Abstract
Hepatitis B virus (HBV) capsids play an important role in viral nucleic acid metabolism and other elements of the virus life cycle. Misdirection of capsid assembly (leading to formation of aberrant particles) may be a powerful approach to interfere with virus production. HBV capsids can be assembled in vitro from the dimeric capsid protein. We show that a small molecule, bis-ANS, binds to capsid protein, inhibiting assembly of normal capsids and promoting assembly of noncapsid polymers. Using equilibrium dialysis to investigate binding of bis-ANS to free capsid protein, we found that only one bis-ANS molecule binds per capsid protein dimer, with an association energy of −28.0 ± 2.0 kJ/mol (−6.7 ± 0.5 kcal/mol). Bis-ANS inhibited in vitro capsid assembly induced by ionic strength as observed by light scattering and size exclusion chromatography. The binding energy of bis-ANS for capsid protein calculated from assembly inhibition data was −24.5 ± 0.9 kJ/mol (−5.9 ± 0.2 kcal/mol), essentially the same binding energy observed in studies of unassembled protein. These data indicate that capsid protein bound to bis-ANS did not participate in assembly; this mechanism of assembly inhibition is analogous to competitive or noncompetitive inhibition of enzymes. While assembly of normal capsids is inhibited, our data suggest that bis-ANS leads to formation of noncapsid polymers. Evidence of aberrant polymers was identified by light scattering and electron microscopy. We propose that bis-ANS acts as a molecular “wedge” that interferes with normal capsid protein geometry and capsid formation; such wedges may represent a new class of antiviral agent.
Hepatitis B virus (HBV) causes chronic and acute infections. Worldwide, more than 350 million people suffer from chronic infection, with an annual mortality rate of approximately 1 million people (21). HBV is an enveloped DNA virus with an icosahedral core, or capsid. In vivo, HBV capsids assemble around an RNA-reverse transcriptase complex (2). Assembly of the capsid is required for reverse transcription of the RNA pregenome to the mature DNA form (reviewed in references 7 and 21). In HBV, the dominant form of capsid (25) is composed of 120 copies of the capsid protein dimer (6, 10, 29). Though assembly is robust in vitro (3, 23, 29, 35), even modest mutations of the capsid protein can have dramatic effects on the viability of progeny virus (12, 18, 19, 30, 31). This suggests that capsid assembly could be an effective therapeutic target for chronic HBV. Though interfering with assembly is a likely strategy for antiviral intervention, to the authors' knowledge this approach has not been used with any virus.
Capsid formation using the assembly domain of the HBV capsid protein has been examined (29, 34). The truncated capsid protein consists of the first 149 amino acids and lacks the C-terminal, 34-residue RNA-binding domain. The assembly domain forms a stable dimer (29, 32), hereafter referred to as Cp1492. Cp1492 assembles rapidly and efficiently in response to increased levels of ionic strength, mainly forming T=4 icosahedral particles (34). The yield of particles depends on the protein and salt concentrations (35).
The HBV capsid protein, as well as Cp1492, appears to undergo a modest conformational transition concomitant with assembly. An epitope, located at the dimer interface on the exterior of the capsid, reacts with different antibodies depending on whether the protein is part of a capsid or is a free dimer (5, 17, 22). Nonetheless, circular dichroism spectra for capsids and dimers are virtually identical (29).
To probe this conformational change, we examined Cp1492 interaction with bis-ANS (5,5′-bis[8-(phenylamino)-1-naphthalenesulfonate]). Bis-ANS is a fluorescent probe that binds hydrophobic sites on proteins and in particular to molten globules (13, 16, 20, 24, 26). It inhibits microtubule polymerization by binding tubulin (9) and also inhibits bacteriophage P22 capsid formation by binding capsid and scaffold proteins (28).
In this work, we show that bis-ANS binds HBV capsid protein but not capsids, thus inhibiting and misdirecting assembly. Based on our growing understanding of capsid assembly, we identified the characteristics of assembly inhibitors and related them to their effect on the assembly process—inhibiting and/or misdirecting polymerization of capsid protein. Assembly reactions may be accessible to inhibition in a broad spectrum of viruses. We hope that this demonstration using HBV encourages efforts to discover antivirals that target capsid assembly.
MATERIALS AND METHODS
Preparation of samples and spectroscopy.
Cp1492 was purified from an Escherichia coli expression system as previously described (34) except that an additional assembly and dissociation step was added to remove inactive protein. The assembly-disassembly process was accomplished by first dialyzing freshly prepared 1-mg/ml dimer into reaction buffer (50 mM HEPES [pH 7.5], 2 mM dithiothreitol [DTT]). Assembly was initiated by adding NaCl to reach a final concentration of 0.5 M NaCl. Assembled capsids were separated from dimers by size exclusion chromatography (SEC) by using a Sephacryl S300 column equilibrated with reaction buffer plus 0.5 M NaCl (all chromatography equipment was from Amersham-Pharmacia, Piscataway, N.J.). The capsid fractions were pooled and concentrated to 1 mg/ml by using a pressure concentrator (Millipore Corp., Bedford, Mass.). Capsids were then dissociated by adding solid urea to achieve a final concentration of 3.0 M. After 1.5 h in urea at 4°C, freshly dissociated dimer was isolated by SEC on a Sephacryl S-300 column equilibrated with storage buffer (0.1 M NaHCO3 [pH 9.6], 2 mM DTT). This material was substantially more active and could be stored at −70° for several months without a loss of activity. Protein was quantified by absorbance by using an ɛ280 of 60,900 M−1 cm−1 for Cp149 dimers (Cp1492). Bis-ANS (Sigma, St. Louis, Mo.) was quantified by using an ɛ394 of 23,000 M−1 and an ɛ280 of 47,100 M−1.
