Abstract
Infection with human or simian immunodeficiency virus (SIV) is characterized by the rapid turnover of both viral particles and productively infected cells. It has recently been reported that the clearance of SIV in vivo is exceedingly fast, with half-lives on the order of minutes. The underlying mechanism or site responsible for this rapid clearance, however, remains unknown. To investigate this issue, we chose to infuse infectious SIVmac239 grown from autologous peripheral blood mononuclear cells that were radioactively labeled by [35S]methionine and [35S]cysteine. This approach eliminates from the viral membrane alloantigens that may have a significant impact on viral clearance. In addition, this approach also permits identification of the sites of viral clearance by measuring the radioactive intensity, even if degradation of SIV RNA occurs in tissues. We now report that the half-life of infused SIV in blood is extremely close to estimates from a previous study, in which unlabeled SIV grown in a heterologous cell line was used. The allogeneic effect due to the presence of human antigens on the surfaces of virions may, therefore, play a minimal role in the high rate of virion clearance. Moreover, close to 30% of infused radioactivity was found in the liver and measureable amounts were detected in the lungs (5.4%), lymph nodes (3.0%), and spleen (0.4%). The detection of a significant proportion of infused virus in the liver suggests that viral clearance from circulation is mediated by a common, nonspecific mechanism, such as the phagocytic functions of the reticuloendothelial system. The rapid clearance and degradation of exogenously infused virions may pose a major obstacle for gene therapy with viral vectors, unless strategies to overcome the rapid in vivo elimination of these particles are developed.
Infection with human or simian immunodeficiency virus (HIV or SIV) is characterized by a dynamic equilibrium between virus production and clearance (3, 4, 6, 14, 16-18, 23, 24), and the seemingly stable level of plasma viremia in an infected host is in fact a balance between these two equally rapid processes. Perturbing this equilibrium with antiretroviral therapy or large-volume plasma apheresis has provided fundamental insights into the dynamic nature of this viral infection (6, 18, 19, 23). Mathematical modeling of the decline in plasma viral load after disturbing this equilibrium with antiretroviral therapy or plasma apheresis indicates that HIV type 1 (HIV-1) particles have an estimated half-life of 30 min and that, on average, 1010 to 1012 virions are produced each day (18, 19).
Using the SIV-rhesus macaque model, members of our laboratory and others have recently demonstrated that the clearance of SIV in vivo is also exceedingly fast (7, 24). Two different approaches were used to study virion clearance in rhesus macaques. One approach monitored the disappearance of viral particles from plasma after intravenous bolus injection; the other tracked the level of plasma viremia during and following a constant intravenous infusion of SIV particles. Both of these approaches yielded similar results; that is, exogenously infused virions were cleared from plasma extremely rapidly, with half-lives on the order of minutes (7, 24). This new estimate is approximately 10- to 100-fold faster than that previously reported for HIV-1 in infected humans. These results suggested that if the rapid clearance of virions observed in rhesus monkeys is applicable to infected humans, then HIV-1 production must be proportionally higher than previous minimal estimates (6, 17, 18, 23).
The underlying mechanisms or sites responsible for this rapid clearance, however, remain unknown. In a previous experiment, four animals were euthanized after the completion of particle infusion and various organs were collected for measurement of SIV (24). Although the majority of SIV RNA was detected in lymph nodes, spleen, lung, and liver, only ∼1 to ∼10% or less of the infused virions were accounted for in each of the four animals (24). Most likely, the vast majority of viral particles were degraded prior to tissue sampling or were cleared in tissues other than those studied.
There are two caveats associated with the previous experiment which may have direct implications on our final estimates of virion half-lives and possible sites of viral clearance. One involves the SIV stock used in the infusion studies. The SIV stock was generated by transfecting human 293 cells and then purifying the transfected cells by sucrose gradient centrifugation (24). Human molecules incorporated on the SIV surface during the process of virus budding (5, 15, 22) may have led to an allogeneic effect which resulted in rapid clearance following infusion into rhesus monkeys. The other caveat concerns the measurement of SIV RNA after tissue homogenization. If SIV RNA was rapidly degraded in tissues, the measurement of SIV RNA would not have accurately reflected the sites of virus clearance, since sites where active virus degradation occurred would have had low, rather than high, SIV RNA levels.
