Abstract
Xenotransplantation of porcine organs carries the risk of reactivation of latent virus in donor and recipient tissues as well as transmission of viruses between species. We have investigated the activation of baboon cytomegalovirus (BCMV) and porcine CMV (PCMV) in a pig-to-primate model of xenotransplantation. Tissues originating from a series of six swine-to-baboon composite thymokidney xenotransplants were investigated. Four immunosuppressed baboons died (survival range, 7 to 27 days) with the graft in situ. Increases in BCMV DNA copy numbers occurred in three (75%) of these baboons and was thought to be responsible for pneumonitis and the death of one animal. In two baboons, disseminated intravascular coagulation was successfully treated by graftectomy and discontinuation of immunosuppression. PCMV was upregulated in five of six xenografts (83%). PCMV infection was associated with ureteric necrosis in one xenograft. Although significantly increased in native tissues, low levels of BCMV and PCMV were also detected in tissues other than that of the native viral host species. The cross-species presence of CMV did not appear to cause clinical or histological signs of invasive disease. Thus, viral infections with clinical disease were restricted to tissues of the native species of each virus. Intensive immune suppression currently required for xenotransplantation results in a significant risk of reactivation of latent infections by BCMV and PCMV. It is not yet known whether viral DNA detected across species lines represents cellular microchimerism, ongoing viral infection, or uptake of free virus. The observation of graft injury by PCMV demonstrates that CMV will be an important pathogen in immunosuppressed xenograft recipients. Strategies must be developed to exclude CMV from porcine organ donors.
Infection is a major problem in transplantation (7). In particular, cytomegalovirus (CMV) is the most significant posttransplant infection in human allotransplantation. CMV is activated from latency by the allo-immune response and by the immune suppression needed to maintain graft function (7, 17-19). CMV is a betaherpesvirus that causes invasive disease and lifelong latent infection in many mammalian species. Xenotransplantation of swine tissues has been proposed to alleviate the shortage of human organs available for allotransplantation. Swine are considered the organ donors of choice for xenotransplantation for reasons of physiological compatibility, breeding characteristics, and ethical considerations (13).
We have investigated the induction of xenograft tolerance in a pig-to-baboon model based on a previously described pig-to-mouse model involving thymectomy, T-cell depletion, and the transplantation of donor thymic tissue (14, 24). Allotransplant studies of miniature swine have demonstrated that composite thymic tissue-renal allografts with a limited course of immune suppression, thymectomy, and T-cell depletion can induce tolerance across class I and fully-mismatched barriers (23). However, xenotransplantation in the pig-to-baboon model requires intensive immune suppression. This suppression enhances the risk of reactivation of latent CMV in the baboon recipient as well as in the transplanted porcine organ. Consequently, there may be an enhanced risk of transmission of these viruses between donor and recipient (5-7, 16). Techniques which readily distinguish active infection from passive acquisition of virus in vivo are not yet available.
We have developed quantitative molecular assays specific for baboon CMV (BCMV) and porcine CMV (PCMV) to assess the potential for activation of CMV replication and to investigate the potential for interspecies transmission of CMV in this pig-to-baboon model.
MATERIALS AND METHODS
Pig-to-baboon xenotransplantation.
Landrace pigs (n = 5; approximate weight on date of transplantation, 40 kg) transgenic for human decay-accelerating factor were provided by Novartis Pharmaceuticals, Inc. (East Hanover, N.J.). One animal served as the donor for two xenografts. Sera of two pig donors (for baboons 69-144 and 69-222) were available for PCMV serologic testing by an immune fluorescence assay, and they demonstrated titers of 1:1,024 and 1:64 (Animal Disease Diagnostic Laboratory, Purdue University, West Lafayette, Ind.). A composite thymokidney graft was created by the autologous transplantation of porcine thymic tissue from the native thymus under the renal capsule (22). After a period of 1 to 2 months, the thymic tissue developed a new blood supply from renal vessels with normal thymic architecture. These thymokidneys were used for xenotransplantation studies of baboons. Baboons (Papio anubis; n = 6) weighing between 8 and 20 kg were purchased from Biological Resources Foundation (Houston, Tex.). At the time of thymokidney transplantation, all the baboons had additional porcine thymic tissue transplanted into the omentum. In each recipient, one native kidney remained in situ. All baboons used in these studies were BCMV seropositive (Esoterix Inc., San Antonio, Tex.).
