Abstract
There is currently intensive research on the design of novel human immunodeficiency virus type 1 (HIV-1) vaccine immunogens that can elicit potent neutralizing antibodies. A prerequisite for comparing and optimizing these strategies is the ability to precisely measure neutralizing antibody responses. To this end, we sought to develop an assay that directly quantifies single-round HIV-1 infection of peripheral blood mononuclear cells (PBMC). Initial experiments demonstrated that essentially all productively infected PBMC could be identified by flow cytometric detection of intracellular p24 antigen (p24-Ag). After infection of PBMC with HIV-1, p24+ lymphocytes could be distinguished beginning 1 day postinfection, and the majority of CD8− T cells were p24-Ag positive by 3 to 4 days postinfection. To directly quantify first-round infection, we included a protease inhibitor in PBMC cultures. The resulting 2-day assay was highly sensitive and specific for the detection of HIV-1-infected PBMC. Serial dilutions of virus stocks demonstrated that the number of target cells infected was directly related to the amount of infectious virus input into the assay. In neutralization assays, the flow cytometric enumeration of first-round infection of PBMC provided quantitative data on the number of target cells infected and on the inactivation of infectious virus due to reaction with antibody. We also used this single-round assay to compare the percentage of cells expressing p24-Ag to the number of copies of HIV-1 gag per 100 PBMC. The precision and reproducibility of this assay will facilitate the measurement of HIV-1 neutralization, particularly incrementally improved neutralizing antibody responses generated by new candidate vaccines.
Virus neutralization assays are designed to measure a reduction in virus infectious titer mediated by exposure to antibody. Initial human immunodeficiency virus type 1 (HIV-1) neutralization assays used prototypic viral isolates (e.g., HIV-IIIB) grown in continuous T-cell lines (e.g., H9 and CEM-SS), with infection monitored by counting virus-induced syncytia (30, 48, 57, 59, 76, 77). Since T-cell syncytia corresponded to infection of a single cell, the fraction of virus neutralized was directly related to a reduction in the number of syncytia (12, 47). These assays were shown to be quantitative and reproducible but were limited to syncytium-forming viruses that used the CXCR4 coreceptor present on T-cell lines (6). This led to the use of primary mononuclear cells as target cells, because all HIV-1 isolates could be propagated in activated peripheral blood mononuclear cells (PBMC) (1, 41, 46, 56, 60). In addition, studies have shown that the in vivo protection mediated by passive transfer of anti-HIV-1 neutralizing antibodies to severe combined immunodeficiency mice reconstituted with human PBMC (24) or to rhesus macaques (5, 38, 43, 53, 64) could be predicted by the potency of neutralization measured by in vitro assays using PBMC target cells.
Since HIV-1 isolates do not consistently form syncytia in primary T cells, it is difficult to directly enumerate the number of PBMC infected in culture (11, 21, 61, 71). Most PBMC neutralization assays monitor virus growth by assaying for extracellular expression of viral proteins such as p24 antigen (p24-Ag) or reverse transcriptase (1, 3, 41, 46, 75). These assays require several rounds of virus replication before expressed proteins can be quantified and thus only indirectly measure the actual number of target cells infected. We have commonly used enzyme-linked immunosorbent assay (ELISA) measurement of p24-Ag expressed into culture supernatants as an endpoint in PBMC neutralization assays (40-42). Although this assay does not measure single-round virus replication, we closely monitor virus growth kinetics to measure secreted p24-Ag expression during the early viral growth phase (36). If this is done for each virus prior to performing the neutralization assay, a single day (varying from day 3 to day 7) can be found for which the amount of soluble p24-Ag varies proportionally with virus input. Although this allows comparisons among HIV-1 strains with varied growth kinetics, the preneutralization steps are labor intensive and time-consuming. In addition, the reproducibility of the Ag-capture neutralization assays is limited by factors such as the numerous cell washes required to remove viral antigens and serum anti-p24 antibody, varied viral growth in culture, death of cells and release of p24-Ag, and manipulations required to harvest and measure p24-Ag (7, 8, 16, 21, 37, 62, 69).
The accurate measurement of antibody-mediated neutralization of primary HIV-1 isolates is important for studies of the mechanism of virus neutralization and for the assessment of immune responses to candidate vaccines. The variability inherent in neutralization assays that quantify infection indirectly by measurement of secreted p24-Ag led us to develop an assay that directly enumerates the first-round infection of individual lymphocytes. Numerous prior studies have demonstrated the flow cytometric detection of infected lymphocytes in culture and in HIV-1-infected patients by intracellular staining with an anti-p24 antibody (14, 15, 31, 32, 44, 67, 73). Folghera et al. used flow cytometric detection of intracellular p24 to measure neutralization of HIV-IIIB in H9 target cells (22). More recently, Darden and colleagues reported the use of a flow cytometric primary isolate HIV-1 neutralization assay that enumerated the number of HIV-1-infected PBMC after 4 to 7 days in culture (16).
By including a protease inhibitor in the culture with target PBMC, we developed a flow cytometric neutralization assay that measures single-round infection of individual PBMC. The enumeration of first-round infection of PBMC provided quantitative data on the number of infectious virus particles, as measured by the number of target cells infected. Thus, this in vitro PBMC neutralization assay can directly quantify the inactivation of infectious virus mediated by exposure to antibody.
MATERIALS AND METHODS
Antibodies.
Anti-HIV-1 human monoclonal antibody (MAb) IgG1b12 was provided by Dennis Burton (Scripps, La Jolla, Calif.), and IgG1 human MAbs 2F5 and 2G12 were provided by Hermann Katinger (Polymun Scientific, Vienna, Va.). MAb b12 binds to the CD4 binding region of gp120, 2G12 recognizes a carbohydrate-dependent, conformationally sensitive region of gp120 outside the CD4 binding site (72), and 2F5 binds to a linear epitope in the extracellular region of gp41 (55). HIVIG (manufactured as HIV-IG by NABI, Boca Raton, Fla.) is a preparation of purified immunoglobulin G (IgG) derived from the pooled plasma of multiple HIV-1-seropositive donors as previously described (34).
Cells and virus stocks.
Leukocytes were obtained by leukapheresis of HIV-seronegative donors, and PBMC were isolated by Ficoll-Hypaque gradient centrifugation. Prior to HIV-1 infection, PBMC were activated by incubation in intereukin-2 (IL-2) cell culture medium containing 10 μg of phytohemagglutinin (PHA) (PHA-P; Difco Laboratories, Detroit, Mich.) per ml. IL-2 culture medium was RPMI 1640 medium containing 100 U of penicillin, 100 μg of streptomycin, 2 mM l-glutamine, 10% heat-inactivated fetal calf serum, and 20 U of recombinant IL-2 (Roche Molecular Biochemicals, Indianapolis, Ind.) per ml. After overnight incubation with PHA, cells were washed and continued in culture with IL-2 for 3 to 5 days (36). All cell cultures were maintained in 5% CO2 incubators at 37°C.
HIV-1 isolates were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases, NIH (IIIB, ADA, JRCSF, BaL, and SF162), and were expanded by two or three cycles of growth on PHA- and IL-2-stimulated PBMC. To produce the final virus stock, PBMC were exposed to undiluted virus for 2 h at a cell concentration of 107/ml, and IL-2 culture medium was added to bring the cell concentration to 106/ml. IL-2 culture medium was exchanged every 2 days, and supernatants were collected during the peak of p24-Ag expression, usually 5 to 10 days after infection. Virus stocks were made cell free by centrifugation at 1,000 × g and filtration though a 0.45-μm filter.
In some cases, viral stocks were concentrated by as much as 10-fold through a 100-kDa cutoff polyethersulfone filter (Centricon Plus Biomax filter; Millipore, Bedford, Mass.), according to the manufacturer's instructions. Virus aliquots were stored in the vapor phase of liquid nitrogen. HIV-1 strains JRCSF, ADA, BaL, and SF162 are macrophage-tropic isolates that use the CCR5 coreceptor. HIV-1 IIIB is a T-cell line-adapted isolate that uses the CXCR4 coreceptor. Virus 50% tissue culture infectious doses (TCID50) were determined by a sensitive 14-day endpoint titration assay using PHA- and IL-2-stimulated PBMC as previously described (39).
The recombinant green fluorescent protein (GFP) reporter virus was generated by cotransfection of 293T cells with the pNL4-3env− plasmid (full-length NL4-3 HIV-1 proviral DNA with a frameshift in env and encoding GFP in place of nef) and the pSVIIIenv plasmid, encoding the HXB2 Env protein. The env plasmid and pNL4-3env− were constructed as previously described (27). Supernatant containing reporter virus was collected 48 h after transfection, clarified by centrifugation and 0.45-μm filtration, and stored at −80°C.
Viral infection and flow cytometric analysis for intracellular expression of p24-Ag.
