Abstract
Human cytomegalovirus (HCMV) is a prototypic member of the betaherpesvirus family. The HCMV virion is composed of a large DNA genome encapsidated within a nucleocapsid, which is wrapped within an inner proteinaceous tegument and an outer lipid envelope containing viral glycoproteins. Although genome encapsidation clearly occurs in the nucleus, the subsequent steps in the virion assembly process are unclear. HCMV glycoprotein B (gB) is a major component of the virion envelope that plays a critical role in virus entry and is essential for the production of infectious virus progeny. The aim of our present study was to identify the secretory compartment to which HCMV gB was localized and to investigate the role of endocytosis in mediating gB localization and HCMV biogenesis. We show that HCMV gB is localized to the trans-Golgi network (TGN) in HCMV-infected cells and that gB contains all of the trafficking information necessary for TGN localization. Endocytosis of gB was shown to play a role in mediating TGN localization of gB and in targeting of the protein to the site of virus envelopment. However, inhibition of endocytosis with a dominant-negative dynamin I molecule did not affect the production of infectious virus. These observations indicate that, although endocytosis is involved in the trafficking of gB to the site of glycoprotein accumulation in the TGN, endocytosis of gB is not required for the production of infectious HCMV.
Human cytomegalovirus (HCMV) is a ubiquitous betaherpesvirus that causes debilitatingdisease in immunosuppressed individuals (4). The virion of HCMV consistsof an encapsidated genome wrapped within an inner proteinaceous tegument and an outer lipid envelope studded with viral glycoproteins (11). Although two distinct models have been proposed to describe the assembly process of HCMV and other herpesviruses, most recent studies support a “re-envelopment” model. In this model, nucleocapsids assembled within the nucleus enter the cytoplasm by a process of envelopment followed by deenvelopment at the inner and outer nuclear membrane. Final envelopment of the naked cytoplasmic nucleocapsid then occurs at specific membranes of the cellular secretory system. For the most extensively studied herpesviruses, i.e., varicella-zoster virus (VZV), pseudorabies virus (PRV), and herpes simplex virus type 1 (HSV-1), final envelopment is thought to occur at the trans-Golgi network (TGN) (5, 8, 9, 16, 24, 25). This proposed TGN site of envelopment corresponds to accumulation of major viral envelope proteins in the TGN, indicating that these viruses target components of the virion to the site of assembly (1, 2, 27).
Results from a number of studies support a re-envelopment model to describe HCMV assembly. First, treatment of HCMV-infected cells with brefeldin A (BFA; which blocks secretory transport to the TGN) was shown to result in the accumulation of naked nucleocapsids in the cytoplasm, suggesting that naked cytoplasmic capsids represent an intermediate species in HCMV assembly (6). Second, a number of major components of the HCMV virion have been shown to accumulate in a compartment that displays considerable colocalization with a cellular TGN-localized protein (TGN46), suggesting that final envelopment of HCMV may occur at the TGN as observed for VZV and PRV (15).
Localization of many proteins to the TGN (e.g., furin endoprotease, VZV gE, and HSV gE) has been shown to be mediated, at least in part, by endocytosis from the cell surface (1, 2, 12, 27). For HCMV gB, previous studies indicate that endocytosis is a common characteristic of gB trafficking in all permissive cell types studied (human fibroblasts [HF], an astroglioma cell line [U373], and human retinal pigment epithelial cells [ARPE-19]) (7, 14, 21). Furthermore, studies in HF cells suggested that a horseradish peroxidase-accessible endosomal compartment was a major site of virus envelopment (20) and that gB was transported to the site(s) of virus assembly in these cells by retrieval from the cell surface (14). Together, these studies suggest that endocytosis plays a role in the intracellular trafficking of HCMV gB in a wide variety of HCMV permissive cell types, as well as in the targeting of gB to a cytoplasmic site of virus envelopment in HF cells.
The aim of the present study was to determine the subcellular site of gB accumulation and the role of endocytosis in the transport of gB to this site and in the production of normal levels of HCMV progeny. First, we show that gB expressed during HCMV infection of two permissive cell types (HF and U373 cells) accumulates in the TGN. Second, we demonstrate that HCMV gB contains all of the cis-acting information necessary for TGN localization. Finally, we show that endocytosis is involved in the targeting of gB to the TGN as well as to an undefined cytoplasmic site of virus assembly. However, inhibition of endocytosis with a dominant-negative dynamin I molecule had no effect on the level of infectious progeny virus. This result indicates that levels of gB sufficient for the production of normal levels of infectious progeny virus can be achieved in the absence of gB retrieval from the cell surface.
MATERIALS AND METHODS
Cell lines and virus.
HCMV AD169 strain was propagated in normal human dermal fibroblast cells (NHDF; Clonetics, Walkersville, Md.) by standard methods. U373 cells were obtained from the American Type Culture Collection (Rockville, Md.) and cultured at 37°C in an atmosphere of 5% CO2 in complete medium (Dulbecco modified essential medium [BioWhittaker, Md.] containing 10% fetal bovine serum and supplemented with 4 mM l-glutamine, 200 μg of penicillin G/ml, and 200 μg of streptomycin sulfate/ml).
