Abstract
Although the transmissible spongiform encephalopathies (TSEs) are neurodegenerative diseases, their agents usually replicate and accumulate in lymphoid tissues long before infection spreads to the central nervous system (CNS). Studies of a mouse scrapie model have shown that mature follicular dendritic cells (FDCs), which express the host prion protein (PrPc), are critical for replication of infection in lymphoid tissues. In the absence of mature FDCs, the spread of infection to the CNS is significantly impaired. Tumor necrosis factor alpha (TNF-α) secretion by lymphocytes is important for maintaining FDC networks, and signaling is mediated through TNF receptor 1 (TNFR-1) expressed on FDCs and/or their precursors. A treatment that blocks TNFR signaling leads to the temporary dedifferentiation of mature FDCs, raising the hypothesis that a similar treatment would significantly delay the peripheral pathogenesis of scrapie. Here, specific neutralization of the TNFR signaling pathway was achieved through treatment with a fusion protein consisting of two soluble human TNFR (huTNFR) (p80) domains linked to the Fc portion of human immunoglobulin G1 (huTNFR:Fc). A single treatment of mice with huTNFR:Fc before or shortly after intraperitoneal injection with the ME7 scrapie strain significantly delayed the onset of disease in the CNS and reduced the early accumulation of disease-specific PrP in the spleen. These effects coincided with a temporary dedifferentiation of mature FDCs within 5 days of huTNFR:Fc treatment. We conclude that treatments that specifically inhibit the TNFR signaling pathway may present an opportunity for early intervention in peripherally transmitted TSEs.
The transmissible spongiform encephalopathies (TSEs), or “prion diseases,” are infectious neurodegenerative diseases that affect humans and both wild and domestic animals. The host prion protein (PrPc) is critical for TSE agent replication (8) and accumulates in diseased tissues as an abnormal, detergent-insoluble, relatively proteinase-resistant isoform, PrPSc (4). Although the precise nature of the TSE agent is uncertain (13), PrPSc copurifies with infectivity and is considered to be a major component of the infectious agent (41).
Natural TSEs, including sheep scrapie, bovine spongiform encephalopathy (BSE), chronic wasting disease in mule deer and elk, and variant Creutzfeldt-Jakob disease (vCJD) in humans, are considered to be acquired peripherally. For example, the emergence of vCJD in the United Kingdom population is almost certainly due to consumption of BSE-contaminated tissues (7). Following peripheral exposure, TSE agents usually accumulate in lymphoid tissues long before infection spreads to the central nervous system (CNS). For example, after intragastric or oral challenge of rodents with scrapie, the infectious agent first accumulates in Peyer's patches and gut-associated lymphoid tissues (2, 24). The detection of PrPSc in Peyer's patches and gut-associated lymphoid tissues of sheep with natural scrapie (1, 20) prior to detection in other lymphoid tissues and the CNS (46) implies that this disease is also acquired orally. Lymphoid tissues play an important role in transmission in some TSE models (17), but this tissue tropism may be agent strain dependent. Although acquired peripherally, BSE in cattle (43) and iatrogenic Creutzfeldt-Jakob disease in humans (21) appear to be confined to nervous tissues. However, within the lymphoid tissues of patients with vCJD (21) and most sheep with natural scrapie (45) or following experimental peripheral infection of rodents with scrapie (5, 29, 30, 33), early PrPSc accumulation takes place on follicular dendritic cells (FDCs). Studies of mouse scrapie models have shown that mature FDCs are critical for replication in lymphoid tissues and that in their absence, neuroinvasion following peripheral challenge is significantly impaired (5, 29, 30, 35). From the lymphoid tissues, infectious agents spread to the CNS via peripheral nerves (19).
The FDC therefore presents a potential target for therapeutic intervention in peripherally acquired TSEs such as natural sheep scrapie and vCJD. Indeed, recent studies have demonstrated that treatments that temporarily interfere with the integrity (29, 35) or function (28) of FDCs also interfere with TSE pathogenesis. Signaling through lymphocyte-derived tumor necrosis factor alpha (TNF-α) is critical for FDC development, as mice deficient in TNF-α (TNF-α−/− mice) lack mature FDCs in lymphoid tissues (38). The effects of TNF-α on FDC development are mediated via signaling through TNF receptor 1 (TNFR-1) expressed on FDCs and/or their precursors (44). Specific neutralization of the TNF-α signaling pathway leads to the temporary inactivation of FDCs (31), suggesting that FDCs require constant stimulation from this cytokine to maintain their differentiated state. It has previously been shown that in the absence of mature FDCs in the lymphoid tissues of TNF-α−/− mice, susceptibility to peripheral challenge with scrapie is reduced (30). Therefore, in this study we sought to determine whether a treatment that temporarily blocks the TNF-α signaling pathway would delay the spread of scrapie to the CNS.
MATERIALS AND METHODS
huTNFR:Fc treatment.
At the times indicated, C57BL mice (8 to 12 weeks old) were given a single intraperitoneal (i.p.) injection of 100 μg of a dimeric fusion protein containing the soluble human TNFR (huTNFR) (p80) domain linked to the Fc portion of human immunoglobulin G1 (huTNFR:Fc; Immunex Corp., Seattle, Wash.) (34) or 100 μg of polyclonal human immunoglobulin G (hu-Ig) (Sandoglobulin; provided by J. Browning, Biogen Inc., Cambridge, Mass.) as a control. To monitor the effects of treatment on FDC status, at the times indicated following treatment, two spleens were taken from each group and halved. One half was fixed in periodate-lysine-paraformaldehyde and embedded in paraffin wax for immunocytochemical detection of PrP (33) with the PrP-specific antiserum 1B3 (15). The other half was snap-frozen at the temperature of liquid nitrogen, and 6-μm-thick sections were cut on a cryostat. FDCs were visualized by staining with the FDC-specific monoclonal antiserum FDC-M2 (27) or 8C12 monoclonal antiserum to detect CD35 (Pharmingen, San Diego, Calif.). Immunolabeling was carried out with alkaline phosphatase coupled to the avidin-biotin complex (Vector Laboratories Inc., Burlingame, Calif.). Vector Red (Vector Laboratories) was used as a substrate.
