Abstract
The surface glycoprotein S of transmissible gastroenteritis virus (TGEV) has two binding activities. (i) Binding to porcine aminopeptidase N (pAPN) is essential for the initiation of infection. (ii) Binding to sialic acid residues on glycoproteins is dispensable for the infection of cultured cells but is required for enteropathogenicity. By comparing parental TGEV with mutant viruses deficient in the sialic acid binding activity, we determined the contributions of both binding activities to the attachment of TGEV to cultured cells. In the presence of a functional sialic acid binding activity, the amount of virus bound to two different porcine cell lines was increased sixfold compared to the binding of the mutant viruses. The attachment of parental virus was reduced to levels observed with the mutants when sialic acid containing inhibitors was present or when the cells were pretreated with neuraminidase. In virus overlay binding assays with immobilized cell surface proteins, the mutant virus only recognized pAPN. In addition, the parental virus bound to a high-molecular-mass sialoglycoprotein. The recognition of pAPN was sensitive to reducing conditions and was not dependent on sialic acid residues. On the other hand, binding to the sialic acid residues of the high-molecular-mass glycoprotein was observed regardless of whether the cellular proteins had been separated under reducing or nonreducing conditions. We propose that binding to a surface sialoglycoprotein is required for TGEV as a primary attachment site to initiate infection of intestinal cells. This concept is discussed in the context of other viruses that use two different receptors to infect cells.
Transmissible gastroenteritis coronavirus (TGEV) is an enteropathogenic coronavirus that causes diarrhea in pigs. While older animals generally recover, piglets under the age of 3 weeks usually die from the infection. TGEV is a positive-stranded RNA virus surrounded by a lipid envelope (10). The viral membrane contains three transmembrane proteins: the S (220-kDa), M (29- to 36-kDa), and minor E (10-kDa) proteins. The M protein adopts two conformations, one with the amino terminus outside of the virion and the carboxy terminus inside and the other with both the amino and carboxy termini exposed on the viral surface (11, 12). The surface protein S initiates the infection by binding to the cell surface; it also mediates the subsequent fusion between the viral and cellular membranes. The S protein has two binding activities. Binding to aminopeptidase N is required for TGEV to initiate the infection of cells (7). In addition, the S protein has a sialic acid binding activity which enables TGEV to recognize terminal sialic acid residues on glycoproteins and glycolipids (29). As a consequence of the latter binding activity, TGEV can agglutinate erythrocytes. The two binding activities are located on different domains of the S protein. Studies with mutants of TGEV indicated that residues within a short stretch of amino acids (145 to 209) are important for the recognition of sialic acids (17, 18). Some of the mutants had been selected for resistance to a monoclonal antibody. The point mutations that were responsible for the lack of antibody reactivity also resulted in the loss of both the hemagglutinating activity and the enteropathogenicity (17). These results indicate that the sialic acid binding activity is correlated with the enteropathogenicity of TGEV. This view is consistent with data demonstrating that the enteric tropism of TGEV requires a factor (possibly the binding to a coreceptor) that maps around amino acid 219 of the S protein (1, 27), a position that is distal from the binding site for aminopeptidase N located between residues 522 and 744 (1, 14). Other factors may also be required to render TGEV enteropathogenic, but they have not been identified in terms of a molecular interaction. Porcine respiratory coronavirus (PRCoV), which is closely related to TGEV, also shows the importance of the sialic acid binding activity for enteropathogenicity. This virus replicates with high efficiency in the respiratory tract but with very low efficiency in the gut (5). Like the mutants mentioned above, PRCoV has no hemagglutinating activity (29). In the case of PRCoV, the lack of sialic acid binding activity is explained by a large deletion in the S gene that results in a truncated spike protein (24, 26). The point mutations that result in the loss of hemagglutinating activity and enteropathogenicity are located in the portion of the S protein that is present in the TGEV S protein but absent from the PRCoV S protein.
The available data suggest that sialic acid binding activity is required for enteropathogenicity but dispensable for the growth of TGEV in cell culture. In the present study, we investigated whether the binding of TGEV to cultured cells is mediated only by the interaction with aminopeptidase N or whether the sialic acid binding activity may also contribute to the attachment of TGEV to cells. We report that TGEV binds more efficiently to cells than do mutants that lack sialic acid binding activity. In addition to aminopeptidase N, a high-molecular-mass sialoglycoprotein on the surface of the cells is recognized by TGEV.
(This work was performed by C. Schwegmann-Wessels in partial fulfillment of the requirements for the Dr. Med. Vet. degree from the Tierärztliche Hochschule Hannover.)
MATERIALS AND METHODS
Virus.
The Purdue strain of TGEV (PUR46-MAD) (25) was used throughout this study. Stock virus was propagated in swine testicular (ST) cells. After incubation for 20 to 24 h at 37°C, the supernatant was harvested, clarified by centrifugation, and stored at −80°C after the addition of 1% fetal calf serum.
Cells.
