Abstract
BACKGROUND
Grafting of testicular tissue into immunodeficient mice has become an interesting and promising scientific tool for the generation of gametes and the study of testicular function. This technique might potentially be used to generate sperm from patients whose testes need to be removed or are destroyed due to therapeutic intervention or as a consequence of disease. Here we explore whether adult human testicular tissue from patients with different testicular pathologies survives as xenograft.
METHODS AND RESULTS
Testis tissue from adult patients with varying degrees of spermatogenesis was grafted into two strains of immunodeficient mice (severe combined immunodeficiency, Nu/Nu). Tissue with active spermatogenesis prior to grafting largely regressed. However, testicular tissue survival was better in cases where spermatogenesis was suppressed prior to grafting and occasionally spermatogonial stem cells survived. Cases with spermatogenic disruption were not corrected by the xenografting.
CONCLUSION
Superior survival of the germinal epithelium and spermatogonia when spermatogenesis was suppressed prior to grafting could provide a novel strategy for germline preservation in pre-pubertal cancer patients. This approach could also be valuable to study the early stages of human spermatogenesis.
Keywords: fertility preservation, grafting, spermatogenesis, steroidogenesis, testis
Introduction
In recent years, grafting of immature testicular tissue into immundeficient mice has been introduced as a strategy to grow and differentiate immature testicular tissue up to the stage of fertile sperm (Honaramooz et al., 2002; Schlatt et al., 1999). Similarly successful was the generation of sperm from xenografts of immature testes from hamster, monkey, bull, pig and cat in mouse hosts (Schlatt et al., 2002a; Snedaker et al., 2004; Honaramooz et al., 2004; Oatley et al., 2005). Spermatogenesis in a xenograft might provide a model for the study of human spermatogenesis and could represent an alternative approach for fertility preservation in cancer patients. To date, options for safeguarding future fertility are limited in patients for whom sperm cryopreservation prior to treatment is not possible (Nordhoff and Schlatt, 2003; Orwig and Schlatt, 2005). It is estimated that 1 in 650 children will be diagnosed with cancer by age 16 years and that 80% will be cured. The oncological treatment has severe side-effects putting male and female patients at risk for infertility. Of these, a significant proportion will be permanently unable to reproduce because of the irreversible damage from chemo- or radiotherapy to the germinal epithelium, specifically to the spermatogonial stem cells (for review: Wallace et al., 2005). Autologous germ cell transplantation has been suggested as a potential means of restoring fertility after cancer treatment (Radford, 2003). In this approach, germ cells will be collected before cytotoxic treatment and cryopreserved as a single cell supsension. If the patient does not recover fertility, these cells can be infused back into the seminiferous tubules where they are expected to re-initiate spermatogenesis. While this approach has been shown in principle to be feasible in pre-clinical studies (Schlatt et al., 1999; 2002b), it carries the risk of re-introduction of cancer cells to the patient as has been demonstrated in a rodent model (Jahnukainen et al., 2001) that potentially could be addressed by cell sorting prior to transplantation (Fujita et al., 2005).
Since the xenografting of primate testes was successful in allowing induction of spermatogenesis from a non-human primate to occur in a mouse host (Honaramooz et al., 2004) even after cryopreservation of the tissue (Orwig and Schlatt, 2005), it seemed likely that it could be applied to human testis tissue. The objective of the present study was to examine the potential of adult human testis tissue, from patients with normal or abnormal spermatogenesis, to survive as ectopic xenografts in mice.
