Abstract
Simian virus 40 (SV40) capsid assembly occurs in the nucleus. All three capsid proteins bind DNA nonspecifically, raising the dilemma of how they attain specificity to the SV40 minichromosome in the presence of a large excess of genomic DNA. The SV40 packaging signal, ses, which is required for assembly, is composed of multiple DNA elements that bind transcription factor Sp1. Our previous studies showed that Sp1 participates in SV40 assembly and that it cooperates in DNA binding with VP2/3. We hypothesized that Sp1 recruits the capsid proteins to the viral minichromosome, conferring upon them specific DNA recognition. Here, we have tested the hypothesis. Computer analysis showed that the combination of six tandem GC boxes at ses is not found at cellular promoters and therefore is unique to SV40. Cooperativity in DNA binding between Sp1 and VP2/3 was not abolished at even a 1,000-fold excess of cellular DNA, providing strong support for the recruitment hypothesis. Sp1 also binds VP1 and cooperates with VP1 in DNA binding. VP1 pentamers (VP15) avidly interact with VP2/3, utilizing the same VP2/3 domain as described for polyomavirus. We conclude that VP15-VP2/3 building blocks are recruited by Sp1 to ses, where they form the nucleation center for capsid assembly. By this mechanism the virus ensures that capsid formation is initiated at a single site around its minichromosome. Sp1 enhances the formation of SV40 pseudovirions in vitro, providing additional support for the model. Analyses of Sp1 and VP3 deletion mutants showed that Sp1 and VP2/3 bind one another and cooperate in DNA binding through their DNA-binding domains, with additional contacts outside these domains. VP1 contacts Sp1 at residues outside the Sp1 DNA-binding domain. These and additional data allowed us to propose a molecular model for the VP15-VP2/3-DNA-Sp1 complex.
Simian virus 40 (SV40) is a papovavirus, with a small double-stranded circular DNA genome of 5.2 kb. The viral capsid, surrounding the viral minichromosome, is composed of three virus-encoded proteins, VP1, VP2, and VP3. Seventy-two pentamers of VP1 form the outer shell, while VP2 and VP3 bridge the VP1 shell and the chromatin core. VP3 translation initiates from an internal AUG within the VP2 coding sequence, utilizing the same translational frame (Fig. 1A). Thus, both proteins are identical at their carboxy part and will be referred to as VP2/3.
FIG. 1.
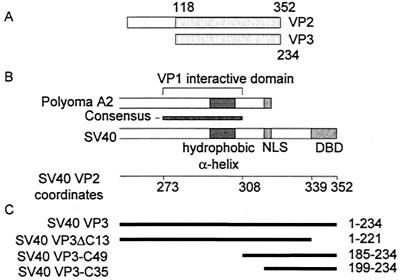
Schematic representation of VP2 and VP3. (A) Linear alignment of SV40 VP2 and VP3, showing the extent of a common segment and the N-terminal VP2-unique region. The numbers refer to amino acids of VP2 and VP3, respectively. (B) Alignment of the carboxy parts of polyomavirus A2 and SV40 VP2/3. SV40 amino acid coordinates (Swiss-Prot accession number P03093) are shown below the map. The consensus region (SV40 amino acids 273 to 308) is based on an alignment of eight polyomaviruses. The hydrophobic alpha-helix is almost identical in the polyomaviruses (4). The VP1 interactive domain of polyomavirus VP2/3 was determined by biochemical analysis (2) and X-ray crystallography (4). Abbreviations: NLS, nuclear localization signal (14); DBD, SV40 DNA-binding domain (amino acids 339 to 352) (5). (C) Schematic representation of VP3 truncation mutants. The heavy lines, aligned according to the schematic map in panel B, represent the amino acids included in the mutant proteins. The amino acids are designated at the right.
The VP1 monomers are tightly bound in pentamers, forming structures of fivefold symmetry with an inward-facing cavity (21, 34). The pentamers are tied together through their carboxy-terminal arms. Five arms extend from each pentamer and insert into the neighboring pentamers in three distinct kinds of interactions. This unique type of bonding underlies the variability in contacts between the identical building blocks, allowing the flexibility required for the packing geometry (21).
X-ray crystallography for the closely related murine polyomavirus A2 (4) has shown that a single molecule of VP2 or VP3 is firmly anchored in the inner cavity of the VP1 pentamer (VP15) by hydrophobic interactions, through a region near the C terminus of VP2/3 (Fig. 1B). Alignment of several papovavirus VP2/3 amino acid sequences, including murine polyomavirus A2 and SV40, demonstrated a long stretch of homology that contains the region that interacts with VP1 (4) (Fig. 1B). Furthermore, the inner core of the SV40 pentamer has hydrophobic residues at positions equivalent to those that contact VP2/3 in polyomavirus. Thus, it was suggested that the VP15-VP2/3 contacts in SV40 are similar to those in polyomavirus (4). Studies of the nuclear transport of the capsid proteins of SV40 (14) and of polyomavirus (8) suggested that immediately after translation the VP15-VP2/3 complex is formed in the cytoplasm. Taken together, these finding strongly suggest that a complex of a VP1 pentamer bound to a VP2/3 monomer (VP15-VP2/3) is the basic building block of the viral capsid.
SV40 assembly is thought to occur by a gradual addition and organization of the capsid proteins around the minichromosome (reviewed in reference 3). All three capsid proteins were found to bind DNA nonspecifically (5, 33). This raised the question of how they find the SV40 minichromosome in the presence of excess genomic DNA.
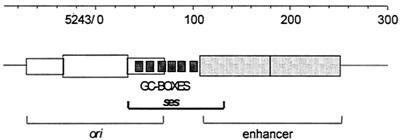
The SV40 packaging signal, ses, is required in cis and is present within the SV40 regulatory region overlapping the six GC boxes (22) (Fig. 2) known to bind the cellular transcription factor Sp1. Genetic studies showed that ses is composed of redundant DNA elements which are partly interchangeable (7). DNA motifs that bind transcription factor Sp1 are critical for ses function in packaging, suggesting that Sp1 may have a role in virus assembly, although it is not known to be present in the mature virion.
FIG. 2.
Physical map of the SV40 regulatory region. The SV40 ori, ses, and enhancer (composed of two 72-bp repeats) are designated. ses overlaps the GC boxes (six GGGCGG elements) and 26 bp of the 5′ enhancer (22). Sequence coordinates (GenBank accession number J02400) are shown above the map.
Sp1 is a ubiquitous transcription factor with a three-zinc-finger DNA-binding domain and two glutamine-rich activation domains (6). It binds to GC boxes present at ses (11), activating both early and late SV40 transcription. Cellular Sp1 transcription is upregulated early in SV40 infection, suggesting that it is required for the virus life cycle (28).
Our previous studies of the role of Sp1 in SV40 assembly focused on the interaction between Sp1 and the internal capsid proteins VP2 and VP3. We found significant cooperativity between Sp1 and VP2/3 in DNA binding at ses and the formation of a ternary complex between Sp1, VP2/3, and ses DNA. Protein-protein interactions between VP2/3 and Sp1 also occur in the absence of DNA (13). We hypothesized that Sp1 recruits VP2/3 to the SV40 minichromosome during assembly, thus conferring specific DNA recognition upon a protein which otherwise binds to DNA nonspecifically.
In the present study we showed that the VP1 interactive domain of SV40 VP2/3 is analogous to that of polyomavirus. We tested the hypothesis that Sp1 recruits the VP15-VP2/3 building blocks to ses. In addition, experiments with truncation mutants allowed us to propose a molecular model for the structure of the Sp1-DNA-VP15-VP2/3 complex.
MATERIALS AND METHODS
Plasmid construction.
pGEX-VP3 (13) and pQE-VP1 (37) have been previously described. pGEX-VP3ΔC13 (5) was obtained from Harumi Kasamatsu.
Table 1 summarizes the cloning of the plasmids constructed in this project. The cloning vectors and restriction sites used for cloning appear in the second column. The inserts were produced by PCR, using as a template either SV40 DNA (Gibco-BRL) or an Sp1 expression vector, which was obtained from Jim Kadonaga (18). Appropriate restriction sites for cloning were introduced in the primers (Table 1). When no site is indicated the PCR product was amplified by PwoI enzyme, which produces blunt ends, and inserted by blunt end ligation.
