Abstract
Crude decoction, aqueous and ethanolic extracts of two medicinal plants (Psidium guajava and Diospyros mespiliformis), widely used in the central plateau of Burkina Faso to treat many diseases were evaluated for their antagonistic effects on caffeine induced calcium release from sarcoplasmic reticulum of rat skeletal muscle cells. These different extracts showed a decrease of caffeine induced calcium release in a dose dependent manner. Comparison of the results showed that Psidium guajava leaf extracts are more active than extracts of Diospyros mespiliformis and that crude decoctions show better inhibitory activity. The observed results could explaine their use as antihypertensive and antidiarrhoeal agents in traditional medicine, by inhibiting intracellular calcium release.
Keywords: Psidium guajava, Diospyros mespiliformis, Myrtaceae, Ebenaceae, Medicinal plants, Intracellular calcium, Sarcoplasmic reticulum, Caffeine
INTRODUCTION
In Africa, people use natural resources for medicinal purposes. Psidium guajava and Diospyros mespiliformis are medicinal plants used in tropical and subtropical countries to treat many disorders such as diarrhoea, cough and gastrointestinal disorders.
It was reported that Psidium guajava leaf extract has a wide spectrum of biological activities such as anticough, antibacterial, haemostasis (Jaiarj et al., 1999; 2000), antidiarrhoeal and narcotic properties (Lozoya et al., 1990), and antioxidant properties (Qian and Nihorimbere, 2004).
According to Lutterodt and Maleque (1988) and Meckes et al.(1996), the leaf extract is used to treat diarrhoea, abdominal pain, convulsions, epilepsy, cholera, insomnia and has hypnotic effect.
Some studies reported that the leaf extract and its derivative identified as quercetin has effect on the intracellular calcium levels in gastrointestinal smooth muscle (Lutterodt, 1989; Lozoya et al., 1990), in cardiac muscle cell (Morales et al., 1994; Apisariyakul et al., 1999) and in neuromuscular junction (Re et al., 1999; Chaichana and Apisariyakul, 1996).
More than twenty identified compounds from Psidium guajava leaf have been reported in (Seshadri and Vasishta, 1965; Osman et al., 1974; Lutterodt and Maleque, 1988). The major components are: β-selinene, β-caryophyllene, caryophyllene oxide, squalene, selin-11-en-4α-ol (Meckes et al., 1996), guaijavarin, isoquercetin, hyperin, quercitrin and quercetin-3-O-gentobioside (Lozoya et al., 1994), morin-3-O-α-L-lyxopyranoside and morin-3-O-α-L-arabopyranoside (Arima and Danno, 2002), β-sitosterol, uvaol, oleanolic acid and ursolic acid (Begum et al., 2004).
Our recent phytochemical screening of Psidium guajava leaf showed tannins in aqueous extract, anthocyans, alkaloids, flavonoids, tannins and steroids/terpenoids in ethanolic extract.
Two studies showing that Diospyros mespiliformis exhibits antimicrobial activity (Adeniyi et al., 1996; Sanogo et al., 1998) have been reported. Anthraquinones, tannins, triterpene, saponins, steroids and sugars in the leaf extract of this plant had been reported (Adeniyi et al., 1996).
According to our recent phytochemical screening of Diospyros mespiliformis, flavonoids, tannins and saponosides were found in aqueous extract, anthocyans, flavonoids tannins and steroids/terpenoids exists in ethanolic extract.
Psidium guajava and Diospyros mespiliformis are used to treat many diseases, especially hypertension and diarrhoea in the central plateau of Burkina Faso (1Nacoulma-Ouédraogo, 1996). Indeed, in developing countries, hypertension is now considered as a cause of mortality in adults. In spite of the presence of known antihypertensive modern medicines in the pharmaceutical market, among medicinal plants used to treat this disease are Psidium guajava and Diospyros mespiliformis. Few of these plants are biologically and chemically investigated in order to determine their effectiveness and active constituents. Some reports indicated that the extracts of these plants could interfere with calcium influx and/or calcium release from an intracellular store. So the study was done in mammalian skeletal muscle cells in culture (Belemtougri et al., 2001). Skeletal muscle cells have well characterized calcium release processes which could be the target of these plant components.
The present paper reports the inhibition of calcium release from sarcoplasmic reticulum by these plant leaves extracts on rat skeletal muscle cells. This could indicate one mechanism underlying their actions.
MATERIALS AND METHODS
Plant collection
Fresh leaves of Psidium guajava and Diospyros mespiliformis were collected from Gampéla (Burkina Faso, West Africa) in July 1997. The plants were authenticated by Prof. Millogo-Rasolodimby, Department of Botany, University of Ouagadougou. The herbarium specimens are deposited in this Department.
Preparation of plant extracts
Crude decoctions were prepared from the shade dried leave powder. Six grams of leaf powder of each plant were macerated in deionized water for 24 h at room temperature and then boiled for 10 min to mimic the traditional preparation methods. After cooling, the resulting extract was filtered through Whatman n° 2 filter and evaporated to give crude extracts.
