Abstract
We previously demonstrated that immunization of mice with plasmid DNAs (pDNAs) expressing the murine cytomegalovirus (MCMV) genes IE1-pp89 and M84 provided synergistic protection against sublethal viral challenge, while immunization with plasmids expressing putative virion proteins provided no or inconsistent protection. In this report, we sought to augment protection by increasing the breadth of the immune response. We identified another MCMV gene (m04 encoding gp34) that provided strong and consistent protection against viral replication in the spleen. We also found that immunization with a DNA pool containing 10 MCMV genes that individually were nonprotective elicited reproducible protection against low to intermediate doses of challenge virus. Moreover, inclusion of these plasmids into a mixture with gp34, pp89, and M84 DNAs provided even greater protection than did coimmunization with pp89 and M84. The highest level of protection was achieved by immunization of mice with the pool of 13 pDNAs, followed by formalin-inactivated MCMV (FI-MCMV). Immunization with FI-MCMV elicited neutralizing antibodies against salivary gland-derived MCMV, and of greatest importance, mice immunized with both the combined pDNA pool and FI-MCMV had undetectable levels of virus in the spleen and salivary glands after challenge. Intracellular cytokine staining of splenocytes from pDNA- and FI-MCMV-immunized mice showed that pDNA immunization elicited high levels of pp89- and M83-specific CD8+ T cells, whereas both pDNA and FI-MCMV immunizations generated strong CD8+-T-cell responses against virion-associated antigens. Taken together, these results show that immunization with pDNA and inactivated virus provides strong antibody and cell-mediated immunity against CMV infection.
Whereas infection of immunocompetent individuals with human cytomegalovirus (HCMV) is usually asymptomatic, hosts with deficient cell-mediated immunity (CMI)—such as newborns and solid organ or bone marrow allograft recipients receiving immunosuppressive therapy—are at serious risk for disseminated HCMV infection and disease (3). In addition, human immunodeficiency virus (HIV)-infected individuals are susceptible to life-threatening HCMV disease if uncontrolled HIV replication sufficiently depletes the CD4+-T-lymphocyte pool. The severe morbidity and mortality observed in these immunocompromised groups underscore the important role that CMI plays in controlling HCMV infection and highlight the necessity for an effective vaccine that provides strong antiviral CMI.
Studies of immunity to murine CMV (MCMV) have both confirmed the observations made regarding HCMV-specific cell-mediated and humoral immunity and provided important groundwork for the development of CD8+-T-cell immunotherapy and vaccine strategies. The cell-mediated response to MCMV plays a critical role in resolving the acute infection and limiting the reactivation and dissemination of latent virus. Natural killer cells provide an important first line of defense to early MCMV infection before the accumulation of virus-specific CD8+ cytotoxic T lymphocytes (CTLs) (39). The acquired cell-mediated response, which includes both CD8+ and CD4+ T lymphocytes, then functions to resolve the acute CMV infection. In adoptive-transfer experiments, it was demonstrated that CD8+ T lymphocytes limit viral dissemination and protect whole-body gamma-irradiated mice from lethal infection (43, 46). In addition, CD4+, but not CD8+, T lymphocytes appear to be important for clearance of MCMV from the salivary glands and can take on an antiviral role if CD8+ cells are depleted (23, 38).
Infection of BALB/c mice leads to generation of CD8+ CTLs directed against both structural and nonstructural antigens of MCMV, with major subsets being specific for the IE1 gene product, pp89 (28, 44, 45), as well as the m164 gene product (21). It was recently demonstrated that ca. 80% of all memory CD8+ T cells in the spleen and lung are specific for either of these two antigens (21). Immunization of BALB/c mice with a recombinant vaccinia virus expressing either full-length pp89 or an unrelated antigen modified to contain the Ld-restricted epitope of pp89, 168YPHFMPTNL179, elicits a CD8+ CTL response that is protective against lethal challenge but not against infection and morbidity (8, 54). In contrast, exposure of BALB/c mice to a sublethal dose of MCMV appears to induce additional protective mechanisms, suggesting that other MCMV proteins play a role in the protective response. Recently, it has been found that gp34, the 34-kDa glycoprotein product of the m04 gene, generates a CD8+-T-cell response during infection (20), even though gp34 may play a role in immune evasion by forming a complex with major histocompatibility complex (MHC) class I molecules in the endoplasmic reticulum that is transported to the cell surface (27). Importantly, a CTL line specific for the Dd epitope of gp34, 243YGPSLYRRF251, was found to protect immunoablated mice against viral replication in target organs (20). Finally, another early protein, encoded by m18, was found to contain a Dd-associated epitope, 346SGPSRGRII354 that elicits both acute and memory CD8+-T-cell frequencies similar to those of m04 (17).
We used a plasmid DNA (pDNA) immunization model to help identify MCMV gene products that can confer protective cell-mediated responses. We first demonstrated that intradermal (i.d.) immunization of BALB/c mice with a plasmid expressing pp89 elicits pp89-specific CTL levels similar to those occurring after viral infection and that these responses conferred protection against subsequent lethal or sublethal intraperitoneal (i.p.) viral challenge (13). Based upon the reported existence of CTLs specific for virion-associated antigens during infection, we next tested the protective efficacies of MCMV genes encoding the homologs of HCMV matrix (M32, M48, M56, M69, M82, M83, and M99) and capsid (M85 and M86) genes, as well as the nonstructural gene M84 (35). After i.p. challenge of mice immunized with single plasmids or a pool of up to four plasmids, we identified a new viral gene, M84, that conferred protection against viral replication in the spleen, a target organ important for primary viral amplification leading to lethal hepatitis (25). In the course of our studies, we found that the M84 gene, like M83, is a homolog of HCMV UL83-pp65 (6), a key target for HCMV-specific CD8+ T cells (26, 55). Characterization of these MCMV homologs revealed that like UL83-pp65, the M83 gene product is a late, virion-associated phosphoprotein that is dispensable for replication in tissue culture (6, 34). In contrast, the M84 gene product was found to be a nonstructural early protein that was also dispensable for growth in culture (34). Importantly, coimmunization of mice with pp89 and M84 genes was found to confer a synergistic level of protection after a high sublethal challenge dose (35). We also found that although protection against low sublethal viral doses could be elicited by a plasmid pool containing M83, M85, M86, and M99 genes, immunization with these antigens individually as either pDNA or recombinant vaccinia virus vectors was not protective (35).
Subsequent studies by the Matthias J. Reddehase group identified an MHC class I Kd-restricted epitope in the M84 gene product of MCMV strain Smith, 297AYAGLFTPL305 (22). In addition, a nonapeptide epitope of M83 that is presented on the molecule Ld was identified (761YPSKEPFNF769) (19). Although the acute and memory CD8+-T-cell responses to these epitopes were found to be just above the limits of detection by ELISPOT assay, CTL lines specific for each epitope were found to confer protection against viral replication in the spleen, liver, and lungs after transfer to immunoablated mice (19). Taken together, these results suggest that, while both M83 and M84 genes encode protective MHC class I-restricted epitopes, pDNA immunization with these genes reveals differences in their relative efficiencies for the priming of specific CD8+ T cells and/or the ability of these CD8+ T cells to confer protection in immunocompetent mice. In recent studies, we have shown that pDNA immunization with an M84 plasmid elicits a specific CD8+-T-cell response that is readily amplified upon subsequent viral challenge (57). Notably, the M84 epitope-specific CD8+-T-cell response primed by pDNA immunization was ∼5-fold higher than that elicited by MCMV infection. These results highlight the importance of determining which of the MCMV antigens confer protective responses and what parameters of these immune responses determine whether protection is achieved in the immunocytotherapy or pDNA immunization models.
In contrast to the highly protective role demonstrated for T lymphocytes, the humoral response to MCMV likely does not alter the course of the acute infection or affect the latent viral load, as the neutralizing antibody titers peak ca. 14 days after infection, a time when infectious virus had already disseminated to the salivary glands and other target organs (24). However, the antiviral antibodies elicited during the acute infection can help to limit the dissemination of reactivated virus (24, 37, 42). In addition, it has also been shown that antiviral antibody prophylaxis with either adoptively transferred MCMV antiserum or vaccination with chemically inactivated MCMV can reduce the severity of the acute infection (11, 12, 50, 53). As with HCMV, the neutralizing antibody activities against MCMV have been shown to be directed against their respective glycoprotein B (gB) and gH homologs. In addition, immunization of mice with either gB subunit vaccine (56) or gB- or gH-expressing recombinant vaccinia viruses (40) conferred some protection against subsequent challenge. Several clinical studies examining the correlation of the total or HCMV-neutralizing antibody titers with viral load and/or disease severity have implicated a role for the antibody response for limiting HCMV disease. However, the levels of HCMV-specific antibodies are not always predictive of disease outcome, and the therapeutic efficacy of anti-HCMV antibodies in allograft recipients is still not well defined (3).
By pDNA immunization, we have been able to significantly suppress viral replication in the spleen after viral challenge. However, titers in the salivary glands were only moderately reduced. In this report, we asked whether increased protection against MCMV replication could be achieved (i) by broadening the CD8+-T-cell-mediated immune response to the pDNA vaccine and (ii) by complementing the CD8+-T-cell immunity with protective antibody responses against multiple epitopes. Our hypothesis was that better protection could be conferred to the host through the generation of immune responses that more closely resembled those following the acute infection, both in terms of the number of antigens delivered and the generation of both cell-mediated and humoral immunity. In the studies presented here, we found that immunization with a DNA pool containing both individually protective and nonprotective plasmids, followed by immunization with formalin-killed virus, is an effective means of generating complete protection against viral replication and dissemination.
MATERIALS AND METHODS
Mice, cells, and viruses.
Three- to four-week-old pathogen-free female BALB/c (H-2d haplotype) mice were purchased from Simonsen Laboratories or Harlan Sprague-Dawley, Inc. Mice were housed in microisolator covered cages and allowed to acclimate to the University of California, San Diego, vivarium for 1 week prior to immunization.
All media contained 0.29 mg of l-glutamine, 100 U of penicillin, and 0.1 mg of streptomycin per ml, and all calf sera (CS) and fetal bovine sera (FBS) were heat inactivated at 56°C for 1 h. NIH 3T3 cells (ATCC CRL 1658) were grown in Dulbecco modified Eagle medium (DMEM; low glucose) supplemented with 10% CS. COS-7 (ATCC CRL 1651) and J774A.1 (ATCC CRL TIB-67) macrophages (H-2d) were grown in DMEM (high glucose) supplemented with 10% FBS and 1 mM sodium pyruvate. Mouse embryonic cells (MECs) were prepared by standard methods from BALB/c embryos, propagated in DMEM (low glucose) plus 10% FBS, and used for virus preparation as previously described (4).