Materials and methods
Sources of human testicular tissue
In experiment 1, testicular tissue was obtained from six men (between 32 and 40 years of age, Table I). Four men (donor 1–4, Table I) were diagnosed with obstructive azoospermia [one with congenital absence of the vas deferens, three with epididymal blockage]. One man (donor 6, Table I) was a tumour survivor showing non-obstructive azoospermia (Hodgkin lymphoma, 6 years after completing chemotherapy cycles). These five men were recruited as patients undergoing infertility treatment. As part of their treatment, these patients were biopsied. The four men with obstructive azoospermia underwent open testicular biopsy in order to retrieve sperm for ICSI, while the patient with azoospermia post-chemotherapy underwent a diagnostic biopsy. The sixth patient (donor 5, Table I) was diagnosed with testicular cancer. He underwent hemi-orchidectomy. In this patient, the grafted pieces of tissue were dissected from ‘normal’ areas of the testes clearly distinct and located away from the cancerous parts of the testis. All patients agreed to donate their testis tissue for this research and signed an informed consent. The procedure was approved by the Institutional Review Boards. Testis biopsies and dissected pieces of testicular tissue were maintained in ice-cold Dulbecco’s modified Eagle’s medium (DMEM Mediatech, Herndon, VA, USA) to avoid extended periods of warm ischaemia until grafting was performed within 3 h.
Table I.
Histopathological diagnosis of testis tissue samples (experiment 1)
| General | Germ cells | Sertoli cells | Peritubular cells | Leydig cells | Diagnosis | |
|---|---|---|---|---|---|---|
| Donor 1 | Normal testicular morphology and spermatogenesis | Normal | Normal | Normal | Normal | Obstructive azoospermia |
| Graft 1 week | Mostly degenerated testicular tissue with hyalinized seminiferous tubules | Two tubules filled with necrotic germ cells. Few elongating spermatids and sperm present | High degree of fibrosis | Few Leydig cells | ||
| Graft 5 weeks | Fully degenerated testicular tissue with hyalinized seminiferous tubules | Few surviving Sertoli cells, high degree of hyalinization | None | Some Leydig cells present | ||
| Donor 2 | Severe hypospermatogenesis | Most tubules show spermatocytes, some with round spermatids | About 50% of tubules show fully hyalinized Sertoli cells. In tubules with spermatogenesis, Sertoli cell morphology is normal | Low degree of fibrosis | Normal to high number of Leydig cells with normal morphology | Obstructive azoospermia |
| Grafts 10 weeks | Fully degenerated testicular tissue with hyalinized seminiferous tubules | None | None | Some normal Leydig cells | ||
| Donor 3 | Normal testicular morphology with qualitatively and quantitatively normal spermatogenesis | Normal | Normal | Normal | Normal | Obstructive azoospermia |
| Grafts 14 weeks | Nearly completely degenerated testicular tissue | None | Few Sertoli cell-only (SCO) tubules | High degree of fibrosis | Some Leydig cells | |
| Donor 4 | Mild hypospermatogenesis with Leydig cell hyperplasia | Mixed appearance from SCO tubules to normal number of germ cells | Normal | Normal | Large clusters of hyperplastic Leydig cells | Obstructive azoospermia |
| Grafts 2 weeks | Degenerated testicular tissue, immune cell infiltration | None | Few SCO tubules, high degree of hyalinization | Mostly degenerated.When present high degree of fibrosis | Few normal Leydig cells | |
| Grafts 19 weeks | Fully degenerated testicular tissue with hyalinized seminiferous tubules | None | None | Some morphologically normal Leydig cells present | ||
| Donor 5 | Testicular cancer, regions of non- cancerous tissue with qualitatively normal spermatogenesis | All types present | Normal | Normal | Normal | Testicular cancer. Normospermia in non-cancerous tissue |
| Grafts 10 weeks | Focal SCO, mild to heavy fibrosis | Type A spermatogonia | Normal | Variable degree of fibrosis | Normal | |
| Donor 6 | SCO syndrome with mild fibrosis | None | Many normal and few degenerating Sertoli cells. Vacuolization indicates low degree of secretion | Normal morphology, some fibrosis in degenerating tubules | Normal to low number of morphologically normal Leydig cells | Survivor of cancer after chemotherapy, SCO |
| Grafts 2 weeks | Complete SCO syndrome with focal fibrosis and hyalinization | None | Some normal and some degenerating Sertoli cells. Lumen formation indicates secretory activity | Variable degree of fibrosis | Normal number and normal morphology | |
| Graft 19 weeks | Complete SCO syndrome | None | Normal, few cells degenerating | Normal | Normal |
In experiment 2, testes were obtained from three patients who underwent gender-transforming surgery. Prior to surgery, all of these patients received therapy with cyproterone acetate for ≥2 years to suppress pituitary gonadotrophic function. The dosage varied from 50 to 100 mg/day, based on tolerability of side-effects. In addition, either estradiol valerate (10 mg/week) or ethinylestradiol (0.02 mg/day) was administered as estrogen supplementation. All hormones were supplied as oral tablets. All patients gave written informed consent for the use of their tissue and a minimal set of anonymized clinical data for research purposes. The testes were dissected and immediately transferred into ice-cold Leibovitz medium (Mediatech, Herndon, VA, USA), until grafting within 3 h.
