Abstract
α-Dystroglycan (α-DG) has been identified as a major receptor for lymphocytic choriomeningitis virus (LCMV) and Lassa virus, two Old World arenaviruses. The situation with New World arenaviruses is less clear: previous studies demonstrated that Oliveros virus also exhibited high-affinity binding to α-DG but that Guanarito virus did not. To extend these initial studies, several additional Old and New World arenaviruses were screened for entry into mouse embryonic stem cells possessing or lacking α-DG. In addition, representative viruses were further analyzed for direct binding to α-DG by means of a virus overlay protein blot assay technique. These studies indicate that Old World arenaviruses use α-DG as a major receptor, whereas, of the New World arenaviruses, only clade C viruses (i.e., Oliveros and Latino viruses) use α-DG as a major receptor. New World clade A and B arenaviruses, which include the highly pathogenic Machupo, Guanarito, Junin, and Sabia viruses, appear to use a different receptor or coreceptor for binding. Previous studies with LCMV have suggested the need for a small aliphatic amino acid at LCMV GP1 glycoprotein amino acid position 260 to allow high-affinity binding to α-DG. As reported herein, this requirement appears to be broadly applicable to the arenaviruses as determined by more extensive analysis of α-DG receptor usage and GP1 sequences of Old and New World arenaviruses. In addition, GP1 amino acid position 259 also appears to be important, since all arenaviruses showing high-affinity α-DG binding possess a bulky aromatic amino acid (tyrosine or phenylalanine) at this position.
Arenaviruses are enveloped, single-stranded RNA viruses with a bisegmented and ambisense genome (8). Two groups of arenaviruses are presently recognized: the Old World arenaviruses, with lymphocytic choriomeningitis virus (LCMV) and Lassa virus as the prototype members, and the New World arenaviruses, a more extensive group, with Tacaribe virus as the prototype. In both groups, a substantial number of the viruses are associated with severe hemorrhagic fever (HF), yet some of the viruses are not known to cause human disease. In the Old World group, Lassa virus is the cause of Lassa fever, a severe disease with a mortality of approximately 15%. The New World arenaviruses have previously been divided into three major clades, A, B, and C, with all of the HF viruses being members of clade B (4). These include Junin, Machupo, Guanarito, and Sabia viruses, which are the cause of Argentinian HF, Bolivian HF, Venezuelan HF, and a single fatal HF case in Brazil, respectively (8). These New World HF viruses generally result in disease with higher mortality (20 to 30%) than Lassa fever. Rodents are the natural hosts of arenaviruses, and human infections occur by exposure to rodent excreta.
It seems likely that the considerable variation seen among these viruses in their pathogenicity and tropism is linked, at least in part, to variation in host cell receptor usage (16, 23). The selective attachment and binding of enveloped RNA viruses to host cells are often a complex process involving various receptors and coreceptors (2, 20). To date, only α-dystroglycan (α-DG) has been identified as a receptor for the arenaviruses (10). However, some New World arenaviruses and variants of Old World arenaviruses appear to have low-affinity binding to α-DG, suggesting the potential role of additional receptors or coreceptors (10, 24). To examine this further, we analyzed the interaction of most of the New World arenaviruses and an additional Old World virus, Ippy, with α-DG. Initial screening was done using stem cell lines lacking (null mutant) or possessing α-DG (+/+). This was followed by use of a virus overlay protein blot assay (VOPBA) technique to measure differences in the affinity of binding of the viruses to α-DG. Last, we determined the nucleotide sequence of the region of the genome of the New World arenaviruses encoding the GP1 glycoprotein domain equivalent to that suspected to be critical for LCMV binding to α-DG (10, 15, 24).
Our binding studies show that, while virtually all isolates of the Old World arenaviruses exhibit high-affinity binding to α-DG, New World arenaviruses in clades A and B do not. Comparison of the deduced GP1 amino acid sequences indicate that the amino acids at positions 259 and 260 influence virus-host cell receptor interactions. This study highlights the importance of α-DG in binding of Old World arenaviruses and clade C New World arenaviruses to host cells and indicates that the other New World arenaviruses, including those associated with HFs, utilize a different major receptor or coreceptor.
MATERIALS AND METHODS
Proteins and antibodies.
α-DG was purified from rabbit skeletal muscle (12). Monoclonal antibodies to LCMV-GP, WE36.1 (GP1), and 86.3 (GP2) have been described earlier (7). Horseradish peroxidase (HRP)-conjugated anti-mouse immunoglobulin G (IgG) was from Pierce Chemical Co. (Rockford, Ill.). Mouse hyperimmune serum against the Old World and New World arenaviruses was from the Special Pathogens Branch of the Centers for Disease Control and Prevention (Atlanta, Ga.).