Scrapie infection.
At the times indicated relative to treatment, mice were injected intracerebrally (i.c.) or i.p. with 20 μl of a 1.0, 0.1, or 0.01% (wt/vol) dilution of unspun brain homogenate from C57BL mice terminally affected with ME7 scrapie (20 μl of a 1.0% homogenate represents a dose of approximately 1 × 104.5 i.c. 50% infectious dose [ID50] units). Following challenge, animals were coded and assessed weekly for signs of clinical disease and killed at a standard clinical end point (16). Scrapie diagnosis was confirmed by histopathological assessment of vacuolation in the brain. Where indicated, some mice were sacrificed 70 days postchallenge, and their spleens were taken for further analysis. For the bioassay of scrapie infectivity, individual half spleens were prepared as 10% (wt/vol) homogenates in physiological saline and 20 μl was injected i.c. into groups of 12 C57BL indicator mice. The scrapie titer in each spleen was determined from the mean incubation period for the assay mice with reference to established dose-incubation period response curves for scrapie-infected spleen tissue (11).
Immunoblot detection of PrPSc.
The remaining half of each spleen collected 70 days postchallenge was prepared as previously described (12, 28, 30). In brief, before immunoblot analysis, spleen tissue homogenates were treated with 20 μg of proteinase K (to confirm the presence of PrPSc) and subsequently partially purified by treatment with 2% (wt/vol) N-lauroylsarcosine (in 0.1 M Tris [pH 7.4]), allowing sedimentation of only the proteinase-K-resistant, detergent-insoluble fraction of PrP (PrPSc). Samples were subjected to electrophoresis through sodium dodecyl sulfate-12% polyacrylamide gels (Bio-Rad, Hemel Hempstead, United Kingdom) and transferred to polyvinylidene difluoride membranes (Bio-Rad) by semidry blotting. PrP was detected with the PrP-specific rabbit polyclonal antiserum 1B3 (15) followed by alkaline phosphatase-conjugated goat anti-rabbit antiserum (Jackson ImmunoResearch Laboratories Inc., West Grove, Pa.), and bound alkaline phosphatase activity was detected with SigmaFast NBT/BCIP solution (Sigma, Poole, Dorset, United Kingdom).
Ultrastructural immunohistochemistry.
Spleen fragments were immersion fixed in 0.5% paraformaldehyde-0.5% glutaraldehyde for 24 h at 4°C, postfixed in osmium tetroxide, dehydrated, and embedded in araldite. Serial 65-nm-thick sections were then placed on 300-mesh nickel grids and prepared as previously described (23). Briefly, PrP was detected by staining with the PrP-specific rabbit polyclonal antiserum 1A8 (14) followed by Auroprobe 1-nm colloidal gold. Sections were then postfixed in 2.5% glutaraldehyde in phosphate-buffered saline, and staining was enhanced with immunogold silver stain. The grids were then counterstained with uranyl acetate and lead citrate.
Previous studies have shown that the combination of fixatives and pretreatments required for the preparation of tissue for electron microscopy by this method destroys PrPc immunoreactivity and reveals only disease-specific PrP accumulations (23).
Statistical analysis.
Incubation period data are expressed as means ± standard errors of the mean, and significant differences between incubation periods were sought by one-way analysis of variance with Minitab for Windows (Minitab Inc., State College, Pa.).
RESULTS
Effect of huTNFR:Fc treatment on FDC status.
A blockade of the TNF-α signaling pathway was achieved by a single i.p. injection of 100 μg of huTNFR:Fc (34). This fusion protein binds TNF-α with high affinity and acts as an antagonist of TNF-α biological activity in in vivo assays in mice (34, 47). Here, within 2 days of treatment of mice with huTNFR:Fc, a significant reduction in staining for the FDC markers FDC-M2 and CD35 was observed in lymphoid follicles of the spleen (Fig. 1). Furthermore, FDC-M2 and CD35 expression was absent 5 (data not shown) and 7 (Fig. 1) days after treatment with huTNFR:Fc. The cellular isomer of the prion protein, PrPc, is expressed by FDCs in uninfected mice (Fig. 1) (5, 29, 33). Likewise, PrPc expression was also markedly reduced within 2 days of treatment and undetectable 5 (data not shown) and 7 (Fig. 1) days after treatment with huTNFR:Fc. The effects of huTNFR:Fc treatment on FDC status were temporary, as PrP-expressing FDC networks were detected in the spleen 14 days after treatment (Fig. 1). Treatment of mice with 100 μg of polyclonal hu-Ig as a control had no adverse effect on FDC status (Fig. 1). In some follicles from hu-Ig-treated mice, there appeared to be increases in the size of the FDC network and the level of PrPc expression 14 days after treatment compared to values for follicles analyzed 2 and 7 days after treatment (Fig. 1). This may be indicative of a germinal-center response to hu-Ig.
FIG. 1.
Effect of huTNFR:Fc treatment on FDC status in spleens of uninfected mice. Tissues were taken on the indicated days postinjection (d.p.i.) with hu-Ig (control) or huTNFR:Fc, and adjacent frozen sections were stained with FDC-M2 monoclonal antiserum to detect FDCs (upper row; red) or with 8C12 monoclonal antiserum to detect CD35 (middle row; red). PrP was detected on paraffin-embedded sections with the PrP-specific polyclonal antiserum 1B3 (bottom row; red). Expression of FDC-M2, CD35, and PrP in the spleen was undetectable 7 days after treatment with huTNFR:Fc. Original magnification, ×400.