Except as noted, Cp1492 was dialyzed from storage buffer into reaction buffer before examination of bis-ANS binding and capsid assembly. Assembly was driven by dilution of the sample with an equal volume of buffered NaCl.
Fluorescence and circular dichroism spectroscopy.
Fluorescence spectra were recorded at 20°C with a SPEX Fluoromax fluorometer by using a 3-mm-path-length cuvette (Hellma, Forest Hills, N.Y.). All slits were set for a 1-nm band pass. Circular dichroism spectra were recorded at 20°C on a Jasco J-715 spectropolarimeter equipped with a thermostatted cell holder. All spectra were recorded with 10 μM Cp1492 in 25 mM HEPES at 20°C by using a 1-mm-path cuvette (Hellma, Forest Hills, N.Y.).
Equilibrium dialysis.
Equilibrium dialysis experiments were performed in a Hoeffer rotating equilibrium dialysis apparatus with eight 500-μl chambers per cassette. Chambers were divided into two halves by a 10-kDa-nominal-molecular-weight-cutoff dialysis membrane (Amersham-Pharmacia). Samples were given 72 h to equilibrate, based on control chambers with bis-ANS and no protein.
Capsid assembly at equilibrium.
Cp1492 at 20 μM in reaction buffer was incubated for 1 h with bis-ANS prior to assembly. Assembly was initiated by adding 1 volume of buffered NaCl solution at the appropriate concentration. Assembly reactions were allowed to equilibrate for 24 h. Longer incubation, up to 72 h, did not detectably increase the yield of capsid. Samples were separated into capsid and dimer fractions by SEC using a 26-ml Sephacryl S-300 column on an AKTA-FPLC chromatography workstation. Peaks were quantified at 280 nm and integrated by using Unicorn software. Both DTT and bis-ANS adsorbed weakly to the column and eluted later than the salt peak. Experiments were performed at 21°C.
Assembly kinetics.
Assembly kinetic experiments observing changes in light scattering at 320 nm were performed in a 0.3-cm fluorescence cuvette, as previously described (35). Samples were preincubated and assembly was initiated as described above. The bis-ANS inner-filter effect was corrected with a coefficient based on bis-ANS absorbance at 320 nm (ɛ320 = 8,400 M−1 cm−1) (11); cf. the inner-filter correction for 16 μM bis-ANS, which was 1.09.
Electron microscopy (EM).
Samples were adsorbed to freshly glow-discharged grids and stained with 2% uranyl acetate (8). Grids were carbon-coated collodion (EM Science, Fort Washington, Pa.) on a 300-mesh copper substrate. Samples were visualized on a JEOL 1200SX transmission electron microscope retrofitted with an AMT 2 kx2k (Danvers, Mass.) charge-coupled device camera. Images were typically recorded with a magnification of ×60,000.
Analysis of assembly inhibition.
Our model of assembly inhibition is summarized in Fig. 1. Calculations of dissociation constants Kcapsid and Kbis-ANS are based on assuming two equilibria (equations 1 and 2) and a mass-conservation law (equation 3).
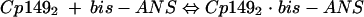 |
(1) |
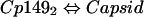 |
(2) |
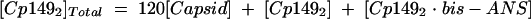 |
(3) |
FIG. 1.
Competing equilibria between binding bis-ANS (bA) and capsid assembly. Cp1493 with bound bis-ANS does not participate in capsid assembly. This diagram does not depict the possible presence of noncapsid polymer.
The equilibrium between Cp1492 and capsid was described by a dissociation constant, Kcapsid (equation 4). Though Kcapsid values are in the unusual units of M−119, the equilibrium expression is appropriate for spherical polymers such as capsids (33) and micelles (27). Because of the magnitude of the exponent, Kcapsid and all equations incorporating a 120th power were manipulated in logarithmic form. This approach to calculating Kcapsid yielded consistent results for dimer concentrations from 1 to 20 μM.
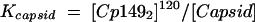 |
(4) |
Equation 3 was recast in terms of capsid concentration by substituting equation 4 for Cp1492 to yield equation 5. Solving equation 5 for the [Cp1492 · bis-ANS] complex requires values for Kcapsid and for [Capsid]. These were determined by SEC of reactions with and without bis-ANS.