TABLE 1.
Plasmid construction
| Plasmid | Cloning vector (restriction sites used) | PCR templatec | PCR primerc |
|---|---|---|---|
| PGEX-VP1 | pGEX-2T (BamHI, filled-in EcoRI) | SV40a | 5′-CTCTAAAGATCTATGAAGATGGCCC-3′ |
| 5′-GCAATAGCATCACAAATTTCAC-3′ | |||
| pGEX-VP3C49 | pGEX-2T (BamHI, EcoRI) | SV40 | 5′-GGCCTGTACGGATCCGTTACTTCT-3′ |
| 5′-TTGAGGAATTCAAAAGCACTCC-3′ | |||
| pGEX-VP3C35 | pGEX-2T (BamHI, EcoRI) | SV40 | 5′-GATGGATCCAACAAAAAGAAAAGG-3′ |
| 5′-TTGAGGAATTCAAAAGCACTCC-3′ | |||
| pQE-VP1ΔC60 | pQE30 (BamHI, SmaI) | SV40 | 5′-CTCTAAAGATCTATGAAGATGGCCC-3′ |
| 5′-TTAAGTCGACTGGTTAGGGGTTTTTCAC-3′ | |||
| pBac-Sp1 | pFastBacHT1 (EcoRI, HindIII) | pSp1778Cb | 5′-CCAAGATGAATTCATGGATGAAATG-3′ |
| 5′-CCCGGAAGCTTGTTATCAGAAGC-3′ | |||
| pQE-ZnD | pQE30 (BamHI, filled-in HindIII) | pSp1778C | 5′-CGATCCTGGCAAAAAGAAACAG-3′ |
| 5′-CCCGGAAGCTTGTTATCAGAAGC-3′ | |||
| pQE-Zn | pQE30 (BamHI, SmaI) | pSp1778C | 5′-GGGCGGATCCGATCCTGGCAAAAAG-3′ |
| 5′-CTCAGCCTCACTTCTTATTCTG-3′ |
Gibco-BRL.
Obtained from Jim Kadonaga (18).
Underlined nucleotides represent restriction enzyme sites inserted in the sequence for cloning. In the absence of such sites, the blunt end of the PCR product was used for ligation.
Protein purification.
Purified wild-type human Sp1 prepared from HeLa cells infected with recombinant vaccinia virus containing a full-length Sp1 cDNA was purchased from Promega. One footprint unit (fpu), as defined by the manufacturer, is the amount of protein required to yield a complete footprint on a 35-fmol fragment of the SV40 early promoter. One footprint unit is approximately 50 ng.
Glutathione S-transferase (GST), GST-VP1, GST-VP3, GST-VP3ΔC13, GST-VP3C49, and GST-VP3C35 were expressed in Escherichia coli from pGEX2T, pGEX-VP1, pGEX-VP3, pGEX-VP3ΔC13, pGEX-VP3C49, and pGEX-VP3C35, respectively. The cultures were grown at 30°C (37°C for GST, GST-VP3C49, and GST-VP3C35) to an optical density of ∼0.6. Expression of the fusion protein was induced by the addition of 0.1 mM isopropyl-β-d-thiogalactopyranoside (1 mM for GST-VP3C49 and GST-VP3C35). The cultures were further incubated for 90 min, and the bacteria were collected by centrifugation and sonicated in a solution of phosphate-buffered saline (PBS). The supernatant was incubated with 0.5 ml of glutathione (GSH)-agarose resin for 10 min at room temperature. After being washed several times with PBS, the fusion protein was eluted with 0.5 ml of 50 mM Tris (pH 8.0)-15 mM GSH. The purified protein was then dialyzed against 50 mM Tris (pH 8.0)-2 mM EDTA-10% glycerol. The fusion proteins were of the expected size as determined by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Coomassie staining.
His-VP1 (expressed from pQE-VP1) was produced in a strain deficient for the GroELS chaperone machine and purified using Ni-nitrilotriacetic acid (NTA) chromatography under native conditions (37). His-VP1ΔC60 (expressed from pQE-VP1ΔC60) was produced in E. coli transformed with pRARE (Novagen) and purified using Ni-NTA chromatography as described (37).
ZnD and Zn (expressed from pQE-ZnD and pQE30-Zn, respectively) were produced in E. coli and purified by Ni-NTA chromatography under denaturing conditions (8.0 M urea), as recommended by the manufacturer (Qiagen). Refolding was carried out by stepwise dialysis as described for full-length Sp1 (17). Briefly, the eluate from the Ni-NTA chromatography was dialyzed against buffer A (20 mM Tris-HCl, pH 7.7; 50 mM KCl; 10 mM MgCl2; 1 mM EDTA; 1 mM dithiothreitol [DTT]; 0.2 mM phenylmethylsulfonyl fluoride; 1 mM sodium metabisulfite) containing 6 M urea for 90 min. The mixture was then dialyzed against buffer B (20 mM Tris-HCl, pH 7.7; 50 mM KCl; 10 mM MgCl2; 1 mM EDTA; 20% [vol/vol] glycerol; 1 mM DTT; 0.2 mM phenylmethylsulfonyl fluoride; 1 mM sodium metabisulfite; 10 μM ZnSO4) containing 1 M urea for 90 min. This was followed by overnight dialysis against buffer B, and the protein was stored at −70°C.
His-Sp1 was produced in Sf9 cells. Recombination between the Bacmid and pBac-Sp1 and recombinant baculovirus production were carried out as recommended by the manufacturer of the Bac-To-Bac Baculovirus Expression System (Invitrogen). Sf9 cells were infected with this recombinant baculovirus at multiplicity of infection 10, as specified by the manufacturer (Invitrogen). The nuclear proteins were extracted after 2 days according to the method of Jackson and Tjian (15) and purified by Ni-NTA chromatography under native conditions. Nuclear extract (1.5 ml) was centrifuged at 80,000 × g and incubated with 0.2 ml of Ni-NTA resin, which had been preequilibrated with buffer C (50 mM Tris, pH 7.5; 0.42 M KCl; 20% [vol/vol] glycerol; 10% [wt/vol] sucrose; 5 mM MgCl2; 1 mM sodium metabisulfite; 2 mM 2-mercaptoethanol) containing 20 mM imidazole. The mixture was incubated at 4°C for 15 min on a rotary shaker and transferred to a column. The column was washed with 60 ml of buffer C containing 25 mM imidazole. His-Sp1 was eluted with 0.6 ml of buffer C containing 250 mM imidazole. The eluate was dialyzed against buffer D (0.1 M KCl, 25 mM HEPES KOH, 12.5 mM MgCl2, 20% [vol/vol] glycerol, 0.1% [vol/vol] NP-40, 1 mM DTT, 10 μM ZnSO4).
GST-VP3 affinity chromatography.
Affinity chromatography experiments were carried out as described by Barouch and Harrison (2). Approximately equal amounts (∼0.4 mg) of GST-VP3 or its derivatives were bound to 0.5 ml of GSH-agarose affinity resin. All subsequent steps were carried out at 4°C. The resin loaded with the fusion protein was added to a solution of purified His-VP1-ΔC60 (15 μg) in 0.5 ml of buffer I (50 mM Tris-HCl, pH 8.0; 150 mM NaCl; 0.1% β-mercaptoethanol; 1 mM EDTA; 1% Triton X-100) with 50 mM imidazole. After 20 min of mixing on a rotary shaker the resin was set in a column. The flowthrough was collected, and the column was washed with 5 volumes (2.5 ml) of buffer I. The proteins were eluted three times, each with 0.5 ml of buffer I containing 10 mM GSH. The fractions were analyzed by SDS-PAGE and visualized by Western blotting with anti-His antibody and by Coomassie staining to identify the fractions that contained the GST-VP3 derivatives eluted off the column.
GST-pull down assay.
