Abstract
For this report, the rapid identification and characterization of human immunodeficiency virus type 1 (HIV-1)-derived broadly cross-subtype-reactive CD8 cytotoxic T lymphocyte (CTL) epitopes were performed. Using a gamma interferon (IFN-γ) Elispot assay-based approach and a panel of recombinant vaccinia viruses expressing gag, env, pol, and nef genes representing the seven most predominant subtypes and one circulating recombinant form of HIV-1, the subtype specificity and cross-subtype reactivity of a CD8 response were directly measured from circulating peripheral blood mononuclear cells (PBMC). Enhanced sensitivity of detection of CD8 responses from cryopreserved PBMC was achieved using autologous vaccinia virus-infected B-lymphoblastoid cell lines as supplemental antigen-presenting cells. Of eleven subjects studied, six exhibited broadly cross-subtype-reactive CD8-mediated IFN-γ production (at least seven of eight subtypes recognized) to at least one major gene product from HIV-1. Screening of subjects showing broadly cross-subtype-specific responses in the vaccinia virus-based enzyme-linked immunospot (Elispot) assay using a panel of overlapping peptides resulted in the identification of cross-subtype responses down to the 20-mer peptide level in less than 3 days. Three subjects showed broad cross-subtype reactivity in both the IFN-γ Elispot assay and the standard chromium release cytotoxicity assay. Fine mapping and HLA restriction analysis of the response from three subjects demonstrated that this technique can be used to define epitopes restricted by HLA-A, -B, and -C alleles. In addition, the ability of all three epitopes to be processed from multiple subtypes of their parent proteins and presented in the context of HLA class I molecules following de novo synthesis is shown. While all three minimal epitopes mapped here had previously been defined as HIV-1 epitopes, two are shown to have novel HLA restriction alleles and therefore exhibit degenerate HLA binding capacity. These findings provide biological validation of HLA supertypes in HIV-1 CTL recognition and support earlier studies of cross-subtype CTL responses during HIV-1 infection.
An efficacious prophylactic vaccine is currently the only rational answer for the global human immunodeficiency virus type 1 (HIV-1) pandemic. Of the many obstacles which continue to confound the development of such a vaccine, the genetic diversity of the virus is foremost (75). Geographically defined epidemics can be characterized by the dominance of distinct genetic subtypes of HIV-1. At least nine subtypes and 12 circulating recombinant forms (CRFs) of HIV-1 are currently recognized (56). With the identification of intersubtype genetic recombinants of HIV-1 occurring in regions where multiple subtypes are coendemic, the true extent of genetic diversity of the virus is only now becoming clear (17, 18, 35, 63). Despite causing profound immunodeficiency, acute infection with HIV-1 stimulates strong cellular and humoral immune responses against the virus (57). Although the precise correlates of protective immunity against HIV-1 infection are not clearly defined, there is a growing consensus that vaccine efficacy will depend on the generation of broadly cross-reactive neutralizing antibody and CD8 cytotoxic T lymphocyte (CTL) responses (33, 54).
Evidence for the central role of HIV-1-specific CTL in the control of initial viremia immediately following infection and the establishment of long-term AIDS-free survival has been accumulating rapidly. CTL are readily detectable in the peripheral blood of asymptomatic chronically HIV-1-infected individuals (74), but decline rapidly in association with progression to AIDS (16, 43). In untreated primary HIV-1 infection, the appearance of CTL is temporally associated with the control and reduction of initial viremia (10, 46). During chronic infection, the magnitude of a dominant HLA-A0201-restricted gag-specific CTL response was inversely associated with HIV-1 plasma viral load (60).
The strongest evidence for the in vivo efficacy of the CTL response during HIV-1 infection comes from studies of viral escape mutations within immunodominant epitopes. Viral escape mutations have been associated with the failure to control viremia in both primary and chronic HIV-1 infection, with the escape mutations involving amino acid substitutions at critical HLA binding residues and complete deletion of the epitope sequence (11, 31, 44, 61, 62). Recently, studies of vertical transmission of HIV-1 from mother to child have shown selective transmission of viral escape mutants from immunodominant CTL epitopes (30, 77). In addition, detectable but low CTL activity has been documented in high-risk multiply exposed but uninfected individuals and in uninfected perinatally exposed infants (19, 41, 65, 66).
The simian immunodeficiency virus (SIV)-infected rhesus macaque model of HIV-1 infection has provided a unique insight into the critical functional role of CD8 CTL in control and protection from lentiviral infections. In both acute and chronic infection of rhesus macaques with SIV, large numbers of CTL are demonstrable in peripheral blood and in lymph nodes (51). Compelling evidence suggests that CTL activity during acute SIV infection of rhesus macaques provides a strong selective pressure for escape mutations within SIV structural and regulatory proteins (1, 27). Studies using major histocompatibility complex (MHC) class I/peptide tetramer technology show a strong negative correlation between plasma virus load and the number of CTL in acute SIV infection (47, 48). The infusion of anti-CD8 monoclonal antibodies into chronically SIV-infected macaques results in a profound depletion of CD8 T cells both in the periphery and in lymphoid tissue and is accompanied by a rapid and dramatic rise in plasma viremia and rapid progression to symptomatic disease and death (37). In animals in which monoclonal antibody infusion was halted, SIV-specific CD8 cell numbers rebounded, and there was a coincident decline in plasma viral load (67). Taken together, these data confirm the importance of CTL in controlling HIV-1 infection and indicate that HIV-specific CTL may constitute an important component of both an effective prophylactic vaccine and immunotherapy of infected individuals.
Given the global distribution of the epidemic, the genetic diversity of the virus, and the importance of HIV-specific CD8 CTL, it is imperative to understand the relative degrees of cross-recognition of the different subtypes. The degree and strength of CTL recognition are profoundly influenced by minor sequence variations within the specific epitope and by the restricting HLA allele (12, 68). Traditionally, the magnitude and frequency of cross-subtype HIV-specific CTL responses have been studied in chromium release assays based on the ability of effector CTL from in vitro-stimulated cultures of peripheral blood mononuclear cells (PBMC) to lyse peptide-pulsed or recombinant vaccinia virus-infected target cells. This technique has been employed in a number of studies to demonstrate the presence and cross-subtype reactivity of CTL in HIV-1-infected individuals (8, 14, 15, 28, 53, 55, 78, 79).
An inherent drawback to this approach is that it measures a single parameter; the cytolytic capacity of CD8 effector T cells. The in vitro stimulation (IVS) conditions used may skew the relative frequencies of different HIV-specific CD8 cells in the initial PBMC sample. In vitro studies have demonstrated that potent inhibition of viral replication by HIV-specific CD8 CTL can be mediated by both lytic and nonlytic mechanisms (80, 81). Therefore, detection of parameters other than cytolytic activity following IVS needs to be performed to obtain a meaningful measure of the frequency of circulating HIV-specific CD8 T cells as well as the relative degree of cross-recognition of different subtypes of HIV-1 by these cells. The gamma interferon (IFN-γ) enzyme-linked immunospot (Elispot) assay is the current assay of choice for this purpose, as it permits the rapid identification of function (upregulation of IFN-γ gene transcription) from both memory and effector CTL within a heterogeneous population of cells and is amenable to high throughput with a relatively small number of cells (34, 49).
In this report, a study designed to measure the quantity of HIV subtype and cross-subtype-specific CD8 T cells directly from the PBMC of HIV-seropositive individuals is presented. By using recombinant vaccinia viruses expressing gag, env, and nef gene products from HIV-1 subtypes A through H and pol gene products from subtypes A and B, CD8 T cells specific for a particular HIV-1 gene product from any subtype can be directly enumerated from a PBMC sample. To increase the sensitivity of the IFN-γ Elispot assay, autologous B-lymphoblastoid cell lines (BLCL) were infected with the recombinant vaccinia viruses and then added as supplemental antigen-presenting cells (APC) to the PBMC. The magnitude of the CD8 response between heterologous subtypes can be directly compared, and the degree of cross-recognition can be assessed.
The results from the study presented here show that broadly cross-subtype-reactive and weakly cross-subtype-reactive CD8 CTLs can be detected in subtype B-infected individuals from the United States and from subtype A/E (CRF01_AE)-infected Thais. CTL activity from cross-subtype responders, as measured by chromium release effected by CD8 cells from in vitro-expanded cultures, was concordant with the Elispot results. Subsequent screening of Gag- and Env-specific cross-subtype responders using a matrix of pooled peptides allowed rapid identification of cross-subtype epitopes from both gene products. Further characterization of two of the broadly cross-subtype-reactive epitopes revealed that while they had previously been defined, they exhibit degenerate HLA binding capacity. This assay format will be useful in future studies of the breadth of specificity of immune responses generated by natural infection with HIV-1 and in the assessment of HIV-1 vaccine candidates.
MATERIALS AND METHODS
Study subjects.
PBMC from Thai subjects were obtained from discarded units of donated blood which were determined to be HIV-1 positive by serology following donation at the Army Institute of Pathology, Bangkok, Thailand. HIV serology was performed by enzyme-linked immunosorbent assay (ELISA) (Vironostika Uniform II; Organon Teknika, Turnhout, Belgium), and reactive specimens were confirmed by Western blot (HIV Blot 2.2; Genelabs Diagnostics, Singapore). Since the heterosexual epidemic in Thailand is characterized as >95% CRF01_AE (29, 76), HIV genotyping was performed as described (3).
Briefly, crude cell lysates of PBMC were subjected to nested PCR using primers amplifying within the viral gp41 coding region, capable of differentiating HIV subtype B and CRF01_AE. Four of the eight Thai subjects were infected with CRF01_AE strains (VAIP-4, AIHP-6, AIHP-8, and AIHP-9), while the remaining four were not able to be subtyped (AIHP-3, AIHP-4, AIHP-5, and AIHP-200). Viral load data were not available for these subjects. For the three subtype B-infected subjects from the United States, aliquots of PBMCs were obtained from patients enrolled in one of two Institutional Review Board-approved clinical trials at the National Naval Medical Center, Bethesda, Md. Subjects underwent apheresis on a Fenwal CS3000 Plus cell separator, which was used to process approximately 6 to 10 liters of whole blood. PBMC were then isolated by Ficoll gradient centrifugation of the leukopacks. Viral load determinations were made on plasma by using the Roche-Amplicor assay, version 1.0. Viral load (VL) and CD4 counts at the time of PBMC collection for each of these samples were as follows: US101, VL = 128, 223 RNA copies/ml and CD4 = 290 cells/μl; US102, VL = 1183 RNA copies/ml and CD4 = 413 cells/μl; and US103, 564 RNA copies/ml and CD4 = 409 cells/μl.
Recombinant vaccinia viruses.
Recombinant vaccinia viruses expressing HIV-1 gag, env, and nef gene products from subtypes A through H and HIV-1 pol products from subtypes A and B were obtained from the National Institutes of Health (NIH) AIDS Research and Reference Reagent Program (ARRRP), Division of AIDS, National Institute of Allergy and Infectious Disease, NIH. Table 1 shows the vaccinia virus recombinants used in the study, outlining the clone of origin and the subtype and gene products that each recombinant expresses. The recombinant vaccinia virus vSC8 (NIH ARRRP, catalog number 357), which expresses β-galactosidase, and the wild-type WR strain vP1170 (NIH ARRRP, catalog number 3928) were used as controls for background recombinant vaccinia virus-specific responses. Quality control analysis for the efficiency of BLCL infection was performed on three separate occasions using the entire panel of recombinant vaccinia viruses and revealed infection levels ranging from 35 to 85% of the BLCL (data not shown).
TABLE 1.