HCMV titration.
Cells were infected with HCMV by the addition of virus to cell monolayers at the multiplicity of infection (MOI) indicated, followed by incubation for 1 h at 37°C in an atmosphere of 5% CO2. Monolayers were then washed three times with Dulbecco phosphate-buffered saline (DPBS), fresh complete medium was added, and cells were cultured at 37°C in an atmosphere of 5% CO2. Supernatant and cell fractions were harvested at various times postinfection (p.i.), and the level of virus in each fraction was determined by titration on NHDF cells, using standard methodology.
Generation and use of adenovirus vectors.
Construction and characterization of the adenovirus expressing gBFLAG have been previously described (10). Recombinant adenoviruses expressing dynamin I wild-type (dynWT) and the dominant-negative dynamin I (dynK44A) were produced by previously described methods (17). Recombinant viruses were screened by PCR and protein expression was confirmed by Western analysis of infected cell lysates with the appropriate antibody. All recombinant adenoviruses were plaque purified, and viral stocks were grown and the titers were determined on 293 cells. Recombinant protein expression is under the control of a tet-responsive promoter-enhancer element with protein expression driven by coinfection with an adenovirus (AdtTa) expressing the “tet-off” transactivator (tTa), and protein levels were regulated by altering the MOI of AdtTa used to infect cells. For expression of recombinant proteins, adenoviruses expressing the protein of interest and AdtTa were added to cells at the MOI indicated and then incubated for 2 h at 37°C. Monolayers were then washed twice with DPBS, and cells were cultured for the indicated time prior to harvest.
Immunofluorescence microscopy.
Localization of viral and cellular proteins in U373 and NHDF cells was determined by indirect immunofluorescence by a modification of the method of Molloy et al. (12). All procedures were performed at room temperature. Cells grown on chamber slides were rinsed in DPBS and fixed for 15 min in DPBS containing 4% paraformaldehyde, 0.1 mM CaCl2, and 0.1 mM MgCl2. Cells were washed three times in DPBS and then permeabilized and blocked by incubation for 15 min in DPBS containing 2% normal goat serum and 0.4% Triton X-100. Cells were then washed three times in wash buffer (DPBS containing 0.2% Triton X-100 and 0.2% bovine serum albumin) and incubated in primary antibody diluted in DPBS containing 0.1% Triton X-100 for 1 h. Primary antibodies used were a mouse anti-gB monoclonal antibody (MAb) 27-156 (used at a dilution of 1/150), rabbit anti-gB polyclonal antibody R2448 (used at a dilution of 1/50), mouse anti-FLAG MAbs M1 and M2 (Sigma; used at a dilution of 1/200), a mouse anti-γ adaptin MAb AP-1 (Sigma; used at a dilution of 1/150), a rabbit polyclonal anti-TGN46 antibody (used at a dilution of 1/300) (13), and a mouse anti-p115 antibody (used at a dilution of 1/300; BD Transduction Laboratories). After incubation, cells were washed three times with wash buffer and incubated in DPBS containing the appropriate fluorophore-conjugated species-specific secondary antibodies. Epifluorescence was visualized with a Nikon Optiphot fluorescence microscope.
Antibody uptake assay.
Antibody uptake experiments were performed as previously described (12). Briefly, cells were infected with AdgB and AdtTa at MOIs of 100 and 10, respectively. At 2 days p.i., cells were incubated with either MAb M1 or a control MAb of an identical isotype in the media for 4 h at 37°C prior to fixation and staining. The gB that had cycled to the cell surface and was retrieved intracellularly was then visualized using an MAb M1 isotype-specific secondary antibody. Total gB was visualized by incubation of fixed cells with MAb M2 and the respective isotype-specific antibody. Epifluorescence was visualized using a Nikon Optiphot fluorescence microscope.
Surface biotinylation.
Levels of gB incorporated in the virion after endocytosis from the cell surface of HCMV-infected U373 cells were determined by surface biotinylation as described previously (7). At 3 days p.i., HCMV-infected U373 cells stably expressing tTa (U373 tTa cells) were pulse-labeled with [35S]methionine and [35S]cysteine (20 μCi/ml). After a 1-h labeling period, cells were surface biotinylated at 4°C for 30 min, followed by a chase at 37°C for 2 days. Virus was then harvested from cultures (supernatant and cell associated), and total antigen (gB or pp65 control) was immunoprecipitated from detergent-solubilized virus lysates, using anti-gB MAb 27-156 or anti-pp65 MAb 28-17 and protein A-Sepharose beads. Immunoprecipitated antigen was eluted, and a fraction of the eluate was removed to enable quantitation of total viral gB or pp65. Biotinylated antigen was recovered from the remaining eluate by a second round of precipitation with immobilized Avidin (Pierce). Protein fractions were then analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
RESULTS
gB expressed during HCMV infection is localized to the Golgi compartment.