Virus strains, virus purification, and virus quantification.
LCMV Armstrong ARM53b is a triple plaque-purified isolate of ARM CA 1371 (11). LCMV WE54 has been described elsewhere (27). Seed stocks of LCMV stains were prepared by growth in baby hamster kidney (BHK-21) cells. Purified virus stocks were produced and titers were determined as described earlier (10, 11). The rest of the virus strains used in this study were obtained from the collection at the Special Pathogens Branch, Centers for Disease Control and Prevention. The virus passage history of each is as follows: Parana SHB5, V1, E6 + 1; Oliveros, E6 + 2; Tamiami SMB7, SHB1, V1, E6 + 2; Latino SHB6, V1, E6 + 1; Pichinde SM14, SHB1, V1, E6 + 1; Amapari SM13, V1, E6 + 1; Sabia SMB, E6 + 2; and Machupo SM80, E6 + 3. Ippy virus was obtained from R. Shope, University of Texas Medical Branch at Galveston. The exact history of this virus is unclear, but it had been passed in suckling mice and had not been tissue culture adapted prior to this study. Allpahuayo virus was obtained from R. B. Tesh, University of Texas Medical Branch at Galveston. Purified virus stocks for Amapari, Parana, Oliveros, and Latino viruses were prepared as previously reported (10). The work with most of the infectious viruses was done at biosafety level 3 (BSL-3), with the exception of the Machupo, Sabia, Flexal, and Guanarito viruses, which were handled in the BSL-4 laboratories at the Special Pathogens Branch, and the LCMV strains, which were handled at BSL-2.
Cells.
African green monkey kidney (Vero-E6) and BHK-21 cells were maintained in Dulbecco's modified Eagle medium (Gibco BRL, Grand Island, N.Y.) containing 10% fetal calf serum (HyClone, Logan, Utah). The α-DG knockout embryonic stem (ES) cells (B11) and the wild-type (R1) cells were maintained in Dulbecco's modified Eagle medium containing 20% fetal calf serum, 1% glutamine, 1% essential amino acids (Gibco BRL), 0.001% β-mercaptoethanol (Sigma, St. Louis, Mo.), and 103 U of leukemia inhibitory factor (Gibco BRL)/ml. The cells were seeded in tissue culture flasks pretreated with 0.1% gelatin.
Immunofluorescence.
α-DG+/+ and α-DG−/− ES cells were grown on gelatin-pretreated glass coverslips and were infected with the arenaviruses at a multiplicity of infection of 0.1 to 1, with the exception of Ippy virus, which was used at an apparent low multiplicity of infection because it had previously only been passed in suckling mice. At 24 h postinfection, the infected cells were washed twice with phosphate-buffered saline (PBS) and were fixed with acetone at room temperature for 10 min. After fixation, the cells were washed three times with PBS and mouse hyperimmune serum against New World arenaviruses was added at a 1:200 dilution in 1% bovine serum albumin in PBS for 30 min. The cells were then washed three times with PBS and incubated for 30 min with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (Sigma), diluted 1:200 in 1% bovine serum albumin in PBS. Multiple final washes were done, and the cells were mounted on microscope slides and viewed using a Zeiss microscope.
VOPBA.
VOPBA was performed as described earlier (10, 25). In brief, α-DG from rabbit skeletal muscle was subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and the protein was transferred to nitrocellulose. Virus binding was tested, using 107 PFU of Lassa virus (LFV) and LCMV (WE54 and ARM53b) per ml. Bound virus was detected by monoclonal antibodies 33.6 (undiluted hybridoma supernatant) and 86.6 (dilution 1:500), using an HRP-conjugated secondary antibody. Blots were developed using Super Signal West Pico ECL Substrate (Pierce), and signals were recorded on autoradiographic film (Kodak, Rochester, N.Y.). For VOPBA with Oliveros, Latino, Amapari, and Parana viruses, purified α-DG from rabbit skeletal muscle (5 μg/lane) was separated by SDS-PAGE, blotted to nitrocellulose by electrotransfer, and incubated with 107 PFU of polyethylene glycol-precipitated Amapari, Oliveros, Latino, and Parana viruses per ml. For detection of bound virus, a 1:1,000 dilution of mouse hyperimmune serum against New World arenaviruses and an HRP-coupled anti-mouse IgG secondary antibody were used. Blots were developed with enhanced chemiluminescence (ECL) as described above.
Detection of Oliveros, Latino, Amapari, and Parana viruses in dot blot.