Panel of recombinant vaccinia viruses expressing HIV-1 gene products used in this study
| Clone | Derivation | Subtype | Gene |
|---|---|---|---|
| vT135 | 92UG037 | A | gag |
| vT143 | 92UG037 | A | pol |
| vT183 | 92UG037 | A | env |
| vT139 | 92UG037 | A | nef |
| vP1287 | IIIB | B | gag |
| vP1288 | IIIB | B | pol |
| vP1174 | MN | B | env |
| vTF-Nef | pNLXho | B | nef |
| vT196 | 96ZM651 | C | gag |
| vP1488 | 92BR025 | C | env |
| vT197 | 96ZM651 | C | nef |
| vT157 | 94UG114 | D | gag |
| vT173 | 94UG114 | D | env |
| vT158 | 94UG114 | D | nef |
| vT142 | 90CR402.1 | CRF01_AE | gag |
| vP1536 | TH023 | CRF01_AE | env |
| vCM235-Nef | CM235-32 | CRF01_AE | nef |
| vT177 | 93BR020 | F | gag |
| vT234 | 93BR020 | F | env |
| vT178 | 93BR020 | F | nef |
| vT243 | 92NG83.2 | G | gag |
| vT245R | 92NG83.2 | G | env |
| vT244 | 92NG83.2 | G | nef |
| vT180 | 90CR056 | H | gag |
| vT239R | 90CR056 | H | env |
| vT179 | 90CR056 | H | nef |
Synthetic HIV peptides.
Overlapping synthetic peptide sets were obtained from the NIH ARRRP. Peptide sets consisted of 49 peptides 20 amino acids in length, overlapping by 10 amino acids spanning the HIV-1 subtype A gag 92UG037 sequence; 80 peptides 20 amino acids in length, overlapping by 10 amino acids spanning the HIV-1 subtype B env MN sequence; and 122 peptides 15 amino acids in length overlapping by 11 amino acids spanning the HIV-1 subtype B gag HXB2 sequence. One overlapping peptide set, 166 peptides 15 amino acids in length, overlapping by 11 amino acids and spanning the HIV-1 subtype E env TH023 sequence, was obtained from Natural and Medical Sciences Institute (University of Tübingen, Germany). Peptide purity was greater than 80%, and all were reconstituted in dimethyl sulfoxide prior to storage. The 7 by 7 peptide matrix for the subtype A gag 92UG037 20-mer set was prepared by creating seven linear pools of seven peptides and seven pools of every seventh peptide in the series. Minimal epitope peptides 9 and 10 amino acids in length with free amino termini were synthesized using FMOC (9-fluorenylmethoxy carbonyl) chemistry and standard solid-phase techniques (Excel automated synthesizer; Waters, Milford, Mass.). Purity (>90%) was determined by high-pressure liquid chromatography (HPLC), mass spectrophotometry, amino acid analysis, and N-terminal sequencing.
Recombinant vaccinia virus-based IFN-γ Elispot assay.
All assays were performed using RPMI 1640 medium containing 10% normal human serum, 100 U of penicillin, and 100 μg of streptomycin per ml (complete medium). The IFN-γ Elispot assay was employed to measure directly the circulating frequency of HIV-specific CD8 T cells in the PBMC of infected individuals. Autologous BLCL were infected with 10 PFU/cell of recombinant vaccinia virus and incubated for 14 to 16 h. PBMC were recovered from liquid nitrogen, thawed rapidly, washed twice, and incubated at 2 × 106 cells/ml in complete medium for 14 to 16 h. After the overnight rest, the PBMC were recounted prior to addition to the Elispot assay plates at 105 cells per well. Ninety-six-well Elispot plates (Multiscreen-IP MAIP-type plates; Millipore) were prepared by precoating with mouse anti-human IFN-γ monoclonal antibody (MAb) 1-D1K (Mabtech AB, Stockholm, Sweden) at 5 μg/ml in 50 μl of phosphate-buffered saline (PBS) overnight at 4°C. Plates were washed five times with PBS and blocked with 100 μl of complete medium for 1 h at 37°C.
For evaluation of CD8 T-cell dependence, the overnight-rested PBMC were split into two aliquots and treated with either Dynabeads M-450 CD8 (for CD8 depletions) or Dynabeads M-450 sheep anti-mouse immunoglobulin G (IgG) (for sham depletions) (Dynal Biotech ASA, Oslo, Norway). The immunomagnetic beads and cells adhered to the beads were removed with a magnet, and the resulting cell populations were washed twice and resuspended in complete medium. Based on the cell count in the sham depletion, between 5 × 104 and 1 × 105 PBMC were added to each well of the precoated and blocked Elispot plate. Vaccinia virus-infected BLCL were washed once after the overnight infection and distributed in duplicate wells at between 1 × 104 and 2 × 104 cells per well (ratio of 5:1, PBMC to BLCL). The negative control and vaccinia virus control wells (vSC8 infection) were all performed in quadruplicate. As a positive control for functional integrity of the cells, either phytohemagglutinin-P (PHA) or staphylococcal enterotoxin B (SEB) was added to duplicate wells at a 5 μg/ml final concentration.
For the direct vaccinia virus addition experiments 2 × 105 PBMC were added to each well of the Elispot plates, and vaccinia virus was added directly to the wells at 2 PFU/cell. Plates were incubated for 20 to 24 h at 37°C (5% CO2) and were then washed with PBS-0.05% Tween 20 buffer and incubated for 2 h with mouse anti-human IFN-γ antibody conjugated with biotin (7B6-1-biotin; Mabtech AB). Development consisted of a 1-h incubation with an avidin-horseradish peroxidase complex (Vectastain ABC kit; Vector Laboratories, Burlingame, Calif.) followed by washing (PBS-0.05% Tween 20 buffer) and incubation with peroxidase substrate AEC for 4 min (Vector Laboratories, Burlingame, Calif.).
Peptide-based IFN-γ Elispot assay.
For the evaluation of peptide-specific responses, PBMC preparation, CD8 cell depletion, and Elispot plate preparation and development were performed as described above. Peptides, either individually or in the indicated pools, were added to duplicate wells at a final concentration of 2 μg/ml in complete medium. Postdepletion PBMC were added at 105 cells/well based on the isotype-matched IgG-bead control depletion, and the plates were incubated for 20 to 24 h before development. Negative control wells consisted of complete medium containing 0.1% dimethyl sulfoxide (peptide solvent), and functional integrity of the cells was assessed by using PHA or SEB as described above.
The HLA restriction Elispot was performed using PBMC depleted of CD4 T cells by using Dynabeads M-450 CD4 (Dynal Biotech ASA, Oslo, Norway) as described above. Autologous and partial HLA-matched BLCL were pulsed overnight with relevant peptide at 10 μg/ml, washed three times to remove excess peptide, and added to the Elispot plate at 104 cells per well in triplicate. CD4-depleted PBMC were added at 104 cells per well, and plates were incubated for 18 to 20 h at 37°C (5% CO2) before development and counting as described above.
Elispot assay analysis.
Elispot plates were examined under a stereomicroscope, and spots were evaluated with an Automated Elispot Reader System with KS 4.3 software (Carl Zeiss, Thornwood, N.Y.) by an independent scientist in a blinded fashion (Henry M. Jackson Foundation, Rockville, Md., or Zellnet Consulting, New York, N.Y.). Positive IFN-γ spot-forming units (SFU) representing single cells were counted and expressed as SFU per 106 input PBMC. The cutoff for positivity was the 99% confidence interval for the quadruplicate negative control wells. In some peptide-stimulated assays, the negative controls were performed in duplicate only, and positivity for the test wells was defined as mean SFU greater than two times the mean SFU of the negative control wells.
HLA typing.
HLA typing was performed on all subjects using DNA extracted from autologous BLCL by the American Red Cross National Histocompatibility Laboratory at the University of Maryland Medical Center, Baltimore, Md., and at Duke University, Durham, N.C.
CTL assay.
Effector cells were prepared by IVS of freshly thawed cryopreserved PBMC with relevant peptide (10 μg/ml final concentration). Briefly, 10 × 106 to 20 × 106 PBMC were incubated with peptide (50 μg/ml) in 2 ml of complete medium for 2 h. Peptide IVS cells were resuspended to 10 ml with complete medium supplemented with 330 U of recombinant human interleukin-7 (IL-7) (R&D Systems, Minneapolis, Minn.) per ml. After incubation for 4 days, 20 U of recombinant human IL-2 (Boehringer-Mannheim GmbH, Mannheim, Germany) per ml was added to the IVS cultures, and fresh medium and IL-2 were added after a further 7 days. After 14 to 20 days of IVS with the peptide, CTL activity against recombinant vaccinia virus-infected or peptide-pulsed autologous or partially HLA-matched BLCL was measured. BLCL (106) were incubated overnight with 10 μg of relevant peptide per ml or 10 PFU of relevant recombinant vaccinia virus per cell in the presence of 100 μCi of 51Cr-labeled sodium chromate (NEN, Boston, Mass.). Non-peptide-pulsed BLCL and BLCL infected with the vSC8 vaccinia virus served as negative controls. CTL activity was titrated at several effector to target cell (E:T) ratios and expressed as a percentage of maximal specific lysis.
RESULTS
Optimization of IFN-γ Elispot assay conditions using vaccinia virus-infected BLCL as supplemental APC for cryopreserved PBMC.
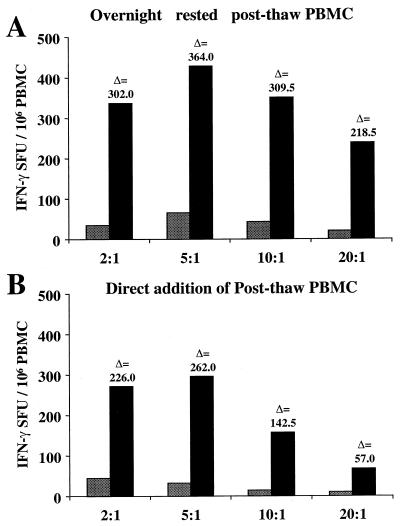
Initially, the optimal conditions were determined for antigen-specific stimulation of IFN-γ-producing T cells from cryopreserved PBMC of HIV-1-seropositive subjects using vaccinia virus-infected BLCL as supplemental APC. First, the optimal ratio of responder to stimulator cells was determined in a subject previously determined to have a CD8 CTL response to the HIV-1 pol gene detectable by standard chromium release assay following IVS. Control vSC8- and vP1288 (subtype B HIV-1 pol gene-expressing)-infected autologous BLCL were cocultured with thawed and overnight rested or freshly thawed PBMC and tested for IFN-γ secretion in an Elispot assay. The thawed and rested PBMC were added to the assay wells following incubation in complete medium for 16 h and recounted and assessed for viability. The freshly thawed PBMC were added to the assay wells within 2 h of thawing.
PBMC were added to the assay wells at 105 per well, and responder-to-stimulator cell ratios (PBMC-BLCL) of 20, 10, 5, and 2 to 1 were tested. The optimal ratio for responder-to-stimulator cells was determined to be 5:1 when using 105 PBMC per well of a standard 96-well Elispot plate (Fig. 1A and B). This ratio was used throughout the study, and when limited numbers of PBMC were available, as few as 5 × 104 were added to each well. Overnight-rested PBMC yielded higher frequencies of antigen-specific IFN-γ production at all cell ratios tested and provided greater sensitivity and better delta values between control and test conditions (Fig. 1A and B).
FIG. 1.
Determination of optimal ratio of PBMC to supplemental recombinant vaccinia virus-infected autologous BLCL for maximizing the sensitivity of IFN-γ-producing cell detection. Vertical bars represent the SFU/106 PBMC for control wells (vP1170, vaccinia virus WR; stippled bars) and test wells (vP1288, expressing the IIIB pol gene; solid bars) performed in duplicate. (A) Cryopreserved PBMC thawed and rested overnight (as outlined in Results) prior to addition to assay. (B) Cryopreserved PBMC added directly to assay postthaw.