ST and LLC-PK1 (pig kidney) cells were grown in Dulbecco's modified Eagle medium supplemented with 10% fetal calf serum.
Sucrose gradient centrifugation.
Sucrose gradient centrifugation was performed as described by Krempl and Herrler (19).
Neuraminidase treatment of virus.
Virus sedimented by ultracentrifugation was resuspended in 200 μl of phosphate-buffered saline (PBS) per tube and treated with 50 mU of Vibrio cholerae neuraminidase/ml for 30 min at 37°C. After enzyme treatment, virus was purified by sucrose gradient centrifugation as indicated above.
Isolation of the S protein.
Purified TGEV—either pretreated with neuraminidase or untreated—suspended in 400 μl of PBS was incubated in the presence of 1% n-octylglucopyranoside for 10 min at room temperature. After centrifugation for 30 min at 16,000 × g and 4°C, the supernatant was layered onto a sucrose gradient (10 to 30% [wt/wt] above a 0.5-ml cushion of 60% sucrose) in PBS containing 1% n-octylglucopyranoside. After centrifugation for 18 h at 214,000 × g, fractions of 0.5 ml were collected. The samples were analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and silver staining. Samples containing S protein were pooled and dialyzed overnight against H2O and PBS (4:1). Aliquots were stored at −20°C and used like the purified virus for overlay binding assays.
Binding of TGEV to cells.
Cell monolayers in microtiter plates were washed three times with PBS, and each well was incubated for 1 h at 37°C either with 25 mU of V. cholerae neuraminidase in MES (morpholineethanesulfonic acid) buffer or with buffer alone (0.05 M MES, 0.1 M NaCl, 0.02 M CaCl2; pH 6.5). After being washed, the cells were incubated with purified TGEV or TGEV mutants for 1 h at 4°C (11 μg of viral protein per well). The amount of added viral protein was determined via photometrical measurement of the UV absorption at 280 nm. The cells were washed three times with PBS and fixed with 3% paraformaldehyde for 20 min at room temperature, rinsed with PBS containing 0.1 M glycine, and incubated with glycine for 5 min. The fixed cells were incubated with the monoclonal antibody against the S protein and then with a peroxidase-conjugated rabbit antimouse antibody. Between all incubation steps, cells were rinsed three times with PBS. For the detection of bound antibody, the ABTS [2,2′-azinobis(3-ethylbenzthiazolinesulfonic acid)] peroxidase substrate was used. The reaction was stopped with 1% SDS. Extinction was measured at 405 nm.
Cell surface labeling.
The cellular surface proteins were biotinylated as described by Zimmer et al. (33).
Streptavidin and lectin precipitation.
Biotinylated cell monolayers were scraped with a rubber policeman from the petri dishes into ice-cold PBS (pH 7.4). The cells were pelleted by centrifugation (693 × g, 5 min, 4°C) and resuspended in 1 ml of NP-40 lysis buffer (1% 4-nonylphenolpolyethyleneglycol, 0.5% deoxycholate, 50 mM Tris-HCl [pH 7.5], 150 mM NaCl) containing protease inhibitors (protease inhibitor cocktail Complete; Roche). Following incubation on ice for 15 min, insoluble material was removed by centrifugation (16,000 × g, 30 min, 4°C). The cell lysate (1 ml) received 100 μl of a 50% slurry of streptavidin-agarose (prewashed three times with NP-40 lysis buffer) and was incubated overnight at 4°C while rotating. The streptavidin-agarose was pelleted by centrifugation (16,000 × g, 3 min, 4°C) and washed three times with NP-40 lysis buffer. A volume of 50 μl of V. cholerae neuraminidase (1 U/ml) in sodium acetate buffer with protease inhibitors [1 μM pepstatin, 1 μM leupeptin, 1 mM 4-(2-aminoethyl)-benzolsulfonylfluoride hydrochloride] was added to one part of the pellets; the other portion received only buffer with protease inhibitors. The samples were incubated on a rotating apparatus for 1 h at 37°C. The precipitated proteins were eluted by heating the streptavidin-agarose in 50 μl of 2× concentrated SDS sample buffer (100 mM Tris-HCl [pH 6.8], 4% SDS, 10% glycerol, 0.02% bromphenol blue) at 96°C for 10 min. For reducing conditions, 100 mM dithiothreitol was added to the samples followed by heating at 96°C for 5 min.
Isolation of cellular glycoproteins by the lectin wheat germ agglutinin was performed in principle like the streptavidin precipitation without the biotinylation step.
Virus overlay binding assay.
The cellular proteins were separated by SDS-PAGE and blotted to nitrocellulose by using a semidry Western blotting method (20). Nonspecific binding sites were blocked by incubation with blocking reagent (Roche) overnight at 4°C. After three washes with PBS-0.1% Tween, the membrane was incubated with purified TGEV or TGEV mutants for 1 h at 4°C (∼8 μg of protein per blot). Between each of the following incubation steps, the nitrocellulose was washed three times for 10 min each time with PBS-0.1% Tween. Incubation with a monoclonal antibody (6A.C3) against the viral S protein (13) for 1 h at 4°C was followed by incubation with a peroxidase-conjugated second antibody (goat antimouse). A chemiluminescent substrate (Pierce) was used for the detection of bound antibody.