Preparations of Oliveros, Latino, Amapari, and Parana viruses were diluted to 107 PFU/ml and dotted on nitrocellulose. After being air dried, the membrane was blocked in 5% skim milk powder-PBS for 1 h. As primary antibody, a 1:1,000 dilution of mouse hyperimmune serum against New World arenaviruses was used in 2% (wt/vol) skim milk-PBS and was incubated for 1 h at 6°C. After three washes in PBS-0.1% (wt/vol) Tween 20, the secondary antibody, goat anti-mouse IgG coupled to peroxidase 1:5,000 in PBS-0.1% (wt/vol) Tween 20, was applied for 1 h at room temperature. The blot was developed using Super Signal West Pico chemiluminescence substrate.
RNA extraction, amplification, and sequence analysis.
RNA was extracted from supernatants of Vero-E6 cells infected with the virus isolates Latino, Amapari, Guanarito, Parana, Machupo, and Tamiami. TriPure reagent (1 ml) was added to 200 μl of tissue culture medium followed by chloroform extraction and matrix purification (Rnaid kit; Bio 101, La Jolla, Calif.). For reverse transcriptase PCR amplification, three main primer pairs were used. A conserved reverse primer with sequence neg933 (CTCTAAACAGTATCCACCTGG) (numbering based on Allpahuayo virus S segment) was used in all three pairs. The forward primer of the first pair was designed based on the Oliveros virus S genome segment sequence. This primer, pos861 (TACAAAACACCACTTGGGA), and reverse primer neg933 were used to amplify the S segment fragment of Latino, Guanarito, and Amapari viruses. The forward primer of the second pair was designed based on the Allpahuayo virus S genome. This primer, pos757 (TTATCATTCAGAACACAACATGG), and neg933 were used to amplify the S segment fragment of Parana and Tamiami viruses. The forward primer of the third pair was designed based on the Junin (Parodi strain) virus S segment sequences. This primer, pos450 (GTGGGGCATGATTGGT), and neg913 were used to amplify the S segment fragment of Machupo virus. The PCR products were purified using the Qiagen (Valencia, Calif.) PCR purification kit. A fraction of the purified product was used to perform cycle sequence analysis as previously described (4). The primers used in the sequence reaction were the same as those used in the PCR. All sequences were verified by sequencing of both strands of the PCR products.
Phylogenetic analysis.
Phylogenetic analysis was done using the PAUP* (version 4.0b4a) Macintosh computer software program (26). Nucleotide sequence differences were analyzed by the maximum-parsimony method, using the heuristic search option and a 3:1 weighting of transversions over transitions.
RESULTS
Is α-DG the major receptor for the New World arenaviruses?
The initial study on the identification of α-DG as the host cell receptor for Old World arenaviruses (LCMV and the Lassa fever, Mopeia, and Mobala viruses) included only two members of the diverse New World arenavirus group, namely, Oliveros and Guanarito (10). Oliveros virus showed high-affinity binding to α-DG, whereas Guanarito showed only low-affinity binding. On the basis of these results, we anticipated that the receptor usage for the New World arenaviruses might not be as uniform as that for the Old World arenaviruses. To examine this prospect, most of the New World arenavirus were screened for their ability to bind to α-DG. In this study we also included Ippy virus, an Old World arenavirus not included in the earlier study.
Screening was performed on two mouse ES cell lines. These included the parental line, which expressed α-DG (α-DG+/+ stem cell line), and a knockout version lacking α-DG (α-DG−/−) (14). Cells were infected and assayed by immunofluorescence for virus uptake and replication 24 h postinfection. Of the 11 New World arenaviruses tested, only Oliveros and Latino viruses showed high levels of virus antigen in the α-DG+/+ stem cells and negligible virus antigen in the α-DG knockout cells, indicating clear use of α-DG as their major functional receptor (Fig. 1). As expected, the Old World virus Ippy showed evidence of infection on α-DG+/+ stem cells but none on the α-DG knockout, a finding similar to the results seen with virtually all of the Old World arenaviruses tested previously (10). Direct virus binding to α-DG was tested by VOPBA, using α-DG purified from rabbit skeletal muscle immobilized on nitrocellulose membranes. As seen in Fig. 2A, the Old World arenaviruses LCMV WE54 and Lassa virus bind to α-DG with comparable affinity, whereas LCMV ARM53b exhibits a markedly lower binding affinity. Among the New World arenaviruses tested, Oliveros and Latino but not Amapari and Parana showed binding to α-DG (Fig. 2B). The intensity of the signals obtained with Oliveros and Latino suggests binding affinities in the range of the high-affinity binders among the Old World arenaviruses, such as LCMV WE54 and Lassa virus. A dot blot assay was used to show that similar amounts of each purified virus were used in the affinity-binding assay (Fig. 3).