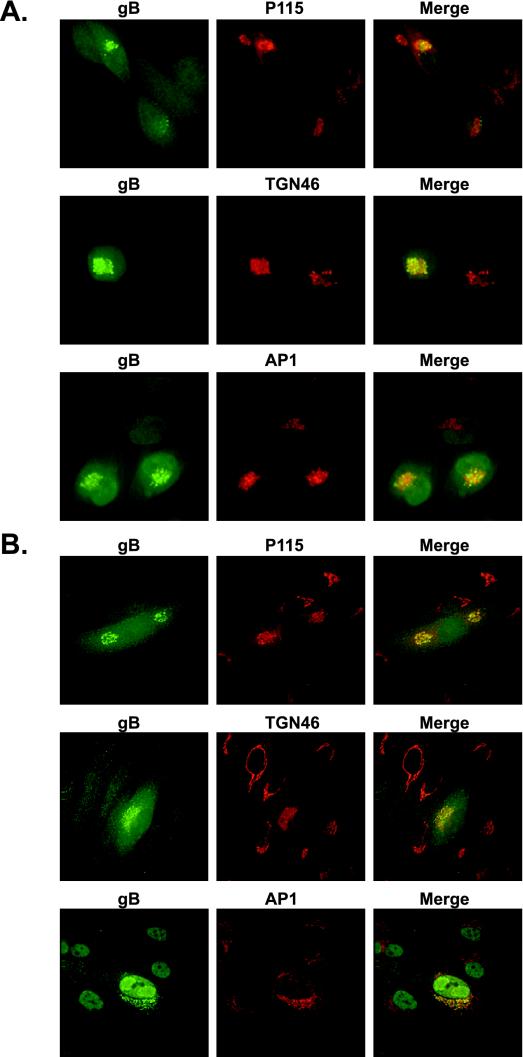
The first series of experiments was designed to identify the secretory compartment to which gB was localized during HCMV infection. U373 and NHDF cells were infected with HCMV strain AD169 (MOI = 0.3) and harvested at day 3 p.i. for analysis by immunofluorescence. As shown in Fig. 1, gB expressed during HCMV infection of both U373 (Fig. 1A) and NHDF (Fig. 1B) cells displayed a vesicular, perinuclear distribution that colocalized to a large degree with markers of the Golgi (p115) and TGN (AP-1 and TGN46). HCMV is known to encode an Fc receptor that binds to rabbit immunoglobulin G. To ensure that the TGN46 staining pattern was due to a specific interaction between the rabbit anti-TGN46 antibody and TGN46, HCMV-infected cells were stained with polyclonal rabbit antibody (R638) against the nuclear HCMV IE86 protein. R638 was shown to localize only to the nucleus with no staining in the Golgi region (data not shown). To ensure that the Golgi localization of gB represented a steady-state distribution of the protein and was not merely a result of the itinerant passage of gB through this compartment, cycloheximide was added for various periods of time prior to fixation. Cycloheximide pretreatment for periods as long as 6 h had no effect on the distribution of gB (data not shown). These results demonstrate that HCMV gB is localized to either the Golgi or TGN compartment during HCMV infection in two distinct permissive cell types.
FIG.1.
gB expressed during HCMV infection is localized to the Golgi compartment. Immunofluorescence microscopy of HCMV-infected U373 (A) and NHDF (B) cells. Cells were infected with AD169 at an MOI of 0.3 and harvested at day 3 p.i. The localization of viral and cellular proteins in U373 and NHDF cells was determined by indirect immunofluorescence by a modification of the method of Molloy et al. (12). gB expressed during HCMV infection of both U373 (A) and NHDF (B) cells displayed a vesicular, perinuclear distribution that colocalized to a large degree with markers of the Golgi (p115) and TGN (AP-1 and TGN46), demonstrating that HCMV gB is localized to either the Golgi or TGN compartments during HCMV infection in two distinct permissive cell types.
HCMV gB contains cis-acting trafficking information necessary to mediate TGN localization.
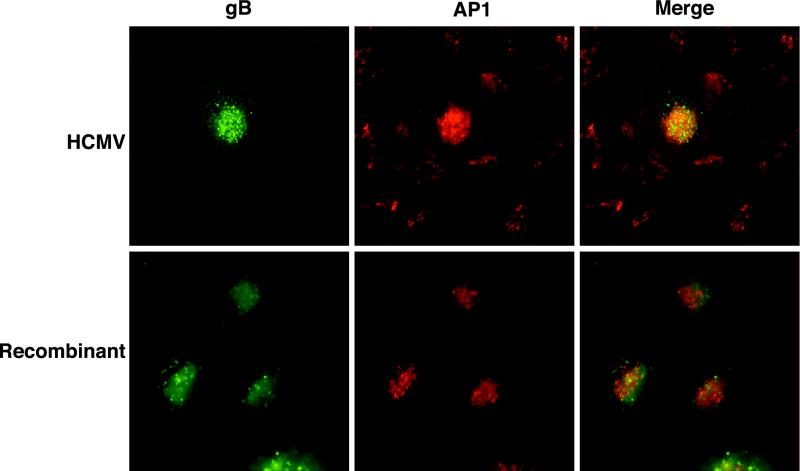
To determine whether gB contained all of the cis-acting trafficking information necessary to mediate localization of the viral glycoprotein to this secretory compartment, we determined the localization of recombinant gB in the absence of HCMV infection using an adenovirus expression system. To facilitate these studies, the recombinant gB contained a FLAG epitope inserted immediately downstream from the furin cleavage site at position 461 of the gB molecule. The presence of the FLAG epitope at this position enables identification of the mature, cleaved virion-associated form of gB based on the presence of M1 MAb reactivity. The presence of the FLAG epitope at this position has no effect on the processing, half-life, or subcellular distribution of HCMV gB (10). U373 cells were coinfected with adenoviruses expressing recombinant gB and tTa. At day 2 p.i., cells were fixed and stained as described above. Recombinant gB (Fig. 2) had a vesicular perinuclear staining pattern that colocalized with AP-1 comparable to that observed for gB expressed during HCMV infection. This comparable localization of recombinant- and HCMV-expressed gB demonstrates that HCMV gB contains all of the cis-acting motifs necessary to mediate localization to the Golgi or TGN compartment.