Baboons were treated with the immunosuppressive regimen outlined in Table 1. This regimen is described in detail elsewhere (R. N. Barth et al., unpublished data). In brief, recipients underwent either thymectomy or thymic irradiation (9), splenectomy, T-cell depletion, adsorption of anti-Galα1-3Gal (Gal) antibody (12, 21), complement depletion (11), and treatment with anti-CD154 monoclonal antibody (2). T-cell depletion was accomplished by administering combinations of anti-thymocyte globulin (Pharmacia/Upjohn, Peapack, N.J.), murine anti-human CD3 antibody immunotoxin conjugate (FN18-CRM9; kindly provided by D. Neville, National Institutes of Health [NIH], Bethesda, Md.) (10), cyclophosphamide (Mead Johnson Oncology Products, Princeton, N.J.), and LoCD2b (rat anti-primate CD2b monoclonal antibody; BioTransplant Incorporated, Charlestown, Mass.). In all baboons, T-cell depletion was >90% on the day of transplantation (measured by fluorescence-activated cell sorting analysis of peripheral blood samples) (23).
TABLE 1.
Induction therapy for pig-to-baboon thymokidney xenotransplants
| Baboon | Transplanted organs | Thymectomy | Amt of thymic irradiation (CGy) | Antibodya | Cyclophosphamide concn (mg/kg) | Date of graftectomy (POD) | Date of death (POD) | Cause of death |
|---|---|---|---|---|---|---|---|---|
| B69-161 | TK + thymus | <@000a> Yes | 0 | FN18 | 120 | 27 | CMV disease | |
| B69-144 | TK + thymus | Yes | 0 | ATG | 70 | 15 | Alive | |
| B69-128 | TK + thymus | Yes | 0 | ATG | 120 | 27 | CVb collapse | |
| B69-222 | TK + thymus | Yes | 0 | FN18 | 60 | 11 | Myocardial infarction | |
| B69-268 | TK + thymus | No | 700 | ATG | 100 | 18 | >200 | Euthanatized |
| B81-99 | TK + thymus | No | 700 | ATG | 120 | 13 | CV collapse |
FN18, FN18-CRM9, anti-CD3 antibody; ATG, antithymocyte globulin; CGy, centi-Gray.
CV, cardiovascular.
Baboons were monitored clinically for graft function and evidence of rejection or infection. Blood chemistry and hematologic parameters were followed daily and surveillance blood cultures for aerobic and anaerobic organisms were taken twice weekly. Animals received prophylaxis with cefazolin sodium for 48 h at the time of surgical procedures and levofloxacin during the posttransplantation course. Antimicrobials were adjusted according to culture results and/or clinical condition. Animals were transfused as necessary with irradiated packed red blood cells, platelets (collected by apheresis), and Gal-depleted fresh frozen plasma from donor baboons.
Care of animals was in accordance with the Guide for the Care and Use of Laboratory Animals prepared by the National Academy of Sciences and published by the National Institutes of Health. Protocols were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care.
Histological examinations.
Biopsies of the transplanted porcine tissues were carried out on postoperative day (POD) 15 and at the time of graftectomy or autopsy. Both donor and recipient tissues were biopsied at autopsy. Tissue samples were fixed in 1% formaldehyde solution, embedded in paraffin, and sectioned. Tissues were then stained using hematoxylin and eosin and periodic acid-Schiff. Coded samples were examined by light microscopy by a renal pathologist, and rejection or infection was diagnosed according to a standardized grading system (3).
DNA extraction.
Tissue samples were snap frozen at time of biopsy or autopsy. DNA was isolated using the Purgene DNA Isolation Kit (Purgene, Minneapolis, Minn.). Quantitation of total DNA was performed by Hoechst dye fluorescence assay on a DNA fluorometer (Hoefer Scientific Instruments, San Francisco, Calif.). DNA extracted from the same tissue was used for all quantitative experiments.
Quantitative PCR.
Target DNA sequences were quantified by real-time PCR using the ABI PRISM 7700 Sequence Detection System (Perkin-Elmer, Foster City, Calif.). Sequence-specific primers were generated for each gene target using Primer Express software (Perkin-Elmer) and are summarized in Table 2. PCR conditions were identical for all assays with the exception of the initial BCMV assays; both assay conditions yielded comparable results. Each PCR mixture consisted of 250 to 500 ng of DNA, 900 nM primers, 200 nM probe, and TaqMan Universal PCR 2× MasterMix (Applied Biosystems, Branchburg, N.J.) containing the passive reference dye ROX in a 50-μl final mixture reaction volume loaded into a 96-well plate. The PCR conditions were as follows: an initial cycle at 50°C for 2 min, 95°C for 10 min, 50 cycles of denaturation at 95°C for 15 s, and annealing/extension at 60°C for 1 min. Under these conditions, target DNA was detected with a linear dynamic range from 100 to 106 copies (data not shown). The threshold cycle, or CT, values of PCR amplification of the standards were used to generate a standard curve for the quantitation of target DNA. Samples from which reproducible amplification signals were detected at levels below the lowest standard quantitation limit are referred to as “below x copies” , with x being the lower limit of quantitative detection. Copy numbers are presented as means ± standard errors of the mean SEM of at least two quantitative experiments.