HIV-1 infection of PHA- and IL-2-stimulated PBMC was performed in 96-well round-bottomed culture plates by combining 40 μl of virus stock with 20 μl of PBMC (1.5 × 105 cells). The multiplicity of infection (MOI) is indicated for individual experiments. Polycationic substances such as DEAE-dextran and Polybrene were not used in any of the experiments. After overnight incubation at 37°C, 150 μl of IL-2 culture medium was added, and the cells were continued in culture. PBMC were harvested for intracellular p24-Ag staining on specific days as described for individual experiments. For experiments performed with indinavir, PBMC were resuspended in IL-2 culture medium containing indinavir prior to being distributed into culture plates. The final concentration of indinavir was 1 μM, and this concentration was maintained throughout. Zidovudine (AZT) was included in some experiments as specifically described. AZT and indinavir were obtained from the NIH AIDS Research and Reference Reagent Program.
For intracellular p24-Ag staining, cells were transferred to V-bottomed plates and washed once in phosphate-buffered saline (PBS) containing 1% fetal calf serum (FCS). Cells were fixed and permeabilized using the Cytofix/Cytoperm Kit (BD-PharMingen, San Diego, Calif.). Permeabilized cells were washed twice in V-bottomed plates using the wash buffer provided by the manufacturer and resuspended for 20 min at 4°C with 50 μl of a 1:160 dilution of a phycoerythrin (PE)-conjugated mouse anti-p24 MAb (KC57-RD1; Beckman Coulter, Inc.), or a mouse IgG1 isotypic control antibody. After two additional washes, HIV-1- or mock-infected PBMC were analyzed with a FACSCalibur flow cytometer (Becton Dickinson), and data analysis was performed with FlowJo software (Tree Star, Inc., San Carlos, Calif.). Live cells initially gated by forward and side scatter were analyzed for intracellular expression of p24-Ag. The number of p24-Ag-positive cells was determined using a bivariate plot of fluorescence versus forward scatter; the gate was set on mock-infected cells. For surface staining, the MAbs used were PE- or fluorescein isothiocyanate (FITC)-conjugated anti-CD4 and anti-CD8 and isotype-matched controls (all from BD-PharMingen). Flow cytometric data in the figures are shown as contour plots with outliers. As indicated in some experiments, dead cells were identified by lack of exclusion of ethidium monoazide bromide (EMA) prior to permeabilization (45).
Quantitative PCR for proviral DNA.
HIV DNA was quantified by quantitative PCR with an ABI7700 (Perkin-Elmer, Norwalk, Conn.). HIV gag primers and probe were designed against the Los Alamos HIV database. Conserved primers and probe sequences were trimmed to optimal sequences that matched >98% of all sequences in the database at 100% identity. The gag primer positions and sequences were 795gagF, GGTGCGAGAGCGTCAGTATTAAG; 911gagR, AGCTCCCTGCTTGCCCATA; and probe 841gagP, FAM-AAAATTCGGTTAAGGCCAGGGGGAAAGAA-QSY7 (MegaBases, Chicago, Ill.).
To quantify cell number in each reaction, quantitative PCR was performed simultaneously for albumin gene copy number. Albumin primer and probe sequences were AlbF, TGCATGAGAAAACGCCAGTAA; AlbR, ATGGTCGCCTGTTCACCAA; and AlbP, FAM-TGACAGAGTCACCAAATGCTGCACAGAA-QSY7. PHA- and IL-2-stimulated PBMC, exposed to serial dilutions of virus in the presence of indinavir, were harvested for quantitative PCR on day 2. PBMC were lysed in proteinase K (100 μg/ml) (Boehringer, Indianapolis, Ind.). Quantitative PCR was performed on 5 μl of cell lysate for 45 cycles using Platinum Taq (Invitrogen) using the conditions described previously (20). Plasmid standards were constructed for absolute quantification of gag and albumin copy number and were validated with sequential dilutions of 8E5/LAV cells containing a single integrated copy of proviral DNA directing synthesis of defective viral particles. 8E5/LAV cells were obtained from Thomas Folks through the AIDS Research and Reference Reagent Program (23). Duplicate reactions were run, and template copies were calculated by the ABI7700 software.
Neutralization assays.
The intracellular p24-Ag neutralization assays were performed in 96-well culture plates by incubating 40 μl of virus stock with 10 μl of antibody. Antibody concentration was defined at this step. Approximately 20,000 TCID50 (determined by a 14-day titration assay as described above) of HIV-1 was added to each well. After incubation for 30 min at 37°C, 20 μl of PBMC (1.5 × 105 cells) was added to each well. PBMC were maintained in IL-2 culture medium containing 1 μM indinavir, and the cells were fed on day 1 with 150 μl of IL-2 culture medium containing indinavir. Since there were 150,000 PBMC per well, the resulting MOI was approximately 0.1.
PBMC were harvested for intracellular p24-Ag staining on day 2. The cells were not washed prior to harvest but were washed prior to staining for p24-Ag, as described above. For each antibody dilution, two wells were set up and were combined to produce enough cells for precise quantitation by flow cytometry. To enumerate infected PBMC, cells were washed, fixed and permeabilized, and stained with the KC57 anti-p24 antibody as described above. After forward and side scatter gating, at least 50,000 events were counted. Final quantitation of p24-Ag-positive cells was done by subtraction of background events in mock-infected PBMC (usually less than 10 positives per 50,000 events). The percent neutralization was defined as reduction in the number of p24-Ag-positive cells compared with the number in control wells with no antibody. Antibody dose-response curves were fit with a nonlinear function, and the inhibitory concentration that neutralized 50, 80, and 90% (IC50, IC80, and IC90, respectively) of virus was calculated by a least-squares regression analysis. Some experiments were performed with PBMC depleted of CD8+ cells. CD8+ T cells were depleted by magnetic separation with anti-CD8-conjugated magnetic beads (Miltenyi Biotec, Auburn, Calif.).
The Ag-capture neutralization assay was performed in a 96-well plate format, similar to the intracellular neutralization assay above. In this assay, ∼1,000 TCID50 of HIV-1 was added to each well, resulting in an MOI of ∼0.01 (36, 40). As previously described, PBMC were washed extensively after overnight incubation with antibody and virus. These washes are necessary to removes virus inoculum, which contains p24-Ag, and to remove residual antibody which can contain anti-p24 antibody that would interfere with the Ag-capture ELISA (37). This assays allows several rounds of virus replication, and therefore virus growth kinetics (measured as extracellular p24-Ag production) was monitored for each virus by serial collection of culture supernatants from days 3 to 7. p24-Ag was measured with a commercial ELISA (Beckman Coulter, Inc.). Neutralization was measured during the early phase of virus growth, defined as the first day that the extracellular p24-Ag level exceeded 10 ng/ml.
RESULTS
HIV-1 infection of PBMC measured by intracellular staining for p24-Ag.
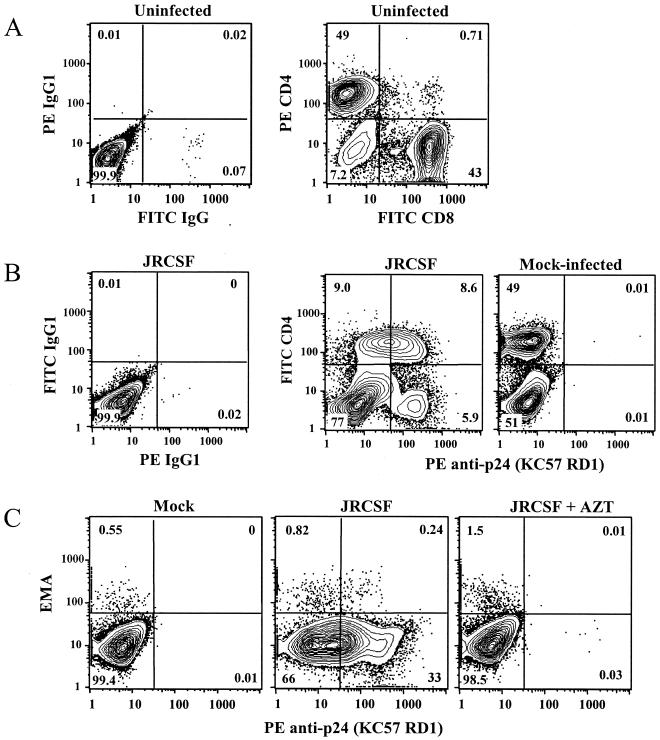
Donor PBMC were exposed to HIV-1 3 to 5 days after PHA and IL-2 stimulation, at which time about 50% of activated cells are CD4+ and likely to be available targets of infection (Fig. 1A). These data were similar for several other donor PBMC; between 40 and 60% of PHA- and IL-2-stimulated PBMC were CD4+ T cells. After exposure to HIV-1, infected PBMC were readily detected by flow cytometric analysis of cells stained for intracellular expression of p24-Ag. However, discrimination of infected cells was hindered by the intermediate expression of p24-Ag, presumably by cells in the early stages of infection (Fig. 1B, second panel). Of note, p24-Ag-positive cells were more clearly distinguished from HIV-negative cells after they had downregulated surface CD4, probably an indication that they are in a later stage of infection (26, 50).
FIG. 1.