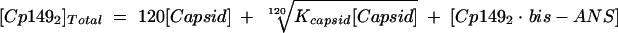 |
(5) |
The concentrations of [Cp1492]Total, known a priori, were consistent with the summed area of SEC capsid and dimer peaks. Values for [Capsid] and Kcapsid were determined experimentally. Given the concentration of the Cp1492 · bis-ANS complex, Kbis-ANS can be calculated from a mass-conservation law and an equilibrium expression (equations 6 and 7, respectively).
 |
(6) |
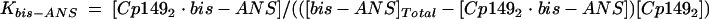 |
(7) |
RESULTS
We can postulate three classes of small molecules that would inhibit capsid assembly: competitive inhibitors, noncompetitive inhibitors, and misdirectors. In considering the process of capsid assembly, it is important to note that the subunits may be oligomeric and are necessarily multivalent. Competitive inhibitors would prevent assembly by binding at sites of subunit-subunit interaction; each multivalent subunit could bind several such molecules. Noncompetitive inhibitors would render subunit assembly incompetent by binding a location other than the contact sites. Misdirectors would function by binding to a (oligomeric) subunit and distorting it, without affecting its contact sites. This would allow a misdirected subunit to bind other subunits but prevent it from conforming to the geometry of the native capsid. In effect, a misdirector should be a molecular wedge. Similar strategies for assembly inhibition and misdirection were hypothesized based on theoretical considerations (14).
bis-ANS binds Cp1492.
Compared to the dye in solution, bis-ANS (30 μM) fluorescence increased by about 12-fold in the presence of 1 μM Cp1492 and the spectrum blue shifted by 20 nm (Fig. 2A). The fluorescence of bis-ANS was virtually the same in solution as in the presence of capsids; slight changes in spectrum could be attributed to contamination of capsids by 1 to 2% (wt/wt) Cp1492.
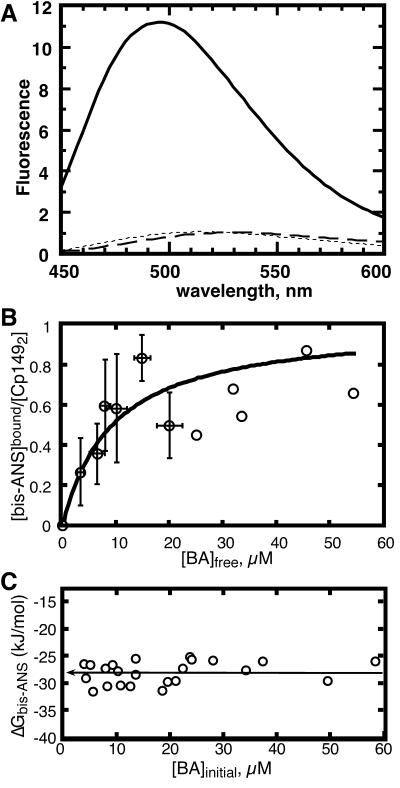
FIG. 2.
bis-ANS binds dimers but not capsids. (A) Spectra of 30 μM bis-ANS in 50 mM HEPES (pH 7.5) (short dashes) and with 1 μM Cp1492 capsids (long dashes) or 1 μM Cp1492 dimers (solid line). (B) The binding isotherm, as determined by equilibrium dialysis, was best fit for a complex of one bis-ANS per dimer with a dissociation constant (Kbis-ANS) of 9.3 ± 1.2 μM, corresponding to −28.3 kJ/mol [ΔG = −RT ln (Keq), where R is the gas constant of 8.31 kJ mol−1 K−1, T is 294 K, and Keq = 1/Kbis-ANS]. (C) The binding energy determined from individual equilibrium dialysis experiments. The average binding energy value for all measurements for concentrations of bis-ANS up to 60 μM was −28.0 ± 2.0 kJ/mol.
The stoichiometry and dissociation constant of bis-ANS for Cp1492 were determined by equilibrium dialysis. Equilibrium dialysis allows direct determination of the stoichiometry of bis-ANS per capsid protein dimer as well as determination of the Kbis-ANS at each concentration tested (Fig. 2C) and curve fitting the binding isotherm (Fig. 2B). The isotherm was best fit for a complex of one bis-ANS per dimer, binding with a Kbis-ANS of 9.3 ± 1.2 μM (ΔG = −28.3 kJ/mol). Despite the difficulty of acquiring consistent data (in large part due to the prolonged incubation at room temperature), the curve fit was in excellent agreement with the average binding energy of −28 ± 2.0 kJ/mol as determined by averaging the individual dialysis experiments. Efforts to accurately measure Kbis-ANS by fluorescence titration were not successful due to the weak binding and inner-filter effect (11) caused by bis-ANS absorbance at high concentrations. We were unable to isolate the complex from free bis-ANS due to the fast off rate of the dye; for example, samples that eluted through spin columns packed with Sepharose G-50 were stripped of all bound bis-ANS.
bis-ANS inhibits capsid assembly.
Binding of bis-ANS by dimer but not capsid led to the expression shown in Fig. 1 and the working hypothesis that bis-ANS is an inhibitor of assembly. The 1:1 stoichiometry of bis-ANS for Cp1492 suggested that it functions as a noncompetitive inhibitor. By comparison, a competitive inhibitor that binds to the intersubunit contact site would be expected to have a stoichiometry of 2:1, or one inhibitor for each protein in the dimer.