Western blotting.
Cellular proteins were separated by SDS-PAGE (21) and blotted to nitrocellulose in the same manner as that described above. Following the blocking and washing steps, the membrane was incubated with a monoclonal antibody (G43) against porcine aminopeptidase N (7) for 1 h at 4°C. After three washes with PBS-0.1% Tween, a peroxidase-conjugated second antibody (goat antimouse) was added. A chemiluminescent substrate (Pierce) was used for the detection of bound antibody.
Silver staining of proteins.
For the detection of proteins in polyacrylamide gels, a silver stain (Bio-Rad) was used according to the instructions of the manufacturer.
Triton X-114 phase separation.
Surface biotinylated ST cells were extracted with the detergent Triton X-114 followed by temperature-induced phase separation (22). Reextracted aqueous and detergent phases were diluted to 1 ml with Tris-buffered saline and used for streptavidin precipitation.
RESULTS
Binding of TGEV to cultured cells.
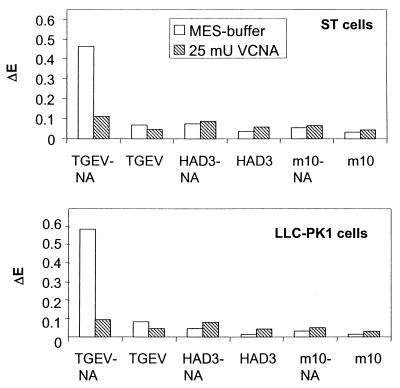
Aminopeptidase N is a crucial receptor for the infection of cultured cells, whereas the sialic acid binding activity is dispensable for virus growth in cell culture. We were interested in knowing whether the binding of TGEV to cultured cells is mediated only by the interaction with aminopeptidase N or whether the sialic acid binding activity of TGEV also contributes to the attachment of virus to cells. For this purpose, ST cells and LLC-PK1 cells were grown in microtiter plates. Purified virus was incubated with the cells at 4°C. After having washed away unbound virus, bound virions were detected by an enzyme-linked immunoassay with a monoclonal antibody directed against the viral surface protein S. In addition to TGEV, two mutants were included in the assay. Mutant HAD3 was selected for its inability to bind to erythrocytes (18). The second mutant, m10, was selected for its resistance to a monoclonal antibody (2). Both mutants are impaired in their sialic acid binding activity, as evidenced by the lack of hemagglutinating activity (17, 18). As shown in Fig. 1, the binding of TGEV to ST as well as to LLC-PK1 cells was measurable (Fig. 1, open bars). When the virus was pretreated with neuraminidase (TGEV-NA), the amount of bound virus increased about sixfold. The enzyme treatment removes sialoglycoconjugates from the virion surface that accumulate in the course of infection. These compounds are derived from the cell surface and are responsible for the lack of hemagglutinating activity of TGEV when harvested late in infection. By contrast, virus harvested earlier in infection is very efficient in agglutinating red blood cells. Virus harvested late in infection can be converted into an efficient agglutinating agent by treatment with neuraminidase. Such virus (TGEV-NA) was used for the binding assay. The difference in the binding efficiency between TGEV (no hemagglutinating activity) and TGEV-NA (high hemagglutinating activity) suggests that the increased binding of TGEV-NA compared to that of TGEV is due to the sialic acid binding activity of TGEV. This conclusion is in agreement with the result obtained with the mutants that lacked sialic acid binding activity. The low levels of bound virus (HAD3 and m10) were only marginally changed by the pretreatment of virions with neuraminidase (HAD3-NA and m10-NA, compare open bars in Fig. 1) and were well below (<0.1) the values obtained with TGEV-NA (>0.4). The importance of the sialic acid binding activity for the binding of TGEV-NA to cells was confirmed by the analysis of cells that had been pretreated with neuraminidase. The enzymatic removal of sialic acid residues from the cell surface resulted in a greater-than-fourfold reduction of bound TGEV-NA in the case of ST cells and in an almost sixfold reduction in the case of LLC-PK1 cells (Fig. 1, dashed bars). Only a slight reduction was observed with TGEV that had not been pretreated with neuraminidase. No reduction was measured with the two mutants. Thus, the sialic acid binding activity may efficiently contribute to the binding of TGEV to cells.
FIG. 1.
Binding of TGEV and the mutants HAD3 and m10 to ST cells (upper panel) and LLC-PK1 cells (lower panel) in microtiter plates. After unbound virus was washed away, cells were fixed and bound virus was detected by an enzyme-linked immunoassay. The designation NA (TGEV-NA, HAD3-NA, m10-NA) indicates virus that has been treated with neuraminidase to inactivate competitive inhibitors that may accumulate on the virion surface during the course of infection. Cells were either mock treated (MES buffer) or pretreated with neuraminidase from V. cholerae (VCNA) to release sialic acids from the cell surface. ΔE indicates the difference between the extinction value of the cells incubated with virus and antibodies and the extinction value of the cells incubated only with antibodies. The amount of added virions was determined by protein analysis as described in Materials and Methods.