To circumvent these potential problems in the present study, we chose to generate and infuse infectious SIVmac239 that has been grown from the autologous peripheral blood mononuclear cells of a rhesus macaque (AR97) and radioactively labeled by incorporation of [35S]methionine and [35S]cysteine in the culture media. The SIV stock was then purified by sucrose gradient centrifugation as described previously (24). The advantage of using this approach was twofold: one, to eliminate alloantigens from the viral membrane so as to avoid their contribution to viral clearance, and two, to trace the sites of viral clearance by measuring the radioactive intensity even if SIV RNA has degraded in tissues. As described previously (24), AR97 was anesthetized prior to the experiment and the virus was infused via a cannula inserted into a femoral vein. Two independent bolus injections of approximately 3.0 × 1010 virus particles each were given 1 h apart. The first injection was administered with virus alone, whereas the second injection was given after the SIV stock was premixed (37°C, 1 h) in vitro with antibodies, primarily immunoglobulin G (SIVIgG), directed against SIVmac251 (24). The amount of SIVIgG used was 2 mg, which, based on in vitro binding results, was sufficient to capture all SIV particles present in the inoculum. The SIVIgG was prepared from the pooled plasma of SIVmac251-infected macaques with midpoint anti-gp120 binding antibody titers of 68,000 to 170,000 and 50% neutralization concentrations of 0.60 to 1.15 μg/ml (1). All macaque plasma samples were heat inactivated at 56°C for 40 min prior to the purification procedure. Two-milliliter blood samples were collected every 2 min for the first 10 min, every 4 min over the next 20 min, and then every 8 min for 1 h. Immediately after blood collection, plasma samples were separated and stored at −80°C and the animal was euthanized by an overdose of sodium pentobarbital (100 to 120 mg/kg of body weight). The plasma viral concentration was determined by the SIV bDNA assay (Bayer Corporation, Emeryville, Calif.). Various tissues, including the liver, lung, spleen, lymph nodes, kidney, heart, muscle, and pancreas, were collected, weighed, and frozen in liquid nitrogen.
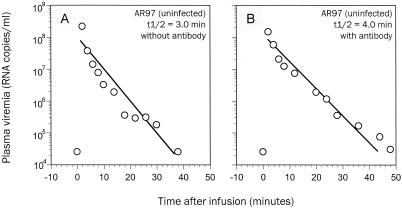
Figure 1 shows the changes in levels of viral RNA in the plasma of animal AR97 during and after the bolus injection in the presence or absence of SIV-specific antibodies. Consistent with a previous report (24), a sharp increase in plasma viral RNA was associated with the bolus injection. Upon completion of the injection, however, a rapid exponential decline in plasma viremia was observed in two independent experiments. Regardless of the presence or absence of SIV-specific antibodies, the clearance rate constants were found to be within a narrow range of 0.17 to 0.23 per min, which in turn yielded decay half-lives of 3.0 to 4.0 min. (To estimate the clearance rate constant, the data points in the midportion of the exponential decay, shown in Fig. 1, were used.) These values are extremely close to previous estimates with unlabeled SIV grown on heterologous human cell lines (24). It should be noted that in vitro treatment of virus with antibody may not be a good experimental model to study the impact of antibody on the clearance of virions, since the binding condition may not be optimal. Passive immunization or preexisting neutralizing antibodies in animals are more likely to have a stronger influence on virion clearance, as demonstrated previously (1, 7).
FIG. 1.
Changes in levels of viral RNA in the plasma of animal AR97 during and after the bolus injection in the absence (A) or presence (B) of SIV-specific antibodies. A regression line based on the exponential decay in viral RNA is shown. All the data points are the geometric means of quadruplicate measurements by the bDNA assay. t1/2, decay half-life.
To identify potential sites of viral clearance, pieces of tissue from each organ, together with those from lymph nodes, muscle, thymus, and tonsil, were measured for radioactive intensity with a liquid scintillation counter (Beckman, Fullerton, Calif.). The total intensity in each organ was then estimated by multiplying the mean of the intensity per milligram of tissue by the total organ weight. It was not feasible to measure the total lymphoid mass; therefore, an upper bound of 1% of body weight was used as the total mass of lymph nodes (24) for these calculations. Table 1 compares the levels of radioactivity in the tested organs as percentages of the total amount of SIV infused with SIV RNA data obtained from one animal (macaque 1336) in the previous experiment. In general, a relatively higher percentage of virus was found in each organ when radioactivity was measured than when the SIV bDNA assay was used. The most distinct difference between these two sets of results, however, was the percentage of virus in the liver. In animal AR97, close to 30% of infused radioactive particles were found in the liver, whereas in animal 1336, only 0.01% of SIV RNA was found. This dramatic difference suggests that the vast majority of viral RNA was degraded prior to tissue sampling. Measurements of radioactivity, however, were not affected, presumably because degraded viral proteins remained in the liver. This finding was not surprising since liver is known to have an efficient ability to clear many foreign pathogens, including bacteria, bacteriophages, and many types of viruses (2, 8-13, 21). In addition, measurable amounts of infused viral particles were also found in the lung (5.4%), lymph nodes (3.0%), and spleen (0.4%) of animal AR97 (Table 1), probably due to a large number of CD4+ T lymphocytes and macrophages residing in these organs to which SIV particles could bind.
