Abstract
To identify the early target cells and tissues in transmucosal feline immunodeficiency virus (FIV) infection, cats were exposed to a clade C FIV isolate via the oral-nasal or vaginal mucosa and multiple tissues were examined by virus isolation coculture (VI), DNA PCR, catalyzed tyramide signal-amplified in situ hybridization (TSA-ISH), and immunohistochemistry between days 1 and 12 postinoculation (p.i.). FIV RNA was detected in tonsil and oral or vaginal mucosa as early as 1 day p.i. by TSA-ISH and in retropharyngeal, tracheobronchial, or external iliac lymph nodes and sometimes in spleen or blood mononuclear cells by day 2, indicating that regional and distant spread of virus-infected cells occurred rapidly after mucosal exposure. By day 8, viral RNA, DNA, and culturable virus were uniformly detected in regional and distant tissues, connoting systemic infection. TSA-ISH proved more sensitive than DNA PCR in detecting early FIV-infected cells. In mucosal tissues, the earliest demonstrable FIV-bearing cells were either within or subjacent to the mucosal epithelium or were in germinal centers of regional lymph nodes. The FIV+ cells were of either of two morphological types, large stellate or small round. Those FIV RNA+ cells which could be colabeled for a phenotype marker, were labeled for either dendritic-cell-associated protein p55 or T-lymphocyte receptor antigen CD3. These studies indicate that FIV crosses mucous membranes within hours after exposure and rapidly traffics via dendritic and T cells to systemic lymphoid tissues, a pathway similar to that thought to occur in the initial phase of infection by the human and simian immunodeficiency viruses.
Human immunodeficiency virus type 1 (HIV-1) has infected >60 million people worldwide, the vast majority transmucosally (81). Although HIV is transmitted most frequently across the genital mucosa, multiple reports suggest that infection also can be transmitted across the oral mucosa (42, 70; M. M. Berrey and T. Shea, Letter, J. Acquir. Immune Defic. Syndr. Hum. Retrovir. 14:475, 1997; H. C. Lane, S. D. Holmberg, and H. W. Jaffe, Letter, Am. J. Public Health 81:658, 1991). How HIV crosses the mucosal barrier, its initial cellular targets, and how virus dissemination ensues are questions difficult to study in humans. Feline immunodeficiency virus (FIV) infection, the feline counterpart of HIV (5-7, 60, 79), also can be transmitted by mucosal exposure, by blood transfer, and vertically from mother to offspring (11, 57, 58, 61, 72, 85).
The initial cells infected in the process of transmucosal lentivirus infection remain to be completely defined. Recent evidence points to dendritic cells (DC). Mucosal and blood DC have been shown to be efficient vectors for HIV transmission to T cells in vitro (3, 12, 31, 65). For macaques chronically infected with simian immunodeficiency virus (SIV) it has been demonstrated that as many as 40% of the SIV-infected cells in the vagina and cervix are Langerhans cells (LC), i.e., mucosal DC (44, 46). Hu and colleagues (37) have demonstrated SIV-infected LC-like cells subjacent to the cervicovaginal epithelium within 24 h and have documented virus entry into mucosal DC within 2 h after vaginal exposure.
Other evidence suggests that epithelial cells may be involved in mucosal lentivirus infection via viral transcytosis (9, 62) and that the virus may rapidly target T cells. After in vivo exposure of the tonsillar mucosa, CD4+ T cells subjacent to the antigen-transporting tonsillar crypt epithelium were the first cells in which SIV infection was detected (76). After intrarectal SIV inoculation (14), the first cells bearing SIV infection detected were T cells in regional lymph nodes (LN).
Still other evidence suggests that a key property of mucosally transmitted viruses may be the ability to infect macrophages (26, 50, 67, 71, 82). HIV RNA and DNA have been demonstrated in macrophages at the mucosal-stromal junction of the endocervical junctional zone (54). The initial SIV-infected cells detected after intravaginal inoculation were identified as primarily submucosal macrophages (45, 46). After ex vivo exposure of human vaginal and cervical mucosal explants to HIV, macrophages were the first cells detectably infected (32). By contrast, several studies have concluded that no selection for macrophage-tropic, non-syncytium-inducing viruses occurs during sexual transmission (24, 52, 69, 91). Moreover, the transmissibility of SIV isolates via the vaginal route does not correlate with in vitro macrophage tropism (20, 44), and mononuclear cells in the cervicovaginal tissues are permissive for both R5- and X4-tropic HIV strains (36).
Thus further studies of the early pathways, tropisms, and kinetics of transmucosal lentivirus infections will be important in understanding how HIV-1 and other lentiviruses breach or exploit the first lines of host defense to establish infection. To this end, we examined the earliest sites of FIV replication following mucosal exposure to a highly replicative, mucosally transmissible clade C FIV (FIV-C) isolate (16, 55, 56).
MATERIALS AND METHODS
Animals, inoculation, and sample collection. (i) Oral-nasal inoculation.
Eight- to 10-week-old weanling specific-pathogen-free (SPF) cats from a breeding colony maintained at Colorado State University (Fort Collins) were exposed to FIV-C by dropwise instillation of either 2 ml of a cell-free infectious culture at 300 50% tissue culture infective doses (TCID50)/ml (n = 18) or 4 × 105 infectious peripheral blood mononuclear cells (PBMC) (n = 8) onto the oral and nasal mucosae while under anesthesia. For oral inoculation, the animals were positioned upright to facilitate oral-nasal exposure and to prevent aspiration.
(ii) Vaginal inoculation.
Female SPF weanling age kittens were exposed to FIV by depositing either 500 μl of a cell-free infectious FIV-C culture at 300 TCID50/ml (n = 7), 500 μl of a cell-free infectious FIV-B culture at 400 TCID50/ml (n = 5), or 2 × 105 FIV-C infectious PBMC (n = 3) into the vagina with a 3[1/2] French Tom Cat catheter (Sherwood Medical, St. Louis, Mo.). Following instillation of virus inoculum, the animals were positioned with hindquarters elevated for 20 min to facilitate retention of inoculum.
Sampling.
At 1- to 2-day intervals during the first 12 days postinoculation (p.i.), one or two cats were necropsied. Multiple mucosal and lymphoid tissues were collected and fixed in 10% buffered formalin for ≤18 h before histologic processing and paraffin embedding. Tissues harvested included spleen; bone marrow; thymus; retropharyngeal, submandibular, gastric, tracheobronchial, mesenteric colic, sacral, iliac, and popliteal LN; tonsils; liver; gastrointestinal tract; lungs; salivary glands; oral-nasal and vaginal mucosae; and brain. Parallel tissue samples were collected in liquid nitrogen for nucleic acid extraction. Small portions of the retropharyngeal and submandibular LN from 11 of the 26 cats subjected to oral-nasal inoculation and 2 control cats were collected in media for virus isolation coculture.
Viral inocula.
Three viral inocula were employed: (i) cell-free virus prepared as supernatant from a coculture of PBMC from SPF donor cats with PBMC from FIV-C-PGammer-infected cats (16), (ii) cell-free virus prepared as supernatant from a coculture of naive PBMC with PBMC from an FIV-B-2542-infected cat (74), and (iii) a cellular inoculum consisting of PBMC isolated from a cat (cat 3823) acutely infected with FIV-C-PGammer. Cats inoculated orally received 4 × 105 infected PBMC in a volume of 1.0 ml, and cats inoculated vaginally received 2 × 105 infected PBMC in a volume of 0.5 ml. The number of infected PBMC inoculated was calculated by dilutional DNA PCR.
Virus isolation.
PBMC and LN cells (LNC) were separated by density gradient centrifugation (Histopaque 1077; Sigma) from heparinized samples and washed twice with phosphate-buffered saline (PBS). LNC were mechanically dispersed by passage through a wire strainer and resuspended in PBS. Naive donor PBMC were obtained from SPF cats and stimulated with concanavalin A (10 μg/ml; Sigma) for 3 to 7 days. One million naive cells were cocultured with either 106 PBMC or LNC from FIV-exposed cats in RPMI medium supplemented with 20% fetal calf serum, 1% penicillin-streptomycin, 2% glutamine, 2 × 10−5 M 2-mercaptoethanol, and 100 U of interleukin-2/ml (Cetus/Roche, Emeryville, Calif.). Cells were cultured in 24-well plates. Supernatants were collected twice weekly and assayed for FIV p24 by a capture enzyme-linked immunosorbent assay (17).
PCR primers and probes.
Primers were selected with the Oligo Primer Analysis Software program (National Biosciences, Inc., Plymouth, Minn.) from the gag nucleotide sequence of FIV-C-PGammer obtained from J. I. Mullins (University of Washington, Seattle). The first-round CgagU1 and CgagL1 primers amplified a fragment 623 bp in length. The primer sequences were as follows: CgagU1 (nucleotides 65 to 80), GGGTAGGGGGAAAGAG; CgagL1 (nucleotides 673 to 656), AGTGAAGTATGGCAATGG. The second-round CgagU2 and CgagL2 primers amplified a fragment 301 bp in length. The primer sequences were as follows: CgagU2 (nucleotides 649 to 631), AAGCCGAGAGGAAAGGAA; CgagL2 (nucleotides 367 to 384), GACCATCAGGAGGGTGAGT. The sensitivity of the nested PCR, determined by using plasmid DNA containing the FIV-C gag gene, was found to be between 1 and 10 copies of target sequence.
Specificity of the PCR products was determined with a 22-mer probe (nucleotides 513 to 534; ATTATGGTTTACAGCCTTTTCG) designed to recognize both first- and second-round products. The probe was labeled with alkaline phosphatase (AP) by using the AP-Oligonucleotide labeling kit (Boehringer Mannheim, Indianapolis, Ind.). Following standard Southern blot transfer of the PCR product from a 1.2% agarose gel to a positively charged nylon membrane and hybridization of the AP-conjugated oligoprobe using QuikHyb hybridization solution (Stratagene, La Jolla, Calif.), the bound probe was visualized with Quantum Yield chemiluminescent substrate (Promega, Madison, Wis.) and XAR autoradiography film (Eastman Kodak Company, New Haven, Conn.).