Binding of TGEV to cell surface proteins.
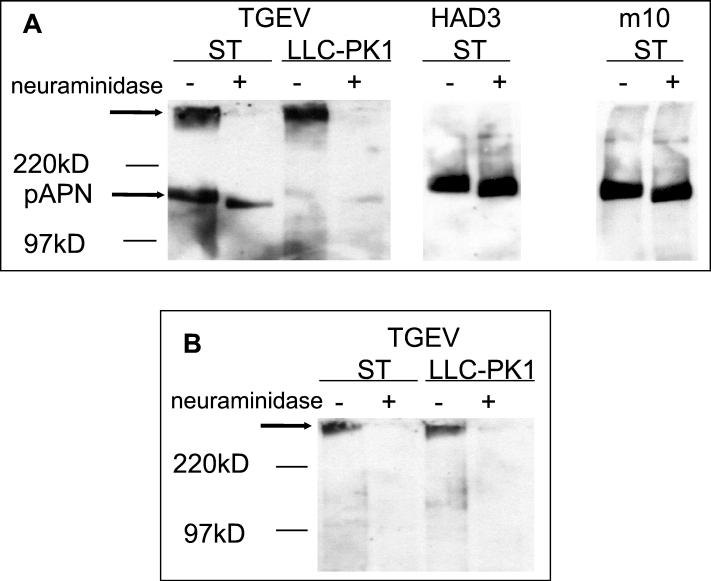
We analyzed cell surface proteins for their ability to mediate virus attachment. After biotinylation of ST and LLC-PK1 cells, cell surface proteins were precipitated from the cell lysates by using immobilized streptavidin. Proteins were separated by SDS-PAGE under nonreducing conditions and blotted to a membrane. The immobilized proteins were used for a virus overlay binding assay to compare TGEV and the mutants HAD3 and m10 (Fig. 2A). All three viruses bound to a protein (pAPN) migrating in a position where aminopeptidase N, the cellular receptor for TGEV, is expected. The pAPN band recognized in ST cells was stronger than the corresponding band from LLC-PK1 cells, suggesting that the latter cells contain less aminopeptidase N on the surface. TGEV, but not the two mutants, recognized an additional band of high molecular mass (Fig. 2A). The binding to this cellular surface component was abolished after neuraminidase treatment of the precipitated cell surface proteins, indicating that the binding was mediated by sialic acid residues present on this high-molecular-mass compound. The fact that the N-hydroxysuccinimide ester of N-hydroxysuccinimide biotin was able to bind to this cellular surface component showed that it has a peptide backbone. Therefore, the high-molecular-mass band represents a sialoglycoprotein. The enzymatic release of sialic acids did not affect the binding to pAPN. When SDS-PAGE was performed in the presence of dithiothreitol, i.e., under reducing conditions (Fig. 2B), pAPN was not recognized by TGEV. This result indicates that the receptor determinant on pAPN that interacts with the S protein of TGEV is a conformational domain rather than a linear stretch of amino acids. In contrast to the result obtained with pAPN, the binding of TGEV to the high-molecular-mass band was not abolished when the electrophoretic separation was performed under reducing conditions (Fig. 2B).
FIG. 2.
Binding of TGEV and the mutants HAD3 and m10 to cell surface proteins. Proteins were isolated from ST or LLC-PK1 cells by surface biotinylation and either mock treated (−) or neuraminidase treated (+). Following electrophoretic separation under nonreducing (A) or reducing (B) conditions, the proteins were transferred to nitrocellulose. The immobilized proteins were incubated with purified virus, and bound virus was detected by an enzyme-linked immunoassay. On the left side, the positions of molecular mass markers are indicated. Arrows point to aminopeptidase N and to the high-molecular-mass sialoglycoprotein recognized by TGEV.