TABLE 2.
Primers and probes for quantitative PCR
| Source | Primer or probe | Sequence |
|---|---|---|
| PCMV | Forward | 5′ GTTCTGGGATTCCGAGGTTG 3′ |
| Reverse | 5′ ACTTCGTCGCAGCTCATCTGA 3′ | |
| Probe | 5′ 6FAM-CAGGGCGGCGGTCGAGCTC-TAMRA 3′ | |
| BCMV | Forward | 5′ GTTTAGGGAACCGCCATTCTG 3′ |
| Reverse | 5′ GTATCCGCGTTCCAATGCA 3′ | |
| Probe | 5′ 6FAM-TCCAGCCTCCATAGCCGGGAAGG-TAMRA 3′ | |
| Pig MHC-I | Forward | 5′ GCCCTGGGCTTCTACCCTAA 3′ |
| Reverse | 5′ TCTCAGGGTGAGTGGCTCCT 3′ | |
| Probe | 5′ 6FAM-CCAGGACCAGAGCCAGGACATGGAGCTCGT-TAMRA 3′ | |
| Baboon CCR5 | Forward | 5′ TACCTGCTCAACCTGGCCAT 3′ |
| Reverse | 5′ TTCCAAAGTCCACTGGGC 3′ | |
| Probe | 5′ 6FAM-TTTCCTTCTTACTGTCCCCTTCTGGGCTC-TAMRA 3′ |
Measurement of baboon and porcine CMV DNA.
BCMV DNA was amplified using primers and probe designed for a 108-bp region of the immediate-early gene of rhesus macaque CMV (1) (8a) (Table 2). The quantitative detection limit was 40 copies per reaction. Sequencing of the product generated by these two primers with DNA isolated from baboon lung infected with BCMV (B69-128 and B69-161) (GenBank accession number AY064547) revealed a sequence homology of 90% (nucleotide) compared with the corresponding sequence of rhesus CMV. BCMV primers and probe had no cross-reactivity with samples derived from cell cultures with known high titers of PCMV. The BCMV assay generated an amplification signal in the subquantitative range in some baboon-exposed xenografts with active PCMV infection. No correlations between BCMV and PCMV amplification signals were found.
Primers and probe specific for PCMV were derived from the PCMV strain B6 DNA polymerase gene (8). The quantitative detection limit for this assay was 20 copies per reaction. No amplification occurred using PCMV primers with nucleic acids from baboon tissues heavily infected with BCMV. Tissues from immunosuppressed baboons exposed to pig tissues and in controls without porcine organ transplantation were studied. Using spiking experiments, sensitivity and specificity were shown to be unchanged in the presence of BCMV.
Measurement of species-specific porcine and baboon DNA.
Pig major histocompatibility class I gene (pig MHC-I) primers and probe were developed as internal controls for porcine cellular DNA. Primers and probe were derived from the pig MHC-I gene (Table 2 [20]). Quantitative sensitivity was 20 copies per reaction. The baboon internal standard was the baboon chemokine receptor 5 gene (baboon CCR5), derived from rhesus macaque CCR5 sequences (kindly provided by P. Johnson, New England Regional Primate Research Center, Southborough, Mass.) (Table 2). Quantitative sensitivity was 25 copies per reaction.
The baboon CCR5 assay was negative in control pig tissues (n = 21). The pig MHC-I assay was negative for normal, control baboon tissues (n = 10) except for the aorta and heart. With these tissues, a low but consistent amplification of target (below quantitation limit) could be observed.
RESULTS
CMV activation was assessed in a series of six pig-to-baboon xenotransplantation experiments. Biopsies and samples from excised xenografts and from autopsies were assessed histologically and by PCR for the presence of PCMV, BCMV, and housekeeping genes of both swine and baboon.
The clinical course for each baboon is summarized in Table 1. Four of six baboons died with the transplant in situ with a functioning graft. Survival times of these baboons ranged from 7 to 27 days. The development of a disseminated intravascular coagulation (DIC) syndrome with thrombocytopenia, decreased serum fibrinogen, increased prothrombin (PT), and/or partial thromboplastin (PTT) times necessitated the removal of the xenografts from two other animals. In these two baboons, residual implants of pig thymic tissue in the omentum were not removed. Immunosuppression was discontinued after graftectomy in both cases, and both baboons recovered from DIC with survival times of >200 days.
BCMV.