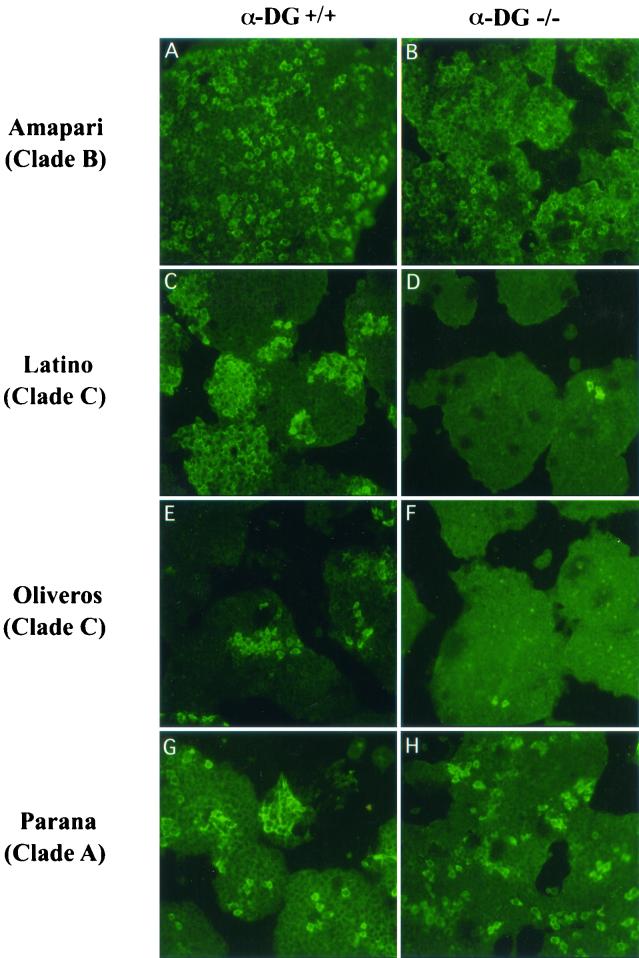
FIG. 1.
New World arenavirus infection of mouse ES cells expressing or not expressing α-DG. Virus-specific immunofluorescence staining of mouse ES cells infected with representative viruses of the different clades of the New World arenaviruses. Cells expressing α-DG (A, C, E, and G) are labeled α-DG+/+. α-DG null mutant cells (B, D, F, and H) are labeled α-DG−/−. Viruses: Amapari (A and B) as a clade B representative, Parana (G and H) as a clade A representative, and Latino (C and D) and Oliveros (E and F) as members of clade C. As would be expected, the percentages of virus-positive cells in α-DG+/+ versus α-DG−/− cells per optical field for α-DG-binding viruses, Oliveros and Latino, were 97 and 98%, respectively, and 47 and 52% for the non-α-DG-binding viruses, Amapari and Parana, respectively.
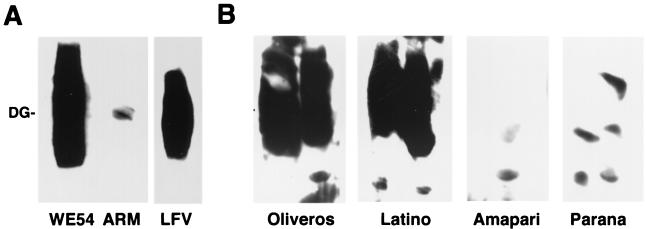
FIG. 2.
Comparison of the binding affinities for α-DG between Old and New World arenaviruses. α-DG purified from rabbit skeletal muscle was separated by SDS-PAGE and blotted to nitrocellulose. The Old World arenaviruses LCMV WE54, LCMV ARM53b, and Lassa virus (LFV) (A) were applied at 107 PFU/ml and detected by the monoclonal anti-LCMV GP2 antibodies 33.6 and 86.6, using an HRP-conjugated anti-mouse IgG secondary antibody and ECL substrate. The New World arenaviruses Oliveros, Latino, Amapari, and Parana (B), each shown in a duplicate lane and used at a concentration of 107 PFU/ml, were detected by a 1:1,000 dilution of mouse hyperimmune serum against New World arenaviruses and an HRP-conjugated secondary antibody as for panel A.
FIG. 3.