TABLE 1.
Percentages of SIV detected in various organs measured either from levels of radioactivity (animal AR97) or from levels of SIV RNA (animal 1336)
| Animal | Type(s) of tissue | % of SIV detected out of total amt infused |
|---|---|---|
| AR97 | Liver | 29.5 |
| Lung | 5.4 | |
| Lymph node | 3.0 | |
| Spleen | 0.4 | |
| Othersa | <2.2 | |
| 1336 | Liver | 0.01 |
| Lung | 0.2 | |
| Lymph node | 1.4 | |
| Spleen | 0.1 | |
| Othersb | <1.9 |
Heart, kidney, muscle, pancreas, thymus, and tonsil.
Heart, kidney, muscle, pancreas, brain, and tonsil.
These observations clearly demonstrate that the half-life of infused SIVmac239 in blood is extremely close to previous estimates for a viral stock produced in heterologous cells. The allogeneic effect due to the presence of human antigens on the virion surface may, therefore, play only a small role in the rate of virion clearance. Consistent with previous findings, the presence of virus-specific antibodies had no significant effect on clearance, suggesting that the high rate of viral clearance is not necessarily immune mediated. The detection of a significant proportion of infused virus in the liver supports this notion and suggests that viral clearance from the circulation may be mediated by a common, nonspecific mechanism, such as the phagocytic functions of the reticuloendothelial system (2, 8-13, 21). Despite the presence of a large number of SIV-specific cytotoxic T lymphocytes in the livers of SIV-infected macaques (20), it is hard to imagine how cytotoxic T lymphocytes can mediate virion clearance at such a fast speed. However, this result does not necessarily mean that antibodies of greater binding affinity or neutralizing activity cannot facilitate the clearance of virions in vivo. In particular, the antibodies used in this study may be less neutralizing than those used in the study conducted by Igarashi and colleagues (7). In addition, the higher virion clearance rate observed in the present or previous study (24), compared to that measured by Igarashi and colleagues, may be due to differences in the speed of viral infusion as well as in the frequency of sample collection. But this discrepancy can be definitively resolved only by additional studies.
At present, we are not sure of the fate of the virus unaccounted for by the current radioactivity data. Other body compartments, such as the gastrointestinal tract, may also play an important role in this process. Given the large lymphoid mass in the gut, it is entirely possible that a significant proportion of infused virions are trapped there. Finally, the rapid clearance and degradation of exogenously infused virions may pose a major obstacle for gene therapy approaches that use viral vectors, unless strategies to overcome the rapid in vivo elimination of viral particles are developed.
Acknowledgments
We thank J. Binley for providing SIVmac251-specific antibodies.
This work was supported by NIH grants AI40387, AI46964, and RR00164.
REFERENCES
- 1.Binley, J. M., B. Clas, A. Gettie, M. Vesanen, D. C. Montefiori, L. Sawyer, J. Booth, M. Lewis, P. A. Marx, S. Bonhoeffer, and J. P. Moore. 2000. Passive infusion of immune serum into simian immunodeficiency virus-infected rhesus macaques undergoing a rapid disease course has minimal effect on plasma viremia. Virology 270:237-249. [DOI] [PubMed] [Google Scholar]
- 2.Brunner, K. T., D. Hures, R. T. McCluskey, and B. Benacerraf. 1960. Blood clearance of P32-labeled vesicular stomatitis and Newcastle disease viruses by the reticuloendothelial system in mice. J. Immunol. 85:99-105. [PubMed] [Google Scholar]
- 3.Cavert, W., D. W. Notermans, K. Staskus, S. W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, Z. Q. Zhang, R. Mills, H. McDade, C. M. Schuwirth, J. Goudsmit, S. A. Danner, and A. T. Haase. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960-964. [DOI] [PubMed] [Google Scholar]
- 4.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 5.Frank, I., H. Stoiber, S. Godar, H. Stockinger, F. Steindl, H. W. Katinger, and M. P. Dierich. 1996. Acquisition of host cell-surface-derived molecules by HIV-1. AIDS 10:1611-1620. [DOI] [PubMed] [Google Scholar]
- 6.Ho, D. D., A. U. Neumann, A. S. Perelson, W. Chen, J. M. Leonard, and M. Markowitz. 1995. Rapid turnover of plasma virions and CD4 lymphocytes in HIV-1 infection. Nature 373:123-126. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi, T., C. Brown, A. Azadegan, N. Haigwood, D. Dimitrov, M. A. Martin, and R. Shibata. 1999. Human immunodeficiency virus type 1 neutralizing antibodies accelerate clearance of cell-free virions from blood plasma. Nat. Med. 5:211-216. [DOI] [PubMed] [Google Scholar]
- 8.Mims, C. A. 1959. The response of mice to large intravenous injections of ectromelia virus, II. The growth of virus in the liver. Br. J. Exp. Pathol. 40:543-550. [PMC free article] [PubMed] [Google Scholar]
- 9.Mims, C. A. 1959. The response of mice to large intravenous injections of ectromelia virus, I. The fate of injected virus. Br. J. Exp. Pathol. 40:533-542. [PMC free article] [PubMed] [Google Scholar]
- 10.Mims, C. A. 1968. The response of mice to the intravenous injection of cowpox virus. Br. J. Exp. Pathol. 49:24-32. [PMC free article] [PubMed] [Google Scholar]
- 11.Mims, C. A. 1956. Rift Valley fever virus in mice. II. Adsorption and multiplication of virus. Br. J. Exp. Pathol. 37:110-128. [PMC free article] [PubMed] [Google Scholar]
- 12.Nathanson, N., and B. Harrington. 1967. Experimental infection of monkeys with Langat virus. II. Turnover of circulating virus. Am. J. Epidemiol. 85:494-502. [DOI] [PubMed] [Google Scholar]
- 13.Nathanson, N., and K. L. Tyler. 1997. Viral pathogenesis. Lippincott-Raven Publishers, Philadelphia, Pa.