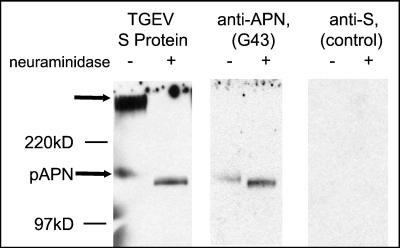
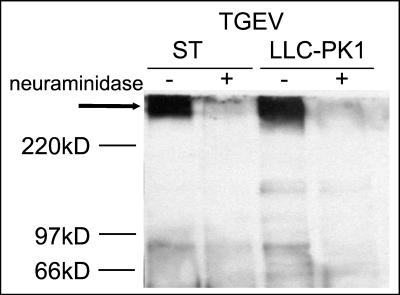
The binding of TGEV to both aminopeptidase N and sialoglycoconjugates is mediated by the viral S protein. In order to find out whether other viral components such as the M or E proteins affect the binding behavior of TGEV, we analyzed whether these binding activities are also observed with isolated S protein. For this purpose, the surface protein was solubilized by detergent treatment of purified virus pretreated with neuraminidase. Following purification of the viral surface glycoprotein by sucrose gradient centrifugation, an overlay binding assay was performed. The S protein resembled TGEV virions, recognizing both a high-molecular-mass band and a band with an apparent molecular mass of about 150 kDa (Fig. 3). The latter band was identified as pAPN by its reaction with a specific monoclonal antibody. Binding to the high-molecular-mass band was abolished by treatment of the sample with neuraminidase. The enzyme treatment was also effective in releasing sialic acid residues from aminopeptidase N, as indicated by a slight increase in the electrophoretic mobility. However, desialylation of aminopeptidase N increased rather than decreased the interactions with the S protein as well as with the monoclonal antibody directed against aminopeptidase N.
FIG. 3.
Binding of the S protein of TGEV to cell surface proteins from ST cells. Mock-treated (−) and neuraminidase-treated (+) proteins were electrophoretically separated under nonreducing conditions and transferred to nitrocellulose. The immobilized proteins were incubated with purified S protein from TGEV (left panel), with a monoclonal antibody directed against aminopeptidase N (middle panel), or as a negative control with monoclonal antibody 6A.C3 directed against the S protein of TGEV (right panel). The S protein was isolated from neuraminidase-treated, purified virions as described in Materials and Methods. The binding of S protein was detected with monoclonal antibody 6A.C3. Bound antibodies were visualized by an enzyme-linked immunoassay.
Characterization of the sialoglycoprotein.
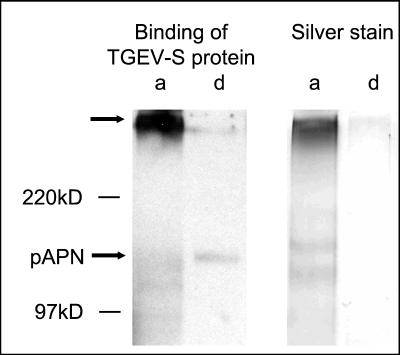
Triton X-114 phase separation was used to study the association of the high-molecular-mass protein with the plasma membrane. Surface biotinylated proteins were extracted with Triton X-114 and precipitated with streptavidin-agarose following phase separation. As shown in Fig. 4, the high-molecular-mass protein was found mainly in the aqueous phase (Fig. 4, lanes a). The protein was detected either by an overlay binding assay with the isolated S protein of TGEV (Fig. 4, left panel) or by silver staining with a modification rendering it more efficient than standard assays in detecting glycosylated macromolecules (Fig. 4, right panel). The behavior of the high-molecular-mass band in the phase separation suggests that it is a membrane-associated rather than a transmembrane protein. But we cannot exclude the possibility that the high-molecular-mass protein is an integral membrane protein and is found in the aqueous phase due to a large hydrophilic ectodomain.
FIG. 4.
Triton X-114 phase separation of surface proteins from ST cells. The proteins recovered from the aqueous (a) and detergent (d) phases were electrophoretically separated and either visualized by silver staining (right panel) or, following transfer to nitrocellulose, analyzed for binding to the S protein of TGEV (left panel). The positions of molecular mass markers are indicated, as are the locations of aminopeptidase N and the high-molecular-mass sialoglycoprotein recognized by S (arrows).
The results described above indicate that among surface biotinylated proteins of ST and LLC-PK1 cells, the high-molecular-mass band is the major sialoglycoprotein recognized by TGEV. To find out whether this also holds true for total sialoglycoproteins, cells were lysed and sialoglycoproteins were precipitated with wheat germ agglutinin. As shown in Fig. 5, with lysates of both ST and LLC-PK1 cells, TGEV bound predominantly to the high-molecular-mass band. Virus binding was abolished after treatment of the cellular proteins with neuraminidase, confirming that this component is a sialylated macromolecule and that the recognition by TGEV is mediated by sialic acid residues. It should be noted that the cellular proteins for this analysis had been separated under reducing conditions. Therefore, the conformation-dependent interaction between TGEV and aminopeptidase N is not detectable in this overlay assay.
FIG. 5.
Binding of TGEV to sialoglycoproteins from total cell lysates. Sialoglycoproteins were isolated from lysates of ST or LLC-PK1 cells by using wheat germ agglutinin-agarose. Mock-treated (−) and neuraminidase-treated (+) proteins were separated by SDS-PAGE under reducing conditions and transferred to nitrocellulose. The immobilized proteins were used for a virus overlay binding assay with neuraminidase-treated TGEV virions. Bound virus was visualized by an enzyme-linked immunoassay. The position of the high-molecular-mass sialoglycoprotein recognized by TGEV is indicated by an arrow.