Since direct addition of recombinant vaccinia viruses to PBMC has proved to be an effective method for measuring IFN-γ Elispot responses from HIV-1-seropositive individuals (50), this method was compared with the addition of supplemental vaccinia virus-infected BLCL. Two HIV-1-seropositive individuals were tested for CD8-mediated HIV-specific IFN-γ responses to recombinant vaccinia virus-expressed gag, pol, env, and nef gene products. For direct addition, recombinant vaccinia viruses were added to each well at 2 PFU per cell and incubated for 20 h. The autologous BLCL addition was performed as described above.
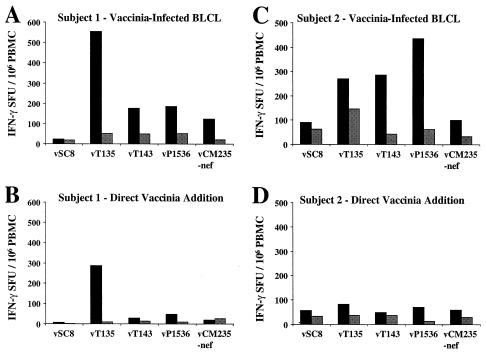
In both subjects tested, cryopreserved PBMC stimulated with vaccinia virus-infected autologous BLCL as APC showed CD8-mediated IFN-γ responses equal to or greater than those of cryopreserved PBMC directly infected and stimulated with recombinant vaccinia viruses (Fig. 2A to 2D). Depletion of CD8 T cells from the PBMC abrogated the response, whereas CD4 depletion did not result in any significant reduction in the response (data not shown). For subject 1 (AIHP-6), there was a twofold increase in the number of gag-specific (vT135, subtype A) CD8 T cells detectable using vaccinia virus-infected autologous BLCL (504 versus 276 IFN-γ SFU/106 PBMC), while for subject 2 (AIHP-9), there was a sixfold increase in the number of detectable env-specific (vP1536 and CRF01_AE) CD8 T cells using vaccinia virus-infected autologous BLCL (374 versus 57 IFN-γ SFU/106 PBMC). The vaccinia virus-infected autologous BLCL assay system was used for the HIV-1 subtype and cross-subtype analyses because of its superior sensitivity.
FIG. 2.
Comparison of sensitivity of HIV-1-specific IFN-γ-producing CD8 T-cell detection from cryopreserved PBMC using either supplemental vaccinia virus-infected BLCL (A and C) or direct addition of vaccinia virus to cryopreserved PBMC (B and D) for two subjects. Results are presented as total numbers of IFN-γ-producing cells (mean of triplicate wells) for either control depletion (solid bars) or CD8 depletion (stippled bars). Recombinant vaccinia viruses representing subtype A (gag and pol) and CRF01_AE (env and nef) were used.
Detection and enumeration of HIV-1 subtype-specific CD8 responses with the IFN-γ Elispot assay.
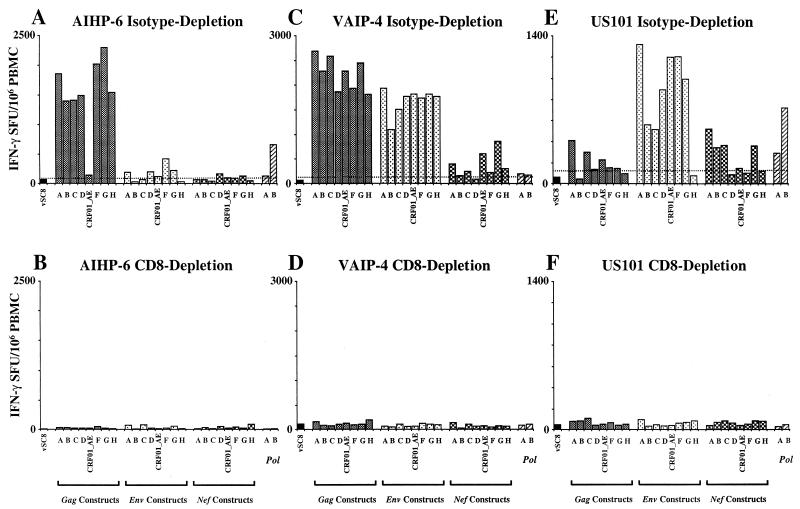
PBMC from three HIV-1 subtype B-infected donors from the United States and eight HIV-1 CRF01_AE-infected donors from Thailand were selected for the cross-subtype study. Figure 3 shows the cross-subtype response of three subjects (two Thai [AIHP-6 and VAIP-4] and one from the United States [US101]) to the panel of gag, env, nef, and pol constructs. A high frequency of IFN-γ-producing cells with cross-subtype reactivity was detectable to at least one gene product from each of the subjects. This response was mediated exclusively by CD8 T cells, as indicated by complete abrogation of the response by depletion of the CD8 cells from the PBMC population prior to performing the assay.
FIG. 3.
Subtype and cross-subtype IFN-γ Elispot analysis for three subjects, AIHP-6 (A and B), VAIP-4 (C and D), and US101 (E and F). Cutoff for positivity (dotted line) is the 99% confidence interval for the negative control vSC8 (WR vaccinia virus, expressing β-galactosidase) performed in quadruplicate. Vertical bars represent the mean IFN-γ SFU/106 PBMC of duplicate wells. Dependence on CD8 T cells is shown by immunomagnetic bead depletion in the lower panel of each pair. Subjects VAIP-4 and AIHP-6 were infected with HIV-1 subtype CRF01_AE, and subject US101 was infected with HIV-1 subtype B.
For two selected donors, CD4 depletion was carried out and had no significant effect on the response in this assay (data not shown). Subject AIHP-6 (Thai CRF01_AE infected) (Fig. 3A and B) responded to seven of the eight gag constructs (subtypes A, B, C, D, F, G, and H) with a frequency of responses ranging from 1,330 to 2,235 specific SFU/106 PBMC. It is notable that while the response to the CRF01_AE construct (vT142) was positive by the criteria used here (145 SFU/106 PBMC versus a 99% confidence interval of 83.2), this response was remarkably lower than the response to the other seven gag constructs. Variable but very low frequency responses were detected to env (five of eight subtypes) and nef (four of eight subtypes) from this subject.
The second Thai CRF01_AE-infected subject (VAIP-4) (Fig. 3C and D) showed a dramatic and broadly cross-subtype-reactive CD8 response directed towards both the gag (specific SFU/106 PBMC range, 1,750 to 2,625) and env (specific SFU/106 PBMC range, 1,040 to 1,875) gene products. All subtypes of gag and env included in the study were recognized at a similar magnitude by this subject. Subject US101 (United States subtype B infected) (Fig. 3E and F) showed a broadly cross-subtype-reactive CD8 response directed towards the env gene product (specific SFU/106 PBMC range, 455 to 1,260). The env gene product from all subtypes except subtype H was recognized by subject US101.
Although responses scored as positive were detected for both gag (six of eight subtypes) and nef (five of eight subtypes) gene products for US101, the magnitude of these responses varied greatly. For the nef constructs, the response from donor US101 was of a similar magnitude for subtypes A, B, C, and G (specific SFU/106 PBMC range, 285 to 460), while the response to the CRF01_AE construct was much lower and barely exceeded the cutoff (145 SFU/106 PBMC versus a 99% confidence interval of 133). Moreover, the US101 response to three of six gag constructs also barely exceeded the cutoff (<100 specific SFU/106 PBMC for subtypes D, F, and G), while the subtype A and C responses were substantially higher (350 and 240 specific SFU/106 PBMC, respectively). While positive responses were detected to the two pol gene products (subtypes A and B) from all three subjects, this response was always lower than the response directed towards other gene products.
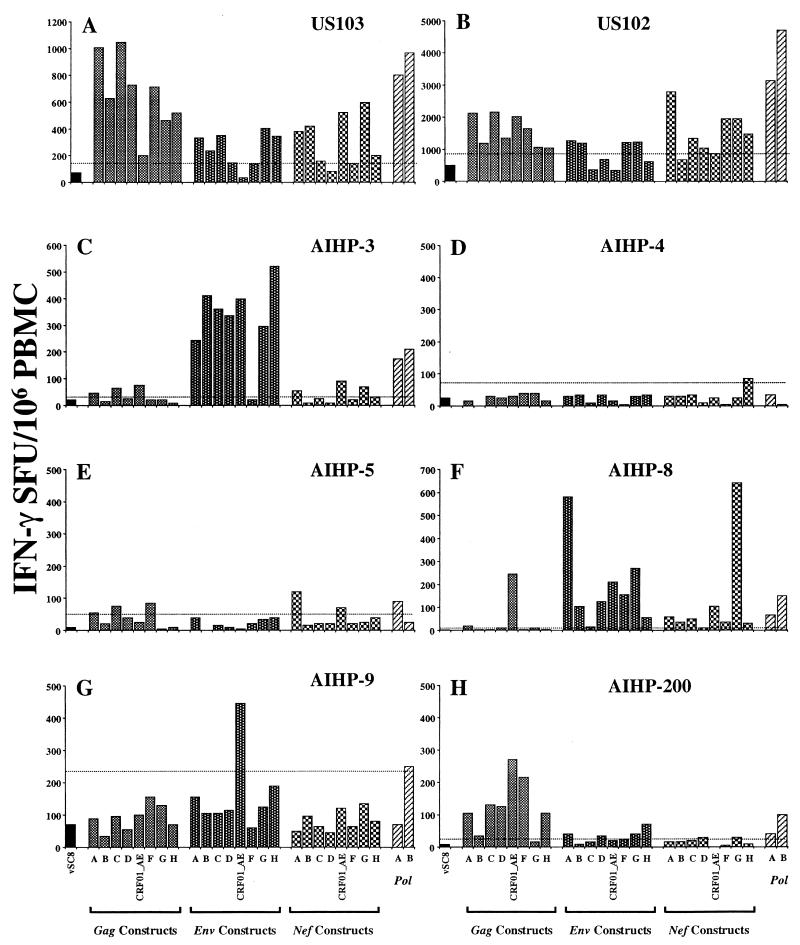
Eight more subjects were subsequently screened with the same assay procedure. Figure 4 shows the pattern of cross-subtype and subtype-specific IFN-γ-secreting responses detected in the PBMC for each of these donors. Three of the donors, two subtype B infected (US103 [Fig. 4A] and US102 [Fig. 4B]) and one Thai CRF01_AE infected (AIHP-3 [Fig. 4C]), exhibited a broad cross-subtype response to gag (US103 and US102) and env (AIHP-3) gene products. Two subjects (AIHP-8 and AIHP-9) exhibited highly subtype-specific responses to multiple gene products. Subject AIHP-9 (Fig. 4G) had a positive CD8 IFN-γ-secreting response to CRF01_AE env and subtype B pol, while for subject AIHP-8 (Fig. 4F), the response was dominated by CRF01_AE gag and subtype G nef reactivity. AIHP-8 exhibited a subtype A env response which was at least twice that of the next most frequently recognized env gene product (567 versus 257 specific SFU/106 PBMC).
FIG. 4.
Cross-subtype IFN-γ Elispot analysis for eight subjects. Subjects US102 and US103 were from the United States, and the remaining six were Thai. Cutoff for positivity (dotted line) is the 99% confidence interval for the negative control vSC8, performed in quadruplicate. Vertical bars represent the mean IFN-γ SFU/106 PBMC of duplicate wells. Subjects AIHP-8 and AIHP-9 were infected with HIV-1 subtype CRF01_AE, and subjects US102 and US103 were infected with HIV-1 subtype B, while the subtype infecting AIHP-3, AIHP-4, AIHP-5, and AIHP-200 was not identified.
