Abstract
Human rhinoviruses (HRVs) are the predominant cause of the common cold. The frequency of HRV infections in industrial countries and the lack of effective therapeutical treatment underline the importance of research for new antiviral substances. As viral infections are often accompanied by the generation of oxidative stress inside the infected cells, several redox-active substances were tested as potential antivirals. In the course of these studies it was discovered that pyrrolidine dithiocarbamate (PDTC) is an extremely potent compound against HRV and poliovirus infection in cell culture. Besides the ability to dramatically reduce HRV production by interfering with viral protein expression, PDTC promotes cell survival and abolishes cytopathic effects in infected cells. PDTC also protects cells against poliovirus infection. These effects were highly specific, as several other antioxidants (vitamin C, Trolox, 2-mercaptoethanol, and N-acetyl-l-cysteine) are inactive against HRV infection. Synthesis of HRV proteins and cleavage of eucaryotic initiation factor 4G responsible for host cell shutoff of cellular protein synthesis are severely inhibited in the presence of PDTC.
Human rhinoviruses (HRVs), the main causative agents of the common cold occurring worldwide (20), constitute the most extensive genus of the Picornaviridae. The frequent appearance of HRV infections and their economic importance in terms of employee absenteeism, physician visits, and medication costs make it a subject of primary importance (17). Despite the frequency of the disease, no cure for the common cold is presently available apart from symptomatic treatment.
Infections of patients with HRVs elicit typical proinflammatory responses accompanied by massive release of inflammatory mediators (53). In particular, cytokines, including interleukin-1 beta (IL-1β), tumor necrosis factor alpha, IL-8, IL-6, and IL-11 (12, 46, 77), and the vasoactive peptides bradykinin and lysyl-bradykinin (45, 54, 64) were found in nasal secretions of patients with colds. There is strong evidence that the activation of these proinflammatory molecules is at least in part mediated by the transcription factor NF-κB (77).
To successfully infect a host, viruses have to both exploit the cellular resources and at the same time avoid defense reactions of the host organism. In tissue culture, morphological changes observed in cells infected with picornaviruses, including cell rounding and detachment from the substrate, are generally termed cytopathic effects (CPE). During picornaviral infection, several essential cellular processes are modified by the action of viral proteins at the levels of both transcription (57, 75) and translation (14, 30). Furthermore, profound changes in cytoskeletal architecture are found to occur (2, 32, 62). A hallmark of infections with rhino- and enteroviruses is the cleavage of the eucaryotic translation initiation factors (eIF) eIF4GI and eIF4GII by viral proteinase 2A, resulting in the shutoff of cap-dependent host cell translation.
There is increasing evidence supporting the view that oxidative stress might play an important role in virus infection. Mechanistically, oxidative stress is characterized by increased levels of reactive oxygen intermediates (ROIs), which act as second messengers for the activation of transactivators such as NF-κB (58, 59), AP-1 (41), Egr-1 (22), p53 (70) and c-fos (37). It has been shown that interference with the generation of ROIs by use of antioxidants can drastically reduce replication of various seemingly unrelated viruses, e.g., bovine diarrhea virus (61), Sindbis virus (36), hepatitis B virus (71), influenza A virus (16), and retroviruses such as the human and feline immunodeficiency viruses (27, 39, 44). It is hypothesized that these viruses induce apoptosis via a pathway involving oxidative stress and the transcription factor NF-κB. Interference with the generation of oxidative stress with antioxidants is believed to inhibit virus-induced apoptosis and thus virus replication.
Although oxidative stress is also known to occur during infection with picornaviruses, little information is available on its role in virus multiplication (28). Therefore, we investigated the effects of several antioxidants including pyrrolidine dithiocarbamate (PDTC), vitamin C, the vitamin E derivative Trolox, 2-mercaptoethanol, and N-acetyl-l-cysteine (NAC) on infections of epithelial cells with several serotypes of HRVs.
In this report, we provide evidence that PDTC has a drastic inhibitory effect on the multiplication of several HRV serotypes in different cell types. In the presence of PDTC, virus growth is greatly reduced and the CPE is absent. Interestingly, these effects are specific for PDTC, as other redox-active compounds do not interfere with virus multiplication. Similarly, PDTC protects cells against the CPE induced by poliovirus infection.
MATERIALS AND METHODS
Media, reagents, and chemicals.
HeLa cells (strain Ohio; European Collection of Cell Cultures, Salisbury, United Kingdom) and A549 alveolar carcinoma cells (American Type Culture Collection) were cultured in Dulbecco modified Eagle's medium (Gibco) supplemented with 10% heat-inactivated fetal calf serum (FCS) (Gibco), 2 mM l-glutamine (Dipro), 100 U of penicillin/ml (Dipro), and 100 μg of streptomycin/ml (Dipro). HeLa S3 cells were grown in RPMI medium (Gibco) for experiments with poliovirus. 16HBE14o− cells (obtained from D. Gruenert, San Francisco, Calif.) were grown in minimal essential medium (Gibco) supplemented with 10% FCS, 2 mM l-glutamine, 100 U of penicillin/ml, and 100 μg of streptomycin/ml. Dishes were coated with 10 μg of bovine serum albumin/ml (Sigma), 30 μg of bovine collagen type I/ml (Promocell) and 10 μg of human fibronectin/ml (Becton Dickinson) in Ham's F-12 medium (HyClone). Human recombinant IL-1α was obtained from Genzyme Diagnostics. PDTC was purchased from Alexis Biochemicals, Lausen, Switzerland. A 0.6 M stock solution in water was stored at −20°C. The cell-permeative vitamin E derivative Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) (Alexis) was stored at 4°C at a concentration of 10 mM. NAC (Sigma) was dissolved in water to a concentration of 1 M and diluted subsequently. 2-Mercaptoethanol was obtained from Calbiochem. Ascorbic acid (vitamin C) (Sigma) was dissolved in water before use. Guanidine HCl (Sigma) was dissolved in water at a concentration of 100 mM.
Virus preparation.
HRV1A, HRV2, HRV14, and HRV16 (American Type Culture Collection) were grown in suspension cultures of HeLa cells (strain Ohio) and purified as described previously (65). Virus titers in 50% tissue culture infectious doses (TCID50)/ml were determined according to Reed and Muench (55). Poliovirus strain Mahoney was cultivated in HeLa S3 cells. Cell supernatant was used for infection without further purification.
Infection of cells with HRV serotypes.
HeLa, 16HBE14o−, and A549 cells seeded into 6-well plates were infected at 70% confluence with HRV serotypes HRV1A, HRV2, HRV14, or HRV16 in infection medium (minimal essential medium [MIM] [Gibco] containing 2% FCS, 2 mM l-glutamine, 30 mM MgCl2, 100 U of penicillin/ml, 100 μg of streptomycin/ml). If not otherwise indicated, input virus was used at 100 TCID50 per cell. PDTC was added simultaneously with HRV preparations. Cellular toxicity was tested by visual inspection and by the proliferation assay (see below) after 24 h with different concentrations of PDTC. The optimal concentrations of PDTC treatment as determined by the toxicity tests differed among various cell types. Routinely, 125 μM PDTC was used for HeLa cells, 60 μM was used for A549 cells, and 6 μM was used for 16HBE14o− cells. Cells were incubated at 37°C and 5% CO2 in a humidified incubator. At 4 h after infection, unbound viral particles were removed by washing three times with 10 mM HEPES (pH 5.3) and 140 mM KCl and once with phosphate-buffered saline (PBS). Cells were then incubated for 2, 4, 6, 8, and 24 h in MIM supplemented with or without PDTC. Cells were investigated by light microscopy, by staining with 0.2% crystal violet in 20% methanol, or by immunofluorescence. At 2, 4, 6, 8, and 24 h after infection, supernatants were collected for the determination of TCID50 (55) or cells were lysed to obtain extracts for Western blotting.
Proliferation assay.
To determine cell viability, the Cell Titer 96 AQueous Non-radioactive Cell Proliferation Assay (Promega) was performed as instructed by the manufacturer. Briefly, 106 cells were seeded into a 96-well plate 1 day prior to infection. Cells were infected using HRV serotypes HRV1A, HRV2, HRV14, and HRV16 at 20 TCID50/cell for HeLa cells. PDTC, guanidine HCl, 2-mercaptoethanol, vitamin C, and Trolox were added as indicated in the figure legends. At 24 h after infection, cell viability was determined by adding the tetrazolium compound followed by incubation for 2 h at 37°C and subsequent measurement of absorbance at 492 nm in a Labsystems Multiscan RC plate reader.
