Abstract
One hallmark of uncontrolled, chronic human immunodeficiency virus type 1 (HIV-1) infection is the absence of strong HIV-1-specific, CD4+ T-cell-proliferative responses, yet the mechanism underlying this T helper (Th)-cell defect remains controversial. To better understand the impact of HIV-1 replication on Th-cell function, we compared the frequency of CD4+ Th-cell responses based on production of gamma interferon to lymphoproliferative responses directed against HIV-1 proteins in HIV-1-infected subjects with active in vivo viral replication versus those on suppressed highly active antiretroviral therapy (HAART). No statistically significant differences in the frequencies of cytokine-secreting, HIV-1-specific CD4+ T cells between the donor groups were found, despite differences in viral load and treatment status. However, HIV-1-specific lymphoproliferative responses were significantly greater in the subjects with HAART suppression than in subjects with active viral replication. Similar levels of HIV-1 RNA were measured in T-cell cultures stimulated with HIV-1 antigens regardless of donor in vivo viral loads, but only HIV-1-specific CD4+ T cells from subjects with HAART suppression proliferated in vitro, suggesting that HIV-1 replication in vitro does not preclude HIV-1-specific lymphoproliferation. This study demonstrates a discordance between the frequency and proliferative capacity of HIV-1-specific CD4+ T cells in subjects with ongoing in vivo viral replication and suggests that in vivo HIV-1 replication contributes to the observed defect in HIV-1-specific CD4+ T-cell proliferation.
Human immunodeficiency virus type 1 (HIV-1)-specific CD4+ T cells are thought to be important for the in vivo control of HIV-1 replication in chronically infected individuals. Support for a key role of CD4+ T helper cells in maintaining effective antiviral immunity originally came from studies of lymphocytic choriomeningitis virus (LCMV) infection in mice. Virus-specific CD4+ T cells were shown to be essential for maintenance of effective cytotoxic T lymphocyte immunity, known to control LCMV replication during chronic infection (25). In accordance with these observations in animal models, the significance of virus-specific CD4+ T cells in the control of human chronic cytomegalovirus (CMV) infection has also been documented (19, 40). In chronic HIV-1 infection, HIV-1 p24-specific CD4+ T-cell lymphoproliferative responses were shown to correlate with slower progression of disease (31). Furthermore, HIV-1-infected individuals with nonprogressive disease and low viral loads, termed long-term nonprogressors (LTNP), were reported to have strong HIV-1 p24-specific lymphoproliferative responses (35). These findings suggest a relationship between HIV-1-specific CD4+ T cells and both control of viral replication and delayed disease progression.
The hallmark of uncontrolled HIV-1 disease is the progressive loss of CD4+ T cells and specific loss of or failure to develop strong HIV-1-specific T helper-cell lymphoproliferative responses (37). As HIV-1 disease progresses, CD4+ T-cell proliferative responses against common recall antigens are gradually lost (11). At the end stages of disease, even responses to mitogens such as phytohemagglutinin are lost in many cases. Impairments in CD4+ T-cell lymphoproliferative responses were among the first functional defects described in chronically HIV-1-infected subjects with ongoing viral replication, yet the mechanism underlying this defect remains controversial.
Two alternative hypotheses have been proposed to explain the lack of HIV-1-specific proliferative responses in the majority of chronically infected individuals. The first maintains that the absence of HIV-1-specific lymphoproliferation is due to the specific deletion of HIV-1-specific CD4+ T cells early in the course of disease. This theory is supported in part by the finding that activated CD4+ T cells are preferentially infected by HIV-1 (36). According to this hypothesis, activation of HIV-1-specific CD4+ T cells early in disease during periods of high-level viremia might lead to their preferential infection and deletion by direct viral cytolysis or might indirectly lead to their death through apoptosis or other bystander mechanisms (10, 15, 18). The second hypothesis holds that HIV-1-specific CD4+ T cells are present during chronic infection but are dysfunctional and thus there is no measurable HIV-1-specific lymphoproliferation. The failure of these cells to proliferate in in vitro assays might reflect an inability of the cells to proliferate in vivo or might arise only in vitro due to cytopathic effects of replicating virus present in activated cultures. Consistent with this “dysfunction” model is the finding that untreated, chronically HIV-1-infected subjects have detectable frequencies of HIV-1-specific CD4+ T cells as determined by intracellular cytokine staining (29). In one study using this technique, subjects with untreated progressive disease were shown to have HIV-1-specific CD4+ T-cell frequencies in blood similar in magnitude to those measured in the blood of LTNP (41). Recently, McNeil et al. demonstrated differences in HIV-1-specific lymphoproliferative responses between HIV-1-infected LTNP and individuals with progressive disease despite similar frequencies of HIV-1 Gag-specific CD4+ T cells. These findings showed that HIV-1-specific CD4+ T-cell proliferation was suppressed in HIV-1-infected subjects with high-level viremia (26). Interestingly, a number of studies have demonstrated that HIV-1-specific lymphoproliferation can be rescued by the addition of factors such as CD40, anti-CD28, and interleukin-12 (IL-12), again suggesting that HIV-1-specific cells are present but dysfunctional in chronically HIV-1-infected subjects (7, 9).
Suppression of viral replication with highly active antiretroviral therapy (HAART) can also lead to the development of HIV-1-specific lymphoproliferative responses in certain clinical settings. It was recently reported that peripheral blood mononuclear cells (PBMC) from subjects who received HAART during acute HIV-1 infection demonstrated strong HIV-1 Gag-specific proliferation once in vivo viral replication was suppressed (34). Some of these subjects were able to maintain high peripheral CD4+ T-cell numbers and low plasma viral loads even with the cessation of antiretroviral therapy, suggesting that intact HIV-1-specific T helper responses might play a role in viral suppression during treatment interruption. In contrast, other studies have indicated that HIV-1-infected subjects treated during chronic or advanced stages of disease generally fail to restore or develop HIV-1-specific proliferative responses, although such treated individuals reconstituted recall and mitogen responses (4, 14, 30, 32). Contrary to these reports, a more recent study demonstrated the restoration or induction of HIV-1-specific lymphoproliferative responses in HIV-1-infected subjects treated with antiretroviral therapy 2 to 8 years after initial infection (1). Surprisingly, another recent study showed that subjects with low CD4+ T-cell nadirs (<75 cells/mm3) developed stronger HIV-1-specific proliferative responses than did those with higher CD4+ T-cell nadirs (>250 cells/mm3) once viral replication was suppressed with HAART (6).
These data taken collectively, along with the findings that HIV-1 p24-specific lymphoproliferation has been shown to inversely correlate with viral load during chronic infection (35), suggest that HIV-1 replication may have a direct effect on the ability of HIV-1-specific CD4+ T cells to proliferate in vitro and maybe in vivo as well. To evaluate the impact of viral replication on HIV-1-specific lymphoproliferation, we first compared the frequency of cytokine-secreting, HIV-1 p24-specific CD4+ T cells in PBMC and their capacity to proliferate in vitro for two groups: HIV-1-infected individuals with active viral replication and individuals receiving effective antiretroviral therapy. Additionally, to more directly address the ability of HIV-1-specific T helper cells to proliferate, in vitro experiments using carboxyfluoroscein succinimidyl ester (CFSE)-labeled PBMC were conducted to track the cells that specifically divided in response to the antigen. Similar frequencies of HIV-1 p24-specific, gamma interferon (IFN-γ)-producing cells were found in the peripheral blood of our cohort of subjects with HAART suppression and in the blood of subjects with active viral replication. By contrast, p24-specific lymphoproliferative responses in subjects with active viral replication were greatly diminished relative to those in subjects receiving effective antiretroviral therapy. Mechanisms underlying this discordance between antigen-specific cytokine secretion and lymphoproliferation were further investigated.
MATERIALS AND METHODS
Study population.
HIV-1-infected study subjects were selected from a cohort of HIV-1-infected individuals monitored in the Adult Infectious Diseases Group Practice at the University of Colorado Health Sciences Center. Inclusion criteria for the cohort of subjects with HAART suppression included receiving a combination of three or more antiretroviral agents with stable suppression of plasma viral load to <400 copies of HIV-1 RNA/ml of plasma for ≥4 months. Subjects were included in the HAART failure group if they had a measured plasma HIV-1 viral load of ≥1,000 copies of RNA/ml for ≥4 months despite a regimen of three or more antiretroviral drugs. Subjects in the HAART-naive group had never received antiretroviral drugs and had a measured plasma HIV-1 viral load of ≥1,000 copies of RNA/ml. Plasma HIV-1 RNA levels were measured with the Roche (Somerville, N.J.) HIV-1 Monitor kit, with analytical sensitivities of 200 and 20 copies/ml for the quantitative and ultraquantitative assays, respectively. HIV-1-negative subjects were healthy adult volunteers. All study subjects participated voluntarily and gave informed consent. The study was approved by the University of Colorado Health Sciences Center Institutional Review Board.