GST fusion proteins were bound to GSH-agarose beads as described above. Beads (0.1 ml) loaded with fusion protein were incubated with approximately 40 μg of K562 nuclear extract (unless otherwise indicated) in a solution of 0.4 ml of PBS, for 20 min in a rotary shaker at 4°C. After centrifugation, the supernatant containing the unbound protein was collected, and the beads were washed several times with PBS. The bound proteins were eluted by boiling in SDS-PAGE sample buffer. The fractions were resolved by SDS-PAGE (NuPage; Invitrogen) following acetone precipitation as required, and the bands were visualized by Western blotting with anti-Sp1 antibody (Santa Cruz). Equivalent amounts of bound and unbound proteins were loaded on the gel. Pretreatment with ethidium bromide was carried out by incubating both the GST-VP1 immobilized on GSH-agarose and the K562 nuclear extract with 3 μM ethidium bromide for 15 min at 4°C.
Gel retardation assays.
The SV40 ses DNA probe (179 bp, which includes SV40 coordinates 5214 to 132, pBR322 coordinates 2379 to 2369, and 6 bp of a BamHI linker) was prepared by PCR of pSOγ-S with the primers S5, 5′-TACTTCTGGAATAGCTC-3′, and S13, 5′-CGAGTCAGTGAGGATCC-3′ (22, 23). The SV40 GC box probe (89 bp; SV40 coordinates 33 to 121) was prepared by PCR of SV40 with the following primers: 5′-TCAGCCATGGGGCGGAGAAT-3′ and 5′-ATTAGTCAGCAACCATAGTCCC-3′. One of the primers was end labeled by kination with [γ-32P]ATP, and the PCR product was subsequently purified using a High Pure Product Purification Kit (Boehringer).
Either purified protein or nuclear extracts (concentrations are indicated in the figure legends) were incubated with approximately 8 ng of probe (unless otherwise indicated). The binding reactions were performed on ice for 20 min in buffer containing the following (per reaction mixture): 10 mM HEPES (pH 7.9), 100 mM KCl, 1 mM DTT, 1 mM EDTA, 1% glycerol, and 1.5 μg of bovine serum albumin. Poly(dI-dC) (1.5 μg/reaction mixture) was added as unlabeled competitor DNA unless otherwise indicated. The bands were resolved on a 5% nondenaturing polyacrylamide gel at 25 mA for 2.5 h.
Cleaved genomic CV-1 DNA was prepared for a competition experiment as follows: genomic DNA was extracted from approximately 6 × 106 cells (12); sonicated for several minutes to approximately 500-bp fragments, as determined by agarose gel electrophoresis; and cleaned with a High Pure Product Purification Kit (Boehringer).
In vitro packaging reaction.
The reaction was carried out as previously described (29). Nuclear extracts of Sf9 cells were prepared according to the method of Schreiber et al. (32), from cells infected with recombinant baculovirus expressing SV40 VP1, VP2, and VP3. Nuclear extracts (1 to 2 μg) were mixed by vortex with 1 μg of DNA (pEGFP-C1; Clontech [accession number U55763]), in the presence of 5 mM ATP, Sp1 (or Sp1 buffer), or ZnD (or ZnD buffer) in a total volume of 6 μl and placed at 37°C for 6 h. CaCl2 and MgCl2 were added to final concentrations of 0.5 and 8 mM, respectively, and the reaction mixtures were incubated for an additional 1 h on ice. DNase I treatment was used to remove DNA that was not stably packaged. DNase I digestion was performed using 0.5 U of enzyme for 10 min on ice and stopped by the addition of EDTA to a final concentration of 5 mM.
The reaction products were assayed for infectious units (IU) as previously described (7, 29) on CMT4 monolayers grown in Dulbecco's modified Eagle's medium with 10% fetal bovine serum, using a standard SV40 infection protocol. CMT4 cells are derived from the cell line CV-1 (African green monkey kidney cells). CMT4 cells harbor the gene for SV40 T antigen expressed from the inducible metallothionein promoter (9). Subconfluent monolayers were incubated with the packaging mixture for 120 min at 37°C, with occasional agitation, followed by the addition of fresh medium containing 100 μM ZnCl2 and 1 μM CdSO4 for induction of T-antigen expression. In situ hybridization was done after 48 h using radioactively labeled pEGFP-C1. The number of infective centers obtained for a 6-μl reaction mixture was used in computing the titer (in infectious units per milliliter).
RESULTS
The arrangement of six Sp1 binding sites at ses is unique.
Sp1 sites are spread throughout the primate genome. Why doesn't packaging occur at cellular GC boxes? We speculated that the arrangement of six tandem, regularly spaced GC boxes is unique to ses, and thus a critical element for the recruitment of the capsid proteins by Sp1.
To test this idea we searched the Eukaryotic Promoter Database (18 October 2001) (27) for adjacent GC boxes, using “findpatterns” in the Wisconsin Genetics Computer Group package. The interval between adjacent GC boxes in SV40 is 3 to 6 bp. The interval in the search was set at 0 to 20 bp. The results of the search (Table 2) show that no cellular promoter contains five or more GC boxes. Many promoters have one or two GC boxes, few have three boxes, and two promoters have four (with larger intervals between them). This demonstrates that the combination of six closely spaced GC boxes is unique to the SV40 regulatory region, supporting the model that ses serves as a recognition signal for the recruitment of the VP15-VP2/3 complex by Sp1.
TABLE 2.
Genomic search for clusters of GC boxes
| No. of adjacent GC boxes | Interval between GC boxes (bp) | Times found in EPDa |
|---|---|---|
| 2 | 0-7 | Many |
| 2 | 8-20 | Many |
| 3 | 0-7 | 4 |
| 3 | 8-20 | 10 |
| 4 | 0-7 | 0 |
| 4 | 8-20 | 2 |
| 5 | 0-7 | 0 |
| 5 | 8-20 | 0 |
| 6 | 0-7 | 1b |
| 6 | 8-20 | 1c |
EPD, Eukaryotic Protein Database.
SV40 GC box region.
Herpes simplex virus.
In addition to SV40, six GC boxes were also found in herpes simplex virus. The interval in herpes simplex virus, 16 bp, is much larger than that in SV40. As this is not a cellular promoter it would not normally compete with the ses region for the recruitment of the capsid proteins.
Sp1-VP2/3 complex is formed preferentially at ses.
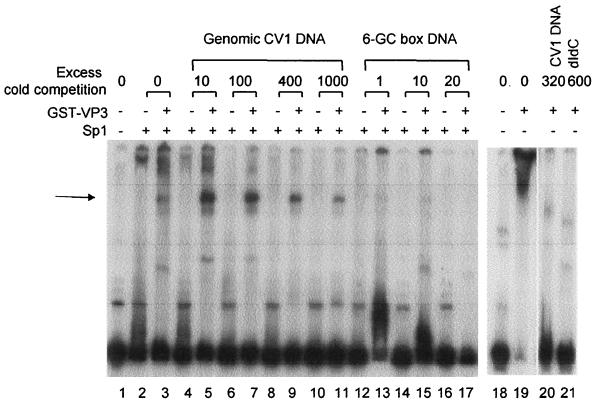
To test the recruitment model, we asked whether excess genomic DNA was capable of competing for the cooperativity in DNA binding between Sp1 and VP2/3. DNA-binding reactions (Fig. 3) were performed with Sp1 and GST-VP3, a 32P-labeled ses probe, and in the presence of increasing concentrations of unlabeled sonicated genomic DNA from CV-1 cells. Our previous studies of the cooperativity between Sp1 and VP2/3 showed, by supershifting with specific antibodies, that both Sp1 and VP2/3 are present in the complex with ses DNA (Fig. 3A and B in reference 13). This complex is seen in the present experiment as a distinct band, designated by the arrow in Fig. 3 (lane 3). The distinct band significantly increases in intensity in the presence of cold genomic DNA. It is observed at all concentrations of cold genomic DNA, even at 1,000-fold excess (lanes 4 to 11). In comparison, the band is almost abolished at as little as an equal molar amount of a cold six-GC-box fragment (lanes 12 to 13). This shows that the Sp1-VP2/3 complex has a much higher affinity for the six-GC-box region than for genomic DNA, providing experimental evidence for the recruitment model.