We tested bis-ANS for an inhibitory effect by examining the yield of capsid from assembly reactions that were allowed to approach equilibrium. Capsid was quantified using SEC (Fig. 3). SEC of HBV showed well-resolved capsid and dimer peaks, in which the capsid eluted shortly after the void volume (Fig. 3A, inset). Bis-ANS binding caused a proportional decrease in capsid production. Capsid peaks were not symmetrical because of nonspecific adsorption of capsids onto the packing; this result was independent of the presence of bis-ANS. However, earlier multiangle light scattering studies (not shown) have shown only capsid-sized protein oligomers eluting in this peak; velocity sedimentation experiments show evidence for capsids and dimers only (29). As expected, higher levels of ionic strength increased the yield of capsids (29, 35) (Fig. 3A and B).
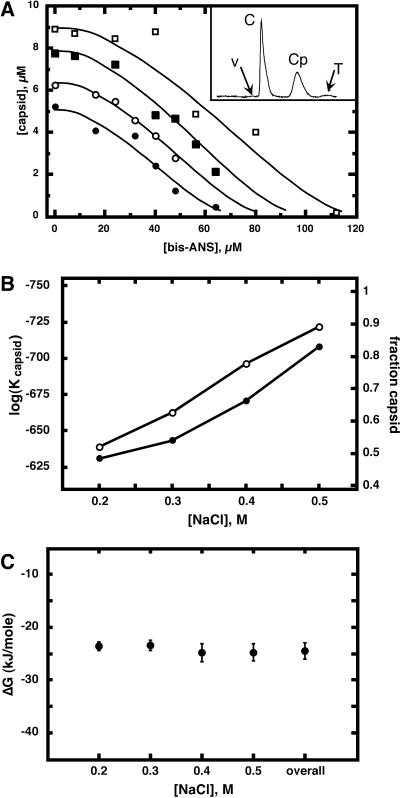
FIG. 3.
bis-ANS can act as a noncompetitive inhibitor of capsid assembly. (A) The yield of capsids, measured by SEC after a 24-h incubation (inset), decreases as a function of the presence of [bis-ANS]. Ionic strength levels affect capsid yield as shown as a function of the following NaCl concentrations: 0.2 M (filled circles), 0.3 M (open circles), 0.4 M (filled squares), and 0.5 M (open squares). In the chromatogram (inset), positions are marked for the void volume (V), capsid peak (C), Cp1492 (Cp), and total (T) volumes. (B) The value of log(Kcapsid) in the absence of bis-ANS is ionic-strength dependent (filled circles). For comparison, the mole fraction of capsids is also shown (open circles). (C) Unlike Kcapsid, Kbis-ANS for Cp1492 is independent of level of ionic strength. Error bars represent results for five determinations.
Both competitive and noncompetitive mechanisms inhibit assembly by forming a subunit-inhibitor complex and decreasing the concentration of assembly-active free subunits. Based on this model, we were able to calculate the affinity of free dimer for bis-ANS (see Materials and Methods). We first had to determine the association constant for the formation of capsid, Kcapsid, in the absence of bis-ANS (equation 4). The capsid association constant, expressed as log(Kcapsid), was seen to vary almost linearly with ionic strength level (Fig. 3B).
Though Kcapsid was sensitive to NaCl concentration, Kbis-ANS was almost independent of it (Fig. 3C). Kbis-ANS was calculated directly from inhibition of capsid assembly, based on the following assumptions: (i) only capsid protein without bis-ANS could assemble into capsids, (ii) an equilibrium exists between capsids and Cp1492 (Fig. 1), and (iii) only capsids eluted in the first SEC peak. As seen by comparison of equation 2 and equation 7, Kbis-ANS is formally a dissociation constant, whether calculated from equilibrium dialysis data or from assembly inhibition experiments. Kbis-ANS derived from assembly inhibition corresponded to an association energy of −24.3 ± 0.9 kJ/mol, only slightly weaker than the −28.0 ± 2.0 kJ/mol determined by equilibrium dialysis. The fact that different analytical methods provide consistent binding energy values for bis-ANS confirms the binding stoichiometry and supports our assertion that capsid assembly is noncompetitively inhibited by bis-ANS (vis-à-vis Fig. 1).
bis-ANS misdirects assembly.
By decreasing the concentration of assembly-active Cp1492 both noncompetitive and competitive assembly inhibitors should slow down assembly kinetics and reduce the yield of capsids. In the absence of bis-ANS, Cp1492 assembly resembles a two-state reaction, with very low concentrations of intermediates (35). Light scattering correlates well with changes in capsid concentration (35). For assembly reactions induced by high salt (≥0.35 M NaCl), bis-ANS had the predicted effect on assembly kinetics (Fig. 4B). However, at lower levels of ionic strength, low concentrations of bis-ANS (Fig. 4A) actually increased the rate of change and amount of light scattering, even though the yield of capsid was attenuated (Fig. 3A). This suggested that Cp1492 interaction with bis-ANS led to the formation of noncapsid polymers, i.e., misdirection.
