Abstract
Drug-resistant mutants with a methionine-to-valine substitution at position 184 of reverse transcriptase (M184V) emerged within 5 weeks of initiation of therapy in four newborn macaques infected with simian immunodeficiency virus (SIVmac251) and treated with lamivudine (3TC) or emtricitabine [(−)-FTC] (two animals per drug). Thus, this animal model mimics the rapid emergence of M184V mutants of HIV-1 during 3TC therapy of human patients. One animal of each treatment group developed fatal immunodeficiency at 12 weeks of age, which is similar to the rapid disease course seen in most untreated SIVmac251-infected infant macaques. To further evaluate the effect of the M184V mutation on viral fitness and virulence, groups of juvenile macaques were inoculated with the molecular clone SIVmac239 with either the wild-type sequence (group A [n = 5]) or the M184V sequence (SIVmac239-184V; group B [n = 5] and group C [n = 2]). The two SIVmac239-184V-infected animals of group C did not receive any drug treatment, and in both animals the virus population reverted to predominantly wild type (184M) by 8 weeks after inoculation. The other five SIVmac239-184V-infected animals (group B) were treated with (−)-FTC to prevent reversion. Although virus levels 1 week after inoculation were lower in the SIVmac239-184V-infected macaques than in the SIVmac239-infected animals, no significant differences were observed from week 2 onwards. Two animals in each group developed AIDS and were euthanized, while all other animals were clinically stable at 46 weeks of infection. These data demonstrate that the M184V mutation in SIV conferred a slightly reduced fitness but did not affect disease outcome.
The emergence of viral mutants with reduced drug susceptibility has been a major barrier to successful drug therapy of human immunodeficiency virus (HIV) infection of humans (49). The main strategy to combat resistance has been the use of drug combinations. The combined use of three or more drugs in highly active antiretroviral therapy has been a major factor in decreasing the number of AIDS-related deaths and the number of new HIV type 1 (HIV-1)-infected individuals in developed countries in the past few years (18). However, the emergence of multidrug-resistant mutants is a growing problem (9), and it is likely that additional strategies to minimize drug resistance will be required to sustain the advances made with highly active antiretroviral therapy.
Drug resistance mutations are expected to reduce the replicative ability of the virus in the absence of drug (8). Indeed, reduced fitness has been demonstrated in vitro for HIV-1 mutants resistant to nucleoside analog inhibitors of reverse transcriptase (RT) or to protease inhibitors (11, 23, 37, 40, 54). A crucial question is whether such reduced fitness will result in attenuated virulence and slower disease progression in HIV-infected patients. If we can identify pathways for resistance that also reduce viral virulence, then it may be possible to take advantage of these in the design of therapeutic strategies. Precedence for attenuation via drug resistance mutations has been provided from work with herpes and influenza viruses. A major reason for the long-term success of the antiherpes drug acyclovir is that most mutants of herpes simplex virus that are resistant to this drug are nonpathogenic and unable to reactivate from nerve tissue (7, 16). Similarly, influenza virus mutants that are resistant to a neuraminidase inhibitor have significantly reduced virulence in mice (55).
The virulence of drug-resistant HIV-1 variants cannot be assessed directly in human patients. Therefore, we use an animal model, simian immunodeficiency virus (SIV) infection of rhesus macaques, to evaluate the virulence of drug-resistant mutants. This model has proven useful for studies of nucleoside analogs, such as 3′-azido-3′-deoxythymidine (AZT; zidovudine) and 9-[2-(phosphonomethoxy)propyl]adenine (PMPA; tenofovir) and for evaluating the emergence, virulence, and clinical implications of drug-resistant viral mutants (59, 60, 62, 67). AZT therapy of SIVmac251-infected newborn macaques resulted in the emergence of SIV mutants highly resistant to AZT; these mutants had a glutamine-to-methionine substitution at position 151 of RT and were fully virulent following inoculation in newborn macaques (62, 67). PMPA treatment of SIVmac251-infected infant macaques resulted in the emergence of mutants with fivefold-reduced susceptibility to PMPA; these mutants had K65R and additional mutations in RT (59). These K65R mutants were as virulent as wild-type SIVmac251 following inoculation into newborn macaques (60, 66).
In the work presented here we utilized this model to study the emergence and clinical implications of SIV mutants resistant to the oxathiolane nucleosides (−)-β-l-2′,3′-deoxy-3′-thiacytidine (3TC; lamivudine) and (−)-β-l-2′,3′-dideoxy-5-fluoro-3′-thiacytidine [(−)-FTC; emtricitabine]. (−)-FTC is a 3TC analog with stronger in vitro activity and an improved pharmacokinetic profile (19, 21, 50). HIV-1 variants resistant to these drugs occur predominantly from a methionine-to-valine mutation in position 184 (M184V) of RT, although a mutation to isoleucine (M184I) also occurs transiently (28, 53). Either of these mutations results in high-level (>100-fold) resistance to 3TC or to (−)-FTC (52, 56). These M184V/I mutants have some unique features in vitro. They have impaired replication kinetics in primary T-lymphocyte cultures but not in established T-cell lines (1, 31, 34, 41). The RT enzymes of these mutants have reduced processivity and enhanced fidelity in biochemical assays (1, 3, 69). And the M184V mutation is known to affect the phenotypic expression of other drug resistance mutations (22, 34, 41, 70).
The SIV macaque model was used to further evaluate the in vivo fitness and virulence of M184V viral mutants. We report here the rapid emergence of M184V variants in 3TC- and (−)-FTC-treated SIVmac251-infected macaques. In addition, we investigated the in vivo fitness and virulence of a variant of the molecular clone SIVmac239 with this M184V mutation in RT.
MATERIALS AND METHODS
Animals and sample collection.
Newborn and juvenile rhesus macaques (Macaca mulatta) were from the type D retrovirus- and SIV-free colony at the California Regional Primate Research Center. Newborn macaques were hand-reared in a primate nursery in accordance with American Association for Accreditation of Laboratory Animal Care Standards. We strictly adhered to the Guide for the Care and Use of Laboratory Animals prepared by the Committee on Care and Use of Laboratory Animals of the Institute of Laboratory Animal Resources, National Research Council (44). When necessary, animals were immobilized with ketamine-HCl (Parke-Davis, Morris Plains, N.J.), 10 mg/kg of body weight, injected intramuscularly. EDTA- and heparin-anticoagulated blood samples were collected regularly to measure viral and immunologic parameters. Complete blood counts were performed on EDTA-anticoagulated blood samples from all animals. Samples were analyzed by using an automated electronic cell counter (Baker 9000; Serono Baker Diagnostics), and differential counts were determined manually. To monitor the immune response to nonviral, nonreplicating antigens, the newborn rhesus macaques were immunized subcutaneously with 0.1 mg of cholera toxin B subunit at 2 weeks of age (i.e., at peak viremia), and a booster immunization was given at 10 weeks of age. The cholera toxin-specific immunoglobulin G (IgG) enzyme-linked immunosorbent assay (ELISA) has been described elsewhere (68).
Viruses.
Strains of SIV used in this study were uncloned SIVmac251 and virus derived from the molecular clone SIVmac239. The SIVmac251 virus stock used in this study was propagated on rhesus peripheral blood mononuclear cells (PBMC) and had a titer of 105 50% tissue culture infectious doses (TCID50) per ml. SIVmac239 containing the M184V mutation in RT (SIVmac239-184V) was generated by site-directed mutagenesis, as previously described (5). The 3′ half of SIVmac239 that was used had the premature stop codon at position 93 of nef repaired (p239SpE3′/nef-open) (51). The SIVmac239 and SIVmac239-184V virus stocks were propagated in CEMx174 cells and had titers of 3.2 × 105 and 1.8 × 105 TCID50 per ml, respectively. Virus titers were determined by limiting-dilution tissue culture methods as previously described (64). Experiments demonstrated that short-term in vitro propagation of M184V virus in the absence of drug does not result in detectable reversion to the wild-type sequence (unpublished data).
Virus inoculation.
In initial studies to monitor drug efficacy and emergence of drug-resistant variants, we utilized newborn macaques infected with SIVmac251. Within 3 days of age, the newborn macaques were inoculated orally under ketamine anesthesia with 1 ml of the uncloned SIVmac251 virus stock, as described previously (65). One of the animals (30579) had received two doses of tenofovir near the time of virus inoculation but became infected with an initial viremia that was indistinguishable from that of untreated control animals and was therefore added to this study (65). For experiments to compare the virulence of SIVmac239 and SIVmac239-184V, we utilized juvenile macaques that were 6 to 8 months of age (∼1.2 to 2.1 kg) at the time of virus inoculation. Animals were inoculated intravenously with 0.5 ml of virus dilution containing 103 TCID50 of either SIVmac239 or SIVmac239-184V.