DISCUSSION
As the sialic acid binding activity of TGEV is dispensable for virus growth in cell culture, its potential in mediating attachment to cultured cells has never been determined. We found that viruses containing sialic acid binding activity are more efficient in binding to ST and LLC-PK1 cells than are mutants lacking this activity. The enzymatic release of sialic acids from the cell surface reduced the amount of bound TGEV more than fourfold. Thus, apart from binding to aminopeptidase N, TGEV has a second binding activity that may mediate attachment to cells.
Binding to more than one surface compound has been demonstrated for several viruses so far, and it reflects the complexity of the process resulting in an infection. The initial stage of an infection can be divided into two steps: virus attachment to the cell surface and penetration of the cell. With enveloped viruses, the latter step involves a fusion event between the viral membrane on one side and the plasma membrane—or endosomal membrane after endocytotic uptake of the virus—on the other side (for a review, see reference 31). The fusion process is induced by a viral surface protein. In order to become fusogenic, the viral surface protein has to undergo a conformational change that exposes a protein domain for the interaction with the lipid bilayer of the cellular target membrane. This process leads via hemifusion (fusion of the external leaflets of both lipid bilayers) to the formation of membrane pores that allow the viral genome to enter the cytoplasm of the cell. With several viruses, the conformational change of the viral fusion protein has been shown to be triggered by the interaction with a specific receptor on the cell surface. In the case of human immunodeficiency virus (HIV), the surface glycoprotein is proteolytically cleaved into the receptor-binding subunit gp120 and the membrane-anchored fusogenic subunit gp41. Virus attachment to the cell is mediated by the binding of gp120 to CD4 (6, 16). After binding to this primary receptor, gp120 interacts with the chemokine receptor CCR5 (9), which in turn induces gp41 to adopt a fusion-active conformation. In an infected individual, variants may evolve that use CXCR4 or other receptors to trigger the fusion reaction. Sequential interaction with cell surface molecules in the initiation of infection has also been reported for members of the herpesvirus family, herpes simplex virus type 1 (HSV-1) and pseudorabies. With these viruses, attachment is mediated by binding of the viral surface glycoprotein gC to cell surface heparan sulfate proteoglycans (23, 28, 32). Virus entry, i.e., fusion of the viral membrane with the plasma membrane, requires the interaction of the viral glycoprotein gD with a member of the nectin family or an alternative cell surface receptor (3). With both HIV and herpesviruses, there exist mutants that replicate efficiently in cultured cells though they lack the ability to bind to the primary attachment receptor, CD4 or heparan sulfate, respectively (15). In this case, the coreceptor or virus entry receptor that usually mediates the fusion process may also be used for attachment. While these mutants can replicate in cultured cells, they are generally not found among natural isolates. Survival in a natural environment obviously requires optimal attachment properties.
These considerations may also apply to TGEV. Binding to aminopeptidase N is obligatory for the initiation of infection. In the case of cultured cells, this interaction is so efficient that no additional attachment receptor is required. The presence of a sialic acid binding activity increases the amount of bound virus, but it does not increase infectivity. The binding of TGEV to sialoglycoconjugates may be compared to the primary attachment of HIV to CD4 or of HSV-1 to heparan sulfate. In the case of TGEV, the sialic acid binding activity may be less important than the primary binding activities of HIV or HSV-1, because there exists a natural variant of TGEV, PRCoV, that lacks sialic acid binding activity. PRCoV is unable to attach to sialoglycoconjugates (29). However, it still can use aminopeptidase N as a receptor to initiate infection (8). PRCoV replicates with high efficiency in the respiratory tract but with very low efficiency in the gut (5). Therefore, the sialic acid binding activity may be required for efficient virus replication in the intestinal epithelium and thus for the enteropathogenicity of TGEV. In agreement with this view, mutants of TGEV that have lost their sialic binding activity due to point mutations in the S protein also have lost their enteropathogenicity (2, 17). These findings are consistent with data reported previously using a different approach (1, 27). Weingartl and Derbyshire also proposed a putative second receptor for TGEV (30). Whether the protein they found is identical to the high-molecular-mass sialoglycoprotein in this study is not known. They did not report an interaction of TGEV with the second receptor protein via binding to sialic acids. It should be noted that the two-step entry mechanism (interaction with a first and a second receptor) proposed for TGEV is somewhat different from the situation reported for HIV and herpesviruses. With the latter viruses, binding to the first receptor increases the efficiency not only of natural infections but also of cell culture infections. By contrast, the sialic acid binding activity of TGEV appears to be required only for intestinal infections.