Phenotype of uninfected and HIV-1-infected PBMC target cells. (A) Uninfected PBMC 4 days after PHA and IL-2 stimulation. Approximately 50% of cells are CD4+ T cells that should be susceptible to HIV-1 infection. (B) Stimulated PBMC were exposed to JRCSF (MOI of 0.01) or to mock virus (culture medium) and harvested for intracellular p24-Ag staining on day 3. Clear discrimination of p24-Ag-positive cells is difficult due to intermediate expression of p24-Ag by some cells (second panel, upper quadrants). Less than 0.01% of mock-infected cells stain positive for p24-Ag (third panel). (C) Mock- and JRCSF-exposed PBMC were stained for intracellular p24-Ag on day 3. Exposure of cells to EMA indicated that 99% of p24-Ag-positive cells were viable. In panel 3, PBMC were exposed to JRCSF in the presence of a reverse transcriptase inhibitor (10 μM AZT), which prevented expression of Gag protein. Values in these and in subsequent contour plots are the percentages of displayed cells falling within the indicated quadrant or gate.
As shown in Fig. 1C, essentially all p24-Ag-expressing cells excluded the EMA dye (i.e., the cells were viable). Also, the KC57 anti-p24 antibody did not stain PBMC that had been exposed to HIV-1 in the presence of a reverse transcriptase inhibitor, indicating that intracellular synthesis of Gag protein was required for detection of infection. Similarly, cells fixed in formaldehyde but not permeabilized did not stain for p24-Ag (data not shown). These results show that the p24-Ag staining we observed was not due to surface-bound virus.
Single-round infection of PBMC by utilizing reverse transcription or protease inhibitors.
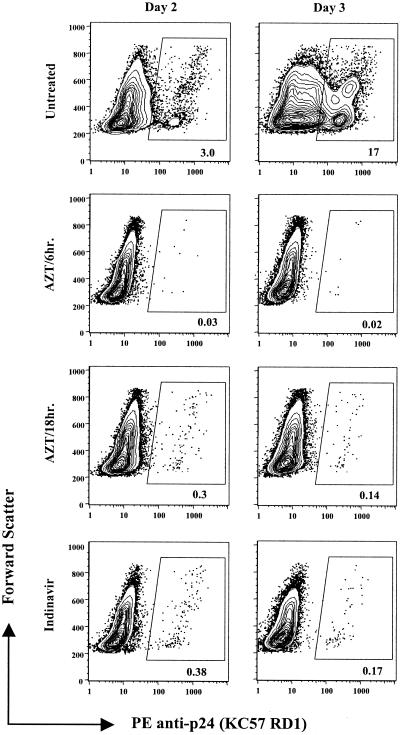
Ag-capture neutralization assays are generally performed by measuring extracellular p24-Ag 3 to 7 days after infection of PBMC. As shown in Fig. 2 (top panels), p24-Ag-positive cells were detected by flow cytometry on day 2 after HIV-1 infection; by day 4, more than 50% of the PBMC in culture and essentially all CD4+ T cells were HIV infected (day 4 data not shown). Additional staining for CD8+ T cells indicated that the large majority of CD8− cells were expressing p24-Ag by 4 days postinfection. Others have observed a similar high percentage of infected PBMC after HIV-1 infection (50, 78).
FIG. 2.
Single-round infection of PBMC in the presence of a reverse transcriptase or protease inhibitor. Stimulated PBMC were exposed to JRCSF for 2 h at an MOI of 0.01 and washed to remove virus. Infection was measured on days 2 and 3 by intracellular staining for p24-Ag. AZT (10 μM) was added to PBMC either 6 h, 12 h (not shown), or 18 h after initial exposure to JRCSF. Indinavir (1 μM) was added to PBMC (bottom panel) prior to exposure to virus as described in Materials and Methods. As expected, an MOI of 0.01 yields few first-round infected cells. Note that the timing of addition of AZT after virus exposure affects the number of positive events, whereas indinavir can be added prior to virus infection of PBMC.
In order to measure single-round infection of PBMC, we tested a reverse transcriptase inhibitor (AZT) and a protease inhibitor (indinavir). In titration experiments, we determined that a concentration of 10 μM AZT or 1 μM indinavir was sufficient to inhibit viral replication without causing cellular toxicity (data not shown). Treatment of PBMC with AZT or indinavir allowed clear discrimination of HIV-1-infected cells compared to untreated PBMC. In the experiment depicted in Fig. 2, 0.38% of indinavir-treated PBMC exposed to JFCSF (MOI of 0.01) were p24-Ag positive on day 2, and there was no increase in infection by day 3 (bottom two panels). Similar data were observed for BaL and IIIB (data not shown).
Of note, we found that the optimal timing of addition of AZT varied among viruses. For JRCSF, addition of AZT to the PBMC culture 18 h after exposure to virus appeared optimal (i.e., similar percent infection as with indinavir), but addition at 6 h was too early (Fig. 2, second panel from top). For HIV-IIIB, addition of AZT 6 h after exposure to virus was optimal, while 18 h was clearly too late to prevent secondary rounds of replication (not shown). Because indinavir does not have virus-specific parameters and can be included in culture with PBMC prior to viral infection, we chose to use it for further experiments.
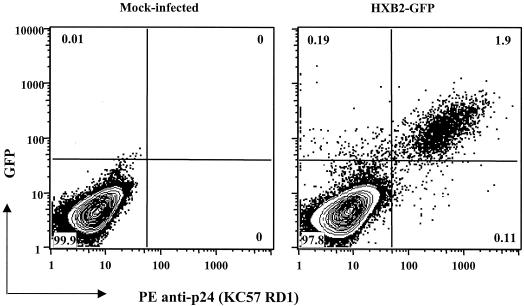
In order to evaluate if the KC57 antibody was detecting the majority of HIV-1-infected cells, we infected PBMC with a single-round GFP reporter virus and monitored cells for expression of GFP and p24-Ag. The contour plots shown in Fig. 3 show that greater than 90% of GFP+ cells also stained for intracellular p24-Ag. This experiment was repeated with indinavir (1 μM) in culture to test if the KC57 antibody would similarly detect unprocessed Gag protein resulting from inhibition of viral protease. Using the same IIIB reporter virus, we observed no difference in p24-Ag detection of PBMC treated with or without indinavir. This is consistent with the manufacturer's literature for the KC57 antibody, which states that it binds to p55 and p24 proteins by Western blotting. Additional studies were performed using 8E5/LAV cells, a biologic subclone of A3.01 cells that express p24-Ag. After staining with KC57, p24-Ag was detected in 80 to 90% of 8E5/LAV cells.
FIG. 3.
PHA- and IL-2-stimulated PBMC were mock infected or infected with a single-round env-pseudotyped HIV-HXB2 GFP reporter virus and evaluated for expression of GFP and p24-Ag on day 2. Approximately 91% of the GFP+ cells were also p24-Ag positive. Similar data were observed in several independent experiments (not shown).
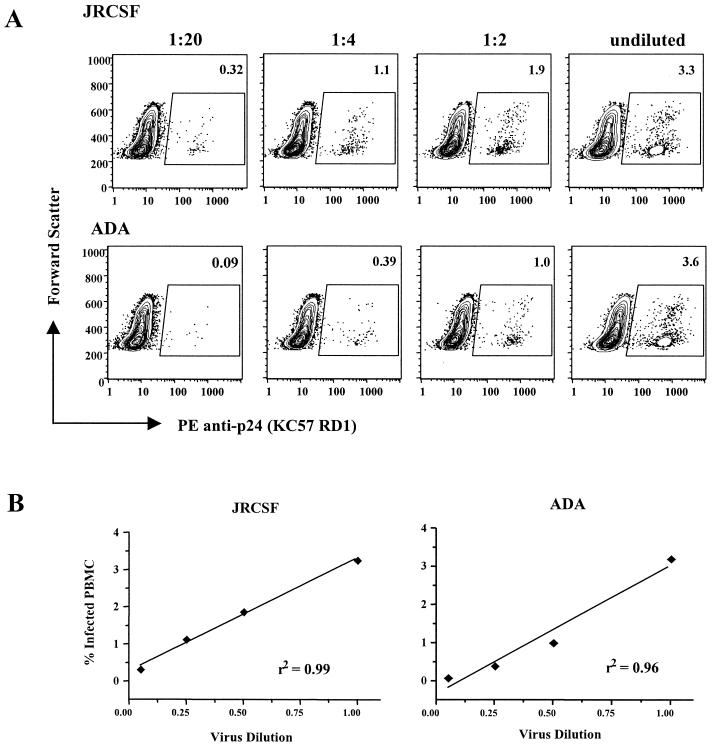
Linear dose response between virus input and number of p24-Ag-positive cells.
The accurate measurement of antibody-mediated virus neutralization is best measured in an assay with a linear relationship between the amount of infectious virus and the number of target cells infected. In order to test this, we exposed indinavir-treated PBMC to serial dilutions of virus. In several independent experiments with JRCSF and ADA, we observed a consistent linear dose response. A representative experiment (Fig. 4A) and corresponding linear regression curves (Fig. 4B) are shown. We have now tested more than 10 primary HIV-1 strains and observed similar findings (not shown). In some cases, there was a plateau of infection at the highest viral inputs (usually at an MOI of >0.5), followed by a linear response as the virus was diluted. Based on these data, we found that an MOI of ∼0.1 generally results in infection of 1 to 4% of PBMC and produces a linear relationship between virus input and the number of target cells infected. This was confirmed for each virus prior to use in neutralization assays.