Lymphocyte proliferation assay (LPA).
PBMC isolated by density gradient centrifugation were resuspended at 106 cells/ml in RPMI medium (Gibco BRL, Rockville, Md.) with 10% human AB serum (Gemini, Woodsland, Calif.), and 100 μl was added to plates containing 100 μl of HIV-1 p24, p66, p55, and gp160 baculovirus-expressed recombinant proteins (NY5, IIIB, LAV, and MN strains, respectively; Protein Sciences Corporation, Meriden, Conn.; final concentration, 1 μg/ml) and baculovirus control protein (final concentration, 0.3 μg/ml). Phytohemagglutinin (final concentration, 5 μg/ml; Remmel, Lenexa, Kans.), CMV (final dilution,1/100; University of Colorado Health Sciences Center Diagnostic Virology Laboratory), and whole Candida albicans protein (10 μg/ml; Greer, Lenoir, N.C.) were used as positive controls in each assay. Cells were incubated at 37°C in a humidified 5% CO2 atmosphere for 6 days. Plates were pulsed with tritiated thymidine for 6 h and harvested, and radioactivity was counted on a Beta-counter (Packard, Meriden, Conn.). The stimulation index (SI) was calculated by dividing thymidine incorporation in the presence of antigen by the incorporation in the absence of antigen. Net counts per minute were calculated by subtracting the mean counts per minute of the control protein from the mean counts per minute in the presence of antigen. Background responses (to medium alone or baculovirus control protein) were less than 1,000 cpm in all assays. Median HIV-1-seronegative lymphoproliferative responses to p24 and p66 were 0.95 ± 0.34 and 1.1 ± 1.5, respectively.
Flow-cytometric detection of antigen-induced intracellular cytokines.
The frequency of antigen-specific, IFN-γ-secreting CD4+ T cells in PBMC was determined by a previously reported method, with minor modifications (29). Briefly, PBMC (1 × 106 to 2 × 106) were placed in 12- by 75-mm culture tubes containing 3 μg of anti-CD28 and -CD49d antibodies (Becton Dickinson, San Diego, Calif.)/ml in RPMI medium-10% human serum and cultured with HIV-1 p24 or p66 (5 μg/ml), CMV (1:10 dilution), staphylococcal enterotoxin B (1 μg/ml; Sigma, St. Louis, Mo.), baculovirus control protein, or medium alone. These cultures were incubated at a 5° slant at 37°C in a humidified 5% CO2 atmosphere for 14 h with brefeldin A (Becton Dickinson) added after the initial 4 h. Cells were surface stained with an anti-CD4 monoclonal antibody (MAb; Caltag, Burlingame, Calif.) for 30 min at 4°C. Cells were washed once with phosphate-buffered saline containing 1% bovine serum albumin and fixed for 15 min at room temperature, washed once again, permeabilized, and stained with anti-IFN-γ and -CD69 MAbs (Caltag) for 30 min at 4°C. Permeabilized cells were washed and resuspended in 1% formaldehyde. Samples were analyzed on a FACScan flow cytometer (Becton Dickinson). Generally 200,000 to 500,000 total events were collected and analyzed with Cell Quest software. The frequencies of antigen-specific CD4+ T cells were determined by subtracting the percentage of IFN-γ+, CD69+, CD4 T cells in PBMC stimulated with control antigen from the percentage of PBMC stimulated with antigen. The median background response to the baculovirus control protein for all HIV-1-infected subjects was 0.03% ± 0.06%.
Expansion of antigen-specific CD4+ T cells.
PBMC (106 PBMC/ml) were cultured with HIV-1 p24 antigen (5 μg/ml) or CMV antigen (1:10 final dilution) or relevant control proteins for 1 week in RPMI medium-10% human serum at 37°C in a humidified 5% CO2 atmosphere. In some experiments, PBMC were labeled with CFSE (Molecular Probes, Eugene, Oreg.) by incubation of 107 cells in a 1.5 μM solution of CFSE in Hanks balanced salt solution for 20 min at 37°C followed by two washes prior to incubation with antigen. The frequency of cultured CD4+ T cells secreting IFN-γ in response to antigenic stimulation was determined as described above, with the exception that autologous antigen-presenting cells (CD3+ T-cell-depleted autologous PBMC or an Epstein-Barr virus-transformed B-cell line) were added 1:1 to cultured cells during the 14-h incubation period. HIV-1 viral replication in cell cultures was measured by using the quantitative Amplicor HIV-1 Monitor Test (Roche) to measure HIV-1 RNA with an analytical sensitivity of 200 copies of RNA/ml. Cell-free supernatants were collected on the last day of culture, stored at −20°C, and thawed immediately prior to use.
Statistics analysis.
The Mann-Whitney test and the Kruskal-Wallis test with pairwise comparison (Dunn multiple comparison test) were utilized to determine differences between subject groups. A Spearman correlation test was performed to analyze the association between antigen-specific lymphoproliferation, IFN-γ frequencies, and plasma viral load.
RESULTS
HIV-1-specific PBMC lymphoproliferative responses in subjects with HAART suppression.
HIV-1-specific CD4+ T-cell function was first determined by measuring PBMC proliferation in response to HIV-1 antigens (p24, p66, and gp160) and Candida in 33 HIV-1-infected subjects with HAART suppression and 13 seronegative donors (Fig. 1). The HIV-1-infected subjects were all chronically infected and receiving combination antiretroviral therapy (three or more agents) and had peripheral CD4 counts ranging from 168 to 1,098 cells/mm3 (median, 488 cells/mm3) and plasma viral loads under 400 copies of HIV-1 RNA/ml of plasma. All of these HAART-treated subjects had been fully suppressed for at least 4 months, and none had begun receiving therapy during acute or early infection. Of the three HIV-1 antigens tested, PBMC proliferative responses to HIV-1 p24 in the HIV-1-infected cohort differed the most (P < 0.0001) from those in the HIV-1-seronegative controls (Fig. 1). Of the 33 donors with HAART suppression tested, 15 (45%) had significant PBMC proliferative responses (SI ≥ 5) to HIV-1 p24 antigen; the median SI was 4, and mean SI was 13.6. A similar number of significant responses to HIV-1 p66 antigen were observed (14 of 33 donors, or 42%; median SI, 4.1; mean SI, 14.8), and these responses were also significantly higher than those of the seronegative controls (P = 0.006). Median responses to HIV-1 gp160 in the HIV-1-infected subjects did not differ significantly from those of seronegative controls (P = 0.28), although 7 of 32 (21%) HIV-1-infected donors had positive lymphoproliferative responses (SI > 5) to gp160. There was no significant difference in the distribution of Candida-specific lymphoproliferative responses between the HIV-1-infected and uninfected groups, with 87% (median SI = 58) of the HIV-1-infected subjects and 92% (median SI = 34) of the seronegative donors having positive lymphoproliferative responses.
FIG. 1.
Lymphoproliferative responses to HIV-1 recombinant antigens (p24, p66, and gp160) and Candida antigen in subjects with HAART suppression and HIV-1-seronegative subjects. PBMC from HIV-1-infected and seronegative control subjects were cultured in the presence of antigen or control protein for 6 days. Lymphoproliferative responses to these antigens were determined by measuring [3H]thymidine incorporation, and the results were expressed as SIs. Each donor is depicted as a separate point, and a solid line represents the median value for each group. The median and mean SIs and numbers of responders for whom SI was ≥5 for each protein are shown at the bottom. Statistical significance was determined by the Mann-Whitney test.
Similar frequencies of HIV-1- and CMV-specific IFN-γ-producing CD4+ T cells are detectable in PBMC from HIV-1-infected subjects with HAART suppression and in those from subjects with active viral replication.