Increases in the copy numbers of BCMV and PCMV in host and donor tissues are summarized for each baboon in Table 3. Of the four baboons which died while immunosuppressed, reactivation of BCMV was observed for three (75%) (B69-161, 69-128, and 69-222). In these baboons, upregulation was present in all tissues examined (including lung, liver, native kidney, colon, and ileum). On histological examination, inclusion bodies were detected in the lungs of two baboons (B69-161 and B69-128) (Fig. 1).
TABLE 3.
Summary of BCMV and PCMV in host and graft tissue
| Baboon | Presence of CMV disease by histology | Copy no. of CMV DNA/1 μg of DNA of:
|
Presence of CMV upregulation
|
||||
|---|---|---|---|---|---|---|---|
| BCMV in baboon tissue (lung) | BCMV in porcine xenograft | PCMV in baboon tissue (lung) | PCMV in porcine xenograft | BCMV | PCMV | ||
| B69-161 | + | >5 × 105 | <500 | >1 × 103 | >1 × 105 | + | + |
| B69-144a | + | <40 | <40 | Absent | >1 × 105 | NA | + |
| B69-128 | + | >5 × 103 | <40 | >2 × 103 | >1 × 105 | + | + |
| B69-222 | − | >1 × 105 | <100 | <20 | <20 | + | − |
| B69-268 | − | Absent | <40 | <20 | >1 × 105 | − | + |
| B81-99 | − | <40 | <40 | >1 × 104 | >1 × 105 | − | + |
NA, not applicable. For B69-144, numbers shown are for lymph node biopsy at time of graftectomy (baboon still alive).
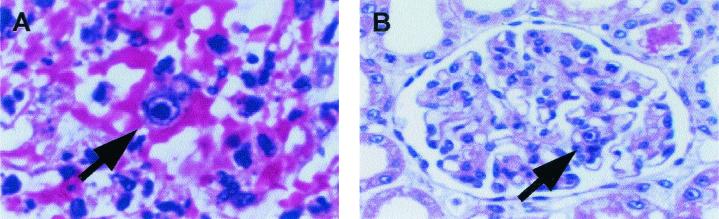
FIG. 1.
Histopathology of lung and kidney of baboon 69-161. (A) Hematoxylin and eosin staining of the lung revealed a significant pneumonia with evidence of intranuclear and intracytoplasmic inclusion bodies (arrow). (B) The native kidney also demonstrated inclusion bodies (arrow).
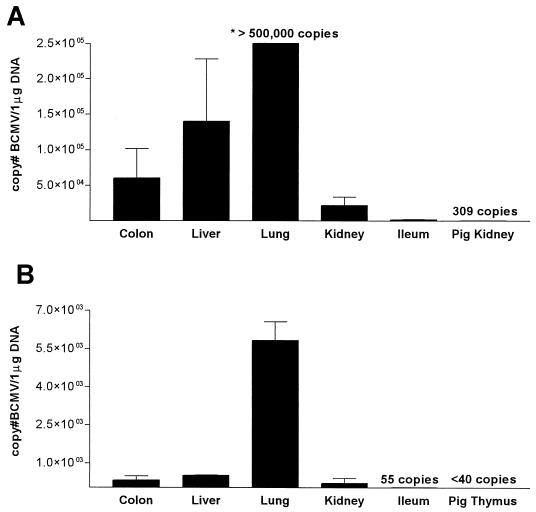
B69-161 developed progressive respiratory failure and expired on POD 27. Histology from the baboon lung revealed evidence of a diffuse pneumonia with intracellular inclusion bodies present within the lung parenchyma (Fig. 1A). Inclusion bodies were also evident throughout the native baboon kidney (Fig. 1B) and liver. No inclusion bodies were observed in the transplanted swine thymokidney or thymic grafts. Based on clinical and histological data, CMV infection was considered to be the cause of death in this animal. BCMV DNA was detected in a high copy number in lung and liver and at somewhat lower levels in colon and native kidney (Fig. 2A). This pattern corresponded to the histological findings described above.
FIG. 2.
BCMV copy numbers in baboon tissues and porcine xenograft from baboon 69-161 (A) and baboon 69-128 (B). The copy number in 1 μg of total DNA extracted from tissues frozen at autopsy is shown. Means ± SEM of at least two independent assays are shown.
B69-128 expired on POD 27 from cardiopulmonary arrest secondary to bacterial sepsis. This baboon had received intravenous ganciclovir (5 mg/kg of body weight/intravenously [i.v.]/twice a day [b.i.d.]) from POD 22 to POD 27. Despite antiviral therapy, viral inclusion bodies were identified in the lung but not in other tissues. PCR analysis detected BCMV in all baboon tissues (Fig. 2B).