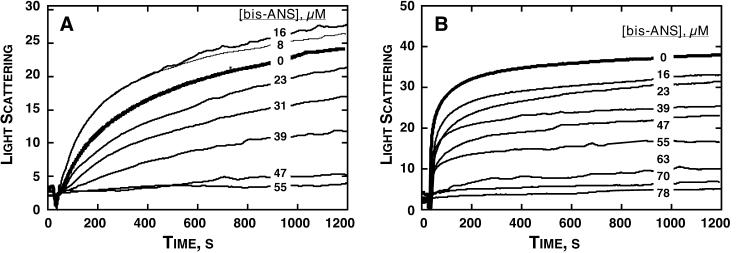
FIG. 4.
Capsid assembly kinetics show evidence for both inhibition and misdirection by bis-ANS. (A) In assembly at a low level of ionic strength (0.25 M NaCl), increased light scatter observed with low concentrations of bis-ANS suggests changes in assembly path and products. Samples contained 10 μM Cp1492 with various bis-ANS concentrations. (B) At a high level of ionic strength (0.5 M NaCl), bis-ANS showed only inhibitory effects. Kinetics of capsid assembly as induced by NaCl were observed in real time by monitoring light scattering. In the absence of bis-ANS, there was an excellent correlation between light scattering and capsid concentration (35).
Examination of assembly reaction products by EM provided additional evidence for assembly misdirection. Negative stain micrographs of HBV usually show a mixture of 28- and 32-nm-diameter particles (Fig. 5A) that have a tendency to self-associate. Two hours after initiating assembly of a sample of 10 μM Cp1492 with bis-ANS present by addition of 0.25 M NaCl, the micrographs show many normal capsids, capsids with “tabs” of aggregated protein, and large disordered aggregates of protein (Fig. 5B to D). Qualitatively, the amount of noncapsid material correlated with the concentration of bis-ANS. In rare cases, we observed tube-like structures, about 20 nm in diameter, with the same punctate pattern seen on capsids.
FIG. 5.
EM shows that noncapsid polymers are affected by bis-ANS concentration. Samples are from 0.25 M NaCl assembly reactions. Capsids, but not noncapsid polymers, are found in reactions without bis-ANS (A), though only 50% of the Cp1492 in the reaction is assembled. Capsids, and noncapsid oligomers induced by misdirection, are seen in the presence of 16 μM (B), 23 μM (C), and 39 μM (D) bis-ANS. Arrows identify examples of noncapsid polymers. Small tabs of protein are associated with capsids in low concentrations of bis-ANS (B).
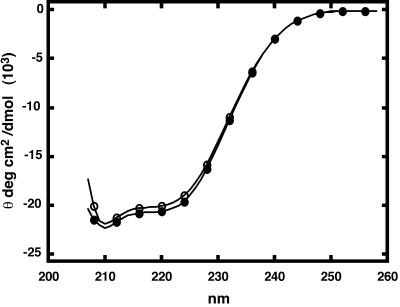
Are noncapsid polymers of Cp1492 examples of misdirected assembly or are they simply amorphous aggregates of misfolded protein? We used circular dichroism spectroscopy to look for evidence of protein refolding induced by bis-ANS, analogous to the misfolded proteins associated with prion or amyloid formation. Circular dichroism spectra of Cp1492, in the presence of bis-ANS, are nearly identical to the spectrum of Cp1492 without any dye, i.e., the secondary structure of the protein is essentially unchanged by binding bis-ANS (Fig. 6). Similarly, given a high enough ionic strength level, Cp1492 that was incubated with excess bis-ANS is capable of efficiently forming capsids (Fig. 3A).
FIG. 6.
Circular dichroism spectra of Cp1492 with and without bis-ANS. Samples of Cp1492 (at 10 μM in 25 mM HEPES, pH 7.5) without (closed circles) and with 30 μM (open circles) bis-ANS. In the presence of 30 μM bis-ANS, more than half of the Cp1492 is bound to the dye. Bis-ANS absorbance prevented collection of shorter-wavelength data.
DISCUSSION
We have shown that HBV capsid assembly is inhibited by a small molecule, bis-ANS. Bis-ANS binds HBV capsid protein with a 10- to 40-μM dissociation constant based on binding to free dimer in solution and on the effect of bis-ANS on capsid assembly. The binding stoichiometry was 1 dye molecule per capsid protein dimer, which leads us to speculate that bis-ANS might bind at the dimer interface, an ideal location for a molecular wedge. On one level, bis-ANS is a noncompetitive inhibitor, described by simple competing equilibria (Fig. 1). Because a single bis-ANS molecule cannot bind to both of the contact surfaces of a dimeric subunit, we deduce that bis-ANS is acting as a noncompetitive inhibitor.
The simple inhibition of capsid assembly has limited utility. Competitive and noncompetitive mechanisms would require near-stoichiometric concentrations of inhibitor to halt capsid assembly. Because of their extreme dependence on concentration, virus assembly reactions are predicted to have a pseudo-critical concentration (33). At low concentrations, almost all of the subunits are in the form of free subunits, but above a threshold concentration it appears that nearly all further subunits form capsids. This behavior has been observed with HBV (23) as well as with other spherical viruses (cf. cowpea chlorotic virus [1] and bacteriophage P22 [15]). Consequently, low concentrations of inhibitor will not have an overwhelming effect on the extent of assembly, though the decreased protein concentration will have a modest affect on the kinetics of assembly.