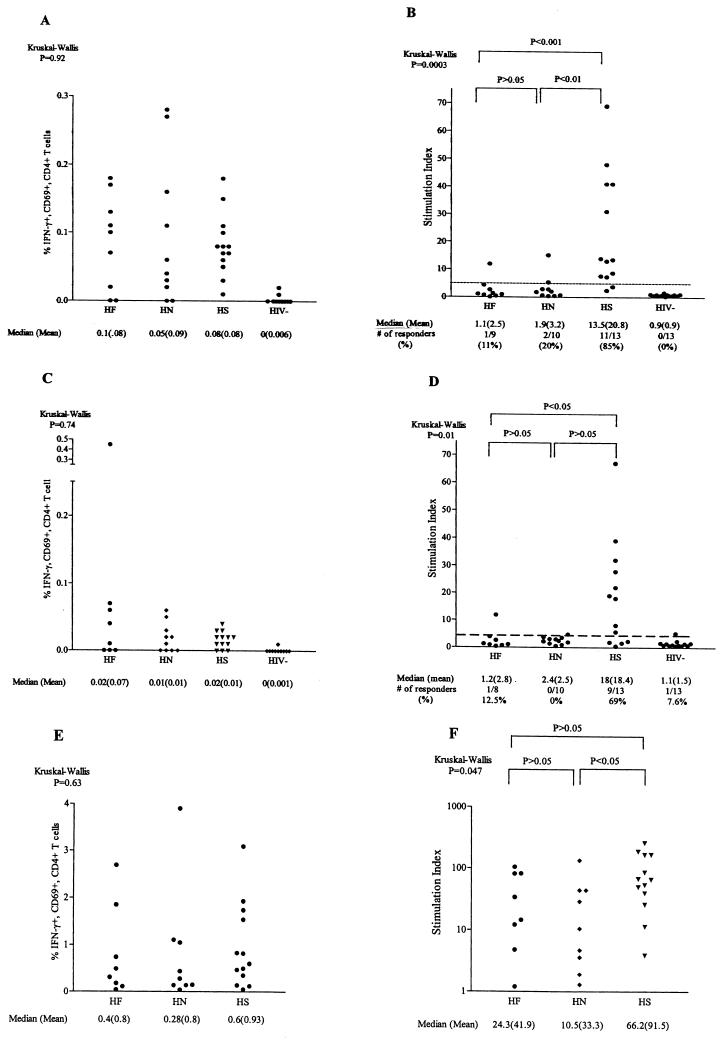
As noted above, a significant proportion of our successfully treated HIV-1-infected subjects displayed PBMC proliferative responses to HIV-1 proteins, responses that historically have been rarely observed in chronically infected subjects with active viral replication (11, 24). To determine whether in vivo HIV-1 replication negatively impacts the ability of HIV-1-specific CD4+ T cells to proliferate in vitro, we prospectively compared the IFN-γ frequency and lymphoproliferative capacity of HIV-1-specific CD4+ T cells in 19 subjects with active viral replication (10 HAART naive and 9 HAART failures) to those in 13 subjects stably receiving HAART with plasma HIV-1 RNA levels of <400 copies/ml. The clinical characteristics of the HIV-1-infected subjects are shown in Table 1. HAART-naive subjects were evaluated independently of HAART failure subjects, despite similarities in viral loads, due to the possibility of immunologic differences resulting from antiretroviral therapy. Subjects with HAART suppression were all chronically treated, with a median duration of viral suppression of 28 months. We elected to focus on responses to HIV-1 p24 and p66 antigens rather than other HIV-1 antigens as a result of our findings that proliferation in response to these two proteins was significantly greater in HIV-1-infected donors with HAART suppression than in seronegative controls (Fig. 1). We first determined the frequency of HIV-1 p24-, p66-, and CMV-specific CD4+ T cells in the subject groups using a recently developed flow cytometry-based assay that detects antigen-specific CD4+ T cells by measuring intracellular IFN-γ production and CD69 coexpression (Fig. 2 A, C, and E). All three of the HIV-1-infected donor groups had statistically higher p24-specific CD4+ T-cell frequencies than did seronegative controls (Fig. 2A). Analysis of variance (Kruskal-Wallis test) revealed that there was no statistically significant difference (P = 0.92) between the three groups of HIV-1-infected subjects in their frequencies of HIV-1 p24-specific IFN-γ-producing CD4+ T cells. The median frequencies of IFN-γ-producing cells in response to HIV-1 p24 were 0.1% (range, 0 to 0.18%) for HAART-naive subjects, 0.05% (range, 0 to 0.28%) for HAART failure subjects, and 0.08% (range, 0.01 to 0.17%) for subjects with HAART suppression. The median frequencies of p24-specific IFN-γ-producing CD4+ T cells were four- to fivefold higher than were p66-specific frequencies in all three subject groups (Fig. 2A and C). Likewise, for individual subjects, the frequency of p24-specific cells usually equaled or exceeded that of p66-specific cells (data not shown). Frequencies of HIV-1 gp160-specific cells were generally lower than those of p24- or p66-specific cells except in one subject. This subject experienced long-term HAART failure, and 3.9% of his total CD4+ T cells produced IFN-γ in response to gp160 (data not shown).
TABLE 1.
Clinical characteristics of HIV-1-infected donor groups
| HAART group | No. of subjects | Median age (range) (yr) | Median T-cell count (range) (cells/mm3) | Median T-cell count at nadir (range) (cells/mm3) | Median no. of copies of HIV mRNA/ml of plasma (range) |
|---|---|---|---|---|---|
| Suppresseda | 13 | 42 (31-48) | 461 (130-947) | 225 (0-581) | 20 (20-346) |
| Failureb | 9 | 40 (32-47) | 141 (48-500) | 64 (0-267) | 11,166 (4,469-1.61 × 106) |
| Naive | 10 | 32 (28-54) | 397 (97-1,055) | 174 (9-800) | 20,230 (1,985-1.07 × 106) |
Viral load, <400 copies of HIV-1 RNA/ml of plasma for ≥4 months.
Viral load, >1,000 copies of HIV-1 RNA/ml of plasma for ≥4 months.
FIG. 2.
Responder IFN-γ frequencies and lymphoproliferation of HIV-1 p24-, p66-, and CMV-specific CD4+ T cells in subjects receiving HAART with suppressed viral loads (HS), subjects failing HAART (HF), HAART-naive subjects (HN), and seronegative control subjects (HIV−). (A, C, and E) Responder frequencies of IFNγ+ CD69+ (percentage of CD4 T cells) CD4+ T cells for HIV-1 p24, p66, and CMV antigens based on intracellular cytokine staining, respectively. (B, D, and F) Lymphoproliferative responses (SIs) to HIV-1 p24, p66, and CMV antigen in HS, HF, HN, and seronegative control subjects, respectively. PBMC from HS, HF, HN, and seronegative subjects were stimulated with the respective antigen and assessed for intracellular IFN-g production within the CD4 1 CD69 1 T-cell population by flow cytometry and for lymphoproliferation by measuring [ 3 H]thymidine incorporation. Responder frequencies were corrected by subtracting the background from PBMC stimulated with control antigens (baculovirus for HIV-1 antigens or CMV-infected cell control lysate). Responses of individual subjects are depicted as separate points. A positive lymphoproliferative response is defined as a SI of $5 (dashed line). Where applicable the median and mean values and number of responders for each protein are shown. Statistical significance was determined by using the Kruskal-Wallis and Dunn multiple-comparison tests.
The frequencies of IFN-γ-producing CD4+ T cells responding to CMV in these same HIV-1-infected donor groups were also evaluated (Fig. 2E). As was the case with IFN-γ responses to p24 antigen, no statistically significant differences in the distribution of IFN-γ responses to CMV between the three HIV-infected subject groups were noted (P = 0.63). The median CMV-specific IFN-γ frequencies were significantly higher than the median p24-specific CD4+ T-cell frequencies in all subject groups. Even when the frequencies of CD4+ T cells reactive against all the HIV-1 antigens tested (p24, p66, and gp160) were summed for each individual donor, the responses to CMV were still greater (data not shown). These results show that IFN-γ-producing CD4+ T cells recognizing HIV-1 and CMV antigens were found in the peripheral blood of this cohort of chronically HIV-1-infected subjects regardless of differences in treatment status or levels of plasma viral RNA and that the frequency of these cells in PBMC did not significantly differ between groups.
Discordance between frequencies of HIV-1-specific IFN-γ-producing CD4+ T cells and lymphoproliferation in HIV-1-infected subjects with active viral replication.
The lymphoproliferative capacity of PBMC in response to HIV-1 antigens and CMV antigen from the subjects in the above clinical subgroups was also assessed (Fig. 2B, D, and F). Based on the responses of our seronegative donor, an SI of ≥5 is considered a positive antigen-specific response. In the group of subjects with HAART suppression, 11 of 13 (85%) responded to HIV-1 p24 antigen (median SI, 13.5; range, 2 to 69), whereas in the subject groups with active viral replication, little p24-specific proliferation of PBMC was observed (Fig. 2B). Only 1 in 9 (11%) subjects in the HAART failure group responded to p24 antigen (median SI, 1.1; range, 0 to 12), and only 2 in 10 (20%) subjects in the HAART-naive group responded (median SI, 1.9; range, 0 to 15). The distributions of HIV-1 p24-specific proliferative responses for both of the HIV-1-infected subject groups with active viral replication were significantly different from that for the group with HAART suppression (Fig. 2B), yet the frequencies of p24-specific IFN-γ-producing CD4+ T cells for the three subject groups were not statistically different (Fig. 2A).