Ultrastructural analysis of the effect of huTNFR:Fc treatment on FDC status.
Mice were treated with huTNFR:Fc or hu-Ig 38 days after i.p. injection with scrapie, and PrP deposition in the spleen was analyzed 7 days later by light-microscopical and ultrastructural immunohistochemical methods. As expected, in spleens of control-treated mice, abundant disease-specific PrP staining in association with FDCs was detected by light microscopy (Fig. 2a and c). Immunoelectron microscopic analysis confirmed that these PrP accumulations were disease specific and were found in association with electron-dense material at the surface of highly convoluted FDC dendrites (Fig. 2e and f), as previously reported (23). In some follicles, individual fibrils consistent with the dimensions of amyloid fibrils were present in association with FDCs (data not shown). Disease-specific PrP accumulations were also detected within secondary lysosomes of tingible body macrophages (Fig. 2e).
FIG. 2.
Immunohistological analysis of the effects of huTNFR:Fc treatment on FDC status and PrP labeling in spleens of mice already incubating scrapie. (a to d) Light-microscopical analysis. Mice were injected i.p. with scrapie and 38 days later given a single i.p. injection of huTNFR:Fc (b and d) or hu-Ig as a control (a and c). Spleens were obtained 7 days later; frozen sections were stained for FDCs with FDC-M2 monoclonal antiserum (a and b; red), and PrP was detected on paraffin-embedded sections with the PrP-specific polyclonal antiserum 1B3 (c and d; red). Abundant abnormal PrP was detected in association with FDCs in the spleens of control mice 45 days after scrapie injection. However, when mice were treated with huTNFR:Fc 38 days after scrapie injection, heavy PrP labeling was still apparent in the spleen 7 days after treatment despite a temporary absence of FDC-M2 expression. The arrow in panel D indicates a tingible body macrophage containing apoptotic B lymphocytes. Magnification, ×400. (e to i) Ultrastructural analysis. Mice were injected i.p. with scrapie and 38 days later given a single i.p. injection of huTNFR:Fc (g to i) or hu-Ig as a control (e and f). Spleens were obtained 7 days later, and araldite sections were immunostained with the PrP-specific polyclonal antiserum 1A8. (e) An area of immunogold reactivity (indicated with an asterisk) is present between lymphocytes in the germinal center of a scrapie-injected, hu-Ig-treated control mouse. This focal immunoreaction is associated with a complex knot of mature FDC processes. Part of a tingible body macrophage is present at the bottom left (arrow). Bar = 1.76 μm. (f) High magnification of the FDC complex indicated with an asterisk in panel e. Highly convoluted FDC processes are associated with the immunogold reaction. Many short linear and curvilinear structures are immunolabeled. Bar = 0.35 μm. (g) Edge of a germinal center showing marked apoptosis (black arrows) in the spleen of a scrapie-injected, huTNFR:Fc-treated mouse. Immature FDC dendritic processes surround the germinal center (white arrows). Bar = 1.87 μm. (h) Tingible body macrophage (arrow) in the spleen of a scrapie-injected, huTNFR:Fc-treated mouse showing intralysosomal PrP accumulation. Moderately reactive FDC dendrites lie adjacent to the macrophage. Bar = 1.31 μm. (i) Foci of immunoreactivity associated with immature FDC dendritic processes in the spleen of a scrapie-injected, huTNFR:Fc-treated mouse. The immunogold reaction is associated with electron-dense material in the extracellular space surrounding FDC dendrites. Bar = 0.56 μm.
Mice were treated with huTNFR:Fc 38 days after scrapie injection, and abundant PrP labeling was still apparent in the spleen 7 days after treatment (Fig. 2d) despite a temporary absence of mature FDCs (Fig. 2b). Ultrastructural analysis revealed that the centers of secondary lymphoid follicles in spleens from huTNFR:Fc-treated mice showed marked degenerative changes compared with those from hu-Ig-treated control mice. Severe and extensive lymphocyte apoptosis was noted (Fig. 2g), and large numbers of highly reactive macrophages containing degenerative cellular material were also present in these sites (Fig. 2h). In many cases, whole apoptotic B lymphocytes could also be identified within these macrophages. At the ultrastructural level, FDC networks were identified but their processes appeared immature (Fig. 2i) and lacked the highly convoluted characteristics observed for those of hu-Ig-treated control mice (Fig. 2f). The immature nature of these FDC dendrites was consistent with the loss of expression of FDC-specific markers following huTNFR:Fc treatment (Fig. 1 and 2). Although a few mature FDC processes were identified at the ultrastructural level, it was not possible to determine whether these were regenerating FDCs or mature FDCs unaffected by the TNFR signaling blockade (31).
Effect of huTNFR:Fc treatment on scrapie pathogenesis.