FIG. 4.
Linear dose response between virus input and number of p24-Ag-positive cells. (A) PHA- and IL-2-stimulated PBMC were infected with decreasing dilutions of JRCSF or ADA, starting with undiluted virus stock (MOI of ∼0.2). (B) Regression analysis of virus dilution data demonstrates the linear relationship.
Reproducibility of neutralization measured by the intracellular p24-Ag assay.
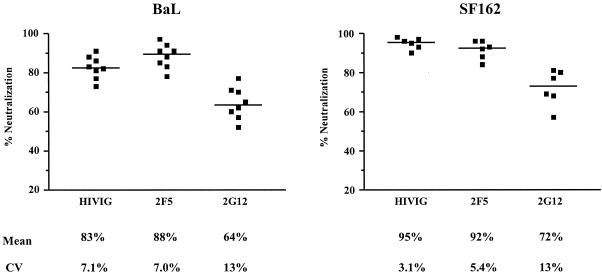
MAbs 2F5 and 2G12 and HIVIG were used to measure the interassay reproducibility of neutralization of two viral isolates (BaL and SF162). A single dose of each antibody was used in repetitive experiments. The results from eight independent experiments with BaL and six independent experiments with SF162 are shown in Fig. 5. When the potency of the antibody produced 80% or more virus neutralization (i.e, HIVIG and 2F5 against each virus), the coefficient of variation (CV) among experiments was less than 10%. When the less potent MAb 2G12 was used, neutralization in the range of 60 to 70% could still be reproducibly measured, though the CV was somewhat higher (range, 10 to 15%). Thus, the intracellular p24-Ag assay can precisely measure even modest levels of neutralization.
FIG. 5.
Reproducibility of the intracellular p24-Ag neutralization assay. A single concentration of MAbs 2F5 and 2G12 (50 μg/ml each) and HIVIG (1,000 μg/ml) was used in replicate experiments with viruses BaL and SF162. For each antibody, each symbol represents an independent experiment. The arithmetic mean and CV are shown below the graphs.
Comparison of intracellular p24-Ag neutralization assay and extracellular Ag-capture assay.
Since most of our published data on HIV-1 neutralization made use of the extracellular p24-Ag capture assay, we performed several experiments to evaluate if the single-round intracellular p24-Ag assay produced data similar to the p24-Ag capture assay. Although the Ag-capture assay does not measure single-round virus replication, we carefully monitor virus growth kinetics to measure extracellular p24-Ag during the early viral growth phase, when there is a linear relationship between extracellular p24-Ag and virus input.
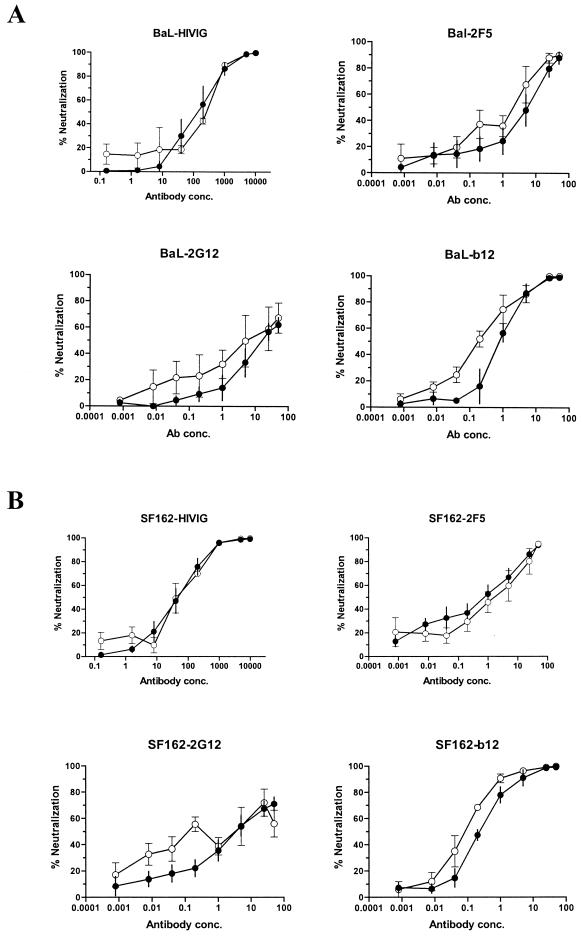
In order to test the antibody dose response for key neutralization epitopes, serial dilutions of MAbs b12, 2F5, and 2G12 and polyclonal HIVIG were tested against two HIV-1 isolates, BaL and SF162, using both assays. As shown in Fig. 6, both assays produced similar neutralization dose-response curves for each antibody against viruses BaL and SF162. The neutralization curves for the p24-Ag capture assay often displayed slightly higher neutralization at lower antibody concentrations, suggesting the possibility that this assay is somewhat more sensitive at low antibody levels. However, the variability in the p24-Ag capture assay makes it difficult to reproducibly measure low levels of neutralization.
FIG. 6.
Comparison of antibody neutralization curves for the intracellular p24-Ag assay (solid circles) and the extracellular Ag-capture assay (open circles). Neutralization of HIV-1 isolates BaL (A) and SF162 (B) by MAbs b12, 2G12, and 2F5 and by polyclonal HIVIG was compared in the two assays. The intracellular p24-Ag assay was performed with a viral MOI of ∼0.1, and infected cells were measured on day 2. The Ag-capture assay was performed with an MOI of ∼0.01, and extracellular p24 was measured on day 3 for BaL and day 4 for SF162. Each of the neutralization curves is an average (± standard error of the mean) of three independent experiments.
Table 1 shows that the corresponding mean IC50, IC80, and IC90 were generally similar for the two assays. Thus, despite a virus input that was 20 times higher than that used in the Ag-capture assay, the intracellular p24-Ag assay appears to measure similar levels of antibody-mediated virus neutralization. These limited data are not yet sufficient to make quantitative comparisons about intra- and interassay variability of the two assays. However, we did observe that the CV in quadruplicate control wells (i.e., virus without antibody) was less than 10% for each of the intracellular p24-Ag assays. Comparatively, the replicate wells in the p24-Ag capture assay displayed a CV of greater than 15%, usually in the range of 20 to 60%.
TABLE 1.
Comparison of inhibitory antibody concentrations in the intracellular p24-Ag assay and the Ag-capture assaya
| Virus | Antibody | Concn (μg/ml)
|
|||||
|---|---|---|---|---|---|---|---|
| p24-Ag assay
|
Ag-capture assay
|
||||||
| IC50 | IC80 | IC90 | IC50 | IC80 | IC90 | ||
| BaL | HIVIG | 95 | 322 | 678 | 106 | 355 | 737 |
| 2F5 | 23 | 27 | >50 | 0.9 | 12 | >50 | |
| 2G12 | 15 | >50 | >50 | 6.0 | >50 | >50 | |
| b12 | 0.5 | 1.6 | 3.2 | 0.3 | 1.0 | 2.2 | |
| SF162 | HIVIG | 32 | 197 | 569 | 41 | 185 | 445 |
| 2F5 | 0.6 | 8.6 | 38 | 1.1 | 11 | 42 | |
| 2G12 | 3.5 | >50 | >50 | 0.9 | >50 | >50 | |
| b12 | 0.2 | 1.2 | 3.1 | 0.1 | 0.4 | 1.2 | |
IC50, IC80, and IC90 are the concentration of antibody that produced 50, 80, and 90% virus neutralization, respectively. Values are averages of three independent experiments for each antibody against each virus.
Quantitation of single-round infection of PBMC using quantitative PCR for viral gag.
Using indinavir to maintain single-round infection of PBMC, we compared the percentage of cells expressing p24-Ag to the number of copies of HIV-1 gag per 100 PBMC. PHA- and IL-2-stimulated PBMC were infected with twofold serial dilutions of HIV-1 BaL or SF162. As shown in Table 2, the number of gag copies per 100 PBMC exceeded the number of cells expressing p24-Ag by three- to sixfold. Similar data were observed using a single round of replications of GFP reporter virus (data not shown). Of note, the dose response to virus input reached a plateau at high virus input. At virus concentrations below this, both p24-Ag expression and copies of HIV-1 gag were proportionately related to viral input. Thus, given appropriate standardization, quantitation of viral DNA could be used as a rapid endpoint readout in neutralization assays.
TABLE 2.