FIG. 2.
HCMV gB contains cis-acting trafficking information necessary to mediate TGN localization. U373 cells were coinfected with adenoviruses expressing recombinant gB and tTa. At day 2 p.i., cells were fixed and stained as described above. Recombinant gB had a vesicular perinuclear staining pattern that colocalized with AP-1 comparable to that observed for gB expressed during HCMV infection. This comparable localization of recombinant- and HCMV-expressed gB demonstrates that HCMV gB contains all of the cis-acting motifs necessary to mediate localization to the Golgi or TGN compartment.
BFA treatment results in the redistribution of HCMV gB to the MTOC characteristic of a TGN-localized protein.
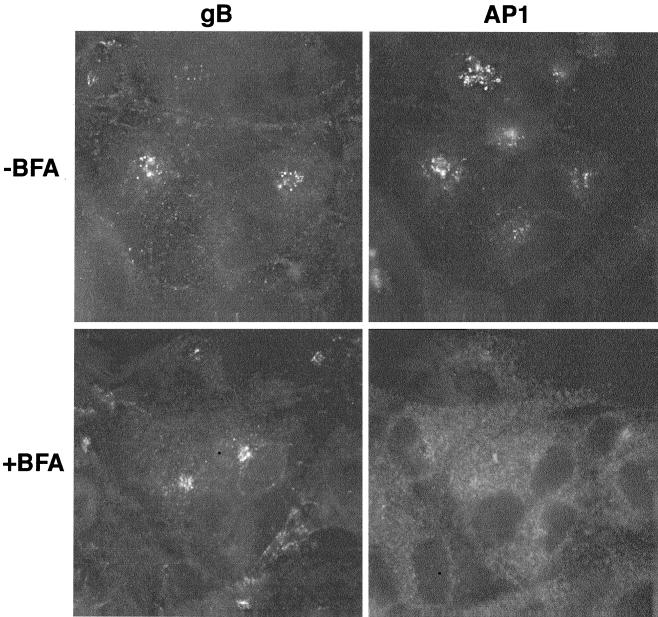
Within the cell, the Golgi and TGN compartments are closely juxtaposed. To distinguish which compartment was the site of gB localization, we assessed the effect of BFA on gB localization; BFA is a fungal metabolite that can be used to distinguish between the TGN and earlier Golgi compartments by its ability to redistribute pre-TGN localized proteins to the endoplasmic reticulum and TGN proteins to the microtubule-organizing center (MTOC). U373 cells expressing recombinant gB were incubated with BFA for 1 h prior to fixation and staining. In the absence of BFA, recombinant gB displayed the normal vesicular perinuclear distribution which overlapped with AP-1. However, in the presence of BFA, gB and AP-1 were redistributed to the MTOC and cytoplasm, respectively, demonstrating that HCMV gB is localized to the TGN compartment (Fig. 3); the γ-adaptin recognized by AP-1 is a cytosolic component of the cellular secretory machinery that associates with the cytosolic face of the TGN. This interaction is inhibited by BFA resulting in redistribution of γ-adaptin into the cytoplasm.
FIG. 3.
BFA treatment results in the redistribution of HCMV gB to the MTOC characteristic of a TGN-localized protein. To distinguish which compartment was the site of gB localization, we assessed the effect of BFA on gB localization. U373 cells expressing recombinant gB were incubated with BFA for 1 h prior to fixation and staining. In the absence of BFA, recombinant gB displayed the normal vesicular perinuclear distribution which overlapped with AP-1. However, in the presence of BFA, gB and AP-1 were redistributed to the MTOC and cytoplasm, respectively, demonstrating that HCMV gB is localized to the TGN compartment.
HCMV gB recycles between the TGN and the cell surface.