A further two subjects, AIHP-4 (Fig. 4D) and AIHP-5 (Fig. 4E), exhibited very low-level (<100 specific SFU/106 PBMC) yet subtype-specific responses. Responses to the pol gene products from subtypes A and B were readily detectable and of comparable magnitude in six subjects (five responders and one nonresponder). Only two subjects (AIHP-5, positive subtype A pol response, and AIHP-9, positive subtype B pol response) were disparate with respect to their pol gene reactivity. Of the 11 subjects tested, all demonstrated IFN-γ responses to at least one gene product from HIV-1, while 6 of 11 exhibited broadly cross-subtype-reactive IFN-γ production (at least seven of eight subtypes recognized) to at least one gene product.
Mapping of cross-subtype responses using overlapping peptide pools.
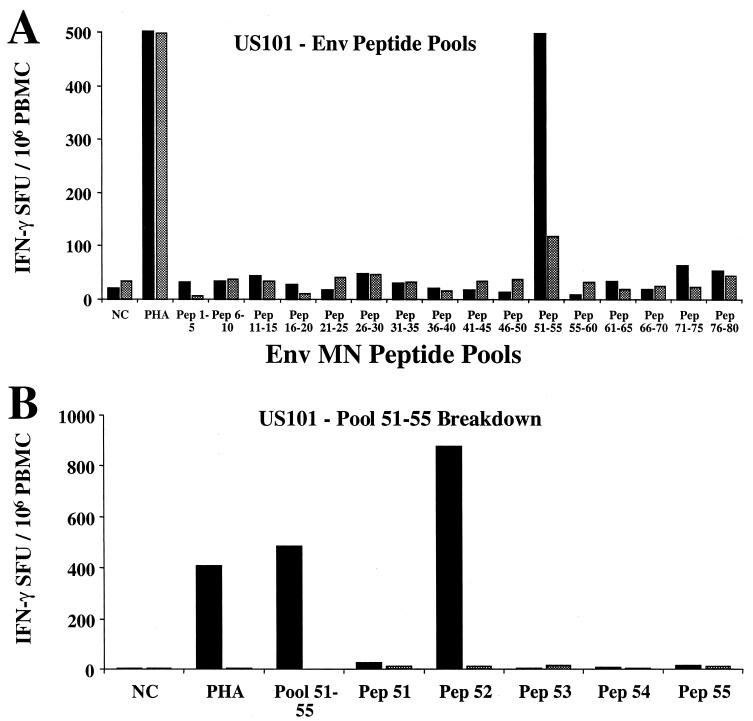
Three donors with broad cross-subtype IFN-γ-producing CD8 cell reactivity and with adequate numbers of cryopreserved PBMC were selected for epitope mapping. To map the cross-subtype-reactive env response from subject US101, a panel of 16 pools of five 20-mer peptides (corresponding to the subtype B env MN sequence) was first used to stimulate cryopreserved PBMC and subsequently broken down into individual peptides from the responding pools. Figure 5A shows that peptide pool 51-55 stimulated a CD8-mediated IFN-γ response, while Fig. 5B shows that peptide 52 was the predominant stimulating 20-mer peptide within this pool. In both assays, CD8 depletion dramatically reduced the IFN-γ SFU response.
FIG. 5.
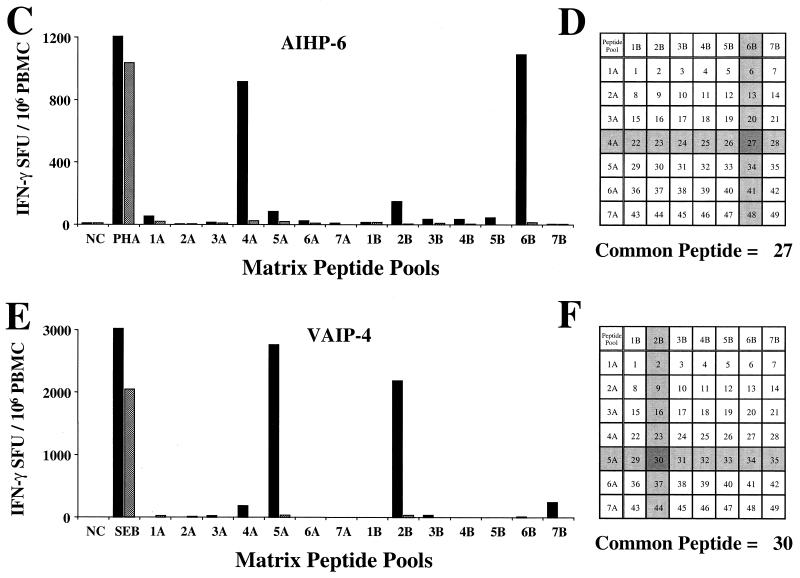
Identification of immunodominant epitopes from three subjects showing broadly cross-subtype-reactive CD8 T-cell responses. Vertical bars represent the mean IFN-γ SFU/106 PBMC of duplicate wells for both control depletion (solid bars) and CD8 depletion (stippled bars). Subject US101 was screened with 16 pools of five peptides representing the env gene from subtype B isolate MN (A), and the positive pool was subsequently broken down to individual peptides (B). Subjects AIHP-6 (C) and VAIP-4 (E) were screened with a 7 by 7 matrix of overlapping 20-mer peptides representing the gag gene from subtype A isolate 92UG037. Panel D shows the determination of peptide 27 (shaded, in pools 4A and 6B) as the immunodominant peptide for AIHP-6, and panel F shows peptide 30 (shaded, in pools 5A and 2B) as the immunodominant peptide for VAIP-4.
Since this method required the performance of at least two Elispot assays, an alternative format was next used to reduce both assay time and the total number of cryopreserved PBMC used. A 7 by 7 matrix format of overlapping 20-mer peptides corresponding to the 92UG037 gag gene (subtype A gag expressed by recombinant vaccinia virus vT135) was used to map the CD8 response in donors AIHP-6 and VAIP-4, both of whom demonstrated a strong cross-subtype response directed towards the gag gene. Figures 5C to 5F shows the IFN-γ Elispot response for both subjects and the peptide matrix format used to map the response. The AIHP-6 response was directed predominantly towards peptide 27 of 92UG037 gag, since this is the common peptide from pools 4A (920 SFU/106 PBMC) and 6B (1093 SFU/106 PBMC), which show a close concordance of CD8-mediated IFN-γ SFU (Fig. 5C and 5D). A less frequent but readily detectable CD8 response was also directed at peptide 30 from this subject (pools 5A and 2B).
For subject VAIP-4, the response was directed primarily towards peptide 30 of 92UG037 gag. This is the common peptide from pools 5A (2765 SFU/106 PBMC) and 2B (2190 SFU/106 PBMC), which show a close concordance of CD8-mediated IFN-γ SFU (Fig. 5E and 5F). VAIP-4 also exhibited a less dominant CD8 response to a second peptide within gag, peptide 28 from pools 4A and 7B. By first screening with a panel of recombinant vaccinia viruses expressing HIV-1 gene products followed by a matrix of overlapping peptides from the gene of interest, the IFN-γ Elispot assay can be used to identify CD8 epitopes with broad cross-subtype reactivity down to the 20-mer level in 3 days.
Concordance of IFN-γ Elispot assay and CTL activity for monitoring cross-subtype reactivity.
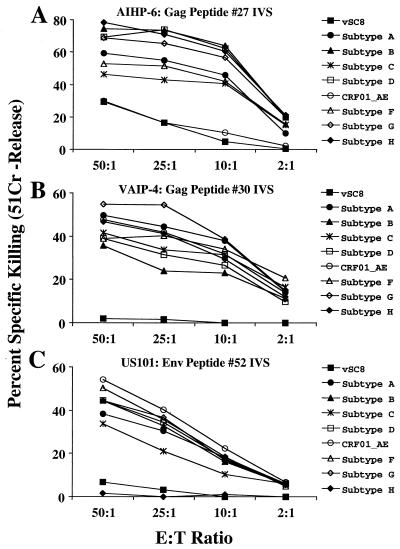
Standard chromium release CTL assays were conducted to determine whether IFN-γ production by CD8 T cells detected directly from PBMC concords with cytolytic activity following an in vitro stimulation. Effector cells from subjects AIHP-6, VAIP-4, and US101 were prepared by 14 to 20 days of culture in vitro with the 20-mer peptide to which it had been determined that each responded (see previous section). Figure 6A shows the cytotoxic activity of the T cells generated by culture of AIHP-6 PBMC with 20-mer peptide 27 from 92UG037 gag against autologous BLCL infected with each of the gag-expressing vaccinia virus constructs. Significant specific killing of autologous BLCL infected with subtypes A, B, C, D, F, G, and H gag-expressing vaccinia virus was observed: >27% specific killing at at least two E:T ratios and titration of the response with decreasing E:T ratio. No specific killing of the CRF01_AE gag vaccinia virus vT142 was observed above that of the control vaccinia virus vSC8. This is concordant with the IFN-γ Elispot response for this donor (Fig. 3A), in whom a strong IFN-γ-producing response to all of the gag constructs except vT142 (CRF01_AE) was observed.
FIG. 6.
Cytotoxic activity of peptide-stimulated cultures of cryopreserved PBMC. The identified immunodominant 20-mer peptides were used to generate effector cells from subjects AIHP-6 (A), VAIP-4 (B), and US101 (C). Vaccinia virus-infected autologous BLCL served as target cells. Percent specific killing is shown for target cells infected with the gag constructs for AIHP-6 and VAIP-4 and the env constructs for US101 at E:T ratios of 50:1 to 2:1. Autologous BLCL infected with vSC8 served as the negative controls.
Subject VAIP-4, who demonstrated a gag-specific IFN-γ Elispot response to all subtypes tested (Fig. 3B), also exhibited cytotoxic activity against autologous BLCL infected with vaccinia virus expressing the gag gene from subtypes A through H and CRF01_AE following in vitro stimulation with the 20-mer peptide (Fig. 6B). Specific killing was >23% at three E:T ratios and titrated with decreasing E:T ratio and concorded well with the IFN-γ Elispot performed directly from the PBMC. For subject US101, a similar concordance of the IFN-γ Elispot response detected directly from PBMC (Fig. 3C) and cytolytic activity from an in vitro-expanded effector CTL population was observed (Fig. 6C). Effector CTL derived by in vitro stimulation with 20-mer peptide 52 from MN env efficiently lysed autologous BLCL infected with subtypes A, B, C, D, F, and G and CRF01_AE env-expressing vaccinia virus: >18% specific killing at at least two E:T ratios and titration of the response with decreasing E:T ratio.
Fine mapping and HLA restriction analysis of cross-subtype conserved epitopes.