The K181 strain of MCMV was obtained from Michael Oldstone. Salivary gland-derived stocks of MCMV (SG-MCMV) and tissue culture-derived MCMV were prepared as previously described (35). For some challenge experiments, an SG-MCMV stock was prepared with modifications to produce a more homogeneous stock. After Dounce homogenization, the salivary gland homogenate was clarified by centrifugation at 500 × g for 5 min at 4°C. The resulting pellet was Dounce homogenized again in fresh freezing medium (DMEM, 10% CS, 10% dimethyl sulfoxide) and then frozen and thawed. After they were reclarified as described above, the supernatants were combined from the supernatants obtained from the first spin. The combined supernatants were then clarified for 35 min at 4,000 × g, and the resulting supernatants were clarified at 10,000 × g for 10 min at 4°C. The preparation was stored at −80°C until use and was found to have a titer on NIH 3T3 cells of 1.9 × 107 PFU/ml and a 50% lethal dose (LD50) of ca. 8 × 105 PFU in BALB/c mice. This LD50 value was ca. three- to fourfold higher than those previously reported, possibly due to the removal of large virion aggregates that would individually score as 1 PFU by plaque assay but would contribute to a virulence higher than that produced by a single infectious virion.
Preparation of FI-MCMV.
Tissue culture-derived MCMV was propagated as described above and partially purified by ultracentrifugation as previously described (9) except that a 25% sorbitol-Tris-buffered saline cushion was used. Viral pellets were resuspended in phosphate-buffered saline (PBS), titers were determined in triplicate on NIH 3T3 cells, and then the pellets were formalin inactivated as previously described (53). The resulting preparation was confirmed to be completely inactivated by high-sensitivity plaque assay (see below) and had a final titer of ca. 1 × 109 to 2 × 109 PFU equivalents (PFU eq) per ml. Mock FI-MCMV was prepared from the supernatants of uninfected NIH 3T3 cells or MECs and formalin treated as described above.
Plasmids.
Cloning of the following MCMV genes into the pcDNA3 vector was previously described: the IE1 gene pp89, M84, seven matrix protein genes (M32, M48, M56, M69, M82, M83, and M99), two capsid genes (M85 and M86), and the early gene e1 (M112-113) (35). In addition, the construction of amino-terminal ubiquitin fusions of pp89 and M84 (U-pp89 and U-M84, respectively) was recently described (57).
The gp34 gene (m04) was PCR amplified from MCMV-infected NIH 3T3 DNA (prepared by Qiagen Blood Kit) and FLAG tagged at its carboxy terminus with the following primers: sense, 5′-ATG TCT CTC GTA TGT CGG CTG GTG TTG GTG-3′; and antisense, 5′-TTA CTT GTC GTC GTC ATC CTT GTA GTC GTT ACT CTT AAG CGG TTT GAA GTT C-3′ (the FLAG tag-encoding sequence is underlined). Primer sequences were based upon the published m04 sequence of MCMV strain Smith (41). After amplification with cloned Pfu polymerase (Stratagene), the 825-bp product was agarose gel purified and blunt end ligated into EcoRV-digested, calf intestinal alkaline phosphatase (CIAP)-treated pc3 vector (57). Resultant clones were screened by restriction analysis, and a clone containing the insert in the desired orientation, designated pc3-gp34, was propagated; the entire insert was then sequenced by using Sequenase (Amersham). Alignment of the putative amino acid sequences of K181 and Smith strains was performed by using DNAMAN 4.0 software (Lynnon BioSoft, Inc.). Expression of the clone was examined by transient transfection of COS-7 cells with pc3-gp34, (expressing the FLAG-tagged gp34 from MCMV K181) or with pcDNA3-m04 (expressing the wild-type m04 open reading frame [ORF] from MCMV Smith [a gift from Ann Hill]). After transfection, cells were lysed in reducing sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) buffer, lysates were electrophoresed on an SDS-10% PAGE gel and electroblotted onto nitrocellulose, and the gp34 proteins were detected by using either a BALB/c anti-MCMV hyperimmune serum (5), or a FLAG-specific mouse monoclonal antibody (Kodak-IBI) and Pierce SuperSignal West Pico substrate.
Immunization and virus challenge.
Plasmid DNAs for injection were propagated in Escherichia coli DH5α (Life Technologies) and purified by using either Qiagen Mega or Giga columns, followed by four Triton X-114 extractions as previously described (2), or by using Qiagen EndoFree Mega or Giga columns. The backs of the mice were shaved and i.d. injected three times within 2 to 4 weeks with plasmid DNA in endotoxin-free Tris-buffered saline (pH 8) by using a U-100 tuberculin syringe (Becton Dickinson, Inc.).
For immunization with inactivated MCMV, FI-MCMV was diluted in PBS, and an equal volume of either PBS or Imject Alum (Pierce) was added dropwise while the mixture was swirling. FI-MCMV was mixed with alum or PBS for 30 min and then injected i.p. (at 107 PFU eq in 0.2 ml per mouse). Mock FI-MCMV-immunized mice were injected with formalin-inactivated mock preparation plus alum.
At various intervals after the last immunization, mice were i.p. challenged with either a single sublethal dose of MCMV, typically 0.5 to 0.6 LD50, or one of three challenge doses over a range of 0.3 to 0.7 LD50 in 0.5 ml of PBS (pH 7.4). Actual challenge doses used were dependent upon the virulence of the viral preparation.
Virus titration.
Spleen and salivary gland homogenates (10% [wt/vol]) were prepared in DMEM-10% CS-10% dimethyl sulfoxide and stored at −70°C. Infectious MCMV present in the organ homogenates was quantified by plaque assay on NIH 3T3 monolayers in 24-well dishes (13). For the detection of very low levels of infectious virus in the spleen, a more sensitive protocol was used. Spleen homogenates were thawed in a 37°C bath and clarified by microcentrifugation for 2 min at 16,000 × g. One hundred microliters of clarified homogenate (0.1 spleen equivalent) was mixed with 5 ml of DMEM plus 6% CS, and then the mixture was added to a 75% confluent monolayer of NIH 3T3 cells in a T-75 flask. As a control for the presence of inhibitors of plaque formation in the homogenate, another 100 μl of the homogenate supernatant was added to 5 ml of DMEM plus 6% CS containing 10 PFU of MCMV. After adsorption for 3 h, the 5 ml of inoculum was diluted by the addition of 15 ml of DMEM plus 6% CS, and the flasks were incubated overnight at 37°C and 10% CO2. The diluted inoculum was then removed, the monolayers were washed twice with warm PBS, and a 25-ml overlay of DMEM-6% CS-0.5% agarose was added. Flasks were incubated until day 5 or 6 postinfection, and then the monolayers were formalin fixed and stained with crystal violet before the plaques were counted. This protocol was found to reliably detect ≥10 PFU per spleen without inhibition of the exogenously added MCMV in each positive control. Of note, we found that infection with centrifugal enhancement (800 × g, 40 min) did not significantly increase the number of plaques formed when known amounts of virus of <10 PFU, as measured by standard 24-well plaque assay, were used (data not shown). While we did see infectivity enhancement that increased with increasing amounts of input virus at >10 PFU, we chose to use overnight adsorption of virus to provide consistent comparisons between high and low levels of infectious virus.
Neutralization assay.
Blood was collected from the retro-orbital plexus prior to challenge, and sera were stored at −20°C until use. The neutralization activity against SG-MCMV was subsequently determined as previously described (12), except that 20 μl of each decomplemented serum (heated at 56°C for 30 min) was mixed with 50 PFU of SG-MCMV and rabbit complement (Sigma). The positive control serum was a pool of sera from mice 6 months after i.p. injection with 2.25 × 105 PFU of SG-MCMV (0.3 LD50). The neutralization titer was calculated as the highest reciprocal twofold dilution of serum that resulted in a ≥50% reduction in the number of PFU of input virus.
ICCS for detection of MCMV antigen-specific CD8+ T lymphocytes.
Intracellular cytokine staining (ICCS) was performed as previously described with modifications (1, 15, 51, 57). Synthetic peptides of the two defined, Ld-restricted epitopes of MCMV—pp89 168YPHFMPTNL176 and M83 761YPSKEPFNF769—were synthesized and purified by high-pressure liquid chromatography by PeptidoGenic Research and Co., Inc. (Livermore, Calif.). Splenocytes were harvested, blood cells were lysed, and the resulting splenocytes were washed with RP-10 (RPMI 1640, 10% FBS, 50 μM 2-mercaptoethanol) and resuspended in RP-10 to 2 × 107 cells per ml. To stimulate antigen-specific CD8+ T cells, 100 μl of splenocytes was mixed with 100 μl of RP-10 containing a 2 μM concentration of the appropriate epitope peptide and 1:500 diluted GolgiPlug containing brefeldin A (Pharmingen). Alternatively, splenocytes were stimulated with J774A.1 macrophages (H-2d) infected with UV-inactivated MCMV (UV-MCMV) with centrifugal enhancement (800 × g, 40 min) at an equivalent multiplicity of infection (MOI) of 10 for 5 h. Stimulator cells were then harvested, washed three times with RP-10, and resuspended in RP-10 containing GolgiPlug as described above; 100 μl of splenocytes was then mixed with 100 μl of stimulator cells (3.3 × 105 cells). After incubation of splenocytes with peptide or stimulator cells at 37°C and 7% CO2 for 8 h, cells were pelleted and resuspended in fluorescence-activated cell sorting buffer containing Fc Block (Pharmingen). After incubation on ice, cells were pelleted, CD8 labeled, fixed, and permeabilized, and gamma interferon (IFN-γ) labeled as recommended by the manufacturer (Pharmingen). The double-stained cells were analyzed by flow cytometry on a Coulter Epics Elite counter by the CORE Flow Cytometry Laboratory, Veterans Affairs Medical Center, San Diego, Calif., and the number of antigen-specific CD8+ T cells, enumerated by using B. D. Flow Cytomete software (Elite), was expressed as a percentage of the total CD8+ T cells.
RESULTS
The m04 (gp34) ORF of MCMV K181 possesses amino-terminal sequence heterogeneity compared with the Smith strain but contains the defined MHC class I Dd-restricted epitope and elicits strong protection against sublethal challenge after pDNA immunization
Although pp89 has long been known to be a key target of the CD8+-T-lymphocyte response during MCMV infection, until recently little information existed about the identities of additional MCMV antigens that elicit CD8+-T-cell responses. In order to further define the CD8+-T-cell specificities in the MCMV-infected BALB/c mouse, Holtappels et al. screened titrating concentrations of 35 MCMV genome-spanning candidate MHC class I Dd-binding nonapeptides for their ability to restimulate MCMV-specific memory spleen cells and lyse peptide-loaded P815 cells (20). This enormous study serendipitously identified a nonapeptide epitope contained within the gp34-encoding m04 ORF of MCMV Smith strain. Although adoptive transfer of a CTL line specific for this epitope was protective against MCMV infection in whole-body gamma-irradiated mice, the CTL line was unable to lyse MCMV-infected MECs in vitro. We therefore sought to determine whether an gp34-specific immune response could protect immunocompetent mice against subsequent viral replication.