For analysis of DNA extracted from formalin-fixed paraffin-embedded tissues a second set of nested FIV-C gag gene-specific PCR primers and a complementary probe were designed as described above. The first-round PCgagU1 and PCgagL1 primers amplified a fragment 393 bp in length. The primer sequences were as follows: PCgagU1 (nucleotides 475 to 495), GAAAAGGCAAGAGAAGGGTT; PCgagL1 (nucleotides 867 to 847), GGCATAATCTGAAGGTCC. The second-round PCgagU2 and PCgagL2 primers amplified a fragment 144 bp in length. The primer sequences were as follows: PCgagU2 (nucleotides 671 to 692), GACCATTGCCATACTTCACTGC; PCgagL2 (nucleotides 815 to 793), CTTTTATAGCCGCCAACTTTCC. The sensitivity of the nested PCR, determined by using plasmid DNA containing the FIV-C gag gene, was found to be between 1 and 10 copies of the target sequence. A 25-mer probe (nucleotides 723 to 698; TGAGTCAGCCCTATCCCCATTATCT) was designed to recognize both first- and second-round products.
PCR protocol.
DNA was extracted from PBMC, frozen tissue samples, and formalin-fixed paraffin-embedded tissues with a QIAamp tissue kit (Qiagen, Inc., Chatsworth, Calif.). One-microgram DNA samples were amplified in duplicate by nested PCR using FIV-C-specific gag primers. Replicates with discordant results were repeated in duplicate. For DNA extracted from PBMC or frozen tissue samples the first set of nested FIV-C gag primers were used to amplify a final product of 301 bp. For both first- and second-round reactions, hot-start PCR was performed with Ampliwax PCR Gems (Perkin-Elmer Corp., Norwalk, Conn.) for 35 cycles of 94, 57, and 72°C for 30 s each. The first- and second-round reaction mixtures contained 3 mM MgCl2, 200 μM (each) deoxynucleoside triphosphate (dNTP), 1.25× Gene Amp 10× PCR buffer II (Perkin-Elmer Corp.), 2.5 U of Ampli Taq DNA polymerase (Perkin-Elmer Corp.), and 0.1 μM (each) first-round primer or 0.05 μM (each) second-round primer, respectively. For DNA extracted from formalin-fixed paraffin-embedded tissues the second set of nested FIV-C gag primers were used to amplify a final product of 144 bp. For both first- and second-round reactions, hot-start PCR was performed with Ampliwax PCR Gems (Perkin-Elmer Corp.) for 35 cycles of 94, 54, and 72°C for 30 s each. The first- and second-round reaction mixtures contained 3 mM MgCl2, 200 μM (each) dNTP, 1.25× Gene Amp 10× PCR buffer II (Perkin-Elmer Corp.), 2.5 U of Ampli Taq DNA polymerase (Perkin-Elmer Corp.), and 0.5 μM (each) first-round primer or second-round primer. Products were visualized on a 1.2% agarose gel stained with GelStar (FMC Bioproducts, Rockland, Maine). Amplimer specificity was confirmed by Southern blotting using the AP-labeled internal oligoprobe described above.
RNA in situ hybridization (ISH) with tyramide signal amplification (TSA).
Plasmids containing either the FIV-C gag or env gene were obtained from J. I. Mullins (84). The FIV-C genes were isolated via restriction enzyme digestion, 0.7% agarose gel electrophoresis, and Qiaex II gel purification (Qiagen, Inc.). The purified, isolated gene fragments (gag, 1,353 bp; envA, 1,255 bp; envB, 776 bp) were subcloned into the pGEM-7Zf(+) vector (Promega) containing T7 and SP6 transcription promoter sequences. Digoxigenin-labeled sense and antisense riboprobes were transcribed using their respective promoters and either AmpliScribe T7 or SP6 transcription kits (Epicentre Technologies Corp., Madison, Wis.).
ISH was performed in a manner similar to that described by Hirsch et al. (35). Formalin-fixed paraffin-embedded tissues were sectioned (4 to 5 μm thick) and placed on positively charged glass slides (Fischer Scientific, Pittsburgh, Pa.). Tissue sections were deparaffinized and rehydrated sequentially with xylene, xylene-ethanol, 100% ethanol, 95% ethanol, and RNase-free water for 5 min each at room temperature. The slides were then incubated with 5 mM levamisole for 20 min, washed with SSC buffer (0.15 M NaCl, 0.015 M sodium citrate), incubated in 0.2 N HCl for 20 min, and washed again with SSC buffer. The sections were digested with 20 μg of proteinase K/ml in buffer containing 10 mM Tris (pH 7.4) and 2 mM CaCl2 for 10 min at 37°C. Digestion was stopped with 0.1 M glycine in PBS. Slides were washed with PBS, and endogenous peroxidase was blocked with 5% H2O2 in methanol for 30 min. The slides were then incubated in 0.1 M triethanolamine-0.25% acetic anhydride solution for 10 min, washed in 2× SSC, incubated in 0.1 M Tris (pH 7.4)-0.1 M glycine solution for 15 min, and washed in 2× SSC. Prehybridization was done at 50°C for 10 min with hybridization solution containing 50% deionized formamide, 1× SSC, 1× Denhardt's solution, 5 mM NaPO4 (pH 6.8), 0.1% sodium dodecyl sulfate, 250 μg of salmon sperm DNA/ml, 5% dextran sulfate, and 250 μg of tRNA/ml. Riboprobes were added at 1 ng/μl, and the sections were placed onto coverslips, heated to 65°C for 5 min, chilled for 10 min on ice, and hybridized overnight at 55°C. Following hybridization, the coverslips were removed and the slides were washed with 4× SSC-50% formamide for 1 h at 50°C and then in 2× SSC for 5 min. An RNase mixture (1 unit of RNase T1 and 20 μg of RNase A/ml for 30 min at 37°C) was used to digest excess probe, and wash steps were repeated. Slides were blocked for 1 h in a buffer containing 2% horse serum, 0.3% Tween 20, 0.5% blocking agent (Boehringer Mannheim), 100 mM Tris (pH 7.4), and 150 mM NaCl. The hybridized probe was detected with a sheep anti-digoxigenin peroxidase-conjugated antibody (Boehringer Mannheim) diluted 1:100 in blocking buffer for 1 h at room temperature. The sections were washed in TNT buffer (0.1 M Tris-HCl [pH 7.5], 0.15 M NaCl, 0.05% Tween 20) three times for 5 min each. At this point, the Renaissance TSA indirect ISH kit (NEN Life Science Products, Boston, Mass.) was employed according to manufacturer's directions. Briefly, the tyramide reagent, diluted 1:50 in the diluent provided in the NEN kit, was applied to the slides for 10 min. After three washes with TNT buffer, streptavidin-AP, diluted 1:2,500 in the provided diluent, was applied for 30 min. After three more washes with TNT buffer, the sections were incubated with 5-bromo-4-chloro-3-indolylphosphate (BCIP)-nitroblue tetrazolium substrate for 2 h in the dark or with Vector red (Vector Laboratories) as described by the manufacturer. Finally, the sections were washed in 10 mM Tris-HCl-10 mM EDTA solution (pH 8.1), counterstained with methyl green, dehydrated in graded ethanols, cleared in xylene, and mounted with Clear*Mount (American Master Reagent Company Inc., Lodi, Calif.). Negative controls included tissue sections from uninfected cats and application of sense riboprobes on tissue sections from FIV-infected and uninfected cats.
RNA TSA-ISH combined with immunohistochemistry.
The TSA-ISH portion of this technique was performed as described above with addition of immunohistochemistry for cell phenotype identification. Instead of the proteinase K permeabilization, the slides were heated for 25 min at 95°C in a citrate buffer (Dimension Laboratories Inc., Mississauga, Ontario, Canada) and then cooled to room temperature in the buffer for an additional 20 min. Following the posthybridization washes and blocking steps, the primary antibody was applied. The slides were incubated for 1 h at room temperature with one of the following primary antibodies in 0.1 M Tris-HCl, pH 7.5-0.15 M NaCl: (i) a polyclonal rabbit anti-human CD3 antibody (Dako Corp., Carpinteria, Calif.) at a 1:40 dilution to identify T cells, (ii) monoclonal mouse anti-human MAC387 antibody (Dako Corp.) at a 1:100 dilution to identify macrophages, or (iii) mouse anti-human p55 actin bundling protein (49, 89) (AIDS Research and Reference Reagent Program, National Institutes of Health, Rockville, Md.) at a 1:100 dilution to identify DC. The primary phenotyping antibodies were detected with colloidal-gold-conjugated secondary antibodies goat anti-mouse immunoglobulin G (IgG)-gold (MAC387 and p55) and goat anti-rabbit IgG-gold (CD3) (Pierce, Rockford, Ill.). After detection of the FIV probe with an anti-digoxigenin peroxidase-conjugated antibody and Vector red chromogen, silver enhancement (Boehringer Mannheim) was performed to visualize the bound colloidal gold antibody. Negative controls included serial sections of tissue to which either irrelevant mouse IgG1 or rabbit IgG at a concentration matching that of the primary antibody had been applied and which were processed in parallel with experimental sections.
RESULTS
Comparison of assay sensitivities.
Mucosal and lymphoid tissues from cats inoculated via the oral-nasal route or vaginal route were examined by (i) replicate nested DNA PCR, (ii) virus isolation coculture (VI), and (iii) TSA-ISH using FIV-C-specific gag and env riboprobes (Table 1). Overall, the correlation between VI and PCR was 90%, with the sensitivity of PCR slightly greater than that of VI. In 4 of 39 instances, VI was negative while DNA PCR was positive, although, in 2 of the 4 instances, only one of three replicate PCRs yielded positive results, suggesting very low proviral load. The overall correlation of PCR and TSA-ISH was 68%. Unexpectedly, TSA-ISH was more sensitive than nested DNA PCR. In 97% of the noncorrelating samples (148 of 153), TSA-ISH revealed FIV+ cells when nested DNA PCR was negative. While the discordance could be due to the detection of input virion RNA, we considered it unlikely that the sensitivity of TSA-ISH would permit detection of non-transcriptionally active nonmulticopy viral RNA. The cell equivalents examined by TSA-ISH (104 to 105 cells per slide) and nested DNA PCR (1 μg of DNA or 1.5 × 105 cells per reaction) were approximately congruent. Each sample was examined at minimum in duplicate by both assays.