Administration of drugs.
Stock solutions of 3TC or (−)-FTC (16 mg/ml) were prepared in phosphate-buffered saline (pH 7.4; Sigma-Aldrich, St. Louis, Mo.), filter sterilized (pore size, 0.2 μm; Nalgene, Rochester, N.Y.), and stored at 4°C. Drugs were administered subcutaneously into the backs of animals at a regimen of 8 mg per kg of body weight once daily. The dosage regimen of 3TC was selected because it is expected to give areas under the concentration-time curve for adult macaques similar to those of the daily dose of 3TC used in human pediatric patients (4 mg/kg twice daily) (36, 42) or adult patients (150 mg orally twice daily) (24, 57), except that for our macaques, it was administered in a single daily dose. Drug dosages were adjusted weekly according to body weight. The untreated control animals did not receive daily sham inoculations.
Quantitative virus isolation (cell-associated and cell-free).
Levels of infectious virus in cells and plasma of peripheral blood were determined regularly by a limiting-dilution assay (four replicates per dilution) of PBMC and plasma, respectively, in cultures with CEMx174 cells in 24-well plates and subsequent p27 core antigen measurement, according to methods previously described (64). Virus levels in fresh lymphoid tissues (lymph nodes, spleen, and thymus) collected from the animals at the time of necropsy were determined by aseptically teasing tissues into single-cell suspensions of mononuclear cells and by a limiting-dilution culture assay similar to the one described above for PBMC.
Plasma viral RNA levels.
Quantitative assays for the measurement of SIVmac251 RNA were performed by using a branched-DNA signal amplification assay specific for SIV (P. J. Dailey, M. Zamroud, R. Kelso, J. Kolberg, and M. Urdea, Abstr. 13th Annu. Symp. Nonhuman Primate Models AIDS, abstr. 99, 1995). The lower quantification limit of this assay was 1,500 copies of SIV RNA per ml of sample. A real-time TaqMan PCR assay was used to quantitate SIVmac239 RNA as previously described (35). This assay has a sensitivity of 50 copies of viral RNA per ml of plasma (35).
Anti-SIV class-specific antibody determination.
The whole-virus anti-SIV IgG antibody ELISA was described previously (63).
Lymphocyte phenotyping by three-color flow cytometry.
T-lymphocyte antigens were detected by direct labeling of whole blood with peridinin chlorophyll protein-conjugated anti-human CD8 (clone SK1; Becton Dickinson Immunocytometry Inc., San Jose, Calif.), phycoerythrin-conjugated anti-human CD4 (clone M-T477; Pharmingen), and fluorescein-conjugated anti-human CD3 (clone SP34; Pharmingen). A separate aliquot of blood was labeled with fluorescein-conjugated anti-human CD3 and peridinin chlorophyll protein-conjugated anti-human CD20 (clone L27; Becton Dickinson). Red blood cells were lysed, and the samples were fixed in paraformaldehyde with the Coulter Q-prep system (Coulter Corporation, Hialeah, Fla.). Lymphocytes were gated by forward and side light scatter and were then analyzed with a FACScan flow cytometer (Becton Dickinson). CD4+ and CD8+ T lymphocytes were defined as CD3+ CD4+ and CD3+ CD8+ lymphocyte populations, respectively. B lymphocytes were defined as CD3− CD20+ lymphocytes.
Drug susceptibility assays.
Phenotypic drug susceptibilities of SIVmac isolates were characterized by a previously described assay based on a dose-dependent reduction of viral infectivity. This assay is able to detect SIV mutants with decreased susceptibility to several antiviral drugs (59, 67).
Sequence analysis of the SIV RT-encoding region.
DNA sequence analyses were performed on proviral DNA obtained from 5.0 × 105 CEMx174 cells infected with virus isolated from the SIV-infected animals. Infected cells were harvested as soon as culture supernatants were positive by antigen capture ELISA. Genomic DNA was extracted by a silica-guanidine thiocyanate extraction protocol (6) in a 50-μl elution volume (10 μM Tris, pH 8.5). A 2-μl aliquot of each DNA preparation was amplified by nested PCR. The primers used for each round are described in Table 1. Each round of the nested PCR was carried out under the following conditions: incubation at 94°C for 45 s, 30 cycles of 94°C for 45 s, 57°C for 40 s, and 72°C 120 s, and incubation at 72°C for 5 min. All PCRs used RedTaq polymerase (Sigma-Aldrich) under the manufacturer's recommended conditions. Amplicons were sequenced by Davis Sequencing (Davis, Calif.) with the primers listed in Table 1, and data were compared to published sequences of SIVmac251 and SIVmac239 (accession numbers M19499 and M33262, respectively.)
TABLE 1.
Primer sequences for PCR and sequencing of SIV RT
| Primer | 3′ base | Sequence (5′-3′) |
|---|---|---|
| PCR primers | ||
| First round | ||
| 239-2459 | 2459 | ACC CAG CTG TGG ATC TG |
| 239-4751 (R) | 4751 | TCC ACT AGC TAC ATG TAC TGC AAC |
| Second round | ||
| 239-2675 | 2675 | GTA ACA GGA ATA GAG TTA GGT CCA C |
| 239-4615 (R) | 4615 | TCT GTC TGG CCA CTA TTC TG |
| Sequencing primers | ||
| 239-2786 | 2786 | ATT AAA GGG ACA ATC ATG ACA G |
| SIV-RT3 | 3153 | GAA TAC CAC ACC CTG CAG GAC TAG C |
| SIV-RT5 | 3658 | ATT GGG CAG CTC AAA TTT ATC CAG G |
| SIV-RT9 | 3838 | GGC AAG CCA TTA GAA GCC ACG GTA |
Necropsy and collection and preparation of tissue samples.
Animals were euthanized when it became apparent that their condition was terminal, according to criteria described previously (68). Tissues collected at necropsy were fixed in 10% buffered formalin, embedded in paraffin, sectioned at 6 μm, stained with hematoxylin and eosin, and examined by light microscopy.
Statistical analysis.
Statistical analysis was used to compare treated and untreated SIV-infected animals with regard to survival and virus levels. Survival was compared with the generalized Wilcoxon test (12). Virus levels in peripheral blood were compared by calculating the area under the curve for each animal, followed by analysis according to the Wilcoxon rank-sum test (12). It was previously shown that these analyses can distinguish biologically relevant differences (39, 60, 67).
RESULTS
The major goal of this project was to evaluate the effect of the M184V mutation in RT on the virulence and fitness of SIV in vivo. In order to do this we had to first establish whether the M184V mutation emerges following 3TC or (−)-FTC therapy of SIV-infected macaques in a manner similar to the emergence of M184V mutants of HIV-1 in humans. Therefore, we utilized the model of SIVmac251 infection of newborn macaques. Next, the effect of M184V on fitness and virulence was studied by comparing juvenile macaques inoculated with the wild-type molecular clone of SIVmac239 or with SIVmac239 containing the M184V mutation.
Emergence of M184V variants of SIVmac251 in newborn macaques.
Within 3 days after birth, four newborn macaques were inoculated orally with virulent, wild-type (i.e., drug-susceptible) uncloned SIVmac251 as described previously (65). All four animals became persistently infected following oral SIVmac251 inoculation and had peak viremia levels at 2 weeks (Fig. 1). Three weeks after virus inoculation, two animals each were started on 3TC and on (−)-FTC (8 mg/kg of body weight, subcutaneously once daily) (Table 2).
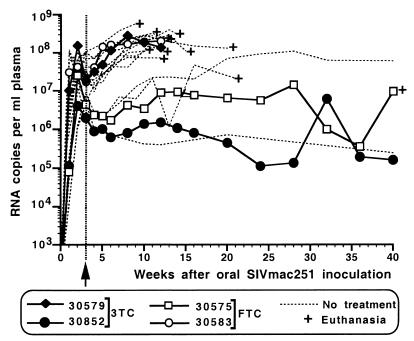
FIG. 1.
Virus levels in SIVmac251-infected infant macaques receiving 3TC or (−)-FTC treatment. All newborn macaques were inoculated at birth orally with SIVmac251. The dashed lines represent 14 untreated SIVmac251-infected infant macaques from a number of previous studies. Four animals were started on 3TC or FTC treatment at 3 weeks of age (arrow). Plasma RNA levels were measured by branched-DNA assay.
TABLE 2.