Environmental conditions in the gastrointestinal tract appear quite unfavorable for virus infection. Low pH, proteases, and especially detergent-like bile salts might explain why enveloped viruses usually do not cause intestinal infections via the gastrointestinal route. Analyzing cell culture-grown virus, we did not find any difference in the sensitivity to low pH and protease inactivation between TGEV and mutants lacking sialic acid binding activity (18). The resistance of TGEV to inactivation by detergent was only slightly increased compared to that of the mutants (18). Therefore, the importance of the sialic acid binding activity of TGEV may be to help the virus get access to the target cells. In order to infect an enterocyte, the virus has to pass through a mucus blanket that may be as thick as 100 μm and through the glycocalix (about 100 nm thick) covering the apical membrane of the intestinal cells (for a review, see reference 4). Both layers are rich in carbohydrates, including sialic acid. Binding to sialoglycoproteins may allow the virus to stay longer in the intestine and make it easier to find the aminopeptidase N receptor for initiating infection. It will be interesting to apply the methods described in the present report to intestinal cells and to identify sialoglycoproteins that interact with TGEV. In this way, a pathogenicity factor may be elucidated that is functional in an organism but not in cell culture.
Acknowledgments
Financial support was provided by the Deutsche Forschungsgemeinschaft (SFB 280).
REFERENCES
- 1.Ballesteros, M. L., C. M. Sánchez, and L. Enjuanes. 1997. Two amino acid changes at the N-terminus of transmissible gastroenteritis coronavirus spike protein result in the loss of enteric tropism. Virology 227:378-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bernard, S., and H. Laude. 1995. Site-specific alteration of transmissible gastroenteritis virus spike protein results in markedly reduced pathogenicity. J. Gen. Virol. 76:2235-2241. [DOI] [PubMed] [Google Scholar]
- 3.Campadelli-Fiume, G., F. Cocchi, L. Menotti, and M. Lopez. 2000. The novel receptors that mediate the entry of herpes simplex viruses and animal alphaherpesviruses into cells. Rev. Med. Virol. 10:305-319. [DOI] [PubMed] [Google Scholar]
- 4.Cone, R. A. 1999. Mucus, p. 43-64. In P. L. Ogra, J. Mestecky, M. E. Lamm, W. Strober, J. Bienenstock, and J. R. McGhee (ed.), Mucosal immunology. Academic Press, San Diego, Calif.
- 5.Cox, E., M. B. Pensaert, P. Callebaut, and K. Van Deun. 1990. Intestinal replication of a porcine respiratory coronavirus closely related antigenically to the enteric transmissible gastroenteritis virus. Vet. Microbiol. 23:237-243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dalgleish, A. G., P. C. Beverley, P. R. Clapham, D. H. Crawford, M. F. Greaves, and R. A. Weiss. 1984. The CD4 (T4) antigen is an essential component of the receptor for the AIDS retrovirus. Nature 312:763-767. [DOI] [PubMed] [Google Scholar]
- 7.Delmas, B., J. Gelfi, R. L'Haridon, L. K. Vogel, H. Sjostrom, O. Noren, and H. Laude. 1992. Aminopeptidase N is a major receptor for the entero-pathogenic coronavirus TGEV. Nature 357:417-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delmas, B., J. Gelfi, H. Sjostrom, O. Noren, and H. Laude. 1993. Further characterization of aminopeptidase-N as a receptor for coronaviruses. Adv. Exp. Med. Biol. 342:293-298. [DOI] [PubMed] [Google Scholar]
- 9.Deng, H., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major coreceptor for primary isolates of HIV-1. Nature 381:647-648. [DOI] [PubMed] [Google Scholar]
- 10.Enjuanes, L., D. Brian, D. Cavanagh, K. Holmes, M. M. C. Lai, H. Laude, P. Masters, P. Rottier, S. G. Siddell, W. J. M. Spaan, F. Taguchi, and P. Talbot. 2000. Coronaviridae, p. 835-849. In M. H. V. van Regenmortel, C. M. Fauquet, D. H. L. Bishop, E. B. Carsten, M. K. Estes, S. M. Lemon, M. A. Mayo, D. J. McGeoch, C. R. Pringle, and R. B. Wickner (ed.), Virus taxonomy. Academic Press, New York, N.Y.