Comparison of percentage of PBMC expressing p24-Ag to number of viral gag copies per 100 cellsa
| Virus | Virus dilution | % of cells positive for p24-Ag | No. of copies of gag DNA per 100 PBMC | Fold difference |
|---|---|---|---|---|
| BaL | — | 2.83 | 8.97 | 3.17 |
| 1:2 | 2.05 | 7.40 | 3.61 | |
| 1:4 | 1.74 | 6.73 | 3.87 | |
| 1:8 | 1.14 | 4.20 | 3.69 | |
| 1:16 | 0.85 | 2.47 | 2.91 | |
| 1:32 | 0.48 | 1.64 | 3.42 | |
| SF162 | — | 3.89 | 18.11 | 4.66 |
| 1:2 | 2.30 | 13.28 | 5.78 | |
| 1:4 | 1.46 | 6.97 | 4.78 | |
| 1:8 | 0.94 | 5.36 | 5.71 | |
| 1:16 | 0.52 | 2.78 | 5.36 | |
| 1:32 | 0.24 | 1.40 | 5.88 |
PHA- and IL-2-stimulated PBMC were exposed to serial dilutions of virus in medium containing indinavir. On day 2, aliquots were split and evaluated by flow cytometry for intracellular p24-Ag expression and by quantitative PCR for viral gag DNA. The MOI of undiluted virus was ∼1.0.
DISCUSSION
The ability to make accurate and precise measurements of antibody-mediated virus neutralization is important for the evaluation of mechanisms of neutralization and for the assessment of antibody responses elicited by immunization. For HIV-1, the use of primary virus isolates and human target cells, such as PBMC, is an in vitro assay system that is believed to measure physiologically relevant virus neutralization. As in the plaque reduction neutralization assays described for many viruses, we wanted to measure the amount of infectious virus in culture and the reduction in infectious titer due to reaction with antibody. To achieve this, we developed a single-round-of-replication PBMC neutralization assay that enumerates infected cells by the flow cytometric detection of cells expressing p24-Ag. Compared to our Ag-capture neutralization assay, the intracellular p24-Ag assay directly identifies infected target cells and provides a precise and reproducible measurement of antibody-mediated inactivation of infectious virus.
We and others commonly perform HIV-1 neutralization assays that measure the amount of viral protein (e.g., p24-Ag) secreted in culture as an assay endpoint. Ideally, the amount of secreted p24-Ag would be directly related to the number of PBMC infected, but this is not necessarily the case. Factors such as multiple rounds of virus replication, cell death and release of p24-Ag, and release of noninfectious virions can each affect the total amount of cell-free p24-Ag measured in culture (7, 8, 16, 21, 37, 62, 69). Also, primary virus isolates have varied replication kinetics in activated PBMC. Some viruses show peak p24-Ag expression in 3 to 4 days, while others peak at 7 to 10 days (4, 7, 21, 62, 69, 70). Ag-capture neutralization assays generally quantify p24-Ag on one particular day after infection. Therefore, endpoint p24-Ag measurement on day 7 postinfection may represent the early phase of growth for one virus isolate and multiple rounds of replication for a more rapidly growing virus. This makes comparisons of neutralization among viruses problematic unless the assays take into account the viral growth kinetics (7, 36).
Several additional factors limit the precision and reproducibility of p24-Ag capture assays. If the amount of virus neutralized is small (e.g., <50%), the multiple rounds of replication may allow the nonneutralized virus fraction to multiply and overshadow the detection of neutralization. Also, PBMC must be washed extensively after exposure to virus in order to remove residual viral p24-Ag and serum anti-p24 antibody, which can interfere with the Ag-capture ELISA (37). Cells are then left in culture for a variable number of days (usually between 4 and 7) before supernatants are collected for measurement of p24-Ag. Our own experience and that of others suggest that this assay format does not generate reproducible measurements when antibody neutralizes less than 80% of infectious virus (7).
Our Ag-capture neutralization assay was typically done with an MOI of ∼0.01; i.e., we would infect 1.5 × 105 PBMC with up to 1,000 TCID50 of virus. Since this ratio indicates that less than 1 in 100 PBMC would be infected, it is not surprising that several rounds of viral replication are required before secreted p24-Ag can be detected. Therefore, to develop a robust single-round replication assay, we increased the viral MOI (to ∼0.1) to allow initial infection of a larger number of PBMC. Rather than 1,000 TCID50 of HIV-1 per well, we added approximately 20,000 TCID50 per well (containing 150,000 PBMC) in the intracellular p24-Ag assay. To achieve this, we concentrated most PBMC-derived virus stocks 5- to 10-fold by using a 100-kDa cutoff Millipore filter. Virus stocks were quantified by a sensitive 14-day endpoint virus dilution assay to determine the TCID50 per milliliter. Using this estimation of infectious virus, we observed that an MOI of ∼0.1 produced first-round infection of 1 to 2% of PBMC.
Since the KC57 anti-p24 antibody appears to be detecting the large majority of productively infected cells, the infection of less than the expected 10% of PBMC is likely due to an infectious process that is less than 100% efficient. This is commonly observed for virus tissue culture assays and has been documented for retroviral infection of various cell types (33, 49, 51, 63, 68, 74). Of note, we could increase the number of infected PBMC by two- to threefold by inclusion of a polycationic substance such as Polybrene or DEAE-dextran, which appears to promote infection by overcoming the normal electrostatic repulsion between the virion and the anionic extracellular cell matrix (51, 65). Due to the potential for interference with physiologic virus-host cell interactions, we chose not to use such molecules in our neutralization assay. We found that the use of an MOI of at least 0.1 and minimization of the initial volume of virus and cells (i.e., 70 μl in our neutralization assay) were important for efficient infection. The use of PBMC depleted of CD8+ T cells (i.e., 90% CD4+ T cells) improved the efficiency of HIV-1 infection, such that 1.5 to 2 times as many cells were HIV-1 infected at a given MOI. The reason for this is not clear, but the higher CD4+ target cell density may provide more opportunities for virus-cell interactions. Since depletion of CD8+ T cells results in a more uniform population of CD4+ target cells, we have recently begun to routinely use PBMC targets depleted of CD8+ T cells.
Our intracellular p24-Ag assay, using the PE-conjugated KC57 anti-p24 antibody, produced sensitive and specific detection of HIV-1-infected cells. Staining of HIV-1-infected PBMC with an isotypic PE-conjugated control antibody or staining of mock-infected cells with KC57 resulted in less than 0.02% positive cells (Fig. 1). The KC57 anti-p24 antibody also did not stain PBMC that had been exposed to HIV-1 in the presence of a reverse transcriptase inhibitor, indicating that intracellular synthesis of Gag protein is required for detection of infection. Similarly, HIV-1-infected cells were not detected unless they were permeabilized prior to KC57 staining. These results showed that the p24-Ag staining we observed was not due to surface-bound virus.
The sensitivity of detection of HIV-1-infected cells was evaluated by use of a GFP reporter virus; these data confirmed that the flow cytometric detection of p24-Ag identifies over 90% of productively HIV-1-infected cells. Intracellular p24-Ag staining was able to detect HIV-infected cells beginning 1 day after exposure to virus. Without a protease inhibitor in the culture, the infection spread rapidly over 3 to 4 days and resulted in a spectrum of fluorescence intensity of p24-positive cells that made discrimination of HIV-1-infected cells difficult. The addition of indinavir to PBMC cultures effectively prevented secondary rounds of replication and allowed clear discrimination of infected cells. AZT could also be used to prevent secondary rounds of virus replication but had to be added to the culture 6 to 18 h after the cells were exposed to virus, whereas indinavir could be included with PBMC prior to exposure to virus (Fig. 2).
Since quantitative PCR assays for viral DNA can also be used as an endpoint in neutralization assays (56), we used indinavir to maintain single-round infection of PBMC and compared the percentage of cells expressing p24-Ag to the number of copies of HIV-1 gag per 100 PBMC. We observed that the number of gag copies per 100 cells was three- to sixfold greater than the percentage of cells expressing p24-Ag. This could result from an insensitivity of flow cytometric methods to low levels of p24-Ag expression in some cells. Alternatively, it is likely that only a consistent minority of PBMC containing viral gag DNA go on to express viral proteins. Of note, below the viral levels that reach saturation in the assay, there is a linear relationship between virus input and the number of copies of HIV-1 gag. Thus, similar to p24-Ag expression, quantitative PCR assays for viral DNA can be standardized to accurately measure virus neutralization.
The combination of flow cytometric detection of p24-Ag-expressing cells and indinavir in culture produced a neutralization assay that directly quantifies the number of productively infected PBMC during first-round infection. This produces a linear relationship between virus input and the number of target cells infected (Fig. 4). There are several additional advantages to this assay format. Compared to our multiple-round Ag-capture assay, there is no need to monitor virus growth kinetics and sample p24-Ag on various days; rather, the assay endpoint is determined on day 2, when infected PBMC can be readily discriminated. This short time in culture and minimal cell manipulation lead to much lower variability in infection of target cells. The coefficient of variation among replicate virus-infected control wells in this assay is usually less than 10%, versus a much wider range (10 to 60%) in our Ag-capture assay. This low intra-assay variation, along with a low background in mock-infected PBMC, allows precise quantitation of neutralization.
While we do not provide evidence that the intracellular p24-Ag assay is more sensitive for detecting neutralizing antibody than prior assays, we have shown that moderate levels of neutralization (e.g., 60 to 70% neutralization of BaL and SF162 by MAb 2G12) can be reproducibly measured (Fig. 5). The precision and reproducibility of this assay may be important in comparing the antibody response generated by various HIV-1 immunogens and should facilitate the detection of incrementally improved neutralizing antibody responses generated by new candidate vaccines.