Recipient animals and surgical procedures
In experiment 1, the donor testis biopsy tissue (donors 1–4 and 6) was cut into small fragments to be used as grafts and one fragment was fixed for reference histology (~0.2–0.5 mm in diameter). Two recipient male ICR/severe combined immunodeficiency (SCID) mice (Taconic, Germantown, NY, USA) per donor were anaesthetized and castrated. During the same surgery, each mouse received one or two incisions (~5 mm each) on each side of the back and one fragment of testis tissue was sutured to the subcutaneous tissue through each skin incision using 6–0 braided silk (two to four grafts per mouse). For donor 5, the tissue was dissected from non-cancerous areas of the testis and dissected into small fragments similar in size to the fragments obtained from biopsies. Following castration, eight small pieces of testicular tissue (0.5–1 mm3) were placed s.c. under the back skin of two nude mice (strain: Nu/Nu) using tumour transfer needles. Animal experimentation was approved by the University of Pittsburgh and University of Pennsylvania Institutional Animal Care and Use Committees.
In experiment 2, each donor testis was decapsulated and the testicular tissue was dissected into small fragments (0.5–1 mm3). Eight randomly selected fragments were grafted through small skin incisions under the back skin of two nude mice (strain: Nu/Nu) for each patient. Six recipients were used carrying a total number of 48 testis grafts. The animals were castrated during the same surgical procedure. Additional fragments of tissue were fixed in Bouin’s solution and embedded in paraffin for reference histology. Animal maintenance and handling in experiment 2 were in accordance with the German Federal Law for care and use of laboratory animals and were approved by the Regierungspräsident in Münster, Germany.
Histological analysis
In experiment 1, recipient mice were killed between 2 and 19 weeks after grafting, and in experiment 2, between 6 and 10 weeks after grafting. The grafts were dissected, fixed in Bouin’s solution and embedded routinely in paraffin or methacrylate resin (Technovit, Kulzer, Germany) in accordance with the protocol supplied by the manufacturer. Periodic acid–Schiff-stained sections were analysed. In both experiments, analysis of the histopathology and determination of the status of spermatogenesis of donor and graft testis tissue was performed in a blinded fashion by one trained observer (S.S.). In each graft, all seminiferous tubules present in the widest cross-section of each graft were examined. Tubules were qualitatively scored for the presence of spermatogonia or more advanced germ cell types and for the survival of Sertoli cells. Furthermore the degree of fibrosis and hyalinization was determined. In all animals, weight of the seminal vesicles was recorded as an indicator of bioactive testosterone secretion from the xenografts.
A semiquantitative analysis was performed on samples from experiment 2. All seminiferous tubules which were not fully hyalinized were evaluated in the grafted tissue samples. Each cross-section of seminiferous tubules was either scored as: (i) Sertoli cell only (SCO); (ii) Type A spermatogonia present; or (iii) Type B spermatogonia present. At least three different regions of the pre-grafting samples were analysed and an average of 170 seminiferous tubules per patient was scored to determine the degree of spermatogenic activity in the testicular tissue prior to grafting.