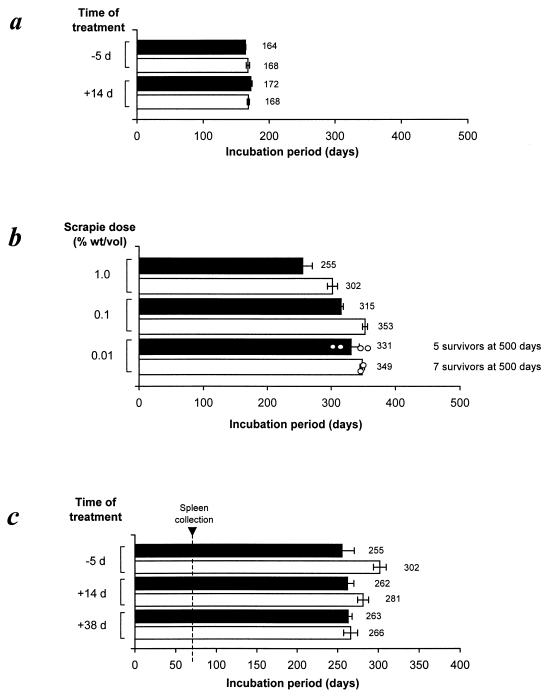
Mice were given a single i.p. injection of huTNFR:Fc (or hu-Ig as a control) at one of three different times relative to scrapie challenge: 5 days before scrapie injection, so mature FDCs would be absent in lymphoid tissues at the time of scrapie injection; 14 days after scrapie injection, soon after the onset of scrapie replication in lymphoid tissues; or 38 days after scrapie injection, when high levels of scrapie infectivity are present in lymphoid tissues (5, 30). When groups of six mice were challenged with the ME7 scrapie strain by direct i.c. injection into the CNS, treatment with huTNFR:Fc 5 days before or 14 days after scrapie challenge had no effect on the incubation period of the disease (∼164 to 172 days; Fig. 3a) or pathology within the brain (data not shown) compared with those of controls. These findings demonstrate that the blockade of the TNFR signaling pathway did not affect scrapie pathogenesis once disease was established in the CNS.
FIG. 3.
Blockade of the TNFR signaling pathway significantly extends the incubation period of disease following peripheral injection with ME7 scrapie. (a) Mice were treated with huTNFR:Fc (□) or hu-Ig as a control (▪) 5 days before or 14 days after i.c. injection with a moderate dose of scrapie (1.0% [wt/vol] scrapie brain homogenate). (b) Mice were treated with huTNFR:Fc (□) or hu-Ig as a control (▪) 5 days before i.p. injection with a moderate (1.0%) or limiting (0.1% or 0.01%) dose of scrapie. ○, incubation periods for individual mice. (c) Mice were treated with huTNFR:Fc (□) or hu-Ig (▪) 5 days before or 14 or 38 days after i.p. injection with a moderate dose of scrapie. Each bar represents a mean ± standard error of the mean for six to nine mice. The vertical broken line represents the time at which two spleens were taken from each treatment group for subsequent analysis of scrapie infectivity and PrPSc accumulation (see Fig. 4). d, days.
When treated with huTNFR:Fc before or shortly after peripheral (i.p.) injection with scrapie, mice developed neurological disease much later than did the hu-Ig-treated controls. The most significant effect was observed when mice were treated 5 days before i.p. scrapie injection (Fig. 3b). For example, following injection with a moderate dose of scrapie (20 μl of a 1.0% scrapie brain homogenate), all control-treated mice succumbed to disease, with a mean incubation period of 255 ± 15 days (n = 8), whereas those treated with huTNFR:Fc developed disease 47 days later, with a mean incubation period of 302 ± 7 days (P < 0.01; n = 8; Fig. 3b). Likewise, when treated with huTNFR:Fc before injection with a 10-fold-lower scrapie dose (20 μl of a 0.1% scrapie brain homogenate), mice developed neurological disease with a mean incubation period of 353 ± 4 days (n = 9), which was 38 days longer than the mean incubation period of the hu-Ig-treated controls (315 ± 7 days; P < 0.001; n = 9; Fig. 3b). Despite these highly significant prolongations of the incubation period, little effect on disease susceptibility was observed following treatment with huTNFR:Fc prior to injection with a low dose of scrapie (20 μl of a 0.01% scrapie brain homogenate): 7 of 9 huTNFR:Fc-treated mice remained free of scrapie disease 500 days after inoculation, compared to 5 of 9 control mice (Fig. 3b).
An increase in survival time was also observed when treatment with huTNFR:Fc was delayed until 14 days after i.p. injection with a moderate dose of scrapie (Fig. 3c). In this instance, mice developed neurological disease with a mean incubation period of 281 ± 7 days (n = 8), which was 19 days longer than the mean incubation period of the hu-Ig-treated controls (262 ± 8.0 days; n = 8). However, treatment with huTNFR:Fc 38 days after injection, a time when high levels of infectivity agents have already accumulated in the spleen (5, 30), had no effect on the incubation period compared with that of control-treated mice (Fig. 3c).
Scrapie infectivity and PrPSc accumulation in the spleen.
Within 70 days of a peripheral injection of untreated mice with the ME7 scrapie strain, high levels of infectivity and the disease-specific isomer of the prion protein, PrPSc, accumulate within lymphoid tissues (5, 12, 28, 30). In this study, spleens were taken from each control and huTNFR:Fc treatment group 70 days after i.p. injection with a moderate dose of scrapie and halved. PrPSc accumulation was determined in one half by immunoblot analysis, while the scrapie infectivity titer was estimated in the other half by bioassay in groups of indicator mice. As expected, all spleens from control mice treated with hu-Ig 5 days before or 14 or 38 days after scrapie challenge contained high infectivity titers (5.0 to 5.3 log i.c. ID50/g, as estimated by incubation period assay; Fig. 4) and abundant detergent-insoluble, relatively proteinase-K-resistant PrPSc (Fig. 4). However, following treatment of mice with huTNFR:Fc 5 days before scrapie challenge, PrPSc was less abundant in the spleen 70 days postinfection (Fig. 4a, lanes 4 and 6). In contrast, the infectivity titers were as high as those detected in spleens from hu-Ig-treated controls, suggesting that the accumulation of PrPSc in the spleen lags behind replication of infectivity during the early stages of infection, as observed in previous studies (12, 28).
FIG. 4.