In B69-222, BCMV DNA was detected at the highest copy numbers in lung (1.2 × 105) and liver (5.3 × 104) and in smaller amounts in kidney (3.6 × 103), heart (1.4 × 103), and transplanted kidney (160 copies). Histologically, no evidence of BCMV disease was found in these tissues (data not shown). In the remaining baboons, including the two long-term survivors after graftectomy, BCMV DNA was detectable in <40 copies in most of the tissues tested.
BCMV DNA was present in all of the porcine xenografts. In two grafts (B69-161 and B69-222), BCMV was quantifiable. These two animals had high levels of BCMV in all tissues studied. Baboon-specific DNA (baboon CCR5) was also detectable in all six xenografts.
PCMV.
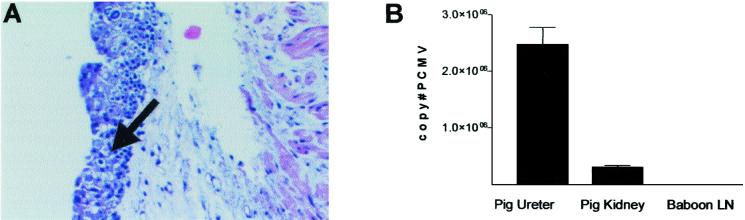
In healthy pigs, PCMV was present in less than 100 copies per μg of DNA in a variety of tissues (n = 21). In particular, all tested donor pig tissues for baboons 69-128, 69-161, 69-222, and 69-144 were negative or below the detection limit at the time of transplantation. PCMV was increased in five of six xenografts (Table 3). For one animal (69-144), the porcine thymokidney xenograft was removed on POD 15 due to DIC. This baboon recovered with normalization of coagulation parameters and without further complications. The distal ureter of this pig revealed gross evidence of hemorrhagic urothelium and histological evidence of inclusion bodies within the urothelium of the distal pig ureter (Fig. 3A). PCMV DNA was found in high copy numbers in the pig ureter and the pig kidney (Fig. 3B). In contrast, PCMV DNA was undetectable in a baboon lymph node biopsy taken from the same recipient at the time of graftectomy.
FIG. 3.
Histopathology of the pig ureter (A) and PCMV viral load of the graft and of a baboon lymph node biopsy (B) from baboon 69-144. Baboon 69-144 developed hematuria and signs of a progressive coagulopathy requiring graftectomy of the porcine thymokidney on POD 15. Hematoxylin and eosin staining revealed the presence of inclusion bodies within the pig urothelium (A, arrow). (B) PCMV DNA copies in 1 μg of total DNA extracted from the pig ureter, pig kidney, and baboon lymph node biopsy. Means ± SEM of at least two independent assays are shown. LN, lymph node.
Histologic examination of the remaining xenografts with high levels of PCMV showed no abnormalities associated with the presence of CMV. Only one xenograft did not contain high levels of PCMV DNA (B69-222). Serologic testing of serum of the pig donor for B69-222 revealed a PCMV antibody titer of 1:64.
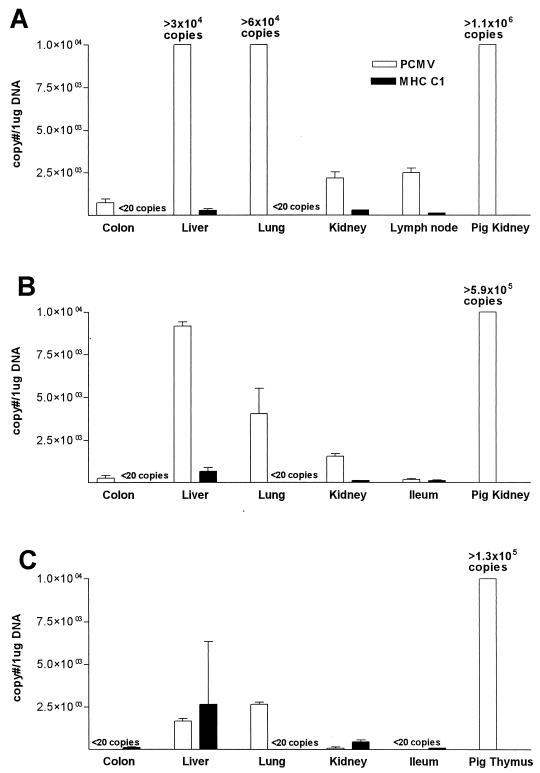
Baboon tissues showed a wide range of levels of PCMV DNA. In three baboons, including the two long-term survivors after graftectomy, PCMV was absent or detected in low copy numbers (<20 copies). In three baboons (B81-99, B69-161, and B69-128), PCMV was present in baboon tissues in higher copy numbers. There was no histologic evidence of viral infection despite detection of PCMV in baboon tissues. In baboon 81-99, PCMV was present in high titers in the baboon liver (3.3 × 104 copies/μg of total DNA) and lung (6.0 × 104 copies/μg of total DNA) and in measurable amounts in mesenteric lymph node, colon, and native kidney (Fig. 4A). DNA sequencing of the product amplified by PCMV primers from the lung and liver of B81-99 confirmed the presence of PCMV DNA in these tissues. PCMV was also detected in significant amounts in multiple baboon tissues from other animals (Fig. 4B and C). In all of the baboon tissues with detectable PCMV, pig-specific cellular DNA (porcine MHC-I) was also detected. While levels of pig MHCC1 were comparable between all the baboons, no correlation was found with the levels of PCMV in the same baboon tissues (Fig. 4A to C).