FIG. 3.
The Sp1-VP3 complex is formed preferentially at ses. The panel at left shows a gel retardation assay that was performed with Sp1 purified from HeLa cells (Promega) and GST-VP3 using an SV40 ses DNA fragment (179 bp; 2.5 ng). Even-numbered lanes contain Sp1 only (4 ng; ∼4 pmol); odd-numbered lanes except lane 1 contain Sp1 (4 ng) and GST-VP3 (200 ng). Sonicated genomic DNA from CV-1 cells (which was approximately 500 bp) or a six-GC-box fragment (89 bp; SV40 coordinates 33 to 121) was added at the molar ratio indicated above the lanes. The arrow indicates the major Sp1-VP3 complex formed in the cooperativity reaction. The panel at right shows a parallel gel retardation assay using the SV40 ses probe (lane 18) depicting DNA binding of GST-VP3 only, without Sp1, in the absence of competitor (lane 19) and in the presence of a molar ratio of genomic DNA (lane 20) or poly(dI-dC) (lane 21). The faint bands in lanes 20 and 21, with a slightly higher mobility than the Sp1-GST-VP3-DNA complex, are most likely due to binding of GST-VP3 to contaminants in the DNA probe (see faint bands in lanes 1 and 18). We have no explanation for the different mobilities of these bands in lanes 20 and 21.
Previous studies (13) were carried out in the presence of poly(dI-dC) to compete for the nonspecific DNA-binding activity of VP3. In the presence of a 600-fold molar excess of poly(dI-dC), there are several distinct bands (Fig. 5B, lanes 5 and 6). In Fig. 3, in the absence of poly(dI-dC), we see one distinct band and a smear (lane 3). The smear is most likely due to nonspecific binding of VP2/3 to DNA in the absence of competitor DNA, as seen in lane 19. The smear is competed for either by genomic DNA (lane 20) or by poly(dI-dC) (lane 21).
FIG. 5.
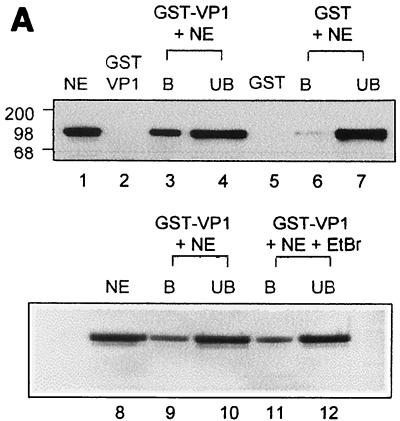
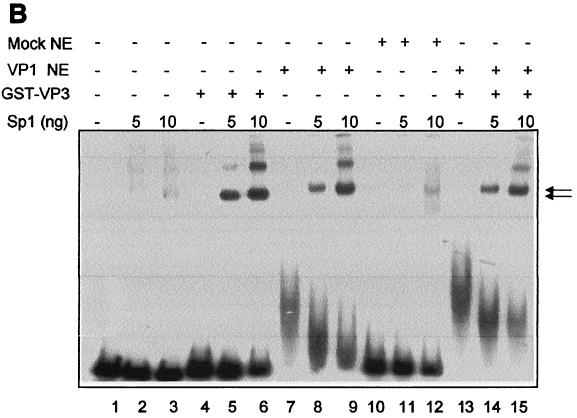
Sp1 interacts with VP1. (A) Binding of Sp1 present in K562 nuclear extracts to GST-VP1 and GST. The top panel shows fractions, bound protein (B), and unbound protein (UB) that were analyzed by SDS-PAGE analysis and Western blotting with anti-Sp1 antibody (Santa Cruz). Twenty micrograms of K562 nuclear extracts was used in each experiment. Lane 1, K562 nuclear extracts (NE); lanes 2, 3, and 4, GST-VP1 immobilized on GSH-agarose resin; lanes 5, 6, and 7, GST immobilized on GSH-agarose resin. The bottom panel shows the effect of ethidium bromide (EtBr) treatment; before GST-VP1 (immobilized on GSH-agarose resin) and K562 NE were allowed to interact they were each incubated in either the absence (lanes 9 and 10) or presence (lanes 11 and 12) of EtBr. (B) Cooperativity between Sp1 and VP1 in DNA binding. A radioactively labeled SV40 ses DNA fragment (179 bp, lane 1) was incubated with the following proteins as indicated: Sp1 (5 or 10 ng as indicated; 5 ng ≈ 5 pmol), GST-VP3 (200 ng), VP1 from Sf9 NE (10 μg of total proteins; ∼1 μg of VP1), and mock-infected Sf9 NE (10 μg of total proteins).
Why does the Sp1-GST-VP3-DNA complex increase in intensity in the presence of genomic DNA? A likely explanation is that in the absence of genomic DNA VP2/3 extensively sequesters ses DNA, occluding specific Sp1 binding and specific cooperativity of Sp1 with VP2/3. Genomic DNA [or poly(dI-dC)] that competes for the nonspecific binding of VP2/3 allows Sp1 to access ses, to specifically bind, and to recruit VP2/3.
Interaction of VP1 with VP2/3.
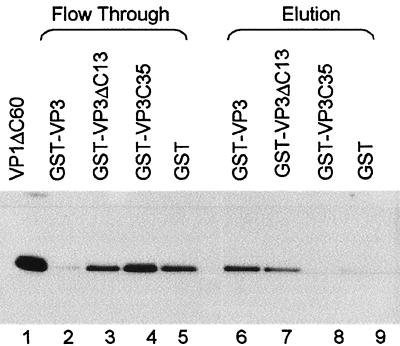
The crystal structure of the polyomavirus VP15-VP2/3 complex predicts that the VP1 interactive domain of SV40 is at the polyomavirus family consensus sequence (Fig. 1B), near but not at the C terminus. However, it was reported that the C-terminal 13 amino acids of SV40 VP2/3 are required to bind SV40 VP1 (10). To resolve this question, we carried out protein interaction studies using GST-VP3 and the following GST-VP3 mutants (Fig. 1C): GST-VP3ΔC13, from which the C-terminal 13 amino acids are deleted (5) but which carries the consensus, putative VP1 interactive domain, and GST-VP3C35, which contains only the 35 C-terminal amino acids and does not carry the consensus domain. Equal amounts of these GST fusion proteins were bound to GSH-agarose resin and allowed to interact with His-VP1ΔC60. This VP1 mutant has a deletion of the 60 amino acids that comprise the C-terminal arm and therefore cannot assemble into capsids and remains as pentamers. The unbound material was collected in the column flowthrough. After extensive washing the bound proteins were eluted with GSH. The results (Fig. 4) showed that His-VP1ΔC60 bound to GST-VP3 (lane 6) and to a lesser extent also to GST-VP3ΔC13 (lane 7). His-VP1ΔC60 did not bind to GST-VP3C35 or to the GST control (lanes 8 and 9). These results corroborate the hypothesis that the VP1 interactive domain in SV40 VP2/3 is at the consensus sequence.
FIG. 4.
Interaction between VP2/3 and VP1. GST fusion proteins (GST-VP3, GST-VP3ΔC13, GST-VP3C35, and GST) were immobilized on GSH-agarose resin and then incubated with His-VP1ΔC60. The flowthrough was collected, and after extensive washing the proteins were eluted with GSH. Coomassie staining analysis of this experiment (not shown) showed that most of the GST fusion protein was always present in the second elution. The flowthrough (lanes 2 to 5) and second elution (lanes 6 to 9) from each experiment were analyzed by SDS-PAGE analysis followed by Western blotting with anti-six-His antibody. Lane 1, His-VP1ΔC60 starting material. The amount shown in the flowthrough is equivalent to half of that shown in the elutions and in lane 1.
It was reported that the VP3ΔC13 mutant does not bind VP1 at all (10), in experiments that were performed using VP3ΔC13 and VP1 that were prepared by in vitro transcription-translation. This method of preparing the proteins may have caused one or both of the partners to be improperly folded and to form inappropriate interactions, leading to results that are inconsistent with ours and with the prediction from the structure.