TABLE 1.
Comparison of virus detection assay sensitivities
| Assay results | No. of observations/total | Correlation (%) |
|---|---|---|
| VI+ PCR+ | 9/39 | 35/39 (90) |
| VI− PCR− | 26/39 | |
| VI+ PCR− | 0/39 | 4/39 (10) |
| VI− PCR+ | 4/39 | |
| ISH+ PCR+ | 81/482 | 329/482 (68) |
| ISH− PCR− | 248/482 | |
| ISH+ PCR− | 148/482 | 153/482 (32) |
| ISH− PCR+ | 5/482 |
Oral-nasal transmission. (i) Exposure to cell-free virus. (a) TSA-ISH.
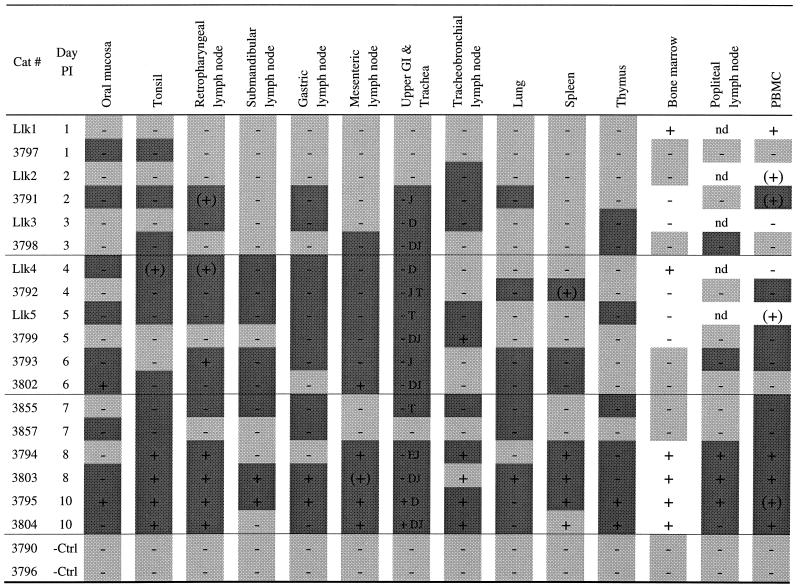
FIV-C RNA was detected in the tonsil and oral mucosa as early as day 1 p.i. and in regional draining LN (retropharyngeal, gastric, mesenteric, or tracheobronchial) by days 2 to 3 p.i. (Table 2). However, in two animals (Llk3 and 3798) viral RNA was detected in other lymphoid issues (thymus and popliteal LN) within 3 days p.i., indicating that systemic viral dissemination had already occurred. Overall, FIV RNA was most frequently detected in tonsil mucosa (13 of 18 animals examined between days 1 and 10 p.i.). The next most frequent sites were retropharyngeal LN, gastric LN, and mucosal tissues of the upper gastrointestinal tract (12 of 18 animals each) (Table 2). At all time points but one, TSA-ISH either correlated with or exceeded nested PCR (or VI) in identifying rare virus-bearing cells (Table 2).
TABLE 2.
Earliest detection by DNA PCR and TSA-ISH after oral-nasal exposure of cats to cell-free FIV-Ca
−Ctrl, negative control; GI, gastrointestinal tract; nd, PCR not done; +, PCR positive; −, PCR negative; (+), PCR+ only once; dark shading, ISH+; light shading, ISH−; no shading, ISH not done; E, esophagus; D, duodenum; J, jejunum; T, trachea.
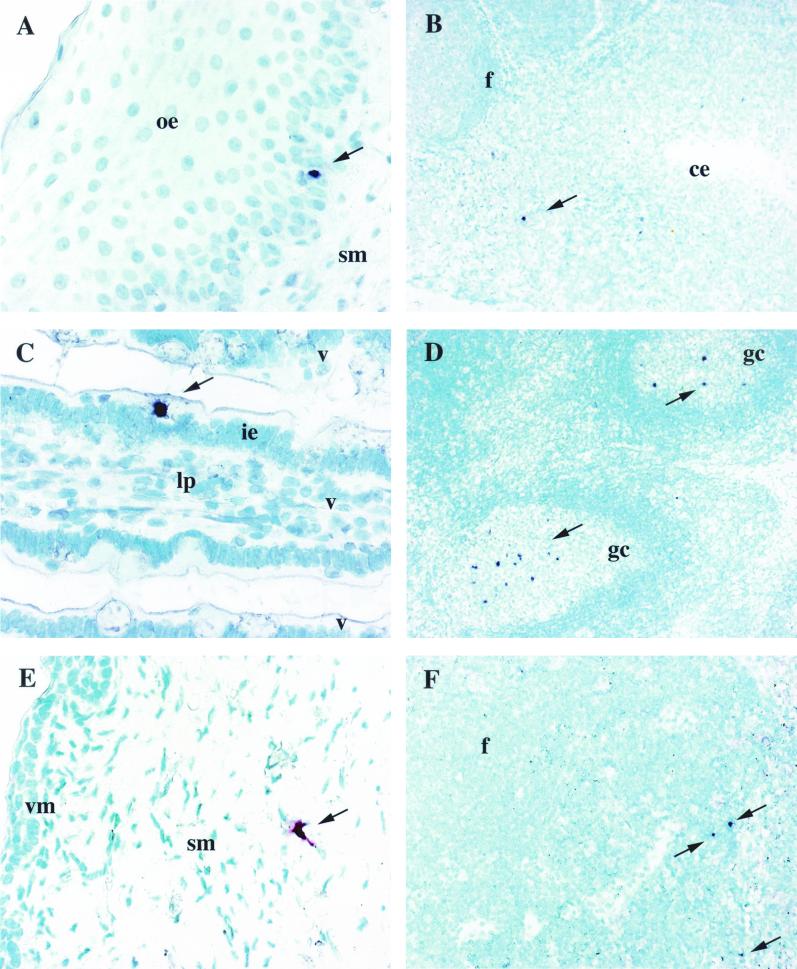
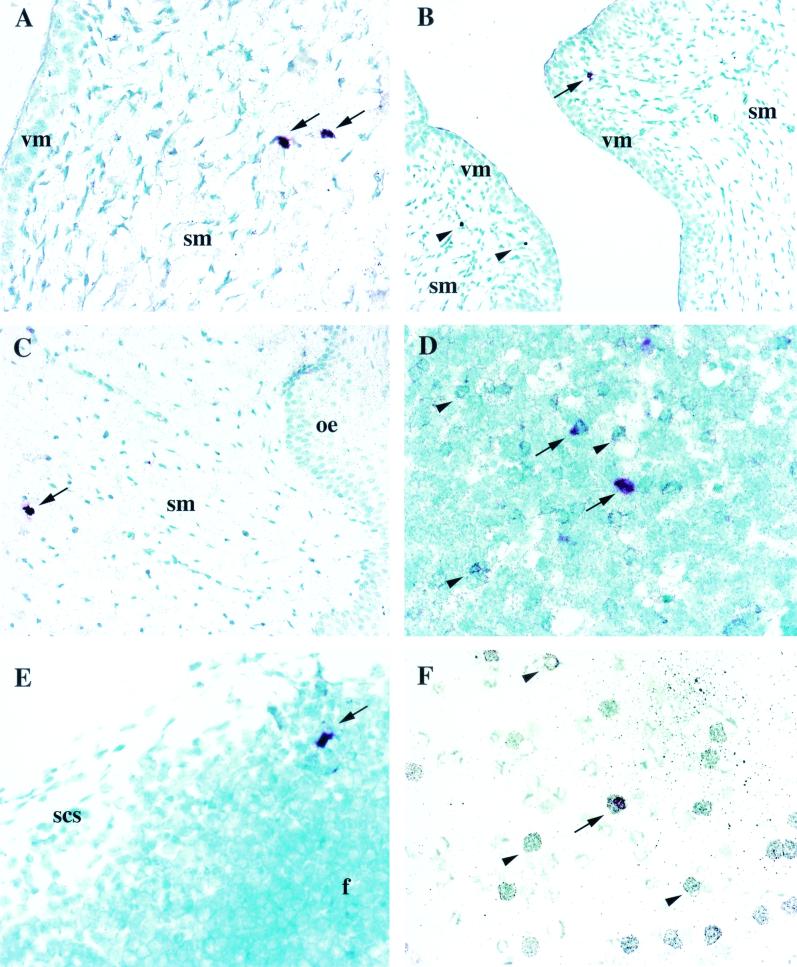
FIV-bearing cells were solitary cells with either stellate/dendritic or small round morphology within the epithelial and subepithelial mucosae (Fig. 1A). In the tonsil, FIV-positive cells were usually individual and in the interfollicular regions and on occasion were subjacent to the crypt lymphoepithelium (Fig. 1B). In the upper gastrointestinal tract isolated FIV RNA-bearing cells were detected within the intestinal epithelium (Fig. 1C), lamina propria, and/or submucosa. In LN, single FIV RNA+ cells or clusters of two to five FIV RNA+ cells were within follicle germinal centers and less commonly in interfollicular areas (Fig. 1D). In more than one-half of the instances in which the contralateral LN also was available for examination (e.g., mandibular and pharyngeal LN), the paired node was negative for FIV RNA+ cells, emphasizing the sparse distribution of infected cells in the first week postexposure.
FIG. 1.
RNA ISH utilizing either Vector red or BCIP-nitroblue tetrazolium chromogen and methyl green counterstain. (A) FIV+ cell (arrow) in the oral epithelium (oe) of cat 3797 1 day p.i. Magnification, ×400. (B) FIV+ cell (arrow) in the tonsillar parenchyma adjacent to the crypt epithelium (ce) of cat 3791 2 days p.i. Magnification, ×100. (C) FIV+ cell (arrow) in the intestinal epithelium (ie) of a villus (v) from the jejunum of cat 3791 2 days p.i. Magnification, ×400. lp, lamina propria. (D) Numerous FIV+ cells (arrows) in the germinal center (gc) of a follicle (f) from the retropharyngeal LN from cat 3795 10 days p.i. Magnification, 100. (E) FIV+ cell (arrow) with DC morphology in the vaginal submucosa (sm) of cat 3598 2 days p.i. Magnification, ×400. vm, vaginal mucosa. (F) Several FIV+ cells (arrows) in the parafollicular region of the medial iliac LN from cat 3853 4 days p.i. Magnification, ×200.