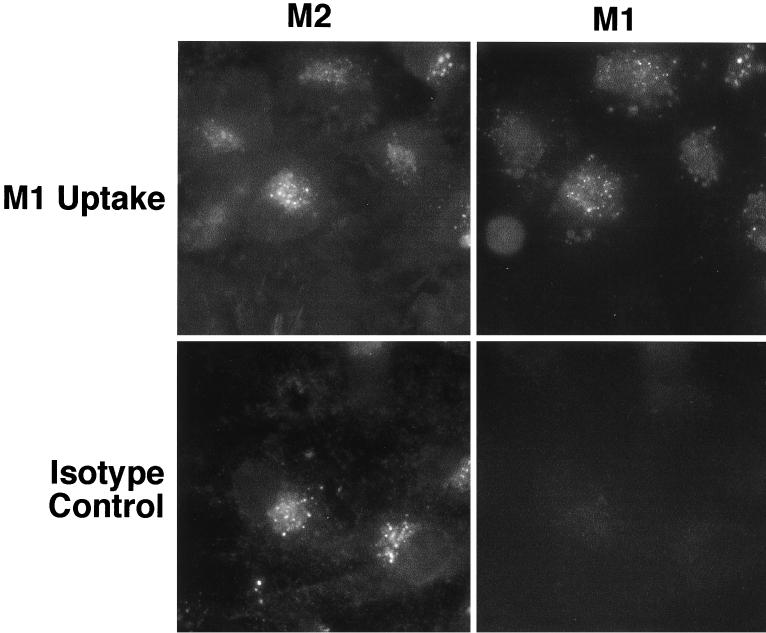
Localization of proteins to the TGN can be mediated either by static retention within this compartment or by a retrieval process of proteins from the cell surface (1, 2, 12, 26). To determine whether retrieval from the cell surface played a role in mediating TGN localization of gB, we performed antibody uptake experiments. Briefly, U373 cells expressing recombinant gB were incubated with MAb M1 for a period of 4 h prior to fixation and staining. The gB that cycled to the cell surface and had been retrieved into the cytoplasm was then visualized using an M1 isotype-specific secondary antibody. Total gB was visualized by incubation of fixed cells with MAb M2 and the respective isotype-specific antibody. As shown in Fig. 4, cells expressing recombinant gB that had been incubated with MAb M1 prior to fixation showed a vesicular perinuclear distribution of M1 reactivity (cell surface-retrieved gB) that was superimposable upon the postfixed M2 staining pattern (total gB). These results demonstrate that retrieval from the cell surface plays a role in the TGN localization of HCMV gB.
FIG. 4.
HCMV gB recycles between the TGN and the cell surface. To determine whether retrieval from the cell surface played a role in mediating TGN localization of gB, we performed antibody uptake experiments. U373 cells expressing recombinant gB were incubated with MAb M1 for a period of 4 h prior to fixation and staining. The gB that cycled to the cell surface and had been retrieved into the cytoplasm was then visualized using an M1 isotype-specific secondary antibody. Total gB was visualized by incubation of fixed cells with MAb M2 and the respective isotype-specific antibody. Cells expressing recombinant gB that had been incubated with MAb M1 prior to fixation show a vesicular perinuclear distribution of M1 reactivity (cell surface-retrieved gB) that was superimposable upon the postfixed M2 staining pattern (total gB). These results demonstrate that retrieval from the cell surface plays a role in the TGN localization of HCMV gB.
Retrieval of gB from the cell surface represents a major pathway for transport of gB to the site of virus assembly.
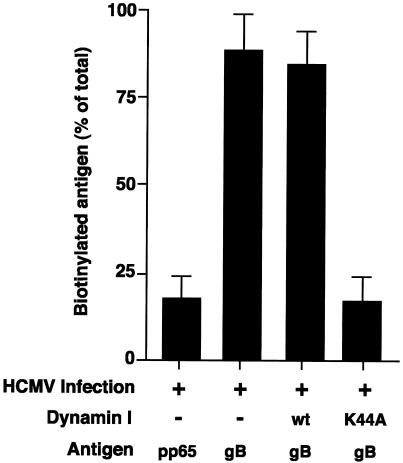
In HCMV-infected HF cells, the majority of gB undergoes endocytosis from the cell surface prior to incorporation into the virion (14). To determine whether gB retrieved from the cell surface was similarly incorporated into the virion in U373 cells, surface biotinylation experiments were performed as described previously (7). As shown in Fig. 5, at day 3 p.i. the majority of gB retrieved from the cell surface during a 30-min biotinylation period was incorporated into virus particles in HCMV-infected U373 cells. The ability of an adenovirus-expressed dominant-negative dynamin I mutant (dynK44A) to block biotinylated gB incorporation into virus particles demonstrated that the biotinylated protein was being retrieved specifically from the cell surface (endocytosis of HCMV gB is clathrin dependent, and we have shown previously that the process can be efficiently blocked by dynK44A [7]); the low level of biotinylated gB found in the virion in the presence of the dynamin I block is at a “background” level comparable to the level of biotinylation that was observed for a cytosolic non-surface-expressed HCMV protein, pp65. These biotinylation studies show that the majority of gB in HCMV-infected U373 cells is transported to the site of virus assembly by retrieval from the cell surface, as has been observed previously for gB in HCMV-infected HF cells (14).
FIG. 5.