Detection of Oliveros, Latino, Amapari, and Parana viruses by dot blot assay. Five microliters (upper row) and 2.5 μl (lower row) of 107-PFU/ml Oliveros, Latino, Amapari, and Parana viruses were immobilized in nitrocellulose and detected with a 1:1,000 dilution of mouse hyperimmune serum against New World arenaviruses, using a peroxidase-conjugated secondary antibody and ECL substrate for detection.
Examination of the relationship of arenavirus phylogeny and ability to infect α-DG-expressing or non-α-DG-expressing stem cells.
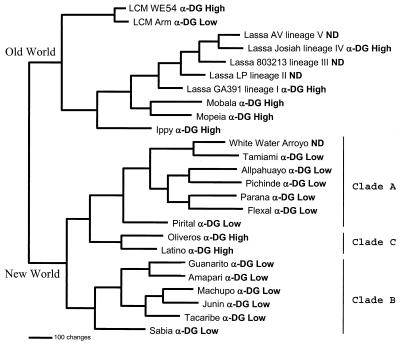
As shown previously, phylogenetic analysis of the arenaviruses shows the clear division between the Old and New World arenaviruses and defines the three major clades of New World viruses, with Oliveros and Latino representing the only currently defined members of clade C (Fig. 4). The extensive variety of viruses making up clades A and B includes the most pathogenic members of the New World arenaviruses, and all appear not to use α-DG as their receptor. Similar to the extensive phylogenetic analysis carried out by others (6, 8), we found no support for the earlier suggestion by Albariño et al. (1) that, of the New World arenaviruses, Pichinde and Oliveros viruses are the most closely related to the Old World arenaviruses.
FIG. 4.
Arenavirus phylogenetic analysis. An arenavirus phylogenetic tree was generated based on maximum-parsimony analysis of the sequence differences present among an aligned 637-nucleotide region of the virus genome S segments (6, 21). Analysis using the heuristic search option and a 3:1 weighting of transversions over transitions generated a single most-parsimonious tree. Horizontal distances represent nucleotide step differences (see bar scale), while vertical branches are for visual clarity only. The arenavirus S segment sequences included the following viruses: LCMV WE54 (GenBank accession number M22017), LCM Armstrong (M20869), Lassa AV (Af246121), Lassa Josiah (J04324), Lassa 803213 (x52400), Lassa LP (af181854), Lassa GA391 (af181853), Mobala (af012530), Mopeia (m33879), Ippy (u80003), White Water Arroyo (af228063), Tamiami (u43690), Allpahuayo (ay012687), Pichinde (k02734), Parana (u43689), Flexal (u43687), Pirital (af277659), Oliveros (u34248), Latino (u43688), Guanarito (u43686), Amapari (u43685), Machupo (x62616), Junin (d10072), Tacaribe (m20304), and Sabia (u41071). Two major clades are seen, corresponding to the Old and New World arenaviruses. The New World viruses form three major clades: A, B, and C. The high- or low-affinity α-DG binding of each virus tested is indicated adjacent to the virus label. ND, not done.
Possible sequence requirements in GP1 determine α-DG receptor usage.
Extensive studies of LCMV variants had suggested that amino acid 260 may play an important role in determining usage of α-DG as a receptor (15, 24, 25). The parental LCMV Armstrong strain and its variant, clone 13, differ considerably in their pathogenicity for mice and their affinity for α-DG. Clone 13 virus binds α-DG at a 2.5-log-higher affinity than does the parental Armstrong virus. Further, clone 13 binding can be blocked by 4 to 6 nM soluble α-DG, whereas more than 400 nM is required to block LCMV Armstrong. Reassortments between LCMV clone 13 and LCMV Armstrong mapped the genes involved in binding to the S RNA, which encodes the GP and NP proteins (25). There are only two amino acid differences identified between Armstrong and clone 13. There are amino acid change in the surface GP1 at position 260 from an F (Armstrong) to an L (clone 13) and a second change in the L viral polymerase at position 1079 from K (Armstrong) to Q (clone 13) (22). Thus, the altered use of α-DG as a receptor would appear to be linked to the change at GP1 amino acid 260, as noted by sequence analysis of more than 35 LCMV variants and strains that showed different binding affinities to α-DG (24).
Sequence analysis of the Old and New World arenaviruses (Fig. 5) shows that the presence of a leucine or isoleucine at position 260 would be generalized as required for the interaction of the virus surface glycoproteins with their receptor usage. However, it is noteworthy that all the viruses that utilize α-DG have a bulky amino acid possessing an aromatic ring, such as F or Y, at amino acid position 259. It appears that the combination of a bulky aromatic amino acid at 259 and a small aliphatic amino acid at 260 may be required for α-DG binding. The presence of a second bulky amino acid at position 260 (as in the case of LCMV Armstrong and other strains and variants of LCMV not containing a bulky aromatic amino acid at this position and which fail to bind to α-DG [24]) must sterically hinder virus-α-DG interaction or alter the secondary or tertiary structure and thereby lower the affinity of binding of such viruses to α-DG.