(b) DNA PCR.
In 14 animals assayed between days 1 and 7 p.i., FIV-C DNA was first detected by PCR in either blood (n = 4), retropharyngeal LN (n = 3), bone marrow (n = 2), or tonsil, spleen, tracheobronchial LN, mesenteric LN, or oral mucosa (n = 1 each) of individual animals (Table 2). Despite multiple solution phase DNA PCR assays on multiple regional and systemic lymphoid tissues, FIV was detectable only sporadically before day 8 p.i. In 9 of 275 tissue samples, FIV DNA was detected only in one of multiple PCRs, connoting a very low proviral level.
(ii) Exposure to cell-associated virus. (a) TSA-ISH.
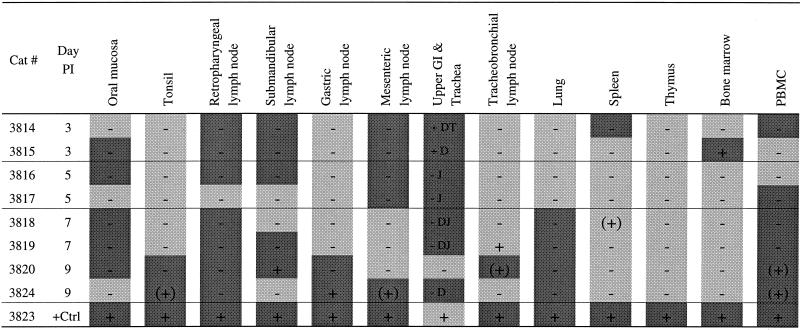
FIV-C RNA-bearing cells were detected by 3 days p.i. in the oral mucosa; retropharyngeal, popliteal, submandibular, and mesenteric LN; upper gastrointestinal tract; trachea; spleen; bone marrow; PBMC; and thymus; this suggests early systemic viral dissemination. Overall, viral RNA was most frequently detected in the small intestine and retropharyngeal LN (seven of eight animals examined between days 3 and 9 p.i.). The oral mucosa and PBMC contained the next-highest number of RNA-bearing cells (six of eight animals). For all assays except two, TSA-ISH either correlated with or exceeded nested DNA PCR in identifying rare virus-bearing cells. In 37 cases, TSA-ISH was positive when PCR was negative (Table 3).
TABLE 3.
Earliest detection by DNA PCR and TSA-ISH after oral-nasal exposure of cats to PBMC from an FIV-C-infected donor cata
+Ctrl, positive control; GI, gastrointestinal tract; +, PCR positive; −, PCR negative; (+), PCR+ only once; dark shading, ISH+; light shading, ISH−; D, duodenum; J, jejunum; T, trachea.
As with cell-free infection, FIV-C mRNA+ cells were localized to follicles and less often interfollicular areas adjacent to the subcapsular sinus in the retropharyngeal LN. In the tonsils virus-positive cells were in follicles and beneath the crypt lymphoepithelium (Fig. 1B). Thus the localization of early FIV-infected cells in cell-free viral inocula did not differ from that in cell-associated viral inocula (data not shown).
(b) DNA PCR.
FIV-C DNA was detected sporadically in the duodenum, trachea, tracheobronchial LN, spleen, or bone marrow in four of six cats examined between days 3 and 7 p.i. (Table 3). By day 9, viral DNA was detected in local and/or regional lymphoid tissues and PBMC in each of two cats examined. In 6 of 12 PCR+ tissue samples, FIV DNA was detected once (Table 3) in three or more replicate PCRs, indicating DNA levels at the margin of assay sensitivity.
(iii) Tissue tropism after oral-nasal infection.
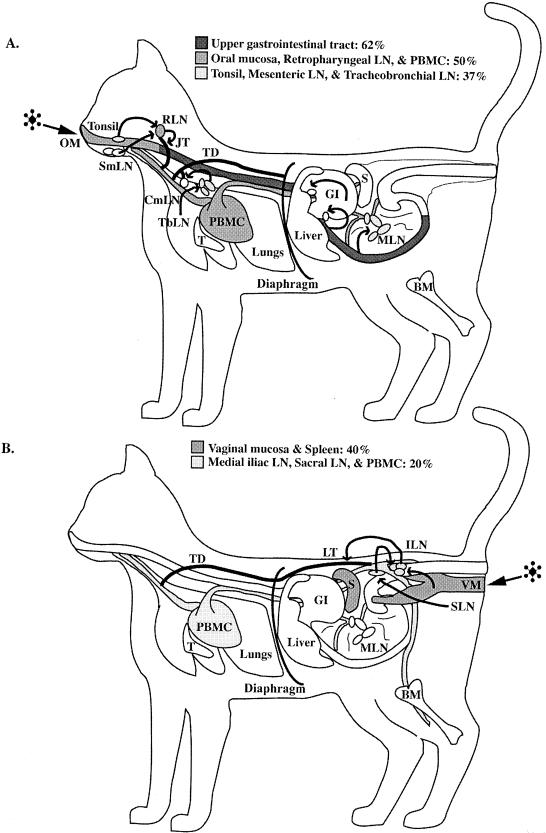
In 88% of animals (n = 23), virus-infected cells were detected in mucosal tissues and lymphoid tissues concurrently (Tables 2 and 3). Only in an animal sacrificed at 1 day p.i. (3797; Table 2) were virus-infected cells limited to the oral mucosa and tonsil. In all but two animals (Llk1 and Llk2; Table 2), when virus was detectable in at least one lymphoid tissue, virus-infected cells also were detected in mucosal tissues. FIV-positive cells were most frequently identified in the upper gastrointestinal tract mucosa (62%) or oral mucosa, retropharyngeal LN, or, surprisingly, PBMC (each 50% of animals) (Fig. 2A and Table 4). Between 4 and 6 days p.i., the mesenteric LN was the most common FIV-positive tissue (100% of animals) (Table 4), followed by retropharyngeal and submandibular LN. By 7 to 10 days, in addition to being present in lymphoid tissues, FIV-infected cells were present in PBMC in all animals and in the lungs of 90% of animals (Table 4). Thus rapid dissemination of virus and virus-infected cells from mucosal entry sites to regional and then systemic lymphoid tissues occurred after oral-nasal FIV exposure.
FIG. 2.
(A) Illustration demonstrating target tissue infection frequency between 1 and 3 days after oral-nasal FIV exposure as determined by DNA PCR and RNA ISH. FIV was most frequently identified in tissues of the upper gastrointestinal tract (GI) (62% of the animals). The next most frequently observed FIV-infected tissues were oral mucosa (OM), retropharyngeal LN (RLN), and PBMC (50% of the animals). (B) Illustration demonstrating target tissue infection frequency between 1 and 3 days after vaginal FIV exposure as determined by DNA PCR and RNA ISH. FIV was most frequently identified in tissues of the vaginal mucosa (VM) and spleen (S) (40% of the animals). The next most frequently observed FIV-infected tissues were medial iliac LN (ILN), sacral LN (SLN), and PBMC (20% of the animals). Arrows (both panels), flow of lymphatic drainage from the mucosal tissues to regional lymphoid tissues to lymphatic trunks and/or ducts, which ultimately empty into the venous circulation (PBMC). BM, bone marrow; CmLN, cranial mediastinal LN; JT, jugular trunk; LT, lumbar trunk; SmLN, submandibular LN; T, thymus; TbLN, tracheobronchial LN; TD, thoracic duct.
TABLE 4.
Frequency of FIV+ tissues 1 to 10 days after oral-nasal exposure of cats to virus
| Tissue | Frequency of FIV+ tissues (%a) at days:
|
||
|---|---|---|---|
| 1-3 | 4-6 | 7-10 | |
| Oral mucosa | 4/8 (50) | 5/8 (62) | 7/10 (70) |
| Tonsil | 3/8 (37) | 4/8 (50) | 8/10 (80) |
| Retropharyngeal LN | 4/8 (50) | 6/8 (75) | 9/10 (90) |
| Submandibular LN | 2/8 (25) | 6/8 (75) | 5/10 (50) |
| Gastric LN | 2/8 (25) | 5/8 (62) | 7/10 (70) |
| Mesenteric LN | 3/8 (37) | 8/8 (100) | 5/10 (50) |
| Upper gastrointestinal tract | 5/8 (62) | 7/8 (87) | 7/10 (70) |
| Tracheobronchial LN | 3/8 (37) | 2/8 (25) | 7/10 (70) |
| Lung | 1/8 (12) | 3/8 (37) | 9/10 (90) |
| Spleen | 1/8 (12) | 3/8 (37) | 5/10 (50) |
| Thymus | 2/8 (25) | 1/8 (12) | 5/10 (50) |
| Bone marrow | 2/8 (25) | 1/8 (12) | 4/10 (40) |
| Popliteal LN | 1/6 (17) | 1/4 (25) | 4/6 (67) |
| PBMC | 4/8 (50) | 5/8 (62) | 10/10 (100) |
Percentages in boldface indicate tissues most frequently FIV+.
Vaginal transmission. (i) TSA-ISH.
FIV RNA was detected in cells of either large (dendritic/cell-like) or small round (lymphocyte-like) morphology in the vaginal mucosa as early as 1 and 2 days p.i. (Fig. 1E) and in the medial iliac LN as early as days 3 and 4 (Fig. 1F). However, virus-bearing cells commonly were not identified in vaginal tissue from the same animals with positive draining LN, connoting the relative rarity of virus-positive cells in the mucosa. In addition, viral dissemination to systemic sites (spleen, PBMC) occurred early (days 2 to 3). As in animals with oral-nasal infection, in 27 instances TSA-ISH proved more sensitive in revealing rare virus-positive cells. FIV+ cells were most often in the subepithelial region (Fig. 1E) and less commonly in either intraepithelial or mucosa-associated lymphoid tissue. In LN, virus-bearing cells were in follicles and/or adjacent to the subcapsular sinus (Fig. 1F).
(ii) DNA PCR.