Forty grams of leaf powder of each plant were successively extracted with ethanol and deionized water to give ethanolic and aqueous extracts (Belemtougri et al., 2001).
On the other hand, the results of screening the compounds summarized in Tables 1 and 2 were obtained according to 2Samaté (2001). Briefly 1 g of each plant leaf powder was macerated in 10 ml of chlorhydric acid or 5% phosphoric acid. The alkaloids were detected using Mayer and Dragendorff method followed by thin layer chromatography. Steroids/terpenoids, phenolic components (tannins and flavonoids) were determined using Liebermann-Burchard method. The infusion previously prepared becomes blue when it is mixed with ferric chloride indicating the presence of tannins while it is red when mixed with vanillic chloride, indicating the presence of flavonoids.
Table 1.
Summary of results after chemical screening of Psidium guajava leaf extracts
| Extracts | Anthocyans | Anthracenosides | Alkaloids | Flavonoids | Quinones | Tannins | Steroids/Terpenoids | Saponosides |
| Aqueous extract | − | − | − | − | − | + | − | − |
| Ethanolic extract | + | − | + | + | − | + | + | − |
| Dichloromethanolic extract | − | − | + | − | − | − | + | − |
+: Presence; −: Absence
Table 2.
Summary of results after chemical screening of Diospyros mespiliformis leaf extracts
| Extracts | Anthocyans | Anthracenosides | Alkaloids | Flavonoids | Quinones | Tannins | Steroids/Terpenoids | Saponosides |
| Aqueous extract | − | − | + | − | − | + | − | + |
| Ethanolic extract | + | − | + | − | − | + | + | − |
| Dichloromethanolic extract | ND | − | − | ND | ND | − | + | ND |
+: Presence; −: Absence; ND: Not determined
Cell culture
Primary cultures of rat skeletal muscle cells were initiated from neonatal progenitors. These myogenic cells were obtained by trypsinisation of small muscle pieces of 1 to 2 day-old rat hindlimbs (Cognard et al., 1993a). Briefly, the cells were maintained for 96 h in growth medium (Ham F12, Gibco) supplemented with 10% fetal calf serum (Boehringer, Mannheim, Germany), 10% heat-inactivated horse serum (Gibco) and 1% antibiotics: (Penicillin G 100 U/ml, Sigma and Streptomycin 50 U/ml, Sigma).
The growth medium was exchanged for a fusion medium constituted by Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 5% heat-inactivated horse serum and 1% antibiotics. This medium exchange was considered as time zero to age differentiated cells in culture.
Experiments were carried out on 3 to 5 day-old myotubes after induction of fusion, at stages exhibiting well characterized calcium release processes.
Intracellular free calcium measurements
Intracellular free calcium was measured with the fluorescent dye Indo-1/AM (Sigma Chemical Co., St. Louis, MO, USA). Cells were loaded with 3 μmol/L of the lipophilic form of Indo-1 (dissolved in dimethylsulfoxide) diluted in control solution. The cells were incubated for 45 min in the dark at room temperature, washed with control solution and incubated 10 min at 37 °C to obtain complete de-esterification of the probe. Fluorescence was recorded at room temperature using an OSP100 CA microscopic photometry device (Olympus).
Excitation of Indo-1 was set in UV range (350 nm) by means of a xenon lamp. Fluorescence emission was acquired through a dichroic filter (455 nm) by means of two photomultiplier tubes with two band pass filters at 405 nm for the emission of the calcium bound form and at 485 nm for the calcium free form fluorescence of the probe.
Experimental solutions
The experiments were performed in a control solution containing (mmol/L): NaCl 130; KCl 5.4; CaCl2 2.5; MgCl2 0.8; Glucose 5.6; HEPES 10 and adjusted at pH 7.4 with Tris.
The test solutions were made by adding caffeine (final concentration 10 mmol/L) (caffeine solution) or adding 1 mg/ml to 10 mg/ml of the plant extracts to caffeine solution (caffeine+Psidium or Diospyros solution). They were added to the control solution just before experiments. Rapid changes of the solutions in the surrounding of interrogated cells were achieved by means of a home made microperfusion system.
Statistical analysis
Data analysis was performed by means of Software Prism 3.0 (GraphPad Software, San Diego, CA, USA) and Origin 5.0 (Microcal Software Inc., Northampton, MA, USA). Results of the experiments were expressed as means±SEM and paired or unpaired Student’s t-test was used to test for significance difference between the means. P<0.05 was considered significant.