Sp1 binds to the major capsid protein VP1 and cooperates with it in DNA binding.
We asked whether Sp1 also recruits VP1 to ses. We tested for protein-protein interactions between VP1 and Sp1. Figure 5A shows that GST-VP1 immobilized on GSH-agarose pulls down Sp1 present in nuclear extracts of K562 cells (lane 3). Only trace amounts of Sp1 bound to the GST control (lane 6). A silver stain analysis of a similar gel demonstrated that the vast majority of the proteins in the nuclear extract did not bind to the GST-VP1 resin (not shown), indicating specificity of the binding. These results indicated that Sp1 binds to VP1. A separate experiment (bottom) showed that the addition of ethidium bromide to the binding reaction mixture had no effect (compare lanes 11 and 12 to lanes 9 and 10), suggesting that the interaction is not mediated by DNA. We cannot exclude the possibility that the interactions are mediated via another protein present in the nuclear extracts. However, this seems to be unlikely, as purified His-VP1ΔC60 cooperated in DNA binding with purified Sp1 (see below).
Cooperativity in DNA binding was tested using ses DNA (Fig. 5B). As a source of VP1 we used nuclear extracts of Sf9 cells infected with a recombinant baculovirus that expresses VP1 (30). The reaction was performed in the presence of double-stranded poly(dI-dC) to compete for the nonspecific binding activity of VP1 (33) and at a low concentration of Sp1, conditions at which Sp1 alone and VP1 alone each bound poorly to the DNA probe (lanes 2 and 3 and lane 7, respectively). A dramatic increase in gel retardation was observed when both Sp1 and VP1 were present in the DNA-binding reaction, producing several discrete bands (lanes 8 and 9). The complexes are similar in intensity to the bands produced by Sp1 and GST-VP3 (lanes 5 and 6) and with somewhat lower mobility. The control for VP1, mock-infected Sf9 nuclear extracts that do not contain VP1, did not affect the binding of Sp1 (lanes 10 to 12). A comparable degree of cooperativity was observed when Sp1 was incubated with both VP1 and GST-VP3 (lanes 14 and 15). VP1 and GST-VP3 were added at a molar ratio of 5:1. We interpret these results to indicate that the complex contains a VP15-VP2/3 unit, Sp1, and DNA.
We also carried out DNA-binding experiments with purified His-VP1ΔC60, produced in E. coli, with similar results. Supershifting experiment with anti-His antibody showed that His-VP1ΔC60 was present in the complex with Sp1 and DNA (not shown).
The C terminus of VP2/3 is required and sufficient for the interactions with Sp1.
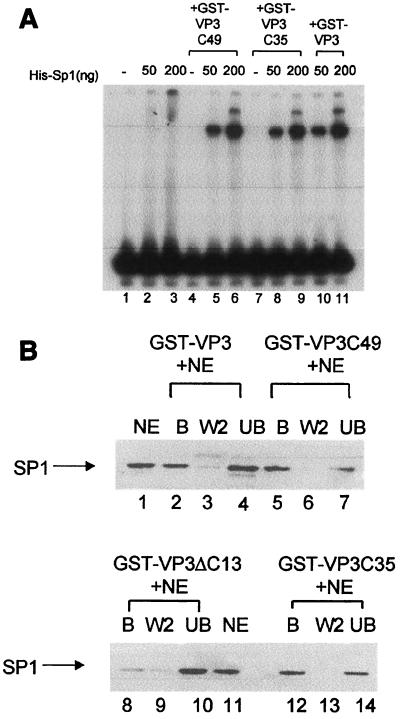
Previous studies (13) showed that the VP2/3 C-terminal DNA-binding domain (located at the 13 C-terminal amino acids) is required both for the cooperativity with Sp1 in specific binding to ses and for Sp1-VP2/3 protein-protein interactions in the absence of DNA. To define the minimal domain sufficient for these interactions, VP2/3 truncation mutants were constructed, produced in E. coli, and purified as GST fusion proteins. Figure 1C depicts a schematic diagram of the mutants. GST-VP3ΔC13 and GST-VP3C35 are described above. GST-VP3C49 carries sequences including several amino acids of the VP1 interactive domain, as defined for polyomavirus. Previously, it was demonstrated that GST alone and GST-VP3ΔC13 do not cooperate with Sp1 (13). Here, GST-VP3C49 and GST-VP3C35 were used in a DNA-binding assay with His-Sp1 and a six-GC-box probe. The results (Fig. 6A) demonstrate that both GST-VP3C49 and GST-VP3C35 cooperate with Sp1 in DNA binding to the GC box region (compare lanes 2 and 3 with lanes 5 and 6 and lanes 8 and 9). The degree of cooperativity is similar to that of full-length GST-VP3 (lanes 10 and 11). We conclude that the 35 C-terminal amino acids are sufficient for cooperativity between VP2/3 and Sp1. The results of these experiments are summarized in Table 3.
FIG. 6.
The VP3 C-terminal amino acids are sufficient for interactions with Sp1. (A) Cooperativity between Sp1 and VP3 mutants in DNA binding. A radioactively labeled six-GC-box DNA fragment (89 bp) (lane 1) was incubated with purified His-Sp1 and different VP3 mutants as indicated above the lanes. His-Sp1 produced in insect cells has lower DNA-binding activity than native Sp1; therefore, larger quantities of Sp1 were used in this experiment than in the experiment shown in Fig. 5B (50 ng of His-Sp1 ∼50 pmol). The 89-bp GC box probe produces only two bands in these assays. (B) Binding of Sp1 to VP3 mutants. GST-VP3, GST-VP3C49, and GST-VP3C35, as indicated above the lanes, all specifically interact with Sp1 present in nuclear extracts of K562 cells. The fractions—bound protein (B), wash (W), and unbound protein (UB)—were analyzed by Western blotting with anti-Sp1 antibody. Lanes 1 and 11 contain K562 nuclear extract (NE). The aliquots shown in each of the experimental lanes correspond to equivalent amounts of the input NE. GST-VP3ΔC13 does not interact with Sp1.
TABLE 3.
Domains of Sp1 and capsid proteins that participate in cooperativity in DNA binding
| Capsid protein and mutant | Participation in cooperativity in DNA binding
|
||
|---|---|---|---|
| Sp1 | ZnD | Zn | |
| GST-VP3 | + | + | + |
| GST-VP3ΔC13 | − | − | NDb |
| GST-VP3C49 | + | − | − |
| GST-VP3C35 | + | − | − |
| GST | − | − | − |
| VP1a | + | − | ND |
| VP1ΔC60 | + | − | − |
The same results were obtained for VP1 produced in insect cells and His-VP1 produced in E. coli.
ND, not done.
It has previously been shown that full-length VP3 binds Sp1 in the absence of DNA and that the C terminus is required for the binding (13). Is the C terminus of VP2/3 sufficient for binding Sp1? GST-pull down assays using GST-VP2/3 and their derivatives immobilized on GSH-agarose resin (Fig. 6B) show that GST-VP3, GST-VP3C49, and GST-VP3C35 all “fished” Sp1 out of K562 nuclear extracts (lanes 2, 5, and 12, respectively). In agreement with previous results (13), GST-VP3ΔC13 bound Sp1 poorly (lane 8). These findings demonstrate that the C terminus of VP2/3 is required and sufficient for the interactions with Sp1. However, we cannot exclude the possibility that the interactions seen in this experiment were mediated via DNA or another protein in the nuclear extract.
The zinc finger domain of Sp1 is required and sufficient for the interaction with VP3.