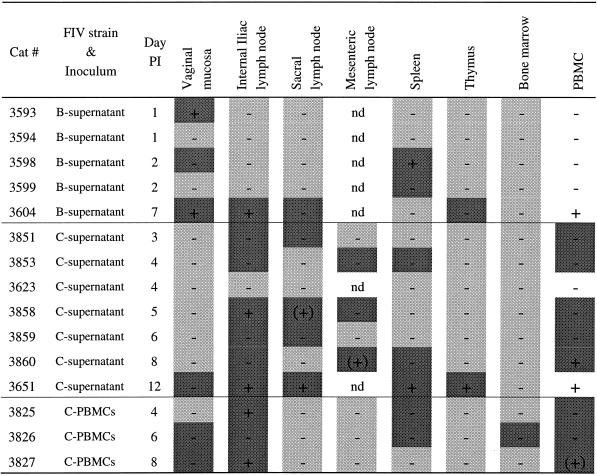
FIV DNA was first detected in vaginal mucosa and spleen of two of four cats between days 1 and 2 p.i. (Table 5). Viral DNA was detected in the medial iliac LN (which drains the vaginal vault) from three of seven cats between days 4 and 8 but was not detected in vaginal tissues of any of these animals (Table 5).
TABLE 5.
Detection of FIV in tissues after vaginal exposure to different FIV inoculaa
nd, not done; +, PCR positive; −, PCR negative; (+), PCR+ only once; dark shading, ISH+; light shading, ISH−; no shading, ISH not done.
(iii) Tissue tropism after vaginal FIV infection.
Between days 1 and 3 p.i. FIV was most frequently identified (40% of animals) in the vaginal mucosa and, surprisingly, spleen (Table 6 and Fig. 2B). Between 4 and 6 days p.i., the medial iliac LN and PBMC were the tissues most frequently containing FIV-infected cells (83% of animals) (Table 6). However, systemic spread of virus infection was evident as well, e.g., the spleen was virus positive in 50% of animals. In 54% of the animals (n = 7) virus-infected cells were detectable only in regional LN and not in the vagina. In five cats (38%) examined between 2 and 12 days p.i. FIV-positive cells were present in lymphoid tissues and PBMC in addition to the vaginal mucosa (Table 5). By 7 to 12 days p.i., PBMC and medial iliac LN infections were observed for 100% of the animals. In only one animal (examined at day 1 p.i.) was virus detectable only in the vagina (Table 5).
TABLE 6.
Frequency of FIV+ tissues 1 to 12 days after vaginal exposure of cats to virus
| Tissue | Frequency of FIV+ tissues (%a) at days:
|
||
|---|---|---|---|
| 1-3 | 4-6 | 7-12 | |
| Vaginal mucosa | 2/5 (40) | 1/6 (17) | 3/4 (75) |
| Medial iliac LN | 1/5 (20) | 5/6 (83) | 4/4 (100) |
| Sacral LN | 1/5 (20) | 2/6 (33) | 2/4 (50) |
| Mesenteric LN | 0/1 (0) | 2/5 (40) | 1/2 (50) |
| Spleen | 2/5 (40) | 3/6 (50) | 2/4 (50) |
| Thymus | 0/5 (0) | 0/6 (0) | 2/4 (50) |
| Bone marrow | 0/5 (0) | 1/6 (17) | 0/4 (0) |
| PBMC | 1/5 (20) | 5/6 (83) | 4/4 (100) |
Percentages in boldface indicate tissues most frequently FIV+.
Phenotype of FIV+ cells.
To maximize the feasibility of colabeling FIV target cells, only tissues with the highest frequency of virus-positive cells, as determined by ISH-TSA results (Tables 2, 3, and 5) were selected for phenotyping (Table 7). Three cell markers for which antibodies useful in formalin-fixed tissues were available were used: anti-CD3, anti-p55/fascin, and anti-MAC387, which label T cells, DC, and macrophages, respectively. FIV+ p55+ DC were found in the subepithelial vaginal or oral mucosa (Fig. 3A and C) when no CD3+ FIV+ or MAC387+ FIV+ cells could be identified. FIV+ CD3+ T cells were detected in the tonsil (Fig. 3D). An FIV+ MAC387+ cell (macrophage) was identified in the retropharyngeal LN of a cat 3 days p.i. (Fig. 3E). FIV+ CD3+ T cells were detected in the PBMC of one cat (at 10 days p.i.) (Fig. 3F). There were also FIV+ cells, usually of small round cell morphology, that were not labeled with any of the phenotype markers examined. Thus, FIV+ cells had phenotype markers consistent with a DC, T-cell, and, in one instance, macrophage lineage.
TABLE 7.
Phenotypic identification of early mucosal FIV+ target cells
| Cat | Day p.i. | Tissue | Presence of cells with phenotype:
|
||
|---|---|---|---|---|---|
| CD3+ ISH+ | p55+ ISH+ | MAC387+ ISH+ | |||
| 3797 | 1 | Oral mucosa | − | − | NDa |
| Tonsil | − | − | − | ||
| 3815 | 3 | Tonsil | − | − | − |
| Retropharyngeal LN | − | − | + | ||
| 3795 | 10 | Oral mucosa | − | − | ND |
| Tonsil | + | − | − | ||
| 3804 | 10 | Oral mucosa | − | + | − |
| PBMC | + | ND | ND | ||
| 3826 | 6 | Vaginal mucosa | − | + | − |
| Medial iliac LN | − | − | − | ||
ND, not done.
FIG. 3.
Dual RNA ISH and immunohistochemistry utilizing Vector red for FIV RNA probe detection, silver-enhanced immunogold for phenotypic identification, and methyl green counterstain. (A) p55+ FIV+ DC (arrows) in the vaginal submucosa (sm) of cat 3826 6 days p.i. Magnification, ×400. vm, vaginal mucosa. (B) FIV+ cell (arrow) and two FIV-uninfected MAC387+ macrophages (arrowheads) in the vaginal submucosa of cat 3826 6 days p.i. Magnification, ×200. (C) p55+ FIV+ cell (arrow) in the oral submucosa of cat 3804 10 days p.i. Magnification, ×200. oe, oral epithelium. (D) CD3+ FIV+ T cells (arrows) and several FIV-uninfected CD3+ cells (arrowheads) in the tonsillar parenchyma of cat 3795 10 days p.i. Magnification, ×400. (E) MAC387+ FIV+ macrophage (arrow) in a follicle (f) adjacent to the subcapsular sinus (scs) region of the retropharyngeal LN from cat 3815 3 days p.i. Magnification, ×400. (F) CD3+ FIV+ T cell (arrow) and several FIV-uninfected CD3+ T cells (arrowheads) in the PBMC of cat 3804 10 days p.i. Magnification, ×400.
DISCUSSION
The early pathogenesis of mucosal FIV infection appears consistent with what is known regarding the early aspects of SIV and HIV infections. In 37 out of 41 (90%) cats studied, virus-infected cells were demonstrable in the mucosa and/or regional LN between 1 and 12 days after oral-nasal inoculation. Between 1 and 3 days after oral-nasal exposure, virus-infected cells were detectable in mucosal tissues of 39% (5 of 13) of animals and in regional (pharyngeal, gastric, mesenteric, and tracheobronchial) LN in 62%. The FIV-infected cells were in the oral mucosa, tonsil, and small intestine. Likewise, after vaginal inoculation, FIV-infected cells were identified in the epithelial and subepithelial regions of the mucosa and/or in the mucosal lymphoid nodules by 1 to 2 days p.i. and in the regional LN (medial iliac and sacral) by 3 days p.i.
The early FIV-bearing cells were either large stellate or small round in morphology. Of the cells in which colabeling of phenotype and virus was successful, the larger stellate cells labeled for DC marker p55 and the smaller round cells labeled for CD3, the T-cell receptor-associated antigen. In studies in which HIV-infected cells were detected at the lymphoepithelial interface of the tonsil and adenoids, most of the virus-bearing cells were colabeled for viral RNA and S100, a DC marker (29, 68). In buccal biopsy samples from HIV-infected individuals, provirus was identified in lymphocytes, LC, and epithelial cells (66). As early as 3 days after application of SIV to macaque tonsillar mucosa, rare SIV-infected CD4+ T cells abutting the antigen-transporting epithelium of the tonsillar crypts were identified (76). Thus, the detection of FIV-positive cells beneath the tonsillar lymphoepithelium after oral exposure to virus is consistent with findings in HIV and SIV infection and suggests that the tonsillar mucosa is one portal of entry.
Several scenarios for lentivirus mucosal infection have been postulated, i.e., mucosal membrane lesion (59), M-cell translocation (2), epithelial cell transcytosis (9), epithelial cell endocytosis (10), epithelial cell infection (1, 8, 34, 39, 41, 51), and LC/DC infection (37; T. Lehner, L. Hussain, J. Wilson, and M. Chapman, Letter, Nature 353:709, 1991). Studies with macaques support the concept that DC are an initial cell infected by SIV in the vaginal mucosa but have also shown that CD4+ T cells in the subepithelial tonsillar or vaginal mucosa are infected very early postexposure (37, 44, 75, 76). Frankel et al. (30) demonstrated CD3+ T cells in contact with multinucleated syncytia coexpressing S100 and p55 (DC markers) and HIV-1 Gag at the mucosal surface of adenoids from HIV-infected patients. FIV infection of mucosal DC has not yet been shown, although FIV RNA and antigen have been found in association with lymphoid follicular DC (5, 38, 80), as has been observed in HIV-infected people (19, 28, 78). Taken together the above observations support the concept that mucosal DC may vector HIV to T cells during primary infection.
Our findings after vaginal inoculation of FIV are consistent with those of Spira et al. (75), who reported that the first SIV-infected cells were in the vaginal mucosa and were morphologically compatible with DC. Within 2 days, virus was identified in the draining internal iliac LN, and by 5 days systemic dissemination was detected by PBMC coculture (37, 75). The time between mucosal exposure to HIV and initial viremia is estimated to be 4 to 11 days, an interval consistent with experimental studies in the FIV and SIV systems (53). Two days following oral-nasal exposure to cell-free virus, FIV-bearing cells were detected in parafollicular zones and germinal centers of draining LN. Similarly, after intrarectal SIV inoculation, virus-infected cells were identified in the germinal centers of the paracolic LN draining the rectum (14). Transit time of lentiviruses or virus-infected cells from mucosa to initial sites of replication may be even more rapid. Within 24 h following vaginal inoculation of mice with murine DC pulsed with heat-inactivated HIV-1, virus was detected in the iliac and sacral LN by RNA PCR (43).