RESULTS
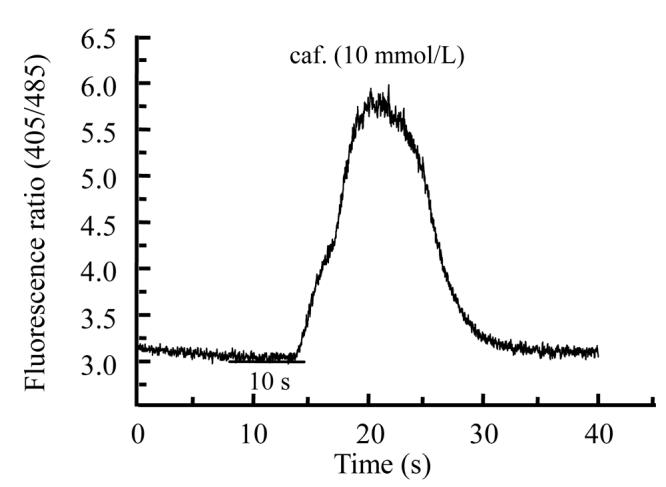
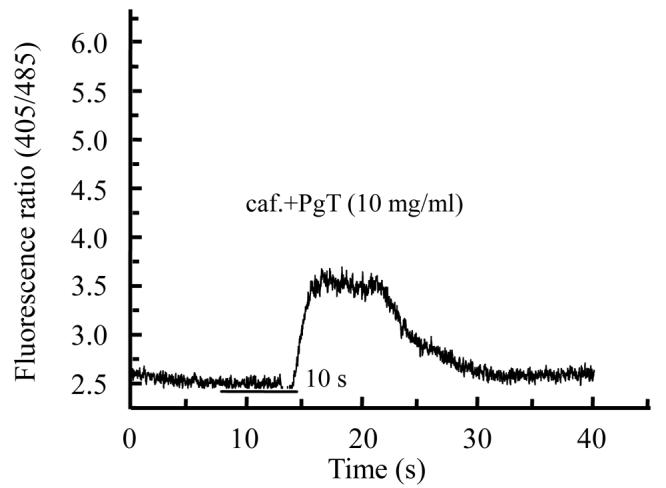
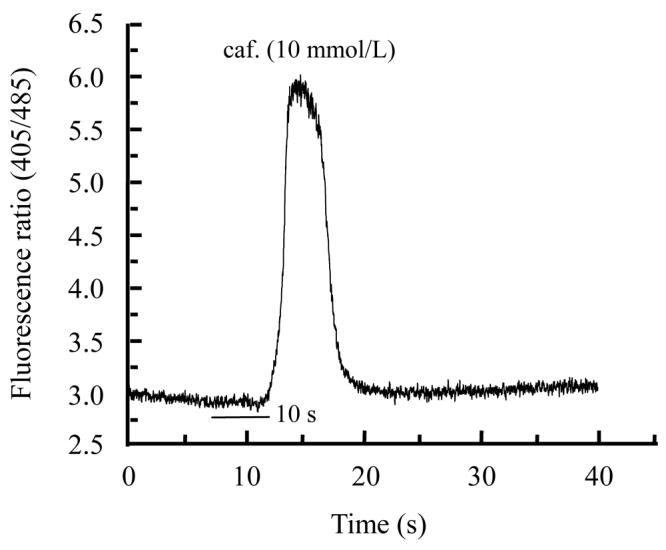
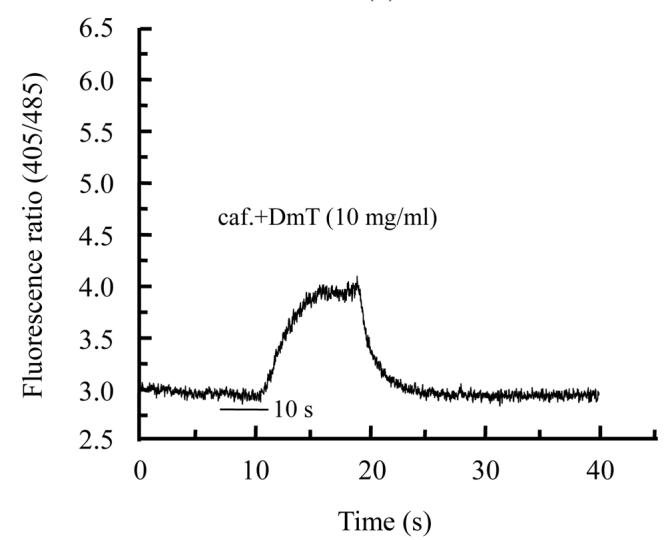
Different extracts of Psidium guajava and Diospyros mespiliformis failed to display any activity on rat skeletal muscle cells when applied alone (data not shown). This indicated their ineffectiveness on resting calcium in skeletal muscle cell. Then we used caffeinic solution to investigate some activity on intracellular calcium release from sarcoplasmic reticulum (SR). Caffeine was applied in order to directly stimulate calcium release through ryanodine receptor channels (Cognard et al., 1993b). Application of caffeine (10 mmol/L) on myotubes induced Ca2+ release from SR. For each cell 10 mmol/L caffeine solution was applied and the response was considered as control (Fig.1a) and another was interrogated with caffeine+plants extracts. As illustrated in Fig.1b and Fig.2b, crude extracts of Psidium guajava and Diospyros mespiliformis at 10 mg/ml decreased the amplitude of Ca2+ release from SR compared with the controls. This indicated that the extracts have significant antagonistic effect on caffeine sensitive Ca2+ release from SR.