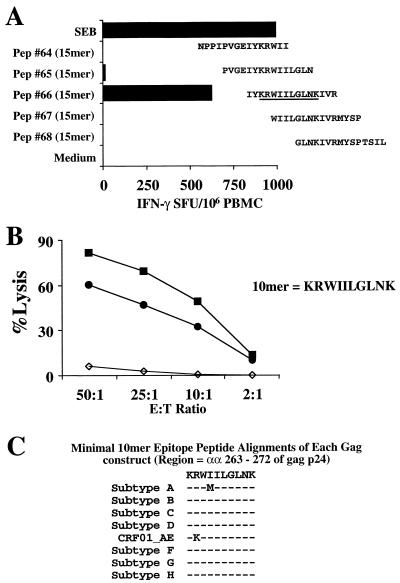
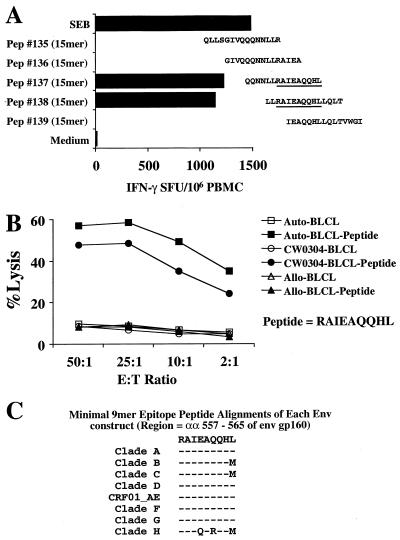
Overlapping gag 15-mer peptide IFN-γ Elispot mapping of the PBMC response of AIHP-6 in the region of the gag 20-mer peptide 27 was performed (Fig. 7A). Only the single 15-mer peptide 66, matching the subtype B isolate HXB2 sequence, demonstrated the ability to stimulate IFN-γ production from PBMC of AIHP-6. HLA class I typing of AIHP-6 revealed that this donor expressed the HLA-B27 allele.
FIG. 7.
Minimal epitope mapping of the gag-specific cross-subtype CD8 response of subject AIHP-6. (A) Overlapping 15-mer peptides (Pep) (representing HXB2 gag) were used to minimally map the epitope directly from cryopreserved PBMC. Only one 15-mer peptide (peptide 66) contained the 10-mer peptide KRWIILGLNK (gag 263-272) (underlined). (B) Percent specific killing of the parent 20-mer peptide (peptide 27 from 92UG037 gag, solid squares) and the minimal 10-mer peptide (solid circles) representing the immunodominant HLA-B27 epitope (predicted from the subject's HLA type) are compared to non-peptide-pulsed autologous BLCL (open diamonds). Effector cells were generated by 20-mer peptide stimulation of cryopreserved PBMC, and target cells (autologous BLCL) were pulsed overnight with each peptide at 10 μg/ml. (C) Alignment of the amino acid sequences for each of the gag constructs used in the study covering the minimal epitope. A critical arginine (R) to lysine (K) substitution is present at the P2 HLA-B27 binding site of the CRF01_AE (vT142) construct sequence.
Since the dominant stimulating peptides for this donor contained a highly conserved HLA-B27-restricted epitope (amino acids 263 to 272 of gag p24) associated with good clinical outcome (30, 31, 40), the ability of the effector cells generated above to recognize both gag peptide 27 and the minimal HLA-B27-restricted epitope was tested. As shown in Fig. 7B, the effector cells efficiently recognized and lysed autologous BLCL pulsed with both the 20-mer peptide and the minimal 10-mer peptide. Figure 7C shows the sequence of peptide 27 and the minimal epitope together with the HLA class I type of AIHP-6. Of note is the peptide sequence alignment for each of the gag constructs covering the 10-mer HLA-B27 epitope region. The CRF01_AE (vT142) sequence differs from all others by the substitution of lysine (K) for arginine (R) at the previously defined critical P2 anchor residue (30, 31, 40). Therefore, both the vaccinia virus-based Elispot assay and the CTL assay concord directly with the molecular sequence data.
The fine specificity of the CD8 response was also mapped in subject US101, who showed broad cross-subtype reactivity to the HIV-1 env gene product. Screening of the IFN-γ Elispot response of PBMC from this subject with overlapping 15-mer peptides in the region of the env 20-mer peptide 52 was performed (Fig. 8A). Two 15-mer peptides (137 and 138, matching the CRF01_AE isolate TH023 sequence) demonstrated the ability to stimulate IFN-γ production from PBMC of US101. The 11-mer sequence common to these two peptides was noted to contain a previously defined HLA-B51-restricted 9-mer peptide epitope (70), RAIEAQQHL (amino acids 557 to 565, HXB2 numbering system [45]). HLA typing revealed that this subject did not express HLA-B51, but did express HLA CW0304, for which the peptide binding motif would accommodate this epitope (73).
FIG. 8.
Minimal epitope mapping and HLA restriction of the env-specific cross-subtype CD8 response of subject US101. (A) Overlapping 15-mer peptides (Pep) (representing TH023 env) were used to minimally map the epitope directly from cryopreserved PBMC. Two 15-mer peptides (peptides 137 and 138) contained the 9-mer peptide RAIEAQQHL (gag 557-565) (underlined). (B) Percent specific killing of the predicted minimal epitope RAIEAQQHL based on the HLA-CW0304 motif. Effector cells were generated by 20-mer peptide stimulation (peptide 52 from MN env) of cryopreserved PBMC and target cells (autologous BLCL, allogeneic BLCL, and HLA-CW0304-only matched BLCL) were pulsed overnight with each peptide at 10 μg/ml. (C) Alignment of the amino acid sequences for each of the env constructs used in the study covering the minimal epitope. Two critical central amino acid substitutions are present in the subtype H (vT239R) construct sequence.
Effector cells from the MN env 20-mer peptide 52 (subtype B MN amino acids 551 to 570)-driven IVS were used to test for HLA restriction. When autologous BLCL, BLCL matched only for HLA CW0304, and BLCL expressing no common HLA class I alleles were pulsed with the 9-mer peptide, only the autologous BLCL and the BLCL expressing HLA CW0304 were efficiently lysed by the env-specific effector CTL (Fig. 8B). Therefore, the 9-mer peptide RAIEAQQHL from the HIV-1 envelope is also an HLA-CW0304-restricted epitope. Alignment of the amino acid sequences of each of the env constructs in this region showed sequence conservation for subtypes A through G (and CRF01_AE) but two central amino acid substitutions in the subtype H vT239R env gene product corresponding to a lack of reactivity to this construct in both the Elispot and CTL assays (Fig. 8C).
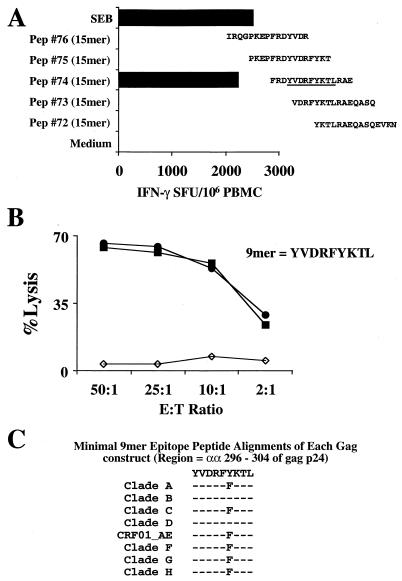
The CD8 response of subject VAIP-4, who showed a gag-specific response across all subtypes in both the Elispot and the post-in-vitro-stimulation CTL assays, was also mapped using minimal peptides. Using a panel of 15-mer peptides overlapping by 11 amino acids (subtype B gag HXB2 sequence), which covers the region of the 20-mer peptide (92UG037 gag 291-310), a single 15-mer peptide was shown to stimulate IFN-γ production directly from PBMC in a 20-h Elispot assay (Fig. 9A). This peptide alone contained a 9-mer sequence which fits well with the described HLA-A0207 peptide binding motif (69).
FIG. 9.
Minimal epitope mapping of the gag-specific cross-subtype CD8 response of subject VAIP-4. (A) Overlapping 15-mer peptides (Pep) (representing HXB2 gag) were used to minimally map the epitope directly from cryopreserved PBMC. Only one 15-mer (peptide 74) contains the 9-mer peptide YVDRFYKTL (gag 296-304) (underlined). (B) Percent specific killing of the parent 15-mer peptide (peptide 74, solid squares) and the minimal 9-mer peptide (solid circles). Effector cells were generated by 20-mer peptide stimulation (peptide 30 from 92UG037 gag) of cryopreserved PBMC, and target cells (autologous BLCL) were pulsed overnight with each peptide at 10 μg/ml. (C) Alignment of the amino acid sequences for each of the gag constructs used in the study covering the minimal epitope.
Since this subject was homozygous for HLA-A0207, the 9-mer peptide YVDRFYKTL (HXB2 gag 296-304) was synthesized and tested for specificity in a CTL assay following in vitro stimulation of PBMC with the 20-mer gag peptide from 92UG037 (specificity shown in Fig. 5E). As shown in Fig. 9B, the 9-mer (HXB2 gag 296-304)-pulsed autologous BLCL targets were lysed as efficiently as the parent 15-mer (HXB2 gag 293-307)-pulsed targets, demonstrating that it is indeed the minimal epitope. An alignment of the amino acid sequences of each of the gag constructs in this region showed sequence conservation for all subtypes except for a phenylalanine to tyrosine substitution at amino acid position 301 (Fig. 9C).
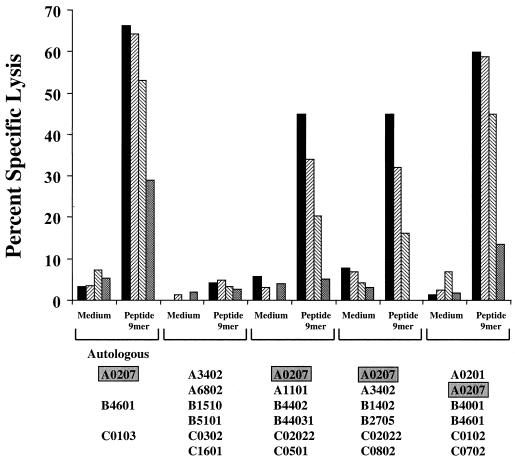
Data in Fig. 10 confirm that the restricting allele for this epitope is HLA-A0207. Effector cells from the same gag 20-mer (92UG037 gag 291-310)-driven IVS culture as above efficiently lysed only partially HLA-matched BLCL which express the HLA-A0207 gene product.
FIG. 10.
HLA restriction analysis of the gag-specific cross-subtype CD8 response of subject VAIP-4 mapped to peptide YVDRFYKTL (gag 296-304). Effector cells were generated by 20-mer peptide stimulation (peptide 30 from 92UG037 gag) of cryopreserved PBMC, and target cells were pulsed overnight with peptide at 10 μg/ml. Percent specific killing of autologous and partially HLA class I-matched BLCL is shown at E:T ratios of 50:1 (solid bars), 25:1 (Z-hatched bars), 10:1 (S-hatched bars), and 2:1 (stippled bars). The HLA class I type of each target cell is shown along the x axis.
DISCUSSION
The identification of CTL epitopes is a costly, time-consuming, labor-intensive, and difficult process. In the case of HIV-1, this procedure is made all the more arduous by the genetic diversity of the virus. Using overlapping peptides (usually 15 to 20 amino acids in length) spanning a given protein, a response can be mapped to a single peptide or two overlapping peptides. Truncated peptides are then used to map the minimal or optimal epitope. If multiple proteins are to be mapped, the cost of peptides and the number of available cells (usually PBMC) make the procedure almost impossible. Even when applying a method based on overlapping pools of peptides in a matrix format, it is difficult to test all the expressed proteins from even a single genetic isolate of HIV-1.
The use of computer-assisted bioinformatics approaches based on HLA peptide binding motifs and available virus sequence data has been shown to be a useful tool for identifying CTL epitopes derived from HIV-1 (2, 9, 38). While these methods may ultimately prove to be the technique of choice for epitope screening, they still require knowledge-based algorithms. Since binding motifs have not yet been defined for all HLA alleles and peptide binding is not the sole determinant of epitope dominance, not all CTL epitopes will be identified by HLA motif-screening algorithms. Therefore, empirical experimental-based procedures still have a role in the elucidation of CTL epitopes.