Additionally, the forward and side scatter plots of the PBMC allow determination of the viability of the target cells. In some cases, serum or plasma from certain animal species can impair cell growth or viability, which would lead to less p24-Ag secretion and the possibility of falsely inferring antibody-mediated neutralization. This potential problem can be more readily monitored due to the flow cytometric phenotypic characterization of the cells. Overall, this assay is less labor intensive and substantially less expensive than the Ag-capture assay. While a flow cytometer is required, the cost of the KC57 MAb and related staining reagents is about fivefold less than the per-well cost of a commercial Ag-capture ELISA. We have also recently begun to use a multiwell autosampler attachment to the FACSCalibur (Multiwell autosampler system; Becton Dickinson). This allows the neutralization assay to be set up in a 96-well plate and analyzed by automated flow cytometric determinations in a 96-well format.
Since there are many published reports using Ag-capture PBMC neutralization assays, we compared antibody-mediated neutralization in our Ag-capture assay (MOI of ∼0.01) to our single-round intracellular p24-Ag assay (MOI of ∼0.1). Using MAbs that bind to the three best-characterized neutralization epitopes (b12, 2F5, and 2G12) and a polyclonal HIVIG, we found similar antibody dose-response curves for virus neutralization in the two assays (Fig. 6). This is not surprising, as a 20-fold difference in virus input is small compared to the molar excess of antibody at neutralizing concentrations. Numerous studies of virus neutralization have suggested that, in antibody excess, the same fraction of virus is neutralized per unit time irrespective of the amount of virus added (2, 17, 19, 35, 48). However, this is only strictly true during high antibody excess, and it is possible that the amount of neutralization measured in the presence of a low amount of HIV-specific antibody could be affected by the concentration of virus used (18, 52, 66). For the p24-Ag capture assay with a lower MOI, the data in Fig. 6 suggest a trend toward slightly greater neutralization at lower antibody concentrations. However, in our experience, the variability in the p24-Ag capture assay makes it difficult to reproducibly measure low levels of neutralization. Ongoing experiments are evaluating the impact of virus concentration on neutralization in our intracellular p24-Ag assay.
This neutralization assay uses primary strains of HIV-1 and primary human PBMC target cells. Most other single-round infection neutralization assays utilize env-pseudotyped viruses that express reporter proteins such as chloramphenicol acetyltransferase, alkaline phosphatase, β-galactosidase, luciferase, or GFP (10, 13, 28, 29). Although restricted to recombinant viruses, these assays can quantify single-round infection and have been used extensively to evaluate the effect of antibody on HIV-1 infection of target cells. Among these reporter systems, alkaline phosphatase, β-galactosidase and GFP have the advantage of allowing direct visualization and enumeration of the number of infected target cells (27, 50). In contrast, quantitating chloramphenicol acetyltransferase and luciferase activity involves lysis of cells, and the data generated reflect relative amounts of the reporter gene produced in the entire culture.
Flow cytometric methods have also been used to reproducibly measure HIV-1 neutralization in human osteosarcoma (HOS) cells engineered to express HIV-1 coreceptors and to express GFP upon HIV-1 infection (9). This assay has the advantage of directly enumerating infected cells, though the target cells are a nonlymphocytic cell line and infection is not limited to a single round. T-cell lines such as CEM cells have also been engineered to express GFP or luciferase upon HIV-1 infection (25, 58, 65).
Pinter and colleagues have employed a neutralization assay that identifies HIV-1-infected PBMC on polylysine-coated slides by indirect staining with polyclonal HIVIG; infection was quantified after a period of 4 to 7 days (54). Though quantitative, the visual inspection of infected cells on slides is cumbersome and does not readily permit the evaluation of large numbers of samples. More recently, Darden and colleagues reported the development of a primary-isolate HIV-1 neutralization assay that identified infected PBMC in culture by flow cytometric detection of intracellular p24-Ag (16). They quantified infected cells 4 to 8 days after HIV-1 infection, thus measuring HIV-1 infection after several rounds of replication. Nonetheless, they showed that neutralization measured by intracellular p24-Ag expression was roughly similar to that measured by extracellular p24-Ag. They also reported that the KC57 anti-p24 antibody (also used in this report) could detect viruses from clades A to G, confirming the general utility of this antibody for detection of HIV-1-infected cells.
In summary, by including a protease inhibitor in the culture with activated PBMC, we developed a flow cytometric HIV-1 neutralization assay that quantifies first-round infection of primary human lymphocytes by staining for intracellular expression of p24-Ag. The assay requires a higher MOI than traditional PBMC neutralization assays but provides accurate data on antibody-mediated virus neutralization. The enumeration of first-round infection of target cells provides quantitative data on the number of infectious virus particles in culture. Thus, this PBMC neutralization assay can directly quantify the reduction of infectious virus mediated by exposure to antibody. The precision and reproducibility of this assay should facilitate the comparison of antibody response generated by various HIV-1 immunogens and the detection of incrementally improved neutralizing antibody responses generated by new candidate vaccines.
Acknowledgments
We thank Dennis Burton for providing MAb b12, Hermann Katinger and Gabriela Stiegler for MAbs 2F5 and 2G12, and Chris Sapan of NABI, Inc., for HIVIG. We are appreciative of the technical advice and assistance from Steve Perfetto and Phil Erhenberg, and we thank Nelson Michael, Richard Koup, Richard Wyatt, Nancy Sullivan, David Montefiori, and Gary Nabel for helpful discussions.
D.G. was supported by NIH NS37277 and by an Elizabeth Glaser Scientist Award from the Pediatric AIDS Foundation.
REFERENCES
- 1.Albert, J., B. Abrahamsson, K. Nagy, E. Aurelius, H. Gaines, G. Nystrom, and E. M. Fenyo. 1990. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS 4:107-112. [DOI] [PubMed] [Google Scholar]
- 2.Andrewes, C. H., and W. J. Elford. 1933. Observations on anti-phage-1: the percentage lab. Br. J. Exp. Pathol. 14:367-374. [Google Scholar]
- 3.Arendrup, M., A. Sonnerborg, B. Svennerholm, L. Akerblom, C. Nielsen, H. Clausen, S. Olofsson, J. O. Nielsen, and J. E. Hansen. 1993. Neutralizing antibody response during human immunodeficiency virus type 1 infection: type and group specificity and viral escape. J. Gen. Virol. 74:855-863. [DOI] [PubMed] [Google Scholar]
- 4.Asjo, B., L. Morfeldt-Manson, J. Albert, G. Biberfeld, A. Karlsson, K. Lidman, and E. M. Fenyo. 1986. Replicative capacity of human immunodeficiency virus from patients with varying severity of HIV infection. Lancet 2:660-662. [PubMed] [Google Scholar]
- 5.Baba, T. W., V. Liska, R. Hofmann-Lehmann, J. Vlasak, W. Xu, S. Ayehunie, L. A. Cavacini, M. R. Posner, H. Katinger, G. Stiegler, B. J. Bernacky, T. A. Rizvi, R. Schmidt, L. R. Hill, M. E. Keeling, Y. Lu, J. E. Wright, T. C. Chou, and R. M. Ruprecht. 2000. Human neutralizing monoclonal antibodies of the IgG1 subtype protect against mucosal simian-human immunodeficiency virus infection. Nat. Med. 6:200-206. [DOI] [PubMed] [Google Scholar]
- 6.Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. [DOI] [PubMed] [Google Scholar]
- 7.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 8.Burns, D. P., and R. C. Desrosiers. 1992. A caution on the use of SIV/HIV gag antigen detection systems in neutralization assays. AIDS Res. Hum. Retrovir. 8:1189-1192. [DOI] [PubMed] [Google Scholar]
- 9.Cecilia, D., V. N. KewalRamani, J. O'Leary, B. Volsky, P. Nyambi, S. Burda, S. Xu, D. R. Littman, and S. Zolla-Pazner. 1998. Neutralization profiles of primary human immunodeficiency virus type 1 isolates in the context of coreceptor usage. J. Virol. 72:6988-6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen, B. K., K. Saksela, R. Andino, and D. Baltimore. 1994. Distinct modes of human immunodeficiency virus type 1 proviral latency revealed by superinfection of nonproductively infected cell lines with recombinant luciferase-encoding viruses. J. Virol. 68:654-660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cheng-Mayer, C., D. Seto, M. Tateno, and J. A. Levy. 1988. Biologic features of HIV-1 that correlate with virulence in the host. Science 240:80-82. [DOI] [PubMed] [Google Scholar]