PrPSc accumulation and infectivity titers in the spleen 70 days after i.p. injection with scrapie. Immunoblots show the accumulation of detergent-insoluble, relatively proteinase-K-resistant PrPSc. Treatment of tissue in the presence (+) or absence (−) of proteinase K before electrophoresis is indicated. Following proteinase K treatment, a typical three-band pattern was observed between molecular mass values of 20 and 30 kDa, representing unglycosylated, monoglycosylated, and diglycosylated isomers of PrP (in order of increasing molecular mass). Scrapie infectivity titers are expressed as log i.c. ID50 units per gram. Mice were treated with hu-Ig (control) or huTNFR:Fc 5 days before (a) or 14 (b) or 38 (c) days after scrapie injection. Lane M contained molecular mass markers. d, days; PK, proteinase K.
When treatment was delayed until 14 or 38 days after scrapie challenge, no differences in the accumulation of infectivity or abundance of PrPSc in the spleen were detected between control- and huTNFR:Fc-treated mice when measured 70 days after scrapie challenge (Fig. 4b and c).
DISCUSSION
Here we have shown that a single treatment of mice with huTNFR:Fc before or shortly after a peripheral scrapie injection significantly extended survival time compared to that of control-treated mice. Our studies also demonstrated that treatment prior to peripheral exposure decreased the early accumulation of disease-specific PrPSc within the spleen. These effects coincided with a temporary dedifferentiation of mature PrP-expressing FDCs in the spleen following treatment with huTNFR:Fc. Taken together, these results are consistent with previous findings that in the absence of mature FDCs in lymphoid tissues, neuroinvasion following peripheral injection with scrapie is impaired (5, 29, 30). Surprisingly, a single treatment with huTNFR:Fc had little influence on disease susceptibility following low-dose scrapie challenge. Nevertheless, TNF-α blockade over longer periods may present a potential strategy for intervention in peripherally acquired TSEs.
Secretion of TNF-α has been implicated in the development of neuropathology in several human inflammatory, infectious, and autoimmune disorders (40). Although TNF-α expression has been reported to occur in the brains of mice showing clinical signs of scrapie (9), studies using TNF-α−/− mice (30) and TNFR-1-deficient mice (25) suggest that this cytokine signaling pathway alone is not directly involved in the development of neuropathology in TSEs. Due to its high molecular weight, huTNFR:Fc would be unlikely to cross the blood-brain barrier and inhibit TNF-α signaling within the brain. Treatment with huTNFR:Fc in this study had no effect on survival time or neuropathology when mice were injected with scrapie directly into the CNS, confirming that the effects of treatment on TSE pathogenesis operate at a peripheral stage prior to neuroinvasion. Our studies suggest this is most likely due to a temporary interference with the integrity of FDCs, although effects of huTNFR:Fc treatment on other cell types in the spleen cannot be entirely excluded. However, the increased survival time following treatment with huTNFR:Fc 14 days after scrapie injection (Fig. 3b) and the recent demonstration that membrane lymphotoxin, not TNF-α, regulates the migration of dendritic cells in the spleen (48) suggest that it is unlikely that the effects of huTNFR:Fc treatment on scrapie pathogenesis are due to impaired cell trafficking from the site of scrapie challenge to the spleen.
Light-microscopical analysis demonstrated that mature PrP-expressing FDCs were temporarily absent in the spleen soon after treatment with huTNFR:Fc. Several hypotheses could explain the fate of FDCs following huTNFR:Fc treatment: (i) FDCs temporarily revert to an immature state that affects their function and phenotype; (ii) the chemokine gradients responsible for the organization of cell populations within the germinal center are altered (36), and as a consequence the FDCs disperse; or (iii) in the absence of stimulation from TNF-α, FDCs undergo apoptosis. We consider the first hypothesis most likely, as despite a temporary absence of FDC-M2, CD35, and PrPc expression by FDCs, immature FDC processes were detected at the ultrastructural level, suggesting that these cells had reverted to a dedifferentiated state. Antigens are trapped and retained on the surface of FDCs through interactions between complement components and cellular complement receptors (37, 39). The loss of expression of complement receptor 1 (CD35; Fig. 1) and substantially decreased abundance of complement component C3 (data not shown) in lymphoid follicles of treated mice implied that these immature FDC processes had an impaired ability to retain antigens (31). Recent studies have demonstrated that C1q, C3, and complement receptors play an important role in the localization of TSE infectious agents to FDCs (26, 28). Therefore, it is unlikely that during the period of dedifferentiation following treatment with huTNFR:Fc, these immature FDC processes would have the potential to acquire TSE infectivity. Occasionally a few mature FDC processes were detected in the spleen by ultrastructural analysis 7 days after treatment (data not shown). These may represent FDCs in the process of regeneration, but it is also plausible that these were FDCs that were participating in strong antigenic responses and whose state of differentiation was unaffected by treatments which inhibit the TNFR signaling pathway (31).
Ultrastructural analysis of secondary lymphoid follicles from huTNFR:Fc-treated mice revealed other associated degenerative changes. FDCs provide important costimulatory factors which prevent B lymphocytes from undergoing apoptosis (18). Therefore, the detection of severe and extensive lymphocyte apoptosis following treatment with huTNFR:Fc suggested that this was most likely due to a loss of mature FDCs. Many of these apoptotic B lymphocytes were identified whole within tingible body macrophages which scavenge apoptotic lymphocytes and are considered to regulate the germinal-center reaction (42). The increased survival time following treatment with huTNFR:Fc is unlikely to be directly related to a loss of B lymphocytes by apoptosis, as ME7 scrapie pathogenesis is unaffected in mice with impaired germinal-center B-lymphocyte development (30). However, the effects of treatment on disease susceptibility could be indirectly related to a loss of cytokine stimuli from B lymphocytes, which leads to FDC dedifferentiation.