Western blot analysis.
Cells were infected in 6-well plates as described for virus infection. At 0, 2, 4, 6, 8, and 24 h after infection, the medium was removed and the cells were lysed by the addition of 100 μl of protein sample buffer (8% sodium dodecyl sulfate [SDS], 20% β-mercaptoethanol, 20% glycerol, 0.04% bromophenol blue). Protein extract (20 μl per lane) was subjected to SDS-polyacrylamide gel electrophoresis and electroblotted onto polyvinylidene difluoride membranes. Blocking and incubation with antibodies was done using 5% nonfat dry milk and 0.1% Tween 20 in Tris-buffered saline (50 mM Tris-HCl [pH 7.4], 150 mM NaCl). For immunodetection, a rabbit polyclonal antibody against eIF4GI (76) and an antiserum against HRV2 prepared by conventional methods were used. Secondary antibodies were alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin (Sigma). Staining using an alkaline phosphatase reaction was performed as described previously (35). Molecular sizes were determined using prestained molecular weight marker SDS-7B (Sigma).
Immunofluorescence.
Immunofluorescence was essentially performed as described elsewhere (3). Briefly, cells were grown on sterile coverslips in 6-well tissue culture dishes. Fixation was done with 4% paraformaldehyde-PBS for 10 min at room temperature. Cells were permeabilized by incubation in 0.5% Triton X-100-PBS for 5 min. Blocking was done at 4°C using 1% goat serum-PBS. The primary antibody was a rabbit polyclonal antiserum raised against human p65 (Santa Cruz) diluted 1:500 in PBS-1% bovine serum albumin. As secondary antibody, Alexa 488-labeled goat anti-rabbit immunoglobulin G conjugates were used at 1:800 dilution (Molecular Probes). Finally, slides were mounted with DAKO fluorescent mounting medium and examined with a Leica TCS-NT confocal microscope using fluorescein isothiocyanate settings.
RESULTS
PDTC drastically reduces production of infectious HRV particles.
Viral infections frequently result in the generation of oxidative stress in the infected cells (16, 23, 28, 61). To study the influence of antioxidants on HRV infections, the effect of PDTC on the replication was investigated in epithelial cells by using several serotypes of HRV. HeLa cells, A549 alveolar carcinoma cells, and 16HBE14o− human bronchial epithelial cells were infected with HRV2 or HRV14 by using 100 TCID50 per cell. At the same time, PDTC was added at concentrations of 125 μM for HeLa cells, 60 μM for A549 cells, and 6 μM for 16HBE14o− cells. At 4 h after infection, input virus was removed and the MIM was replaced by growth medium containing the same concentrations of PDTC. Supernatants were collected 24 h postinfection (p.i.), and the amount of progeny virus was determined by titration using TCID50 assays on HeLa cells (Fig. 1).
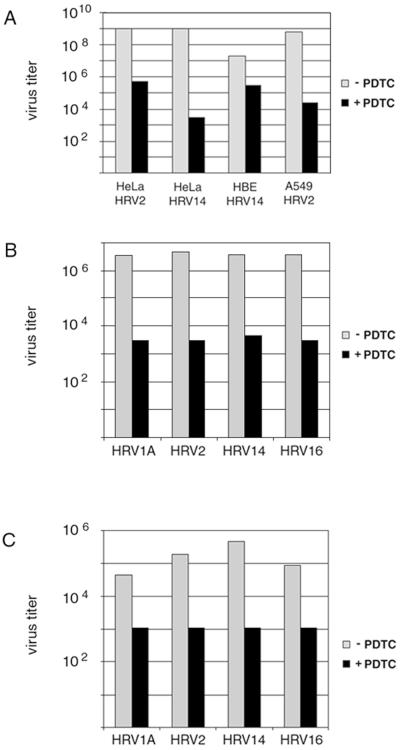
FIG. 1.
PDTC inhibits HRV replication in cell culture. (A) HeLa cells, 16HBE14o− cells, and A549 cells were infected with HRV2 or HRV14 using 100 TCID50/cell as described in Materials and Methods. PDTC was added at the time of infection. Viral titers in the supernatants of infected cells were determined after 24 h by TCID50 assay. Representative experiments are shown. (B and C) HeLa cells were infected with HRV serotypes HRV1A, HRV2, HRV14, and HRV16 using 20 TCID50/cell. At the same time, 125 μM PDTC was added. Virus titers of supernatants collected from 0 to 24 h p.i. (B) and from 24 to 48 h p.i. (C) as determined by TCID50 assay are shown.
Under these conditions, HRV replication in untreated cells resulted in the generation of a viral titer between 107 and 109 TCID50/ml after 24 h p.i. (Fig. 1A). Presence of PDTC during infection dramatically changes the outcome of viral infection: depending on the cell type, viral titers decrease between 2 orders of magnitude in the case of HRV14-infected 16HBE14o− cells and nearly 6 orders of magnitude in the case of HRV14-infected HeLa cells (Fig. 1A). Although there are differences in the amount of virus reduction, the inhibitory effect of PDTC does not appear to be restricted to a particular cell type.
To investigate whether this effect was serotype specific, both minor group viruses HRV1A and HRV2 and major group viruses HRV14 and HRV16 were used to infect HeLa cells. In this experiment, the amount of input virus was reduced to 20 TCID50/cell. Concurrent treatment of cells with PDTC reduced viral titers after 24 h by 3 orders of magnitude for all HRV serotypes tested (Fig. 1B). Viral titers in the supernatants harvested from 24 to 48 h p.i. were also significantly reduced by PDTC treatment (Fig. 1C), implying that the effect is due to a reduction in virus growth rather than to a delay in virus release. As a lower amount of input virus (20 TCID50/cell) was used in this experiment, the absolute number of virus particles produced was reduced compared to that for the results presented in Fig. 1A. Nevertheless, the antiviral effect of PDTC was in a similar range to that observed in the experiments using a higher amount of input virus.
These experiments demonstrate that PDTC has strong antiviral properties against several serotypes of HRV independent of cell types. There is also no dependence on the viral receptor, as major group HRVs bind to intercellular adhesion molecule 1 whereas minor group HRVs use the low-density lipoprotein receptor and/or related proteins for viral entry. Different amounts of input virus change the absolute amount of progeny virus but do not affect significantly the percentage of reduction of viral titers, indicating that the antiviral properties of PDTC are independent of the multiplicity of infection.
Search for PDTC-resistant HRV variants.
Following a protocol similar to that described for the generation of guanidine-resistant mutants of poliovirus (68), HRV14 was propagated through five passages in HeLa cells in the presence of increasing concentrations of 15 to 30 μM PDTC. This resulted in a complete loss of viral titer (data not shown), whereas under similar conditions guanidine-resistant poliovirus variants had been isolated previously (68). In another experiment, HRV14 was carried through nine passages at increasing concentrations from 4 to 12 μM PDTC. Again no PDTC-resistant HRV14 variants were obtained (data not shown). Under similar conditions, HRV14 variants resistant to the inhibitor enviroxime have been produced (21). From these experiments it can be concluded that mutations in HRV14 conferring resistance against PDTC do not occur with high frequency.
PDTC inhibits HRV-induced CPE.
Many viruses are capable of inducing cell death, leading to lysis of infected cells (1, 10, 33). In late stages of HRV infections, morphological changes, commonly known as CPE, can be microscopically observed. HRV-induced CPE is characterized by cell rounding, shrinkage, deformation of nuclei, and chromatin condensation.
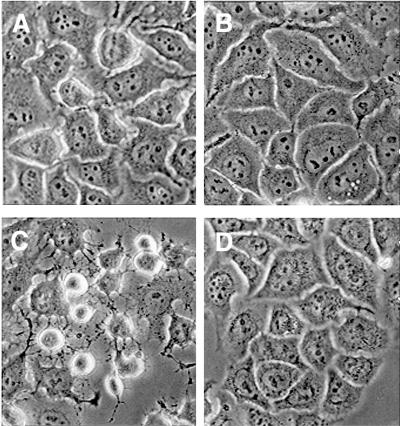
During infections of HeLa cells with HRV1A, HRV2, HRV14, and HRV16 using 100 TCID50/cell, a clear CPE was visible within 8 h p.i. Figure 2 shows the morphology of HeLa cells infected with HRV14. Mock-infected cells (Fig. 2A) or cells treated with 125 μM PDTC (Fig. 2B) showed typical spread-out shapes and normal morphology. At this concentration, no signs of cytotoxicity of PDTC were seen. Infection with HRV14 in the absence of PDTC resulted in a severe CPE (Fig. 2C). Addition of PDTC upon infection inhibited the formation of a visible CPE. As shown in Fig. 2D, the morphology of cells 8 h after infection with HRV14 in the presence of PDTC was virtually undistinguishable from that of mock-infected cells (Fig. 2A). A similar protective effect was shown in 16HBE14o− cells infected with HRV14 and in A549 cells infected with HRV2 (data not shown). Thus, the CPE of the virus infection is prevented by the presence of PDTC.