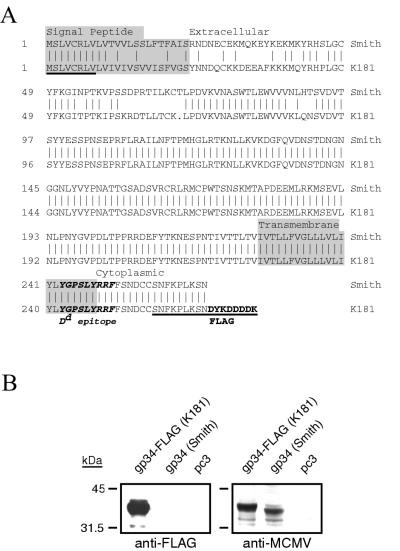
The m04 ORF from MCMV K181-infected NIH 3T3 cell DNA was amplified and placed into a pcDNA3-derived plasmid vaccine vector, pc3 (see Materials and Methods). A carboxy-terminal FLAG tag was added to the m04-gp34 sequence for ease in detection of the gene product. Figure 1A shows the sequence alignment of the putative amino acid sequence of the resultant K181 m04-gp34 clone, pc3-gp34, compared with the published m04 sequence of strain Smith (41). We found that the two sequences share an overall 86% amino acid identity and that the amino-terminal 90 to 91 amino acids of these proteins share at most a 60% identity level with a single amino acid deletion found in the m04 ORF of K181. These identity values are approximate because the 8 amino-terminal and 9 carboxy-terminal amino acids of our m04 clone are encoded by the Smith sequence-derived PCR primers (underlined sequences in Fig. 1A). Nevertheless, the amino acid sequence heterogeneity between the two strains was located in both the putative signal peptide region and the membrane-distal portion of the putative extracellular domain. Within the heterogeneous extracellular region, a consensus N-glycosylation sequence (amino acids 77 to 79 in the Smith strain) was found to be intact in the K181 sequence. Most notably, the Dd-restricted epitope identified in Smith strain is identical in the transmembrane-cytoplasmic domain junction of our K181 clone. To confirm that the sequence heterogeneity found in the K181-derived m04 clone was not due to mutations introduced during PCR amplification, the heterogeneous region of m04 (beginning downstream of the 5′ sense cloning primer) was directly sequenced in the restriction nuclease-cloned EcoRI X fragment of K181 (33). Both sequences were found to be identical, demonstrating the true heterogeneity of m04 sequences in these two strains of MCMV. Whether this sequence heterogeneity contributes to differences in the immune evasion or virulence characteristics of strains Smith and K181 is remains to be determined.
FIG. 1.
Expression of the m04 (gp34) gene of MCMV strain K181. (A) Amino acid sequence alignment of the m04 (gp34) ORF of K181 and Smith strains. PCR primers derived from the Smith strain were used to amplify the m04 ORF of strain K181, and the resulting FLAG-tagged gene fusion was cloned and dideoxy sequenced. Shown is the putative amino acid sequence of the K181 clone compared to the published Smith strain sequence. Underlined are the PCR primer-encoded amino acids, including the carboxy-terminal FLAG tag. Indicated by alternate shaded and nonshaded sequence are the putative signal peptide, extracellular, transmembrane, and cytoplasmic domains. The Dd-restricted nonapeptide epitope defined for strain Smith is indicated in boldface italic letters. (B) Expression of m04 in COS-7 cells after transient transfection. Cells were transfected with the empty vector pc3 or plasmids expressing either the FLAG-tagged gp34 from K181 or the wild-type gp34 from Smith strain, and 48 h later, cells were lysed in reducing SDS-PAGE buffer. Lysates were analyzed by Western blotting with either an anti-FLAG monoclonal antibody (left) or an anti-MCMV hyperimmune serum (right) and enhanced-chemiluminescence detection.
To test the expression of the m04 clone, COS-7 cells were transiently transfected with either the FLAG-tagged gp34 plasmid or a pcDNA3-based plasmid expressing gp34 from Smith strain (a generous gift from Ann Hill). Cell lysates were subjected to SDS-PAGE and Western blot analysis with an anti-FLAG monoclonal antibody. The left panel of Fig. 1B shows a strongly immunoreactive 35-kDa band that was detectable only in the K181 gp34-FLAG-transfected cells, demonstrating the expression of an appropriately sized protein with an in-frame FLAG tag. In addition, an identical blot was probed with a BALB/c mouse anti-MCMV hyperimmune serum. This serum was found to detect the FLAG-reactive protein in the gp34-FLAG lane, as well as a slightly faster migrating protein in the cells transfected with the Smith strain gp34 plasmid. The migration of the Smith strain gp34 is consistent with its lack of an epitope tag. These results not only confirm that the K181 gp34 protein is appropriately expressed from the plasmid construct but also show that MCMV infection of the host elicits a vigorous humoral response against gp34.
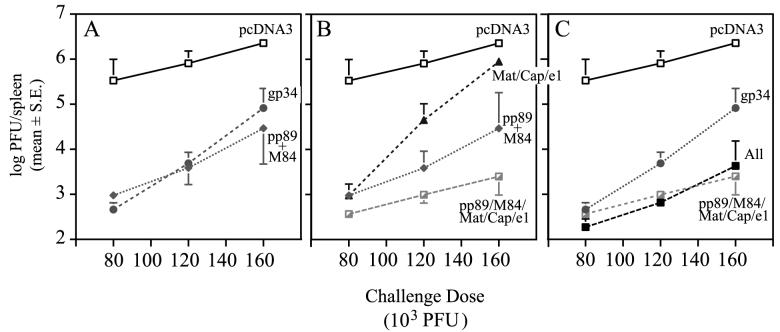
To determine whether immunization with the gp34 plasmid could elicit a protective response in immunocompetent mice, groups of BALB/c female mice were i.d. immunized three times within 3 weeks with either 26 μg of pcDNA3, 10 μg of pc3-gp34 (K181), or 2 μg each of pp89 and M84 plasmids. The DNA masses of all injections were normalized to 26 μg by the addition of empty pcDNA3 vector. Four weeks after the last immunization, four mice per group were i.p. challenged with 80 × 103, 120 × 103, or 160 × 103 PFU of SG-MCMV K181 (corresponding to ca. 0.3, 0.5, and 0.6 LD50 for this viral stock). On day 6 postchallenge, spleens were harvested and homogenized for MCMV titer determination. We found that coimmunization with pp89 plus M84 elicited responses resulting in 80- to 350-fold reductions in spleen viral titers compared to the mice immunized with vector alone (Fig. 2A). These reductions in viral titers were slightly lower than those previously observed after pp89 plus M84 coimmunization, possibly due to the longer-than-usual period before challenge (4 weeks versus 2 weeks) and/or the use of 2 μg each of MCMV antigen-expressing pDNA (instead of 15 μg). Most importantly, immunization with the gp34-expressing plasmid consistently resulted in 30- to 730-fold reductions in viral titers after challenge with all three challenge doses (Fig. 2A). It should be noted that 10 μg of the gp34-expressing plasmid was used in this group compared to the 2 μg of each of pp89 and M84. The higher mass of the gp34 plasmid was used to help prevent it from being limiting, since it had not been titrated for protective efficacy (data not shown). Nonetheless, these results demonstrate that pDNA-delivered gp34 provides protection against viral replication in mice with intact CMI.
FIG. 2.
Protection against viral replication in BALB/c mice immunized with the gp34 plasmid or various pools of MCMV antigen-expressing plasmids. Mice were i.d. immunized three times within 3 weeks with 26 μg of plasmid DNA mixtures containing either (i) empty vector (pcDNA3); (ii) the gp34-FLAG plasmid (gp34); (iii) pp89- and M84-expressing plasmids (pp89+M84); (iv) a pool of 10 plasmids expressing genes encoding putative matrix (M32, M48, M56, M69, M82, M83, and M99) and capsid (M85 and M86) antigens, as well as the nonstructural e1 gene product (Mat/Cap/e1); (v) a combined pool containing pp89, M84, and the Mat/Cap/e1 plasmids (pp89/M84/Mat/Cap/e1); or (vi) a combined pool containing the gp34 plasmid and the pp89/M84/Mat/Cap/e1 plasmid pool (All). Four weeks after the last immunization, mice were i.p. challenged with either 80 × 103, 120 × 103, or 160 × 103 PFU of SG-MCMV (corresponding to 0.3, 0.5, and 0.6 LD50 for this viral stock), and on day 6 postchallenge, spleens were harvested for MCMV titer determination on NIH 3T3 cells. The resulting spleen titers are shown as the means of the log10 PFU per spleen for four mice with the standard error (S.E.) of the mean indicated by error bars. For display purposes, the data are presented in three panels with the pcDNA3 control included in all panels for comparison. MCMV titers in the spleens of mice immunized with the following plasmids are shown: pcDNA3, gp34, or pp89+M84 (A); pcDNA3, pp89+M84, Mat/Cap/e1, or pp89/M84/Mat/Cap/e1 (B); and pcDNA3, gp34, pp89/M84/Mat/Cap/e1, or the All plasmid pool (C).
Coimmunization with a pool of minor MCMV antigens that are not protective when injected individually provides protection against low to intermediate challenge doses and augments the protection elicited by pp89 plus M84 coimmunization
During our previous pDNA immunization experiments directed at identifying novel protective MCMV antigens, we subcloned nine MCMV ORFs encoding putative tegument and capsid antigens and tested their ability to protect against subsequent challenge (35). None of these antigens elicited consistent protection either when individually delivered by pDNA (35; data not shown) or recombinant vaccinia virus (35). However, when mice were immunized with a pool of M83, M85, M86, and M99 plasmids, a low level of protection was observed after challenge with the lower viral doses. In view of these results and an earlier published report suggesting that a large number of MCMV antigens other than pp89 may contribute to CD8+-T-cell immunity (18), we tested whether the combined CMI generated against several individually nonprotective antigens could lead to a significant level of protection against viral replication.
To address this question, groups of mice from the same gp34 immunization experiment described above were i.d. immunized with a pool of 10 plasmids consisting of 2 μg each of plasmids expressing seven putative matrix (M32, M48, M56, M69, M82, M83, and M99) and two putative capsid (M85 and M86) antigens, as well as one nonstructural (e1) antigen: a plasmid pool designated Mat/Cap/e1. In addition, we tested whether this combination of individually nonprotective plasmids could augment the protective responses elicited by pp89-M84 coimmunization by including 2 μg each of the pp89 and M84 plasmids into Mat/Cap/e1 to yield the vaccine pp89/M84/Mat/Cap/e1. Finally, 2 μg of the gp34-expressing plasmid was added to the pp89/M84/Mat/Cap/e1 mixture to yield the “All” vaccine, a mixture of 13 plasmids expressing MCMV genes. As described above, DNA masses in all vaccines were normalized by the addition of empty pcDNA3 vector. These mice were immunized and challenged in the same experiment described above, and all subsequent splenic viral titers were measured in the same plaque assay as described above.
Interestingly, after challenge with the lowest viral dose of 80 × 103 PFU, immunization with the plasmid mixture consisting of the 10 individually nonprotective plasmids (Mat/Cap/e1) resulted in a 350-fold average reduction in viral titers in the spleen relative to the pcDNA3-immunized controls (Fig. 2B). This level of protection was similar to that observed in the pp89- and M84-coimmunized mice challenged with the same viral dose. Furthermore, a significant reduction in viral titer (20-fold) was observed in the Mat/Cap/e1-immunized mice after challenge with 120 × 103 PFU. By comparison, the reduction level in the pp89- and M84-coimmunized mice was ca. 200-fold at this viral dose. Finally, at the highest challenge dose, the viral titer reductions in the spleens of the Mat/Cap/e1 group were only threefold. Taken together, these results demonstrate that MCMV antigens that individually do not elicit protective responses can contribute to a strong protective response when combined into a multivalent vaccine. Compared to the “major” antigens of MCMV that are individually protective in our pDNA immunization assay (pp89, M84, and gp34), however, the combined response to the Mat/Cap/e1 group was more easily overwhelmed by increases in the viral challenge dose. Thus, these individually nonprotective antigens may represent minor antigens that can contribute to the breadth of CMI to the virus.