FIG. 4.
PCMV and pig MHC-I DNA in baboon tissues and pig xenograft. (A) Baboon 81-99; (B) baboon 69-161; (C) baboon 69-128. Numbers of copies of PCMV and porcine MHCC1 DNA in 1 μg of total extracted DNA at autopsy are shown. Means ± SEM of at least two independent assays are shown. For the xenografts, only PCMV is shown.
DISCUSSION
Infection is a central concern in xenotransplantation, both due to novel agents (such as porcine endogenous retrovirus) and to known pathogens (such as CMV) (5, 7). The activation of CMV is related to the intensity of immune suppression, the amount and strain of virus, the intensity of graft rejection, and the presence of a proinflammatory state (e.g., tumor necrosis factor) (7, 17-19). Thus, a regimen such as the one used in these studies, with T-cell depletion by antilymphocyte antisera and graft rejection, is likely to carry a significant risk of CMV activation.
We have developed a series of quantitative molecular assays for PCMV and BCMV and for the presence of porcine and baboon cellular DNA. The present study demonstrates that intense immunosuppression, possibly coupled with a xenogeneic immune response, resulted in activation of CMV in both the recipient and in the porcine xenograft. While clinically evident disease was restricted to the native host species for each virus, low levels of PCMV were detectable in baboon tissue (and vice versa). Both PCMV and BCMV species were activated and contributed to xenograft failure and recipient disease.
Immune suppression resulted in increased tissue burden of BCMV in three out of four (75%) thymokidney recipients who died while under immune suppression. Given the use of T-cell-depleting regimens (including antithymocyte globulins), this is consistent with clinical observations. The reactivation of BCMV was observed in most tissues studied and contributed to the death of one animal from CMV pneumonitis. A single animal known to have active BCMV infection received ganciclovir in a therapeutic dose (5 mg/kg/i.v./b.i.d.) for 5 days. Despite therapy, BCMV inclusion bodies were present in the lung at autopsy. Prophylactic and therapeutic antivirals for BCMV and PCMV are under investigation.
PCMV was increased in five of six thymokidney xenografts, most without evidence of renal dysfunction. PCMV upregulation was associated with ureteric necrosis in one xenograft. This CMV infection resulted in hematuria and viral inclusion bodies and was accompanied by systemic coagulopathy. This coagulopathy resolved upon removal of the xenograft without specific antiviral therapy. This xenograft did not show evidence of rejection to explain the development of graft failure and coagulopathy. This suggests that PCMV infection may have contributed to DIC. However, one baboon with evidence of both humoral rejection and PCMV infection also developed DIC, which reversed upon graft removal. Thus, the relative contributions of PCMV and rejection to endothelial activation and to the DIC syndrome requires further investigation. It would be expected that PCMV infection of the graft might also contribute to the incidence of graft rejection, further enhancing endothelial activation (7). The roles of these factors in the DIC syndrome (e.g., release of tissue factor) are under study.
All animals demonstrated porcine genomic DNA in baboon tissues and baboon DNA in the porcine xenograft. Whether this reflects infection, microchimerism, or uptake of DNA by resident phagocytic cells is unknown. Given that both PCMV and pig genomic DNA were found in baboon tissues, it is likely that there was some systemic spread of PCMV-infected pig cells (i.e., microchimerism). PCMV was also found in the peripheral blood mononuclear cell fraction of baboon blood. Thus, the risk of PCMV infection of baboon cells was increased. The PCMV and porcine cellular DNA found in baboon tissues varied in amount and ratio to a large degree. However, such data cannot distinguish between an active infection and microchimerism or passive uptake of virus. Earlier studies have suggested that CMV is relatively species specific (15). However, recent in vitro findings of human CMV infection of porcine cells have put this conclusion into question (4). Studies of pig-to-primate xenotransplantation offer the opportunity to study this problem directly.