The recombinant vaccinia virus-based Elispot assay employed here has several advantages when screening PBMC for cross-subtype CTL responses. The assay is optimized for using cryopreserved PBMC, which permits retrospective analysis of samples of interest in a batch format. A minimum of 15 × 106 PBMC—with CD8 depletion to determine the responding phenotype (screening gag, env, and nef from seven subtypes and one CRF)—are required to perform the assay as presented here. In agreement with the results of Huang et al. (36), the use of vaccinia virus-infected BLCL as supplemental APC increases the sensitivity of detecting CD8 responses from cryopreserved PBMC over the direct vaccinia virus addition method (50). The greater sensitivity and reproducibility achieved by this method may be attributed to the addition of graded numbers of APC expressing high levels of viral antigen directly available to the class I processing pathway, rather than relying on the infectibility and functional integrity of APC present in the cryopreserved PBMC. Importantly, the negative immunoregulatory effects of vaccinia virus on IFN-γ production from PBMC and on dendritic cell maturation may be avoided (26, 39). In addition, it is important to note that resting the postthaw cryopreserved PBMC overnight also increases the sensitivity of the assay. While an important caveat for the method used here is the generation of autologous BLCL prior to performing the assay, this is not a serious drawback. It is foreseeable that high background responses to vaccinia virus or to Epstein-Barr virus (EBV) antigens expressed by the BLCL could in some cases conceal low-frequency CD8 responses.
The results presented here are an important extension of previous studies aimed at determining and characterizing the breadth of subtype recognition of HIV-specific CD8 responses. Here it is shown that broadly cross-subtype-reactive CD8 cells are detectable at a high frequency in the circulating PBMC of HIV-1-seropositive subjects. While the sample size here is small (11 subjects), more than half (six) had a CD8 response to at least one major HIV-1 gene product which was conserved across at least seven of the eight genetically distinct constructs tested. Previous studies of CD8 cross-subtype responses to HIV-1 have used traditional chromium release cytotoxicity assays following IVS to measure the responses (8, 28, 53, 55, 78, 79). While these studies have been important in showing functional cross-reactivity between some subtypes, the outcome is dependent on relative outgrowth of effector CTL clones in the in vitro culture system.
In the system used here, CD8 function was measured after only 20 to 24 h of culture and directly quantitated for multiple proteins from multiple HIV-1 subtypes. Critically, it was shown in three subjects that the cross-subtype reactivity was not limited to functional upregulation of IFN-γ production, but was also mediated by cytotoxic mechanisms from in vitro-expanded effector CTL. The cytotoxic activity showed direct concordance with the Elispot responses, highlighting the tight linkage of these two functions of CTL. The number of different subtypes tested in this study exceeds that used in other cross-subtype studies and provides good coverage of the global diversity of the virus. These results suggest that within North Americans and Thais at least, cross-recognition of geographically diverse subtypes of HIV-1 is a common feature of CD8 T cells. Therefore, the subtype that an immunogen is based on may not be the deciding factor for vaccine efficacy.
Another important finding of this study is that once detected using the panel of recombinant vaccinia viruses, cross-subtype CD8 responses can be rapidly mapped down to the 20-mer peptide level. By applying the matrix pool peptide method described for flow cytometric analysis (6, 7, 42) directly following the recombinant vaccinia virus screening assays, cross-subtype-reactive epitopes may be mapped in 3 days. Of note was the observation that the cross-subtype responses mapped here for each of the three subjects were all mediated by a single peptide. While the Elispot responses from the PBMC to the recombinant vaccinia viruses showed a similar magnitude between the different subtypes, it was necessary to show that these responses were against the same epitope(s). Since the in vitro stimulation cultures for generating effector CTL were performed with the identified 20-mer peptides, it was clear that the responses were most likely against the same epitope present in each subtype. This was confirmed by minimal epitope mapping with 15-mer and optimal-length peptides of 9 to 10 amino acids. This ability to rapidly map cross-subtype CTL epitope-containing regions may be of benefit to the design of vaccine constructs which contain fusions of CTL epitope regions (21, 32).
Although each of the three optimal epitopes mapped in this study had been described previously, two displayed novel HLA restrictions. The gag 296-304 epitope YVDRFYKTL (HXB2 numbering system [45]) had been described in two previous studies as being restricted by either HLA-A2601 or HLA-B70 (24, 59). A recent study also identified the same epitope but with a single substitution, YVDRF FKTL (mutation in italics), which was restricted by HLA-B1510 (58). Here it is shown conclusively, using partially HLA class I-matched APC, that HLA-A0207 also presents this epitope and that both major variants of the epitope are recognized equally well. It should be noted that this epitope matches precisely the published HLA-A0207 motif, with a valine at position 2, an aspartic acid at position 3, and a leucine at position 9 (69, 72). Since the subject studied here was Thai and the allele frequency of HLA-A0207 is relatively high (≈10%) in the Thai population (64), it may be prudent to study further the recognition of this epitope in the Thai population.
To date there have been relatively few studies which have focused on epitope identification in Thailand (9, 53, 71), and this study highlights the need for further such work to be performed. The second epitope to demonstrate a novel HLA restriction was env 557-565 (HXB2 numbering system [45]). This epitope is shown here to be restricted by HLA-CW0304, as opposed to results of a previous study which showed it to be restricted by HLA-B51 (70). Taken together, these findings raise the possibility of different populations' being able to recognize the same epitopes from HIV-1 restricted by different HLA alleles. Such degeneracy of peptide binding and subsequent CTL recognition in humans has been described for the malaria parasite Plasmodium falciparum (23) and for EBV (52), but not for HIV-1. Further identification of such epitopes will be of benefit to the design of globally effective HIV-1 vaccines.
It is significant that each of the epitopes defined here is not only conserved, but processed and presented from the context of high intersubtype sequence diversity. All responses measured here are based on the de novo synthesis of protein in vaccinia virus-infected cells and the natural antigen processing and presentation of these epitopes in the context of HLA class I molecules. It has been suggested by several studies that the amino acid residues flanking an epitope may impact the efficiency of epitope processing, the availability of that peptide epitope for loading into nascent HLA class I molecules, and hence subsequent CTL recognition (4, 5, 22, 25, 82, 83). In HIV-1 infection, there have been mixed results concerning the ability of flanking sequence to affect epitope availability and presentation. The central immunogenic region of the nef protein was reported to elicit impaired CTL recognition due to non-epitope-flanking sequence variation (20), while the dominant HLA-A0201 epitope SLYNTVATL (gag amino acids 77 to 85) is efficiently processed and presented despite multiple variations in the flanking sequences (13). The present study, however, provides no evidence that CTL epitope-flanking residues affect epitope availability or provide an escape mechanism from CTL recognition. In the case of the three epitopes mapped in detail here, the epitopes should in theory be within the context of maximal sequence variation present in HIV-1, since the recombinant vaccinia viruses used represent the major divergent virus subtypes.
There is a growing consensus that the generation of broadly cross-subtype-reactive CTL responses will constitute an important function of an efficacious HIV-1 vaccine (33, 54, 57). Therefore, identification of broadly cross-subtype-reactive CTL epitopes from naturally infected HIV-1-seropositive individuals will help define the precise sequence content of such a vaccine product. The use of a panel of recombinant vaccinia viruses expressing the HIV-1 gag, env, and nef gene products from the principal subtypes of the virus representing the global pandemic and an Elispot format assay allow rapid elucidation of cross-subtype CTL reactivity. By coupling such an assay with a matrix format of overlapping 20-mer peptides, broadly cross-subtype-reactive immunodominant CTL epitopes can be mapped in 3 days for any given individual independent of the HLA type. This assay format will be useful for identifying broadly cross-reactive CTL epitopes for incorporation into a globally efficacious HIV-1 vaccine. In addition, this format assay may be useful for screening CTL responses in current HIV vaccine trials to assess their global applicability.
Acknowledgments
We thank Bruce Levine and Carl June (University of Pennsylvania) for kindly providing the leukopheresis PBMC. We are also grateful to Nancy Reinsmoen (Duke University) for HLA typing of some specimens.
Financial support was provided by Department of Defense collaborative agreement DAMD17-98-2-8007.
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of Army or Defense.
REFERENCES
- 1.Allen, T. M., D. H. O'Connor, P. Jing, J. L. Dzuris, B. R. Mothe, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viraemia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 2.Altfeld, M. A., B. Livingston, N. Reshamwala, P. T. Nguyen, M. M. Addo, A. Shea, M. Newman, J. Fikes, J. Sidney, P. Wentworth, R. Chesnut, R. L. Eldridge, E. S. Rosenberg, G. K. Robbins, C. Brander, P. E. Sax, S. Boswell, T. Flynn, S. Buchbinder, P. J. Goulder, B. D. Walker, A. Sette, and S. A. Kalams. 2001. Identification of novel HLA-A2-restricted human immunodeficiency virus type 1-specific cytotoxic T-lymphocyte epitopes predicted by the HLA-A2 supertype peptide-binding motif. J. Virol. 75:1301-1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Artenstein, A. W., T. C. VanCott, J. R. Mascola, J. K. Carr, P. A. Hegerich, J. Gaywee, E. Sanders-Buell, M. L. Robb, D. E. Dayhoff, S. Thitivichianlert, S. Nitayaphan, J. G. McNeil, D. L. Birx, R. A. Michael, D. S. Burke, and F. E. McCutchan. 1995. Dual infection with human immunodeficiency virus type 1 of distinct envelope subtypes in humans. J. Infect. Dis. 171:805-810. [DOI] [PubMed] [Google Scholar]