FIG. 2.
PDTC inhibits HRV-induced CPE. HeLa cells were infected with HRV14 in the absence or presence of 125 μM PDTC. Cells were examined by light microscopy 8 h p.i. (A) Noninfected cells; (B) noninfected cells with PDTC; (C) HRV14-infected cells without PDTC; (D) HRV14-infected cells with PDTC.
PDTC promotes viability of infected cells.
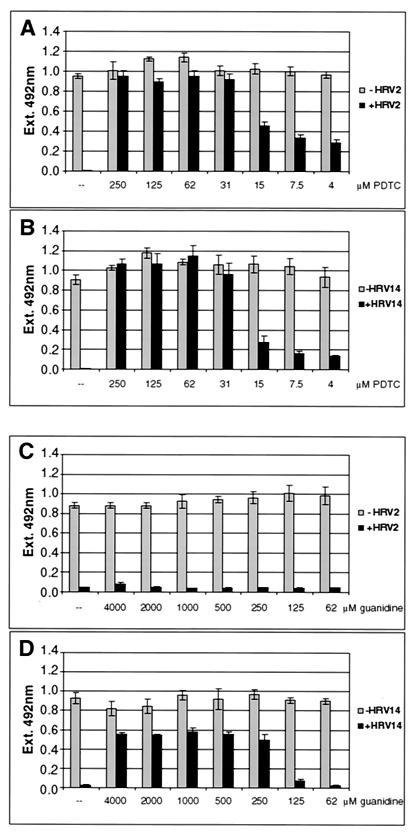
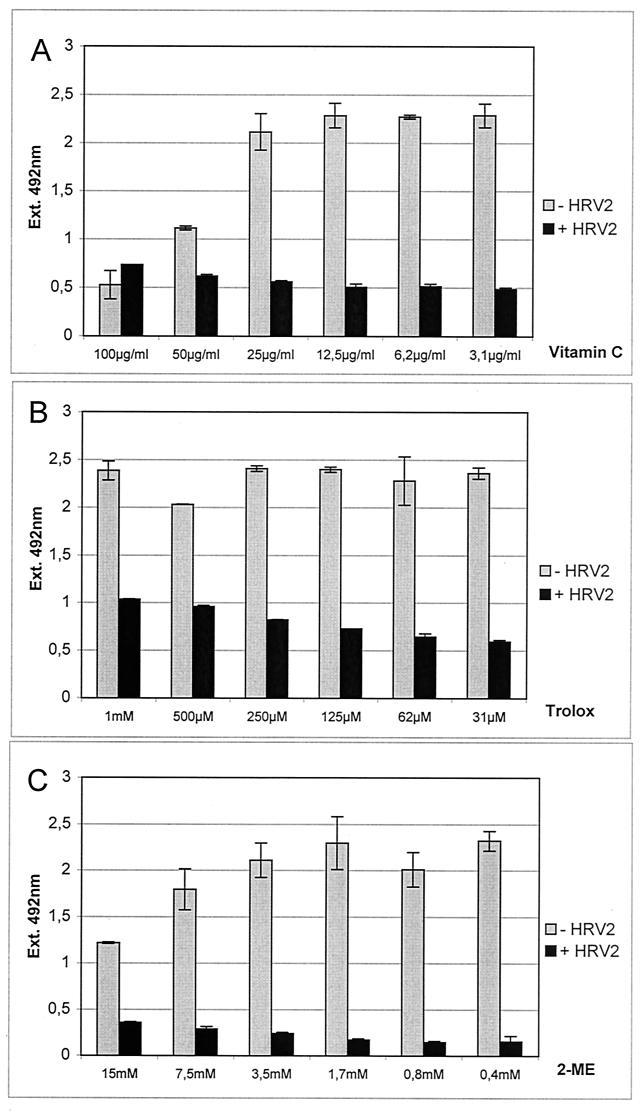
To quantify the effect of PDTC on cells during viral infection, a proliferation assay was employed. Conversion of the tetrazolium substrate into a formazan dye is accomplished by dehydrogenase enzymes. The observed optical density therefore reflected the metabolic activity of cells. HeLa cells infected with HRV2 (Fig. 3A) and with HRV14 (Fig. 3B) showed a complete loss of metabolic activity after 24 h of infection. However, PDTC concentrations as low as 31 μM protected cells efficiently against virus-induced cell death. Even at lower concentrations of PDTC, cellular survival was significantly improved. PDTC did not show cytotoxic effects on uninfected cells in the range of concentrations used. In fact, a weak stimulatory effect upon PDTC treatment was seen in the proliferation assay in comparison to that for untreated cells (Fig. 3A and B). It is also noteworthy that under certain conditions of cellular stress, PDTC can exert cytotoxic effects (data not shown; see reference 13). Similar experiments have been carried out using guanidine HCl, a well-known inhibitor of enterovirus multiplication (56). As seen in Fig. 3C, guanidine had essentially no effect on HRV2 infection even at high concentrations. In contrast, HeLa cells were protected significantly against HRV14 infection at concentrations of guanidine between 250 μM and 4 mM (Fig. 3D). However, the fact that PDTC inhibited all HRV serotypes tested whereas guanidine did not prevent HRV2 infection indicates that the mechanisms of action of these two inhibitors are different.
FIG. 3.
PDTC promotes cell viability and survival of HRV-infected cells. Proliferation assays of cells 24 h after mock infection or infection of cells with HRV2 (A) and HRV14 (B). No PDTC (-) or a PDTC concentration of between 250 μM and 4 μM was added. Results using guanidine HCl as inhibitor in the concentration range of 4,000 μM to 62 μM are presented for HRV2 (C) and for HRV14 (D). Absorption values at 492 nm are shown, corresponding to relative mitochondrial activities. The values represent the means (± standard deviations [SD]) for at least four samples.
PDTC inhibits poliovirus-induced CPE.
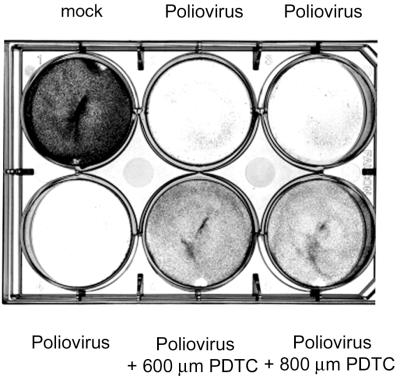
In terms of genomic structure and sequence identity, HRV and enteroviruses are closely related. It was therefore of interest to investigate whether PDTC is specific for HRV or whether the drug exhibits a more general antiviral action. Figure 4 presents the results of experiments in which PDTC was tested for its inhibitory activity against poliovirus. A clear reduction of the poliovirus-induced CPE can be seen. Interestingly, the HeLa S3 subtype used for infection experiments with poliovirus had a much higher tolerance for PDTC. The poliovirus-inhibiting effect was found to occur at a 5- to 20-fold higher concentration of PDTC compared to that of HRV2 (Fig. 4; see Fig. 9).
FIG. 4.
PDTC promotes cell survival following poliovirus infection. HeLa S3 cells grown in RPMI medium were infected with poliovirus strain Mahoney at a multiplicity of infection of 1. After 3 h, the virus inoculum was removed and replaced by RPMI medium containing the indicated amounts of PDTC. At 24 h after infection, cells were stained with crystal violet.
FIG. 9.
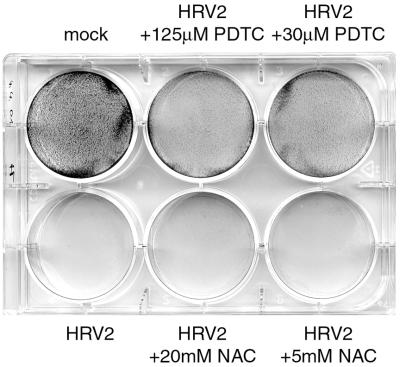
NAC does not show an antiviral effect. Cell viability of NAC-treated HeLa cells was determined by crystal violet staining and compared to that of PDTC-treated cells.
Early steps of viral life cycle are unaffected by PDTC treatment.