FIG. 5.
Possible sequence requirements on the GP1 for high-affinity binding to α-DG. Arenaviruses using α-DG as their major receptor have the small aliphatic amino acid leucine or isoleucine at position 260 (position numbering relative to LCMV) and a bulky aromatic amino acid, phenylalanine or tyrosine, at position 259 (presented in boldface). The consensus sequence cleavage site (tetrapeptide) between GP1 and GP2 of each arenavirus is shown underlined in italics.
DISCUSSION
The receptor of the Old World arenaviruses has been identified recently as α-DG. Utilizing the same tools used in the initial identification of the α-DG receptor (i.e., mouse stem cells expressing α-DG or null mutants lacking α-DG and α-DG-binding assays), we extended the screening to include most of the New World arenaviruses and Ippy virus, an Old World arenavirus not included in the earlier study. The present study shows that all of the New World arenaviruses tested, with the exception of the clade C viruses Oliveros and Latino, do not use the same receptor as the Old World arenaviruses. The use of different receptors or coreceptors by members of the same virus family is not surprising, as this has often been observed in viruses belonging to the same genus (3, 13). On the other hand, the extent of the α-DG usage as a receptor by many of the arenaviruses is quite surprising, given the diverse tropism (e.g., LCMV versus Lassa) and pathogenicity (Lassa versus Mopeia) of many of these viruses.
Analysis of the phylogenetic relationship of the arenaviruses relative to their α-DG receptor usage indicates that the Old World arenaviruses and the clade C New World arenaviruses share this property. The rodent hosts of the Old World arenaviruses are evolutionarily more ancient than the hosts of the New World arenaviruses, strongly suggesting that the Old World arenaviruses are ancestral to the New World arenaviruses (5). It would appear then that the use of α-DG as a receptor may be an ancestral feature of the arenaviruses that has been lost by clades A and B but retained by clade C New World arenaviruses during the evolution of this diverse virus family. However, based on virus-host ecologic and phylogenetic studies, it is very difficult to explain why only clade C of the New World arenaviruses utilizes the same receptor as the Old World arenaviruses. An earlier phylogenetic study suggested that Oliveros (clade C) and Pichinde (clade A) New World arenaviruses may be more closely related to the Old World arenaviruses and ancestral to the clade B New World arenaviruses (1). This would have correlated to some extent with Old World and clade C arenaviruses sharing the ancestral property of α-DG receptor usage. However, a recently done, more rigorous phylogenetic analysis (6, 8) showed that the ancestral placing of Oliveros and Pichinde relative to other New World arenaviruses in the earlier study (1) was an artifact of the method used. Similarly, there is no clear correlation relative to primary rodent reservoir host species. For example, the natural host for clade C viruses Latino and Oliveros are Calomys callosus and Bolomys obscurus, respectively (i.e., rodents of two different rodent genera within the family Muridae). However, C. callosus is also the natural host of Machupo virus (clade B).
Earlier studies indicated the importance of the GP1 carboxy terminus in arenavirus-host cell interactions. More specifically, previous sequence analysis of LCMV variants has clearly shown that aliphatic amino acid L or I at GP1 position 260 is critical for α-DG high-affinity binding and receptor usage (24, 25). The present study further defines the amino acid requirement for α-DG receptor usage. Comparison of the GP1 amino acid sequences and α-DG receptor usage of a broad array of Old and New World arenaviruses indicates that, despite also being aliphatic, a V instead of L or I at position 260 (e.g., Pirital, Pichinde, Allpahuayo, Guanarito, Parana, and Tacaribe viruses) prevents high-affinity α-DG binding.
In addition to L or I at position 260, we observed that the presence of the bulky aromatic amino acid F or Y at position 259 may be critical for high-affinity α-DG binding. This suggests that the GP1 amino acid motif corresponding to α-DG high-affinity binding may be a bulky aromatic (F or Y) at position 259 followed by a small aliphatic (L or I) at position 260. This would be consistent with the low-affinity binding seen for LCMV Armstrong, in which a second bulky aromatic amino acid (F) replaces the small aliphatic amino acid at position 260. This presumably causes a steric hindrance of the high-affinity α-DG binding. Similarly, the absence of α-DG binding by Pirital, Pichinde, Allpahuayo, Guanarito, Parana, and Tacaribe viruses, despite the presence of the small aliphatic V at position 260, may be related to the absence of a bulky aromatic at position 259 in these viruses. Interestingly, the amino acid region amino-terminal to positions 259 and 260 is highly variable, reminiscent of binding domains and neutralization sites seen in influenza variants and other well-characterized segmented negative-strand RNA viruses (17).