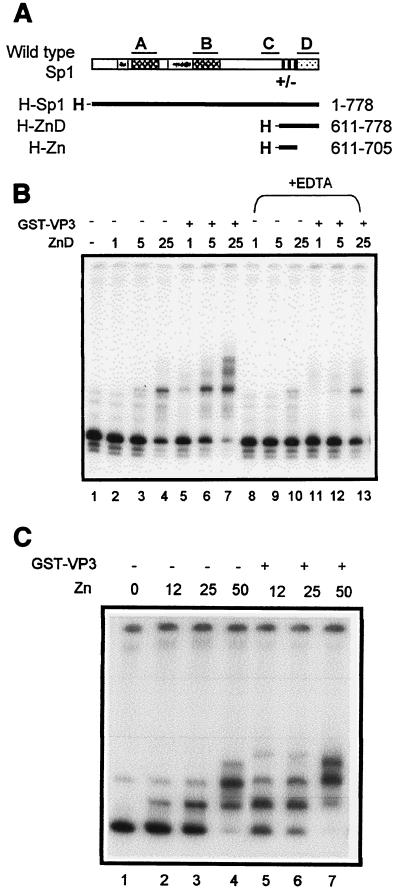
Since the VP2/3 DNA-binding domain is responsible for interactions with Sp1, we speculated that both proteins contact one another via their respective DNA-binding domains (13). Sp1 has a three-zinc-finger DNA-binding domain which lies close to the C terminus, between two domains involved in transcription activation, domains C and D (Fig. 7A) (6). The D domain mediates transcription by forming high-order structures between oligomers of Sp1 (25).
FIG. 7.
Identification of the VP2/3 interactive domain in Sp1. (A) Schematic representation of Sp1. The four regions, A, B, C, and D, that contribute to transcription activation (6) are marked above the diagram. A and B are glutamine-rich domains; gray boxes represent regions rich in serine/threonine residues. The region preceding the first zinc finger (+/−) is rich in charged amino acids. The black boxes represent the zinc fingers. Domain D mediates the formation of higher-order complexes between Sp1 oligomers (25). Below, heavy lines represent His-Sp1 (produced in Sf9 cells) and His-Sp1 mutants (produced in E. coli). H, six-His tag. The amino acids included are shown at right. (B) Gel shift with a ses DNA fragment. GST-VP3 (200 ng) and ZnD (amounts shown in nanograms) were added as indicated above the figure (1 ng of ZnD ≈ 0.05 pmol). In lanes 8 to 13, ZnD waspreincubated with EDTA to disrupt the zinc finger DNA-binding domain. (C) Gel shift experiment with a ses DNA fragment. GST-VP3 (200 ng) and Zn (amounts shown in nanograms) were added as indicated above the lanes (12 ng of Zn ≈ 0.6 pmol).
Two Sp1 truncation mutants (cloned as His-tagged proteins) were produced and purified from E. coli: one includes both the zinc finger and D domains (ZnD), and the other contains just the zinc finger domain (Zn) (Fig. 7A). In agreement with previous reports (18, 19), gel shift assays with a ses probe showed that both mutants were active in DNA binding (Fig. 7B and C, lanes 2 to 4), indicating that they were properly folded. Both mutants exhibited cooperativity with GST-VP3 in DNA binding to ses DNA (Fig. 7B and C, compare lanes 5 to 7 with lanes 2 to 4). These results, which are summarized in Table 3, demonstrate that the zinc finger domain mediates the interaction between Sp1 and VP3 in DNA binding. Furthermore, treatment with EDTA, which disrupts the zinc fingers of Sp1 (36), interfered with the DNA binding of ZnD (Fig. 7B, lanes 8 to 10) and inhibited the cooperativity between ZnD and VP3 (lanes 11 to 13).
To test whether the zinc finger domain is also responsible for the protein-protein interactions with VP3, we carried out GST-VP3 pull down assays with K562 cell nuclear extracts, which contain Sp1, in the presence and absence of EDTA. The results demonstrated that in the presence of EDTA Sp1 was no longer able to bind to GST-VP3 (data not shown). We conclude the Sp1 zinc finger domain is essential for the formation of both the Sp1-VP2/3 and Sp1-VP2/3-DNA complexes.
The minimal interactive domains of VP2/3 and Sp1 are not sufficient to cooperate with one another.
A simple prediction of the experiments described above is that the minimal interactive domains of VP2/3 and of Sp1, contained in the C terminus of VP2/3 and the zinc finger domain of Sp1, are sufficient for cooperativity with one another. However, gel shift experiments with a ses probe showed that ZnD and Zn did not cooperate with either GST-VP3C35 or GST-VP3C49 (Table 3), indicating that there are additional contact points between the two proteins outside the minimal domains.
VP1 and VP2/3 bind to different domains of Sp1.
We used the Sp1 truncation mutants to identify the VP1 interactive domain in Sp1. The results of gel retardation assays with the Sp1 mutants and VP1 (from insect cells), His-VP1, or His-VP1ΔC60 (produced in E. coli) are summarized in Table 3. Both the VP1 preparations cooperated in DNA binding with wild-type Sp1, but not with the ZnD mutant. Likewise, the VP1ΔC60 mutant did not cooperate with either the ZnD or Zn mutants. This indicates that for cooperativity in DNA binding VP1 contacts Sp1 at residues outside the zinc finger and D domains.
Sp1 participates in SV40 packaging in vitro.
SV40 virions and pseudovirions can be prepared in vitro. The three capsid proteins VP1, VP2, and VP3, produced as recombinant proteins in insect cells, localize to the nucleus where they assemble spontaneously to form virus-like particles (30). Virus-like particles are capable of incorporating in vitro either SV40 or plasmid DNA, producing infectious particles (29). Assembly can be assayed by infecting CMT4 cells and detecting infectious centers using in situ hybridization.
We have used this experimental system to ask whether Sp1 participates in packaging in vitro. Table 4 shows the results of representative experiments, performed with pEGFP-C1 that contains the SV40 ses element. It can be seen that the addition of Sp1 to the in vitro packaging reaction mixture increased the number of infectious particles produced. The effect of Sp1 on packaging was concentration dependent, with an optimum at 1 fpu. These data provide strong support for the hypothesis that Sp1 participates in SV40 assembly in vivo. High concentrations of Sp1 may be inhibitory due to sequestering of VP15-VP2/3.
TABLE 4.
Sp1 enhances SV40 packaging in vitro
| Sp1 [fpu (pmol)a] | ZnD [ng (pmol)] | IU/ml | Fold increaseb |
|---|---|---|---|
| 0 | 1.5 × 104 | 1 | |
| 1 (0.5) | 1.4 × 105 | 9.3 | |
| 5 (2.5) | 8.9 × 104 | 5.9 | |
| 0 | 1.2 × 104 | 1 | |
| 16 (0.8) | 1.1 × 104 | 0.9 | |
| 40 (2) | 1.48 × 104 | 1.2 | |
| 80 (4) | 3.6 × 104 | 3 |
1 fpu ≈ 50 ng.
Fold increase over control without the addition of Sp1 or ZnD.
As the Sp1 zinc finger domain is sufficient for the interaction with VP3, we asked whether this domain enhances in vitro packaging. The results (Table 4) showed that ZnD did increase the efficiency of packaging, but to a lower extent than the full-length protein: while 0.5 pmol of Sp1 increased the titer almost 10-fold, 4 pmol of ZnD, the highest concentration used (for technical limitations of the assay), increased the titer by only 3-fold. This result suggests that the ZnD mutant is sufficient to mediate recruitment of the capsid proteins to ses, but to a lesser extent than the full-length protein.
DISCUSSION
Viruses are known to usurp the cellular machinery for their gene expression and propagation. We previously reported that SV40 utilizes the host transcription factor Sp1 to facilitate its assembly. Here, we demonstrate that the underlying mechanism is by the recruitment of the capsid building block to the SV40 minichromosome. Thus, Sp1 confers the specificity of recognition of the packaging signal ses.
Previous biochemical studies have shown that Sp1 interacts with VP2/3 and forms a ternary complex with VP2/3 at ses (13). This led us to propose that Sp1 recruits VP2/3 to ses. The findings in this study provide strong support for the recruitment hypothesis. We demonstrate that cellular DNA did not compete with binding of the complex to ses. The specificity of ses is explained by the arrangement of six tandem GC boxes, which is unique to SV40. As VP15 and VP2/3 avidly bind to each other, most likely soon after their translation, we propose that Sp1 recruits the VP15-VP2/3 building block to ses. Supporting this hypothesis, we show here that Sp1 interacts with both VP1 and VP2/3 and that VP15-VP2/3 complexes are formed in vitro. The recruitment model explains how the capsid proteins find the minichromosome in the nucleus in a vast excess of cellular DNA. Additional support for the model comes from the finding that Sp1 greatly enhances the efficiency of packaging in vitro.