Attempts at successful xenotransplantation currently require ablation of both cellular and humoral immunity. The use of composite thymokidney xenografts to “reeducate” the immune system may ultimately reduce the need for long-term exogenous immune suppression. The present study supports the need to exclude CMV from xenograft donors. In addition, antiviral agents must be identified to protect recipients from xenogeneic infection. The absence of interspecies infection due to common viruses would be a potential advantage to xenotransplantation in the form of possible protection of the grafted organ from common human pathogens. These might include human CMV, Epstein-Barr virus, other human herpes viruses, hepatitis viruses, and possibly human immunodeficiency virus. However, at present, CMV is likely to remain a significant pathogen in clinical xenotransplantation. Further studies are in progress to elucidate the potential risk of xenogeneic infection with PCMV.
Acknowledgments
This study was supported by Public Health Services grant RO1-AI43890 (A.K.), NIH-NIAID 5T32-AI07529-04 (N.J.M.), and NIH-NIAID PO1-AI45897 (J.A.F., D.H.S., and D.K.C.C.). R.N.B. was supported by a Roche Laboratories Surgical Scientist Scholarship and Duke University Department of Surgery, Durham, N.C.
We acknowledge Dan Shaffer for designing the rhesus CMV-specific primers and probe used to detect baboon CMV by real-time PCR, Peter Barry for providing plasmid with the rhesus CMV immediate-early gene insert, and Paul Johnson for providing the sequences for the baboon chemokine receptor 5 assay.
REFERENCES
- 1.Barry, P. A., D. J. Alcendor, M. D. Power, H. Kerr, and P. A. Luciw. 1996. Nucleotide sequence and molecular analysis of the rhesus cytomegalovirus immediate-early gene and the UL121-117 open reading frames. Virology 215:61-72. [DOI] [PubMed] [Google Scholar]
- 2.Buhler, L., K. Yamada, H. Kitamura, I. P. J. Alwayn, M. Basker, J. Z. Appel, R. B. Colvin, M. E. White-Scharf, D. H. Sachs, S. C. Robson, M. Awwad, and D. K. C. Cooper. 2001. Pig kidney transplantation in baboons: anti-Galα1-3Gal IgM alone is associated with acute humoral xenograft rejection and disseminated intravascular coagulation. Transplantation 72:1743-1752. [DOI] [PubMed] [Google Scholar]
- 3.Colvin, R. B. 1996. The renal allograft biopsy. Kidney Int. 50:1069-1082. [DOI] [PubMed] [Google Scholar]
- 4.Degre, M., T. Ranneberg-Nilsen, S. Beck, H. Rollag, and A. E. Fiane. 2001. Human cytomegalovirus productively infects porcine endothelial cells in vitro. Transplantation 72:1334-1337. [DOI] [PubMed] [Google Scholar]
- 5.Fishman, J. A. 1998. Infection and xenotransplantation. Developing strategies to minimize risk. Ann. N. Y. Acad. Sci. 862:52-66. [DOI] [PubMed] [Google Scholar]
- 6.Fishman, J. A. 1998. The risk of infection in xenotransplantation. Ann. N. Y. Acad. Sci. 862:45-51. [DOI] [PubMed] [Google Scholar]
- 7.Fishman, J. A., and R. H. Rubin. 1998. Infection in organ-transplant recipients. N. Engl. J. Med. 338:1741-1751. [DOI] [PubMed] [Google Scholar]
- 8.Goltz, M., F. Widen, M. Banks, S. Belak, and B. Ehlers. 2000. Characterization of the DNA polymerase loci of porcine cytomegaloviruses from diverse geographic origins. Virus Genes 21:249-255. [DOI] [PubMed] [Google Scholar]
- 8a.Kaur, A., C. L. Hale, B. Noren, N. Kassis, M. A. Simon, and R. P. Johnson. 2002. Decreased frequency of cytomegalovirus (CMV)-specific CD4+ T lymphocytes in simian immunodeficiency virus-infected rhesus macaques: inverse relationship with CMV viremia. J. Virol. 76:3646-3658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kawai, T., A. B. Cosimi, R. B. Colvin, J. Powelson, J. Eason, T. Kozlowski, M. Sykes, R. Monroy, M. Tanaka, and D. H. Sachs. 1995. Mixed allogeneic chimerism and renal allograft tolerance in cynomolgus monkeys. Transplantation 59:256-262. [PubMed] [Google Scholar]