Our data indicate that bis-ANS is both a noncompetitive inhibitor and an assembly misdirector. As noncompetitive inhibitors of assembly, bound molecules would not be expected to trap partially assembled intermediates by poisoning assembly; noncompetitive inhibition is an alternative explanation for the effect of bis-ANS on bacteriophage P22 assembly (28). Assembly misdirection may be caused by distortion of subunit geometry. A misdirector should allow subunits to self-associate but prevent them from forming spherical particles by the sporadic distortion of the growing lattice by misdirector-bound subunits. In ideal cases, a misdirector could lead to the formation of regular nonnative structures, but this is not a necessary result of misdirection. Though we cannot determine the characteristics of the noncapsid polymer directly, we can deduce many of its features. We speculate that the bis-ANS binding site is located at the dimer interface, an ideal position for a molecular wedge. The noncapsid Cp1492 polymer is assembled from folded protein. The value of Kbis-ANS will be the same under equilibrium dialysis and assembly conditions only if the noncapsid polymer includes protein with and without bound bis-ANS, i.e., the concentration of Cp1492 · bis-ANS complex in the noncapsid polymer must be about the same as that in solution. Along with the observation that noncapsid polymer is only found at lower levels of ionic strength, these features help us to describe the characteristics of an ideal assembly misdirector. We next discuss these points individually.
The noncapsid polymers are not aggregates of misfolded protein. Circular dichroism studies indicate that modest concentrations of bis-ANS do not affect Cp1492 secondary structure. Cp1492 (10 mM) that was incubated with (and bound to) bis-ANS prior to assembly was still competent to form capsids; i.e., 50 μM bis-ANS almost eliminated assembly at 0.2 M NaCl but barely affected the yield of capsid in reactions driven by 0.5 M NaCl (Fig. 3A). In general, aggregation of unfolded protein is irreversible under mild solution conditions. If bis-ANS unfolded the protein and caused it to aggregate, then higher salt concentrations would have little effect on the yield of capsid. It is unlikely that aggregated misfolded protein could rapidly dissociate and refold and participate in such equilibria.
The binding analysis, based on the equilibria in Fig. 1, led to the prediction that misassembled protein would include both free dimer and dimer with bound bis-ANS. If protein with bis-ANS alone were aggregated, high bis-ANS concentrations would increase aggregation and light scatter. If bis-ANS-bound dimer were excluded from the aggregate, then one would predict the presence of amorphous protein aggregates in the absence of bis-ANS. Noncapsid polymers were not observed in the absence of bis-ANS, though only 50% of the Cp1492 was in capsid form (Fig. 5A). Neither were they observed when there was a great excess of bis-ANS. Instead, the greatest excess light scatter, when compared to the measured amount of capsid, occurred at low levels of ionic strength when 25% to 50% of the noncapsid protein was bound to bis-ANS.
The instability of noncapsid polymer and its presence only at low levels of ionic strength were consistent with the energetics of bis-ANS binding and capsid assembly. Bound bis-ANS is at equilibrium and can freely dissociate. At high levels of ionic strength, where subunit-subunit interaction energy is high, formation of an icosahedral lattice with several intersubunit contacts is expected to be strong enough to exclude bound bis-ANS (i.e., at high salt concentrations, bis-ANS can act as a weak noncompetitive inhibitor but not as a misdirector). At lower levels of ionic strength, subunits with bis-ANS could polymerize, because a large fraction of subunits were able to form all or most polyvalent contacts. So why is excess light scattering observed when bis-ANS is present at lower concentrations? We suggest that when all subunits have bound misdirector, there are no sites with contacts properly positioned for multivalent binding. This explains the decreased light scattering at elevated concentrations of bis-ANS at all salt concentrations (Fig. 2A). It is no surprise that an assembly misdirector can also act as a noncompetitive inhibitor.
A spherical polymer is most stable when all subunits are fully ligated (33). A misassembled capsid will have many loose ends for addition or loss of subunits. The power of a misdirector is that it converts a growing spherical polymer, which will incorporate only a few hundred subunits, into a polymer that can incorporate an unlimited number. Hypothetically, only a few misdirectors are required for the initiation and maintenance of aberrant growth (14). How will misdirectors behave in vivo, in which the full-length capsid protein is present and nucleic acid has a role in assembly? If the major effect of the presence of viral nucleic acid were to increase the effective concentration of subunits, then we would anticipate that misdirection would be enhanced.
There is a fundamental difference between the behaviors of capsids and noncapsid polymers of Cp1492. Though large aggregates were observed by EM, they were not seen in SEC studies: no excess material eluted in the void volume which would demonstrate the presence of material much larger than a 4-MDa capsid. Large aggregates and polymers may be rare or may be artifacts of the staining process (8). However, the increased light scatter at low bis-ANS concentrations correlates with the presence of small tabs on capsids, not large aggregates, as the most common EM evidence for misdirection. We suggest that the misdirected polymers dissociate during elution from SEC. A stable spherical polymer can be constructed based on weak association energy per contact (33, 35); the stability comes from the complete ligation of the multivalent subunits. However, subunits at the ends of a noncapsid polymer are partially ligated and are labile; the polymer can dissociate when the concentration of free subunit is decreased, i.e., during SEC. This appears to be the case for HBV, for which bis-ANS decreased the yield of capsid under the same conditions in which it increased light scattering.