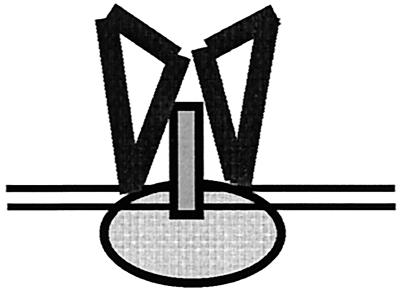
A diagram depicting our understanding of the molecular structure of the recruitment complex is depicted in Fig. 8. In the crystal structure VP2/3 enters the VP1 pentamer from the base, continuing to the upper part of the conical hollow. It then loops back to interact specifically with the inner face of VP1, forming a hairpin-like structure (4). In our schematic diagram, a cross section of a VP1 pentamer is depicted with a molecule of VP2/3 contacting both the inner core of VP1 and the DNA. Sp1 is shown bound to the DNA. We do not know if Sp1 participates in assembly as a tetramer or as a higher-order oligomer. Our diagram is based on the following experimental results: (i) previous findings that showed that a single GC box is sufficient to mediate the cooperativity between Sp1 and VP2/3 (albeit at a lower degree than full-length ses) and that binding of the Sp1-VP2/3 complex produces the same DNase I protection pattern as Sp1 alone (13); (ii) evidence that the C terminus of VP2/3, which contains the DNA-binding domain, is required and sufficient for the interactions with Sp1 (Table 3); and (iii) evidence that the zinc-finger domain of Sp1 is required and sufficient for the interaction with VP2/3, but not with VP1 (Table 3). As Sp1 and VP2/3 contact one another and the DNA via their respective DNA-binding domains, we propose that the DNA is sandwiched between the two proteins. It has been shown that Sp1 binds to DNA in the major groove (11, 26) and that the minor groove of the GC boxes remains free and appears to be widened upon Sp1 binding (35). Thus, it is possible that VP2/3 binds to the minor groove.
FIG. 8.
Diagram model of SP1-DNA-VP15-VP2/3 complex. A cross section of a VP1 pentamer (each triangle represents a VP1 monomer) complexed with a VP2/3 monomer (rectangle) is shown. GC box DNA (two parallel lines) is bound by both Sp1 (oval) and VP2/3, with the DNA sandwiched in between. Additional contacts are made between VP2/3 and Sp1 via their respective DNA-binding domains. VP1 contacts Sp1 outside the Sp1 zinc finger and D domains.
Our results show that cooperativity between VP1 and Sp1 is mediated by amino acids outside the zinc finger and D domains. The first 15 residues of the VP1 N terminus are not visible in the crystal structure. They probably extend inward (21) and were shown to interact with the minichromosome (20). It is possible that these residues also interact with Sp1. According to this molecular model, the interaction between Sp1 and VP2/3 may be sufficient to recruit the VP15-VP2/3 building blocks to ses. We propose that the additional contact points between Sp1 and VP1 increase the affinity of Sp1 for the VP15-VP2/3 complex. Note that Sp1 and VP1 bind to one another in the absence of DNA.
The data show that the C terminus of VP2/3 and the zinc finger domain of Sp1 are not sufficient for interactions with one another, indicating the presence of additional contact points between the two proteins. This is consistent with the data from in vitro packaging experiments. The Sp1 mutant ZnD enhanced packaging in vitro, but to a lesser extent than full-length Sp1.
Our studies substantiate the argument that the VP15 interactive domain of VP2/3 of SV40 is in the polyomavirus family consensus sequence, near but not at the carboxy terminus. This is demonstrated by the binding of GST-VP3ΔC13, but not GST-VP3C35, to VP15. The C terminus deletion mutant bound VP1 to a lesser extent than the complete VP3. A possible explanation is that, as previously suggested (2, 14), the truncation of 13 amino acids resulted in a conformational change of this protein that reduced its binding to VP1. An alternative possibility is that in SV40, the C terminus of VP2/3, which is not present in polyomavirus, make additional contacts with the VP1 pentamer that stabilizes the complex. Regardless, our results confirm that the three-dimensional structure of the SV40 building block is similar to that of polyomavirus, as previously suggested (4), although there may be additional interactions not found in polyomavirus.
SV40 minichromosomes that are active in replication and transcription have a nucleosome-free region at the regulatory region (16, 31), presumably due to the binding of transcription and replication factors. Before packaging this gap is closed (3), allowing condensation of the minichromosome, a prerequisite for viral assembly. A functional ses is required in cis for nucleosomal rearrangement (23). The viral late proteins participate in this process in trans (1, 23). In addition, the viral late proteins turn off the viral promoters (13; our unpublished results), presumably through their interaction with Sp1. Thus, ses appears to have multiple functions in the SV40 assembly: in the switch from transcription (and presumably also replication) to assembly, as a unique recognition signal for VP15-VP2/3, and in nucleosomal rearrangement. Turnoff of promoter activity, nucleosomal rearrangement, and viral assembly appear to be tightly coupled. Sp1 is not known to be present in the mature virion and is most likely displaced from the minichromosome as part of the process of nucleosomal rearrangement. However, the possibility that Sp1 remains in the capsid bound to ses cannot be excluded.
According to our model, the VP15-VP2/3 building blocks recruited to ses by Sp1 serve as the nucleation center for capsid propagation. Size considerations suggest that probably no more than three such complexes can form along the six GC boxes of ses, which face the same side of the double helix. By this mechanism the virus ensures that capsid formation is initiated at only one site around its minichromosome. Several such initiation sites would most likely lead to improperly formed capsids and/or premature termination. It has been proposed (34) that the first step in the assembly of polyomaviruses is the formation of a six-pentamer complex: a single pentavalent pentamer surrounded by five others with a fivefold axis of symmetry. The pentavalent pentamer makes identical contacts with the surrounding pentamers. In the fully formed capsid there are 12 such pentamers that lie at the fivefold rotation axis of the icosahedron.
One would predict that all polyomaviruses that have similar life cycles, with assembly occurring in the nucleus, face the same problem of recognition of their minichromosome by the capsid proteins. Although a requirement for a specific packaging signal has not been shown for other members of the polyomavirus family, it is attractive to speculate that they also use similar recruitment mechanisms. It has been recently reported that YY1, a transcription and nuclear matrix factor, interacts with polyomavirus VP1. As YY1 has two consensus binding sequences in the polyomavirus enhancer, it might have a role in conferring DNA-binding specificity to VP1 (24). As murine polyomavirus VP2 and VP3 do not have a DNA-binding domain (4), the specific molecular structure of the recruitment complex must be different.
Acknowledgments
We thank Amos B. Oppenheim for helpful discussions and critical reading of the manuscript, Jim Kadonaga for kindly providing pSp1778C, Harumi Kasamatsu for kindly providing pGEX-VP3ΔC13, and Guy Tomer for cloning pGEX-VP1.
This research was supported by The Israel Science Foundation founded by The Academy of Sciences and Humanities.