These data raised the question of whether PDTC exerts its antiviral activity by a direct mode of action or whether cells are converted into an antiviral state, e.g., by formation of stable protective proteins. However, preincubation of the cells with PDTC for 1 h followed by removal of the drug did not protect the cells against subsequent HRV infection (data not shown). This suggests that the PDTC pretreatment did not induce a long-lasting antiviral state.
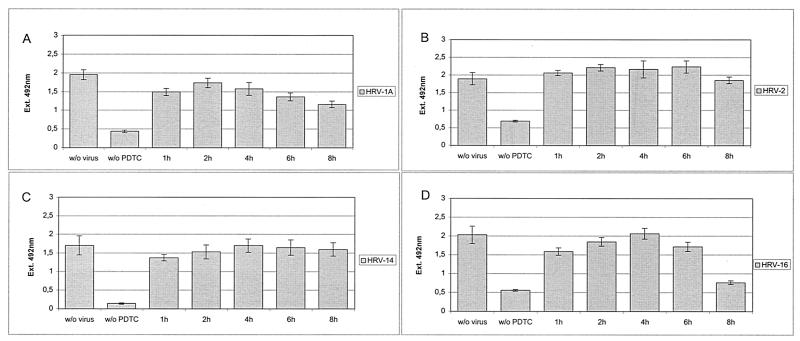
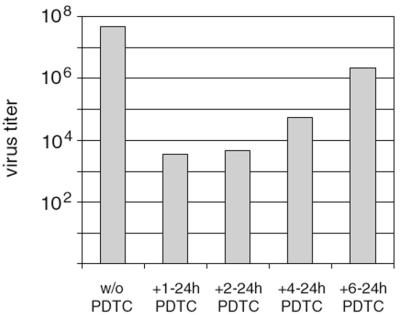
To clarify at which step of the viral life cycle PDTC interferes, proliferation assays were performed by adding PDTC at different time points after virus infection (Fig. 5). Under the conditions used, a complete life cycle lasts approximately 10 h. Addition of PDTC within the first 6 h p.i. resulted in the best protection from virus-induced loss of proliferation. Again, this effect is not specific for a single serotype, as HRV serotypes HRV1A, HRV2, HRV14, and HRV16 show similar results (Fig. 5). If PDTC treatment started as late as 8 h p.i., there was a decrease of inhibition but still significant protection, particularly for HRV2 and HRV14. Similar results were obtained by determining viral titers in the supernatants of infected and PDTC-treated cells (Fig. 6). Presence of PDTC from 4 h to 24 h p.i. reduced the titers of newly produced HRV2 by 3 orders of magnitude. Even if PDTC treatment started as late as 6 h p.i., the viral titers were significantly decreased. Both in proliferation assays and in titers of progeny virus, a striking antiviral effect of PDTC can be seen. We therefore conclude that early events such as receptor binding and internalization of the virus are not affected by the drug but that PDTC acts at a later stage of infection. This is in line with experiments employing freezing and thawing of infected cells, which show a drastic reduction in the number of infectious particles in PDTC-treated cells compared to that in untreated controls. This supports the notion that PDTC inhibits virus multiplication directly rather than indirectly by blocking the CPE and/or by preventing the release of infectious viral particles from the cells (data not shown).
FIG. 5.
Early steps in the viral life cycle are unaffected by PDTC treatment. HeLa cells were infected with HRV1A (A), HRV2 (B), HRV14 (C), or HRV16 (D). PDTC (125 μM) was added at different time points between 1 and 8 h after infection (w/o PDTC, no PDTC added). Proliferation assays were performed 24 h after infection. All values represent the means (± SD) for at least four samples.
FIG. 6.
Addition of PDTC at later stages still reduces viral multiplication. HeLa cells were infected with HRV2, and PDTC was added for the indicated intervals. Viral titers in the supernatants of cells after 24 h are shown for a representative experiment (in TCID50 per milliliter).
PDTC treatment greatly reduces cleavage of cellular substrates during HRV infection.
To follow the progress of rhinoviral infection in the presence and absence of PDTC in more detail, we analyzed the proteolytic cleavage events mediated by the viral 2A proteinase. A distinctive feature of infections with rhino- and enteroviruses is the early and efficient cleavage of the cellular translation initiation factors eIF4GI and eIF4GII by the viral 2A proteinase (18, 19, 30, 31) leading to the shutoff of host cell protein synthesis (15). Another recently described cleavage event during rhinoviral infection is the cleavage of the intermediate filament protein cytokeratin 8. This cleavage is also mediated by the viral 2A proteinase and occurs late in the infection (62). To study the influence of PDTC treatment on the progress of the HRV infection, Western blotting was performed to analyze the cleavage of these cellular 2A proteinase substrates (Fig. 7).
FIG. 7.
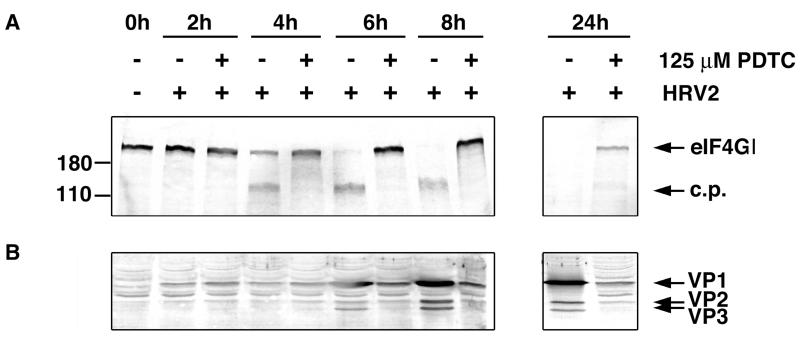
PDTC inhibits HRV-mediated cleavage of eIF4GI and reduces the expression of rhinoviral proteins. Protein extracts from HRV2-infected HeLa cells in the absence or presence of 125 μM PDTC were collected at the indicated time points after infection. (A) Western blot analyses were performed to detect cleavage of eIF4GI. c.p., cleavage products. (B) Western blot showing the expression of the rhinoviral proteins VP1, VP2, and VP3. Mock-infected cells served as controls.
Under the conditions used, cleavage of eIF4GI in the absence of PDTC was seen at 4 h p.i. in HRV2-infected HeLa cells and complete cleavage was accomplished at 8 h p.i. (Fig. 7A). At these time points, cells infected in the presence of PDTC did not show any detectable cleavage of eIF4GI. Only at a very late stage (24 h p.i.) was a partial eIF4GI cleavage detected in PDTC treated cells. Similarly, cleavage of eIF4GII was greatly reduced (data not shown). In untreated cells, cleavage of cytokeratin 8 was visible at 6 h p.i. In infected cells treated with PDTC, cytokeratin cleavage was completely absent, indicating either that 2A proteinase function was blocked or that the expression level of the viral 2A proteinase was greatly diminished (data not shown). However, there was also no effect of PDTC on cleavage of synthetic peptide substrates by highly purified HRV2 2A proteinase (66) or on eIF4GI cleavage activity of in vitro synthesized 2A proteinase (data not shown) in a coupled transcription-translation system (TnT; Promega) (18, 52).
PDTC treatment reduces expression of viral coat proteins.
To study the interference of PDTC with the expression of viral proteins, capsid proteins of HRV2 were analyzed in protein extracts of HRV2-infected HeLa cells by Western blotting (Fig. 7B). Significant amounts of the rhinoviral proteins VP1, VP2, and VP3 were detectable starting at 6 h p.i. Treatment with PDTC strongly reduces the expression of the capsid proteins within the first 8 h of infection. Low expression of VP1, VP2, and VP3 was found at the very late time point of 24 h p.i.
Other antioxidants fail to inhibit HRV replication.
From the preceding data, the mechanism of the antirhinoviral action of PDTC cannot be explained. One widely accepted property of PDTC is its function as an antioxidant (41). In order to determine whether the observed antiviral activity is a specific effect of PDTC or a general feature of antioxidants, other commonly used antioxidative substances were tested during infection of HeLa cells with HRV2 (Fig. 8). Vitamin C (Fig. 8A), the vitamin E derivate Trolox (Fig. 8B), and 2-mercaptoethanol (Fig. 8C) did not show any antiviral effect in proliferation assays. The range of concentrations used was based on previously published data about the biological effects of the corresponding substance. In the absence of virus, toxic effects of the applied substances were seen only in the case of vitamin C at concentrations of 50 μg/ml or higher (Fig. 8A). In addition, the redox-active compound NAC was tested. As NAC reacts with the formazan dye of the proliferation assay, its potential antiviral property was tested in a different assay (Fig. 9). Cells were infected with HRV2 in the presence or absence of NAC or PDTC. After 24 h, the monolayer was stained with crystal violet. Figure 9 shows that untreated infected cells were lysed completely. PDTC reduced the CPE, whereas NAC had no effect. Based on these results, it can be concluded that the inhibition of HRV production by PDTC is not simply due to a general action as an antioxidant.