- 4.Bergmann, C. C., L. Tong, R. Cua, J. Sensintaffar, and S. Stohlman. 1994. Differential effects of flanking residues on presentation of epitopes from chimeric peptides. J. Virol. 68:5306-5310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergmann, C. C., Q. Yao, C. K. Ho, and S. L. Buckwold. 1996. Flanking residues alter antigenicity and immunogenicity of multi-unit CTL epitopes. J. Immunol. 157:3242-3249. [PubMed] [Google Scholar]
- 6.Betts, M. R., J. P. Casazza, and R. A. Koup. 2001. Monitoring HIV-specific CD8+ T cell responses by intracellular cytokine production. Immunol. Lett. 79:117-125. [DOI] [PubMed] [Google Scholar]
- 7.Betts, M. R., J. P. Casazza, B. A. Patterson, S. Waldrop, W. Trigona, T. M. Fu, F. Kern, L. J. Picker, and R. A. Koup. 2000. Putative immunodominant human immunodeficiency virus-specific CD8+ T-cell responses cannot be predicted by major histocompatibility complex class I haplotype. J. Virol. 74:9144-9151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Betts, M. R., J. Krowka, C. Santamaria, K. Balsamo, F. Gao, G. Mulundu, C. Luo, N. N"Gandu, H. Sheppard, B. H. Hahn, S. Allen, and J. A. Frelinger. 1997. Cross-clade human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte responses in HIV-infected Zambians. J. Virol. 71:8908-8911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bond, K. B., B. Sriwanthana, T. W. Hodge, A. S. De Groot, T. D. Mastro, N. L. Young, N. Promadej, J. D. Altman, K. Limpakarnjanarat, and J. M. McNicholl. 2001. An HLA directed molecular and bioinformatics approach identifies new HLA-A11 HIV-1 subtype E cytotoxic T lymphocyte epitopes in HIV-1-infected Thais. AIDS Res. Hum. Retrovir. 17:703-717. [DOI] [PubMed] [Google Scholar]
- 10.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 12.Brander, C., and B. D. Walker. 1999. T lymphocyte responses in HIV-1 infection: implications for vaccine development. Curr. Opin. Immunol. 11:451-459. [DOI] [PubMed] [Google Scholar]
- 13.Brander, C., O. O. Yang, N. G. Jones, Y. Lee, P. Goulder, R. P. Johnson, A. Trocha, D. Colbert, C. Hay, S. Buchbinder, C. C. Bergmann, H. J. Zweerink, S. Wolinsky, W. A. Blattner, S. A. Kalams, and B. D. Walker. 1999. Efficient processing of the immunodominant, HLA-A*0201-restricted human immunodeficiency virus type 1 cytotoxic T-lymphocyte epitope despite multiple variations in the epitope flanking sequences. J. Virol. 73:10191-10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao, H., P. Kanki, J. L. Sankale, A. Dieng-Sarr, G. P. Mazzara, S. A. Kalams, B. Korber, S. Mboup, and B. D. Walker. 1997. Cytotoxic T-lymphocyte cross-reactivity among different human immunodeficiency virus type 1 clades: implications for vaccine development. J. Virol. 71:8615-8623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cao, H., I. Mani, R. Vincent, R. Mugerwa, P. Mugyenyi, P. Kanki, J. Ellner, and B. D. Walker. 2000. Cellular immunity to human immunodeficiency virus type 1 (HIV-1) clades: relevance to HIV-1 vaccine trials in Uganda. J. Infect. Dis. 182:1350-1356. [DOI] [PubMed] [Google Scholar]
- 16.Carmichael, A., X. Jin, P. Sissons, and L. Borysiewicz. 1993. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein-Barr virus in late disease. J. Exp. Med. 177:249-256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carr, J. K., M. Avila, M. Gomez Carrillo, H. Salomon, J. Hierholzer, V. Watanaveeradej, M. A. Pando, M. Negrete, K. L. Russell, J. Sanchez, D. L. Birx, R. Andrade, J. Vinoles, and F. E. McCutchan. 2001. Diverse BF recombinants have spread widely since the introduction of HIV-1 into South America. AIDS 15:F41-F47. [DOI] [PubMed] [Google Scholar]
- 18.Carr, J. K., J. N. Torimiro, N. D. Wolfe, M. N. Eitel, B. Kim, E. Sanders-Buell, L. L. Jagodzinski, D. Gotte, D. S. Burke, D. L. Birx, and F. E. McCutchan. 2001. The AG recombinant IbNG and novel strains of group M HIV-1 are common in Cameroon. Virology 286:168-181. [DOI] [PubMed] [Google Scholar]
- 19.Cheynier, R., P. Langlade-Demoyen, M. R. Marescot, S. Blanche, G. Blondin, S. Wain-Hobson, C. Griscelli, E. Vilmer, and F. Plata. 1992. Cytotoxic T lymphocyte responses in the peripheral blood of children born to human immunodeficiency virus-1-infected mothers. Eur. J. Immunol. 22:2211-2217. [DOI] [PubMed] [Google Scholar]
- 20.Couillin, I., B. Culmann-Penciolelli, E. Gomard, J. Choppin, J. P. Levy, J. G. Guillet, and S. Saragosti. 1994. Impaired cytotoxic T lymphocyte recognition due to genetic variations in the main immunogenic region of the human immunodeficiency virus 1 NEF protein. J. Exp. Med. 180:1129-1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Groot, A. S., A. Bosma, N. Chinai, J. Frost, B. M. Jesdale, M. A. Gonzalez, W. Martin, and C. Saint-Aubin. 2001. From genome to vaccine: in silico predictions, ex vivo verification. Vaccine 19:4385-4395. [DOI] [PubMed] [Google Scholar]
- 22.Del Val, M., H. J. Schlicht, T. Ruppert, M. J. Reddehase, and U. H. Koszinowski. 1991. Efficient processing of an antigenic sequence for presentation by MHC class I molecules depends on its neighboring residues in the protein. Cell 66:1145-1153. [DOI] [PubMed] [Google Scholar]
- 23.Doolan, D. L., S. L. Hoffman, S. Southwood, P. A. Wentworth, J. Sidney, R. W. Chesnut, E. Keogh, E. Appella, T. B. Nutman, A. A. Lal, D. M. Gordon, A. Oloo, and A. Sette. 1997. Degenerate cytotoxic T cell epitopes from P. falciparum restricted by multiple HLA-A and HLA-B supertype alleles. Immunity 7:97-112. [DOI] [PubMed] [Google Scholar]
- 24.Dorrell, L., T. Dong, G. S. Ogg, S. Lister, S. McAdam, T. Rostron, C. Conlon, A. J. McMichael, and S. L. Rowland-Jones. 1999. Distinct recognition of non-clade B human immunodeficiency virus type 1 epitopes by cytotoxic T lymphocytes generated from donors infected in Africa. J. Virol. 73:1708-1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Eisenlohr, L. C., J. W. Yewdell, and J. R. Bennink. 1992. Flanking sequences influence the presentation of an endogenously synthesized peptide to cytotoxic T lymphocytes. J. Exp. Med. 175:481-487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Engelmayer, J., M. Larsson, M. Subklewe, A. Chahroudi, W. I. Cox, R. M. Steinman, and N. Bhardwaj. 1999. Vaccinia virus inhibits the maturation of human dendritic cells: a novel mechanism of immune evasion. J. Immunol. 163:6762-6768. [PubMed] [Google Scholar]
- 27.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 28.Ferrari, G., W. Humphrey, M. J. McElrath, J. L. Excler, A. M. Duliege, M. L. Clements, L. C. Corey, D. P. Bolognesi, and K. J. Weinhold. 1997. Clade B-based HIV-1 vaccines elicit cross-clade cytotoxic T lymphocyte reactivities in uninfected volunteers. Proc. Natl. Acad. Sci. USA 94:1396-1401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao, F., D. L. Robertson, S. G. Morrison, H. Hui, S. Craig, J. Decker, P. N. Fultz, M. Girard, G. M. Shaw, B. H. Hahn, and P. M. Sharp. 1996. The heterosexual human immunodeficiency virus type 1 epidemic in Thailand is caused by an intersubtype (A/E) recombinant of African origin. J. Virol. 70:7013-7029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goulder, P. J., C. Brander, Y. Tang, C. Tremblay, R. A. Colbert, M. M. Addo, E. S. Rosenberg, T. Nguyen, R. Allen, A. Trocha, M. Altfeld, S. He, M. Bunce, R. Funkhouser, S. I. Pelton, S. K. Burchett, K. McIntosh, B. T. Korber, and B. D. Walker. 2001. Evolution and transmission of stable CTL escape mutations in HIV infection. Nature 412:334-338. [DOI] [PubMed] [Google Scholar]
- 31.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 32.Hanke, T., and A. J. McMichael. 2000. Design and construction of an experimental HIV-1 vaccine for a year 2000 clinical trial in Kenya. Nat. Med. 6:951-955. [DOI] [PubMed] [Google Scholar]
- 33.Heeney, J. L., and B. H. Hahn. 2000. Vaccines and immunology: elucidating immunity to HIV-1 and current prospects for AIDS vaccine development. AIDS 14:S125-S127. [PubMed] [Google Scholar]
- 34.Hickling, J. 1998. Measuring human T-lymphocyte function. Exp. Rev. Mol. Med. http://www-ermm.cbcu.cam.uk/jhc/txt001.htm. [Online.] [DOI] [PubMed]
- 35.Hoelscher, M., B. Kim, L. Maboko, F. Mhalu, F. von Sonnenburg, D. L. Birx, and F. E. McCutchan. 2001. High proportion of unrelated HIV-1 intersubtype recombinants in the Mbeya region of southwest Tanzania. AIDS 15:1461-1470. [DOI] [PubMed] [Google Scholar]
- 36.Huang, X. L., Z. Fan, C. Kalinyak, J. W. Mellors, and C. R. Rinaldo, Jr. 2000. CD8+ T-cell gamma interferon production specific for human immunodeficiency virus type 1 (HIV-1) in HIV-1-infected subjects. Clin. Diagn. Lab. Immunol. 7:279-287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jin, X., C. G. Roberts, D. F. Nixon, J. T. Safrit, L. Q. Zhang, Y. X. Huang, N. Bhardwaj, B. Jesdale, A. S. DeGroot, and R. A. Koup. 2000. Identification of subdominant cytotoxic T lymphocyte epitopes encoded by autologous HIV type 1 sequences, using dendritic cell stimulation and computer-driven algorithm. AIDS Res. Hum. Retrovir. 16:67-76. [DOI] [PubMed] [Google Scholar]
- 39.Kalvakolanu, D. V. 1999. Virus interception of cytokine-regulated pathways. Trends Microbiol. 7:166-171. [DOI] [PubMed] [Google Scholar]
- 40.Kaslow, R. A., C. Rivers, J. Tang, T. J. Bender, P. A. Goepfert, R. El Habib, K. Weinhold, and M. J. Mulligan. 2001. Polymorphisms in HLA class I genes associated with both favorable prognosis of human immunodeficiency virus (HIV) type 1 infection and positive cytotoxic T-lymphocyte responses to ALVAC-HIV recombinant canarypox vaccines. J. Virol. 75:8681-8689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaul, R., T. Dong, F. A. Plummer, J. Kimani, T. Rostron, P. Kiama, E. Njagi, E. Irungu, B. Farah, J. Oyugi, R. Chakraborty, K. S. MacDonald, J. J. Bwayo, A. McMichael, and S. L. Rowland-Jones. 2001. CD8(+) lymphocytes respond to different HIV epitopes in seronegative and infected subjects. J. Clin. Investig. 107:1303-1310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kern, F., I. P. Surel, C. Brock, B. Freistedt, H. Radtke, A. Scheffold, R. Blasczyk, P. Reinke, J. Schneider-Mergener, A. Radbruch, P. Walden, and H. D. Volk. 1998. T-cell epitope mapping by flow cytometry. Nat. Med. 4:975-978. [DOI] [PubMed] [Google Scholar]
- 43.Klein, M., B. C. van, A. Holwerda, G. S. Kerkhof, R. Bende, I. Keet, J. Eeftinck-Schattenkerk, A. Osterhaus, H. Schuitemaker, and F. Miedema. 1995. Kinetics of Gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J. Exp. Med. 181:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koenig, S., A. J. Conley, Y. A. Brewah, G. M. Jones, S. Leath, L. J. Boots, V. Davey, G. Pantaleo, J. F. Demarest, C. Carter, C. Wannebo, J. R. Yanelli, S. A. Rosenberg, and H. C. Lane. 1995. Transfer of HIV-1-specific cytotoxic T lymphocytes to an AIDS patient leads to selection for mutant HIV variants and subsequent disease progression. Nat. Med. 1:330-336. [DOI] [PubMed] [Google Scholar]
- 45.Korber, B. T., B. T. Foley, C. L. Kuiken, S. K. Pillai, and J. G. Sodroski. 1998. Numbering positions in HIV relative to HXB2CG, p. IV-27-IV-35. In B. T. Korber, C. L. Kuiken, B. T. Foley, B. H. Hahn, F. McCutchan, J. W. Mellors, and J. G. Sodroski (ed.), Human retroviruses and AIDS 1998. Theoretical Biology and Biophysics Group, Los Alamos National Laboratory, Los Alamos, N.Mex.