HIV-1 p66-specific lymphoproliferation was also greater in the group with HAART suppression than in either group with active viral replication (Fig. 2D). PBMC from only 1 of 8 subjects experiencing HAART failure and from 0 of 10 HAART-naive subjects responded to HIV-1 p66, whereas positive responses to p66 were seen in 9 of 13 subjects with HAART suppression. These differences between the cohort with HAART suppression and that experiencing HAART failure were statistically significant, but those between subjects with HAART suppression and naive subjects only trended toward significance. These results point to an HIV-1-specific proliferative defect in PBMC from subjects with active viral replication and show that there is a discordance between the frequency of HIV-1-specific CD4+ T cells based on IFN-γ production and HIV-1-specific lymphoproliferation in this same subset of subjects.
Lymphoproliferative responses to whole CMV antigen in these subject groups were also assessed. As shown in Fig. 2F, the median CMV-specific SIs in the two cohorts with active viral replication were also lower than those in the cohort with suppression, but only differences between the group with HAART suppression and the HAART-naive group reached statistical significance. However, a comparison of the median CMV-specific proliferative responses to the median frequencies of CMV-specific, IFN-γ-producing CD4+ T cells revealed that the relative response patterns for the three groups for both assays were similar. Unlike p24-specific proliferative responses in subjects with active viral replication, CMV-specific proliferative responses in these subjects were reduced but still detectable. However, when net counts per minute were analyzed rather than SI, it was found that CMV-specific proliferative responses in both groups with active viral replication were significantly lower than those in the group with HAART suppression (data not shown), suggesting that CMV-specific proliferation is also adversely affected by HIV-1 replication in vivo.
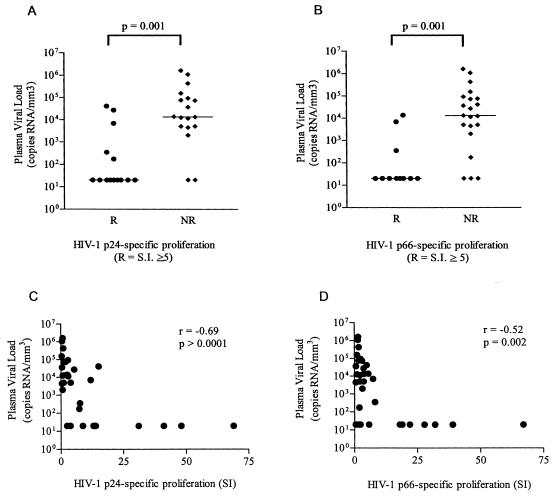
To evaluate the effect of plasma viral load on HIV-1-specific lymphoproliferation, all 32 HIV-1-infected subjects were categorized based on lymphoproliferative responses against HIV-1 p24 (Fig. 3A) and p66 (Fig. 3B) as either responders (SI ≥ 5) or nonresponders (SI < 5), with HIV-1 plasma viral loads plotted as the variable. Plasma HIV-1 viral loads were found to be significantly higher in subjects whose PBMC failed to respond by proliferation to p24 (P = 0.001) or p66 (P = 0.001) antigens. To evaluate whether there was a direct relationship between p24 (Fig. 3C) and p66 (Fig. 3D) lymphoproliferation and plasma viral load, a correlative analysis was performed. Strong, and statistically significant inverse correlations between both p24-specific (r = −0.69, P > 0.0001) and p66-specific (r = −0.52, P = 0.002) lymphoproliferation and plasma HIV-1 RNA levels were observed. These results further support the hypothesis that in vivo viral replication is associated with the inhibition of HIV-1-specific lymphoproliferative responses.
FIG. 3.
Plasma viral loads of HIV-1-infected subjects that responded (responders, R) or failed to respond (nonresponders, NR) to HIV-1 p24 (A) or p66 (B) proteins in a standard LPA and correlations of HIV-1 p24 (C) and HIV-1 p66 (D) SIs and viral loads for all patients. Responders were defined as subjects who had SIs of ≥5 for HIV-1 proteins. PBMC from HIV-infected subjects were pulsed with either HIV-1 p24 or p66 or baculovirus control protein, and proliferation was measured using a 6-day [3H]thymidine incorporation proliferation assay. Plasma viral loads were determined by using the Roche HIV-1 RNA Monitor kit. Each subject is depicted as a separate point, and the bar represents the median for each group. Statistical significance was determined by using the Mann-Whitney T test (A and B) or the Spearman correlation test (C and D).
Correlation between numbers of HIV-1 p24-specific, IFN-γ+ CD69+ CD4+ T cells and p24-specific lymphoproliferation.
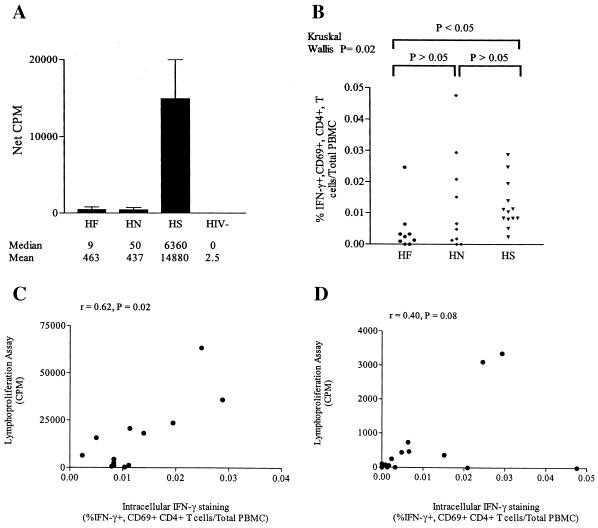
To determine whether a direct relationship between the frequency of antigen-specific, cytokine-producing CD4+ T cells and lymphoproliferation existed, we analyzed each parameter of CD4+ T-cell function in more-absolute terms, which allows for a more direct comparison between assays for each individual study subject. When HIV-1 p24-specific lymphoproliferation was expressed as net counts per minute rather than SI, statistically greater proliferation in subjects with HAART suppression than in those with active viral replication was again observed (Fig. 4A) and the median net counts per minute for subjects with active viral replication (HAART naive and HAART failure) was more than 100-fold lower than that for the cohort with HAART suppression. As was also observed with the evaluation of SIs, there was no significant difference between the cohort experiencing HAART failure and the HAART-naive cohort in terms of p24-specific proliferation evaluated by using net counts per minute.
FIG. 4.
Correlations between p24-specific proliferation and absolute numbers of p24-specific IFN-γ-producing CD4+ T cells in peripheral blood of HIV-1-infected subjects. (A) HIV-1 p24-specific lymphoproliferative responses in net counts per minute (CPM). Net values of counts per minute from LPAs were calculated by subtracting the mean CPM from wells containing baculovirus-stimulated PBMC from the mean counts per minute of PBMC stimulated with HIV-1 p24 protein. A statistically significant difference between the net counts per minute for both cohorts with active viral replication (HF and HN) versus the HS group was found (P = 0.001). (B) Absolute number of HIV-1 p24-specific, IFN-γ+ CD69+ CD4+ T cells per milliliter of blood in each HIV-1-infected cohort. This number was obtained by multiplying the p24-specific IFN-γ+ CD69+ CD4+ T-cell frequency by the peripheral blood CD4 count per milliliter. (C and D) Correlations between the number of HIV-1 p24-specific IFN-γ-producing CD4+ T cells per milliliter of blood in subjects with HAART suppression (C) and subjects with active viral replication (D). The correlation coefficients for the subjects with HAART suppression and subjects with active viral replication were 0.62 and 0.40, respectively. HF, HN, HS, and HIV− are as defined for Fig. 2.
To account for differences in the number of antigen-specific CD4+ T cells plated in the LPA, in which a constant number of PBMC are plated per well, the percentage of p24-specific, IFN-γ+ CD69+ CD4+ T cells per total PBMC for each subject was determined (Fig. 4B). By this more accurate reflection of relative p24-specific, cytokine-producing cells plated in the LPA, no statistical difference in the percentage of HIV-1 p24-specific CD4+ T cells per total PBMC between the HAART-naive group and the group with HAART suppression was found (P > 0.05). However, the group experiencing HAART failure had a statistically lower percentage of p24-specific CD4+ T cells per total PBMC than did the group with HAART suppression (P < 0.05). This was not unexpected, based on the relatively lower peripheral CD4 counts of subjects in the HAART failure group (Table 1). These same relationships between clinical groups were also observed when the absolute numbers of HIV-1 p24-specific, IFN-γ+ CD69+ CD4+ T cells per milliliter of whole blood were calculated (data not shown).
When the association between the percentage of IFN-γ-producing, p24-specific CD4+ T cells per total PBMC and p24-specific net counts per minute (proliferation) was assessed, a strong, statistically significant positive correlation (r = 0.62, P = 0.02) between these parameters for the cohort with HAART suppression was found (Fig. 4C). Interestingly, a positive correlation between IFN-γ-producing, p24-specific CD4+ T cells per total PBMC and p24-specific net counts per minute in subjects with active viral replication was also observed (Fig. 4D), although this did not reach statistical significance. It is also clear from these results that for a given percentage of HIV-1 p24-specific, IFN-γ+ CD69+ CD4+ T cells per PBMC, there was significantly less proliferation in subjects with active viral replication than in those with viral suppression.