Results
Experiment 1
The donor tissue from the six patients used for the grafting showed different testicular pathologies and a variable degree of spermatogenesis. Overall, 28 out of 37 grafts (76%) could be recovered, representing grafted tissue from each donor. The recovered xenografts had all established blood supply. All grafts obtained from biopsies which had germ cells at the time of grafting (donors 1–4; Figure 1A, C) showed degenerative changes of the seminiferous tubules at all time points analysed (Figure 1B, D). In the grafted tissue from the testis of the testicular cancer patient who showed qualitatively normal spermatogenesis prior to grafting (donor 5), the majority of seminiferous tubules survived after 10 weeks (Figure 1E, F). The grafted tissue revealed histopathological changes similar to those observed in infertile patients such as fibrosis of the lamina propria and a high number of SCO tubules, but also contained some Type A spermatogonia. The testicular tissue of the patient with non-obstructive azoospermia, whose histological diagnosis was SCO syndrome as a consequence of chemotherapy (donor 6; Figure 1G), was recovered both at 2 and 19 weeks after xenografting and had maintained intact tubular morphology present at the time of grafting (Figure 1H). The qualitative histological appearance of the testicular tissue before and after xenografting is shown in Table I.
Figure 1.
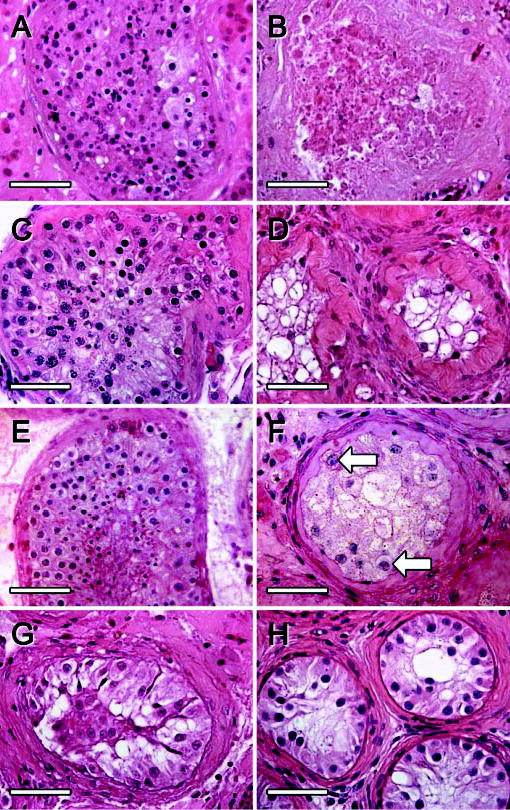
Histological appearance of human testis tissue from infertility patients [A, donor 4 (obstructive azoospermia); C, donor 3 (obstructive azoospermia); G, donor 6 (after chemotherapy)] and a testicular cancer patient (E, donor 5, testicular cancer patient) before and 2 weeks (B), 14 weeks (D), 10 weeks (F) and 19 weeks (H) after grafting into mouse hosts. Germ cells were lost from grafts of biopsies that presented with active spermatogenesis (A and B, C and D), whereas some A spermatogonia remained in grafts of normal testicular tissue from the cancer patient (E and F; arrows: Type A spermatogonia). Tubular morphology was maintained in quiescent seminiferous tubules (G, H). Bars = 50 μm.
Although Leydig cells could be encountered in the grafted tissue, the low weight of the seminal vesicles in all recipient animals (range: <10–35 mg) indicates that no or only small amounts of androgens were secreted by the xenografts. Normal weight of the seminal vesicles in intact male mice is >100 mg.
Experiment 2
As expected due to the long exposure to high levels of estrogens, the testicular tissue of all trans-sexual patients showed severe, although variable, degrees of testicular degeneration. In the first trans-sexual patient, most of the seminiferous tubules were fully hyalinized and did not contain living cells prior to grafting (Figure 2A). Of those seminiferous tubules which contained living cells, only 13% contained also Type A spermatogonia. All other seminiferous tubules were SCO (Figure 2B). No Type B spermatogonia were observed in the testis of this patient. Twelve testicular grafts from this donor were recovered from mice recipients after 6 weeks. The majority of these grafts (58%) contained only fully degenerated seminiferous tubules. Five grafts (42%) contained some SCO tubules (Figure 2E). No spermatogonia were observed in the grafted tissue of this patient.
Figure 2.