Treatment of SIVmac251-infected infant macaques with 3TC or FTC: experimental design and summary of outcomea
| Animal (sex) | Treatment | Clinical outcome | Histopathology |
|---|---|---|---|
| 30579 (F) | 3TC | AIDS at 12 wk | Lymphoid depletion, chronic fibrosing pancreatitis with adenovirus infection, enterocolitis, hypoproteinemia (plasma protein, 4 gm/dl), bronchitis |
| 30852 (M) | 3TC | Healthy at 22 mob | Lymphoid hyperplasia, cryptosporidium-positive cholecystitis, and pancreatitis with fibrosis and acinar atrophy; pneumonia |
| 30583 (M) | (−)-FTC | AIDS at 12 wk | Enterocolitis, thymic atrophy, cheilitis, hypoproteinemia (plasma protein, 3.1 gm/dl) |
| 30575 (F) | (−)-FTC | Disease at 40 wk | Chronic fibrosing pancreatitis; serositis; mucinous degeneration and fibrosis of intima of pulmonary blood vessels with thrombosis; thymic atrophy; lymphoid depletion |
Animals were inoculated at birth orally with SIVmac251; 3 weeks later, they were started on 3TC or (−)-FTC treatment (8 mg/kg, administered subcutaneously once daily).
Animal 30852 was euthanized while still clinically stable at 22 months of age.
Treatment of the four SIVmac251-infected infant macaques with 3TC or (−)-FTC had no significant effect on viral RNA levels in plasma (Fig. 1) or PBMC-associated infectious-virus titers, determined by comparing these data to those of a large number of historical untreated newborn macaques that had previously been inoculated orally with the same virus stock (58, 61, 63). Virus isolates obtained from PBMC and plasma of these animals were cultured in CEMx174 cells and used for phenotypic and genotypic analyses. Population sequence analysis of PCR products of the RT region revealed that after 3 weeks of treatment, all virus isolates still had wild-type sequence but that in the next sample, collected 5 weeks after the start of treatment (i.e., 8 weeks after virus inoculation), the M184V mutation (ATG to GTG) was present in all four animals and the wild-type sequence was no longer detected. No M184I mutation was detected, although we cannot exclude the possibility that such mutants may have been present transiently between 3 and 5 weeks of treatment but were replaced rapidly by M184V mutants due to the high viral turnover rates in these animals. Phenotypic analyses also revealed that the M184V virus isolates from each of these four animals were >100-fold resistant to 3TC. To detect any other mutations in RT that may have emerged during drug treatment, the whole RT-encoding region was sequenced from virus isolated from PBMC of these four animals at the time of death or at 50 weeks of age (for animal 30852). Mutations additional to M184V that were found were R35K (animal 30575), V90I (animal 30852), I341 M (animal 30579), R394K (animals 30579 and 30583), P420L (animal 30852), and E434K (animal 30852). The significance of these mutations is unclear, but they are unlikely to contribute to 3TC resistance; except for 341M and 420L (which were found only in a single animal), all of the amino acid substitutions are found in other wild-type SIV or HIV-2 isolates (43).
Two animals, one in each treatment group (30579 and 30583), had persistently high viral RNA levels (>107 RNA copies/ml) (Fig. 1). These animals failed to make strong and persistent SIV-specific IgG antibody responses (peak titers of 1:400 and 1:25,600 for animals 30853 and 30579, respectively) (65), and their antibody responses following immunizations with cholera toxin B subunit were reduced compared to those in uninfected age-matched control animals (data not shown). These two infants developed fatal immunodeficiency at 12 weeks of age, with widespread virus dissemination in lymphoid tissues (including thymus, spleen, and lymph nodes), reduced percentages of CD4+ T lymphocytes over total lymphocytes, and reduced CD4+/CD8+ T-lymphocyte ratios. Histopathological findings in these animals were consistent with terminal SIV disease (Table 2). Sequence analysis was performed on virus isolated from different lymphoid compartments (PBMC, plasma, spleen, and axillary, mesenteric, and inguinal lymph nodes) collected from these animals at time of euthanasia. For all compartments, only M184V sequence was detected; the only exception was the thymus of animal 30583, for which the virus isolate had only wild-type sequence. This fulminant disease course in animals 30579 and 30583, characterized by rapid immunosuppression and widespread virus dissemination, is similar to that seen in the majority of untreated SIVmac251-infected infant macaques, which have persistently high viremia and poor antiviral immune responses due to rapid immunosuppression and develop disease in 2 to 4 months (58, 59, 61, 63, 67).
The other two animals (30575 and 30852) had a delayed and lower primary viremia prior to week 3, and in comparison to untreated SIV-infected animals, drug treatment did not induce a significant reduction in the virus levels in these animals; these animals were able to make a strong and persistent antiviral IgG antibody response (titers > 1:102,400), and their time course resembled that of a minority of untreated SIVmac251-infected infant macaques which have a delayed disease course (survival for 1 to 2 years) (Fig. 1). Animal 30575, which was treated with (−)-FTC, had to be euthanized at 40 weeks of age due to rapidly deteriorating health; the most striking gross lesion found during autopsy was a severely enlarged (∼5-cm diameter) and inflamed pancreas and pulmonary thrombosis. Histopathological examination revealed chronic pancreatitis with severe fibrosis and degeneration of the wall of pulmonary blood vessels (Table 2). Pancreatitis has also been considered a serious side effect of 3TC treatment in HIV-infected children with advanced disease (36; product information for 3TC [Epivir]; GlaxoSmithKline). Thus, it is possible that SIV-induced immunosuppression may have predisposed this animal to a similar pancreatic toxicity.
The fitness of a drug-resistant virus can be determined by the rate of reversion following drug withdrawal. Because virus levels in the 3TC-treated animal 30852 were relatively low after the initial viremia (105 to 106 copies of SIV RNA per ml of plasma) (Fig. 1), treatment was stopped at 50 weeks of age. In virus isolated from PBMC of this animal up to 59 weeks of age (9 weeks after 3TC withdrawal), only mutant sequence (M184V) was detected. During these first 9 weeks after 3TC withdrawal, virus levels in this animal remained relatively constant (between 0.6 × 106 and 0.8 × 106 copies of viral RNA/ml, at all six times when blood samples were collected). However, virus isolated from PBMC at 66 weeks of age (16 weeks after 3TC withdrawal) was wild-type (M) at position 184. Viral RNA levels in plasma at 66 and 76 weeks of age were 1.7 × 106 and 2.0 × 106 copies per ml, respectively, which indicated an approximately threefold increase compared to those at week 59, when M184V mutant was still dominant. Animal 30852 was clinically stable at 22 months of age but, when euthanized at that time, showed evidence of immune dysfunction (Table 2).
In vivo fitness and virulence of SIVmac239-184V.
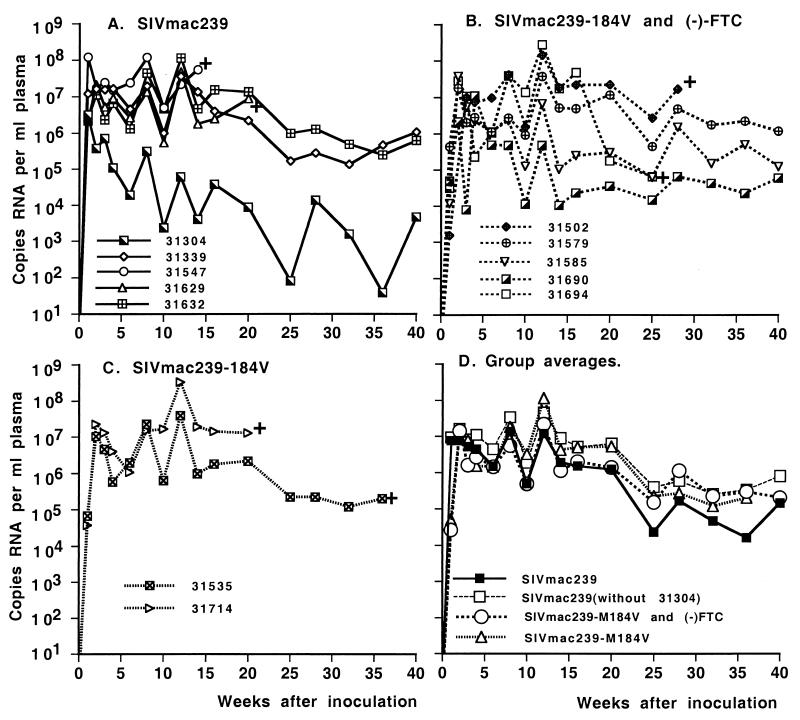
We used juvenile macaques to compare the fitness and virulence of wild-type SIVmac239 to those of SIVmac239-184V. For these studies, juvenile macaques were inoculated intravenously with 1,000 TCID50 of SIVmac239 (group A [n = 5]) or SIVmac239-184V (groups B [n = 5] and C [n = 2]) (Table 3). The SIVmac239-184V-infected animals of group B were treated with (−)-FTC (8 mg/kg of body weight, administered subcutaneously once daily), beginning 1 day prior to virus inoculation, in order to prevent reversion of the mutant virus. All animals became persistently infected. Virus levels reached peak levels by 1 week postinoculation in the five animals infected with SIVmac239 (>106 RNA copies/ml; average ± standard deviation, log = 6.88 ± 0.74) (Fig. 2). In contrast, all seven SIVmac239-184V-inoculated animals had significantly lower (∼2 log) viral RNA levels 1 week after inoculation (log = 4.42 ± 0.90 and 4.70 ± 0.18 for groups B and C, respectively; P < 0.001, Wilcoxon rank-sum test). But from week 2 on, there was no difference among the groups (Fig. 2), and animals showed similar individual variation over time. Beyond 20 weeks postinoculation, most surviving animals had virus loads greater than 104 copies of viral RNA per ml of plasma; the main exception was one animal in group A (number 31304) for which virus levels remained lower. There were no significant differences in virus loads between the groups regardless of whether data from animal 31304 were included (Fig. 2D).