HCMV gB is transported to the site of virus assembly by retrieval from the cell surface in HCMV-infected U373 cells. At 3 days p.i., HCMV-infected U373 tTa cells (MOI = 10) were pulse-labeled with [35S]methionine and [35S]cysteine (20 μCi/ml) for 1 h, surface biotinylated at 4°C for 30 min, and chased at 37°C for 2 days. Virus was then harvested from cultures (supernatant and cell associated), and total gB or pp65 (control) was immunoprecipitated from virus lysates. Immunoprecipitated antigen was eluted, and a fraction of eluate was removed to enable quantitation of total viral gB or pp65. Biotinylated antigen was recovered from the remaining eluate by a second round of precipitation. Protein fractions were analyzed by SDS-PAGE. The level of biotinylated gB or pp65 present in the virion is presented as percentage of total virion-associated antigen. The dynK44A-mediated block of biotinylated gB incorporation into the virus particle shows that the biotinylated gB in the virion has been retrieved specifically from the cell surface.
The gB retrieval pathway is unnecessary for the production of normal levels of infectious virus progeny.
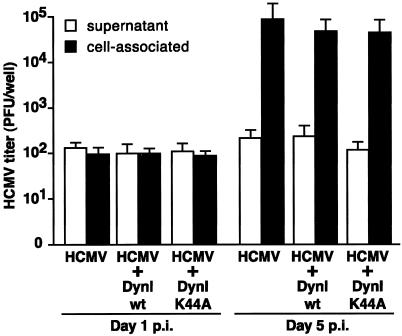
Together with the study by Radsak et al. (14), our demonstration that surface gB is incorporated into the virion in U373 cells suggests that a common “cell surface” retrieval pathway is used in a number of different cell types to direct gB to the site of virion assembly (14). To determine the role of the gB retrieval pathway for the production of infectious HCMV progeny, we assessed the effect of dynK44A-mediated inhibition of endocytosis on virus production in HCMV-infected U373 cells. U373 tTa cells were coinfected with HCMV and either an adenovirus expressing dynWT or an adenovirus expressing dynK44A. Cell lysates or supernatants were prepared from the coinfected U373 cells at day 5 p.i. and assayed for the production of cell-associated or extracellular virus by plaque assay. As shown in Fig. 6, inhibition of gB endocytosis did not affect the levels of infectious virus produced in cell-associated or supernatant fractions. These results suggest that, although gB can be retrieved from the cell surface for incorporation within the virion, this pathway is not necessary for the production of normal levels of infectious virus progeny.
FIG. 6.
The gB retrieval from the cell surface is not necessary for the production of normal levels of infectious virus progeny. U373 tTa were coinfected with HCMV (MOI = 10) and either the adenovirus expressing dynWT or dynK44A (MOI = 10). Cell lysates and supernatants were prepared from the coinfected U373 cells at either day 1 or day 5 p.i. and then assayed for the production of cell-associated or extracellular virus using a standard plaque assay. Inhibition of gB endocytosis did not affect the levels of infectious virus produced in cell-associated or supernatant fractions. These results demonstrate that, although gB can be retrieved from the cell surface for incorporation within the virion, this pathway is not necessary for the production of normal levels of infectious virus progeny.
DISCUSSION
We have shown that gB is localized to the TGN during HCMV infection of U373 and NHDF cells. gB was able to accumulate in the TGN in the absence of other HCMV proteins, demonstrating that gB contains all of the cis-acting elements necessary to direct TGN accumulation. Further investigation into the gB trafficking itinerary revealed that gB was localized to the TGN, at least in part, by a mechanism of retrieval from the cell surface. This endocytic pathway was shown to direct gB from the cell surface to a cytoplasmic site of virion assembly in HCMV-infected cells. However, gB endocytosis was not required for the production of normal levels of infectious virus progeny, indicating that sufficient levels of gB can be transported to the site of virion assembly via a direct trafficking pathway without requiring retrieval of the protein from the cell surface.
These results reveal a startling similarity between the intracellular trafficking of HCMV gB and the trafficking of the major envelope proteins of the alphaherpesviruses (PRV, VZV, and HSV). Presumably, this similarity reflects a requirement of these viruses to target components of the virion to the site of virus assembly and suggests a similarity between the assembly process of HCMV and other herpesviruses. Similar to gE of HSV and VZV and to Us9 of PRV, the colocalization of HCMV gB with markers of the TGN (AP-1 and TGN46) combined with the demonstrated relocalization of gB to the MTOC after BFA treatment shows that gB is localized to the TGN or closely juxtaposed region (1-3). In the alphaherpesviruses, in which the virion assembly process has been better defined, the accumulation of gE and Us9 in the TGN corresponds with the proposed site of final envelopment of these viruses in the TGN (1, 2, 5, 8, 9, 16, 24, 25, 27). Consequently, the accumulation of HCMV gB in the TGN suggests that the TGN may also be a major site of assembly for HCMV. Furthermore, the localization of HCMV gB to the TGN in two distinct cell types indicates that the TGN may be a site of HCMV assembly that is common to many, if not all, HCMV permissive cell types.