Histological appearance of human testis tissue from three trans-sexual patients before (A–D) and 6 weeks after xenografting into mouse hosts (E–H). Testicular tissue from patient 1 (A, B) showing severe degeneration with mainly or fully hyalinized seminiferous and Sertoli cell-only tubules. Testicular tissue from patient 2 (C, D) had viable seminiferous tubules and about half of them contained Type A spermatogonia, some multinucleated (white arrow) (E) Grafted testicular tissue from patient 1. No germ cells were observed in the seminiferous tubules. Grafted testicular tissue from patients 2 (F, G) and 3 (H): Some spermatogonia are observed in the seminiferous tubules (black arrow). In all grafts Sertoli cell morphology appears to be normal and no strong fibrosis is observed in the lamina propria of the seminiferous tubules. Bars = 50 μm.
The testicular tissue of the second patient demonstrated better survival of the Sertoli cells following estrogen therapy (Figure 2C). Only few fully hyalinized tubules were observed and 50% of the seminiferous tubules contained not only Sertoli cells but also Type A spermatogonia. A substantial proportion of the Type A spermatogonia were multinucleated and some Type B spermatogonia were encountered in two of the >300 analysed seminiferous tubules (Figure 2D). From the 13 testicular tissue grafts which were recovered and analysed 6 weeks after grafting, only one graft showed complete degeneration of all seminiferous tubules. About half of the seminiferous tubules in the remaining 12 grafts were fully hyalinized. From 186 analysed seminiferous tubules, 11% contained Type A spermatogonia (Figure 2F, G). The remaining 89% were SCO tubules. No multinucleated Type A spermatogonia or Type B spermatogonia were encountered in the grafted tissue.
The testicular tissue obtained from the third trans-sexual patient resembled the degree of estrogen-induced damage of the seminiferous tubules observed in patient 2: 60% of the seminiferous tubules contained spermatogonia and the remaining 40% of the tubules were SCO. No Type B spermatogonia were observed in this patient. In the grafted tissue degeneration was more pronounced when compared to patient 2 as 50% of the 24 recovered grafts after 6 weeks contained exclusively fully hyalinized seminiferous tubules. However, 5% of the analysed seminiferous tubules contained Type A spermatogonia (Figure 2H).
All recipients of testicular tissue from trans-sexual patients, like those in experiment 1, showed small seminal vesicles <30 mg. This indicates that the release of androgens from the graft was absent or very low.
Discussion
Our data reveal that xenografting of adult human testicular tissue into immunodeficient mice is not likely to correct the pathophysiological conditions leading to spermatogenic disruption. These findings are in agreement with previous observations that spermatogenesis cannot be maintained in testis xenografts from mature rats (Schlatt et al., 2002a), whereas xenografting of neonatal or immature testis tissue from a variety of species resulted in testicular development, completion of spermatogenesis and production of fertilization-competent sperm (Honaramooz et al., 2002, 2004; Schlatt et al., 2002a, 2003). In this study, we unequivocally show that spermatogonia can survive in adult human xenografts. Although under the current conditions most of the human testicular tissue undergoes complete regression of spermatogenesis, it appears that when sufficient testicular tissue is transplanted few grafts show a more favourable chance of germ cell survival and will contain undifferentiated germ cells even after extended periods following grafting. The widespread degeneration and almost full regression of the grafted tissue limits the use of biopsies as donor material. However, when larger quantities of testicular tissue are available, e.g. from orchidectomies, establishment of a larger number of grafts in several recipients offers a realistic chance to maintain germline stem cells. Thus, xenografting of human testis tissue has the potential for germline conservation.
In the present study, we used two different strains of immunocompromised mice. Both nude mice and SCID mice have been used previously for testicular xenografting experiments, and so far no obvious differences in regard to the survival and recovery of ectopic testicular grafts between strains have been reported. Due to the fact that the immune system in nude mice is less compromised when compared to SCID mice, it might be possible that some testicular grafts are lost due to more prominent tissue rejection. On the other hand, nude mice colonies are easier to maintain and the loss of hair minimizes the complications due to infections and wound healing after the grafting procedure. To our knowledge, no systematic comparison has yet been performed to determine the optimal animal model to be used as a recipient for xenografts in a clinical setting.