- 12.Chesebro, B., and K. Wehrly. 1988. Development of a sensitive quantitative focal assay for human immunodeficiency virus infectivity. J. Virol. 62:3779-3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Connor, R. I., B. K. Chen, S. Choe, and N. R. Landau. 1995. Vpr is required for efficient replication of human immunodeficiency virus type-1 in mononuclear phagocytes. Virology 206:935-944. [DOI] [PubMed] [Google Scholar]
- 14.Cory, J. M., B. M. Ohlsson-Wilhelm, E. J. Brock, N. A. Sheaffer, M. E. Steck, M. E. Eyster, and F. Rapp. 1987. Detection of human immunodeficiency virus-infected lymphoid cells at low frequency by flow cytometry. J. Immunol. Methods 105:71-78. [DOI] [PubMed] [Google Scholar]
- 15.Costigliola, P., F. Tumietto, E. Ricchi, and F. Chiodo. 1992. Detection of circulating p24 antigen-positive CD4+ cells during HIV infection by flow cytometry. AIDS 6:1121-1125. [DOI] [PubMed] [Google Scholar]
- 16.Darden, J. M., V. R. Polonis, M. S. deSouza, S. Chantakulkij, A. E. Brown, D. L. Birx, and K. Pattanapanyasat. 2000. A flow cytometric method for measuring neutralization of HIV-1 subtype B and E primary isolates. Cytometry 40:141-150. [PubMed] [Google Scholar]
- 17.Delbecco, R., M. Vogt, and A. G. R. Strickland. 1956. A study of the basic aspects of neutralization of two animal viruses, Western equine encephalitis virus and poliomyelitis virus. Virology 2:162-205. [DOI] [PubMed] [Google Scholar]
- 18.Della-Porta, A. J., and E. G. Westaway. 1978. A multi-hit model for the neutralization of animal viruses. J. Gen. Virol. 38:1-19. [DOI] [PubMed] [Google Scholar]
- 19.Dimmock, N. J. 1993. Neutralization of animal viruses. Curr. Top. Microbiol. Immunol. 183:1-149. [DOI] [PubMed] [Google Scholar]
- 20.Douek, D. C., R. A. Vescio, M. R. Betts, J. M. Brenchley, B. J. Hill, L. Zhang, J. R. Berenson, R. H. Collins, and R. A. Koup. 2000. Assessment of thymic output in adults after haematopoietic stem-cell transplantation and prediction of T-cell reconstitution. Lancet 355:1875-1881. [DOI] [PubMed] [Google Scholar]
- 21.Fenyo, E. M., J. Albert, and B. Asjo. 1989. Replicative capacity, cytopathic effect and cell tropism of HIV. AIDS 3:S5-12. [DOI] [PubMed] [Google Scholar]
- 22.Folghera, S., S. Fiorentini, F. Martinelli, G. Ravizzola, F. Gargiulo, L. Terlenghi, M. De Francesco, A. Caruso, and A. Turano. 1994. Development of a flow cytometric assay for the detection and measurement of neutralizing antibodies against human immunodeficiency virus. New Microbiol. 17:21-28. [PubMed] [Google Scholar]
- 23.Folks, T. M., D. Powell, M. Lightfoote, S. Koenig, A. S. Fauci, S. Benn, A. Rabson, D. Daugherty, H. E. Gendelman, M. D. Hoggan, et al. 1986. Biological and biochemical characterization of a cloned Leu-3-cell surviving infection with the acquired immune deficiency syndrome retrovirus. J. Exp. Med. 164:280-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gauduin, M. C., P. W. Parren, R. Weir, C. F. Barbas, D. R. Burton, and R. A. Koup. 1997. Passive immunization with a human monoclonal antibody protects hu-PBL-SCID mice against challenge by primary isolates of HIV-1. Nat. Med. 3:1389-1393. [DOI] [PubMed] [Google Scholar]
- 25.Gervaix, A., D. West, L. M. Leoni, D. D. Richman, F. Wong-Staal, and J. Corbeil. 1997. A new reporter cell line to monitor HIV infection and drug susceptibility in vitro. Proc. Natl. Acad. Sci. USA 94:4653-4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Glushakova, S., J. Munch, S. Carl, T. C. Greenough, J. L. Sullivan, L. Margolis, and F. Kirchhoff. 2001. CD4 down-modulation by human immunodeficiency virus type 1 Nef correlates with the efficiency of viral replication and with CD4(+) T-cell depletion in human lymphoid tissue ex vivo. J. Virol. 75:10113-10117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Busciglio, X. Yang, W. Hofmann, W. Newman, C. R. Mackay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 28.He, J., and N. R. Landau. 1995. Use of a novel human immunodeficiency virus type 1 reporter virus expressing human placental alkaline phosphatase to detect an alternative viral receptor. J. Virol. 69:4587-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Helseth, E., M. Kowalski, D. Gabuzda, U. Olshevsky, W. Haseltine, and J. Sodroski. 1990. Rapid complementation assays measuring replicative potential of human immunodeficiency virus type 1 envelope glycoprotein mutants. J. Virol. 64:2416-2420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho, D. D., M. G. Sarngadharan, M. S. Hirsch, R. T. Schooley, T. R. Rota, R. C. Kennedy, T. C. Chanh, and V. L. Sato. 1987. Human immunodeficiency virus neutralizing antibodies recognize several conserved domains on the envelope glycoproteins. J. Virol. 61:2024-2028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jason, J., and K. L. Inge. 1999. Increased expression of CD80 and CD86 in in vitro-infected CD3+ cells producing cytoplasmic HIV type 1 p24. AIDS Res. Hum. Retrovir. 15:173-181. [DOI] [PubMed] [Google Scholar]
- 32.Kux, A., S. Bertram, F. T. Hufert, H. Schmitz, and D. von Laer. 1996. Antibodies to p24 antigen do not specifically detect HIV-infected lymphocytes in AIDS patients. J. Immunol. Methods 191:179-186. [DOI] [PubMed] [Google Scholar]
- 33.LaBonte, J. A., T. Patel, W. Hofmann, and J. Sodroski. 2000. Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J. Virol. 74:10690-10698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lambert, J. S., L. M. Mofenson, C. V. Fletcher, J. Moye, Jr., E. R. Stiehm, W. A. Meyer III, G. J. Nemo, B. J. Mathieson, G. Hirsch, C. V. Sapan, L. M. Cummins, E. Jimenez, E. O'Neill, A. Kovacs, and A. Stek. 1997. Safety and pharmacokinetics of hyperimmune anti-human immunodeficiency virus (HIV) immunoglobulin administered to HIV-infected pregnant women and their newborns. Pediatric AIDS Clinical Trials Group Protocol 185. Pharmacokinetic Study Group. J. Infect. Dis. 175:283-291. [DOI] [PubMed] [Google Scholar]
- 35.Mandel, B. 1978. Neutralization of animal viruses. Adv. Virus Res. 23:205-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mascola, J. R. 1999. Neutralization of HIV-1 infection of human peripheral blood mononuclear cells: antibody dilution method, p. 309-315. In N. L. Michael and J. H. Kim (ed.), HIV protocols, vol. 17. Humana Press Inc., Totowa, N.J. [DOI] [PubMed] [Google Scholar]
- 37.Mascola, J. R., and D. S. Burke. 1993. Antigen detection in neutralization assays: high levels of interfering anti-p24 antibodies in some plasma. AIDS Res. Hum. Retrovir. 9:1173-1174. [DOI] [PubMed] [Google Scholar]
- 38.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mascola, J. R., M. K. Louder, S. R. Surman, T. C. Vancott, X. F. Yu, J. Bradac, K. R. Porter, K. E. Nelson, M. Girard, J. G. McNeil, F. E. McCutchan, D. L. Birx, and D. S. Burke. 1996. Human immunodeficiency virus type 1 neutralizing antibody serotyping using serum pools and an infectivity reduction assay. AIDS Res. Hum. Retrovir. 12:1319-1328. [DOI] [PubMed] [Google Scholar]
- 40.Mascola, J. R., M. K. Louder, T. C. VanCott, C. V. Sapan, J. S. Lambert, L. R. Muenz, B. Bunow, D. L. Birx, and M. L. Robb. 1997. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 71:7198-7206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mascola, J. R., J. Louwagie, F. E. McCutchan, C. L. Fischer, P. A. Hegerich, K. F. Wagner, A. K. Fowler, J. G. McNeil, and D. S. Burke. 1994. Two antigenically distinct subtypes of human immunodeficiency virus type 1: viral genotype predicts neutralization serotype. J. Infect. Dis. 169:48-54. [DOI] [PubMed] [Google Scholar]
- 42.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, and D. S. Burke. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. The National Institute of Allergy and Infectious Diseases AIDS Vaccine Evaluation Group. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 43.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 44.McSharry, J. J., R. Costantino, E. Robbiano, R. Echols, R. Stevens, and J. M. Lehman. 1990. Detection and quantitation of human immunodeficiency virus-infected peripheral blood mononuclear cells by flow cytometry. J. Clin. Microbiol. 28:724-733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mitra, D. K., S. C. De Rosa, A. Luke, A. Balamurugan, B. K. Khaitan, J. Tung, N. K. Mehra, A. I. Terr, A. O'Garra, L. A. Herzenberg, and M. Roederer. 1999. Differential representations of memory T cell subsets are characteristic of polarized immunity in leprosy and atopic diseases. Int. Immunol. 11:1801-1810. [DOI] [PubMed] [Google Scholar]