Our proposal that the TGN localization of HCMV gB reflects the targeting of this protein to a TGN site of virus assembly is consistent with results from a recent study by Sanchez et al. (15). In this earlier study, critical virion tegument proteins (pp28, pp65, and pp150) and glycoproteins (gB and gH) accumulated in an intracellular region that contained high levels of infectious virus and displayed considerable colocalization with TGN46. However, a complete colocalization of virion components with TGN46 was not observed, which was hypothesized to result from exclusion of normal cellular glycoproteins from regions of the TGN by the large amounts of virion glycoproteins. This alteration in the morphology of the TGN has also been observed in VZV-infected cells and is thought to be due to the establishment of subdomains within the TGN that are relatively enriched in virion compared to cellular glycoproteins. This asymmetrical redistribution of viral and cellular glycoproteins within the TGN is believed to be critical for the correct assembly and subsequent release of the mature VZV virion particle (23). During HCMV infection of both NHDF and U373 cells, we consistently observed an expansion of the TGN from the normal ribbon-like staining pattern of TGN46 in uninfected cells to a more expanded mesh-like morphology after infection (Fig. 1). In addition, the staining intensity of TGN46 was frequently diminished in infected cells, which may be a consequence of dispersion of TGN46 in a TGN that has been enlarged by the incorporation of high levels of virion glycoproteins. Whether this alteration in the TGN46 staining pattern corresponds to a segregation of viral from cellular glycoproteins within the TGN awaits the necessary immunoelectron microscopic analysis. Alternatively, the change in TGN morphology may be induced by virion factors that modify the secretory system to facilitate HCMV assembly. Our preliminary studies have shown that HCMV infection alters the normal function of components of the cellular trafficking machinery (including AP-1 and β-COP) (data not shown). Although the biological significance of these alterations for the replication of HCMV is currently unclear, their occurrence does indicate that HCMV modulates the cellular secretory system.
The ability of recombinantly expressed gB to mediate its own localization to the TGN demonstrates that, similar to VZV and HSV gE and to PrV Us9, HCMV gB contains all of the cis-acting elements necessary for TGN localization. Inspection of the HCMV gB cytoplasmic domain for the presence of trafficking motifs that are known to function in these other viral envelope proteins reveals a number of motifs that may be involved in the intracellular trafficking of HCMV gB. An acidic cluster (AC) motif has been shown to play a critical role in mediating endocytosis and TGN localization of all of the alphaherpesvirus envelope proteins studied (1-3, 18, 27). This motif appears to direct TGN localization by binding to a cellular connector protein, PACS-1, which connects the glycoprotein to the AP-1 complex (22). In HCMV gB, although the ability of the AC motif to mediate TGN localization of HCMV gB has not been investigated, the motif has been shown to modulate endocytosis in a number of different cell types (7, 21). Furthermore, our preliminary studies have shown that the HCMV gB AC does bind to PACS-1 in vitro, suggesting that this motif may be important for the accumulation of gB in the TGN (data not shown). In alphaherpesviruses, additional tyrosine- and dileucine-based motifs have also been shown to function in protein trafficking (1-3, 18, 27). Although function has not been demonstrated, the HCMV gB cytoplasmic domain does contain a number of similar motifs that may be involved in trafficking, and we are currently using a site-directed mutagenesis approach to assess the function of these motifs in gB trafficking.
Our results from the antibody uptake studies demonstrate that HCMV gB is localized to the TGN, at least in part, by a process of recycling from the cell surface. This dynamic recycling between the TGN and cell surface via endosomal intermediates is another similarity shared between the trafficking of HCMV gB and envelope proteins of the alphaherpesviruses. Previously, endocytosis was shown to target HCMV gB to a cytoplasmic site of virus assembly in HF cells (20), and in the present study we show that this endocytic pathway can similarly target gB to the site of virus assembly in HCMV-infected U373 cells. However, the inability of the dynK44A-mediated inhibition of endocytosis to decrease levels of infectious progeny produced during HCMV infection of U373 cells indicates that sufficient levels of gB can be transported to the site of virus assembly in the absence of gB retrieval from the cell surface. This observation that endocytosis plays a minimal role, if any, in viral biogenesis is consistent with studies of other herpesviruses. In PRV, gC is not retrieved from the cell surface (where the protein accumulates) but is incorporated at high levels into the virus envelope (19). Additionally, biotinylation studies have shown that, although PRV gE is retrieved from the cell surface, this population of gE molecules is not incorporated within the virion (19), and in recombinant PRV expressing either gE or UL9 proteins defective in endocytosis the mutant proteins are effectively incorporated within the virion and the viruses replicate at normal levels (3, 19). Consequently, the redundancy of the glycoprotein “cell surface” retrieval pathway for virus biogenesis may be a further characteristic of the assembly pathway shared by many of the herpesviruses.
In summary, we have shown that HCMV gB, like alphaherpesvirus gE and Us9, accumulates in the TGN. Since the site of glycoprotein accumulation corresponds to the site of virus envelopment in alphaherpesviruses, this accumulation of gB in the TGN strongly suggests that HCMV may undergo final envelopment in the TGN. Similar to the alphaherpesviruses, TGN localization of HCMV gB was mediated, at least in part, by a process of retrieval from the cell surface. However, the endocytosis of HCMV gB from the cell surface was not required for the production of normal levels of infectious virus. This observation indicates that, as observed for the envelope proteins of the alphaherpesviruses, sufficient levels of HCMV gB can be achieved at the site of virus assembly in the absence of the gB retrieval pathway.