TABLE 3.
Inoculation of juvenile macaques with wild-type SIVmac239 or SIVmac239-184V: experimental design and summary of outcomea
| Group | Inoculum | Drug | Animal | Outcome | Histopathology |
|---|---|---|---|---|---|
| A | SIVmac239 | None | 31547 | AIDS at 14 wk | Mixed pattern of lymphoid depletion and hyperplasia; thymic atrophy; interstitial pneumonia; pleuritis; fibrosing pancreatitis with amphophilic intranuclear inclusion bodies (possible papovavirus or adenovirus); cryptosporidium-positive enteritis and cholecystitis |
| 31629 | AIDS at 20 wk | Cryptosporidium-positive cholecystitis, gastroenterocolitis, and pancreatic ductitis; Pneumocystis carinii-positive pneumonia | |||
| 31304 | Alive at 46 wk | NA | |||
| 31339 | Alive at 46 wk | NA | |||
| 31632 | Alive at 46 wk | NA | |||
| B | SIVmac239-184V | FTC | 31694 | AIDS at 26 wk | Pneumonia; cryptosporidium-positive gastroenterocolitis, oral and esophageal candidiasis; thymic atrophy; lymphoid hyperplasia |
| 31502 | AIDS at 30 wk | Cryptosporidium-positive cholecystitis, cholangitis, and gastroenteritis; lymphoid hyperplasia; thymic atrophy; fibrosing pancreatitis; oral and esophageal candidiasis; histiocytic alveolar pneumonia (possible P. carinii) | |||
| 31690 | Alive at 46 wk | NA | |||
| 31579 | Alive at 46 wk | NA | |||
| 31585 | Alive at 46 wk | NA | |||
| C | SIVmac239-184V | None | 31714 | AIDS at 24 wk | Thymic atrophy; P. carinii-positive pneumonia; cryptosporidium-positive gastritis and colitis; cholangitis, lymphoid hyperplasia |
| 31535 | AIDS at 39 wk | Lymphoid depletion; protozoan-positive enteritis (possible Trichomonas); amphophilic intranuclear inclusion bodies in kidney (possible papovavirus or adenovirus infection) |
Juvenile rhesus macaques (6 to 8 months of age) were inoculated intravenously with 1,000 TCID50 of SIVmac239 or SIVmac239-184V. Animals of group B were started on (−)-FTC treatment (8 mg/kg subcutaneously once daily) 1 day prior to the virus inoculation. NA, not applicable.
FIG. 2.
Virus levels in juvenile macaques infected with SIVmac239 or SIVmac239-184V. Plasma RNA levels, measured by real-time PCR (TaqMan), are presented for animals inoculated intravenously with SIVmac239 (A) or SIVmac239-184V (B and C). Some animals (B) also received (−)-FTC treatment to prevent reversion. +, euthanasia because of simian AIDS. (D) Averages for the different groups, including the average for group A when animal 31304 is excluded. There were no statistically significant differences in virus loads between the groups regardless of whether data from animal 31304 were included.
The two SIVmac239-184V-infected animals of group C did not receive any (−)-FTC treatment and were used to monitor reversion. In both animals, mutant M184V virus was detected until 6 weeks postinfection, but wild-type virus was predominant by 8 weeks postinfection. DNA sequence analyses confirmed that the M184V mutation in the (−)-FTC-treated SIVmac239-184V-infected animals (group B) did not revert to wild type. This suggests that the (−)-FTC dosage regimen exerted sufficient selection pressure to maintain the M184V mutation.
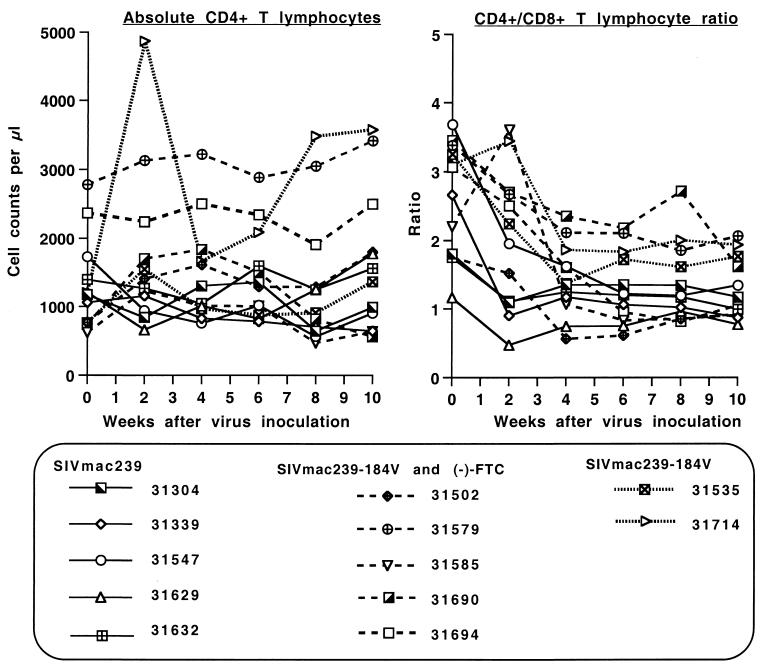
Absolute CD4+-T-lymphocyte and CD8+-T-lymphocyte counts in peripheral blood were quite variable over time. At the time of virus inoculation, there were no significant differences in these lymphocyte subsets between the different groups (P > 0.9). At 2 weeks after virus inoculation, however, four of the five SIVmac239-infected animals had a decrease, while six of the seven SIVmac239-184V-inoculated animals demonstrated an increase in absolute CD4+-T-lymphocyte counts; accordingly, the SIVmac239-infected animals had lower absolute CD4+ T lymphocyte counts than the SIVmac239-184V-infected animals at 2 and 4 weeks after virus inoculation (P ≤ 0.05, two-tailed t test) (Fig. 3). This difference in absolute CD4+-T-lymphocyte counts disappeared from 6 weeks on. No differences were observed in absolute CD8+-T-lymphocyte counts. The CD4/CD8 T-lymphocyte ratio was significantly lower in the SIVmac239-infected animals than in the SIVmac239-184V-infected animals only 2 weeks after virus inoculation (P = 0.002; two-tailed t test) (Fig. 3). After the primary viremia stage there were no significant differences in any of these parameters between the groups of animals. Individual animals in each group showed a decline of CD4+-T-lymphocyte counts to <500/μl and CD4/CD8 T-lymphocyte ratios of <1 during disease progression.
FIG. 3.
Changes in absolute CD4+-T-lymphocyte counts and CD4/CD8 T-lymphocyte ratios. Animals were inoculated intravenously with SIVmac239 or SIVmac239-184V. Five of the SIVmac239-184V-infected animals also received (−)-FTC treatment. Absolute CD4+ CD3+ T lymphocytes and the ratio of CD4+ CD3+ to CD8+ CD3+ T lymphocytes are presented for the first 10 weeks of infection.
Two animals in group A and two in group B developed fatal immunodeficiency within 30 weeks of infection (Table 3), while the remaining animals in these groups remained clinically stable throughout the observation period of 46 weeks. The two animals of group C succumbed to disease at 24 and 39 weeks of age. Accordingly, there were no statistically significant differences in survival between groups (P > 0.1, generalized Wilcoxon test). Limiting-dilution culture assays performed on plasma, PBMC, and mononuclear cells from several tissues collected during necropsy (spleen, thymus, and mesenteric lymph node) revealed that all groups had similarly extensive virus dissemination. Histopathological findings in all animals were consistent with terminal SIV disease and immunodeficiency (Table 3).