Based on our observations, we can summarize the requirements for assembly misdirection. First, the system to be misdirected must have high levels of intersubunit association energy or assembly will be inhibited instead of misdirected. This was what happened when there was a great excess of bis-ANS with HBV. Second, the level of association energy of the misdirector for subunits must be stronger than that of the energy of subunits for a growing capsid or the misdirector will be displaced in favor of correct assembly. Third, a misdirector must not interfere with the intersubunit contact site. Because the assembly subunit for many capsids is an oligomer of capsid proteins, the interface between subunit components is an attractive target for a molecular wedge, i.e., the dimer interface for HBV.
Misdirectors are postulated to disturb the geometry of intersubunit contacts. The theory of quasi-equivalence (4) is based on the assumption that capsid protein interactions are constrained to a few conformations. Assembly misdirectors distort capsid protein oligomers, breaking these constraints. In a chronically infected cell, this could lead to production of large aggregates of capsid protein and many fewer competent progeny viruses. The effect of misdirection could be more dramatic for HBV, where the capsid is a necessary factor for reverse transcription of the RNA pregenome (21).
Acknowledgments
We thank Paul Wingfield and Stephen Stahl for their generous gift of an E. coli strain that overproduces Cp1492. We also acknowledge critical comments from John E. Johnson, Alasdair C. Steven, and Peter E. Prevelige. EM was performed at the Oklahoma Medical Research Foundation Imaging Facility.
This work was supported by Research Project Grant RPG-99-339-01 from the American Cancer Society.
REFERENCES
- 1.Adolph, K. W., and P. J. Butler. 1976. Assembly of a spherical plant virus. Philos. Trans. R. Soc. Lond. B Biol. Sci. 276:113-122. [DOI] [PubMed] [Google Scholar]
- 2.Bartenschlager, R., M. Junker-Niepmann, and H. Schaller. 1990. The P gene product of hepatitis B virus is required as a structural component for genomic RNA encapsidation. J. Virol. 64:5324-5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Birnbaum, F., and M. Nassal. 1990. Hepatitis B virus nucleocapsid assembly: primary structure requirements in the core protein. J. Virol. 64:3319-3330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caspar, D. L. D., and A. Klug. 1962. Physical principles in the construction of regular viruses. Cold Spring Harbor Symp. Quant. Biol. 27:1-24. [DOI] [PubMed] [Google Scholar]
- 5.Conway, J. F., N. Cheng, A. Zlotnick, S. J. Stahl, P. T. Wingfield, D. M. Belnap, U. Kanngiesser, M. Noah, and A. C. Steven. 1998. Hepatitis B virus capsid: localization of the putative immunodominant loop (residues 78 to 83) on the capsid surface, and implications for the distinction between c and e-antigens. J. Mol. Biol. 279:1111-1121. [DOI] [PubMed] [Google Scholar]
- 6.Crowther, R. A., N. A. Kiselev, B. Bottcher, J. A. Berriman, G. P. Borisova, V. Ose, and P. Pumpens. 1994. Three-dimensional structure of hepatitis B virus core particles determined by electron cryomicroscopy. Cell 77:943-950. [DOI] [PubMed] [Google Scholar]
- 7.Ganem, D. 1996. Hepadnaviridae and their replication, p. 2703-2737. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monath, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed. Lippincott-Raven Publishers, Philadelphia, Pa.
- 8.Hayat, M. A., and S. E. Miller. 1990. Negative staining. McGraw-Hill Publishing Co., New York, N.Y.
- 9.Horowitz, P., V. Prasad, and R. F. Luduena. 1984. Bis(1,8-anilinonaphthalenesulfonate). A novel and potent inhibitor of microtubule assembly. J. Biol. Chem. 259:14647-14650. [PubMed] [Google Scholar]
- 10.Kenney, J. M., C. H. von Bonsdorff, M. Nassal, and S. D. Fuller. 1995. Evolutionary conservation in the hepatitis B virus core structure: comparison of human and duck cores. Structure 3:1009-1019. [DOI] [PubMed] [Google Scholar]
- 11.Lakowicz, J. R. 1983. Principles of fluorescence spectroscopy. Plenum Press, New York, N.Y.