Most significantly, we found that the addition of the minor antigen plasmids to the pp89- and M84 plasmid mixture further reduced viral titers to levels 3-, 4-, and 12-fold below those afforded by pp89- and M84 coimmunization alone (Fig. 2B). The net reduction in titer compared to pcDNA3-immunized controls was ca. 3 logs across the challenge doses tested. Inclusion of the protective gp34 plasmid into the highly protective pp89/M84/Mat/Cap/e1 vaccine, however, did not further increase the level of protection (Fig. 2C, pp89/M84/Mat/Cap/e1 versus All).
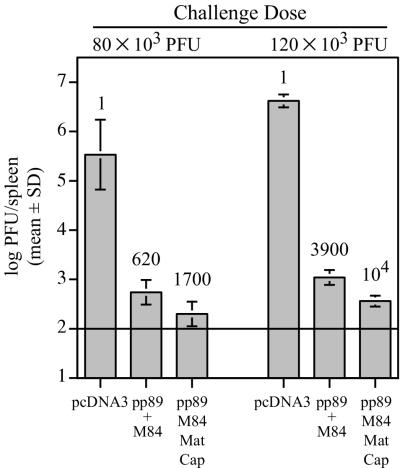
To test whether these modest increases in protection provided by the minor antigens were consistently elicited, we performed a confirmatory small-scale immunization experiment. Eight mice per group were i.d. immunized three times over 3 weeks with either (i) pcDNA3, (ii) pp89 plus M84, or (iii) a pool of pp89, M84, and the structural and putative structural antigens (M32, M48, M56, M69, M82, M83, M85, M86, and M99). The latter vaccine is designated pp89/M84/Mat/Cap. Three weeks after the last immunization, four mice per group were i.p. challenged with either 80 × 103 or 120 × 103 PFU of SG-MCMV (0.3 or 0.5 LD50, respectively), and on day 6 postchallenge, spleens were harvested to assess viral titers. We found that, after either challenge dose, inclusion of the Mat/Cap plasmids into the pp89 plus M84 vaccine provided ∼3-fold reductions in titers below those provided by pp89 plus M84 alone (Fig. 3). Significantly, after the 120 × 103 PFU challenge, MCMV titers in the spleen were reduced by greater than 4 logs relative to the vector-immunized controls. This level represents the strongest protection we have achieved against MCMV replication with our pDNA-based vaccines: a level near the assay detection limit of 102 PFU per spleen. These results suggest that the combined responses to minor antigens can play an additional role in protective immunity. Moreover, DNA immunization with both of these minor antigens and 2 of the major protective antigens of MCMV can generate antiviral immunity that is consistently greater than that afforded by the major antigens alone. However, compared to the protection elicited by the putative matrix, capsid, and e1 plasmids, the individually protective antigens pp89, M84, and gp34 provide the majority of the protective response. At this time, we cannot exclude the possibility that other MCMV genes, such as m164 or m18, could provide protection levels as high or higher than those observed after immunization with the genes shown here. Furthermore, we would predict that compared to the “All” pDNA-immunized mice, mice immunized by prior MCMV infection would possess even stronger protective immunity that would be effective against higher challenge doses. We therefore sought to further augment the protective responses of the pDNA-immunized mice by inclusion of a more complete set of antigens to which both protective cell-mediated and antibody responses could be elicited.
FIG. 3.
Protection against viral replication in BALB/c mice immunized with pp89+M84 plasmids alone or with a pool of plasmids expressing putative matrix and capsid antigens. BALB/c mice were i.d. immunized as described in the legend to Fig. 2 with 26 μg of DNA consisting of (i) pcDNA3 vector, (ii) pp89 and M84 plasmids (pp89+M84), or (iii) pp89 and M84 plasmids combined with the nine plasmids expressing putative matrix and capsid antigens (see Fig. 2). Three weeks after the last immunization, mice were i.p. challenged with either 80 × 103 or 120 × 103 PFU of the SG-MCMV stock described in the text, and on day 6 postchallenge, spleens were harvested and titers were determined as described above. The resulting spleen titers are shown as the mean of the log10 PFU per spleen for four mice with the standard deviation (SD) indicated by error bars. The values above each group indicate the fold reductions of the nonlogarithmic mean titers (i.e., PFU per spleen) of each group relative to the pcDNA3-immunized group for the corresponding challenge dose. The assay limit of sensitivity is indicated by the horizontal line (102 PFU per spleen).
A pDNA prime and FI-MCMV boost strategy confers complete protection against MCMV replication in the spleen and salivary glands.
The studies described above demonstrate that an optimized pDNA vaccine comprised of both dominant and minor antigens consistently reduces the titer of the challenge virus in the spleen to levels 104-fold below those seen in vector-immunized controls. While this robust CMI is important for such cell-disseminated viruses as the CMVs, the low-level viral replication observed in the spleens of vaccinated mice likely results in progeny virus that can seed the salivary glands and thus allow subsequent viral amplification and transmission (35). Since the protection elicited by the pDNA immunization is likely due to CD8+-T-lymphocyte responses, we sought to augment the overall protection with an antibody response that could prevent the dissemination of virus to the salivary glands. To this end, we investigated the protective efficacy of chemically killed MCMV, a multivalent immunogen that potentially provides all possible antigenic sites for neutralizing antibodies.
Tissue culture-derived MCMV was partially purified by ultracentrifugation through a sorbitol cushion and inactivated with formalin as described in Materials and Methods. Each preparation of FI-MCMV was confirmed by plaque assay to be free of infectious virus to a level of <5 PFU per ml, a reduction of more than 2 × 108- to 4 × 108-fold. Prior to the i.p. injection of mice, FI-MCMV was diluted to the desired concentration with sterile, endotoxin-free PBS, and in some cases, an equal volume of aluminum hydroxide solution (Pierce Imject Alum) was added according to the manufacturer's recommendations.
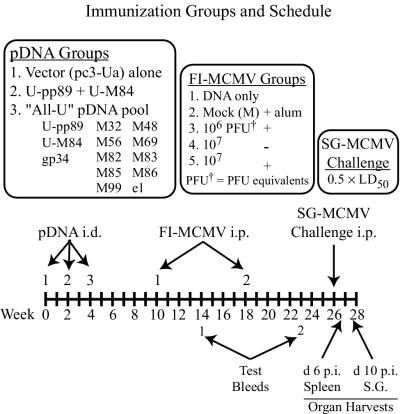
To assess the ability of this killed virus vaccine to induce a protective antibody response and augment the plasmid DNA-generated CTL response, mice were primed by i.d. pDNA immunization and i.p. boosted with FI-MCMV in saline or alum (see Fig. 4 for experimental groups and timeline). Mice first received one of the following pDNA pools: (i) pc3-Ua vector alone, (ii) U-pp89 plus U-M84 (amino-terminal ubiquitinated pp89 and M84 fusion proteins, respectively), or (iii) U-pp89, U-M84, gp34, and the matrix/capsid/e1 pool (together designated the “All-U” pool). The pp89 and M84 ubiquitin fusion proteins were used in this experiment since results from previous experiments with U-pp89 and U-M84 plasmids demonstrated that they may elicit greater protection than the wild-type antigens, especially when the mass of plasmid injected was suboptimal (reference 57 and data not shown). As in the experiment described above, each injection contained 26 μg of DNA consisting of 2 μg of each antigen-expressing plasmid and the appropriate normalization masses of empty vector. Mice were pDNA immunized three times within 4 weeks, and 6 weeks after the last DNA immunization, each pDNA group was i.p. injected with either 106 PFU eq of FI-MCMV in alum, 107 PFU eq of FI-MCMV in saline or alum, or a formalin-treated mock virus preparation (M) in alum or was left untreated. Mock- or FI-MCMV-injected mice were reinjected with the same antigen preparation 8 weeks later, and blood was collected 4 and 5 weeks after the first and second immunizations, respectively, for examination of neutralizing antibody responses.
FIG. 4.
Immunization groups and schedule for vaccination with pDNA and FI-MCMV. Mice were first i.d. immunized three times with 26 μg of one of the three pDNA groups shown. At 6 and 14 weeks after the last pDNA immunization, mice from each pDNA group were either left untreated (DNA only) or were injected with one of the four FI-MCMV or mock preparations shown (FI-MCMV groups 2 to 5). Test bleeds, viral challenge, and organ harvests were performed on the days shown (d, day; p.i., postinfection).
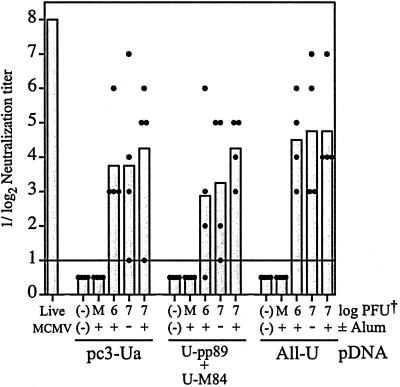
Complement-dependent neutralizing antibody titers were measured with the sera collected after the first and second FI-MCMV injections by standard plaque reduction assay with SG-MCMV. No detectable neutralization activity was detected in sera collected prior to the second FI-MCMV injection (data not shown). After the second injection, however, we found that mice immunized with FI-MCMV had average reciprocal log2 neutralization titers of 3 to 4.75 (Fig. 5). In comparison, a pooled serum from sera of four mice previously i.p. infected with 0.3 LD50 (225 × 103 PFU) of live SG-MCMV had a neutralization titer of 7 or 8 in several assays (Fig. 5 and data not shown). Neutralizing antibody titers of mice within groups were variable, and there were no significant differences in mean neutralizing titers generated after immunization with either of the two doses of FI-MCMV or the inclusion of alum. No neutralizing antibodies were detected in mice immunized with pDNA only or the mock-virion preparation (Fig. 5). In addition to the virus-neutralizing activity, we found by Western blot analysis that mice immunized with FI-MCMV raised antibody responses to gB, a target of neutralization antibodies, as well as to other virion-associated proteins (data not shown).
FIG. 5.
Complement-dependent neutralizing antibody titers in BALB/c mice immunized with pDNA and FI-MCMV. Sera collected on week 23 (test bleed 2) of the experiment (see Fig. 4) were decomplemented, and serial twofold dilutions were mixed with 50 PFU of SG-MCMV and rabbit complement before incubation and standard plaque assay on NIH 3T3 cells. Mice were pDNA and FI-MCMV immunized as shown in Fig. 4. A positive control serum pooled from BALB/c mice 6 months after i.p. injection with 225 × 103 PFU (0.3 LD50) of live SG-MCMV (Live MCMV) was simultaneously tested. Neutralizing titers shown are the greatest reciprocal log2 dilutions of serum that resulted in a ≥50% reduction in the number of PFU of input virus, with bars representing the mean of four mice per group and the closed circles representing the neutralization titers of individual mice. When the neutralization titer of a serum was below the detection limit (indicated by the horizontal line), the titer was arbitrarily set to 0.5 for display purposes and mean calculation. Abbreviations: (−)(−), pDNA-immunized mice only; M, mock FI-MCMV-immunized mice; PFU†, formalin-inactivated PFU equivalents.