Fig. 1.
Effects of caffeine (a) and caffeine plus Psidium guajava crude leaf extract (b) on intracellular calcium release from sarcoplasmic reticulum
Fig. 2.
Effects of caffeine (a) and caffeine plus Diospyros mespiliformis crude leaf extract (b) on intracellular calcium release from reticulum sarcoplasmic
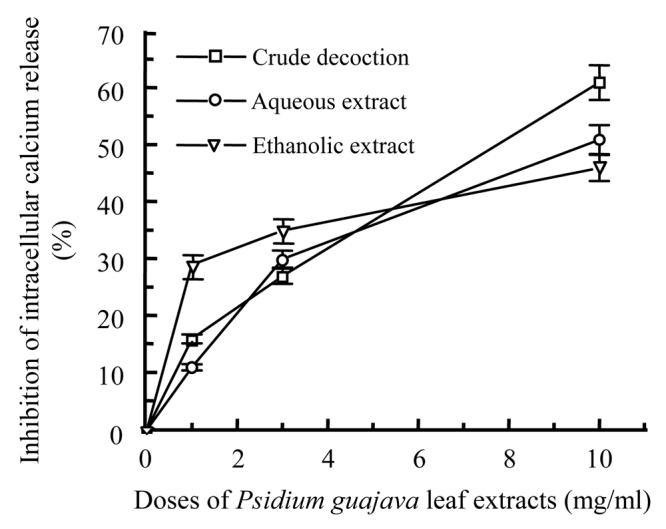
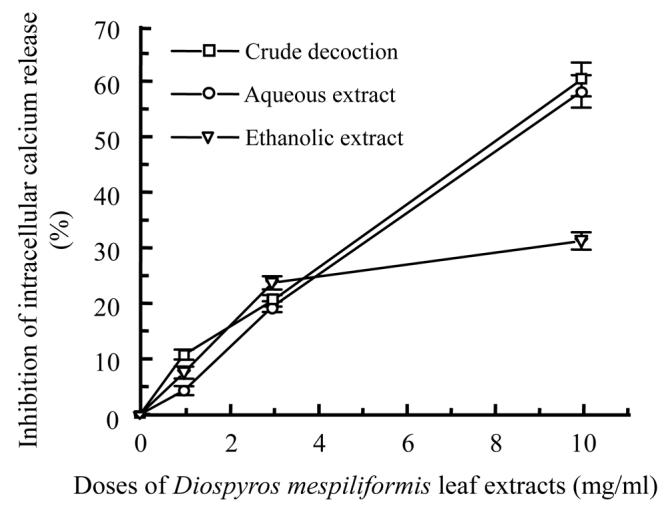
Different extracts of the two plants showed inhibition on intracellular calcium release in dose dependent manner; the crude decoctions were the most active. At 10 mg/ml, crude decoction of Psidium guajava (Fig.3a) was more active than that of Diospyros mespiliformis (Fig.3b).
Fig. 3.
Percentage inhibition of intracellular calcium release from sacroplasmic reticulum by different leaf extracts of Psidium guajava (a) and Diospyros mespiliformis (b) at different doses
At 10 mg/ml the action of different extracts of Psidium guajava could be classified as follows: crude decoction>aqueous extract>ethanolic extract; but those of Diospyros mespiliformis were: crude decoction>ethanolic extract>aqueous extract. At the same dose crude decoction of Psidium guajava showed 61% of inhibition with IC 50≈7.65 mg/ml and that of Diospyros mespiliformis showed 51% with IC 50≈8.84 mg/ml. However, ethanolic extract of Diospyros mespiliformis showed IC 50≈9.23 mg/ml but the other extracts of the two plants showed IC 50 more than 10 mg/ml.
It was interesting to observe that at low doses (3 mg/ml) ethanolic extract of Psidium guajava (Fig.3a) and aqueous extract of Diospyros mespiliformis (Fig.3b) were more active than other extracts.
The extract process used in this study yielded 7.7515 g of ethanolic extract and 1.2501 g of aqueous extract in Psidium guajava leaf extract of forty grams dry powder. Table 1 summarizes the different components of the different extracts of Psidium guajava. The same procedure yielded 5.40 g of ethanolic extract and 1.328 g of aqueous extract in Diospyros mespiliformis leaf extract of forty grams dry powder. Table 2 summarizes the different components of the different extracts of Diospyros mespiliformis.
Ten mg/ml crude decoctions of Psidium and Diospyros showed 61% and 51% inhibition of intracellular calcium release, aqueous extracts exhibited 51% and 29% of inhibition and the last ones, ethanolic extracts showed 46% and 54% inhibition of calcium release from sarcoplasmic reticulum.