DISCUSSION
It is well established that the M184V mutation in RT of HIV-1 results in alteration of several in vitro properties. In particular, the RTs of these mutants have reduced processivity, and M184V viral mutants have impaired replication kinetics in primary T-lymphocyte cultures (1, 3, 31, 34, 41). Although the M184V mutation does not prevent patients from developing HIV-associated illnesses, evidence suggests that the M184V mutation may offer clinical benefits, especially during combination therapy (4, 14, 17, 27, 28, 32, 34, 36, 53). However, many unanswered questions remain regarding the effects of these mutations on viral fitness, virulence, and efficacy of drug therapy in HIV-infected patients. Therefore, in the studies presented here, the SIV macaque model was used to further evaluate the in vivo emergence, fitness, and virulence of M184V mutants.
We have demonstrated that the M184V mutation emerged rapidly in four SIVmac251-infected infant macaques that were started on 3TC or (−)-FTC treatment 3 weeks after virus infection. This emergence of M184V mutants within 5 weeks of drug treatment suggests that drug levels were sufficient to select for the outgrowth of M184V mutants. However, these drug regimens had no significant effect on viral RNA levels in plasma or PBMC-associated infectious virus titers. This finding was unexpected, because in human clinical trials with 3TC or (−)-FTC monotherapy, a 1- to 2-log reduction in viral RNA was observed within 1 to 2 weeks (17, 28, 32, 47, 50, 53). This observation may be the result of a combination of factors. It is unlikely that a higher drug dosage would have been more effective, as oral 3TC dosing of SIV-infected adult macaques at doses up to 20 mg/kg twice daily also did not result in any reduction in viral RNA levels (E. Delwart, personal communication). The high viremia with a highly virulent isolate (SIVmac251) in infants (which do not have fully developed immune systems) may have been overwhelming for these antiviral compounds as monotherapy. In this context, 3TC monotherapy during advanced HIV disease was less effective in suppressing viral RNA for children than for adults (28, 36). Finally, in vitro studies demonstrated that the concentration of 3TC required to inhibit SIV in vitro is slightly higher (about fivefold) than that for HIV-1 (2, 5; A. Giuffre and T. North, unpublished data). It is not known whether this is due to differences in the two RTs, but wild-type SIV isolates have amino acids at two positions in RT (44D and 118I) that differ from those of HIV-1, and these mutations in HIV-1 confer approximately threefold resistance to 3TC in vitro (26). Most importantly for the studies we report here, despite the absence of a transient reduction in virus levels, the drug regimens exerted sufficient selection pressure for the outgrowth of M184V mutants in the SIVmac251-infected macaques.
The rapid disease course in two of the SIVmac251-infected infant macaques that were treated with 3TC or (−)-FTC (animals 30579 and 30853, respectively), characterized by persistently high virus levels, widespread systemic virus dissemination, poor antiviral immune responses, and rapidly fatal immunodeficiency within 12 weeks, was indistinguishable from that of the majority of newborn macaques infected with wild-type SIVmac251. Thus, a shift to a predominant M184V population did not prevent disease in these animals.
To directly evaluate the effect of the M184V mutation on fitness and virulence, we used two molecular clones that differ only at codon 184 of RT, namely, SIVmac239 and SIVmac239-184V. In vitro experiments found that SIVmac239-184V has a slightly reduced replication rate in CEMx174 cells and rhesus macaque PBMC relative to SIVmac239 (J. B. Whitney, M. Oliviera, M. Detorio, Y. Guan, and M. A Wainberg; submitted for publication). To study the effect of the M184V mutation on in vivo fitness and virulence, groups of juvenile macaques were inoculated with SIVmac239 without drug treatment or with SIVmac239-184V in the presence of (−)-FTC treatment (to maintain selection pressure) or absence of drug treatment (to monitor reversion in the absence of selective pressure). Reversion of SIVmac251-184V in animal 30852 occurred between 9 and 16 weeks after 3TC withdrawal. For the two SIVmac239-184V-infected animals which did not receive any (−)-FTC treatment, reversion was detected 8 weeks after virus inoculation. These findings of a reversion from M184V to wild-type sequence confirm that in the absence of drug treatment, SIV isolates with the M184V mutation have reduced fitness relative to wild-type virus, but the rate of reversion suggests that this reduction in fitness is of moderate magnitude. Other mutations (such as a stop codon in nef) that affect the in vivo replication rates of SIV more severely reverted to the wild-type sequence within 2 weeks after inoculation (30, 33).
It was recently reported that the M184V mutation did not revert when macaques were infected with SIVmac239 containing both the M184V and the E89G mutations, whereas the E89G mutation did revert (45). However, the M184V mutation in that study was engineered with two base changes in codon 184, whereas our mutant was constructed to have the single base change observed in drug-resistant SIVmac251 in vivo or SIVmac239 in vitro. Thus, our mutant construct is expected to revert more rapidly than the construct with two base changes. Those authors also reported the appearance of a P272S mutation in virus from animals that had the M184V mutants (45). We did not detect this mutation in virus from any of the animals infected with SIVmac239-184V, including late samples that were collected 40 weeks after infection.
The reduced fitness of M184V mutants may explain why virus levels in the SIVmac239-184V-infected animals 1 week after inoculation were lower than those in animals inoculated with wild-type SIVmac239. Virus levels at 1 week were similarly low in the SIVmac239-184V-infected animals whether they received (−)-FTC treatment or not. Thus, this effect is not due to (−)-FTC inhibition of the M184V virus in vivo, which was predicted to be unlikely based on the >100-fold level of in vitro resistance induced by this mutation. Therefore, the lower virus levels observed in the SIVmac239-184V-infected animals 1 week after virus inoculation are best explained by a reduced fitness of the mutant versus wild-type virus. However, from week 2 on, no difference in virus levels could be observed, as virus levels in the SIVmac239-184V-infected animals reached levels similar to those observed for the wild-type virus. Observations of primary infection of humans with populations of HIV-1 containing M184V mutants also suggest that this mutation does not affect the ability to reach high levels of viremia. In these patients, primary infection with HIV-1 M184V mutant populations led to symptoms of acute infection and peak virus levels similar to those of wild-type HIV-1 (10, 13, 25), although it is unknown whether the time to reach this peak was longer than that for wild-type virus.
An important finding was that, although M184V mutants of SIV were initially less fit (as evident from slower replication during the first week of infection and from reversion in the absence of drug), they were virulent and capable of inducing disease. There was no statistically significant difference in disease-free survival between animals infected with M184V and those with wild-type SIV isolates. The histopathological lesions in the animals with SIVmac239-184V were consistent with SIV-induced fatal immunodeficiency.
The observation that a decreased replicative fitness of the M184V SIV mutants did not cause a significant change in virulence (i.e., their ability to cause disease) or in viremia from week 2 on deserves further attention. Virulence and replicative fitness usually correlate well, as large differences in replicative fitness translate into significant differences in virulence and disease course (15, 38, 39, 48). However, this correlation is not stringent, especially when virus isolates with relatively smaller differences in replicative fitness are compared. Virulence is a broad issue of viral pathogenesis that depends not only on replicative fitness but also on other factors. These factors include the ability of the virus to weaken the immune system and to evade the different antiviral immune responses that the host is mounting in an attempt to control virus replication. Although in vitro studies often provide useful information on replicative fitness, they are currently not able to completely model these complex interactions with the immune system and predict in vivo virulence (30). Animal models are the best tools for providing further insights in fitness and virulence. Our data suggest that the reduction in replicative fitness of SIV caused by the M184V mutation was too small to alter its virulence or was compensated for by other mechanisms. In this context, Frost et al. observed that the drop in viral fitness associated with the M184V mutation in HIV-1 resulted in a drop in virus levels only in individuals with high CD4+-T-cell counts, from which the relative fitness of M184V mutants was estimated to be approximately 10% of that of the wild-type virus prior to therapy. In contrast, in individuals with low CD4+-T-cell counts, this decrease in viral fitness had no effect on the viral load, which suggests that the functional status of the immune system further affects the complex interactions between viral fitness, virus levels, and virulence (20).
There are other mechanisms through which the M184V mutation can alter the pathogenesis for HIV-infected patients which were not addressed in the present macaque studies. As mentioned earlier, the M184V mutation is known to affect the phenotypic expression of some drug resistance mutations. In this regard, addition of M184V to a virus with AZT resistance mutations in RT restores phenotypic susceptibility to AZT by decreasing pyrophosphorolysis (22, 34), and other mutations are required to make the virus coresistant to both drugs (29, 46, 71). The M184V mutation also renders virus more susceptible to inhibition by the nucleotide analogs adefovir [9-(2-phosphonylmethoxyethyl)adenine] and tenofovir (PMPA) (41, 70). Thus, the demonstrated benefits of 3TC in combination therapy, including salvage therapies, even after M184V mutants arise (4) can perhaps be attributed more to its effect on the phenotypic expression of resistance to other drugs than to a direct effect on viral fitness and virulence. Studies in animal models using 3TC or (−)-FTC as part of combination regimens can be performed to further elucidate the role of the M184V mutation on these aspects of viral diversification and pathogenesis.