- 11.Escors, D., J. Ortego, H. Laude, and L. Enjuanes. 2001. The membrane M protein carboxy terminus binds to transmissible gastroenteritis coronavirus core and contributes to core stability. J. Virol. 75:1312-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Escors, D., E. Camafeita, J. Ortego, H. Laude, and L. Enjuanes. 2001. Organization of two transmissible gastroenteritis coronavirus membrane protein topologies within the virion and core. J. Virol. 75:12228-12240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gebauer, F., W. P. Posthumus, I. Correa, C. Sune, C. Smerdou, C. M. Sanchez, J. A. Lenstra, R. H. Meloen, and L. Enjuanes. 1991. Residues involved in the antigenic sites of transmissible gastroenteritis coronavirus S glycoprotein. Virology 183:225-238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Godet, M., J. Grosclaude, B. Delmas, and H. Laude. 1994. Major receptor-binding and neutralization determinants are located within the same domain of the transmissible gastroenteritis virus (coronavirus) spike protein. J. Virol. 68:8008-8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karger, A., and T. C. Mettenleiter. 1993. Glycoproteins gIII and gp50 play dominant roles in the biphasic attachment of pseudorabies virus. Virology 194:654-664. [DOI] [PubMed] [Google Scholar]
- 16.Klatzmann, D., E. Champagne, S. Chamaret, J. Gruest, D. Guetard, T. Hercend, J. C. Gluckman, and L. Montagnier. 1984. T-lymphocyte T4 molecule behaves as the receptor for human retrovirus LAV. Nature 312:767-768. [DOI] [PubMed] [Google Scholar]
- 17.Krempl, C., B. Schultze, H. Laude, and G. Herrler. 1997. Point mutations in the S protein connect the sialic acid binding activity with the enteropathogenicity of transmissible gastroenteritis coronavirus. J. Virol. 71:3285-3287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Krempl, C., M. L. Ballesteros, G. Zimmer, L. Enjuanes, H. D. Klenk, and G. Herrler. 2000. Characterization of the sialic acid binding activity of transmissible gastroenteritis coronavirus by analysis of haemagglutination-deficient mutants. J. Gen. Virol. 81:489-496. [DOI] [PubMed] [Google Scholar]
- 19.Krempl, C., and G. Herrler. 2001. Sialic acid binding activity of transmissible gastroenteritis coronavirus affects sedimentation behavior of virions and solubilized glycoproteins. J. Virol. 75:844-849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kyhse-Anderson, J. 1984. Electroblotting of multiple gels: a simple apparatus without buffer tank for rapid transfer of proteins from polyacrylamide to nitrocellulose. J. Biochem. Biophys. Methods 10:203-209. [DOI] [PubMed] [Google Scholar]
- 21.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 22.Lisanti, M. P., M. Sargiacomo, L. Graeve, A. R. Saltiel, and E. Rodriguez-Boulan. 1988. Polarized apical distribution of glycosyl-phosphatidylinositol-anchored proteins in a renal epithelial cell line. Proc. Natl. Acad. Sci. USA 85:9557-9561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mettenleiter, T. C., L. Zsak, F. Zuckermann, N. Sugg, H. Kern, and T. Ben-Porat. 1990. Interaction of glycoprotein gIII with a cellular heparin-like substance mediates adsorption of pseudorabies virus. J. Virol. 64:278-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rasschaert, D., M. Duarte, and H. Laude. 1990. Porcine respiratory coronavirus differs from transmissible gastroenteritis virus by a few genomic deletions. J. Gen. Virol. 71:2599-2607. [DOI] [PubMed] [Google Scholar]
- 25.Sánchez, C. M., G. Jiménez, M. D. Laviada, I. Correa, C. Suñé, M. J. Bullido, F. Gebauer, C. Smerdou, P. Callebaut, J. M. Escribano, and L. Enjuanes. 1990. Antigenic homology among coronaviruses related to transmissible gastroenteritis virus. Virology 174:410-417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sánchez, C. M., F. Gebauer, C. Suñé, A. Mendez, J. Dopazo, and L. Enjuanes. 1992. Genetic evolution and tropism of transmissible gastroenteritis coronaviruses. Virology 190:92-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sánchez, C. M., A. Izeta, J. M. Sánchez-Morgado, S. Alonso, I. Sola, M. Balasch, J. Plana-Durán, and L. Enjuanes. 1999. Targeted recombination demonstrates that the spike gene of transmissible gastroenteritis coronavirus is a determinant of its enteric tropism and virulence. J. Virol. 73:7607-7618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sawitzky, D., H. Hampl, and K. O. Habermehl. 1990. Comparison of heparin-sensitive attachment of pseudorabies virus (PRV) and herpes simplex virus type 1 and identification of heparin-binding PRV glycoproteins. J. Gen. Virol. 71:1221-1225. [DOI] [PubMed] [Google Scholar]
- 29.Schultze, B., C. Krempl, M. L. Ballesteros, L. Shaw, R. Schauer, L. Enjuanes, and G. Herrler. 1996. Transmissible gastroenteritis coronavirus, but not the related porcine respiratory coronavirus, has a sialic acid (N-glycolylneuraminic acid) binding activity. J. Virol. 70:5634-5637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Weingartl, H. M., and J. B. Derbyshire. 1994. Evidence for a putative second receptor for porcine transmissible gastroenteritis virus on the villous enterocytes of newborn pigs. J. Virol. 68:7253-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Weissenhorn, W., A. Dessen, L. J. Calder, S. C. Harrison, J. J. Skehel, and D. C. Wiley. 1999. Structural basis for membrane fusion by enveloped viruses. Mol. Membr. Biol. 16:3-9. [DOI] [PubMed] [Google Scholar]
- 32.WuDunn, D., and P. G. Spaer. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zimmer, G., H.-D. Klenk, and G. Herrler. 1995. Identification of a 40-kDa cell surface sialoglycoprotein with the characteristics of a major influenza C virus receptor in a Madin-Darby canine kidney cell line. J. Biol. Chem. 270:17815-17822. [DOI] [PubMed] [Google Scholar]