FIG. 8.
Other antioxidative compounds fail to show an antiviral effect. HeLa cells were infected with HRV2 (+ HRV2) at 20 TCID50/cell and treated with a wide range of concentrations of vitamin C (A), the vitamin E derivate Trolox (B), and 2-mercaptoethanol (2-ME) (C). Mock-infected cells (− HRV2) served as controls. All values represent the means (± SD) of at least four samples.
PDTC inhibits HRV-mediated NF-κB activation in infected cells.
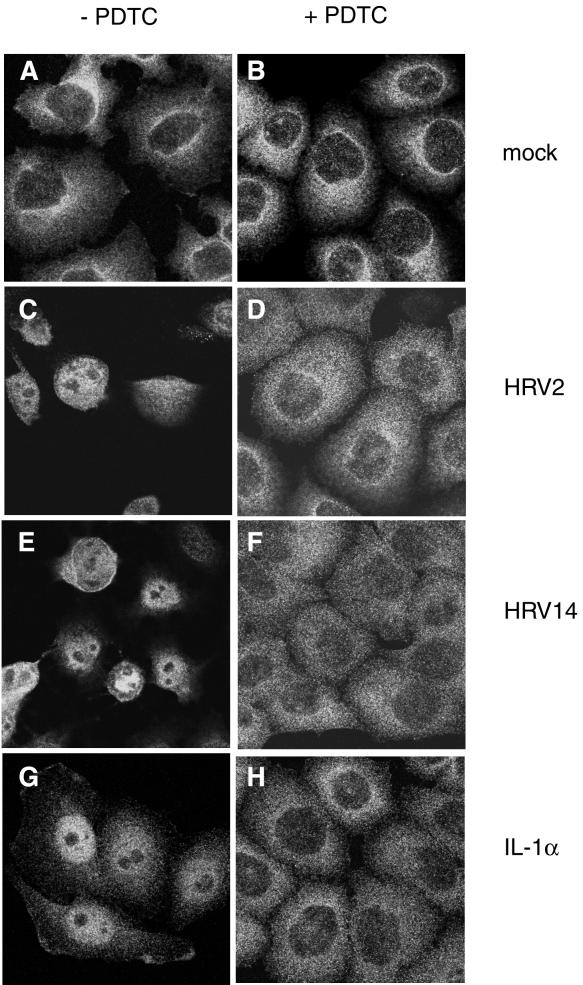
A well-defined function of PDTC is the suppression of activation of the ubiquitous transcription factor NF-κB under various conditions of induction (58, 59). As some reports employing transgenic mice show that activation of NF-κB is essential for picornavirus virulence (60, 63), we analyzed the cellular distribution of the p65 subunit of NF-κB during HRV infection by using immunofluorescence (Fig. 10).
FIG. 10.
PDTC inhibits HRV- and IL-1α-induced NF-κB activation. Immunofluorescence experiments located the NF-κB subunit p65 in HeLa cells. Left column, cells without PDTC; right column, PDTC-treated cells. (A and B) Mock-infected cells. (C and D) Cells infected with HRV2 at 8 h p.i. (E and F) Cells infected with HRV14 at 8 h p.i. (G and H) Cells incubated with IL-1α at 1.5 h after stimulation.
In uninfected HeLa cells, the p65 subunit is localized exclusively in the cytoplasm (Fig. 10A). During infection with HRV2 or HRV14, activation and nuclear translocation of NF-κB were observed. At 8 h p.i., the p65 subunit of NF-κB was detected exclusively in the nucleus (Fig. 10C and E). Compared to direct induction of NF-κB by IL-1α, the activation of NF-κB during rhinovirus infection was a late event. IL-1α stimulation resulted in massive translocation of p65 to the nucleus after 1.5 h (Fig. 10G). IL-1α-mediated translocation was a transient event. At 8 h after induction, NF-κB was again found exclusively in the cytoplasm (data not shown).
Incubation of cells with PDTC completely inhibited NF-κB translocation independent of whether NF-κB was induced by HRV (Fig. 10B, D, and F) or by IL-1α (Fig. 10H). This can be seen both in HeLa cells infected with HRV2 or with HRV14 (Fig. 10) and in 16HBE14o− cells infected with HRV14 (data not shown). Although signaling leading to NF-κB activation is supposed to occur via the generation of ROIs, it is interesting that other antioxidants such as NAC or Trolox failed to prevent HRV-induced NF-κB activation (data not shown).
DISCUSSION
Most of the inhibitors of picornavirus infection act at an early stage of infection, i.e., during virus binding, internalization, and/or uncoating. Only very few of the known inhibitors are effective late in the viral life cycle (8). In this study, we demonstrated novel functions of PDTC during infection with HRV. Treatment of infected cells with PDTC resulted in drastic reduction of rhinoviral replication. Even if the substance was applied several hours after HRV infection, the survival of the infected cells was greatly improved. HRV infection in the presence of PDTC was characterized by a strong reduction in the processing of cellular substrates by the viral 2A proteinase; at the same time, the expression of viral capsid proteins was reduced. The activation of the transcription factor NF-κB usually observed in HRV infections was absent in the PDTC-treated cells.
The events leading to the generation of ROIs during viral infections are still unclear. It was reported previously that the administration of antioxidative compounds during infection can lead to the inhibition of viral replication in a variety of viral systems, e.g., Sindbis virus (36), hepatitis B virus (71), bovine viral diarrhea virus (61), and retroviruses (27). As these viruses are unrelated to each other, it is postulated that these compounds affect a central process required for viral replication.
Mechanistically, it is believed that these viruses induce apoptosis by oxidative stress mediated via ROIs. In several cases a pathway is triggered, leading to activation of the transcription factor NF-κB. Interference with this pathway by antioxidants is believed to inhibit virus-induced apoptosis and thus inhibit efficient virus multiplication. In contrast, there are also reports indicating that under certain conditions PDTC acts as a proapoptotic drug (11, 34, 51). Depending on the viral system analyzed, antioxidative compounds differ in the ability to reduce virus growth. For example, NAC and several other reducing substances like 2-mercaptoethanol and dithiothreitol completely inhibited Sindbis virus-induced cell death whereas other antioxidants such as Trolox and catalase were ineffective (36). NAC was also shown to inhibit hepatitis B virus replication (71). In contrast, NAC and PDTC failed to prevent apoptosis in the case of a cytopathic strain of bovine viral diarrhea virus where butylated hydroxyanisole was effective (61). In this report we present evidence that PDTC, but not other commonly used antioxidants, is able to protect cells from HRV-induced cell death. Similarly, PDTC inhibited cell death following poliovirus infection.
Dithiocarbamates are potent agents and have been shown to be involved in a number of processes, suggesting that the antiviral effect of PDTC might not be due to its antioxidant function alone. Although dithiocarbamates are widely used as antioxidants, they can exert both pro- and antioxidative functions in the cell. Their antioxidant functions include eliminating hydrogen peroxide, scavenging superoxide radicals, peroxynitrite, hydroxyl radicals, and lipid peroxidation products (reference 72 and references therein). These reactions ultimately generate thiuram disulfide, the oxidized form of dithiocarbamates (48). In some cases, the formation of thiurams appears to be metal dependent (6, 47). Thiuram disulfides are responsible for the prooxidant effects of dithiocarbamates characterized by their potent oxidation of glutathione and protein thiols (48, 50). There is evidence that this prooxidative effect inactivates caspases by thiol oxidation and thereby blocks apoptosis (49). As the picornaviral proteinases 2A and 3C are cysteine proteinases, they are highly sensitive to oxidation and are strictly dependent on the reducing environment of the cell. Changes in the reduction-oxidation balance would have detrimental effects on proteinase function and hence on viral replication. However, our preliminary experiments employing peptide cleavage assays by highly purified 2A proteinase as well as eIF4G cleavage by nascent 2A proteinase do not support a mechanism of direct action of PDTC on 2A enzymatic activity.