Acknowledgments
We thank N. Aguirre, S. Au, D. Bennett, I. Bolton, L. Brignolo, R. Buchholz, I. Cazares, K. Christe, T. Dearman, A. Enriquez, A. Giuffre, L. Hirst, M. Hofman, J. Murry, A. Spinner, W. von Morgenland, and Colony Services of the California Regional Primate Research Center for expert technical assistance; P. Dailey and J. Booth (Bayer Reference Testing Laboratory, Bayer Diagnostics, Emeryville, Calif.) for viral RNA measurements by branched-DNA assay; and P. Sharma (Emory University, VA Medical Center, Decatur, Ga.) for helpful discussion and critical review of the manuscript.
This research was supported by NIH grants RR13967 and AI28189 to T.W.N. and RR00169 to the California Regional Primate Research Center and by E. Glaser Pediatric AIDS Foundation grant 50853 to K.K.A.V.R. R.F.S. is supported in part by NIH grant AI41890, the Emory CFAR, and the Department of Veterans Affairs.
REFERENCES
- 1.Back, N. K., M. Nijhuis, W. Keulen, C. A. B. Boucher, B. O. Oude Essink, A. B. van Kuilenberg, A. H. van Gennip, and B. Berkhout. 1996. Reduced replication of 3TC-resistant HIV-1 variants in primary cells due to a processivity defect of the reverse transcriptase enzyme. EMBO J. 15:4040-4049. [PMC free article] [PubMed] [Google Scholar]
- 2.Balzarini, J., M. Weeger, M.-J. Camarasa, E. De Clercq, and K. Überla. 1995. Sensitivity/resistance profile of a simian immunodeficiency virus containing the reverse transcriptase gene of human immunodeficiency virus type 1 (HIV-1) toward the HIV-1 specific non-nucleoside reverse transcriptase inhibitors. Biochem. Biophys. Res. Commun. 211:850-856. [DOI] [PubMed] [Google Scholar]
- 3.Boyer, P. L., and S. H. Hughes. 1995. Analysis of mutations at position 184 in reverse transcriptase of human immunodeficiency virus type 1. Antimicrob. Agents Chemother. 39:1624-1628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Catucci, M., G. Venturi, L. Romano, M. L. Riccio, A. De Milito, P. E. Valensin, and M. Zazzi. 1999. Development and significance of the HIV-1 reverse transcriptase M184V mutation during combination therapy with lamivudine, zidovudine, and protease inhibitors. J. Acquir. Immune Defic. Syndr. 21:203-208. [DOI] [PubMed] [Google Scholar]
- 5.Cherry, E., M. Slater, H. Salomon, E. Rud, and M. A. Wainberg. 1997. Mutations at codon 184 in simian immunodeficiency virus reverse transcriptase confer resistance to the (−) enantiomer of 2′,3′-dideoxy-3′-thiacytidine. Antimicrob. Agents Chemother. 41:2763-2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung, R. C., S. M. Matsui, and H. B. Greenberg. 1994. Rapid and sensitive method for detection of hepatitis C virus RNA by using silica particles. J. Clin. Microbiol. 32:2593-2597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coen, D. M., M. Kosz-Vnenchak, J. G. Jacobsen, D. A. Leib, C. L. Bogard, P. A. Schaffer, K. L. Tylerm, and D. M. Knipe. 1989. Thymidine kinase-negative herpes simplex virus mutants establish latency in mouse trigeminal ganglia but do not reactivate. Proc. Natl. Acad. Sci. USA 86:4736-4740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffin, J. M. 1995. HIV population dynamics in vivo: implications for genetic variation, pathogenesis, and therapy. Science 267:483-489. [DOI] [PubMed] [Google Scholar]
- 9.Condra, J. H. 1998. Resisting resistance: maximizing the durability of antiretroviral therapy. Ann. Intern. Med. 128:951-954. [DOI] [PubMed] [Google Scholar]
- 10.Conway, B., V. Montessori, D. Rouleau, J. S. G. Montaner, M. V. O'Shaughnessy, S. Fransen, A. Shillington, D. Weislow, and D. L. Mayers. 1999. Primary lamivudine resistance in acute/early human immunodeficiency virus infection. Clin. Infect. Dis. 28:910-911. [DOI] [PubMed] [Google Scholar]
- 11.Croteau, G., L. Doyon, D. Thibeault, G. McKercher, L. Pilote, and D. Lamarre. 1997. Impaired fitness of human immunodeficiency virus type 1 variants with high-level resistance to protease inhibitors. J. Virol. 71:1089-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawson-Saunders, B., and R. G. Trapp. 1990. Basic and clinical biostatistics. Appleton & Lange, Norwalk, Conn.
- 13.De Pasquale, M. P., E. Rosenberg, A. Quayle, C. Tremblay, J. Martínez-Picado, J. Allega, L. Sutton, L. Shi, A. V. Savara, S. Boswell, P. Sax, M. Hirsch, A. Caliendo, D. Anderson, B. D. Walker, and R. T. D'Aquila. 1999. Primary HIV infection: in vivo fitness of pretherapy resistant mutants and potential for secondary spread of HIV from semen. Antivir. Ther. 4(Suppl. 1):89.16021878 [Google Scholar]
- 14.Descamps, D., P. Flandre, V. Calvez, G. Peytavin, V. Meiffredy, G. Collin, C. Delaugerre, S. Robert-Delmas, B. Bazin, J.-P. Aboulker, G. Pialoux, F. Raffi, and F. Brun-Vézinet. 2000. Mechanisms of virologic failure in previously untreated HIV-infected patients from a trial of induction-maintenance therapy. JAMA 283:205-211. [DOI] [PubMed] [Google Scholar]
- 15.Desrosiers, R. C., J. D. Lifson, J. S. Gibbs, S. C. Czajak, A. Y. M. Howe, L. O. Arthur, and P. R. Johnson. 1998. Identification of highly attenuated mutants of simian immunodeficiency virus. J. Virol. 72:1431-1437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Efstathiou, D. L., S. Kemp, G. Darby, and A. C. Minson. 1989. The role of herpes simplex virus type 1 thymidine kinase in pathogenesis. J. Gen. Virol. 70:869-879. [DOI] [PubMed] [Google Scholar]
- 17.Eron, J. J., S. L. Benoit, J. Jemsek, R. D. MacArthur, J. Santana, J. B. Quinn, D. R. Kuritzkes, M. A. Fallon, and M. Rubin, for the North American HIV Working Group. 1995. Treatment with lamivudine, zidovudine, or both in HIV-positive patients with 200 to 500 CD4+ cells per cubic millimeter. N. Engl. J. Med. 333:1662-1669. [DOI] [PubMed] [Google Scholar]
- 18.Fauci, A. S. 1999. The AIDS epidemic. N. Engl. J. Med. 341:1046-1050. [DOI] [PubMed] [Google Scholar]
- 19.Feng, J. Y., J. Shi, R. F. Schinazi, and K. S. Anderson. 1999. Mechanistic studies show that (−)-FTC-TP is a better inhibitor of HIV-1 reverse transcriptase than 3TC-TP. FASEB J. 13:1511-1517. [DOI] [PubMed] [Google Scholar]