Since all recipient mice were castrates and had small seminal vesicles (<30 mg) at the time of death, steroidogenesis in the grafts was low, supporting the fact that the recovery of testis function was poor. This low steroidogenic activity is different from the situation after grafting of pre-pubertal donor tissue which supplies normal to elevated levels of androgens to the castrated donors (Honaramooz et al., 2002, 2004; Schlatt et al., 2002).
Seminiferous tubules from donors where spermatogenesis was suppressed by chemotherapy or hormonal treatment showed superior survival in xenografts compared to those with active spermatogenesis. In these involuted testes, the integrity of the seminiferous tubules is largely maintained. It remains to be investigated whether it is possible to improve conditions to allow survival and recovery of spermatogenesis in grafts from adult men with active spermatogenesis. In addition, since the pathological changes observed in xenografts from donors with active spermatogenesis reflected those of infertile patients, xenografting might be a potential model for the study of some aspects of male infertility. It is interesting that hyalinization of seminiferous tubules is already a quite prominent histological feature in testis grafts obtained several weeks after grafting. Since hyalinization of seminiferous tubules is considered the final stage of testicular degeneration (Soderstrom, 1986), it is obvious that it was initiated quite rapidly after the grafting procedure and has occurred during the rather short period of grafting. This indicates that areas of hyalinized seminiferous tubules in diagnostic biopsies of infertile patients may indicate not only a long-term, but also a more recent, damage to the testicular environment.
To gain a better insight into the degenerative events occurring in the grafted tissue, functional markers will be employed in future studies. Immunohistochemical detection of markers for Sertoli cells and peritubular cell differentiation (anti-Müllerian hormone, inhibin, alpha smooth muscle actin; Sharpe et al., 2003; Schlatt et al., 1993) or the integrity of tight junctions and other intercellular connections can reveal changes to the somatic environment. Localization of proliferation markers (proliferating cell nuclear antigen Schlatt and Weinbauer, 1994; bromodeoxyuridine) or markers for spermatogonial differentiation (GFRA-1, C-kit, Notch-1; von Schonfeldt et al., 2004) can reveal the type of germ cells and their mitogenic activity.
Xenografting of small pieces of mature human testis tissue in combination with detection of appropriate markers may provide an accessible and unique approach to elucidate the causes of infertility, test the gonadotoxic effects of new drugs or to evaluate the efficiency of novel male contraceptive treatments. The possibility of cancer reintroduction through testicular tissue cells (Jahnukainen et al., 2001) highlights the necessity of devising reliable strategies to screen gonadal tissue/cells prior to cryopreservation and transplantation (Fujita et al., 2005; Wallace et al., 2005). The potential need to purge tumour cells from the testicular grafts can be addressed more reliably with the xenotransplant in vitro model.
It will be interesting to explore whether immature human testis tissue also survives better as xenograft and has a higher potential for development as was observed in animal studies, thereby providing a potential strategy for germline preservation in pre-pubertal cancer patients. In addition, it remains to be determined whether this technique can be used to produce viable sperm for future potential use in assisted reproduction.
Although the key reason for pursuing fertility preservation is to restore personal autonomy to those who would otherwise be unable to conceive, many regulatory and ethical issues remain. Factors of concern are the risk of the biopsy, transmission of communicable diseases, adequate handling of cells and tissue and the overall clinical safety and effectiveness. Obtaining informed consent from children is not an easy task: parents may initially rule in place of the child, but the child should provide assent to the research without undue pressure or coercion.
In conclusion, xenografting of human testis tissue could provide a novel strategy for germline preservation. This approach could also be valuable to study the early stages of human spermatogenesis.
Acknowledgments
This study was supported by 1R01 RR17359–01 (I.D.), and a Sachbeihilfe from the Deutsche Forschungsgemeinschaft (SCHL394/6) and 1R01 050617-01 (S.S.).