Considering the effectiveness of the different extracts, we can conclude that crude decoctions are more active than other extracts.
DISCUSSION
We examine here the effects of different leaf extracts of Psidium guajava and Diospyros mespiliformis on the caffeine induced intracellular calcium release from sarcoplasmic reticulum of rat skeletal muscle cells. Our different extracts failed to show any effect on resting calcium activity. This suggests no effects of crude extracts on transporters involved in handling of basal calcium activity. We utilized caffeine which is known to induce calcium release from SR following activation of ryanodine receptors (Zimanyi and Pessah, 1994). This release was measured by means of a fluorescent dye Indo-1/AM in this study.
The observed results showed that the different extracts tested were dose-dependently effective in the inhibition of calcium release induced by caffeine. The extracts of Psidium guajava were more active than those of Diospyros mespiliformis. The crude decoctions were more effective than other extracts. This difference could be due to several factors: polarity and chemical nature of the components, differential extraction, partition and quantity of active ingredients present in the different extracts. The crude decoctions appeared to contain the total water soluble active compounds which have synergistic effects on inhibition of calcium release. It suggested that one or several compounds could inhibit ryanodine-sensitive calcium release channels from SR.
The chemical composition of these plants reported in literature showed the presence of various components, especially tannins, flavonoids (quercetin) and triterpenoids (Osman et al., 1974). These results are in a good agreement with our phytochemical screening.
The decrease in the calcium release from SR to myoplasm could be explained by interactions of the extracts with different elements involved in the regulation of calcium release, for example the ryanodine receptors. Our extracts could increase Mg2+ in the cytoplasm and it is well known that this ion has powerful inhibitory effect on calcium release in skeletal muscle (Lamb, 2000). It was also reported that extracts of Psidium guajava could block the L-type calcium membrane channels (Conde-Garcia et al., 2003). Here, this pathway was not explored since depolarizing stimuli were not used, but direct activation of calcium release from SR.
Flavonoids existing in the extracts could play an important role and are phenolic compounds widely distributed in the plant kingdom and have several pharmacological properties such as spasmolytic (Havsteen, 1983; Capasso et al., 1991) and antidiarrhoeal (Tona et al., 1999) activities. Flavonoids have been reported to have free radical scavenger properties (Bharani et al., 1995). van Acker et al.(1995) reported that flavonoids are antioxidants found usually in plants, fruits and vegetable and are known to be excellent scavengers of free radicals and accordingly used in the treatment of vascular endothelial damage and diseases of vascular wall involving inflammation. According to Lutterodt (1989), Morales et al.(1994), Lozoya et al.(1994) and Tona et al.(1999), some flavonoids isolated from Psidium guajava leaves exhibited spasmolytic and antispasmodic activities. It was also shown that flavonoids inhibited certain mammalian enzyme systems such as PKC and PLC and then could reduce Ca2+ release in cytoplasm and lead to muscle relaxation.
It has been reported that quercetin, the main flavonoid of Psidium guajava leaves had many pharmacological activities. According to Alikaridis (1987) and Yuting et al.(1990), quercetin isolated from Ilex species has free radical scavenging activity and lipid antiperoxidation activity, and may relax intact aortic rings (Muccillo Baisch et al., 1998).
Formica and Regelson (1995) reported that quercetin exhibited antioxidant and spasmolytic effects. It induced relaxation of cardiovascular smooth muscle (Duarte et al., 1993; Formica and Regelson, 1995) which could lead to lower blood pressure in vivo. This justifies its use in the treatment of hypertension in the central plateau of Burkina Faso. It has been shown that quercetin has relaxant effects on vascular and intestinal smooth muscle (Duarte et al., 1993; Morales et al., 1994).
Furthermore, quercetin showed inhibition on skeletal muscles contraction (Chaichana and Apisariyakul, 1996; Apisariyakul et al., 1999).
According to Re et al.(1999), quercetin isolated from Psidium guajava leaf induced reduction of presynaptic molecular activity by modulating the cytosolic calcium concentration in mouse neuromuscular junction.
These biological activities of flavonoids and especially those of quercetin showed that flavonoids could play an important role in the inhibition of calcium release from SR.
On the other hand, tannins existing in aqueous and ethanolic extracts of these two plants could also play an important role. It has been reported that they have free radical scavenger properties (Bharani et al., 1995) and antioxidant action (Simeray et al., 1982; Yoshizawa et al., 1987).
Tannins have been reported to have several pharmacological activities such as spasmolytic activity in smooth muscle cells (Tona et al., 1999).
According to Owen and Johns (1999), tannins have protein-binding properties and can interfere with many substances. It was suggested that tannins could reduce the intracellular calcium level by a decrease in the calcium inward current and/or by activation of the calcium pumping system (Chiesi and Schwaller, 1994). Zhu et al.(1997) reported that tannins inhibited calcium channels and induced muscle relaxation.