- 10.Knechtle, S. J., J. H. Fechner, Jr., Y. Dong, S. Stavrou, D. M. Neville, Jr., T. Oberley, P. Buckley, N. Armstrong, K. Rusterholz, X. Hong, M. Tsuchida, and M. M. Hamawy. 1998. Primate renal transplants using immunotoxin. Surgery 124:438-447. [PubMed] [Google Scholar]
- 11.Kobayashi, T., S. Taniguchi, F. A. Neethling, A. G. Rose, W. W. Hancock, Y. Ye, M. Niekrasz, S. Kosanke, L. J. Wright, D. J. White, and D. K. C. Cooper. 1997. Delayed xenograft rejection of pig-to-baboon cardiac transplants after cobra venom factor therapy. Transplantation 64:1255-1261. [DOI] [PubMed] [Google Scholar]
- 12.Kozlowski, T., F. L. Ierino, D. Lambrigts, A. Foley, D. Andrews, M. Awwad, R. Monroy, A. B. Cosimi, D. K. C. Cooper, and D. H. Sachs. 1998. Depletion of anti-Gal(alpha)1-3Gal antibody in baboons by specific alpha-Gal immunoaffinity columns. Xenotransplantation 5:122-131. [DOI] [PubMed] [Google Scholar]
- 13.Lambrigts, D., D. H. Sachs, and D. K. C. Cooper. 1998. Discordant organ xenotransplantation in primates: world experience and current status. Transplantation 66:547-561. [DOI] [PubMed] [Google Scholar]
- 14.Lee, L. A., H. A. Gritsch, J. J. Sergio, J. S. Arn, R. M. Glaser, T. Sablinski, D. H. Sachs, and M. Sykes. 1994. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc. Natl. Acad. Sci. USA 91:10864-10867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaels, M. G., D. J. Alcendor, K. St George, C. R. Rinaldo, Jr., G. D. Ehrlich, M. J. Becich, and G. S. Hayward. 1997. Distinguishing baboon cytomegalovirus from human cytomegalovirus: importance for xenotransplantation. J. Infect. Dis. 176:1476-1483. [DOI] [PubMed] [Google Scholar]
- 16.Michaels, M. G., F. J. Jenkins, K. St. George, M. A. Nalesnik, T. E. Starzl, and C. R. Rinaldo, Jr. 2001. Detection of infectious baboon cytomegalovirus after baboon-to-human liver xenotransplantation. J. Virol. 75:2825-2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olding, L. B., F. C. Jensen, and M. B. Oldstone. 1975. Pathogenesis of cytomegalovirus infection. I. Activation of virus from bone marrow-derived lymphocytes by in vitro allogenic reaction. J. Exp. Med. 141:561-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rinaldo, C. R., Jr., M. S. Hirsch, and P. H. Black. 1976. Activation of latent viruses following bone marrow transplantation. Transplant. Proc. 8:669-672. [PubMed] [Google Scholar]
- 19.Rubin, R. H., A. B. Cosimi, M. S. Hirsch, J. T. Herrin, P. S. Russell, and N. E. Tolkoff-Rubin. 1981. Effects of antithymocyte globulin on cytomegalovirus infection in renal transplant recipients. Transplantation 31:143-145. [DOI] [PubMed] [Google Scholar]
- 20.Sullivan, J. A., H. F. Oettinger, D. H. Sachs, and A. S. Edge. 1997. Analysis of polymorphism in porcine MHC class I genes: alterations in signals recognized by human cytotoxic lymphocytes. J. Immunol. 159:2318-2326. [PubMed] [Google Scholar]
- 21.Xu, Y., T. Lorf, T. Sablinski, P. Gianello, M. Bailin, R. Monroy, T. Kozlowski, M. Awwad, D. K. C. Cooper, and D. H. Sachs. 1998. Removal of anti-porcine natural antibodies from human and nonhuman primate plasma in vitro and in vivo by a Galalpha1-3Galbeta1-4betaGlc-X immunoaffinity column. Transplantation 65:172-179. [DOI] [PubMed] [Google Scholar]
- 22.Yamada, K., A. Shimizu, F. L. Ierino, R. Utsugi, R. N. Barth, N. Esnaola, R. B. Colvin, and D. H. Sachs. 1999. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney.” Transplantation 68:1684-1692. [DOI] [PubMed] [Google Scholar]
- 23.Yamada, K., A. Shimizu, R. Utsugi, F. L. Ierino, P. Gargollo, G. W. Haller, R. B. Colvin, and D. H. Sachs. 2000. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J. Immunol. 164:3079-3086. [DOI] [PubMed] [Google Scholar]
- 24.Zhao, Y., K. Swenson, J. J. Sergio, J. S. Arn, D. H. Sachs, and M. Sykes. 1996. Skin graft tolerance across a discordant xenogeneic barrier. Nat. Med. 2:1211-1216. [DOI] [PubMed] [Google Scholar]