- 46.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuroda, M. J., J. E. Schmitz, D. H. Barouch, A. Craiu, T. M. Allen, A. Sette, D. I. Watkins, M. A. Forman, and N. L. Letvin. 1998. Analysis of Gag-specific cytotoxic T lymphocytes in simian immunodeficiency virus-infected rhesus monkeys by cell staining with a tetrameric major histocompatibility complex class I-peptide complex. J. Exp. Med. 187:1373-1381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kuroda, M. J., J. E. Schmitz, W. A. Charini, C. E. Nickerson, M. A. Lifton, C. I. Lord, M. A. Forman, and N. L. Letvin. 1999. Emergence of CTL coincides with clearance of virus during primary simian immunodeficiency virus infection in rhesus monkeys. J. Immunol. 162:5127-5133. [PubMed] [Google Scholar]
- 49.Lalvani, A., R. Brookes, S. Hambleton, W. J. Britton, A. V. Hill, and A. J. McMichael. 1997. Rapid effector function in CD8+ memory T cells. J. Exp. Med. 186:859-865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Larsson, M., X. Jin, B. Ramratnam, G. S. Ogg, J. Engelmayer, M. A. Demoitie, A. J. McMichael, W. I. Cox, R. M. Steinman, D. Nixon, and N. Bhardwaj. 1999. A recombinant vaccinia virus based Elispot assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS 13:767-777. [DOI] [PubMed] [Google Scholar]
- 51.Letvin, N. L., J. E. Schmitz, H. L. Jordan, A. Seth, V. M. Hirsch, K. A. Reimann, and M. J. Kuroda. 1999. Cytotoxic T lymphocytes specific for the simian immunodeficiency virus. Immunol. Rev. 170:127-134. [DOI] [PubMed] [Google Scholar]
- 52.Levitsky, V., D. Liu, S. Southwood, J. Levitskaya, A. Sette, and M. G. Masucci. 2000. Supermotif peptide binding and degeneracy of MHC: peptide recognition in an EBV peptide-specific CTL response with highly restricted TCR usage. Hum. Immunol. 61:972-984. [DOI] [PubMed] [Google Scholar]
- 53.Lynch, J. A., M. deSouza, M. D. Robb, L. Markowitz, S. Nitayaphan, C. V. Sapan, D. L. Mann, D. L. Birx, and J. H. Cox. 1998. Cross-clade cytotoxic T cell response to human immunodeficiency virus type 1 proteins among HLA disparate North Americans and Thais. J. Infect. Dis. 178:1040-1046. [DOI] [PubMed] [Google Scholar]
- 54.Mascola, J. R., and G. J. Nabel. 2001. Vaccines for the prevention of HIV-1 disease. Curr. Opin. Immunol. 13:489-495. [DOI] [PubMed] [Google Scholar]
- 55.McAdam, S., P. Kaleebu, P. Krausa, P. Goulder, N. French, B. Collin, T. Blanchard, J. Whitworth, A. McMichael, and F. Gotch. 1998. Cross-clade recognition of p55 by cytotoxic T lymphocytes in HIV-1 infection. AIDS 12:571-579. [DOI] [PubMed] [Google Scholar]
- 56.McCutchan, F. E. 2000. Understanding the genetic diversity of HIV-1. AIDS 14:S31-S44. [PubMed] [Google Scholar]
- 57.McMichael, A. J., and S. L. Rowland-Jones. 2001. Cellular immune responses to HIV. Nature 410:980-987. [DOI] [PubMed] [Google Scholar]
- 58.Novitsky, V., N. Rybak, M. F. McLane, P. Gilbert, P. Chigwedere, I. Klein, S. Gaolekwe, S. Y. Chang, T. Peter, I. Thior, T. Ndung'u, F. Vannberg, B. T. Foley, R. Marlink, T. H. Lee, and M. Essex. 2001. Identification of human immunodeficiency virus type 1 subtype C Gag-, Tat-, Rev-, and Nef-specific Elispot-based cytotoxic T-lymphocyte responses for AIDS vaccine design. J. Virol. 75:9210-9228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ogg, G. S., T. Dong, P. Hansasuta, L. Dorrell, J. Clarke, R. Coker, G. Luzzi, C. Conlon, A. P. McMichael, and S. Rowland-Jones. 1998. Four novel cytotoxic T-lymphocyte epitopes in the highly conserved major homology region of HIV-1 Gag, restricted through B*4402, B*1801, A*2601, B*70 (B*1509). AIDS 12:1561-1563. [DOI] [PubMed] [Google Scholar]
- 60.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 61.Phillips, R. E., S. Rowland-Jones, D. F. Nixon, F. M. Gotch, J. P. Edwards, A. O. Ogunlesi, J. G. Elvin, J. A. Rothbard, C. R. Bangham, C. R. Rizza, A. R. M. Townsend, and A. J. McMichael. 1991. Human immunodeficiency virus genetic variation that can escape cytotoxic T cell recognition. Nature 354:453-459. [DOI] [PubMed] [Google Scholar]
- 62.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Renjifo, B., B. Chaplin, D. Mwakagile, P. Shah, F. Vannberg, G. Msamanga, D. Hunter, W. Fawzi, and M. Essex. 1998. Epidemic expansion of HIV type 1 subtype C and recombinant genotypes in Tanzania. AIDS Res. Hum. Retrovir. 14:635-638. [DOI] [PubMed] [Google Scholar]
- 64.Romphruk, A., C. Leelayuwat, S. Barusrux, C. Puapairoj, and Y. Urwijitaroon. 1997. DNA typing of the HLA-A, -B and -C genes: possible MHC class I haplotypes in the northeastern-Thais. J. Med. Assoc. Thai. 80:S13-S19. [PubMed] [Google Scholar]
- 65.Rowland-Jones, S., J. Sutton, K. Ariyoshi, T. Dong, F. Gotch, S. McAdam, D. Whitby, S. Sabally, A. Gallimore, T. Corrah, M. Takiguchi, T. Schultz, A. J. McMichael, and H. Whittle. 1995. HIV-specific cytotoxic T-cells in HIV-exposed but uninfected Gambian women. Nat. Med. 1:59-64. [DOI] [PubMed] [Google Scholar]
- 66.Rowland-Jones, S. L., D. F. Nixon, M. C. Aldhous, F. Gotch, K. Ariyoshi, N. Hallam, J. S. Kroll, K. Froebel, and A. McMichael. 1993. HIV-specific cytotoxic T-cell activity in an HIV-exposed but uninfected infant. Lancet 341:860-861. [DOI] [PubMed] [Google Scholar]
- 67.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 68.Sette, A., and J. Sidney. 1998. HLA supertypes and supermotifs: a functional perspective on HLA polymorphism. Curr. Opin. Immunol. 10:478-482. [DOI] [PubMed] [Google Scholar]
- 69.Sidney, J., M. F. del Guercio, S. Southwood, G. Hermanson, A. Maewal, E. Appella, and A. Sette. 1997. The HLA-A*0207 peptide binding repertoire is limited to a subset of the A*0201 repertoire. Hum. Immunol. 58:12-20. [DOI] [PubMed] [Google Scholar]
- 70.Sipsas, N. V., S. A. Kalams, A. Trocha, S. He, W. A. Blattner, B. D. Walker, and R. P. Johnson. 1997. Identification of type-specific cytotoxic T lymphocyte responses to homologous viral proteins in laboratory workers accidentally infected with HIV-1. J. Clin. Investig. 99:752-762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sriwanthana, B., T. Hodge, T. D. Mastro, C. S. Dezzutti, K. Bond, H. A. Stephens, L. G. Kostrikis, K. Limpakarnjanarat, N. L. Young, S. H. Qari, R. B. Lal, D. Chandanayingyong, and J. M. McNicholl. 2001. HIV-specific cytotoxic T lymphocytes, HLA-A11, and chemokine-related factors may act synergistically to determine HIV resistance in CCR5 delta32-negative female sex workers in Chiang Rai, northern Thailand. AIDS Res. Hum. Retrovir. 17:719-734. [DOI] [PubMed] [Google Scholar]
- 72.Sudo, T., N. Kamikawaji, A. Kimura, Y. Date, C. J. Savoie, H. Nakashima, E. Furuichi, S. Kuhara, and T. Sasazuki. 1995. Differences in MHC class I self peptide repertoires among HLA-A2 subtypes. J. Immunol. 155:4749-4756. [PubMed] [Google Scholar]
- 73.Tzeng, C. M., E. J. Adams, J. E. Gumperz, L. Percival, R. S. Wells, P. Parham, and L. D. Barber. 1996. Peptides bound endogenously by HLA Cw*0304 expressed in LCL 721.221 cells include a peptide derived from HLA E. Tissue Antigens 48:325-328. [DOI] [PubMed] [Google Scholar]
- 74.Walker, B. D., S. Chakrabarti, B. Moss, T. J. Paradis, T. Flynn, A. G. Durno, R. S. Blumberg, J. C. Kaplan, M. S. Hirsch, and R. T. Schooley. 1987. HIV-specific cytotoxic T lymphocytes in seropositive individuals. Nature 328:345-348. [DOI] [PubMed] [Google Scholar]
- 75.Walker, B. D., and B. T. Korber. 2001. Immune control of HIV: the obstacles of HLA and viral diversity. Nat. Immunol. 2:473-475. [DOI] [PubMed] [Google Scholar]
- 76.Weniger, B. G., K. Limpakarnjanarat, K. Ungchusak, S. Thanprasertsuk, K. Choopanya, S. Vanichseni, T. Uneklabh, P. Thongcharoen, and C. Wasi. 1991. The epidemiology of HIV infection and AIDS in Thailand. AIDS 5:S71-S85. [DOI] [PubMed] [Google Scholar]
- 77.Wilson, C. C., R. C. Brown, B. T. Korber, B. M. Wilkes, D. J. Ruhl, D. Sakamoto, K. Kunstman, K. Luzuriaga, I. C. Hanson, S. M. Widmayer, A. Wiznia, S. Clapp, A. J. Ammann, R. A. Koup, S. M. Wolinsky, and B. D. Walker. 1999. Frequent detection of escape from cytotoxic T-lymphocyte recognition in perinatal human immunodeficiency virus (HIV) type 1 transmission: the Ariel project for the prevention of transmission of HIV from mother to infant. J. Virol. 73:3975-3985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wilson, C. C., S. A. Kalams, B. M. Wilkes, D. J. Ruhl, F. Gao, B. H. Hahn, I. C. Hanson, K. Luzuriaga, S. Wolinsky, R. Koup, S. P. Buchbinder, R. P. Johnson, and B. D. Walker. 1997. Overlapping epitopes in human immunodeficiency virus type 1 gp120 presented by HLA-A, B, and C molecules: effects of viral variation on cytotoxic T-lymphocyte recognition. J. Virol. 71:1256-1264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wilson, S. E., S. L. Pedersen, J. C. Kunich, V. L. Wilkins, D. L. Mann, G. P. Mazzara, J. Tartaglia, C. L. Celum, and H. W. Sheppard. 1998. Cross-clade envelope glycoprotein 160-specific CD8+ cytotoxic T lymphocyte responses in early HIV type 1 clade B infection. AIDS Res. Hum. Retrovir. 14:925-937. [DOI] [PubMed] [Google Scholar]
- 80.Yang, O. O., S. A. Kalams, A. Trocha, H. Cao, A. Luster, R. P. Johnson, and B. D. Walker. 1997. Suppression of human immunodeficiency virus type 1 replication by CD8+ cells: evidence for HLA class I-restricted triggering of cytolytic and noncytolytic mechanisms. J. Virol. 71:3120-3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Yang, O. O., and B. D. Walker. 1997. CD8+ cells in human immunodeficiency virus type I pathogenesis: cytolytic and noncytolytic inhibition of viral replication. Adv. Immunol. 66:273-311. [DOI] [PubMed] [Google Scholar]
- 82.Yellen-Shaw, A. J., and L. C. Eisenlohr. 1997. Regulation of class I-restricted epitope processing by local or distal flanking sequence. J. Immunol. 158:1727-1733. [PubMed] [Google Scholar]
- 83.Yellen-Shaw, A. J., E. J. Wherry, G. C. Dubois, and L. C. Eisenlohr. 1997. Point mutation flanking a CTL epitope ablates in vitro and in vivo recognition of a full-length viral protein. J. Immunol. 158:3227-3234. [PubMed] [Google Scholar]