A well-described function of PDTC is the inhibition of activation of transcription factor NF-κB (24, 58, 59). On the one hand, the inhibition of NF-κB activation by PDTC is attributed to its radical scavenging properties (41, 59); on the other hand, it has been suggested that PDTC might exert its inhibitory effect via oxidation of critical thiols in NF-κB (4, 5). This has also been shown for the tumor suppressor and transcription factor p53 (73, 74). Several investigators have demonstrated that PDTC exhibits anti-inflammatory effects through inhibiting NF-κB activation in response to a variety of stimuli including IL-1, tumor necrosis factor alpha, LPS, and H2O2 in several cell types (59).
In this report we provide evidence that during HRV infection NF-κB is translocated to the nucleus at a late time point. Under the conditions used, NF-κB activation was observed at 6 to 8 h p.i. At this stage, host cell protein synthesis is to a large extent shut off due to the cleavage of eIF4GI and eIF4GII. Under these conditions, mRNAs can be translated only by a cap-independent mechanism. However, recently several cellular genes translated under cap-independent conditions were identified (25, 26, 38). Whether NF-κB is involved in activation of these genes is not clear at present.
How can inactivation of NF-κB affect viral multiplication? NF-κB activation is a frequent process during viral infections, e.g., influenza A virus (7), alphavirus (36), dengue virus (40), Newcastle disease virus (69), and human T-cell leukemia virus type 1 (67). In some cases NF-κB has been shown to be required for maximal viral replication. NF-κB activation is thought to provide a cellular environment favorable for efficient viral nucleic acid or protein synthesis, intracellular transport of viral proteins, or assembly and release of progeny virions. Thus, viral replication may be impaired by PDTC inhibition of NF-κB activation.
In the case of encephalomyocarditis virus, it was shown recently that NF-κB knockout mice (p50−/−) survive infections that readily kill normal mice (63). However, NF-κB activation is not essential for virus multiplication in cell culture but is required for the inhibition of apoptosis and for prevention of premature cell death, thus affecting virulence in mice (60). In contrast, no significant apoptosis and/or cell death was detected when NF-κB activation by HRV was inhibited by PDTC treatment (Fig. 10D and F). PDTC was also shown to be involved in activation of pleiotropic transcription factors, e.g., AP-1 (41), transactivator C/EBPβ (9), and heat shock factor 1 (29). Activation of genes regulated by these factors may account for the stimulatory effect of PDTC on uninfected cells as observed in our experiments. Another biological property of PDTC is its activity as a chelator of Zn and Cu (42, 43). As many enzymatic functions are dependent on essential ions, chelation of these ions could lead to inhibition of enzyme function.
To clarify the mechanism of PDTC action, it would be extremely useful to obtain HRV variants resistant to PDTC. However, despite many attempts we have so far been unable to produce any PDTC-resistant HRV. Further long-term experiments are in progress to select for PDTC-resistant variants. Nevertheless, the fact that PDTC equally inhibits all tested HRV serotypes, whereas guanidine inhibits HRV14 but does not affect HRV2, can be taken to indicate that the mechanisms of action of these two inhibitors are different.
In conclusion, PDTC is an extremely potent antirhinoviral substance which reduces HRV growth and inhibits the CPE of infected cells in culture. PDTC also promotes cell viability following poliovirus infection. Elucidation of the antiviral mechanism of PDTC will be the subject of further research and should facilitate understanding of the complex interactions of viruses and cells which may eventually lead to novel approaches for the treatment of picornavirus infections such as the common cold, the most frequent infection of humans.
Acknowledgments
This work was supported by grant F508-MED from the Austrian Science Foundation to E.K. and by EMBO fellowship ASTF 9688 to J.S.
We thank D. Gruenert, San Francisco, Calif., for supplying 16HBE14o− cells, D. Blaas for providing HRV2 antibody, R. Perez-Bercoff for his hospitality, and Z. Rattler for stimulating discussions.
REFERENCES
- 1.Agol, V. I., G. A. Belov, K. Bienz, D. Egger, M. S. Kolesnikova, N. T. Raikhlin, L. I. Romanova, E. A. Smirnova, and E. A. Tolskaya. 1998. Two types of death of poliovirus-infected cells: caspase involvement in the apoptosis but not cytopathic effect. Virology 252:343-353. [DOI] [PubMed] [Google Scholar]
- 2.Badorff, C., G. H. Lee, B. Lamphear, M. E. Martone, K. P. Campbell, R. E. Rhoads, and K. U. Knowlton. 1999. Enteroviral protease 2A cleaves dystrophin: evidence of cytoskeletal disruption in an acquired cardiomyopathy. Nat. Med. 5:320-326. [DOI] [PubMed] [Google Scholar]
- 3.Bonifacino, J. S. (ed.). 1998. Current protocols in cell biology, p. 4.3.1-4.3.4. John Wiley & Sons, Inc., New York, N.Y.
- 4.Brennan, P., and L. A. O'Neill. 1996. 2-Mercaptoethanol restores the ability of nuclear factor kappa B (NF kappa B) to bind DNA in nuclear extracts from interleukin 1-treated cells incubated with pyrrolidine dithiocarbamate (PDTC). Evidence for oxidation of glutathione in the mechanism of inhibition of NF kappa B by PDTC. Biochem. J. 320:975-981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brennan, P., and L. A. O'Neill. 1995. Effects of oxidants and antioxidants on nuclear factor kappa B activation in three different cell lines: evidence against a universal hypothesis involving oxygen radicals. Biochim. Biophys. Acta 1260:167-175. [DOI] [PubMed] [Google Scholar]
- 6.Burkitt, M. J., H. S. Bishop, L. Milne, S. Y. Tsang, G. J. Provan, C. S. Nobel, S. Orrenius, and A. F. Slater. 1998. Dithiocarbamate toxicity toward thymocytes involves their copper-catalyzed conversion to thiuram disulfides, which oxidize glutathione in a redox cycle without the release of reactive oxygen species. Arch. Biochem. Biophys. 353:73-84. [DOI] [PubMed] [Google Scholar]
- 7.Bussfeld, D., M. Bacher, A. Moritz, D. Gemsa, and H. Sprenger. 1997. Expression of transcription factor genes after influenza A virus infection. Immunobiology 198:291-298. [DOI] [PubMed] [Google Scholar]
- 8.Carrasco, L. 1994. Picornavirus inhibitors. Pharmacol. Ther. 64:215-290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chinery, R., J. A. Brockman, D. T. Dransfield, and R. J. Coffey. 1997. Antioxidant-induced nuclear translocation of CCAAT/enhancer-binding protein beta. A critical role for protein kinase A-mediated phosphorylation of Ser299. J. Biol. Chem. 272:30356-30361. [DOI] [PubMed] [Google Scholar]
- 10.Connolly, J. L., S. E. Rodgers, P. Clarke, D. W. Ballard, L. D. Kerr, K. L. Tyler, and T. S. Dermody. 2000. Reovirus-induced apoptosis requires activation of transcription factor NF-κB. J. Virol. 74:2981-2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Della Ragione, F., V. Cucciolla, A. Borriello, V. Della Pietra, C. Manna, P. Galletti, and V. Zappia. 2000. Pyrrolidine dithiocarbamate induces apoptosis by a cytochrome c-dependent mechanism. Biochem. Biophys. Res. Commun. 268:942-946. [DOI] [PubMed] [Google Scholar]
- 12.Einarsson, O., G. P. Geba, Z. Zhu, M. Landry, and J. A. Elias. 1996. Interleukin-11: stimulation in vivo and in vitro by respiratory viruses and induction of airways hyperresponsiveness. J. Clin. Investig. 97:915-924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Erl, W., C. Weber, and G. K. Hansson. 2000. Pyrrolidine dithiocarbamate-induced apoptosis depends on cell type, density, and the presence of Cu2+ and Zn2+. Am. J. Physiol. Cell Physiol. 278:1116-1125. [DOI] [PubMed] [Google Scholar]