- 12.Le Pogam, S., T. T. Yuan, G. K. Sahu, S. Chatterjee, and C. Shih. 2000. Low-level secretion of human hepatitis B virus virions caused by two independent, naturally occurring mutations (P5T and L60V) in the capsid protein. J. Virol. 74:9099-9105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Musci, G., and L. J. Berliner. 1985. Probing different conformational states of bovine alpha-lactalbumin: fluorescence studies with 4,4′-bis[1-(phenylamino)-8-naphthalenesulfonate]. Biochemistry 24:3852-3856. [DOI] [PubMed] [Google Scholar]
- 14.Prevelige, P. E. J. 1998. Inhibiting virus-capsid assembly by altering the polymerisation pathway. Trends Biotechnol. 16:61-65. [DOI] [PubMed] [Google Scholar]
- 15.Prevelige, P. E. J., D. Thomas, and J. King. 1993. Nucleation and growth phases in the polymerization of coat and scaffolding subunits into icosahedral procapsid shells. Biophys. J. 64:824-835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Saha, R., U. Banik, S. Bandopadhyay, N. C. Mandal, B. Bhattacharyya, and S. Roy. 1992. An operator-induced conformational change in the C-terminal domain of the lambda repressor. J. Biol. Chem. 267:5862-5867. [PubMed] [Google Scholar]
- 17.Salfeld, J., E. Pfaff, M. Noah, and H. Schaller. 1989. Antigenic determinants and functional domains in core antigen and e antigen from hepatitis B virus. J. Virol. 63:798-808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Scaglioni, P. P., M. Melegari, and J. R. Wands. 1994. Characterization of hepatitis B virus core mutants that inhibit viral replication. Virology 205:112-120. [DOI] [PubMed] [Google Scholar]
- 19.Scaglioni, P. P., M. Melegari, and J. R. Wands. 1997. Posttranscriptional regulation of hepatitis B virus replication by the precore protein. J. Virol. 71:345-353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Secnik, J., Q. Wang, C. M. Chang, and J. E. Jentoft. 1990. Interactions at the nucleic acid binding site of the avian retroviral nucleocapsid protein: studies utilizing the fluorescent probe 4,4′-bis(phenylamino) (1,1′-binaphthalene)-5,5′-disulfonic acid. Biochemistry 29:7991-7997. [DOI] [PubMed] [Google Scholar]
- 21.Seeger, C., and W. S. Mason. 2000. Hepatitis B virus biology. Microbiol. Mol. Biol. Rev. 64:51-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Seifer, M., and D. N. Standring. 1993. Stability governs the apparent expression of “particulate” hepatitis B e antigen by mutant hepatitis B virus core particles. Virology 196:70-78. [DOI] [PubMed] [Google Scholar]
- 23.Seifer, M., S. Zhou, and D. N. Standring. 1993. A micromolar pool of antigenically distinct precursors is required to initiate cooperative assembly of hepatitis B virus capsids in Xenopus oocytes. J. Virol. 67:249-257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shi, L., D. R. Palleros, and A. L. Fink. 1994. Protein conformational changes induced by 1,1′-bis(4-anilino-5-naphthalenesulfonic acid): preferential binding to the molten globule of DnaK. Biochemistry 33:7536-7546. [DOI] [PubMed] [Google Scholar]
- 25.Stannard, L. M., and M. Hodgkiss. 1979. Morphological irregularities in Dane particle cores. J. Gen. Virol. 45:509-514. [DOI] [PubMed] [Google Scholar]
- 26.Takashi, R., Y. Tonomura, and M. F. Morales. 1977. 4,4′-Bis (1-anilinonaphthalene 8-sulfonate) (bis-ANS): a new probe of the active site of myosin. Proc. Natl. Acad. Sci. USA 74:2334-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tanford, C. 1980. The hydrophobic effect: formation of micelles and biological membranes, 2nd ed. John Wiley and Sons, Inc., New York, N.Y.
- 28.Teschke, C. M., J. King, and P. E. Prevelige, Jr. 1993. Inhibition of viral capsid assembly by 1,1′-bi(4-anilinonaphthalene-5-sulfonic acid). Biochemistry 32:10658-10665. [DOI] [PubMed] [Google Scholar]
- 29.Wingfield, P. T., S. J. Stahl, R. W. Williams, and A. C. Steven. 1995. Hepatitis core antigen produced in Escherichia coli: subunit composition, conformational analysis, and in vitro capsid assembly. Biochemistry 34:4919-4932. [DOI] [PubMed] [Google Scholar]
- 30.Yuan, T. T., M. H. Lin, D. S. Chen, and C. Shih. 1998. A defective interference-like phenomenon of human hepatitis B virus in chronic carriers. J. Virol. 72:578-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuan, T. T., P. C. Tai, and C. Shih. 1999. Subtype-independent immature secretion and subtype-dependent replication deficiency of a highly frequent, naturally occurring mutation of human hepatitis B virus core antigen. J. Virol. 73:10122-10128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou, S., and D. N. Standring. 1992. Hepatitis B virus capsid particles are assembled from core-protein dimer precursors. Proc. Natl. Acad. Sci. USA 89:10046-10050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zlotnick, A. 1994. To build a virus capsid. An equilibrium model of the self assembly of polyhedral protein complexes. J. Mol. Biol. 241:59-67. [DOI] [PubMed] [Google Scholar]
- 34.Zlotnick, A., N. Cheng, J. F. Conway, F. P. Booy, A. C. Steven, S. J. Stahl, and P. T. Wingfield. 1996. Dimorphism of hepatitis B virus capsids is strongly influenced by the C terminus of the capsid protein. Biochemistry. 35:7412-7421. [DOI] [PubMed] [Google Scholar]
- 35.Zlotnick, A., J. M. Johnson, P. W. Wingfield, S. J. Stahl, and D. Endres. 1999. A theoretical model successfully identifies features of hepatitis B virus capsid assembly. Biochemistry 38:14644-14652. [DOI] [PubMed] [Google Scholar]