With regard to these last properties, it can be suggested that tannins could act with proteins involved in the regulation of ryanodine receptors and partly block calcium release from SR in our assays.
Few studies have been reported on Diospyros mespiliformis biological activities. Antibacterial activity has been shown (Adeniyi et al., 1996; Sanogo et al., 1998). Other Diospyros species showed mainly antimalarial (Likhitwatayawuid et al., 1999), antiinflammatory (Recio et al., 1995), hypotensive (Funayama and Hikino, 1979) and oral antimicrobial (Cai et al., 2000) activities.
CONCLUSION
The results reported here showed that different leaf extracts of Psidium guajava and Diospyros mespiliformis inhibited caffeine induced calcium release from sarcoplasmic reticulum in a dose dependent manner. These results could explain their traditional use to treat many diseases. More investigations are in progress to isolate active compounds and vascular smooth muscle experiments will be done to verify their possible relaxant effects.
Acknowledgments
Thanks are due to Prof. Samaté, Prof. Millogo-Rasolodimby and F Mazin for their helpful collaboration.
Footnotes
Nacoulma-Ouédraogo, O.G., 1996. Plantes médicinales et pratiques médicales traditionnelles au Burkina Faso: cas du plateau central. Thèse de Doctorat Es Sciences Naturelles, Université de Ouagadougou, tome II, p.285.
Samaté, A.D., 2001. Compositions chimiques d’huiles essentielles extraites de plantes aromatiques de la zone soudanienne du Burkina Faso: valorisation. Thèse de Doctorat Es Sciences Physiques, Université de Ouagadougou, p.164.
References
- 1.Adeniyi BA, Odelola HA, Oso BA. Antimicrobial potentials of Diospyros mespiliformis (Ebenaceae) Afr J Med Sci. 1996;25:221–224. [PubMed] [Google Scholar]
- 2.Alikaridis F. Natural constituents of Ilex species. J Ethnopharmacology. 1987;20:121–144. doi: 10.1016/0378-8741(87)90084-5. [DOI] [PubMed] [Google Scholar]
- 3.Apisariyakul A, Chaichana N, Takemura H. Dual effects of quercetin on contraction in cardiac and skeletal muscle preparations. Research Communications in Molecular Pathology and Pharmacology. 1999;105:129–138. [PubMed] [Google Scholar]
- 4.Arima H, Danno GI. Isolation of antimicrobial compounds from guava (Psidium guajava L.) and their structural elucidation. Biosci Biotechnol Biochem. 2002;66:1727–1730. doi: 10.1271/bbb.66.1727. [DOI] [PubMed] [Google Scholar]
- 5.Begum S, Hassan SI, Ali SN, Siddiqui BS. Chemical constituents from the leaves of Psidium guajava . Natural Product Research. 2004;18:135–140. doi: 10.1080/14786410310001608019. [DOI] [PubMed] [Google Scholar]
- 6.Belemtougri RG, Constantin B, Cognard C, Raymond G, Sawadogo L. Effects of Sclerocarya birrea (A. rich) hochst (anacardiaceae) leaf extracts on calcium signalling in cultured rat skeletal muscle cells. J Ethnopharmacology. 2001;76:247–252. doi: 10.1016/S0378-8741(01)00248-3. [DOI] [PubMed] [Google Scholar]
- 7.Bharani A, Ganguly A, Bhargava KD. Salutary effect of Terminalia arjuna in patients with severe refractory heart failure. Int J Cardiol. 1995;49:191–199. doi: 10.1016/0167-5273(95)02320-V. [DOI] [PubMed] [Google Scholar]
- 8.Cai L, Wei GX, van der Bijl P, Wu CD. Namibian chewing stick, Diospyros lycioides, contains antibacterial compounds against oral pathogens. J Agric Food Chem. 2000;48:909–914. doi: 10.1021/jf9909914. [DOI] [PubMed] [Google Scholar]
- 9.Capasso A, Pinto A, Mascolo N, Autore G, Capasso F. Reduction of agonist-induced contractions of guinea-pig isolated ileum by flavonoids. Phytother Res. 1991;5:85–87. [Google Scholar]
- 10.Chaichana N, Apisariyakul A. Cholinergic blocking effect of quercetin. Thai J Pharmacol. 1996;18:1–15. [Google Scholar]
- 11.Chiesi M, Schwaller R. Reversal of phospholamban-induced inhibition of cardiac sarcoplasmic reticulum Ca2+-ATPase by tannin. Biochemical and Biophysical Research Communications. 1994;202:1668–1673. doi: 10.1006/bbrc.1994.2126. [DOI] [PubMed] [Google Scholar]