- 14.Etchison, D., and S. Fout. 1985. Human rhinovirus 14 infection of HeLa cells result in the proteolytic cleavage of the p220 cap-binding complex subunit and inactivates globin mRNA translation in vitro. J. Virol. 54:634-638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Etchison, D., S. C. Milburn, I. Edery, N. Sonenberg, and J. W. Hershey. 1982. Inhibition of HeLa cell protein synthesis following poliovirus infection correlates with the proteolysis of a 220,000-dalton polypeptide associated with eucaryotic initiation factor 3 and a cap binding protein complex. J. Biol. Chem. 257:14806-14810. [PubMed] [Google Scholar]
- 16.Flory, E., M. Kunz, C. Scheller, C. Jassoy, R. Stauber, U. R. Rapp, and S. Ludwig. 2000. Influenza virus-induced NF-κB-dependent gene expression is mediated by overexpression of viral proteins and involves oxidative radicals and activation of IκB kinase. J. Biol. Chem. 275:8307-8314. [DOI] [PubMed] [Google Scholar]
- 17.Garibaldi, R. A. 1985. Epidemiology of community-acquired respiratory tract infections in adults. Incidence, etiology, and impact. Am. J. Med. 78:32-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Glaser, W., and T. Skern. 2000. Extremely efficient cleavage of eIF4G by picornaviral proteinases L and 2A in vitro. FEBS Lett. 480:151-155. [DOI] [PubMed] [Google Scholar]
- 19.Gradi, A., Y. V. Svitkin, H. Imataka, and N. Sonenberg. 1998. Proteolysis of human eukaryotic translation initiation factor eIF4GII, but not eIF4GI, coincides with the shutoff of host protein synthesis after poliovirus infection. Proc. Natl. Acad. Sci. USA 95:11089-11094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gwaltney, J. M. 1975. Rhinoviruses. Yale J. Biol. Med. 48:17-45. [PMC free article] [PubMed] [Google Scholar]
- 21.Heinz, B. A., and L. M. Vance. 1995. The antiviral compound enviroxime targets the 3A coding region of rhinovirus and poliovirus. J. Virol. 69:4189-4197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang, R. P., J. X. Wu, Y. Fan, and E. D. Adamson. 1996. UV activates growth factor receptors via reactive oxygen intermediates. J. Cell Biol. 133:211-220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Israel, N., and M. A. Gougerot-Pocidalo. 1997. Oxidative stress in human immunodeficiency virus infection. Cell. Mol. Life Sci. 53:864-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Janssen, Y. M., and C. K. Sen. 1999. Nuclear factor kappa B activity in response to oxidants and antioxidants. Methods Enzymol. 300:363-374. [DOI] [PubMed] [Google Scholar]
- 25.Johannes, G., M. S. Carter, M. B. Eisen, P. O. Brown, and P. Sarnow. 1999. Identification of eukaryotic mRNAs that are translated at reduced cap binding complex eIF4F concentrations using a cDNA microarray. Proc. Natl. Acad. Sci. USA 96:13118-13123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johannes, G., and P. Sarnow. 1998. Cap-independent polysomal association of natural mRNAs encoding c-myc, BiP, and eIF4G conferred by internal ribosome entry sites. RNA 4:1500-1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kalebic, T., A. Kinter, G. Poli, M. E. Anderson, A. Meister, and A. S. Fauci. 1991. Suppression of human immunodeficiency virus expression in chronically infected monocytic cells by glutathione, glutathione ester, and N-acetylcysteine. Proc. Natl. Acad. Sci. USA 88:986-990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaul, P., M. C. Biagioli, I. Singh, and R. B. Turner. 2000. Rhinovirus-induced oxidative stress and interleukin-8 elaboration involves p47-phox but is independent of attachment to intercellular adhesion molecule-1 and viral replication. J. Infect. Dis. 181:1885-1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, S. H., S. I. Han, S. Y. Oh, H. Y. Chung, H. D. Kim, and H. S. Kang. 2001. Activation of heat shock factor 1 by pyrrolidine dithiocarbamate is mediated by its activities as pro-oxidant and thiol modulator. Biochem. Biophys. Res. Commun. 281:367-372. [DOI] [PubMed] [Google Scholar]
- 30.Kräusslich, H. G., M. J. Nicklin, H. Toyoda, D. Etchison, and E. Wimmer. 1987. Poliovirus proteinase 2A induces cleavage of eucaryotic initiation factor 4F polypeptide p220. J. Virol. 61:2711-2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lamphear, B. J., R. Q. Yan, F. Yang, D. Waters, H. D. Liebig, H. Klump, E. Kuechler, T. Skern, and R. E. Rhoads. 1993. Mapping the cleavage site in protein synthesis initiation factor-eIF-4-gamma of the 2A proteases from human coxsackievirus and rhinovirus. J. Biol. Chem. 268:19200-19203. [PubMed] [Google Scholar]
- 32.Lenk, R., and S. Penman. 1979. The cytoskeletal framework and poliovirus metabolism. Cell 16:289-301. [DOI] [PubMed] [Google Scholar]
- 33.Levine, B., Q. Huang, J. T. Isaacs, J. C. Reed, D. E. Griffin, and J. M. Hardwick. 1993. Conversion of lytic to persistent alphavirus infection by the bcl-2 cellular oncogene. Nature 361:739-742. [DOI] [PubMed] [Google Scholar]
- 34.Li, W. G., L. Coppey, R. M. Weiss, and H. J. Oskarsson. 2001. Antioxidant therapy attenuates JNK activation and apoptosis in the remote noninfarcted myocardium after large myocardial infarction. Biochem. Biophys. Res. Commun. 280:353-357. [DOI] [PubMed] [Google Scholar]
- 35.Liebig, H. D., E. Ziegler, R. Yan, K. Hartmuth, H. Klump, H. Kowalski, D. Blaas, W. Sommergruber, L. Frasel, B. Lamphear, R. Rhoads, E. Kuechler, and T. Skern. 1993. Purification of two picornaviral 2A proteinases: interaction with eIF-4 gamma and influence on in vitro translation. Biochemistry 32:7581-7588. [DOI] [PubMed] [Google Scholar]
- 36.Lin, K. I., S. H. Lee, R. Narayanan, J. M. Baraban, J. M. Hardwick, and R. R. Ratan. 1995. Thiol agents and Bcl-2 identify an alphavirus-induced apoptotic pathway that requires activation of the transcription factor NF-kappa B. J. Cell Biol. 131:1149-11461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lo, Y. Y., and T. F. Cruz. 1995. Involvement of reactive oxygen species in cytokine and growth factor induction of c-fos expression in chondrocytes. J. Biol. Chem. 270:11727-11730. [DOI] [PubMed] [Google Scholar]
- 38.Macejak, D. G., and P. Sarnow. 1991. Internal initiation of transcription mediated by the 5′ leader of a cellular mRNA. Nature 353:90-94. [DOI] [PubMed] [Google Scholar]
- 39.Malorni, W., R. Rivabene, M. T. Santini, and G. Donelli. 1993. N-Acetylcysteine inhibits apoptosis and decreases viral particles in HIV-chronically infected U937 cells. FEBS Lett. 327:75-78. [DOI] [PubMed] [Google Scholar]
- 40.Marianneau, P., A. Cardona, L. Edelman, V. Deubel, and P. Despres. 1997. Dengue virus replication in human hepatoma cells activates NF-κB which in turn induces apoptotic cell death. J. Virol. 71:3244-3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Meyer, M., R. Schreck, and P. A. Baeuerle. 1993. H2O2 and antioxidants have opposite effects on activation of NF-kappa B and AP-1 in intact cells: AP-1 as secondary antioxidant-responsive factor. EMBO J. 12:2005-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morpurgo, L., E. Agostinelli, O. Befani, and B. Mondovi. 1987. Reactions of bovine serum amine oxidase with N,N-diethyldithiocarbamate. Selective removal of one copper ion. Biochem. J. 248:865-870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morpurgo, L., A. Desideri, A. Rigo, P. Viglino, and G. Rotilio. 1983. Reaction of N,N-diethyldithiocarbamate and other bidentate ligands with Zn, Co and Cu bovine carbonic anhydrases. Inhibition of the enzyme activity and evidence for stable ternary enzyme-metal-ligand complexes. Biochim. Biophys. Acta 746:168-175. [DOI] [PubMed] [Google Scholar]
- 44.Mortola, E., M. Okuda, K. Ohno, T. Watari, H. Tsujimoto, and A. Hasegawa. 1998. Inhibition of apoptosis and virus replication in feline immunodeficiency virus-infected cells by N-acetylcysteine and ascorbic acid. J. Vet. Med. Sci. 60:1187-1193. [DOI] [PubMed] [Google Scholar]