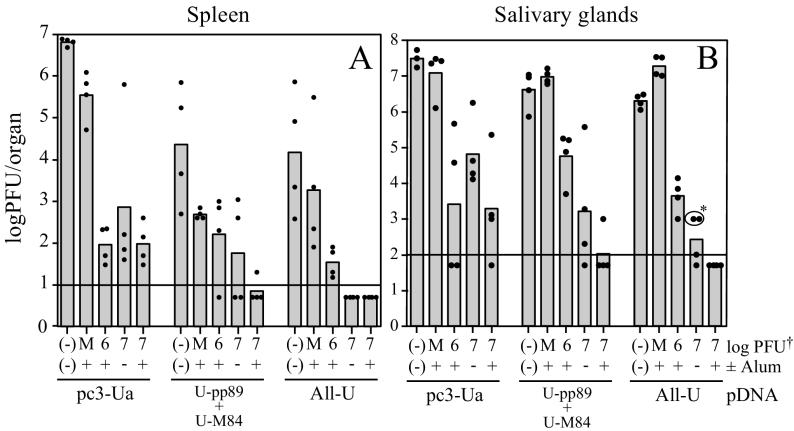
In order to assess the protection elicited by the various pDNA prime and FI-MCMV boost combinations, immunized mice were i.p. challenged with 400 × 103 PFU (0.5 LD50) of SG-MCMV 8 weeks after the second FI-MCMV immunization (22 weeks after the last pDNA immunization). The challenge dose with this viral stock corresponds to 120 × 103 PFU of the more highly virulent stock used for challenge in Fig. 2 and 3 (see Materials and Methods). Spleens and salivary glands were harvested on day 6 and 10 postchallenge, respectively, for MCMV titer determination. When viral titers in the spleen were measured, we found that mice primed with the backbone pc3-Ua vector and boosted with any of the three FI-MCMV-containing preparations had similar protection against viral replication (Fig. 6A). In all cases, MCMV titers in the spleen were reduced to levels of ca. 102 PFU/spleen. Boosting of mice by i.p. injection of the formalin-treated mock virion preparation (M+) resulted in a 1.2-log titer reduction in the spleens compared with the pc3-Ua only immunized controls (Fig. 6A). This reduction may have resulted from nonspecific immunity provided by residual inflammation in the peritoneum caused by previous i.p. injection with alum.
FIG. 6.
MCMV titers in BALB/c mice immunized with pDNA and FI-MCMV. The days of pDNA and FI-MCMV immunization, as well as challenge and organ harvests, are as indicated in Fig. 4. Titers of organ homogenates were determined on NIH 3T3 cells, and the resulting titers are shown as the log10 PFU per organ, with bars representing the means for four mice per group and the closed circles representing individual organ titers. When the viral titer of an organ was below the detection limit of the corresponding plaque assay (indicated by the horizontal line), the titer was arbitrarily set to one-half the detection limit (in PFU per organ) for display purposes and mean calculation. (A) MCMV titers in the spleen on day 6 postchallenge. (B) MCMV titers in the salivary glands on day 10 postchallenge. Note that the pc3-Ua, (−)(−) immunization group contained three mice for salivary gland titer determination. ✽, Circled individual salivary gland titers were arbitrarily set to 103 PFU per organ due to the high toxicity of these homogenates precluding the measure of infectious virus at levels below this titer. Because these homogenates yielded no plaques, these titers and the group mean represent overestimates.
Of particular interest was our finding that coimmunization with U-pp89 plus U-M84 or the All-U plasmid vaccines augmented the protection afforded by FI-MCMV. Most significantly, all of the mice primed with All-U plasmid pool and boosted with 107 PFU eq of FI-MCMV—with or without alum—had no detectable MCMV in their spleens (<10 PFU/spleen). As described in Materials and Methods, the detection limit of 10 PFU/spleen for this assay was based upon a modified plaque assay in which 1/10 of each spleen homogenate was diluted and absorbed to NIH 3T3 cells in a T-75 flask instead of one well of a 24-well dish. This additional dilution of the toxic spleen homogenates was sufficient to ensure that the cell monolayer remained viable and that plaque formation was not inhibited. This was confirmed for each homogenate by adding a small amount of infectious MCMV to a control flask infected with another 1/10 of the same homogenate. Notably, infection with centrifugal enhancement did not increase the number of plaques formed when <10 PFU of virus was exogenously added to the homogenates. In contrast to the undetectable levels of MCMV in the spleens of mice immunized with All-U and FI-MCMV, the mice immunized with only U-pp89 plus U-M84 pDNA or the All-U pDNA pool showed variable 1- to 4-log titer reductions compared to the pc3-Ua immunized controls. This variability, compared to that observed in Fig. 2 and 3, suggests that the plasmid-mediated immune responses had waned somewhat in the 22 weeks that passed between the last pDNA immunization and viral challenge. Note that while the 400 × 103 PFU challenge dose may seem discordant with the doses used in the experiments above (Fig. 2 and 3), 400 × 103 PFU from the viral stock used in for Fig. 6 corresponds to 120 × 103 PFU of the more highly virulent virus stock used in Fig. 2 and 3.
Of greatest importance, we found that i.p. immunization with FI-MCMV provided protection against MCMV replication in the salivary glands (Fig. 6B), a key organ involved in viral transmission. We found with all three pDNA vaccines that further immunization with 107 PFU eq of FI-MCMV with alum generally resulted in protection greater than that elicited by 106 PFU eq with alum or by 107 PFU eq without alum. Importantly, four of four mice immunized with the All-U plasmid pool and with 107 PFU eq of FI-MCMV plus alum had undetectable levels in the salivary glands (<100 PFU/salivary gland). In addition, the titers of 103 PFU per salivary gland shown in the two All-U primed, 107 PFU eq-alum-boosted mice (Fig. 6B, circled with asterisk) represent overestimates of the actual titers, since no plaques developed in any of the wells of this assay, but the toxicity of the homogenates precluded the detection of any virus below the level of 103 PFU per organ. Taken together, a pDNA prime-FI-MCMV boost vaccination strategy provides protection greater than that afforded by either immunization arm alone and can reduce viral titers to undetectable levels in both major target organs examined. Experiments are in progress to determine both the vaccine's ability to limit replication in the liver and lungs, organs relevant to CMV disease in humans, and to compare the protective efficacy with that elicited by prior MCMV infection.
Immunization with the All-U pDNA pool elicits CD8+-T-cell responses against pp89, M83, and virion-associated antigens, whereas FI-MCMV immunization elicits CD8+ T cells specific for M83 and other virion-associated antigens
The above results demonstrate that immunization with FI-MCMV elicits a neutralizing antibody response that may help contribute to the observed protection against subsequent challenge. However, the protection was incomplete unless mice were first primed by i.d. immunization with the pool of 13 MCMV antigen-expressing plasmids. Because the pDNA pool did not itself elicit or contribute to the neutralizing antibody response (Fig. 5), we sought to determine whether CD8+-T-lymphocyte-mediated responses were generated against key antigens in the pDNA and FI-MCMV immunizations.
In order to measure the MCMV-specific CD8+-T-cell responses, a new immunization experiment was initiated in which BALB/c mice were either mock immunized or immunized with the optimal FI-MCMV dose described above but with a condensed immunization schedule. Mice were first i.d. immunized three times within 3 weeks with pc3-Ua or the All-U pDNA pool. At 3 and 8 weeks after the last pDNA injection, mice were i.p. injected with either 107 PFU eq of FI-MCMV plus alum or an equivalent volume of formalin-treated mock virion plus alum. Mice were then sacrificed for ICCS assay-based enumeration of the CD8+ splenocytes specific for key MCMV antigens either expressed by the pDNA pool or contained within the FI-MCMV preparation. In order to measure the response against an antigen delivered only by pDNA immunization, we used the dominant Ld-restricted nonapeptide epitope of pp89 168YPHFMPTNL176 (8). We also examined the CD8+-T-cell responses to an antigen expressed by the All-U pDNA pool, as well as contained in the FI-MCMV, by using the defined Ld-restricted epitope of the M83 gene product (761YPSKEPFNF769) (19). Although M83 is the only virion-associated antigen in which CD8+-T-cell responses have been documented thus far, we were nevertheless interested in measuring the total CD8+-T-cell responses generated against all virion-associated antigens. To this end, we modified the ICCS assay such that the splenocytes from immunized mice were stimulated with permissive J774 macrophage cells (16) that had been previously infected at a high MOI with UV-MCMV and thus present the virion-associated MCMV antigens in the absence of viral gene expression.
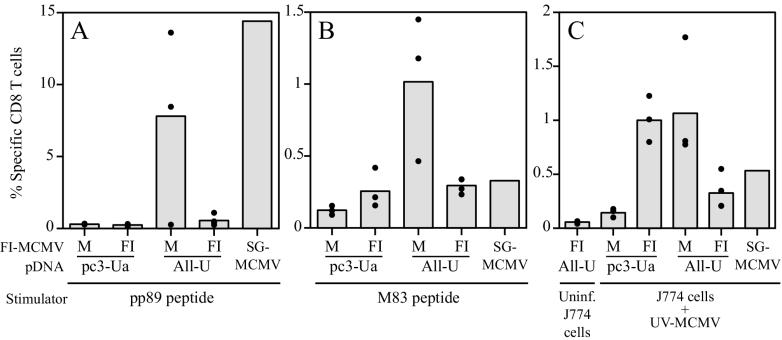
Because MCMV-specific CD8+ T cells were not detectable in an initial ICCS experiment with splenocytes from mice 14 weeks after the second FI-MCMV immunization (data not shown), we measured recall responses after in vivo restimulation with live virus as previously described (57). MCMV-specific recall CD8+-T-cell responses were measured after i.p. challenge of three or four mice per group with l20 × 103 PFU of SG-MCMV (0.15 LD50) 16 weeks after the last FI-MCMV immunization. In addition, an MCMV-immune mouse that had been i.p. infected with MCMV 9 months previously was also i.p. rechallenged as described above to show the recall CD8+-T-cell levels after MCMV infection and reexposure. The levels of pp89 peptide-specific CD8+ T cells in the spleens of immunized mice 5 days after viral challenge (or rechallenge) are shown in Fig. 7A. The mean background staining level in pp89 peptide-stimulated splenocytes from pDNA vector alone and mock-virion-immunized mice after challenge was 0.30%. As expected, mice immunized with empty pDNA vector and FI-MCMV had background levels of pp89-specific CD8+ T cells, since pp89 is a nonstructural antigen. In contrast, when challenged with virus, the mean pp89-specific CD8+-T-cell levels for three mice immunized with the All-U pDNA and mock virion was 7.8%, with a range of 0.3 to 13.6%. Thus, in two of the three mice tested, pp89-specific CD8+-T-cell immunity was generated by the immunizations with 2 μg of U-pp89 pDNA in the All-U pool that included 12 other MCMV antigen-expressing plasmids. In addition, the levels of pp89-specific CD8+ T cells in the two responding mice compared favorably to the 14.4% specific CD8+-T-cell level in the rechallenged MCMV-immune mouse (Fig. 7A, SG-MCMV). While these frequencies of 7.8 and 14.4% pp89-specific CD8+ T cells may appear high in relation to CMI to MCMV, it should be noted that these frequencies were obtained after in vivo restimulation with live virus. In addition, similar, if not higher, frequencies have been demonstrated in several independent experiments (57). Interestingly, after viral challenge, the pp89-specific CD8+-T-cell levels in the four mice immunized with All-U plus FI-MCMV were only slightly higher than the background level, ranging from 0.25% to 1.1% (mean = 0.55%).