REFERENCES
- 1.Ambrose, C., V. Blasquez, and M. Bina. 1986. A block in initiation of simian virus 40 assembly results in the accumulation of minichromosomes containing an exposed regulatory region. Proc. Natl. Acad. Sci. USA 83:3287-3291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barouch, D. H., and S. C. Harrison. 1994. Interactions among the major and minor coat proteins of polyomavirus. J. Virol. 68:3982-3989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bina, M. 1986. Simian virus 40 assembly. Comments Mol. Cell. Biophys. 4:55-62. [Google Scholar]
- 4.Chen, X. S., T. Stehle, and S. C. Harrison. 1998. Interaction of polyomavirus internal protein VP2 with the major capsid protein VP1 and implications for participation of VP2 in viral entry. EMBO J. 17:3233-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clever, J., D. Dean, and H. Kasamatsu. 1993. Identification of a DNA binding domain in simian virus 40 capsid proteins VP2 and VP3. J. Biol. Chem. 268:20877-20883. [PubMed] [Google Scholar]
- 6.Courey, A. J., and R. Tjian. 1988. Analysis of SP1 in vivo reveals multiple transcriptional domains, including a novel glutamine-rich activation motif. Cell 55:887-898. [DOI] [PubMed] [Google Scholar]
- 7.Dalyot-Herman, N., O. Ben-nun-Shaul, A. Gordon-Shaag, and A. Oppenheim. 1996. The simian virus 40 packaging signal ses is composed of redundant DNA elements which are partly interchangeable. J. Mol. Biol. 259:69-80. [DOI] [PubMed] [Google Scholar]
- 8.Forstova, J., N. Krauzewicz, S. Wallace, A. J. Street, S. M. Dilworth, S. Beard, and B. E. Griffin. 1993. Cooperation of structural proteins during late events in the life cycle of polyomavirus. J. Virol. 67:1405-1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerard, R. D., and Y. Gluzman. 1985. New host cell system for regulated simian virus 40 DNA replication. Mol. Cell. Biol. 5:3231-3240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gharakhanian, E., and H. Kasamatsu. 1990. Two independent signals, a nuclear localization signal and a Vp1-interactive signal, reside within the carboxy-35 amino acids of SV40 Vp3. Virology 178:62-71. [DOI] [PubMed] [Google Scholar]
- 11.Gidoni, D., W. S. Dynan, and R. Tjian. 1984. Multiple specific contacts between a mammalian transcription factor and its cognate promoters. Nature 312:409-413. [DOI] [PubMed] [Google Scholar]
- 12.Goossens, M., and Y. Y. Kan. 1981. DNA analysis in the diagnosis of hemoglobin disorders. Methods Enzymol. 76:805-817. [DOI] [PubMed] [Google Scholar]
- 13.Gordon-Shaag, A., O. Ben-Nun-Shaul, H. Kasamatsu, A. Oppenheim, and A. Oppenheim. 1998. The SV40 capsid protein VP3 cooperates with the cellular transcription factor Sp1 in DNA-binding and in regulating viral promoter activity. J. Mol. Biol. 275:187-195. [DOI] [PubMed] [Google Scholar]
- 14.Ishii, N., A. Nakanishi, M. Yamada, M. H. Macalalad, and H. Kasamatsu. 1994. Functional complementation of nuclear targeting-defective mutants of simian virus 40 structural proteins. J. Virol. 68:8209-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackson, S. P., and R. Tjian. 1989. Purification and analysis of RNA polymerase II transcription factors by using wheat germ agglutinin affinity chromatography. Proc. Natl. Acad. Sci. USA 86:1781-1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jakobovits, E. B., S. Bratosin, and Y. Aloni. 1980. A nucleosome-free region in SV40 minichromosomes. Nature 285:263-265. [DOI] [PubMed] [Google Scholar]
- 17.Kadonaga, J. T., K. R. Carner, F. R. Masiarz, and R. Tjian. 1987. Isolation of cDNA encoding transcription factor Sp1 and functional analysis of the DNA binding domain. Cell 51:1079-1090. [DOI] [PubMed] [Google Scholar]
- 18.Kadonaga, J. T., A. J. Courey, J. Ladika, and R. Tjian. 1988. Distinct regions of Sp1 modulate DNA binding and transcriptional activation. Science 242:1566-1570. [DOI] [PubMed] [Google Scholar]
- 19.Kriwacki, R. W., S. C. Schultz, T. A. Steitz, and J. P. Caradonna. 1992. Sequence-specific recognition of DNA by zinc-finger peptides derived from the transcription factor Sp1. Proc. Natl. Acad. Sci. USA 89:9759-9763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li, P. P., A. Nakanishi, D. Shum, P. C. Sun, A. M. Salazar, C. F. Fernandez, S. W. Chan, and H. Kasamatsu. 2001. Simian virus 40 Vp1 DNA-binding domain is functionally separable from the overlapping nuclear localization signal and is required for effective virion formation and full viability. J. Virol. 75:7321-7329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liddington, R. C., Y. Yan, J. Moulai, R. Sahli, T. L. Benjamin, and S. C. Harrison. 1991. Structure of simian virus 40 at 3.8-Å resolution. Nature 354:278-284. [DOI] [PubMed] [Google Scholar]
- 22.Oppenheim, A., Z. Sandalon, A. Peleg, O. Shaul, S. Nicolis, and S. Ottolenghi. 1992. A cis-acting DNA signal for encapsidation of simian virus 40. J. Virol. 66:5320-5328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oppenheim, A., M. Siani, Z. Sandalon, and G. Mengeritsky. 1994. Dynamics of the nucleoprotein structure of simian virus 40 regulatory region during viral development. J. Mol. Biol. 238:501-513. [DOI] [PubMed] [Google Scholar]
- 24.Palkova, Z., H. Spanielova, V. Gottifredi, D. Hollanderova, J. Forstova, and P. Amati. 2000. The polyomavirus major capsid protein VP1 interacts with the nuclear matrix regulatory protein YY1. FEBS Lett. 467:359-364. [DOI] [PubMed] [Google Scholar]
- 25.Pascal, E., and R. Tjian. 1991. Different activation domains of SP1 govern formation of multimers and mediate transcriptional synergism. Genes Dev. 5:1646-1656. [DOI] [PubMed] [Google Scholar]
- 26.Pavletich, N. P., and C. O. Pabo. 1991. Zinc finger-DNA recognition: crystal structure of a Zif268-DNA complex at 2.1 Å. Science 252:809-817. [DOI] [PubMed] [Google Scholar]
- 27.Perier, R. C., V. Praz, T. Junier, C. Bonnard, and P. Bucher. 2000. The eukaryotic promoter database (EPD). Nucleic Acids Res. 28:302-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saffer, J. D., S. P. Jackson, and S. J. Thurston. 1990. SV40 stimulates expression of the transacting factor Sp1 at the mRNA level. Genes Dev. 4:659-666. [DOI] [PubMed] [Google Scholar]
- 29.Sandalon, Z., N. Dalyot-Herman, A. B. Oppenheim, and A. Oppenheim. 1997. In vitro assembly of SV40 virions and pseudovirions: vector development for gene therapy. Hum. Gene Ther. 8:843-849. [DOI] [PubMed] [Google Scholar]
- 30.Sandalon, Z., and A. Oppenheim. 1997. Self assembly and protein-protein interactions between the SV40 capsid proteins produced in insect cells. Virology 237:414-421. [DOI] [PubMed] [Google Scholar]
- 31.Saragosti, S., G. Moyne, and M. Yaniv. 1980. Absence of nucleosomes in a fraction of SV40 chromatin between the origin of replication and the region coding for the late leader RNA. Cell 20:65-73. [DOI] [PubMed] [Google Scholar]
- 32.Schreiber, E., P. Matthias, M. M. Muller, and W. Schaffner. 1989. Rapid detection of octamer binding proteins with ‘mini-extracts’ prepared from a small number of cells. Nucleic Acids Res. 15:6419-6436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soussi, T. 1986. DNA-binding properties of the major structural protein of simian virus 40. J. Virol. 59:740-742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stehle, T., S. J. Gamblin, Y. Yan, and S. C. Harrison. 1996. The structure of simian virus 40 refined at 3.1 Å resolution. Structure 4:165-182. [DOI] [PubMed] [Google Scholar]
- 35.Sun, D., and H. Hurley. 1994. Cooperative bending of the 21-base-pair repeats of the SV40 viral early promoters by human Sp1. Biochemistry 33:9578-9587. [DOI] [PubMed] [Google Scholar]
- 36.Westin, G., and W. Schaffner. 1988. Heavy metal ions in transcription factors from HeLa cells: Sp1, but not octamer transcription factor requires zinc for DNA binding and for activator function. Nucleic Acids Res. 16:5771-5781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wrobel, B., Y. Yosef, A. B. Oppenheim, and A. Oppenheim. 2000. Production and purification of SV40 major capsid protein (VP1) in Escherichia coli strains deficient for the GroELS chaperone machine. J. Biotechnol. 84:285-289. [DOI] [PubMed] [Google Scholar]