Within 70 days of a peripheral injection of immunocompetent mice with the ME7 scrapie strain, high levels of infectivity titers and abundant PrPSc are detected in the spleen (5, 12, 28, 30). Here, when mice were given huTNFR:Fc before scrapie challenge, low levels of PrPSc were detected in the spleen 70 days postinoculation, approximately 50 days after the expected reappearance of mature FDCs. In the absence of mature FDCs at the time of scrapie challenge, it is likely that PrPSc and infectivity from the inoculum persist in the spleen but that a significant proportion is destroyed, for example by macrophages (3, 10). This effect would significantly delay both the onset of replication when the FDCs reappear within 14 days of treatment and the subsequent transfer of infectivity via peripheral nerves (19) into the CNS. Interestingly, infectivity titers in spleens from huTNFR:Fc-treated mice were the same as those from hu-Ig-treated controls, implying that the accumulation of PrPSc in the spleen lags behind the replication of infectivity during the early stages of infection (28). These experiments also suggest that the time interval during which the FDCs were unable to acquire and replicate scrapie was insufficient to allow macrophages adequate time to destroy most of the infectious agents, as huTNFR:Fc treatment had little, if any, effect on disease susceptibility.
Further experiments will show whether it is possible to extend the period of FDC dedifferentiation beyond that described in this report through prolonged treatment with multiple doses of huTNFR:Fc. Such an approach may reduce the accumulation of scrapie infectivity in the spleen and further delay or prevent the development of disease in the CNS. However, a prolonged blockade of proinflammatory cytokines such as TNF-α may cause serious side effects, including increased susceptibility to other infectious microorganisms, increased incidence of malignancies, or induction of autoimmune disease. A therapeutic blockade of TNF-α has been used to successfully treat rheumatoid arthritis and Crohn's disease in humans, where this cytokine plays a critical role in mediating inflammation (22, 32). The experience of long-term treatment of human rheumatoid arthritis patients with TNF-α antagonists suggests that they are safe and well tolerated (22, 32).
The detection of infectivity in lymphoid tissues and of PrPSc in association with FDCs from patients with vCJD (6, 21) and sheep with natural scrapie (1, 20, 45) suggests that these TSEs also share a similar requirement for FDCs. Therefore, the experiments described in this report and those of others suggest that treatments which temporarily interfere with the integrity (29, 35) or immune complex trapping function (28) of FDCs offer a potential approach for early intervention in peripherally acquired TSEs.
Acknowledgments
We thank Irene McConnell, Dawn Drummond, and Emma Murdoch (Institute for Animal Health, Neuropathogenesis Unit, Edinburgh, United Kingdom) for excellent technical support; Immunex Corp. (Seattle, Wash.) for provision of huTNFR:Fc; Christine Farquhar (Institute for Animal Health, Neuropathogenesis Unit) for helpful discussion and provision of 1A8 and 1B3 polyclonal antisera; Marie Kosco-Vilbois (Serono Pharmaceutical Research Institute, Geneva, Switzerland) for provision of FDC-M2 monoclonal antiserum; and Jeffrey Browning (Biogen Inc., Cambridge, Mass.) for provision of hu-Ig.
This work was supported by funding from the Medical Research Council and the Biotechnology and Biological Sciences Research Council.
REFERENCES
- 1.Andreoletti, O., P. Berthon, D. Marc, P. Sarradin, J. Grosclaude, L. van Keulen, F. Schelcher, J.-M. Elsen, and F. Lantier. 2000. Early accumulation of PrPSc in gut-associated lymphoid and nervous tissues of susceptible sheep from a Romanov flock with natural scrapie. J. Gen. Virol. 81:3115-3126. [DOI] [PubMed] [Google Scholar]
- 2.Beekes, M., and P. A. McBride. 2000. Early accumulation of pathological PrP in the enteric nervous system and gut-associated lymphoid tissue of hamsters orally infected with scrapie. Neurosci. Lett. 278:181-184. [DOI] [PubMed] [Google Scholar]
- 3.Beringue, V., M. Demoy, C. I. Lasmezas, B. Gouritin, C. Weingarten, J.-P. Deslys, J.-P. Adreux, P. Couvreur, and D. Dormont. 2000. Role of spleen macrophages in the clearance of scrapie agent early in pathogenesis. J. Pathol. 190:495-502. [DOI] [PubMed] [Google Scholar]
- 4.Bolton, D. C., M. P. McKinley, and S. B. Prusiner. 1982. Identification of a protein that purifies with the scrapie prion. Science 218:1309-1311. [DOI] [PubMed] [Google Scholar]
- 5.Brown, K. L., K. Stewart, D. Ritchie, N. A. Mabbott, A. Williams, H. Fraser, W. I. Morrison, and M. E. Bruce. 1999. Scrapie replication in lymphoid tissues depends on PrP-expressing follicular dendritic cells. Nature Med. 5:1308-1312. [DOI] [PubMed] [Google Scholar]
- 6.Bruce, M. E., I. McConnell, R. G. Will, and J. W. Ironside. 2001. Detection of variant Creutzfeldt-Jakob disease (vCJD) infectivity in extraneural tissues. Lancet 358:208-209. [DOI] [PubMed] [Google Scholar]
- 7.Bruce, M. E., R. G. Will, J. W. Ironside, I. McConnell, D. Drummond, A. Suttie, L. McCardle, A. Chree, J. Hope, C. Birkett, S. Cousens, H. Fraser, and C. J. Bostock. 1997. Transmissions to mice indicate that ‘new variant' CJD is caused by the BSE agent. Nature 389:498-501. [DOI] [PubMed] [Google Scholar]
- 8.Bueler, H., M. Fischer, Y. Lang, H. Bluethmann, H.-P. Lipp, S. J. DeArmond, S. B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577-582. [DOI] [PubMed] [Google Scholar]
- 9.Campbell, I. L., M. Eddleston, P. Kemper, M. B. A. Oldstone, and M. V. Hobbs. 1994. Activation of cerebral cytokine gene expression and its correlation with the onset of reactive and acute-phase response gene expression in scrapie. J. Virol. 68:2383-2387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carp, R. I., and S. M. Callahan. 1982. Effect of mouse peritoneal macrophages on scrapie infectivity during extended in vitro incubation. Intervirology 17:201-207. [DOI] [PubMed] [Google Scholar]
- 11.Dickinson, A. G., V. M. Meikle, and H. Fraser. 1969. Genetical control of the concentration of ME7 scrapie agent in the brain of mice. J. Comp. Pathol. 79:15-22. [DOI] [PubMed] [Google Scholar]
- 12.Farquhar, C. F., J. Dornan, R. A. Somerville, A. M. Tunstall, and J. Hope. 1994. Effect of Sinc genotype, agent isolate and route of infection on the accumulation of protease-resistant PrP in non-central nervous system tissues during the development of murine scrapie. J. Gen. Virol. 75:495-504. [DOI] [PubMed] [Google Scholar]
- 13.Farquhar, C. F., R. A. Somerville, and M. E. Bruce. 1998. Straining the prion hypothesis. Nature 391:345-346. [DOI] [PubMed] [Google Scholar]
- 14.Farquhar, C. F., R. A. Somerville, J. Dornan, D. Armstrong, C. Birkett, and J. Hope. 1994. A review of the detection of PrPsc, p. 301-313. In R. Bradley and B. Marchant (ed.), BSE update. Proceedings of a commission of the European Communities, 14-15 September 1993, Brussels. Working document for the EC Ref: FII.3-JC/003.