References
- Fujita K, Ohta H, Tsujimura A, Takao T, Miyagawa Y, Takada S, Matsumiya K, Wakayama T and Okuyama A (2005) Transplantation of spermatogonial stem cells isolated from leukemic mice restores fertility without inducing leukemia. J Clin Invest doi:10.1172/JCI24189. [DOI] [PMC free article] [PubMed]
- Honaramooz A, Snedaker A, Boiani M, Scholer H, Dobrinski I, Schlatt S. Sperm from neonatal mammalian testes grafted in mice. Nature. 2002;41:778–781. doi: 10.1038/nature00918. [DOI] [PubMed] [Google Scholar]
- Honaramooz A, Li MW, Penedo MC, Meyers S, Dobrinski I. Accelerated maturation of primate testis by xenografting into mice. Biol Reprod. 2004;70:1500–1503. doi: 10.1095/biolreprod.103.025536. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Hou M, Petersen C, Setchell B, Soder O. Intratesticular transplantation of testicular cells from leukemic rats causes transmission of leukemia. Cancer Res. 2001;61:706–710. [PubMed] [Google Scholar]
- Nordhoff V, Schlatt S. Present and future options for preservation of testis tissue and function. Endocr Dev. 2003;5:136–155. doi: 10.1159/000069302. [DOI] [PubMed] [Google Scholar]
- Oatley JM, Reeves JJ, McLean DJ. Establishment of spermatogenesis in neonatal bovine testicular tissue following ectopic xenografting varies with donor age. Biol Reprod. 2005;72:358–364. doi: 10.1095/biolreprod.104.030783. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- Radford J. Restoration of fertility after treatment for cancer. Horm Res. 2003;59(Suppl 1):21–23. doi: 10.1159/000067840. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF. Immunohistochemical detection of proliferating cell nuclear antigen as a tool to study cell proliferation in rodent and primate testes. Int J Androl. 1994;17:214–222. doi: 10.1111/j.1365-2605.1994.tb01245.x. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF, Arslan M, Nieschlag E. Appearance of alpha-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle stimulating hormone and maintained after hormonal withdrawal. J Androl. 1993;14:340–350. [PubMed] [Google Scholar]
- Schlatt S, Rosiepen G, Weinbauer GF, Rolf C, Brook PF, Nieschlag E. Germ cell transfer into rat, bovine, monkey and human testes. Hum Reprod. 1999;14:144–150. doi: 10.1093/humrep/14.1.144. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Kim S, Gosden R. Spermatogenesis and steroidogenesis in mouse, hamster and monkey testicular tissue after cryopreservation and grafting. Reproduction. 2002a;124:323–329. doi: 10.1530/rep.0.1240339. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002b;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Honaramooz A, Boiani M, Schöler HR, Dobrinski I. Progeny from sperm obtained after ectopic grafting of neonatal mouse testes. Biol Reprod. 2003;68:2331–2335. doi: 10.1095/biolreprod.102.014894. [DOI] [PubMed] [Google Scholar]
- Sharpe RM, McKinnell C, Kivlin C, Fisher JS. Proliferation and functional maturation of Sertoli cells, and their relevance to disorders of testis function in adulthood. Reproduction. 2003;125:769–784. doi: 10.1530/rep.0.1250769. [DOI] [PubMed] [Google Scholar]
- Snedaker AK, Honaramooz A, Dobrinski I. A game of cat and mouse: xenografting of testis tissue from domestic kittens results in complete cat spermatogenesis in a mouse host. J Androl. 2004;25:926–930. doi: 10.1002/j.1939-4640.2004.tb03163.x. [DOI] [PubMed] [Google Scholar]
- Soderstrom KO. Tubular hyalinization in human testis. Andrologia. 1986;18:97–103. doi: 10.1111/j.1439-0272.1986.tb01746.x. [DOI] [PubMed] [Google Scholar]
- von Schonfeldt V, Wistuba J, Schlatt S. Notch-1, c-kit and GFRα-1 are developmentally regulated markers of premeiotic germ cells. Cytogenet Genome Res. 2004;105:235–239. doi: 10.1159/000078194. [DOI] [PubMed] [Google Scholar]
- Wallace WH, Anderson RA, Irvine DS. Fertility preservation for young patients with cancer: who is at risk and what can be offered? Lancet Oncol. 2005;6:209–218. doi: 10.1016/S1470-2045(05)70092-9. [DOI] [PubMed] [Google Scholar]