- 46.Montefiori, D. C., I. Y. Zhou, B. Barnes, D. Lake, E. M. Hersh, Y. Masuho, and L. B. Lefkowitz, Jr. 1991. Homotypic antibody responses to fresh clinical isolates of human immunodeficiency virus. Virology 182:635-643. [DOI] [PubMed] [Google Scholar]
- 47.Nara, P. L., and P. J. Fischinger. 1988. Quantitative infectivity assay for HIV-1 and -2. Nature 332:469-470. [DOI] [PubMed] [Google Scholar]
- 48.Nara, P. L., W. C. Hatch, N. M. Dunlop, W. G. Robey, L. O. Arthur, M. A. Gonda, and P. J. Fischinger. 1987. Simple, rapid, quantitative, syncytium-forming microassay for the detection of human immunodeficiency virus neutralizing antibody. AIDS Res. Hum. Retrovir. 3:283-302. [DOI] [PubMed] [Google Scholar]
- 49.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Page, K. A., T. Liegler, and M. B. Feinberg. 1997. Use of a green fluorescent protein as a marker for human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 13:1077-1081. [DOI] [PubMed] [Google Scholar]
- 51.Palsson, B., and S. Andreadis. 1997. The physico-chemical factors that govern retrovirus-mediated gene transfer. Exp. Hematol. 25:94-102. [PubMed] [Google Scholar]
- 52.Parren, P. W., and D. R. Burton. 2001. The antiviral activity of antibodies in vitro and in vivo. Adv. Immunol. 77:195-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Parren, P. W., P. A. Marx, A. J. Hessell, A. Luckay, J. Harouse, C. Cheng-Mayer, J. P. Moore, and D. R. Burton. 2001. Antibody protects macaques against vaginal challenge with a pathogenic R5 simian/human immunodeficiency virus at serum levels giving complete neutralization in vitro. J. Virol. 75:8340-8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pinter, A., W. J. Honnen, S. C. Kayman, O. Trochev, and Z. Wu. 1998. Potent neutralization of primary HIV-1 isolates by antibodies directed against epitopes present in the V1/V2 domain of HIV-1 gp120. Vaccine 16:1803-1811. [DOI] [PubMed] [Google Scholar]
- 55.Purtscher, M., A. Trkola, A. Grassauer, P. M. Schulz, A. Klima, S. Dopper, G. Gruber, A. Buchacher, T. Muster, and H. Katinger. 1996. Restricted antigenic variability of the epitope recognized by the neutralizing gp41 antibody 2F5. AIDS 10:587-593. [DOI] [PubMed] [Google Scholar]
- 56.Robb, M. L., V. Polonis, M. Vahey, S. Gartner, N. Michael, A. Fowler, and R. R. Redfield. 1992. HIV neutralization assay using polymerase chain reaction-derived molecular signals. J. Acquir. Immune Defic. Syndr. 5:1224-1229. [PubMed] [Google Scholar]
- 57.Robert-Guroff, M., M. Brown, and R. C. Gallo. 1985. HTLV-III-neutralizing antibodies in patients with AIDS and AIDS-related complex. Nature 316:72-74. [DOI] [PubMed] [Google Scholar]
- 58.Roos, J. W., M. F. Maughan, Z. Liao, J. E. Hildreth, and J. E. Clements. 2000. LuSIV cells: a reporter cell line for the detection and quantitation of a single cycle of HIV and SIV replication. Virology 273:307-315. [DOI] [PubMed] [Google Scholar]
- 59.Rusche, J. R., K. Javaherian, C. McDanal, J. Petro, D. L. Lynn, R. Grimaila, A. Langlois, R. C. Gallo, L. O. Arthur, P. J. Fischinger, et al. 1988. Antibodies that inhibit fusion of human immunodeficiency virus-infected cells bind a 24-amino acid sequence of the viral envelope, gp120. Proc. Natl. Acad. Sci. USA 85:3198-3202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sawyer, L. S., M. T. Wrin, L. Crawford-Miksza, B. Potts, Y. Wu, P. A. Weber, R. D. Alfonso, and C. V. Hanson. 1994. Neutralization sensitivity of human immunodeficiency virus type 1 is determined in part by the cell in which the virus is propagated. J. Virol. 68:1342-1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schuitemaker, H., N. A. Kootstra, R. E. de Goede, F. de Wolf, F. Miedema, and M. Tersmette. 1991. Monocytotropic human immunodeficiency virus type 1 (HIV-1) variants detectable in all stages of HIV-1 infection lack T-cell line tropism and syncytium-inducing ability in primary T-cell culture. J. Virol. 65:356-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schwartz, S., B. K. Felber, E. M. Fenyo, and G. N. Pavlakis. 1989. Rapidly and slowly replicating human immunodeficiency virus type 1 isolates can be distinguished according to target-cell tropism in T-cell and monocyte cell lines. Proc. Natl. Acad. Sci. USA 86:7200-7203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sharma, S., A. Miyanohara, and T. Friedmann. 2000. Separable mechanisms of attachment and cell uptake during retrovirus infection. J. Virol. 74:10790-10795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shibata, R., T. Igarashi, N. Haigwood, A. Buckler-White, R. Ogert, W. Ross, R. Willey, M. W. Cho, and M. A. Martin. 1999. Neutralizing antibody directed against the HIV-1 envelope glycoprotein can completely block HIV-1/SIV chimeric virus infections of macaque monkeys. Nat. Med. 5:204-210. [DOI] [PubMed] [Google Scholar]
- 65.Spenlehauer, C., C. A. Gordon, A. Trkola, and J. P. Moore. 2001. A luciferase-reporter gene-expressing T-cell line facilitates neutralization and drug-sensitivity assays that use either R5 or X4 strains of human immunodeficiency virus type 1. Virology 280:292-300. [DOI] [PubMed] [Google Scholar]
- 66.Spouge, J. L. 1994. Viral multiplicity of attachment and its implications for human immunodeficiency virus therapies. J. Virol. 68:1782-1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Steele-Mortimer, O. A., H. Meier-Ewert, R. Loser, and M. J. Hasmann. 1990. Flow cytometric analysis of virus-infected cells and its potential use for screening antiviral agents. J. Virol. Methods 27:241-252. [DOI] [PubMed] [Google Scholar]
- 68.Sun, J., B. Barbeau, S. Sato, and M. J. Tremblay. 2001. Neuraminidase from a bacterial source enhances both HIV-1-mediated syncytium formation and the virus binding/entry process. Virology 284:26-36. [DOI] [PubMed] [Google Scholar]
- 69.Sundqvist, V. A., J. Albert, E. Ohlsson, J. Hinkula, E. M. Fenyo, and B. Wahren. 1989. Human immunodeficiency virus type 1 p24 production and antigenic variation in tissue culture of isolates with various growth characteristics. J. Med. Virol. 29:170-175. [DOI] [PubMed] [Google Scholar]
- 70.Tersmette, M., R. A. Gruters, F. de Wolf, R. E. de Goede, J. M. Lange, P. T. Schellekens, J. Goudsmit, H. G. Huisman, and F. Miedema. 1989. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J. Virol. 63:2118-2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tersmette, M., J. J. van Dongen, P. R. Clapham, R. E. de Goede, I. L. Wolvers-Tettero, A. Geurts van Kessel, J. G. Huisman, R. A. Weiss, and F. Miedema. 1989. Human immunodeficiency virus infection studied in CD4-expressing human-murine T-cell hybrids. Virology 168:267-273. [DOI] [PubMed] [Google Scholar]
- 72.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Vanham, G., L. Penne, H. Allemeersch, L. Kestens, B. Willems, G. van der Groen, K. T. Jeang, Z. Toossi, and E. Rich. 2000. Modeling HIV transfer between dendritic cells and T cells: importance of HIV phenotype, dendritic cell-T cell contact and T-cell activation. AIDS 14:2299-2311. [DOI] [PubMed] [Google Scholar]
- 74.Vodicka, M. A., W. C. Goh, L. I. Wu, M. E. Rogel, S. R. Bartz, V. L. Schweickart, C. J. Raport, and M. Emerman. 1997. Indicator cell lines for detection of primary strains of human and simian immunodeficiency viruses. Virology 233:193-198. [DOI] [PubMed] [Google Scholar]
- 75.Von Gegerfelt, A., J. Albert, L. Morfeldt-Manson, K. Broliden, and E. M. Fenyo. 1991. Isolate-specific neutralizing antibodies in patients with progressive HIV-1-related disease. Virology 185:162-168. [DOI] [PubMed] [Google Scholar]
- 76.Vujcic, L., D. Katzenstein, M. Martin, and G. Quinnan. 1990. International collaborative study to compare assays for antibodies that neutralize human immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:847-853. [DOI] [PubMed] [Google Scholar]
- 77.Weiss, R. A., P. R. Clapham, R. Cheingsong-Popov, A. G. Dalgleish, C. A. Carne, I. V. Weller, and R. S. Tedder. 1985. Neutralization of human T-lymphotropic virus type III by sera of AIDS and AIDS-risk patients. Nature 316:69-72. [DOI] [PubMed] [Google Scholar]
- 78.Zolla-Pazner, S., J. O'Leary, S. Burda, M. K. Gorny, M. Kim, J. Mascola, and F. McCutchan. 1995. Serotyping of primary human immunodeficiency virus type 1 isolates from diverse geographic locations by flow cytometry. J. Virol. 69:3807-3815. [DOI] [PMC free article] [PubMed] [Google Scholar]