Expansion of HIV-1-specific CD4+ T cells in vitro.
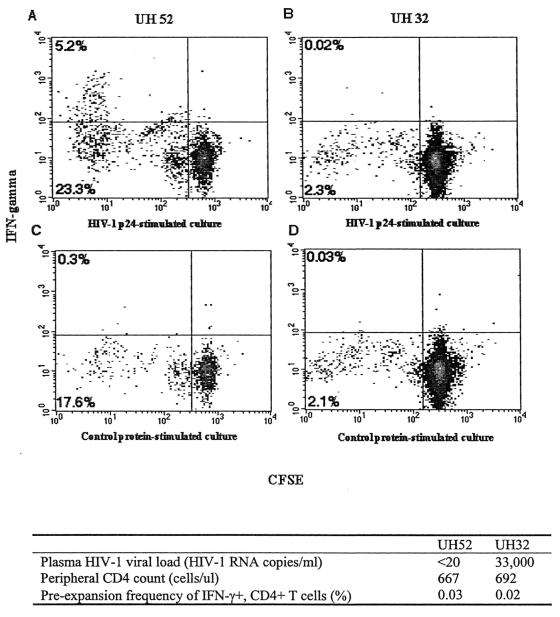
To further investigate the effect of viral replication on proliferation of antigen-specific CD4+ T cells in vitro, PBMC from both donors with HAART suppression and donors experiencing HAART failure were labeled with CFSE and cultured for 8 days in the presence of HIV-1 p24, CMV, or control antigens. On day 8, antigen and antigen-presenting cells were added to cultured T cells, and a standard intracellular IFN-γ assay was performed to assess the frequency of antigen-specific, cytokine-secreting CD4+ T cells. The degree of CD4+ T-cell proliferation was determined on the basis of the fluorescence of CFSE-labeled cells. Of the seven donors studied, four had plasma viral loads of <20 copies of HIV-1 RNA/ml (HAART suppression) and three subjects had viral loads of >4,000 copies of HIV-1 RNA/ml (HAART failure). Two representative examples of CFSE and IFN-γ staining of cultured CD4+ T cells are shown in Fig. 5. These two representative subjects had similar frequencies of HIV-1 p24-specific IFN-γ+ CD4+ T cells in fresh PBMC and similar total peripheral CD4+ T-cell counts, and both were receiving combination antiretroviral therapy. However, they differed markedly with regard to viral suppression: UH52 had a plasma viral load of <20 copies of HIV-1 RNA/ml on therapy, whereas UH32 was failing therapy with a viral load of 33,000 copies/ml. Following the culture period with p24 antigen, more than 10% of CD4+ T cells from UH52 PBMC proliferated specifically in response to p24 antigen, and a significant percentage of those proliferating CD4+ T cells produced IFN-γ in an antigen-specific fashion. HIV-1 p24-specific cells from donor UH32 failed to proliferate in vitro and likewise failed to produce IFN-γ upon antigen-specific stimulation.
FIG. 5.
Expansion of HIV-1 p24-specific IFN-γ+ CD4+ T cells in vitro. CD4+ T cells specific for HIV-1 p24 were detected in fresh PBMC by intracellular IFN-γ staining and flow cytometry. PBMC were labeled with CFSE and stimulated in culture with HIV-1 p24 antigen or baculovirus control protein for 8 days. Cultured cells from each condition were then restimulated with p24 or control antigen in a 6-h assay, and the frequency of IFN-γ producing CD4+ T cells was determined as described in Materials and Methods. Density plots are of gated CD4+ T cells and the percentages of CFSE-low, IFN-γ+ cells are shown in the top left corner or each plot. Density plots and clinical characteristic are shown for representative subjects UH52 and UH32.
Table 2 summarizes the expansion data from all seven subjects tested. All subjects had measurable frequencies of HIV-1 p24-specific IFN-γ-producing cells in their PBMC prior to expansion. After in vitro culture with p24 antigen, 0.67 to 4.5% of the CD4+ T cells in the subjects with viral loads of <20 copies/mm3 produced IFN-γ in response to HIV-1 p24, an increase in frequency of 16- to 100-fold over the frequency in PBMC. Conversely, p24-specfic, IFN-γ-producing CD4+ T cells were rarely observed in cultures from subjects with measurable in vivo HIV-1 viral replication regardless of the initial frequency in PBMC. The failure to detect HIV-1 p24-specific, IFN-γ-producing CD4+ T cells in cultures from subjects failing HAART was associated with very low levels of p24-specific CD4+ T-cell proliferation based on CFSE staining (Fig. 5 and data not shown). These results suggest that p24-specific, IFN-γ-producing CD4+ T cells from subjects with in vivo HIV-1 replication are unable to proliferate in vitro rather than that HIV-1 p24-specific CD4+ T cells can proliferate but simply fail to produce IFN-γ. PBMC from HIV-1-seronegative controls were also cultured with HIV-1 p24 antigen and, as expected, failed to proliferate or produce IFN-γ in response to p24 antigen (data not shown). These results provide direct evidence that HIV-1-specific CD4+ T cells from HIV-1-infected subjects with ongoing in vivo viral replication are impaired in their ability to proliferate and may likely even die in vitro upon antigen-specific stimulation.
TABLE 2.
HIV-1 p24 and CMV antigen-specific CD4+ T-cell expansions from HIV-1-infected donors
| Donor | Peripheral CD4+ T-cell count (cells/mm3) | Copies of HIV-1 RNA/ml of plasma | HIV-1 p24 stimulation
|
CMV stimulation
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % IFN-γ+ CD4+ T cells at daya:
|
Expansionb | Copies of HIV-1 RNAc | % IFN-γ+ CD4+ T cells at day:
|
Expansion | Copies of HIV-1 RNA | |||||
| 0 | 8 | 0 | 8 | |||||||
| UH32 | 826 | 32,681 | 0.02 | 0 | 0 | 5,724 | 0.09 | 2.2 | 24.4 | 39,404 |
| UH90 | 48 | 153,713 | 0.1 | 0 | 0 | 3,314 | NDd | 0 | 0 | 3,154 |
| UH91 | 107 | 4,993 | 0.07 | 0 | 0 | 1,380 | 0.49 | 12.08 | 24.6 | <200 |
| UH20 | 1,273 | <20 | 0.04 | 4.53 | 113 | 8,576 | 3.75 | 7.44 | 1.9 | <200 |
| UH8 | 523 | <20 | 0.04 | 4.08 | 102 | ND | 0.1 | 0 | 0 | ND |
| UH52 | 667 | <20 | 0.03 | 3.80 | 100 | 502 | 0.66 | 13.31 | 20.1 | 200-400 |
| UH64 | 942 | <20 | 0.04 | 0.67 | 16.7 | 2,429 | 0.16 | 0.35 | 2.1 | <200 |
Percentage of CD4+ T cells that produce IFN-γ in fresh PBMC (day 0) and in culture (day 8).
Percentage of IFN-γ+ CD4+ T cells in the culture divided by the percentage in the fresh PBMC.
Copies of HIV-1 RNA/ml of culture supernatant.
ND, not determined.
CMV-specific responses were also examined in order to determine whether the observed proliferative defect in subjects with in vivo HIV-1 replication was limited to HIV-1-specific CD4+ T cells (Table 2). Frequencies of CMV-specific, IFN-γ-producing CD4+ T cells from three subjects with plasma viral loads of <20 copies of HIV-1 RNA/ml (UH20, UH52, and UH64) increased from 2- to 20-fold after in vitro culture with CMV antigen. As opposed to the results for p24-stimulated cultures, CMV-stimulated cultures of PBMC from two subjects with detectable plasma viral loads (UH32 and UH91) also increased significantly, from 0.09 to 2.2% in subject UH32 and from 0.49 to 12% in UH91, with an average expansion from the baseline PBMC frequency of 24-fold. Two of the subjects evaluated, UH90 and UH8, either had low frequencies of CMV-specific IFN-γ-producing CD4+ T cells in PBMC or little CMV-specific PBMC lymphoproliferation at baseline, suggesting a negative CMV serologic status. In both cases, CMV-specific cells failed to expand in vitro, as expected. These results suggest that the plasma HIV-1 viral load may inhibit proliferation of HIV-1-specific CD4+ T cells to a greater extent than CMV-specific CD4+ T cells.
Culture supernatants were examined for HIV-1 replication based on HIV-1 RNA levels at day 8 (Table 2). Interestingly, HIV-1 p24-stimulated cultures from subjects with HAART suppression had detectable levels of HIV-1 RNA, ranging from 500 to 8,500 copies/ml. These numbers were comparable to HIV-1 RNA levels in p24-stimulated cultures from subjects with detectable plasma viral loads who were failing therapy. These data indicate that HIV-1 replication in vitro, as determined by the presence of HIV-1 RNA, does not necessarily prevent HIV-1-specific CD4+ T-cell proliferation. Although levels of HIV-1 RNA in CMV-stimulated cultures were more variable than those in p24-stimulated cultures, at least in one case (UH32) high levels of HIV-1 RNA did not prevent expansion of CMV-specific CD4+ T cells.