- 12.Cognard C, Constantin B, Rivet-Bastide M, Imbert N, Besse C, Raymond G. Appearance and evolution of calcium currents and contraction during the early post-fusional stage of rat skeletal muscle cells developing in primary culture. Development. 1993;117:1153–1161. doi: 10.1242/dev.117.3.1153. [DOI] [PubMed] [Google Scholar]
- 13.Cognard C, Constantin B, Rivet-Bastide M, Raymond G. Intracellular calcium transients induced by different kinds of stimulus during myogenesis of rat skeletal muscle cells studied by laser cytofluorimetry with Indo-1. Cell Calcium. 1993;14:333–348. doi: 10.1016/0143-4160(93)90054-A. [DOI] [PubMed] [Google Scholar]
- 14.Conde-Garcia EA, Nascimento VT, Santiago-Santos AB. Inotropic effects of extracts of Psidium guajava L. (guava) leaves on the guinea pig atrium. Braz J Med Biol Res. 2003;36:661–668. doi: 10.1590/s0100-879x2003000500014. [DOI] [PubMed] [Google Scholar]
- 15.Duarte J, Pérez-Vizcaíno F, Zarzuelo A, Jiménez J, Tamargo J. Vasodilator effects of quercetin in isolated rat vascular smooth muscle. Eur J Pharmacol. 1993;239:1–7. doi: 10.1016/0014-2999(93)90968-N. [DOI] [PubMed] [Google Scholar]
- 16.Formica JV, Regelson W. Review of the biology of quercetin and related bioflavonoids. Food Chem Toxicol. 1995;33:1061–1080. doi: 10.1016/0278-6915(95)00077-1. [DOI] [PubMed] [Google Scholar]
- 17.Funayama S, Hikino H. Hypotensive principles of Diospyros kaki leaves. Chem Pharm Bull (Tokyo) 1979;27:2865–2868. doi: 10.1248/cpb.27.2865. [DOI] [PubMed] [Google Scholar]
- 18.Havsteen B. Flavonoids, a class of natural products of high pharmacological potency. Biochem Pharmacol. 1983;32:1141–1148. doi: 10.1016/0006-2952(83)90262-9. [DOI] [PubMed] [Google Scholar]
- 19.Jaiarj P, Khoohaswan P, Wongkrajang Y, Peungvicha P, Suriyawong P, Saraya MLS, Ruangsomboon O. Anticough and antimicrobial activities of Psidium guajava Linn. leaf extract. J Ethnopharmacology. 1999;67:203–212. doi: 10.1016/S0378-8741(99)00022-7. [DOI] [PubMed] [Google Scholar]
- 20.Jaiarj P, Wongkrajang Y, Thongpraditchote S, Peungvicha P, Bunyapraphatsara N, Opartkiattikul N. Guava leaf extract and topical haemostasis. Phytother Res. 2000;14:388–391. doi: 10.1002/1099-1573(200008)14:5〈388::AID-PTR638〉3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 21.Lamb GD. Excitation-contraction coupling in skeletal muscle: comparison with cardiac muscle. Clinical and Experimental Pharmacology and Physiology. 2000;27:216–224. doi: 10.1046/j.1440-1681.2000.03224.x. [DOI] [PubMed] [Google Scholar]
- 22.Likhitwatayawuid K, Dejadisai S, Jongbunprasert V, Krungkrai J. Antimalarials from Stephania venosa, Prismatomeris sessiliflora, Diospyros montana and Murraya siamensis . Planta Med. 1999;65:754–756. doi: 10.1055/s-2006-960858. [DOI] [PubMed] [Google Scholar]
- 23.Lozoya X, Bercerril G, Martinez M. Intraluminal perfusion model of in vitro guinea pig ileum as a model of study of the antidiarrheic properties of the guava (Psidium guajava) Arch Invest Med (Mex) 1990;21:155–162. [PubMed] [Google Scholar]
- 24.Lozoya X, Meckes M, Abou-Zaid M, Tortoriello J, Nozzolillo C, Amason JT. Quercetin glycosides in Psidium guajava L. leaves and determination of spasmolytic principle. Arch Med Res. 1994;25:11–15. [PubMed] [Google Scholar]
- 25.Lutterodt GD. Inhibition of gastrointestinal release of acetylcholine by quercetin as a possible mode of action of Psidium guajava leaf extracts in the treatment of acute diarrhoeal diseases. J Ethnopharmacology. 1989;25:235–247. doi: 10.1016/0378-8741(89)90030-5. [DOI] [PubMed] [Google Scholar]
- 26.Lutterodt GD, Maleque A. Effects on mice locomotor activity of a narcotic-like principle from Psidium guajava leaves. J Ethnopharmacology. 1988;24:219–231. doi: 10.1016/0378-8741(88)90155-9. [DOI] [PubMed] [Google Scholar]
- 27.Meckes M, Calzada F, Tortoriello J, González JL, Martinez M. Terpenoids isolated from Psidium guajava hexane extract with depressant activity on central nervous system. Phytother Res. 1996;10:600–603. doi: 10.1002/(SICI)1099-1573(199611)10:7〈600::AID-PTR918〉3.0.CO;2-7. [DOI] [Google Scholar]