By both the oral-nasal and vaginal mucosal routes, cell-free FIV was more efficient than was cell-associated virus in establishing infection. This is consistent with the observations in the SIV system in which neonatal and adult rhesus macaques became infected after oral exposure to relatively low titers of cell-free virus (3, 4). However, the cell-associated FIV inoculum (2 × 107 total PBMC from an FIV-infected donor containing 105 infectious PBMC) likely represented a lower virus challenge than did the cell-free inoculum. In another study, two oral inoculations of 2 × 106 and 2 × 107 in vitro infected cells were used to achieve oral transmission of FIV (47).
After mucosal FIV exposure, we found no evidence of epithelial cell infection or transcytosis, as has been described on the basis of in vitro HIV-1 models (1, 9, 10, 21, 22, 48, 62, 77, 88). In contrast to the epithelial cell line studies and in accordance with the present study, in vitro HIV infection studies using cultures of fetal intestine have shown productive infection only in lamina propria mononuclear cells and not in epithelial cells (25). Similarly, most studies of intestinal biopsy samples from HIV-infected patients reveal HIV-infected cells predominantly in the lamina propria and rarely in the epithelial layer (13, 18, 27, 39, 40). However, we detected nonepithelial FIV-bearing cells within the vaginal and oral surface epithelium. In addition, in a few orally exposed cats, virus was detected in gastric and/or mesenteric LN prior to systemic infection, suggesting that FIV can traverse the gastric and/or intestinal mucosa. These findings support the tenet that lentiviruses traverse mucosal barriers via mechanisms not dependent on epithelial infection. Also, vaginal FIV transmission occurred after exposure to either cell-free or cell-associated inocula, which supports the plausibility of multiple pathways for transmucosal lentivirus transit. While cell-free and cell-associated virus may cross mucosal barriers via different pathways, it is clear that FIV can establish systemic infection within days via either route.
We found sparse evidence for early macrophage infection in mucosal FIV infection. However, several lines of circumstantial evidence suggest that an important property of mucosally transmitted virus is the ability to infect macrophages (26, 50, 67, 71, 82). HIV RNA and DNA have been demonstrated in macrophages at the mucosal-stromal junction of the endocervical transformation zone, but virus-positive macrophages were not detected in the vagina or endometrium (54). In the first SIV mucosal challenge experiments, infected cells in the genital tracts of chronically infected macaques were primarily macrophages located in the submucosa of the cervix and vagina (45, 46). Several studies, however, have failed to find evidence for sexual transmission-mediated selection of macrophage-tropic, non-syncytium-inducing HIV strains (24, 52, 69, 91). Hladik et al. (36) demonstrated that mononuclear cells infiltrating the cervicovaginal tissues are permissive for both R5- and X4-tropic HIV-1 variants and that selection of virus variants does not occur by differential expression of HIV-1 coreceptors on genital mononuclear cells. Similarly, in SIV vaginal-transmission studies, the infectivity of the virus in vivo was not correlated with macrophage versus T-cell tropism in vitro (20, 44).
Clinical and laboratory FIV isolates appear to use X4 as a major receptor for cell entry (the feline and human X4 molecules have 94.9% amino acid homology) (64, 87). While chemokine receptors are expressed primarily in hematopoietic cells, many other cell types have been shown to express one or more chemokine receptors, including microglia (31, 73), endothelial cells (33, 83), placental macrophages (23), and colonic, rectal, cervical, and vaginal epithelia (90), making FIV entry into these multiple cell types plausible. The work of Willett and Hosie (86), however, indicates that a second receptor, or equivalent obligate molecule, likely is needed to effect cell infection by many FIV clinical isolates. In this regard, the recent findings of de Parseval and Elder (15) suggest that the heparan receptor and perhaps another molecule can be used by some FIV isolates for cell entry.
In summary, we demonstrate virus-infected cells in mucosae and regional lymph nodes within 1 to 2 days after oral or vaginal exposure of cats to virus. These early FIV-bearing cells were in the tonsillar, vaginal, and intestinal mucosa and were most consistent with DC and T cells. We did not detect infection of epithelial cells. The results contribute to the foundation for further studies of FIV mucosal cell virus receptors, transepithelial passage, and the role of DC and other cells in the transmucosal passage of this and other lentiviruses.
Acknowledgments
We thank Richard Reyes for assistance with riboprobe generation and James Mullins for the FIV-C-PGammer plasmids.
This research was funded by grants K08-A1-01403, RO1-HD-34338, and R01-A1-33773 from DAIDS, NIAID, NIH.
REFERENCES
- 1.Adachi, A., S. Koenig, H. E. Gendelman, D. Daugherty, S. Gattoni-Celli, A. S. Fauci, and M. A. Martin. 1987. Productive, persistent infection of human colorectal cell lines with human immunodeficiency virus. J. Virol. 61:209-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amerongen, H. M., R. Weltzin, C. M. Farnet, P. Michetti, W. A. Haseltine, and M. R. Neutra. 1991. Transepithelial transport of HIV-1 by intestinal M cells: a mechanism for transmission of AIDS. J. Acquir. Immune Defic. Syndr. 4:760-765. [PubMed] [Google Scholar]
- 3.Ayehunie, S., R. W. Groves, A. M. Bruzzese, R. M. Ruprecht, T. S. Kupper, and E. Langhoff. 1995. Acutely infected Langerhans cells are more efficient than T cells in disseminating HIV type 1 to activated T cells following a short cell-cell contact. AIDS Res. Hum. Retrovir. 11:877-884. [DOI] [PubMed] [Google Scholar]
- 4.Baba, T. W., A. M. Trichel, L. An, V. Liska, L. N. Martin, M. Murphey-Corb, and R. M. Ruprecht. 1996. Infection and AIDS in adult macaques after nontraumatic oral exposure to cell-free SIV. Science 272:1486-1489. [DOI] [PubMed] [Google Scholar]
- 5.Bach, J. M., M. Hurtrel, L. Chakrabarti, J. P. Ganiere, L. Montagnier, and B. Hurtrel. 1994. Early stages of feline immunodeficiency virus infection in lymph nodes and spleen. AIDS Res. Hum. Retrovir. 10:1731-1738. [DOI] [PubMed] [Google Scholar]
- 6.Barlough, J. E., C. D. Ackley, J. W. George, N. Levy, R. Acevedo, P. F. Moore, B. A. Rideout, M. D. Cooper, and N. C. Pedersen. 1991. Acquired immune dysfunction in cats with experimentally induced feline immunodeficiency virus infection: comparison of short-term and long-term infections. J. Acquir. Immune Defic. Syndr. 4:219-227. [PubMed] [Google Scholar]
- 7.Beebe, A. M., T. G. Faith, E. E. Sparger, M. Torten, N. C. Pedersen, and S. Dandekar. 1994. Evaluation of in vivo and in vitro interactions of feline immunodeficiency virus and feline leukemia virus. AIDS 8:873-878. [DOI] [PubMed] [Google Scholar]
- 8.Bigornia, E., D. Simon, L. M. Weiss, J. Jones, H. Tanowitz, M. Wittner, and W. Lyman. 1992. Detection of HIV-1 protein and nucleic acid in enterochromaffin cells of HIV-1-seropositive patients. Am. J. Gastroenterol. 87:1624-1628. [PubMed] [Google Scholar]
- 9.Bomsel, M. 1997. Transcytosis of infectious human immunodeficiency virus across a tight human epithelial cell line barrier. Nat. Med. 3:42-47. [DOI] [PubMed] [Google Scholar]
- 10.Bourinbaiar, A. S., and D. M. Phillips. 1991. Transmission of human immunodeficiency virus from monocytes to epithelia. J. Acquir. Immune Defic. Syndr. 4:56-63. [PubMed] [Google Scholar]
- 11.Callanan, J. J., M. J. Hosie, and O. Jarrett. 1991. Transmission of feline immunodeficiency virus from mother to kitten. Vet. Rec. 128:332-333. [DOI] [PubMed] [Google Scholar]
- 12.Cameron, P. U., M. Pope, S. Gezelter, and R. M. Steinman. 1994. Infection and apoptotic cell death of CD4 T cells during an immune response to HIV-1-pulsed dendritic cells. AIDS Res. Hum. Retrovir. 10:61-71. [DOI] [PubMed] [Google Scholar]
- 13.Clayton, F., S. Reka, W. J. Gronin, E. Torlakovic, S. H. Sigal, and D. P. Kolter. 1992. Rectal mucosal pathology varies with human immunodeficiency virus antigen content and disease stage. Gastroenterology 103:919-933. [DOI] [PubMed] [Google Scholar]
- 14.Coudel-Courteille, A., C. Butor, V. Juillard, J. G. Guillet, and A. Venet. 1999. Dissemination of SIV after rectal infection preferentially involves paracolic germinal centers. Virology 260:277-294. [DOI] [PubMed] [Google Scholar]
- 15.de Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Diehl, L. J., C. K. Mathiason-Dubard, L. L. O'Neil, L. A. Obert, and E. A. Hoover. 1995. Induction of accelerated feline immunodeficiency virus disease by acute-phase virus passage. J. Virol. 69:6149-6157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreitz, M. J., S. W. Dow, S. A. Fiscus, and E. A. Hoover. 1995. Development of monoclonal antibodies and capture immunoassays for feline immunodeficiency virus. Am. J. Vet. Res. 56:764-768. [PubMed] [Google Scholar]