- 15.Farquhar, C. F., R. A. Somerville, and L. A. Ritchie. 1989. Post-mortem immunodiagnosis of scrapie and bovine spongiform encephalopathy. J. Virol. Methods 24:215-222. [DOI] [PubMed] [Google Scholar]
- 16.Fraser, H., and A. G. Dickinson. 1973. Agent-strain differences in the distribution and intensity of grey matter vacuolation. J. Comp. Pathol. 83:29-40. [DOI] [PubMed] [Google Scholar]
- 17.Fraser, H., and A. G. Dickinson. 1978. Studies on the lymphoreticular system in the pathogenesis of scrapie: the role of spleen and thymus. J. Comp. Pathol. 88:563-573. [DOI] [PubMed] [Google Scholar]
- 18.Freedman, A. S., D. Wang, J. S. Phifer, and S. N. Manie. 1995. Role of follicular dendritic cells in the regulation of B cell proliferation. Curr. Top. Microbiol. Immunol. 201:83-104. [DOI] [PubMed] [Google Scholar]
- 19.Glatzel, M., F. L. Heppner, K. M. Albers, and A. Aguzzi. 2001. Sympathetic innervation of lymphoreticular organs is rate limiting for prion neuroinvasion. Neuron 31:25-34. [DOI] [PubMed] [Google Scholar]
- 20.Heggebo, R., C. M. Press, G. Gunnes, K. I. Lie, M. A. Tranulis, M. Ulvund, M. H. Groschup, and T. Landsverk. 2000. Distribution of prion protein in the ileal Peyer's patch of scrapie-free lambs and lambs naturally and experimentally exposed to the scrapie agent. J. Gen. Virol. 81:2327-2337. [DOI] [PubMed] [Google Scholar]
- 21.Hill, A. F., R. J. Butterworth, S. Joiner, G. Jackson, M. N. Rossor, D. J. Thomas, A. Frosh, N. Tolley, J. E. Bell, M. Spencer, A. King, S. Al-Sarraj, J. W. Ironside, P. L. Lantos, and J. Collinge. 1999. Investigation of variant Creutzfeldt-Jakob disease and other prion diseases with tonsil biopsy samples. Lancet 353:183-189. [DOI] [PubMed] [Google Scholar]
- 22.Illei, G. G., and P. E. Lipsky. 2000. Novel, non-antigen-specific therapeutic approaches to autoimmune/inflammatory diseases. Curr. Opin. Immunol. 12:712-718. [DOI] [PubMed] [Google Scholar]
- 23.Jeffrey, M., G. McGovern, C. M. Goodsir, K. L. Brown, and M. E. Bruce. 2000. Sites of prion protein accumulation in scrapie-infected mouse spleen revealed by immuno-electron microscopy. J. Pathol. 190:323-332. [DOI] [PubMed] [Google Scholar]
- 24.Kimberlin, R. H., and C. A. Walker. 1989. Pathogenesis of scrapie in mice after intragastric infection. Virus Res. 12:213-220. [DOI] [PubMed] [Google Scholar]
- 25.Klein, M. A., R. Frigg, E. Flechsig, A. J. Raeber, U. Kalinke, H. Bluethman, F. Bootz, M. Suter, R. M. Zinkernagel, and A. Aguzzi. 1997. A crucial role for B cells in neuroinvasive scrapie. Nature 390:687-691. [DOI] [PubMed] [Google Scholar]
- 26.Klein, M. A., P. S. Kaeser, P. Schwarz, H. Weyd, I. Xenarios, R. M. Zinkernagel, M. C. Carroll, J. S. Verbeek, M. Botto, M. J. Walport, H. Molina, U. Kalinke, H. Acha-Orbea, and A. Aguzzi. 2001. Complement facilitates early prion pathogenesis. Nature Med. 7:488-492. [DOI] [PubMed] [Google Scholar]
- 27.Kosco-Vilbois, M. H., H. Zentgraf, J. Gerdes, and J.-Y. Bonnefoy. 1997. To “B” or not to “B” a germinal center? Immunol. Today 18:225-230. [DOI] [PubMed] [Google Scholar]
- 28.Mabbott, N. A., M. E. Bruce, M. Botto, M. J. Walport, and M. B. Pepys. 2001. Temporary depletion of complement component C3 or genetic deficiency of C1q significantly delays onset of scrapie. Nature Med. 7:485-487. [DOI] [PubMed] [Google Scholar]
- 29.Mabbott, N. A., F. Mackay, F. Minns, and M. E. Bruce. 2000. Temporary inactivation of follicular dendritic cells delays neuroinvasion of scrapie. Nature Med. 6:719-720. [DOI] [PubMed] [Google Scholar]
- 30.Mabbott, N. A., A. Williams, C. F. Farquhar, M. Pasparakis, G. Kollias, and M. E. Bruce. 2000. Tumor necrosis factor alpha-deficient, but not interleukin-6-deficient, mice resist peripheral infection with scrapie. J. Virol. 74:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mackay, F., and J. L. Browning. 1998. Turning off follicular dendritic cells. Nature 395:26-27. [DOI] [PubMed] [Google Scholar]
- 32.Maini, R. N., and P. C. Taylor. 2000. Anti-cytokine therapy for rheumatoid arthritis. Annu. Rev. Med. 51:207-229. [DOI] [PubMed] [Google Scholar]