Acknowledgments
We thank Bill Britt and Sean Molloy for their thoughtful advice throughout this study. We are also grateful to Aurelie Snyder at the OHSU Core Laboratory Facility for technical support and to Andrew Townsend at Extreme Images for assistance with graphic illustrations.
This work was supported by research grants from the National Institutes of Health (AI10418 and AI21460 [M.A.J. and J.A.N.] and AI48585 [G.T.]).
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brideau, A. D., T. del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Britt, W. J., and C. A. Alford. 1996. Cytomegalovirus, p. 2493-2523. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 5.Browne, H., S. Bell, T. Minson, and D. W. Wilson. 1996. An endoplasmic reticulum-retained herpes simplex virus glycoprotein H is absent from secreted virions: evidence for reenvelopment during egress. J. Virol. 70:4311-4316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Eggers, M., E. Bogner, B. Agricola, H. F. Kern, and K. Radsak. 1992. Inhibition of human cytomegalovirus maturation by brefeldin A. J. Gen. Virol. 73:2679-2692. [DOI] [PubMed] [Google Scholar]
- 7.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gershon, A. A., D. L. Sherman, Z. Zhu, C. A. Gabel, R. T. Ambron, and M. D. Gershon. 1994. Intracellular transport of newly synthesized varicella-zoster virus: final envelopment in the trans-Golgi network. J. Virol. 68:6372-6390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Granzow, H., F. Weiland, A. Jons, B. G. Klupp, A. Karger, and T. C. Mettenleiter. 1997. Ultrastructural analysis of the replication cycle of pseudorabies virus in cell culture: a reassessment. J. Virol. 71:2072-2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jean, F., L. Thomas, S. S. Molloy, G. Liu, M. A. Jarvis, J. A. Nelson, and G. Thomas. 2000. A protein-based therapeutic for human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 97:2864-2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mocarski, E. S. 1996. Cytomegalovirus and their replication, p. 2447-2492. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 12.Molloy, S. S., L. Thomas, J. K. VanSlyke, P. E. Stenberg, and G. Thomas. 1994. Intracellular trafficking and activation of the furin proprotein convertase: localization to the TGN and recycling from the cell surface. EMBO J. 13:18-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponnambalam, S., C. Rabouille, J. P. Luzio, T. Nilsson, and G. Warren. 1994. The TGN38 glycoprotein contains two non-overlapping signals that mediate localization to the trans-Golgi network. J. Cell Biol. 125:253-268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hubinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from the infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez, V., K. D. Greis, E. Sztul, and W. J. Britt. 2000. Accumulation of virion tegument and envelope proteins in a stable cytoplasmic compartment during human cytomegalovirus replication: characterization of a potential site of virus assembly. J. Virol. 74:975-986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Skepper, J. N., A. Whiteley, H. Browne, and A. Minson. 2001. Herpes simplex virus nucleocapsids mature to progeny virions by an envelopment → deenvelopment → reenvelopment pathway. J. Virol. 75:5697-5702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Streblow, D. N., C. Soderberg-Naucler, J. Vieira, P. Smith, E. Wakabayashi, F. Ruchti, K. Mattison, Y. Altschuler, and J. A. Nelson. 1999. The human cytomegalovirus chemokine receptor US28 mediates vascular smooth muscle cell migration. Cell 99:511-520. [DOI] [PubMed] [Google Scholar]
- 18.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tooze, J., M. Hollinshead, B. Reis, K. Radsak, and H. Kern. 1993. Progeny vaccinia and human cytomegalovirus particles utilize early endosomal cisternae for their envelopes. Eur. J. Cell Biol. 60:163-178. [PubMed] [Google Scholar]
- 21.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wan, L., S. S. Molloy, L. Thomas, G. Liu, Y. Xiang, S. L. Rybak, and G. Thomas. 1998. PACS-1 defines a novel gene family of cytosolic sorting proteins required for trans-Golgi network localization. Cell 94:205-216. [DOI] [PubMed] [Google Scholar]
- 23.Wang, Z. H., M. D. Gershon, O. Lungu, Z. Zhu, S. Mallory, A. M. Arvin, and A. A. Gershon. 2001. Essential role played by the C-terminal domain of glycoprotein I in envelopment of varicella-zoster virus in the trans-Golgi network: interactions of glycoproteins with tegument. J. Virol. 75:323-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Whealy, M. E., J. P. Card, R. P. Meade, A. K. Robbins, and L. W. Enquist. 1991. Effect of brefeldin A on alphaherpesvirus membrane protein glycosylation and virus egress. J. Virol. 65:1066-1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Whiteley, A., B. Bruun, T. Minson, and H. Browne. 1999. Effects of targeting herpes simplex virus type 1 gD to the endoplasmic reticulum and trans-Golgi network. J. Virol. 73:9515-9520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong, S. H., S. H. Low, and W. Hong. 1992. The 17-residue transmembrane domain of β-galactoside α2,6-sialyltransferase is sufficient for Golgi retention. J. Cell Biol. 117:245-258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]