- 20.Frost, S. D. W., M. Nijhuis, R. Schuurman, C. A. B. Boucher, and A. J. Leigh Brown. 2000. Evolution of lamivudine resistance in human immunodeficiency virus type 1-infected individuals: the relative roles of drift and selection. J. Virol. 74:6262-6268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gosselin, G., R. F. Schinazi, J. P. Sommadossi, C. Mathé, M.-C. Bergogne, A.-M. Aubertin, A. Kirn, and J.-L. Imbach. 1994. Anti-human immunodeficiency virus activities of the β-l enantiomer of 2′,3′-dideoxycytidine and its 5-fluoro derivative in vitro. Antimicrob. Agents Chemother. 38:1292-1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Götte, M., D. Arion, M. A. Parniak, and M. A. Wainberg. 2000. The M184V mutation in the reverse transcriptase of human immunodeficiency virus type 1 impairs rescue of chain-terminated DNA synthesis. J. Virol. 74:3579-3585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goudsmit, J., A. De Ronde, D. D. Ho, and A. S. Perelson. 1996. Human immunodeficiency virus fitness in vivo: calculations based on a single zidovudine resistance mutation at codon 215 of reverse transcriptase. J. Virol. 70:5662-5664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heald, A. E., P. H. Hsyu, G. J. Yuen, P. Robinson, P. Mydlow, and J. A. Bartlett. 1996. Pharmacokinetics of lamivudine in human immunodeficiency virus-infected patients with renal dysfunction. Antimicrob. Agents Chemother. 40:1514-1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hecht, F. M., R. M. Grant, C. J. Petropoulos, B. Dillon, M. A. Chesney, H. Tian, N. S. Hellmann, N. I. Bandrapalli, L. Digilio, B. Branson, and J. O. Kahn. 1998. Sexual transmission of an HIV-1 variant resistant to multiple reverse-transcriptase and protease inhibitors. N. Engl. J. Med. 339:307-311. [DOI] [PubMed] [Google Scholar]
- 26.Hertogs, K., S. Bloor, V. De Vroey, C. Van den Eynde, P. Dehertogh, A. Van Cauwenberghe, M. Stürmer, T. Alcorn, S. Wegner, M. Van Houtte, V. Miller, and B. A. Larder. 2000. A novel human immunodeficiency virus type 1 reverse transcriptase mutational pattern confers phenotypic lamivudine resistance in the absence of mutation 184V. Antimicrob. Agents Chemother. 44:568-573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ingrand, D., J. Weber, C. A. B. Boucher, C. Loveday, C. Robert, A. Hill, N. Cammack, and The Lamivudine European Working Group. 1995. Phase I/II study of 3TC (lamivudine) in HIV-positive, asymptomatic or mild AIDS-related complex patients: sustained reduction in viral markers. AIDS 9:1323-1329. [DOI] [PubMed] [Google Scholar]
- 28.Kavlick, M. F., T. Shirasaka, E. Kojima, J. M. Pluda, F. J. Hui, R. Yarchoan, and H. Mitsuya. 1995. Genotypic and phenotypic characterization of HIV-1 isolated from patients receiving (−)-2′,3′-dideoxy-3′-thiacytidine. Antivir. Res. 28:133-146. [DOI] [PubMed] [Google Scholar]
- 29.Kemp, S. D., C. Shi, S. Bloor, P. R. Harrigan, J. W. Mellors, and B. A. Larder. 1998. A novel polymorphism at codon 333 of human immunodeficiency virus type 1 reverse transcriptase can facilitate dual resistance to zidovudine and l-2′,3′-dideoxy-3′-thiacytidine. J. Virol. 72:5093-5098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicalli, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 31.Keulen, W., N. K. T. Back, A. van Wijk, C. A. B. Boucher, and B. Berkhout. 1997. Initial appearance of the 184Ile variant in lamivudine-treated patients is caused by the mutational bias of human immunodeficiency virus type 1 reverse transcriptase. J. Virol. 71:3346-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuritzkes, D. R., J. B. Quinn, S. L. Benoit, D. L. Shugarts, A. Griffin, M. Bakhtiari, D. Poticha, J. J. Eron, M. A. Fallon, and M. Rubin. 1996. Drug resistance and virologic response in NUCA 3001, a randomized trial of lamivudine (3TC) versus zidovudine (ZDV) versus ZDV plus 3TC in previously untreated patients. AIDS 10:975-981. [DOI] [PubMed] [Google Scholar]
- 33.Lang, S. M., M. Weeger, C. Stahl-Hennig, C. Coulibaly, G. Hunsmann, J. Müller, H. Müller-Hermelink, D. Fuchs, H. Wachter, M. M. Daniel, R. C. Desrosiers, and B. Fleckenstein. 1993. Importance of vpr for infection of rhesus monkeys with simian immunodeficiency virus. J. Virol. 67:902-912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Larder, B., S. D. Kemp, and P. R. Harrigan. 1995. Potential mechanism for sustained antiretroviral efficacy of AZT-3TC combination therapy. Science 269:696-699. [DOI] [PubMed] [Google Scholar]
- 35.Leutenegger, C. M., J. Higgins, T. B. Matthews, A. F. Tarantal, P. A. Luciw, N. C. Pedersen, and T. W. North. 2001. Real-time Taqman PCR as a specific and more sensitive alternative to the branched-chain DNA assay for quantitation of simian immunodeficiency virus RNA. AIDS Res. Hum. Retrovir. 17:243-251. [DOI] [PubMed] [Google Scholar]
- 36.Lewis, L. L., D. Venzon, J. Church, M. Farley, S. Wheeler, A. Keller, M. Rubin, G. Yeun, B. Mueller, M. Sloas, L. Wood, F. Balis, G. M. Shearer, P. Brouwers, J. Goldsmith, P. A. Pizzo, B. Anderson, S. Hirschfeld, S. Sei, S. Zeichner, E. Roilides, M. Clerici, M. Wells, and V. Stocker. 1996. Lamivudine in children with human immunodeficiency virus infection: a phase I/II study. J. Infect. Dis. 174:16-25. [DOI] [PubMed] [Google Scholar]
- 37.Maeda, Y., D. J. Venzon, and H. Mitsuya. 1998. Altered drug sensitivity, fitness, and evolution of human immunodeficiency virus type 1 with pol gene mutations conferring multi-dideoxynucleoside resistance. J. Infect. Dis. 177:1207-1213. [DOI] [PubMed] [Google Scholar]
- 38.Marthas, M. L., R. A. Ramos, B. L. Lohman, K. K. A. Van Rompay, R. E. Unger, C. J. Miller, B. Banapour, N. C. Pedersen, and P. A. Luciw. 1993. Viral determinants of simian immunodeficiency virus (SIV) virulence in rhesus macaques assessed by using attenuated and pathogenic molecular clones of SIVmac. J. Virol. 67:6047-6055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marthas, M. L., K. K. A. Van Rompay, M. Otsyula, C. J. Miller, D. Canfield, N. C. Pedersen, and M. B. McChesney. 1995. Viral factors determine progression to AIDS in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 69:4198-4205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Martinez-Picado, J., L. V. Savara, L. Sutton, and R. T. D'Aquila. 1999. Replicative fitness of protease inhibitor-resistant mutants of human immunodeficiency virus type 1. J. Virol. 73:3744-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller, M. D., K. E. Anton, A. S. Mulato, P. D. Lamy, and J. M. Cherrington. 1999. Human immunodeficiency virus type 1 expressing the lamivudine-associated M184V mutation in reverse transcriptase shows increased susceptibility to adefovir and decreased replication capability in vitro. J. Infect. Dis. 179:92-100. [DOI] [PubMed] [Google Scholar]
- 42.Moodley, J., D. Moodley, K. Pillay, H. Coovadia, J. Saba, R. van Leeuwen, C. Goodwin, P. R. Harrigan, K. H. P. Moore, C. Stone, R. Plumb, and M. A. Johnson. 1998. Pharmacokinetics and antiretroviral activity of lamivudine alone or when coadministered with zidovudine in human immunodeficiency virus type 1-infected pregnant women and their offspring. J. Infect. Dis. 178:1327-1333. [DOI] [PubMed] [Google Scholar]
- 43.Myers, G., J. A. Berzofsky, B. Korber, R. F. Smith, and G. N. Pavlakis. 1991. Human retroviruses and AIDS. A compilation and analysis of nucleic acid and amino acid sequences. Los Alamos National Laboratory, Los Alamos, N.Mex.