- 33.McBride, P., P. Eikelenboom, G. Kraal, H. Fraser, and M. E. Bruce. 1992. PrP protein is associated with follicular dendritic cells of spleens and lymph nodes in uninfected and scrapie-infected mice. J. Pathol. 168:413-418. [DOI] [PubMed] [Google Scholar]
- 34.Mohler, K. M., D. S. Torrance, C. A. Smith, R. G. Goodwin, K. E. Stremler, V. P. Fung, H. Madani, and M. B. Widmer. 1993. Soluble tumour necrosis factor (TNF) receptors are effective therapeutic agents in lethal endotoxemia and function simultaneously as both carriers and TNF antagonists. J. Immunol. 151:1548-1561. [PubMed] [Google Scholar]
- 35.Montrasio, F., R. Frigg, M. Glatzel, M. A. Klein, F. Mackay, A. Aguzzi, and C. Weissmann. 2000. Impaired prion replication in spleens of mice lacking functional follicular dendritic cells. Science 288:1257-1259. [DOI] [PubMed] [Google Scholar]
- 36.Ngo, V. N., H. Korner, M. D. Gunn, K. N. Schmidt, D. S. Riminton, M. D. Cooper, J. L. Browning, J. D. Sedgwick, and J. G. Cyster. 1999. Lymphotoxin α/β and tumour necrosis factor are required for stromal cell expression of homing chemokines in B and T cell areas of the spleen. J. Exp. Med. 189:403-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nielsen, C. H., E. M. Fischer, and R. G. Q. Leslie. 2000. The role of complement in the acquired immune response. Immunology 100:4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pasparakis, M., L. Alexopoulo, V. Episkopou, and G. Kollias. 1996. Immune and inflammatory responses in TNFα-deficient mice: a critical requirement for TNFα in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centres, and in the maturation of the humoral immune response. J. Exp. Med. 184:1397-1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pepys, M. B. 1976. Role of complement in the induction of immunological responses. Transplant Rev. 32:93-120. [DOI] [PubMed] [Google Scholar]
- 40.Probert, L., K. Akassoglou, G. Kassiotis, M. Pasparakis, L. Alexopoulou, and G. Kollias. 1997. TNF-alpha transgenic and knockout models of CNS inflammation and degeneration. J. Neuroimmunol. 72:137-141. [DOI] [PubMed] [Google Scholar]
- 41.Prusiner, S. B., D. C. Bolton, D. F. Groth, K. A. Bowman, S. P. Cochran, and M. P. McKinley. 1982. Further purification and characterisation of scrapie prions. Biochemistry 21:6942-6950. [DOI] [PubMed] [Google Scholar]
- 42.Smith, J. P., G. F. Burton, J. G. Tew, and A. K. Szakal. 1998. Tingible body macrophages in regulation of germinal center reactions. Dev. Immunol. 6:285-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Somerville, R. A., C. R. Birkett, C. F. Farquhar, N. Hunter, W. Goldmann, J. Dornan, D. Grover, R. M. Hennion, C. Percy, J. Foster, and M. Jeffrey. 1997. Immunodetection of PrPSc in spleens of some scrapie-infected sheep but not BSE-infected cows. J. Gen. Virol. 78:2389-2396. [DOI] [PubMed] [Google Scholar]
- 44.Tkachuk, M., S. Bolliger, B. Ryffel, G. Pluschke, T. A. Banks, S. Herren, R. H. Gisler, and M. H. Kosco-Vilbois. 1998. Crucial role of tumour necrosis factor receptor 1 expression on nonhematopoietic cells for B cell localization within the splenic white pulp. J. Exp. Med. 187:469-477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Keulen, L. J. M., B. E. C. Schreuder, R. H. Meloen, G. Mooij-Harkes, M. E. W. Vromans, and J. P. M. Langeveld. 1996. Immunohistological detection of prion protein in lymphoid tissues of sheep with natural scrapie. J. Clin. Microbiol. 34:1228-1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.van Keulen, L. J. M., B. E. C. Schreuder, M. E. W. Vromans, J. P. M. Langeveld, and M. A. Smits. 1999. Scrapie-associated prion protein in the gastro-intestinal tract of sheep with scrapie. J. Comp. Pathol. 121:55-63. [DOI] [PubMed] [Google Scholar]
- 47.Wooley, P. H., J. Dutcher, M. B. Widmer, and S. Gillis. 1993. Influence of a recombinant human soluble tumour necrosis factor receptor FC fusion protein on type II collagen-induced arthritis in mice. J. Immunol. 151:6602-6607. [PubMed] [Google Scholar]
- 48.Wu, Q., Y. Wang, E. O. Hedgeman, J. L. Browning, and Y.-X. Fu. 1999. The requirement of membrane lymphotoxin for the presence of dendritic cells in lymphoid tissues. J. Exp. Med. 190:629-638. [DOI] [PMC free article] [PubMed] [Google Scholar]