DISCUSSION
In this study, we observed significant HIV-1-specific lymphoproliferative responses in many HIV-1-infected individuals treated with antiretroviral agents during the chronic phase of HIV-1 infection. Analysis of our cohort of chronically infected subjects with HAART suppression revealed that more than 40% displayed significant lymphoproliferative responses to one or more HIV-1 antigens tested, a finding that contrasts with the results of some earlier studies. Several previous studies reported a failure to reconstitute HIV-1-specific lymphoproliferative responses after initiating antiretroviral therapy in a cohort of chronically infected subjects despite the development of mitogen- and recall antigen-specific proliferative responses in some studies (4, 14, 22, 33). However, results of several more recent studies show some degree of reconstitution of HIV-1-specific proliferation during therapy and are more consistent with our findings (1-3). For instance, Al-Harthi et al. monitored 16 subjects that had been infected with HIV-1 for 2 to 8 years prior to the initiation of HAART. At baseline, 31% of the subjects responded by lymphoproliferation to HIV-1 p24 antigen, and the number of responders increased to 69% following 48 weeks of potent antiretroviral therapy (1). Discrepancies between studies in the degree to which HIV-1-specific lymphoproliferative responses are reconstituted during HAART may be explained by a number of factors, including differences in the clinical characteristics of the donor population, the type of antiretroviral therapy utilized, the degree of virologic response to antiretroviral therapy, and the duration of follow-up.
In contrast to findings for HAART-treated individuals, strong HIV-1-specific lymphoproliferative responses in untreated HIV-1-infected subjects with chronic progressive disease have rarely been observed (5, 20, 37, 39). There are two major alternative explanations that could account for the loss or absence of measurable HIV-1-specific proliferative responses in the clinical setting of active viral replication. The first hypothesis maintains that subjects with active viral replication possess very few HIV-1-specific CD4+ T cells because these cells are preferentially deleted in vivo (24, 36). The second hypothesis holds that HIV-1-specific CD4+ T cells are present in the blood of HIV-1-infected individuals but that these cells fail to proliferate in vitro. The failure of HIV-1-specific CD4+ T cells to proliferate in in vitro assays might be an in vitro phenomenon resulting from HIV-1 replication in culture or other factors. In this study, we observed a marked decrease in HIV-1-specific lymphoproliferation in cells from subjects with active viral replication relative to that in cells from subjects on HAART with low-to-undetectable viral loads.
To determine whether the reduction in HIV-1-specific lymphoproliferation reflected lower frequencies of HIV-1-specific CD4+ T cells in subjects with active viral replication, we concurrently measured the frequency of HIV-1-specific CD4+ T cells using intracellular cytokine staining. Our results did not reveal a statistically significant difference between the frequencies of HIV-1-specific CD4+ T cells in subjects with suppressed HIV-1 replication on HAART versus those in subjects with active HIV-1 replication, despite the significant differences between these groups in the degree of HIV-1-specific lymphoproliferation. These finding are consistent with those of Wilson et al., who reported similar frequencies of HIV-1-specific IFN-γ-producing CD4+ T cells in LTNP and untreated progressors (41). In contrast, in an earlier study, Pitcher et al. found slightly higher frequencies of HIV-1 Gag-specific CD4+ T cells in subjects with untreated infection than in subjects with HAART suppression, suggesting a decline in HIV-specific CD4+ T-cell frequency over time on therapy (29). Our findings are more consistent with the CD4+ T-cell dysfunction hypothesis, in that we observed significant numbers of HIV-1-specific, IFN-γ-producing CD4+ T cells in the blood of HIV-1-infected individuals with active viral replication concurrent with a diminished HIV-1-specific lymphoproliferative response. By directly comparing HIV-1-specific proliferative function and IFN-γ frequencies in HIV-1-infected subjects grouped by treatment status and viral load in this study, these results both support and extend the recently published findings of McNeil et al., who observed a discordance between HIV-1-specific, cytokine-secreting CD4+ T-cell frequencies and lymphoproliferative capacity in untreated HIV-1-infected subjects with high-level HIV-1 viremia (26).
Since there was a possibility that the observed differences in HIV-1-specific lymphoproliferation between the clinical cohorts reflected discrepancies in the number of HIV-1-specific CD4+ T cells plated in the LPA rather than a true cellular proliferative defect, we reasoned that a measurement of the frequency of HIV-1-specific, IFN-γ-producing CD4+ T cells normalized to total PBMC might have more clinical relevance and might more accurately reflect differences in numbers of plated cells than the more relative frequency of CD4+ T cells. We determined that the frequency of Gag-specific CD4+ T cells per total PBMC for subjects failing HAART was significantly lower than that for the group with HAART suppression and HAART-naive group, although the frequencies as a percentage of total CD4+ T cells in the three groups did not differ significantly. These findings suggest that the relative frequency of HIV-1-specific CD4+ T cells does not necessarily change with declining peripheral CD4+ T-cell counts. They also indicate that comparing lymphoproliferative responses between HIV-1-infected subjects with widely differing peripheral CD4 counts, without accounting for those differences in CD4 count or the percentage of CD4 cells per PBMC, might result in inaccurate conclusions about cellular function. No significant differences in the frequencies of Gag-specific CD4+ T cells per total PBMC between the HAART-naive group and the group with HAART suppression were found. The fact that these two clinical groups, which differed markedly in viral loads but not peripheral CD4 counts, displayed major differences in HIV-specific lymphoproliferation further supports the idea that HIV-1 replication inhibits the proliferation of HIV-1-specific CD4+ T cells.
The discordance between the frequency and proliferative capacity of HIV-1-specific CD4+ T cells in subjects with active viral replication suggests that HIV-1-specific CD4+ T cells from subjects with replicating HIV-1 have a defect in their ability to proliferate. This statement, however, is based on the assumption that the cells observed to produce IFN-γ in the intracellular cytokine assay are also responsible for proliferation in the LPA. This assumption is supported by a positive correlation between HIV-1 p24-specific proliferation (net counts per minute) and the frequency of IFN-γ-producing CD4+ T cells responding to HIV-1 p24 in our subjects. In addition, Pitcher et al. demonstrated by double staining that HIV-1-specific CD4+ T cells which produced tumor necrosis factor alpha and IL-2 also produced IFN-γ, suggesting that staining for IFN-γ-producing CD4+ T cells provides comprehensive detection of HIV-1-specific CD4+ cells (29).
However, to more directly and conclusively demonstrate that the IFN-γ+ CD4+ T cells were also the cells that were proliferating in the LPA, we labeled PBMC with CFSE and incubated them for 1 week with HIV-1 p24 protein. Large expansions of p24-specific IFN-γ-producing cells in the CFSE-low cell population from HAART-treated subjects with undetectable viral loads were observed, suggesting that the IFN-γ+ CD4+ T cells detected in peripheral blood had proliferated. These findings confirm the recently published results of McNeil et al., who reported the antigen-specific in vitro proliferation of HIV-1-specific, IFN-γ-producing CD4+ T cells from untreated, HIV-1-infected LTNP with low viral loads (26). By contrast, analysis of subjects with active in vivo HIV-1 replication revealed very little p24-specific CD4+ T-cell proliferation and an absence of IFN-γ+ CD4+ specific T cells in the CFSE-low population, indicating that the HIV-1 p24-specific cells detected in peripheral blood had failed to proliferate upon stimulation with antigen and had possibly died in culture.
The amount of HIV-1 RNA in these expanded cell cultures was measured to investigate the hypothesis that viral replication in vitro might adversely affect HIV-1-specific CD4+ T-cell proliferation via a number of possible mechanisms. HIV-1 replication in vitro might inhibit proliferation in an LPA by direct lysis of activated CD4+ T cells or by more-indirect mechanisms, such as induction of T-cell apoptosis (16, 27, 28). In the small cohort of HAART-treated subjects tested, we observed similar levels of HIV-1 RNA in culture supernatants from subjects regardless of in vivo viral load and regardless of the degree of HIV-1-specific lymphoproliferation achieved in vitro. This finding suggests that active HIV-1 replication in vitro does not necessarily prevent the proliferation of HIV-1-specific CD4+ T cells, but it does not rule out a role for in vitro viral replication in the observed CD4+ T-cell proliferative defect. Although it does not appear that the absolute level of viral replication in culture inversely correlates with HIV-1-specific CD4+ T-cell proliferation, other virologic factors such as viral phenotype (tropism, “fitness,” and virulence) may play a more direct role in the observed proliferation defect. Alternatively, these findings may suggest that in vitro viral replication is not responsible for the observed proliferation defects but that effects of HIV-1 replication on T cells in vivo may persist and are reflected in the observed defects in in vitro proliferation.