- 18.Ehrenpreis, E. D., B. K. Patterson, J. A. Brainer, H. Yokoo, A. W. Rademaker, W. Glogowski, G. A. Noskin, and R. M. Craig. 1992. Histopathologic findings of duodenal biopsy specimens in HIV-infected patients with and without diarrhea and malabsorption. Am. J. Clin. Pathol. 97:21-28. [DOI] [PubMed] [Google Scholar]
- 19.Embretson, J., M. Zupancic, J. L. Ribas, A. Burke, P. Racz, K. Tenner-Racz, and A. T. Haase. 1993. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature 362:359-362. [DOI] [PubMed] [Google Scholar]
- 20.Enose, Y., K. Ibuki, K. Tamaru, M. Ui, T. Kuwata, T. Shimada, and M. Hayami. 1999. Replication capacity of simian immunodeficiency virus in cultured macaque macrophages and dendritic cells is not prerequisite for intravaginal transmission of the virus in macaques. J. Gen. Virol. 80:847-855. [DOI] [PubMed] [Google Scholar]
- 21.Fantini, J., N. Yahi, C. Tourres, O. Delezay, and C. Tamalet. 1997. HIV-1 transmission across the vaginal epithelium. AIDS 11:1663-1664. [PubMed] [Google Scholar]
- 22.Fantini, J., N. Yahi, and J. C. Chermann. 1991. Human immunodeficiency virus can infect the apical and basolateral surfaces of human colonic epithelial cells. Proc. Natl. Acad. Sci. USA 88:9297-9301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fear, W. R., A. M. Kesson, H. Naif, G. W. Lynch, and A. T. Cunningham. 1998. Differential tropism and chemokine receptor expression of human immunodeficiency virus type 1 in neonatal monocytes, monocyte-derived macrophages, and placental macrophages. J. Virol. 72:1334-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fiore, J. R., A. Bjorndal, K. A. Peipke, et al. 1994. The biologic phenotype of HIV-1 is usually retained during and after sexual transmission. Virology 204:297-303. [DOI] [PubMed] [Google Scholar]
- 25.Fleming, S. C., M. S. Kapembwa, T. T. MacDonald, and G. E. Griffin. 1992. Direct in vitro infection of human intestine with HIV-1. AIDS 6:1099-1104. [DOI] [PubMed] [Google Scholar]
- 26.Fouchier, R. A. M., M. Brouwer, N. A. Kootstra, H. G. Huisman, and H. Schuitemaker. 1994. HIV-1 macrophage tropism is determined at multiple levels of the viral replication cycle. J. Clin. Investig. 94:1806-1814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fox, C. H., D. Kotler, A. Tierney, C. S. Wilson, and A. S. Fauci. 1989. Detection of HIV-1 RNA in the lamina propria of patients with AIDS and gastrointestinal disease. J. Infect. Dis. 159:467-471. [DOI] [PubMed] [Google Scholar]
- 28.Fox, C. H., K. Tenner-Racz, P. Racz, A. Firpo, P. A. Pizzo, and A. S. Fauci. 1991. Lymphoid germinal centers are reservoirs of human immunodeficiency virus type 1 RNA. J. Infect. Dis. 164:1051-1057. [DOI] [PubMed] [Google Scholar]
- 29.Frankel, S. S., K. Tenner-Racz, P. Racz, B. M. Wenig, C. H. Hansen, D. Heffner, A. M. Nelson, M. Pope, and R. M. Steinman. 1997. Active replication of HIV-1 at the lymphoepithelial surface of the tonsil. Am. J. Pathol. 151:89-96. [PMC free article] [PubMed] [Google Scholar]
- 30.Frankel, S. S., B. M. Wenig, A. P. Burke, P. Mannan, L. D. R. Thompson, S. L. Abbondanzo, A. M. Nelson, M. Pope, and R. M. Steinman. 1996. Replication of HIV-1 in dendritic cell-derived syncytia at the mucosal surface of the adenoid. Science 272:115-117. [DOI] [PubMed] [Google Scholar]
- 31.Girolomoni, G., M. T. Valle, V. Zacchi, M. G. Costa, A. Giannetti, and F. Manca. 1996. Cultured human Langerhans’ cells are superior to fresh cells at presenting native HIV-1 protein antigens to specific CD4+ T-cell lines. Immunology 87:310-316. [PMC free article] [PubMed] [Google Scholar]
- 32.Greenhead, P., P. Hayes, P. S. Watts, K. G. Laing, G. E. Griffin, and R. J. Shattock. 2000. Parameters of human immunodeficiency virus infection of human cervical tissue and inhibition by vaginal virucides. J. Virol. 74:5577-5586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gupta, S. K., P. G. Lysko, K. Pillarisetti, E. Ohlstein, and J. M. Stadel. 1998. Chemokine receptors in human endothelial cells. J. Biol. Chem. 273:4282-4287. [DOI] [PubMed] [Google Scholar]
- 34.Heise, C., S. Dandekar, P. Kumar, R. Duplantier, R. M. Donovan, and C. H. Halsted. 1991. Human immunodeficiency virus infection of enterocytes and mononuclear cells in human jejunal mucosa. Gastroenterology 100:1521-1537. [DOI] [PubMed] [Google Scholar]
- 35.Hirsch, V. M., G. Dapolito, P. R. Johnson, W. R. Elkins, W. T. London, R. J. Montali, S. Goldstein, and C. Brown. 1995. Induction of AIDS by simian immunodeficiency virus from an African green monkey: species-specific variation in pathogenicity correlates with the extent of in vivo replication. J. Virol. 69:955-967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hladik, F., G. Lentz, R. E. Akridge, G. Peterson, H. Kelley, A. McElroy, and M. J. McElrath. 1999. Dendritic cell-T-cell interactions support coreceptor-independent human immunodeficiency virus type 1 transmission in the human genital tract. J. Virol. 73:5833-5842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hu, J., M. B. Gardener, and C. J. Miller. 2000. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. J. Virol. 74:6087-6095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hurtrel, B., L. Chakrabarti, M. Hurtrel, J. M. Bach, J. P. Ganiere, and L. Montagnier. 1994. Early events in lymph nodes during infection with SIV and FIV. Res. Virol. 145:221-227. [DOI] [PubMed] [Google Scholar]
- 39.Jarry, A., A. Cortez, E. Rene, F. Muzeau, and N. Brousse. 1990. Infected cells and immune cells in the gastrointestinal tract of AIDS patients. An immunohistochemical study of 127 cases. Histopathology 16:133-140. [DOI] [PubMed] [Google Scholar]
- 40.Kotler, D. P., S. Reka, A. Borcich, and W. J. Cronin. 1991. Detection, localisation, and quantitation of HIV-associated antigens in the intestinal biopsies from patients with HIV. Am. J. Pathol. 139:823-830. [PMC free article] [PubMed] [Google Scholar]
- 41.Levy, J. A., W. Margaretten, and J. Nelson. 1989. Detection of HIV in enterochromaffin cells in the rectal mucosa of an AIDS patient. Am. J. Gastroenterol. 84:787-789. [PubMed] [Google Scholar]
- 42.Lifson, A. R., P. M. O'Malley, N. A. Hessol, S. P. Buchbinder, L. Cannon, and G. W. Rutherford. 1990. HIV seroconversion in two homosexual men after receptive oral intercourse with ejaculation: implications for counseling concerning safe sexual practices. Am. J. Public Health 80:1509-1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Masurier, C., B. Salomon, N. Guettari, C. Pioche, F. Lachapelle, M. Guigon, and D. Klatzmann. 1998. Dendritic cells route human immunodeficiency virus to lymph nodes after vaginal or intravenous administration to mice. J. Virol. 72:7822-7829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller, C. J., and J. Hu. 1999. T cell-tropic simian immunodeficiency virus (SIV) and simian-human immunodeficiency viruses are readily transmitted by vaginal inoculation of rhesus macaques, and Langerhan's cells of the female genital tract are infected with SIV. J. Infect. Dis. 179:S413-S417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Miller, C. J., P. Vogel, N. J. Alexander, S. Dandekar, A. G. Hendrickx, and P. A. Marx. 1994. Pathology and localization of simian immunodeficiency virus in the reproductive tract of chronically infected male rhesus macaques. Lab. Investig. 70:255-262. [PubMed] [Google Scholar]
- 46.Miller, C. J., P. Vogel, N. J. Alexander, S. Sutjipto, A. G. Hendrickx, and P. A. Marx. 1992. Localization of SIV in the genital tract of chronically infected female rhesus macaques. Am. J. Pathol. 141:655-660. [PMC free article] [PubMed] [Google Scholar]
- 47.Moench, T. R., K. J. Whaley, T. D. Mandrell, B. D. Bishop, C. J. Witt, and R. A. Cone. 1993. The cat/feline immunodeficiency virus model for transmucosal transmission of AIDS: nonoxynol-9 contraceptive jelly blocks transmission by an infected cell inoculum. AIDS 7:797-802. [DOI] [PubMed] [Google Scholar]
- 48.Morizono, K., and S. Harada. 1998. Human immunodeficiency virus type 1 (HIV-1) infection and transcytosis activity of a HIV-1 susceptible clone from HeLa cell. Microbiol. Immunol. 42:313-320. [DOI] [PubMed] [Google Scholar]
- 49.Mosialos, G., M. Birkenbach, S. Ayehunie, F. Matsumura, G. S. Pinkus, E. Kieff, and E. Langhoff. 1996. Circulating human dendritic cells differentially express high levels of a 55-kd actin-bundling protein. Am. J. Pathol. 148:593-600. [PMC free article] [PubMed] [Google Scholar]
- 50.Mosier, D., and H. Sieburg. 1994. Macrophage-tropic HIV: critical for AIDS pathogenesis? Immunol. Today 15:332-339. [DOI] [PubMed] [Google Scholar]
- 51.Nelson, J. A., C. A. Wiley, C. Reynolds-Kohler, C. E. Reese, W. Margaretten, and J. A. Levy. 1988. Human immunodeficiency virus detected in bowel epithelium from patients with gastrointestinal symptoms. Lancet ii:259-262. [DOI] [PubMed]
- 52.Nielsen, C., C. Pedersen, J. D. Lundgren, and J. Gerstoft. 1993. Biological properties of HIV isolates in primary HIV infection: consequences for the subsequent course of infection. AIDS 7:1035-1040. [DOI] [PubMed] [Google Scholar]
- 53.Niu, M. T., J. A. Jermano, P. Reichelderfer, and S. M. Schnittman. 1993. Summary of the National Institutes of Health workshop on primary human immunodeficiency virus type 1 infection. AIDS Res. Hum. Retrovir. 9:913-924. [DOI] [PubMed] [Google Scholar]