- 45.Naclerio, R. M., D. Proud, L. M. Lichtenstein, A. Kagey-Sobotka, J. O. Hendley, J. Sorrentino, and J. M. Gwaltney. 1988. Kinins are generated during experimental rhinovirus colds. J. Infect. Dis. 157:133-142. [DOI] [PubMed] [Google Scholar]
- 46.Noah, T. L., F. W. Henderson, I. A. Wortman, R. B. Devlin, J. Handy, H. S. Koren, and S. Becker. 1995. Nasal cytokine production in viral acute upper respiratory infection of childhood. J. Infect. Dis. 171:584-592. [DOI] [PubMed] [Google Scholar]
- 47.Nobel, C. I., M. Kimland, B. Lind, S. Orrenius, and A. F. Slater. 1995. Dithiocarbamates induce apoptosis in thymocytes by raising the intracellular level of redox-active copper. J. Biol. Chem. 270:26202-26208. [DOI] [PubMed] [Google Scholar]
- 48.Nobel, C. S., D. H. Burgess, B. Zhivotovsky, M. J. Burkitt, S. Orrenius, and A. F. Slater. 1997. Mechanism of dithiocarbamate inhibition of apoptosis: thiol oxidation by dithiocarbamate disulfides directly inhibits processing of the caspase-3 proenzyme. Chem. Res. Toxicol. 10:636-643. [DOI] [PubMed] [Google Scholar]
- 49.Nobel, C. S., M. Kimland, D. W. Nicholson, S. Orrenius, and A. F. Slater. 1997. Disulfiram is a potent inhibitor of proteases of the caspase family. Chem. Res. Toxicol. 10:1319-1324. [DOI] [PubMed] [Google Scholar]
- 50.Orrenius, S., C. S. Nobel, D. J. van den Dobbelsteen, M. J. Burkitt, and A. F. Slater. 1996. Dithiocarbamates and the redox regulation of cell death. Biochem. Soc. Trans. 24:1032-1038. [DOI] [PubMed] [Google Scholar]
- 51.Ozaki, K., H. Takeda, H. Iwahashi, S. Kitano, and S. Hanazawa. 1997. NF-κB inhibitors stimulate apoptosis of rabbit mature osteoclasts and inhibit bone resorption by these cells. FEBS Lett. 410:297-300. [DOI] [PubMed] [Google Scholar]
- 52.Pelham, H. R., and R. J. Jackson. 1976. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur. J. Biochem. 67:247-256. [DOI] [PubMed] [Google Scholar]
- 53.Pitkaranta, A., and F. G. Hayden. 1998. What's new with common colds? Pathogenesis and diagnosis. Infect. Med. 15:50-59. [Google Scholar]
- 54.Proud, D., C. R. Baumgarten, R. M. Naclerio, and P. E. Ward. 1987. Kinin metabolism in human nasal secretions during experimentally induced allergic rhinitis. J. Immunol. 138:428-434. [PubMed] [Google Scholar]
- 55.Reed, L. J., and H. Muench. 1938. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 27:493-497. [Google Scholar]
- 56.Rightsel, W. A., J. R. Dice, R. J. McAlpine, E. A. Timm, I. W. McLean, G. J. Dixon, and F. M. Schabel. 1961. Antiviral effect of guanidine. Science 134:558-559. [DOI] [PubMed] [Google Scholar]
- 57.Rubinstein, S. J., T. Hammerle, E. Wimmer, and A. Dasgupta. 1992. Infection of HeLa cells with poliovirus results in modification of a complex that binds to the rRNA promoter. J. Virol. 66:3062-3068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schreck, R., K. Albermann, and P. A. Baeuerle. 1992. Nuclear factor kappa B: an oxidative stress-responsive transcription factor of eukaryotic cells. Free Radic. Res. Commun. 17:221-237. [DOI] [PubMed] [Google Scholar]
- 59.Schreck, R., B. Meier, D. N. Mannel, W. Droge, and P. A. Baeuerle. 1992. Dithiocarbamates as potent inhibitors of nuclear factor kappa B activation in intact cells. J. Exp. Med. 175:1181-1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schwarz, E. M., C. Badorff, T. S. Hiura, R. Wessely, A. Badorff, I. M. Verma, and K. U. Knowlton. 1998. NF-κB-mediated inhibition of apoptosis is required for encephalomyocarditis virus virulence: a mechanism of resistance in p50 knockout mice. J. Virol. 72:5654-5660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schweizer, M., and E. Peterhans. 1999. Oxidative stress in cells infected with bovine viral diarrhea virus: a crucial step in the induction of apoptosis. J. Gen. Virol. 80:1147-1155. [DOI] [PubMed] [Google Scholar]
- 62.Seipelt, J., H. D. Liebig, W. Sommergruber, C. Gerner, and E. Kuechler. 2000. 2A proteinase of human rhinovirus cleaves cytokeratin 8 in infected HeLa cells. J. Biol. Chem. 275:20084-20089. [DOI] [PubMed] [Google Scholar]
- 63.Sha, W. C., H. C. Liou, E. I. Tuomanen, and D. Baltimore. 1995. Targeted disruption of the p50 subunit of NF-kappa B leads to multifocal defects in immune responses. Cell 80:321-330. [DOI] [PubMed] [Google Scholar]
- 64.Shibayama, Y., D. Skoner, S. Suehiro, J. E. Konishi, P. Fireman, and A. P. Kaplan. 1996. Bradykinin levels during experimental nasal infection with rhinovirus and attenuated influenza virus. Immunopharmacology 33:311-313. [DOI] [PubMed] [Google Scholar]
- 65.Skern, T., W. Sommergruber, D. Blaas, C. Pieler, and E. Kuechler. 1984. Relationship of human rhinovirus strain 2 and poliovirus as indicated by comparison of the polymerase gene regions. Virology 136:125-132. [DOI] [PubMed] [Google Scholar]
- 66.Sommergruber, W., H. Ahorn, A. Zöphel, I. Maurer-Fogy, F. Fessl, G. Schnorrenberg, H. D. Liebig, D. Blaas, E. Kuechler, and T. Skern. 1992. Cleavage specificity on synthetic peptide substrates of human rhinovirus 2 proteinase 2A. J. Biol. Chem. 267:22639-22644. [PubMed] [Google Scholar]
- 67.Sun, S. C., and D. W. Ballard. 1999. Persistent activation of NF-κB by the tax transforming protein of HTLV-1: hijacking cellular IκB kinases. Oncogene 18:6948-6958. [DOI] [PubMed] [Google Scholar]
- 68.Tershak, D. R. 1982. Guanidine-resistant defective interfering particles of poliovirus. J. Virol. 41:615-625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Umansky, V., V. A. Shatrov, V. Lehmann, and V. Schirrmacher. 1996. Induction of NO synthesis in macrophages by Newcastle disease virus is associated with activation of nuclear factor-kappa B. Int. Immunol. 8:491-498. [DOI] [PubMed] [Google Scholar]
- 70.Vile, G. F. 1997. Active oxygen species mediate the solar ultraviolet radiation-dependent increase in the tumour suppressor protein p53 in human skin fibroblasts. FEBS Lett. 412:70-74. [DOI] [PubMed] [Google Scholar]
- 71.Weiss, L., E. Hildt, and P. H. Hofschneider. 1996. Anti-hepatitis B virus activity of N-acetyl-l-cysteine (NAC): new aspects of a well-established drug. Antivir. Res. 32:43-53. [DOI] [PubMed] [Google Scholar]
- 72.Wild, A. C., and R. T. Mulcahy. 1999. Pyrrolidine dithiocarbamate up-regulates the expression of the genes encoding the catalytic and regulatory subunits of gamma-glutamylcysteine synthetase and increases intracellular glutathione levels. Biochem. J. 338:659-665. [PMC free article] [PubMed] [Google Scholar]
- 73.Wu, H. H., and J. Momand. 1998. Pyrrolidine dithiocarbamate prevents p53 activation and promotes p53 cysteine residue oxidation. J. Biol. Chem. 273:18898-18905. [DOI] [PubMed] [Google Scholar]
- 74.Wu, H. H., J. A. Thomas, and J. Momand. 2000. p53 protein oxidation in cultured cells in response to pyrrolidine dithiocarbamate: a novel method for relating the amount of p53 oxidation in vivo to the regulation of p53-responsive genes. Biochem. J. 351:87-93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yalamanchili, P., K. Harris, E. Wimmer, and A. Dasgupta. 1996. Inhibition of basal transcription by poliovirus: a virus-encoded protease (3Cpro) inhibits formation of TBP-TATA box complex in vitro. J. Virol. 70:2922-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan, R., W. Rychlik, D. Etchison, and R. E. Rhoads. 1992. Amino acid sequence of the human protein synthesis initiation factor eIF-4g. J. Biol. Chem. 267:23226-23231. [PubMed] [Google Scholar]
- 77.Zhu, Z., W. Tang, A. Ray, Y. Wu, O. Einarsson, M. L. Landry, J. Gwaltney, and J. A. Elias. 1996. Rhinovirus stimulation of interleukin-6 in vivo and in vitro. Evidence for nuclear factor kappa B-dependent transcriptional activation. J. Clin. Investig. 97:421-430. [DOI] [PMC free article] [PubMed] [Google Scholar]