI] [PubMed] [Google Scholar]
- 32.Rehermann, B., K. M. Chang, J. G. McHutchison, R. Kokka, M. Houghton, and F. V. Chisari. 1996. Quantitative analysis of the peripheral blood cytotoxic T lymphocyte response in patients with chronic hepatitis C virus infection. J. Clin. Investig. 98:1432-1440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rothenberg, E. V. 1992. The development of functionally responsive T cells. Adv. Immunol. 51:85-214. [DOI] [PubMed] [Google Scholar]
- 34.Salio, M., M. Cella, M. Suter, and A. Lanzavecchia. 1999. Inhibition of dendritic cell maturation by herpes simplex virus. Eur. J. Immunol. 29:3245-3253. [DOI] [PubMed] [Google Scholar]
- 35.Sarobe, P., J. I. Jauregui, J. J. Lasarte, N. Garcia, M. P. Civeira, F. Borras-Cuesta, and J. Prieto. 1996. Production of interleukin-2 in response to synthetic peptides from hepatitis C virus E1 protein in patients with chronic hepatitis C: relationship with the response to interferon treatment. J. Hepatol. 25:1-9. [DOI] [PubMed] [Google Scholar]
- 36.Schwartz, R. H. 1990. A cell culture model for T lymphocyte clonal anergy. Science 248:1349-1356. [DOI] [PubMed] [Google Scholar]
- 37.Shirai, M., H. Okada, M. Nishioka, T. Akatsuka, C. Wychowski, R. Houghten, C. D. Pendleton, S. M. Feinstone, and J. A. Berzofsky. 1994. An epitope in hepatitis C virus core region recognized by cytotoxic T cells in mice and humans. J. Virol. 68:3334-3342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van der Poel, C. L., H. T. Cuypers, H. W. Reesink, A. J. Weiner, S. Quan, R. Di Nello, J. J. Van Boven, I. Winkel, D. Mulder-Folkerts, P. J. Exel-Oehlers, W. Schaasberg, A. Leentvaar-Kuypers, A. Polito, M. Houghton, and P. N. Lelie. 1991. Confirmation of hepatitis C virus infection by new four-antigen recombinant immunoblot assay. Lancet 337:317-319. [DOI] [PubMed] [Google Scholar]
- 39.Wang, J. T., J. C. Sheu, J. T. Lin, T. H. Wang, and D. S. Chen. 1992. Detection of replicative form of hepatitis C virus RNA in peripheral blood mononuclear cells. J. Infect. Dis. 166:1167-1169. [DOI] [PubMed] [Google Scholar]
- 40.Weiner, A., A. L. Erickson, J. Kansopon, K. Crawford, E. Muchmore, A. L. Hughes, M. Houghton, and C. M. Walker. 1995. Persistent hepatitis C virus infection in a chimpanzee is associated with emergence of a cytotoxic T lymphocyte escape variant. Proc. Natl. Acad. Sci. USA 92:2755-2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Weiner, A. J., H. M. Geysen, C. Christopherson, J. E. Hall, T. J. Mason, G. Saracco, F. Bonino, K. Crawford, C. D. Marion, K. A. Crawford, M. Brunetto, P. J. Barr, T. Miyamura, J. McHutchinson, and M. Houghton. 1992. Evidence for immune selection of hepatitis C virus (HCV) putative envelope glycoprotein variants: potential role in chronic HCV infections. Proc. Natl. Acad. Sci. USA 89:3468-3472. [DOI] [PMC free article] [PubMed] [Google Scholar]