FIG. 7.
ICCS assay-based quantification of the MCMV-specific CD8+ T splenocytes of mice immunized with pDNA and FI-MCMV. In a separate pDNA and FI-MCMV immunization experiment, BALB/c mice were i.d. immunized three times within 3 weeks with either empty plasmid vector (pc3-Ua) or the All-U pDNA pool (All-U). At 3 and 8 weeks after the last pDNA immunization, mice were i.p. injected with either mock formalin-inactivated virion in alum (M) or 107 PFU eq of SG-MCMV in alum (FI). At 12 weeks after the last i.p. immunization, mice were i.p. challenged with 120 × 103 PFU (0.15 LD50) of live SG-MCMV. At the same time, a control mouse that had been i.p. injected with live SG-MCMV 9 months previously was challenged (SG-MCMV). Five days after challenge (or rechallenge), splenocytes were harvested and the levels of MCMV-specific CD8+ T lymphocytes were measured by ICCS assay as described in Materials and Methods. The specific levels of CD8+ T cells are shown as the percentages of CD8+ T splenocytes that were IFN-γ positive in response to in vitro stimulation with the peptides or cells specified below. Bars represent the means of three mice per group, and closed circles represent values for individual mice. (A) Stimulation of splenocytes with the pp89 peptide 168YPHFMPTNL176; (B) stimulation of splenocytes with the M83 peptide 761YPSKEPFNF769; (C) stimulation of splenocytes with either uninfected J774A.1 macrophages (Uninf. J774 cells) or J774A.1 macrophages infected with UV-MCMV at an equivalent MOI of 10 for 5 h (J774 cells + UV-MCMV).
Splenocytes from the same mice were also tested for levels of M83 peptide epitope-specific CD8+ T cells. Mice immunized with FI-MCMV (and empty pDNA vector) and challenged with virus had low levels of M83-specific CD8+ T cells, with a mean level of 0.26% (range, 0.16 to 0.42%). This level was approximately twice the background level of 0.12% (range, 0.09 to 0.15%) observed with splenocytes from the pc3-Ua and mock-virion-alum-immunized mice. More significantly, mice immunized with the All-U pDNA pool had a mean M83-specific CD8+-T-cell level of 1.0% (range, 0.46 to 1.4%), a level ∼8-fold higher than the background level and 3-fold higher than that observed in the MCMV-immune mouse (Fig. 7B). Taken together, these data suggest that the M83-expressing pDNA elicits a stronger M83-specific CD8+-T-cell response than the M83 contained within the FI-MCMV preparation. Of note, there appeared to be competition between the pp89 and M83 peptides for Ld, since splenocytes from the All-U, M-immunized mice reacted strongly to one of the two epitopes (Fig. 7A and B). Sequential immunization with the All-U pDNA pool, followed by FI-MCMV, resulted in a mean level of M83-specific CD8+ T cells of 0.29% (range, 0.23 to 0.34%), a level similar to that elicited by FI-MCMV immunization alone, as well as by prior MCMV infection.
Finally, the CD8+-T-cell responses to the virion-associated antigens were measured in the pDNA- and FI-MCMV-immunized mice after challenge. In pilot ICCS experiments with splenocytes from SG-MCMV-infected mice, we found that the optimal stimulator cell-to-splenocyte ratio was 1:6 and that the levels of IFN-γ-positive splenocytes were maximal after stimulation for 8 h (data not shown). Upon ICCS assay of the pDNA- and FI-MCMV-immunized mice described above, an average background level of 0.06% of the CD8+ T cells in the splenocytes of All-U- and FI-MCMV-immunized mice was IFN-γ positive after stimulation with uninfected J774 cells (Fig. 7C). In contrast to the results obtained with the pp89 or M83 peptides, significant levels of UV-MCMV-specific CD8+ T cells were elicited in mice immunized with FI-MCMV alone and then challenged with virus (Fig. 7C). Specifically, an average of 1.0% of CD8+ T cells (range, 0.8 to 1.2%) from the FI-MCMV-immunized mice synthesized IFN-γ in response to the UV-MCMV-infected stimulator cells. This level was ∼7-fold higher than the 0.14% average level in the pDNA vector- and mock virion-immunized mice stimulated with the same cells. UV-MCMV-specific CD8+-T-cell levels elicited after immunization with FI-MCMV alone were similar to those immunized with the All-U pDNA pool alone (mean, 1.1%; range, 0.78 to 1.8%). Taken together, these data demonstrate that immunization with either the All-U pDNA pool or FI-MCMV elicits a CD8+-T-cell response to virion-associated proteins that can be measured after viral challenge. As was the case when pp89- or M83-specific responses were evaluated, in mice primed with the All-U pDNA and boosted with FI-MCMV the recall percentage of UV-MCMV-specific CD8+ T cells was ∼3-fold lower than that obtained with mice immunized with only All-U DNA or FI-MCMV (Fig. 7C). Nevertheless, the resulting levels of CD8+ T cells in All-U plus FI-MCMV-immunized mice were comparable to that seen in the MCMV-immune mouse (0.55%).
DISCUSSION
The goal of this study was to improve a pDNA-based vaccine against CMV by increasing the overall breadth of the humoral and cell-mediated responses elicited by the vaccine. We previously demonstrated that coimmunization of BALB/c mice with pp89- and M84-expressing plasmids resulted in a synergistic level of protection against viral replication in the spleen after challenge, whereas immunization with single plasmids expressing putative matrix and capsid genes was not protective (35). However, although viral titers in the spleens of the pp89 and M84-coimmunized mice were reduced by ∼3 logs relative to controls, viral replication in the salivary glands was not substantially affected. Thus, even low levels of viral replication in the spleens of the pp89 and M84-coimmunized mice resulted in the dissemination of progeny virus to the salivary glands, an immunologically privileged site where the virus replicates even in the presence of strong CD8+-T-cell-mediated immunity (23, 32, 38). We therefore sought to develop a vaccine that (i) further reduced the levels of viral replication in the spleen by providing increased CD8+-T-cell-mediated immunity and (ii) augmented the increased CD8+-T-cell responses with a humoral response that provided immunity against both input virus and low levels of progeny virus and thus prevented viral dissemination to the salivary glands. In the present study, we describe an optimized vaccine strategy that consists of (i) i.d. priming with a pool of pDNAs encoding pp89, M84, and gp34, as well as 10 individually nonprotective plasmids expressing putative matrix, capsid, and nonstructural antigens; and (ii) i.p. boosting with chemically killed virus adsorbed to an alum adjuvant. We found that only when the vaccine contained all of these components was protection sufficient to suppress viral replication in the spleen and salivary glands to undetectable levels.
These results both confirm and extend our previous observation that a pDNA vaccine consisting of M83-, M85-, M86-, and M99-expressing plasmids provides protection against low challenge doses of MCMV (35). We demonstrated here that by increasing the number of individually nonprotective plasmids in the vaccine to 10, a more significant level of protection can be achieved. However, the protective immunity elicited by these plasmids is not as strong as that afforded by the individually protective antigens of MCMV and therefore may represent minor antigens. We also found that inclusion of this limited set of antigens into a pool with the individually protective pp89, M84, and gp34 plasmids resulted in consistently increased protection levels in the spleen. It is possible that inclusion of the nonprotective plasmids may provide helper epitopes that augment the responses to one or more of the individually protective plasmids. Alternatively, the additional protection provided by the minor antigen plasmids may be due to the increase in the breadth of the CD8+-T-cell repertoire for MCMV antigens that may be presented by different cell types or at different stages of the infection. In this regard, Holtappels et al. previously reported that the CD8+ T lymphocytes that infiltrated the lungs of MCMV-infected mice possessed lytic activity against immediate-early, early, and late MCMV antigens and that the peak activities against each of these classes of antigens changed throughout the course of acute infection (18). Furthermore, the pp89-specific CTLs represented a minor fraction of the total CD3ɛ-redirected CD8+-T-cell-mediated cytolytic response. It was later found that CTL lines specific for the defined epitopes of pp89, gp34, M83, and M84 are protective in the spleen and lungs of immunodeficient mice (19, 20).
Immunization with the pDNA pool All-U was found to elicit strong CD8+-T-cell responses against pp89 and M83 Ld peptides, as well as against the virion-associated antigens presented by J774 cells infected with UV-MCMV. However, the detection of CD8+ T cells specific for these antigens was possible only after viral challenge. The inability of our ICCS assay to detect virus-specific CD8+ T cells in the absence of in vivo restimulation with MCMV was likely due at least in part to the prolonged period of time between pDNA immunization and the ICCS assay. Another potential explanation is that the pDNA immunizations were performed with pp89 plus 12 other antigen-expressing plasmids and not just the pp89 plasmid alone. Relevant to this is our finding that coimmunization with pp89 and M84 plasmids results in lower levels of pp89-specific CD8+ T cells compared to immunization with pp89 pDNA alone, although the recall responses were comparable after viral challenge (57). It is therefore conceivable that the addition of the 12 other MCMV antigen-expressing plasmids with the pp89 pDNA further reduced the peak levels of pp89-specific CD8+ T cells. Nevertheless, these results show that a low level of CD8+ T cells prior to challenge may not necessarily predict the strength of the recall response to the challenge virus.
Our studies also showed that immunization with the All-U pDNA pool elicits a CD8+-T-cell response specific for M83 that can be detected after viral challenge. We previously reported that immunization with an M83-expressing recombinant vaccinia virus or pDNA was not protective against subsequent sublethal challenge (35). At that time, it was unknown whether M83 contained any MHC class I-restricted epitopes. Subsequently, Holtappels et al. demonstrated that MCMV infection elicits a CD8+-T-cell response against an Ld-restricted nonapeptide of M83 (19). Interestingly, a CTL line specific for this epitope was protective against viral replication when adoptively transferred to gamma-irradiated recipient mice. Furthermore, the protective ability of this CTL line was greater—on the basis of transferred cell number—than a CTL line specific for M84, an antigen found to be protective in our pDNA immunization assay (19). Although these results may appear to be contradictory, our pDNA vaccine model of protection differs substantially from the adoptive transfer model in two ways. In the latter model, the recipient mice were first ablated of all CMI, allowing for subsequent reconstitution with stimulated CD8+ T cells that not only were specific for the M83 epitope but also possessed lytic activity at the time of transfer. This specific reconstitution may allow for greater protective effects in the absence of competition with CD8+ T cells with T-cell receptors specific for minor or unprotective antigens. Second, the recipient mice were challenged subcutaneously in the footpad, an infection route that provides a more slowly disseminated infection compared to i.p. infection. The subcutaneous infection may provide a longer period for the in vivo proliferation of the transferred M83-specific CD8+ T lymphocytes (29). In the DNA vaccine model, not only must the plasmid-expressed antigen contain an appropriate epitope for MHC class I binding and T-cell-receptor recognition but, after challenge, the epitope must also be presented in the context of MHC class I complexes to the vaccine-primed CD8+ T cells. The resulting stimulation must be sufficient to induce protective lytic activity even in the presence of other presented viral antigens and CD8+ T lymphocytes with different specificities. Because the M83 plasmid was injected with other plasmids, including the three individually protective plasmids pp89, M84, and gp34, it remains to be determined whether the immunization with M83 pDNA or vaccinia virus alone fails to provide protection because they do not prime sufficient numbers of CD8+ T cells or whether primed CD8+ T cells are not sufficiently restimulated in vivo to provide protective lytic activity.