This study provides further evidence that active HIV-1 replication is associated with the inhibition of HIV-1-specific proliferative responses. The exact mechanisms by which viral replication inhibits HIV-1-specific proliferation remain unclear and may be multifactorial. One possible mechanism by which HIV-1 replication could cause persistent T-cell proliferation defects is if high viral antigen loads in vivo were to lead to T-cell hyperactivation, resulting in a subsequent reduction in the expression of costimulatory molecules such as CD28 on CD4+ T cells (7, 8). Support for the role of CD28 in HIV-1-specific responses comes from studies that have shown that the addition of anti-CD28 MAbs can augment HIV-1-specific proliferation (23, 26). Additionally, upregulation of B7 molecules, which bind CD28, by an exogenous CD40 ligand trimer, has been shown to increase IFN-γ production in response to HIV-1 p24 antigen (7, 17). Viral products such as Nef, which would be found at much higher levels in subjects with ongoing in vivo viral replication, also have been shown to inhibit the production of IL-2 by CD4+ T cells (12, 13). An overall reduction in IL-2 production has been observed in persons with HIV-1 infection, and this decrease in IL-2 may lead to decreased T-cell proliferation (11, 21). To further support a role for IL-2, a proportional decrease in the number of CD4+ T cells able to produce IL-2 in HIV-1-infected subjects was recently reported (38).
In summary, strong HIV-1-specific lymphoproliferative responses were detected in HIV-1-infected subjects whose viral loads were suppressed on HAART but not in those subjects with active viral replication. Analysis of the frequency of HIV-1-specific, cytokine-secreting CD4+ T cells in the blood suggests that two factors may be responsible for the loss of proliferation in cells from subjects with active viral replication. In some circumstances, such as more advanced disease and lower peripheral CD4 counts, as reflected in our cohort of subjects experiencing HAART failure, an absolute decrease in number of HIV-1-specific CD4+ T cells might be in part responsible for lower levels of observed HIV-1-specific proliferation. However, our results comparing subjects with similar peripheral CD4 counts but differences in viral loads suggest that the absence of HIV-1-specific proliferative responses in the subjects with active viral replication is not simply due to a loss of HIV-1-specific cells but rather to an inability of these cells to proliferate in response to antigen. Consistent with this was our finding that, although a positive correlation between the frequency of HIV-1 Gag-specific, IFN-γ-producing CD4+ T cells per PBMC and tritiated thymidine incorporation in the LPA was observed for all HIV-1-infected subjects regardless of viral load, the magnitude of proliferation (in counts per minute) per IFN-γ-producing CD4+ T cell was markedly lower in subjects with detectable viral loads. Last, the results of this study also indicate that HIV-1 replication in vitro does not necessarily preclude HIV-1-specific proliferation. This finding may suggest that HIV-1 replication in vivo causes proliferative defects in HIV-1-specific CD4+ T cells that persist in vitro, although further studies to determine the mechanism involved are required.
Taken together, these data support a role for in vivo HIV-1 replication in the suppression of HIV-1-specific CD4+ T-cell proliferation. The fact that a significant number of HIV-1-infected subjects successfully treated with HAART during chronic disease were found to have strong HIV-1-specific CD4+ T-cell responses indicates that HIV-1-induced proliferative defects may be reversible. This suggests that therapeutic HIV-1 vaccines may be able to stimulate or enhance HIV-1-specific T-helper responses even in chronically infected, HAART-treated patients and provides further support for the administration of candidate vaccines to such patients.
Acknowledgments
We thank the participants of this study for their cooperation. We thank Cathi Basler, Michelle Barron, and the physicians and staff of the University Hospital Infectious Diseases Group Practice for assistance in recruiting and enrolling patients to this study. We also thank Elizabeth Connick for helpful input. Reagents were provided through the Vaccine and Prevention Research Program, DAIDS.
This work was supported in part by NIH grants AI01459 and AI48238 (C.C.W.).
REFERENCES
- 1.Al-Harthi, L., J. Siegel, J. Spritzler, J. Pottage, M. Agnoli, and A. Landay. 2000. Maximum suppression of HIV replication leads to the restoration of HIV-specific responses in early HIV disease. AIDS 14:761-770. [DOI] [PubMed] [Google Scholar]
- 2.Angel, J. B., A. Kumar, K. Parato, L. G. Filion, F. Diaz-Mitoma, P. Daftarian, B. Pham, E. Sun, J. M. Leonard, and D. W. Cameron. 1998. Improvement in cell-mediated immune function during potent anti-human immunodeficiency virus therapy with ritonavir plus saquinavir. J. Infect. Dis. 177:898-904. [DOI] [PubMed] [Google Scholar]
- 3.Angel, J. B., K. G. Parato, A. Kumar, S. Kravcik, A. D. Badley, C. Fex, D. Ashby, E. Sun, and D. W. Cameron. 2001. Progressive human immunodeficiency virus-specific immune recovery with prolonged viral suppression. J. Infect. Dis. 183:546-554. [DOI] [PubMed] [Google Scholar]
- 4.Autran, B., G. Carcelain, T. S. Li, C. Blanc, D. Mathez, R. Tubiana, C. Katlama, P. Debre, and J. Leibowitch. 1997. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science 277:112-116. [DOI] [PubMed] [Google Scholar]
- 5.Berzofsky, J. A., A. Bensussan, K. B. Cease, J. F. Bourge, R. Cheynier, Z. Lurhuma, J. J. Salaun, R. C. Gallo, G. M. Shearer, and D. Zagury. 1988. Antigenic peptides recognized by T lymphocytes from AIDS viral envelope-immune humans. Nature 334:706-708. [DOI] [PubMed] [Google Scholar]
- 6.Blankson, J. N., J. E. Gallant, and R. F. Siliciano. 2001. Proliferative responses to human immunodeficiency virus type 1 (HIV-1) antigens in HIV-1-infected patients with immune reconstitution. J. Infect. Dis. 183:657-661. [DOI] [PubMed] [Google Scholar]
- 7.Carlesimo, M., O. Pontesilli, A. R. Varani, M. L. Bernardi, A. M. Mazzone, R. Rosso, E. C. Guerra, A. Cassone, R. Paganelli, and F. Aiuti. 1997. CD28 costimulation and T lymphocyte proliferative responses in HIV-1 infection. Clin. Exp. Immunol. 109:406-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choremi-Papdououlou, H., V. Vigilis, P. Gargalianos, T. Kordossis, A. Iniotaki-Theodoraki, and J. Kosmidis. 1994. Downregulation of CD28 surface antigen on CD4+ and CD8+ T lymohcytes during HIV-1 infection. J. Acquir. Immune Defic. Syndr. 7:245-253. [PubMed] [Google Scholar]
- 9.Chougnet, C., E. Thomas, A. L. Landay, H. A. Kessler, S. Buchbinder, S. Scheer, and G. M. Shearer. 1998. CD40 ligand and IFN-gamma synergistically restore IL-12 production in HIV-infected patients. Eur. J. Immunol. 28:646-656. [DOI] [PubMed] [Google Scholar]
- 10.Clerici, M., A. Sarin, J. A. Berzofsky, A. L. Landay, H. A. Kessler, F. Hashemi, C. W. Hendrix, S. P. Blatt, J. Rusnak, M. J. Dolan, R. L. Coffman, P. A. Henkart, and G. M. Shearer. 1996. Antigen-stimulated apoptotic T-cell death in HIV infection is selective for CD4+ T cells, modulated by cytokines and effected by lymphotoxin. AIDS 10:603-611. [DOI] [PubMed] [Google Scholar]
- 11.Clerici, M., N. I. Stocks, R. A. Zajac, R. N. Boswell, D. R. Lucey, C. S. Via, and G. M. Shearer. 1989. Detection of three distinct patterns of T helper cell dysfunction in asymptomatic, human immunodeficiency virus-seropositive patients. Independence of CD4+ cell numbers and clinical staging. J. Clin. Investig. 84:1892-1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collette, Y., H. L. Chang, C. Cerdan, H. Chambost, M. Algarte, C. Mawas, J. Imbert, A. Burny, and D. Olive. 1996. Specific Th1 cytokine down-regulation associated with primary clinically derived human immunodeficiency virus type 1 Nef gene-induced expression. J. Immunol. 156:360-370. [PubMed] [Google Scholar]