There is only 1 amino acid separating position 260 from the tetrapeptide sequence of the consensus cleavage site (9, 18). For Lassa virus, the consensus cleavage site has been identified as RRLL. Based on mutational analysis, the motif R-X-L/I/V-L has been shown to be essential for cleavage (18). In addition, subtilase SKI1/S1P has been identified recently as the cleavage enzyme of the Lassa virus glycoprotein precursor (19). It is, however, apparent from the sequences reported in this paper that most of the tetrapeptide cleavage site sequences of the New World arenaviruses, including some of the pathogenic ones, such as Machupo, Sabia, and Guanarito, do not possess the consensus motif R-X-L/I/V-L. Cellular proteases other than SKI1/S1P could be responsible for the cleavage of the New World arenaviruses' glycoprotein precursor and their tissue tropism.
In addition to amino acid position 260, a second amino acid at position 153 of GP1 (position numbering relative to LCMV) has been implicated in receptor binding based on earlier studies of virus reassortants between LCMV strain WE variants and the Armstrong strain (27). However, the comparison of the published Old and New World arenavirus GP1 sequences indicates that position 153 is highly variable among these viruses, and no obvious correlation can be seen relative to virus α-DG binding. The ability to make site-directed specific changes in the LCMV GP1 and direct affinity-binding measurements to α-DG using surface plasmon resonance should be informative in this regard.
The virus family Arenaviridae includes an extensive number of severe human pathogens that cause HFs. The identification of the various receptors and coreceptors utilized by arenaviruses and of their relationship relative to viral pathogenesis is clearly important and should lead to a deeper understanding of this virus-host cell interaction and the potential treatment of arenavirus-associated human diseases. This study emphasizes the need for continuing studies on the identification of additional receptors and coreceptors that must be utilized by the New World arenaviruses.
Acknowledgments
We thank Stuart Nichol for helping with the phylogenetic analysis and for extensive discussions and Thomas Ksiazek for helpful discussions and for supplying arenavirus reagents.
This work was supported by Public Health Service grants AI-09484 and AI-45927 from the National Institutes of Health. S. Kunz is a recipient of a fellowship from the Swiss National Science Foundation. K. P. Campbell is an investigator of the Howard Hughes Medical Institute.
REFERENCES
- 1.Albariño, C. G., D. M. Posik, P. D. Ghiringhelli, M. E. Lozano, and V. Romanowski. 1998. Arenavirus phylogeny: a new insight. Virus Genes 16:39-46. [DOI] [PubMed] [Google Scholar]
- 2.Baranowski, E., C. M. Ruiz-Jarabo, and E. Domingo. 2001. Evolution of cell recognition by viruses. Science 292:1102-1105. [DOI] [PubMed] [Google Scholar]
- 3.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 4.Bowen, M. D., C. J. Peters, and S. T. Nichol. 1996. The phylogeny of New World (Tacaribe complex) arenaviruses. Virology 219:285-290. [DOI] [PubMed] [Google Scholar]
- 5.Bowen, M. D., C. J. Peters, and S. T. Nichol. 1997. Phylogenetic analysis of the Arenaviridae: patterns of virus evolution and evidence for co-speciations between arenaviruses and their rodent hosts. Mol. Phylogenet. Evol. 8:301-316. [DOI] [PubMed] [Google Scholar]
- 6.Bowen, M. D., P. E. Rollin, T. G. Ksiazek, H. L. Hustad, D. G. Bausch, A. H. Demby, M. D. Bajani, C. J. Peters, and S. T. Nichol. 2000. Genetic diversity among Lassa virus strains. J. Virol. 74:6992-7004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Buchmeier, M. J., H. A. Lewicki, O. Tonori, and M. B. Oldstone. 1981. Monoclonal antibodies to lymphocytic choriomeningitis and pichinde viruses: generation, characterization, and cross-reactivity with other arenaviruses. Virology 113:73-85. [DOI] [PubMed] [Google Scholar]
- 8.Buchmeier, M. J., M. D. Bowen, and C. J. Peters. 2001. Arenaviridae: the viruses and their replication, p. 1635-1668. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 9.Burns, J. W., and M. J. Buchmeier. 1993. Glycoproteins of the arenaviruses, p. 17-33. In M. S. Salvato (ed.), The Arenaviridae. Plenum Press, New York, N.Y.