One interesting result of these studies was that the recall CD8+-T-cell responses to pp89, M83, and virion-associated antigens in mice immunized with the All-U pDNA pool alone were higher than those in mice that were sequentially immunized with the All-U pDNA pool and FI-MCMV. This reduction at first may appear to be the result of the FI-MCMV immunization interfering with or abrogating the responses to the All-U pDNA. However, this seems unlikely since the mice immunized with pc3-Ua, FI+ had levels of virion-specific CD8+ T cells similar to those of mice immunized by All-U, M+. In addition, mice immunized with both pDNA and FI-MCMV were the only ones to have undetectable levels of virus in their spleens and salivary glands after challenge. Instead, these results suggest that protective immunity was not compromised but augmented. We hypothesize that the apparent decrease in the CD8+-T-cell levels for the mice immunized with pDNA and FI-MCMV may be due to the assay conditions in which recall responses were measured after viral challenge. In order for the memory cells to proliferate, they must be subjected to a threshold level of viral antigen in association with the MHC complexes (29). Because the FI-MCMV immunization elicited neutralizing antibody responses, the restimulating virus may have been neutralized and thus prevented from disseminating to the spleen where subsequent infection and viral antigen presentation would have allowed the proliferation of MCMV-specific memory CD8+ T cells. In addition, in the All-U DNA- and FI-MCMV-immunized mice, it is likely that challenge virus that was not neutralized by the FI-MCMV-induced antibodies was suppressed by the All-U pDNA-induced CD8+-T-cell-mediated immunity at the site of challenge and in the draining lymph nodes. Thus, the local CD8+-T-cell responses may have helped to prevent dissemination of the challenge virus to the spleen, as evidenced by the lack of detectable viral replication in the spleens of the All-U- and FI-MCMV-immunized mice. This hypothesis might also explain why the mice immunized with the FI-MCMV alone had higher recall responses to the virion structural proteins than did mice immunized with the All-U pDNA plus FI-MCMV. In this case, although the FI-MCMV-immunized mice also had high neutralizing antibody titers, some residual infectious virus likely disseminated to and replicated in the spleen, thus resulting in the proliferation of virion-specific CD8+ T cells to higher levels. Future experiments examining the levels of specific CD8+-T-cell responses after the pDNA prime and FI-MCMV boost in the absence of restimulation by challenge virus should help resolve the question.
Two other studies have looked at the efficacies of inactivated virus vaccines for MCMV. Tolpin et al. showed that two i.p. immunizations of CD-1 Swiss mice with 5 × 107 PFU eq of formalin-inactivated SG-MCMV (without adjuvant) provided protection against lethal challenge (80% specific protection against 2 LD50) but not against viral replication and dissemination (53). More recently, Geoffroy et al. showed that i.p. immunization with three doses of 107 PFU eq of sodium periodate-inactivated TC-MCMV completely protected BALB/c mice from death and viral replication when challenged 3 weeks after the last immunization (12). However, when the mice were challenged 3 months after the last immunization, mice were protected from death but not viral replication. In the work presented here, we also found that FI-MCMV immunization alone was unable to completely protect mice against viral infection and dissemination when mice were challenged 2 months after the last immunization. Only when mice were immunized with both pDNA and FI-MCMV was the replication of challenge virus suppressed to levels below detection limits. Taken together, these results show that a protective antibody response to MCMV is an effective means of limiting viral replication and dissemination when used in conjunction with pDNA-induced CD8+-T-cell responses, although the relative contributions that the antibody and CD8+-T-cell responses made in the overall observed protection in the All-U, FI-MCMV-immunized mice remain to be specifically addressed. Further long-term studies are warranted to determine the maintenance of these immune responses and their protective abilities over time.
The use of different prime and boost methods for vaccination is emerging as an effective means for generating high levels of immunity that are stronger than priming and boosting by the same method. Various combinations of different vaccination methods that have been reported thus far include priming or boosting immunizations with pDNA, purified protein subunit, killed whole virus, attenuated or replication-defective recombinant vaccinia virus, adenovirus vectors, and canarypox vectors. Two published reports have specifically examined the efficacy of pDNA prime-killed virus boost vaccinations. In a mouse model of rabies virus vaccination, priming with a pDNA expressing the gG envelope glycoprotein, followed by boosting with human diploid cell killed virus vaccine (HDCV), resulted in ∼10-fold-higher peak and sustained levels of virus-neutralizing antibody titers compared to pDNA prime-pDNA boost (31). In that study, the most effective prime-boost strategy was priming with a gG-expressing recombinant vaccinia virus and boosting with HDCV, since peak levels of neutralizing antibody responses were ∼50-fold higher than those from pDNA prime and boost. In another study it was shown that a pDNA prime-killed influenza virus boost vaccine was more effective in limiting nasal shedding of virus in pigs after intranasal challenge than was a pDNA and boost strategy (30). However, on each of 4 days examined after challenge, the levels of secreted virus in the pDNA prime-killed virus boosted pigs were not significantly different from those receiving killed virus for both the prime and the boost vaccinations. Several other studies have demonstrated that pDNA prime and infectious viral vector boost methods can provide immunity against such pathogens as HIV (14, 47), hepatitis C virus (36), herpes simplex virus (10), influenza virus (7), Plasmodium spp. (48, 49), and Ebola virus (52), and in many cases this prime-boost method resulted in protection superior to the other prime-boost methods tested. Although boosting with live, attenuated, or replication-defective viral vectors has been shown to elicit strong immunity to the delivered antigens, concerns remain regarding the safety of these vectors in immunocompromised individuals, as well as with regard to their relative efficacies in the presence of vector-specific immunity elicited from a previous immunization.
One major benefit of the pDNA prime-killed virus boost strategy used here is that at no time are infectious vectors used, and immune responses to both nonstructural and structural viral antigens can be elicited without developing vector-specific responses. It is our hope that future studies will further demonstrate the effectiveness of this pDNA prime-killed virus boost vaccination against MCMV in providing immunity that is (i) protective against a long-term challenge, (ii) protective against mucosal challenge, (iii) protective in immunologically diverse mouse strains, and (iv) protective against the establishment of or the reactivation from latency. The information gained from these studies should provide the basis for the development of a safe and effective vaccine against HCMV.
Acknowledgments
We thank Ann Hill for providing the Smith strain gp34 plasmid and Matthias J. Reddehase for communicating to us the location of the CD8+-T-cell epitope of gp34 prior to publication. We also thank Veronica Sanchez for critical reading of the manuscript.
This work was supported by research grant number 6-FY99-442 from the March of Dimes Birth Defects Foundation and by NIH training grant T32 AI07036.
REFERENCES
- 1.Badovinac, V. P., and J. T. Harty. 2000. Intracellular staining for TNF AND IFN detects different frequencies of antigen-specific CD8+ T cells. J. Immunol. Methods 238:101-117. [DOI] [PubMed] [Google Scholar]
- 2.Boyle, J. S., J. L. Brady, C. Koniaras, and A. M. Lew. 1998. Inhibitory effect of lipopolysaccharide on immune response after DNA immunization is route dependent. DNA Cell Biol. 17:343-348. [DOI] [PubMed] [Google Scholar]
- 3.Britt, W., and C. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and R. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 4.Brune, W., H. Hengel, and U. H. Koszinowski. 1999. A mouse model for cytomegalovirus infection, p. 19.17.11-19.17.13. In J. E. Coligan, A. M. Kruisbeek, D. H. Margulies, E. M. Shevach, and W. Strober (ed.), Current protocols in immunology, vol. 4. John Wiley & Sons, Inc., New York, N.Y. [DOI] [PubMed] [Google Scholar]
- 5.Cranmer, L., C. Clark, and D. H. Spector. 1994. Cloning, characterization and expression of the murine cytomegalovirus homologue of the human cytomegalovirus 28-kDa matrix phosphoprotein (UL99). Virology 205:417-429. [DOI] [PubMed] [Google Scholar]
- 6.Cranmer, L. D., C. L. Clark, C. S. Morello, H. E. Farrell, W. D. Rawlinson, and D. H. Spector. 1996. Identification, analysis, and evolutionary relationship of the putative murine cytomegalovirus homologues of the human cytomegalovirus UL82 (pp71) and UL83 (pp65) matrix phosphoproteins. J. Virol. 70:7929-7939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dégano, P., J. Schneider, C. M. Hannan, S. C. Gilbert, and A. V. Hill. 1999. Gene gun intradermal DNA immunization followed by boosting with modified vaccinia virus Ankara: enhanced CD8+ T-cell immunogenicity and protective efficacy in the influenza and malaria models. Vaccine 18:623-632. [DOI] [PubMed] [Google Scholar]
- 8.Del Val, M., H. J. Schlicht, H. Volkmer, M. Messerle, M. J. Reddehase, and U. H. Koszinowski. 1991. Protection against lethal cytomegalovirus infection by a recombinant vaccine containing a single nonameric T-cell epitope. J. Virol. 65:3641-3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ebeling, A., G. M. Keil, E. Knust, and U. H. Koszinowski. 1983. Molecular cloning and physical mapping of murine cytomegalovirus DNA. J. Virol. 47:421-433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Eo, S. K., M. Gierynska, A. A. Kamar, and B. T. Rouse. 2001. Prime-boost immunization with DNA vaccine: mucosal route of administration changes the rules. J. Immunol. 166:5473-5479. [DOI] [PubMed] [Google Scholar]
- 11.Farrell, H. E., and G. R. Shellam. 1991. Protection against murine cytomegalovirus infection by passive transfer of neutralizing and non-neutralizing monoclonal antibodies. J. Gen. Virol. 72:149-156. [DOI] [PubMed] [Google Scholar]
- 12.Geoffroy, F., N. Moachon, J. Rodwell, and G. A. Quash. 1996. Murine cytomegalovirus inactivated by sodium periodate is innocuous and immunogenic in mice and protects them against death and infection. Vaccine 14:1686-1694. [DOI] [PubMed] [Google Scholar]
- 13.Gonzalez Armas, J. C., C. S. Morello, L. D. Cranmer, and D. H. Spector. 1996. DNA immunization confers protection against murine cytomegalovirus infection. J. Virol. 70:7921-7928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hanke, T., T. J. Blanchard, J. Schneider, C. M. Hannan, M. Becker, S. C. Gilbert, A. V. Hill, G. L. Smith, and A. McMichael. 1998. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine 16:439-445. [DOI] [PubMed] [Google Scholar]