- 44.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 45.Newstein, M. C., and R. C. Desrosiers. 2001. Effects of reverse-transcriptase mutations M184V and E89G on simian immunodeficiency virus in rhesus monkeys. J. Infect. Dis. 184:1262-1267. [DOI] [PubMed] [Google Scholar]
- 46.Nijhuis, M., R. Schuurman, D. De Jong, R. Van Leeuwen, J. Lange, S. Danner, W. Keulen, T. De Groot, and C. A. B. Boucher. 1997. Lamivudine-resistant human immunodeficiency virus type 1 variants (184V) require multiple amino acid changes to become co-resistant to zidovudine in vivo. J. Infect. Dis. 176:398-405. [DOI] [PubMed] [Google Scholar]
- 47.Pluda, J. M., T. P. Cooley, J. S. G. Montaner, L. E. Shay, N. E. Reinhalter, S. N. Warthan, J. Ruedy, H. M. Hirst, C. A. Vicary, J. B. Quinn, G. J. Yuen, M. A. Wainberg, M. Rubin, and R. Yarchoan. 1995. A phase I/II study of 2′-deoxy-3′-thiacytidine (lamivudine) in patients with advanced human immunodeficiency virus infection. J. Infect. Dis. 171:1438-1447. [DOI] [PubMed] [Google Scholar]
- 48.Quinones-Mateu, M. E., S. C. Ball, A. J. Marozsan, V. S. Torre, J. L. Albright, G. Vanham, G. van der Groen, R. L. Colebunders, and E. J. Arts. 2000. A dual infection/competition assay shows a correlation between ex vivo human immunodeficiency virus type 1 fitness and disease progression. J. Virol. 74:9222-9233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richman, D. D. 1995. Clinical significance of drug resistance in human immunodeficiency virus. Clin. Infect. Dis. 21(Suppl. 2):S166-S169. [DOI] [PubMed] [Google Scholar]
- 50.Rousseau, F. S., J. O. Kahn, M. Thompson, D. Mildvan, D. Shepp, J.-P. Sommadossi, J. Delehanty, J. N. Simpson, L. H. Wang, J. B. Quinn, C. Wakeford, and C. van der Horst. 2001. Prototype trial design for rapid dose selection of antiretroviral drugs: an example using emtricitabine (Coviracil). J. Antimicrob. Chemother. 48:507-513. [DOI] [PubMed] [Google Scholar]
- 51.Sawai, E. T., I. H. Khan, P. M. Montbriand, B. M. Peterlin, C. Cheng-Mayer, and P. A. Luciw. 1996. Activation of PAK by HIV and SIV nef: importance for AIDS in SIV-infected rhesus macaques. Curr. Biol. 6:1519-1527. [DOI] [PubMed] [Google Scholar]
- 52.Schinazi, R. F., R. M. J. Lloyd, M. H. Nguyen, D. L. Cannon, A. McMillan, N. Ilksoy, C. K. Chu, D. C. Liotta, H. Z. Bazmi, and J. W. Mellors. 1993. Characterization of human immunodeficiency viruses resistant to oxathiolane-cytosine nucleosides. Antimicrob. Agents Chemother. 37:875-881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Schuurman, R., M. Nijhuis, R. van Leeuwen, P. Schipper, D. de Jong, P. Collis, S. A. Danner, J. Mulder, C. Loveday, C. Christoperson, S. Kwok, J. Sninsky, and C. A. B. Boucher. 1995. Rapid changes in human immunodeficiency virus type 1 RNA load and appearance of drug-resistant virus populations in persons treated with lamivudine (3TC). J. Infect. Dis. 171:1411-1419. [DOI] [PubMed] [Google Scholar]
- 54.Sharma, P. L., and C. S. Crumpacker. 1997. Attenuated replication of human immunodeficiency virus type 1 with a didanosine-selected reverse transcriptase mutation. J. Virol. 71:8846-8851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tai, C. Y., P. A. Escarpe, R. W. Sidwell, M. A. Williams, W. Lew, H. Wu, C. U. Kim, and D. B. Mendel. 1998. Characterization of human influenza virus variants selected in vitro in the presence of the neuraminidase inhibitor GS 4071. Antimicrob. Agents Chemother. 42:3234-3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tisdale, M., S. D. Kemp, N. R. Parry, and B. A. Larder. 1993. Rapid in vitro selection of human immunodeficiency virus type 1 resistant to 3′-thiacytidine inhibitors due to a mutation in the YMDD region of reverse transcriptase. Proc. Natl. Acad. Sci. USA 90:5653-5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Leeuwen, R., J. M. A. Lange, E. K. Hussey, K. H. Donn, S. T. Hall, A. J. Harker, P. Jonker, and S. A. Danner. 1992. The safety and pharmacokinetics of a reverse transcriptase inhibitor, 3TC, in patients with HIV infection: a phase I study. AIDS 6:1471-1475. [DOI] [PubMed] [Google Scholar]
- 58.Van Rompay, K. K. A., C. J. Berardi, N. L. Aguirre, N. Bischofberger, P. S. Lietman, N. C. Pedersen, and M. L. Marthas. 1998. Two doses of PMPA protect newborn macaques against oral simian immunodeficiency virus infection. AIDS 12:F79-F83. [DOI] [PubMed] [Google Scholar]
- 59.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, C. J. Berardi, A. S. Mulato, A. Spinner, R. P. Tarara, D. R. Canfield, S. Telm, N. Bischofberger, and N. C. Pedersen. 1996. 9-[2-(Phosphonomethoxy)propyl]adenine therapy of established simian immunodeficiency virus infection in infant rhesus macaques. Antimicrob. Agents Chemother. 40:2586-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Van Rompay, K. K. A., J. M. Cherrington, M. L. Marthas, P. D. Lamy, P. J. Dailey, D. R. Canfield, R. P. Tarara, N. Bischofberger, and N. C. Pedersen. 1999. 9-[2-(Phosphonomethoxy)propyl]adenine (PMPA) therapy prolongs survival of infant macaques inoculated with simian immunodeficiency virus with reduced susceptibility to PMPA. Antimicrob. Agents Chemother. 43:802-812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Van Rompay, K. K. A., P. J. Dailey, R. P. Tarara, D. R. Canfield, N. L. Aguirre, J. M. Cherrington, P. D. Lamy, N. Bischofberger, N. C. Pedersen, and M. L. Marthas. 1999. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn rhesus macaques. J. Virol. 73:2947-2955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Van Rompay, K. K. A., J. L. Greenier, M. L. Marthas, M. G. Otsyula, R. P. Tarara, C. J. Miller, and N. C. Pedersen. 1997. A zidovudine-resistant simian immunodeficiency virus mutant with a Q151M mutation in reverse transcriptase causes AIDS in newborn macaques. Antimicrob. Agents Chemother. 41:278-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Van Rompay, K. K. A., M. L. Marthas, J. D. Lifson, C. J. Berardi, G. M. Vasquez, E. Agatep, Z. A. Dehqanzada, K. C. Cundy, N. Bischofberger, and N. C. Pedersen. 1998. Administration of 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) for prevention of perinatal simian immunodeficiency virus infection in rhesus macaques. AIDS Res. Hum. Retrovir. 14:761-773. [DOI] [PubMed] [Google Scholar]
- 64.Van Rompay, K. K. A., M. L. Marthas, R. A. Ramos, C. P. Mandell, E. K. McGowan, S. M. Joye, and N. C. Pedersen. 1992. Simian immunodeficiency virus (SIV) infection of infant rhesus macaques as a model to test antiretroviral drug prophylaxis and therapy: oral 3′-azido-3′-deoxythymidine prevents SIV infection. Antimicrob. Agents Chemother. 36:2381-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Van Rompay, K. K. A., M. B. McChesney, N. L. Aguirre, K. A. Schmidt, N. Bischofberger, and M. L. Marthas. 2001. Two low doses of tenofovir protect newborn macaques against oral simian immunodeficiency virus infection. J. Infect. Dis. 184:429-438. [DOI] [PubMed] [Google Scholar]
- 66.Van Rompay, K. K. A., M. D. Miller, M. L. Marthas, N. A. Margot, P. J. Dailey, R. P. Tarara, D. R. Canfield, J. M. Cherrington, N. L. Aguirre, N. Bischofberger, and N. C. Pedersen. 2000. Prophylactic and therapeutic benefits of short-term 9-[2-(phosphonomethoxy)propyl]adenine (PMPA) administration to newborn macaques following oral inoculation with simian immunodeficiency virus with reduced susceptibility to PMPA. J. Virol. 74:1767-1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Van Rompay, K. K. A., M. G. Otsyula, M. L. Marthas, C. J. Miller, M. B. McChesney, and N. C. Pedersen. 1995. Immediate zidovudine treatment protects simian immunodeficiency virus-infected newborn macaques against rapid onset of AIDS. Antimicrob. Agents Chemother. 39:125-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Van Rompay, K. K. A., M. G. Otsyula, R. P. Tarara, D. R. Canfield, C. J. Berardi, M. B. McChesney, and M. L. Marthas. 1996. Vaccination of pregnant macaques protects newborns against mucosal simian immunodeficiency virus infection. J. Infect. Dis. 173:1327-1335. [DOI] [PubMed] [Google Scholar]
- 69.Wainberg, M. A., W. C. Drosopoulos, H. Salomon, M. Hsu, G. Borkow, M. A. Parniak, Z. Gu, Q. Song, J. Manne, S. Islam, G. Castriota, and V. R. Prasad. 1996. Enhanced fidelity of 3TC-selected mutant HIV-1 reverse transcriptase. Science 271:1282-1285. [DOI] [PubMed] [Google Scholar]
- 70.Wainberg, M. A., M. D. Miller, Y. Quan, H. Salomon, A. S. Mulato, P. D. Lamy, N. A. Margot, K. E. Anton, and J. M. Cherrington. 1999. In vitro selection and characterization of HIV-1 with reduced susceptibility to PMPA. Antivir. Ther. 4:87-94. [DOI] [PubMed] [Google Scholar]
- 71.Winters, M. A., K. L. Coolley, Y. A. Girard, D. J. Levee, H. Hamdan, R. W. Shafer, D. A. Katzenstein, and T. C. Merigan. 1998. A 6-basepair insert in the reverse transcriptase gene of human immunodeficiency virus type 1 confers resistance to multiple nucleoside inhibitors. J. Clin. Investig. 102:1769-1775. [DOI] [PMC free article] [PubMed] [Google Scholar]