- 54.Nuovo, G. J., A. Forde, P. MacConnell, and R. Fahrenwald. 1993. In situ detection of PCR-amplified HIV-1 nucleic acids and tumor necrosis factor cDNA in cervical tissues. Am. J. Pathol. 143:40-48. [PMC free article] [PubMed] [Google Scholar]
- 55.Obert, L. A., and E. A. Hoover. 2000. Relationship of lymphoid lesions to disease course in mucosal feline immunodeficiency virus type C infection. Vet Pathol. 37:386-401. [DOI] [PubMed] [Google Scholar]
- 56.Obert, L. A., and E. A. Hoover. 2000. Feline immunodeficiency virus clade C mucosal transmission and disease courses. AIDS Res Hum. Retrovir. 16:677-688. [DOI] [PubMed] [Google Scholar]
- 57.O'Neil, L. L., M. J. Burkhard, L. J. Diehl, and E. A. Hoover. 1995. Vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 11:171-182. [DOI] [PubMed] [Google Scholar]
- 58.O'Neil, L. L., M. J. Burkhard, and E. A. Hoover. 1996. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J. Virol. 70:2894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Padian, N. 1987. Heterosexual transmission of acquired immunodeficiency syndrome: international perspective and national projections. Rev. Infect. 9:947-960. [DOI] [PubMed] [Google Scholar]
- 60.Pedersen, N. C. 1993. The feline immunodeficiency virus, p. 181-228. In J. A. Levy (ed.), The retroviridae, vol. 2. Plenum Press, New York, N.Y. [Google Scholar]
- 61.Pedersen, N. C., E. W. Ho, M. L. Brown, and J. K. Yamamoto. 1987. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 235:790-793. [DOI] [PubMed] [Google Scholar]
- 62.Phillips, D. M., and A. S. Bourinbaiar. 1992. Mechanism of HIV spread from lymphocytes to epithelia. Virology 186:261-273. [DOI] [PubMed] [Google Scholar]
- 63.Phillips, D. M., X. Tan, M. E. Perotti, and V. R. Zacharopoulos. 1998. Mechanism of monocyte-macrophage-mediated transmission of HIV. AIDS Res. Hum. Retrovir. 14:S67-S70. [PubMed] [Google Scholar]
- 64.Poeschla, E. M., and D. J. Looney. 1998. CXCR4 is required by a nonprimate lentivirus: heterologous expression of feline immunodeficiency virus in human, rodent, and feline cells. J. Virol. 72:6858-6866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pope, M., M. G. H. Betjes, N. Romani, H. Hirmand, P. U. Cameron, L. Hoffman, S. Gezelter, G. Schuler, and R. M. Steinman. 1994. Conjugates of dendritic cells and memory T lymphocytes from skin facilitate productive infection with HIV-1. Cell 78:389-398. [DOI] [PubMed] [Google Scholar]
- 66.Qureshi, M. N., C. E. Barr, I. Hewlitt, R. Boorstein, F. Kong, O. Bagasra, L. E. Bobroski, and B. Joshi. 1997. Detection of HIV in oral mucosal cells. Oral Dis. 3(Suppl. 1):S73-S78. [DOI] [PubMed] [Google Scholar]
- 67.Reinhardt, P. P., B. Reinhardt, J. L. Lathey, and S. A. Spector. 1995. Human cord blood mononuclear cells are preferentially infected by non-syncytium-inducing, macrophage-tropic human immunodeficiency virus type 1 isolates. J. Clin. Microbiol. 33:292-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rinfret, A., H. Latendresse, R. Lefebvre, G. St. Louis, P. Jolicoeur, and L. Lamarre. 1991. Human immunodeficiency virus-infected multinucleated histiocytes in oropharyngeal lymphoid tissues from two asymptomatic patients. Am. J. Pathol. 138:421-426. [PMC free article] [PubMed] [Google Scholar]
- 69.Roos, M. T., J. M. Lange, R. E. de Goed, R. A. Coutinho, P. T. Schellekens, F. Miedema, and M. Tersmette. 1992. Viral phenotype and immune response in primary human immunodeficiency virus type 1 infection. J. Infect. Dis. 165:427-432. [DOI] [PubMed] [Google Scholar]
- 70.Rothenberg, R. B., M. Scarlett, C. del Rio, D. Reznik, and C. O'Daniels. 1998. Oral transmission of HIV. AIDS 12:2095-2105. [DOI] [PubMed] [Google Scholar]
- 71.Schuitemaker, H. 1994. Macrophage-tropic HIV-1 variants: initiators of infection and AIDS pathogenesis? J. Leukoc. Biol. 56:218-224. [DOI] [PubMed] [Google Scholar]
- 72.Sellon, R. K., H. L. Jordan, S. Kennedy-Stoskopf, M. B. Tompkins, and W. A. Tompkins. 1994. Feline immunodeficiency virus can be experimentally transmitted via milk during acute maternal infection. J. Virol. 68:3380-3385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shieh, J. T. C., A. V. Albright, M. Sharron, S. Gartner, J. Strizki, R. W. Doms, and F. Gonzalez-Scarano. 1998. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J. Virol. 72:4243-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Sodora, D. L., E. G. Shpaer, B. E. Kitchell, S. W. Dow, E. A. Hoover, and J. I. Mullins. 1994. Identification of three feline immunodeficiency virus (FIV) env gene subtypes and comparison of the FIV and human immunodeficiency virus type 1 evolutionary patterns. J. Virol. 68:2230-2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spira, A. I., P. A. Marx, B. K. Patterson, J. Mahoney, R. A. Koup, S. M. Wolinsky, and D. D. Ho. 1996. Cellular targets of infection and route of viral dissemination after an intravaginal inoculation of simian immunodeficiency virus into rhesus macaques. J. Exp. Med. 183:215-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Stahl-Hennig, C., R. M. Steinman, K. Tenner-Racz, M. Pope, N. Stolte, K. Matz-Rensing, G. Grobschupff, B. Raschdorff, G. Hunsmann, and P. Racz. 1999. Rapid infection of oral mucosal-associated lymphoid tissue with simian immunodeficiency virus. Science 285:1261-1265. [DOI] [PubMed] [Google Scholar]
- 77.Tan, X., and D. M. Phillips. 1996. Cell-mediated infection of cervix derived epithelial cells with primary isolates of human immunodeficiency virus. Arch. Virol. 141:1177-1189. [DOI] [PubMed] [Google Scholar]
- 78.Tenner-Racz, K., P. Racz, H. Schmidt, M. Dietrich, P. Kern, A. Louie, S. Gartner, and M. Popovic. 1988. Immunohistochemical, electron microscopic and in situ hybridization evidence for the involvement of lymphatics in the spread of HIV-1. AIDS 2:299-309. [DOI] [PubMed] [Google Scholar]
- 79.Tompkins, M. B., P. D. Nelson, R. V. English, and C. Novotney. 1991. Early events in the immunopathogenesis of feline retrovirus infections. J. Am. Vet. Med. Assoc. 199:1311-1315. [PubMed] [Google Scholar]
- 80.Toyosaki, T., T. Miyazawa, T. Furuya, K. Tomonaga, Y. S. Shin, M. Okita, Y. Kawaguchi, C. Kai, S. Mori, and T. Mikami. 1993. Localization of the viral antigen of feline immunodeficiency virus in the lymph nodes of cats at the early stage of infection. Arch. Virol. 131:335-347. [DOI] [PubMed] [Google Scholar]
- 81.UNAIDS/WHO. 2001. AIDS epidemic update: December 2001. Joint United Nations Programme on HIV/AIDS (UNAIDS) World Health Organization (WHO), Geneva, Switzerland.
- 82.van't Wout, A. B., N. A. Kootstra, G. A. Mulder-Kampinga, N. Albrecht-van Lent, H. J. Scherpbier, J. Veenstra, K. Boer, R. A. Coutinho, F. Miedema, and H. Schuitemaker. 1994. Macrophage-tropic variants initiate human immunodeficiency virus type 1 infection after sexual, parenteral, and vertical transmission. J. Clin. Investig. 94:2060-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Volin, M. V., L. Joseph, M. S. Shockley, and P. F. Davies. 1998. Chemokine receptor CXCR4 expression in endothelium. Biochem. Biophys. Res. Commun. 242:46-53. [DOI] [PubMed] [Google Scholar]
- 84.Wang, R. F., and J. I. Mullins. 1995. Mammalian cell/vaccinia virus expression vectors with increased stability in Escherichia coli: production of feline immunodeficiency virus envelope protein. Gene 153:197-202. [DOI] [PubMed] [Google Scholar]
- 85.Weiser, B., S. Nachman, P. Tropper, K. H. Viscosi, R. Grimson, G. Baxter, G. Fang, C. Reyelt, N. Hutcheon, and H. Burger. 1994. Quantitation of human immunodeficiency virus type 1 during pregnancy: relationship of viral titer to mother-to-child transmission and stability of viral load. Proc. Natl. Acad. Sci. USA 91:8037-8041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Willett, B. J., and M. J. Hosie. 1999. The role of the chemokine receptor CXCR4 in infection with feline immunodeficiency virus. Mol. Membr. Biol. 16:67-72. [DOI] [PubMed] [Google Scholar]
- 87.Willett, B. J., L. Picard, M. J. Hosie, J. D. Turner, K. Adema, and P. R. Clapham. 1997. Shared usage of the chemokine receptor CXCR4 by the feline and human immunodeficiency viruses. J. Virol. 71:6407-6415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yahi, N., S. Baghdiguian, H. Moreau, and J. Fantini. 1992. Galactosyl ceramide (or a closely related molecule) is the receptor for human immunodeficiency virus type 1 on colon epithelial cells. J. Virol. 66:4848-4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Yamashiro-Matsamura, S., and F. Matsamura. 1986. Intracellular localization of the 55-kD actin-bundling protein in cultured cells: spatial relationships with actin, alpha-actin, and fimbrin. J. Cell Biol. 103:631-640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang, L., T. He, A. Talal, G. Wang, S. S. Frankel, and D. D. Ho. 1998. In vivo distribution of the human immunodeficiency virus/simian immunodeficiency virus coreceptors: CXCR4, CCR3, and CCR5. J. Virol. 72:5035-5045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zhu, T., H. Mo, N. Wang, D. S. Nam, Y. Cao, R. A. Koup, and D. D. Ho. 1993. Genotypic and phenotypic characterization of HIV-1 in patients with primary infection. Science 261:1179-1181. [DOI] [PubMed] [Google Scholar]