- 13.Collette, Y., H. Dutartre, A. Benziane, and D. Olive. 1997. The role of HIV1 Nef in T-cell activation: Nef impairs induction of Th1 cytokines and interacts with the Src family tyrosine kinase Lck. Res. Virol. 148:52-58. [DOI] [PubMed] [Google Scholar]
- 14.Connick, E., M. M. Lederman, B. L. Kotzin, J. Spritzler, D. R. Kuritzkes, M. St. Clair, A. D. Sevin, L. Fox, M. H. Chiozzi, J. M. Leonard, F. Rousseau, J. D'Arc Roe, A. Martinez, H. Kessler, and A. Landay. 2000. Immune reconstitution in the first year of potent antiretroviral therapy and its relationship to virologic response. J. Infect. Dis. 181:358-363. [DOI] [PubMed] [Google Scholar]
- 15.Cottrez, F., A. Capron, and H. Groux. 1996. Selective CD4+ T cell deletion after specific activation in HIV-infected individuals; protection by anti-CD28 monoclonal antibodies. Clin. Exp. Immunol. 105:31-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dockrell, D. H. 2001. Apoptotic cell death in the pathogenesis of infectious diseases. J. Infect. 42:227-234. [DOI] [PubMed] [Google Scholar]
- 17.Dybul, M., G. Mercier, M. Belson, C. W. Hallahan, S. Liu, C. Perry, B. Herpin, L. Ehler, R. T. Davey, J. A. Metcalf, J. M. Mican, R. A. Seder, and A. S. Fauci. 2000. CD40 ligand trimer and IL-12 enhance peripheral blood mononuclear cells and CD4+ T cell proliferation and production of IFN-gamma in response to p24 antigen in HIV-infected individuals: potential contribution of anergy to HIV-specific unresponsiveness. J. Immunol. 165:1685-1691. [DOI] [PubMed] [Google Scholar]
- 18.Groux, H., G. Torpier, D. Monte, Y. Mouton, A. Capron, and J. C. Ameisen. 1992. Activation-induced death by apoptosis in CD4+ T cells from human immunodeficiency virus-infected asymptomatic individuals. J. Exp. Med. 175:331-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Komanduri, K. V., S. M. Donahoe, W. J. Moretto, D. K. Schmidt, G. Gillespie, G. S. Ogg, M. Roederer, D. F. Nixon, and J. M. McCune. 2001. Direct measurement of CD4+ and CD8+ T-cell responses to CMV in HIV-1-infected subjects. Virology 279:459-470. [DOI] [PubMed] [Google Scholar]
- 20.Krowka, J. F., D. P. Stites, S. Jain, K. S. Steimer, C. George-Nascimento, A. Gyenes, P. J. Barr, H. Hollander, A. R. Moss, J. M. Homsy, et al. 1989. Lymphocyte proliferative responses to human immunodeficiency virus antigens in vitro. J. Clin. Investig. 83:1198-1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane, H. C., J. M. Depper, W. C. Greene, G. Whalen, T. A. Waldmann, and A. S. Fauci. 1985. Qualitative analysis of immune function in patients with the acquired immunodeficiency syndrome. Evidence for a selective defect in soluble antigen recognition. N. Engl. J. Med. 313:79-84. [DOI] [PubMed] [Google Scholar]
- 22.Lederman, M. M., E. Connick, A. Landay, D. R. Kuritzkes, J. Spritzler, M. St. Clair, B. L. Kotzin, L. Fox, M. H. Chiozzi, J. M. Leonard, F. Rousseau, M. Wade, J. D. Roe, A. Martinez, and H. Kessler. 1998. Immunologic responses associated with 12 weeks of combination antiretroviral therapy consisting of zidovudine, lamivudine, and ritonavir: results of AIDS Clinical Trials Group Protocol 315. J. Infect. Dis. 178:70-79. [DOI] [PubMed] [Google Scholar]
- 23.Levine, B. L., J. D. Mosca, J. L. Riley, R. G. Carroll, M. T. Vahey, L. L. Jagodzinski, K. F. Wagner, D. L. Mayers, D. S. Burke, O. S. Weislow, D. C. St. Louis, and C. H. June. 1996. Antiviral effect and ex vivo CD4+ T cell proliferation in HIV-positive patients as a result of CD28 costimulation. Science 272:1939-1943. [DOI] [PubMed] [Google Scholar]
- 24.Levy, J. 1993. Pathogenesis of human immunodeficiency virus infection. Microbiol. Rev. 57:183-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Matloubian, M., R. J. Concepcion, and R. Ahmed. 1994. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 68:8056-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McNeil, A. C., W. L. Shupert, C. A. Iyasere, C. W. Hallahan, J. A. Mican, R. T. Davey, Jr., and M. Connors. 2001. High-level HIV-1 viremia suppresses viral antigen-specific CD4+ T cell proliferation. Proc. Natl. Acad. Sci. USA 98:13878-13883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Meyaard, L., S. A. Otto, R. R. Jonker, M. J. Mijnster, R. P. Keet, and F. Miedema. 1992. Programmed death of T cells in HIV-1 infection. Science 257:217-219. [DOI] [PubMed] [Google Scholar]
- 28.Meyaard, L., S. A. Otto, I. P. Keet, M. T. Roos, and F. Miedema. 1994. Programmed death of T cells in human immunodeficiency virus infection. No correlation with progression to disease. J. Clin. Investig. 93:982-988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitcher, C. J., C. Quittner, D. M. Peterson, M. Connors, R. A. Koup, V. C. Maino, and L. J. Picker. 1999. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat. Med. 5:518-525. [DOI] [PubMed] [Google Scholar]
- 30.Plana, M., F. Garcia, T. Gallart, J. M. Miro, and J. M. Gatell. 1998. Lack of T-cell proliferative response to HIV-1 antigens after 1 year of highly active antiretroviral treatment in early HIV-1 disease. Immunology Study Group of Spanish EARTH-1 study. Lancet 352:1194-1195. [DOI] [PubMed] [Google Scholar]
- 31.Pontesilli, O., P. Carotenuto, S. R. Kerkhof-Garde, M. T. Roos, I. P. Keet, R. A. Coutinho, J. Goudsmit, and F. Miedema. 1999. Lymphoproliferative response to HIV type 1 p24 in long-term survivors of HIV type 1 infection is predictive of persistent AIDS-free infection. AIDS Res. Hum. Retroviruses 15:973-981. [DOI] [PubMed] [Google Scholar]
- 32.Pontesilli, O., S. Kerkhof-Garde, N. G. Pakker, D. W. Notermans, M. T. Roos, M. R. Klein, S. A. Danner, and F. Miedema. 1999. Antigen-specific T-lymphocyte proliferative responses during highly active antiretroviral therapy (HAART) of HIV-1 infection. Immunol. Lett. 66:213-217. [DOI] [PubMed] [Google Scholar]
- 33.Rinaldo, C. R., Jr., J. M. Liebmann, X. L. Huang, Z. Fan, Q. Al-Shboul, D. K. McMahon, R. D. Day, S. A. Riddler, and J. W. Mellors. 1999. Prolonged suppression of human immunodeficiency virus type 1 (HIV-1) viremia in persons with advanced disease results in enhancement of CD4 T cell reactivity to microbial antigens but not to HIV-1 antigens. J. Infect. Dis. 179:329-336. [DOI] [PubMed] [Google Scholar]
- 34.Rosenberg, E. S., M. Altfeld, S. H. Poon, M. N. Phillips, B. M. Wilkes, R. L. Eldridge, G. K. Robbins, R. T. D'Aquila, P. J. Goulder, and B. D. Walker. 2000. Immune control of HIV-1 after early treatment of acute infection. Nature 407:523-526. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 36.Shearer, G. 1998. HIV-induced immuopathogenesis. Immunity 9:578-593. [DOI] [PubMed] [Google Scholar]
- 37.Shearer, G. M., and M. Clerici. 1991. Early T-helper cell defects in HIV infection. AIDS 5:245-253. [DOI] [PubMed] [Google Scholar]
- 38.Sieg, S., D. A. Bazdar, C. Harding, and M. M. Lederman. 2001. Differential expression of interleukin-2 and gamma interferon in human immunodeficiency virus disease. J. Virol. 75:9983-9985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wahren, B., L. Morfeldt-Mansson, G. Biberfeld, L. Moberg, A. Sonnerborg, P. Ljungman, A. Werner, R. Kurth, R. Gallo, and D. Bolognesi. 1987. Characteristics of the specific cell-mediated immune response in human immunodeficiency virus infection. J. Virol. 61:2017-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walter, E. A., P. D. Greenberg, M. J. Gilbert, R. J. Finch, K. S. Watanabe, E. D. Thomas, and S. R. Riddell. 1995. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N. Engl. J. Med. 333:1038-1044. [DOI] [PubMed] [Google Scholar]
- 41.Wilson, J. D., N. Imami, A. Watkins, J. Gill, P. Hay, B. Gazzard, M. Westby, and F. M. Gotch. 2000. Loss of CD4+ T cell proliferative ability but not loss of human immunodeficiency virus type 1 specificity equates with progression to disease. J. Infect. Dis. 182:792-798. [DOI] [PubMed] [Google Scholar]