- 10.Cao, W., M. D. Henry, P. Borrow, H. Yamada, J. H. Elder, E. V. Ravkov, S. T. Nichol, R. W. Compans, K. P. Campbell, and M. B. Oldstone. 1998. Identification of alpha-dystroglycan as a receptor for lymphocytic chriomeningitis virus and Lassa fever virus. Science 282:2079-2081. [DOI] [PubMed] [Google Scholar]
- 11.Dutko, F. J., and M. B. Oldstone. 1983. Genomic and biological variation among commonly used lymphocytic choriomeningitis virus strains. J. Gen. Virol. 64:1689-1698. [DOI] [PubMed] [Google Scholar]
- 12.Ervasti, J. M., and K. P. Campbell. 1991. Membrane organization of the dystrophin-glycoprotein complex. Cell 66:1121-1131. [DOI] [PubMed] [Google Scholar]
- 13.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 14.Henry, M. D., and K. P. Campbell. 1998. A role for dystroglycan in basement membrane assembly. Cell 95:859-870. [DOI] [PubMed] [Google Scholar]
- 15.Kunz, S., N. Sevilla, D. B. McGavern, K. P. Campbell, and M. B. A. Oldstone. 2001. Molecular analysis of the interaction of LCMV with its cellular receptor α-dystroglycan. J. Cell Biol. 155:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuo, L., G. J. Godeke, M. J. Raamsman, P. S. Masters, and P. J. Rottier. 2000. Retargeting of coronavirus by substitution of the spike glycoprotein ectodomain: crossing the host cell species barrier. J. Virol. 74:1393-1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lamb, R. A., and R. M. Krug. 2001. Orthomyxoviridae: the viruses and their replication, p. 1487-1531. In B. N. Fields, D. M. Knipe, P. M. Howley, et al. (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 18.Lenz, O., J. ter Meulen, H. Feldmann, H. D. Klenk, and W. Garten. 2000. Identification of a novel consensus sequence at the cleavage site of the Lassa virus glycoprotein. J. Virol. 74:11418-11421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lenz, O., J. ter Meulen, H. D. Klenk, N. G. Seidah, and W. Garten. 2001. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 98:12701-12705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martin, J., C. C. LaBranche, and F. Gonzalez-Scarano. 2001. Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J. Virol. 75:3568-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moncayo, A. C., C. L. Hice, D. M. Douglas, M. Watts, A. P. A. Travassos de Rosa, H. Guzman, K. L. Russel, C. Calampa, A. Gozalo, V. L. Popov, S. C. Weaver, and R. B. Tesh. 2001. Allpahuayo virus: a newly recognized arenavirus (Arenaviridae) from arboreal rice rats (Oecomys bicolor and Oecomys paricola) in northeastern Peru. Virology 284:277-286. [DOI] [PubMed] [Google Scholar]
- 22.Salvato, M., E. Shimomaye, P. Southern, and M. B. Oldstone. 1988. Virus-lymphocyte interactions. IV. Molecular characterization of LCMV Amstrong (CTL+) small genomic segment and that of its variant, Clone 13 (CTL−). Virology 164:517-522. [DOI] [PubMed] [Google Scholar]
- 23.Schneider-Schaulies, J. 2000. Cellular receptors for viruses: links to tropism and pathogenesis. J. Gen. Virol. 81:1413-1429. [DOI] [PubMed] [Google Scholar]
- 24.Sevilla, N., S. Kunz, A. Holz, H. Lewicki, D. Homann, H. Yamada, K. P. Campbell, J. C. de la Torre, and M. B. A. Oldstone. 2000. Immunosuppression and resultant viral persistence by specific viral targeting of dendritic cells. J. Exp. Med. 192:1249-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smelt, S. C., P. Borrow, S. Kunz, W. Cao, A. Tishon, H. Lewicki, K. P. Campbell, and M. B. A. Oldstone. 2001. Differences in affinity of binding of lymphocytic choriomeningitis virus strains to the cellular receptor α-dystroglycan correlate with viral tropism and disease kinetics. J. Virol. 75:448-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Swofford, D. L. 1998. PAUP*: phylogenetic analysis using parsimony (* and other methods), version 4.0b4a. Sinauer Associates, Sunderland, Mass.
- 27.Teng, M. N., P. Borrow, M. B. Oldstone, and J. C. de la Torre. 1996. A single amino acid change in the glycoprotein of lymphocytic choriomeningitis virus is associated with the ability to cause growth hormone deficiency syndrome. J. Virol. 70:8438-8443. [DOI] [PMC free article] [PubMed] [Google Scholar]