- 15.Hassett, D. E., J. Zhang, M. Slifka, and J. L. Whitton. 2000. Immune responses following neonatal DNA vaccination are long-lived, abundant, and qualitatively similar to those induced by conventional immunization. J. Virol. 74:2620-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hengel, H., U. Reusch, G. Geginat, R. Holtappels, T. Ruppert, E. Hellebrand, and U. H. Koszinowski. 2000. Macrophages escape inhibition of major histocompatibility complex class I-dependent antigen presentation by cytomegalovirus. J. Virol. 74:7861-7868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtappels, R., N. K. Grzimek, D. Thomas, and M. J. Reddehase. 2002. Early gene m18, a novel player in the immune response to murine cytomegalovirus. J. Gen. Virol. 83:311-316. [DOI] [PubMed] [Google Scholar]
- 18.Holtappels, R., J. Podlech, G. Geginat, H. P. Steffens, D. Thomas, and M. J. Reddehase. 1998. Control of murine cytomegalovirus in the lungs: relative but not absolute immunodominance of the immediate-early 1 nonapeptide during the antiviral cytolytic T-lymphocyte response in pulmonary infiltrates. J. Virol. 72:7201-7212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Holtappels, R., J. Podlech, N. K. Grzimek, D. Thomas, M. F. Pahl-Seibert, and M. J. Reddehase. 2001. Experimental preemptive immunotherapy of murine cytomegalovirus disease with CD8 T-cell lines specific for ppM83 and pM84, the two homologs of human cytomegalovirus tegument protein ppUL83 (pp65). J. Virol. 75:6584-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtappels, R., D. Thomas, J. Podlech, G. Geginat, H. P. Steffens, and M. J. Reddehase. 2000. The putative natural killer decoy early gene m04 (gp34) of murine cytomegalovirus encodes an antigenic peptide recognized by protective antiviral CD8 T cells. J. Virol. 74:1871-1884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holtappels, R., D. Thomas, J. Podlech, and M. J. Reddehase. 2002. Two antigenic peptides from genes m123 and m164 of murine cytomegalovirus quantitatively dominate CD8 T-cell memory in the H-2d haplotype. J. Virol. 76:151-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holtappels, R., D. Thomas, and M. J. Reddehase. 2000. Identification of a Kd-restricted antigenic peptide encoded by murine cytomegalovirus early gene M84. J. Gen. Virol. 81(Pt. 12):3037-3042. [DOI] [PubMed] [Google Scholar]
- 23.Jonjić, S., I. Pavić, P. Lučin, D. Rukavina, and U. H. Koszinowski. 1990. Efficacious control of cytomegalovirus infection after long-term depletion of CD8+ T lymphocytes. J. Virol. 64:5457-5464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jonjic, S., I. Pavic, B. Polic, I. Crnkovic, P. Lucin, and U. H. Koszinowski. 1994. Antibodies are not essential for the resolution of primary cytomegalovirus infection but limit dissemination of recurrent virus. J. Exp. Med. 179:1713-1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Katzenstein, D. A., G. S. Yu, and M. C. Jordan. 1983. Lethal infection with murine cytomegalovirus after early viral replication in the spleen. J. Infect. Dis. 148:406-411. [DOI] [PubMed] [Google Scholar]
- 26.Kern, F. I., I. P. Surel, N. Faulhaber, C. Frommel, J. Schneider-Mergener, C. Schonemann, P. Reinke, and H.-D. Vold. 1999. Target structures of the CD8+-T-cell response to human cytomegalovirus: the 72-kilodalton major immediate-early protein revisited. J. Virol. 73:8179-8184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleijnen, M. F., J. B. Hupp, P. Lucin, S. Mukherjee, H. Farrell, A. E. Campbell, U. H. Koszinowski, A. B. Hill, and H. L. Ploegh. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER is not retained but is transported to the cell surface. EMBO J. 16:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koszinowski, U. H., G. M. Keil, H. Schwarz, J. Schickedanz, and M. J. Reddehase. 1987. A nonstructural polypeptide encoded by immediate-early transcription unit 1 of murine cytomegalovirus is recognized by cytolytic T lymphocytes. J. Exp. Med. 166:289-294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lanzavecchia, A., and F. Sallusto. 2000. Dynamics of T lymphocyte responses: intermediates, effectors, and memory cells. Science 290:92-97. [DOI] [PubMed] [Google Scholar]
- 30.Larsen, D. L., A. Karasin, and C. W. Olsen. 2001. Immunization of pigs against influenza virus infection by DNA vaccine priming followed by killed-virus vaccine boosting. Vaccine 19:2842-2853. [DOI] [PubMed] [Google Scholar]
- 31.Lodmell, D. L., and L. C. Ewalt. 2000. Rabies vaccination: comparison of neutralizing antibody responses after priming and boosting with different combinations of DNA, inactivated virus, or recombinant vaccinia virus vaccines. Vaccine 18:2394-2398. [DOI] [PubMed] [Google Scholar]
- 32.Lucin, P., I. Pavic, B. Polic, S. Jonjic, and U. H. Koszinowski. 1992. Gamma interferon-dependent clearance of cytomegalovirus infection in salivary glands. J. Virol. 66:1977-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mercer, J. A., J. R. Marks, and D. H. Spector. 1983. Molecular cloning and restriction endonuclease mapping of the murine cytomegalovirus genome (Smith strain). Virology 129:94-106. [DOI] [PubMed] [Google Scholar]
- 34.Morello, C. S., L. D. Cranmer, and D. H. Spector. 1999. In vivo replication, latency, and immunogenicity of murine cytomegalovirus mutants with deletions in the M83 and M84 genes, the putative homologs of human cytomegalovirus pp65 (UL83). J. Virol. 73:7678-7693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morello, C. S., L. D. Cranmer, and D. H. Spector. 2000. Suppression of murine cytomegalovirus replication with a DNA vaccine encoding the MCMV early nonstructural protein M84 (a homolog of human cytomegalovirus pp65). J. Virol. 74:3696-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pancholi, P., Q. Liu, N. Tricoche, P. Zhang, M. E. Perkus, and A. M. Prince. 2000. DNA prime-canarypox boost with polycistronic hepatitis C virus (HCV) genes generates potent immune responses to HCV structural and nonstructural proteins. J. Infect. Dis. 182:18-27. [DOI] [PubMed] [Google Scholar]
- 37.Polic, B., H. Hengel, A. Krmpotic, J. Trgovcich, I. Pavic, P. Luccaronin, S. Jonjic, and U. H. Koszinowski. 1998. Hierarchical and redundant lymphocyte subset control precludes cytomegalovirus replication during latent infection. J. Exp. Med. 188:1047-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Polic, B., S. Jonjic, I. Pavic, I. Crnkovic, I. Zorica, H. Hengel, P. Lucin, and U. Koszinowski. 1996. Lack of MHC class I complex expression has no effect on spread and control of cytomegalovirus infection in vivo. J. Gen. Virol. 77:217-225. [DOI] [PubMed] [Google Scholar]
- 39.Quinnan, G. V., J. F. Manischewitz, and F. A. Ennis. 1978. Cytotoxic T lymphocyte resonse to murine cytomegalovirus infection. Nature 273:541-543. [DOI] [PubMed] [Google Scholar]
- 40.Rapp, M., M. Messerle, P. Lucin, and U. H. Koszinowski. 1993. In vivo protection studies with MCMV glycoproteins gB and gH expressed by vaccinia virus, p. 327-332. In P. S. A. Michelsons (ed.), A multidisciplinary approach to understanding cytomegalovirus disease. Excerpta Medica, Amsterdam, The Netherlands.
- 41.Rawlinson, W. D., H. E. Farrell, and F. G. Barrell. 1996. Analysis of the complete DNA sequence of murine cytomegalovirus. J. Virol. 70:8833-8849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reddehase, M. J., S. Jonjic, F. Weiland, W. Mutter, and U. H. Koszinowski. 1988. Adoptive immunotherapy of murine cytomegalovirus adrenalitis in the immunocompromised host: CD4-helper-independent antiviral function of CD8-positive memory T lymphocytes derived from latently infected donors. J. Virol. 62:1061-1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Reddehase, M. J., G. M. Keil, and U. H. Koszinowski. 1984. The cytolytic T lymphocyte response to the murine cytomegalovirus. II. Detection of virus replication stage-specific antigens by separate populations of in vivo active cytolytic T lymphocyte precursors. Eur. J. Immunol. 14:56-61. [DOI] [PubMed] [Google Scholar]
- 45.Reddehase, M. J., and U. H. Koszinowski. 1984. Significance of herpesvirus immediate early gene expression in cellular immunity to cytomegalovirus infection. Nature 312:369-371. [DOI] [PubMed] [Google Scholar]
- 46.Reddehase, M. J., W. Mutter, K. Munch, H. J. Buhring, and U. H. Koszinowski. 1987. CD8-positive T lymphocytes specific for murine cytomegalovirus immediate-early antigens mediate protective immunity. J. Virol. 61:3102-3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 48.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. S. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vacination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 49.Sedegah, M., T. R. Jones, M. Kaur, R. Hedstrom, P. Hobart, J. A. Tine, and S. L. Hoffman. 1998. Boosting with recombinant vaccinia increases immunogenicity and protective efficacy of malaria DNA vaccine. Proc. Natl. Acad. Sci. USA 95:7648-7653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shanley, J. D., M. C. Jordan, and J. G. Stevens. 1981. Modification by adoptive humoral immunization of murine cytomegalovirus infection. J. Infect. Dis. 143:231-237. [DOI] [PubMed] [Google Scholar]
- 51.Slifka, M. K., F. Rodriguez, and J. L. Whitton. 1999. 1999. Rapid on/off cycling of cytokine production by virus-specific CD8+ T cells. Nature 401:76-79. [DOI] [PubMed] [Google Scholar]
- 52.Sullivan, N. J., A. Sanchez, P. E. Rollin, Z. Y. Yang, and G. J. Nabel. 2000. Development of a preventive vaccine for Ebola virus infection in primates. Nature 408:605-609. [DOI] [PubMed] [Google Scholar]
- 53.Tolpin, M. D., S. E. Starr, A. M. Arbeter, and S. A. Plotkin. 1980. Inactivated mouse cytomegalovirus vaccine: preparation, immunogenicity, and protective effect. J. Infect. Dis. 142:569-574. [DOI] [PubMed] [Google Scholar]
- 54.Volkmer, H., C. Bertholet, S. Jonjic, R. Wittek, and U. H. Koszinowski. 1987. Cytolytic T lymphocyte recognition of the murine cytomegalovirus nonstructural immediate-early protein pp89 expressed by recombinant vaccinia virus. J. Exp. Med. 166:668-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wills, M. R., A. J. Carmichael, K. Mynard, X. Jin, M. P. Weekes, B. Plachter, and J. G. Patrick Sissons. 1996. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J. Virol. 70:7569-7579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Xu, J., P. A. Lyons, M. D. Carter, W. M. Booth, N. J. Davis-Poynter, G. R. Shellam, and A. A. Scalzo. 1996. Assessment of antigenicity and genetic variation of glycoprotein B of murine cytomegalovirus. J. Gen. Virol. 77:49-59. [DOI] [PubMed] [Google Scholar]
- 57.Ye, M., C. S. Morello, and D. H. Spector. 2002. Strong CD8 T-cell responses following coimmunization with plasmids expressing the dominant pp89 and subdominant M84 antigens of murine cytomegalovirus correlate with long- term protection against subsequent viral challenge. J. Virol. 76:2100-2112. [DOI] [PMC free article] [PubMed] [Google Scholar]