- 28.Morales MA, Tortoriello J, Meckes M, Paz D, Lozoya X. Calcium-antagonist effect of quercetin and its relation with the spasmolytic properties of Psidium guajava L. Arch Med Res. 1994;25:17–21. [PubMed] [Google Scholar]
- 29.Muccillo Baisch AL, Johnston KB, Paganini Stein FL. Endothelium-dependent vasorelaxing activity of aqueous extracts of Ilex paraguariensis on mesenteric arterial bed of rats. J Ethnopharmacology. 1998;60:133–139. doi: 10.1016/S0378-8741(97)00140-2. [DOI] [PubMed] [Google Scholar]
- 30.Osman AM, Younes ME, Sheta AE. Triterpenoids of the leaves of Psidium guajava . Phytochemistry. 1974;13:2015–2016. doi: 10.1016/0031-9422(74)85152-6. [DOI] [Google Scholar]
- 31.Owen PL, Johns T. Xanthine oxidase inhibitory activity of northeastern North American plant remedies used for gout. J Ethnopharmacology. 1999;64:149–160. doi: 10.1016/S0378-8741(98)00119-6. [DOI] [PubMed] [Google Scholar]
- 32.Qian H, Nihorimbere V. Antioxidant power of phytochemicals from Psidium guajava leaf. J Zhejiang Univ Sci. 2004;5(6):676–683. doi: 10.1631/jzus.2004.0676. [DOI] [PubMed] [Google Scholar]
- 33.Re L, Barocci S, Capitani C, Vivani C, Ricci M, Rinaldi L, Paolucci G, Scarpantonio A, Leon-Fernandez OS, Morales MA. Effects of some natural extracts on the acetylcholine release at the mouse neuromuscular junction. Pharmacological Research. 1999;39:239–245. doi: 10.1006/phrs.1998.0433. [DOI] [PubMed] [Google Scholar]
- 34.Recio MC, Giner RM, Manez S, Gueho J, Julien HR, Hostettmann K, Rios JL. Investigations on the steroidal anti-inflammatory activity of triterpenoids from Diospyros leucomelas . Planta Medica. 1995;61:9–12. doi: 10.1055/s-2006-957988. [DOI] [PubMed] [Google Scholar]
- 35.Sanogo R, Crisafi G, Germanò MP, de Pasquale R, Bisignano G. Evaluation of Malian traditional medicines: screening for antimicrobial activity. Phytother Res. 1998;12:S154–S156. doi: 10.1002/(SICI)1099-1573(1998)12:1+〈S154::AID-PTR281〉3.0.CO;2-T. [DOI] [Google Scholar]
- 36.Seshadri TR, Vasishta K. Polyphenols of the leaves of Psidium guajava: quercetin, guaijiaverin, leucocyanidin and amritoside. Phytochemistry. 1965;4:989–992. doi: 10.1016/S0031-9422(00)86281-0. [DOI] [Google Scholar]
- 37.Simeray J, Chaumont JP, Bevalot F, Vaguette J. Les propriétés antifongiques des cistacéas et plus particulièrement de Cistus laurifolius L.: rôle des tannins non hydrolysables. Fitoterapia. 1982;53:45–48. [Google Scholar]
- 38.Tona L, Kambu K, Mesia K, Cimanga K, Apers S, de Bruyne T, Pieters L, Totte J, Vlietinck AJ. Biological screening of traditional preparations from some medicinal plants used as antidiarrhoeal in Kinshasa, Congo. Phytomedicine. 1999;6:59–66. doi: 10.1016/S0944-7113(99)80036-1. [DOI] [PubMed] [Google Scholar]
- 39.van Acker SABE, Tromp MNJL, Haenen GRMMM, van der Vijgh WJF, Bast A. Flavonoids as scavengers of nitric oxide radical. Biochem and Biophys Res Communications. 1995;214:755–759. doi: 10.1006/bbrc.1995.2350. [DOI] [PubMed] [Google Scholar]
- 40.Yoshizawa S, Horiuchi T, Fujiki H, Yoshida T, Okuda T, Sugimura T. Antitumor promoting activity of (−)-Epigallocatechin gallate, the main constituent of tannin in green tea. Phytother Res. 1987;1:44–47. [Google Scholar]
- 41.Yuting C, Rongliang Z, Zhongjian J, Yong J. Flavonoids as superoxide scavengers and antioxidants. Free Radical Biology and Medicine. 1990;19:19–21. doi: 10.1016/0891-5849(90)90045-K. [DOI] [PubMed] [Google Scholar]
- 42.Zhu M, Phillipson JD, Greengrass PM, Bowery NE, Cai Y. Plant polyphenols: biologically active compounds or non-selective binders to proteins? Phytochemistry. 1997;44:441–447. doi: 10.1016/S0031-9422(96)00598-5. [DOI] [PubMed] [Google Scholar]
- 43.Zimanyi I, Pessah IN. Handbook of Membranes Channels. 1994. Pharmacology of Ryanodine-Sensitive Ca2+ Release Channels; pp. 475–494. [Google Scholar]