- 14.Nowak, M. A., A. L. Lloyd, G. M. Vasquez, T. A. Wiltrout, L. M. Wahl, N. Bischofberger, J. Williams, A. Kinter, A. S. Fauci, V. M. Hirsch, and J. D. Lifson. 1997. Viral dynamics of primary viremia and antiretroviral therapy in simian immunodeficiency virus infection. J. Virol. 71:7518-7525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orentas, R. J., and J. E. Hildreth. 1993. Association of host cell surface adhesion receptors and other membrane proteins with HIV and SIV. AIDS Res. Hum. Retrovir. 9:1157-1165. [DOI] [PubMed] [Google Scholar]
- 16.Perelson, A. S., P. Essunger, Y. Cao, M. Vesanen, A. Hurley, K. Saksela, M. Markowitz, and D. D. Ho. 1997. Decay characteristics of HIV-1-infected compartments during combination therapy. Nature 387:188-191. [DOI] [PubMed] [Google Scholar]
- 17.Perelson, A. S., P. Essunger, and D. D. Ho. 1997. Dynamics of HIV-1 and CD4+ lymphocytes in vivo. AIDS 11(Suppl. A):S17-S24. [PubMed] [Google Scholar]
- 18.Perelson, A. S., A. U. Neumann, M. Markowitz, J. M. Leonard, and D. D. Ho. 1996. HIV-1 dynamics in vivo: virion clearance rate, infected cell life-span, and viral generation time. Science 271:1582-1586. [DOI] [PubMed] [Google Scholar]
- 19.Ramratnam, B., S. Bonhoeffer, J. Binley, A. Hurley, L. Zhang, J. E. Mittler, M. Markowitz, J. P. Moore, A. S. Perelson, and D. D. Ho. 1999. Rapid production and clearance of HIV-1 and hepatitis C virus assessed by large volume plasma apheresis. Lancet 354:1782-1785. [DOI] [PubMed] [Google Scholar]
- 20.Schmitz, J. E., M. J. Kuroda, R. S. Veazey, A. Seth, W. M. Taylor, C. E. Nickerson, M. A. Lifton, P. J. Dailey, M. A. Forman, P. Racz, K. Tenner-Racz, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV)-specific CTL are present in large numbers in livers of SIV-infected rhesus monkeys. J. Immunol. 164:6015-6019. [DOI] [PubMed] [Google Scholar]
- 21.Schultz, I., and F. A. Neva. 1965. Relationship between blood clearance and viruria after intravenous injection of mice and rats with bacteriophage and polioviruses. J. Immunol. 94:833-841. [PubMed] [Google Scholar]
- 22.Tremblay, M. J., J. F. Fortin, and R. Cantin. 1998. The acquisition of host-encoded proteins by nascent HIV-1. Immunol. Today 19:346-351. [DOI] [PubMed] [Google Scholar]
- 23.Wei, X., S. K. Ghosh, M. E. Taylor, V. A. Johnson, E. A. Emini, P. Deutsch, J. D. Lifson, S. Bonhoeffer, M. A. Nowak, B. H. Hahn, et al. 1995. Viral dynamics in human immunodeficiency virus type 1 infection. Nature 373:117-122. [DOI] [PubMed] [Google Scholar]
- 24.Zhang, L., P. J. Dailey, T. He, A. Gettie, S. Bonhoeffer, A. S. Perelson, and D. D. Ho. 1999. Rapid clearance of simian immunodeficiency virus particles from plasma of rhesus macaques. J. Virol. 73:855-860. [DOI] [PMC free article] [PubMed] [Google Scholar]