Abstract
Human herpesvirus 6 (HHV-6), a latent lymphotropic and neurotropic virus, has been suspected as an etiologic agent in multiple sclerosis (MS). The study was undertaken to correlate virologic evidence for HHV-6 activity with the state of host immunity to HHV-6 in MS patients and control subjects. The study revealed that cell-free DNA of HHV-6 was detected more frequently in both serum and cerebrospinal fluid of MS patients than in those of control subjects. T cells recognizing the recombinant 101-kDa protein (101K) corresponding to the major immunoreactive region unique to HHV-6 occurred at significantly lower precursor frequency in MS patients than in control subjects. The resulting HHV-6-specific T-cell lines obtained from MS patients exhibited skewed cytokine profiles characterized by the inability to produce interleukin-4 (IL-4) and IL-10. The decreased T-cell responses to HHV-6 and the altered cytokine profile were consistent with significantly declined serum immunoglobulin G (IgG) titers for HHV-6 of MS patients compared to those of control subjects. In contrast, elevated serum IgM titers for HHV-6 were detected in the majority of MS patients, which may reflect frequent exposure of B cells to HHV-6. The findings suggest that the decreased immune responses to HHV-6 may be responsible for ineffective clearance of HHV-6 in MS patients.
It has long been suspected that microbial infections, in particular viral infections, may play an important role in the etiology and pathogenesis of multiple sclerosis (MS). A number of viruses, including human herpesevirus 6 (HHV-6), measles virus, and Epstein-Barr virus, have been implicated as etiologic agents in MS on the basis of epidemiological evidence, geographic pattern, and abnormal immune response to these viruses (10, 11, 16, 17, 18, 45). Some studies demonstrated increased antibody titers to the viruses, whereas others described isolation of viruses from postmortem MS material (29). The potential pathological importance of certain viral infections in patients with MS is thought to involve direct neurotropic properties of the virus that cause tissue damage or their ability to activate autoimmune responses directed at myelin tissue through various mechanisms. Some viruses carry amino-acid-sequence homology with myelin proteins (e.g., myelin basic protein) and can induce activation of autoreactive T cells recognizing myelin antigens through molecular mimicry (15, 39, 47). Neurotropic viruses can also cause direct tissue/myelin damage, resulting in sensitization of autoreactive T cells in response to myelin breakdown products/antigens. Furthermore, viral infections of the central nervous system can also induce autoimmune responses through epitope spreading (27) and superantigen activity (33, 40, 43). However, to date the issues related to the etiologic role of virus in MS remain unresolved.
HHV-6 has predominant tropism for CD4+ T cells and is the etiologic agent for infantile exanthem (7). Genomic analysis places HHV-6 among the betaherpesviruses, along with cytomegalovirus (CMV) and HHV-7. On the basis of DNA restriction analysis, HHV-6 can be separated into two strains, HHV-6A and HHV-6B (1, 22, 34). Only HHV-6B has been definitively proven to cause human disease (44, 49). HHV-6 also has a neurotropic property (3) and was found to cause subacute leukoencephalitis, manifesting as MS and demyelination of the central nervous system (8, 19). Epidemiologic studies have shown that the vast majority of primary HHV-6 infections occur within the first year of life (13, 50). Common to other herpesviruses, latency of HHV-6 is established after primary infection, and its genomic material can be found in T cells of healthy adults. HHV-6 infection can reactivate under certain conditions, such as in immunocompromised patients (34).
The relationship between HHV-6 infection and MS was first suggested by Challoner and colleagues, who detected DNA sequence identical to that of the major DNA binding protein of HHV-6 in postmortem MS brain (9). Expression of HHV-6 virion proteins was found in oligodendrocytes obtained from patients with MS (9). More recent studies demonstrated higher titers of antibodies to HHV-6 and cell-free DNA of HHV-6 in serum and cerebrospinal fluid (CSF) of MS patients, suggesting reactivation of HHV-6 in MS (1-2, 4, 20-23, 29, 35-37, 46, 50). However, other investigators found no differences in these measurements between MS patients and control subjects, failing to confirm such an association (6, 14, 24, 26, 28, 30, 41). It is important to note that the assays reported in some of these studies were based on the whole HHV-6 virus, which contains viral protein components sharing extensive amino-acid-sequence homology with other related viral proteins, such as CMV and HHV-7, thus making the results difficult to interpret. Other studies used various components of HHV-6, whose immunogenicity is unproven for the detection of antibody titers.
This study was undertaken to evaluate the possibility of active viral replication of HHV-6 by detecting cell-free viral DNA released in serum and CSF of MS patients compared to that of control subjects. We then attempted to correlate virologic evidence with the state of host-specific immunity and examined both T-cell responses and the antibody reactivity to HHV-6 in MS patients as well as in control subjects. In this study, oligonucleotide primers and recombinant protein/truncated fragments used to evaluate the virologic as well as immunologic evidence were designed to correspond to the 101-kDa virion protein (101K protein) of HHV-6. The 101K virion protein was originally identified in an HHV-6B strain (Z29) and was found to have 81% sequence homology with HHV-6A (32). It represents the major immunoreactive virion protein of HHV-6 and has been identified as a unique protein component for HHV-6 (32, 48). The recombinant 101K protein and truncated fragments excluded the sequence region homologues from those shared by CMV, HHV-7, and other related viruses, providing the crucial specificity for the assays used in the study. The findings described here have important implications in our understanding of an etiologic role of HHV-6 in MS.
MATERIALS AND METHODS
Patients and specimens.
A group of randomly selected MS patients (n = 33) and a group of control subjects, including 21 healthy individuals and 6 individuals with other inflammatory neurologic diseases (polymyositis and myathenia gravis), were studied. They had not taken any immunosuppressive drugs, including steroids, or immunomodulatory agents (e.g., beta interferon [IFN-β] and Glatiramer Acetate) at least 9 months prior to enrolling in the study and throughout the study. Informed consent was obtained from the patients after the experimental procedures were explained. The protocol was approved by the Institutional Human Subject Committee at Baylor College of Medicine. A panel of CSF specimens derived from patients with MS and other neurologic diseases was obtained from the CSF specimen bank (courtesy of S. Appel and L. Schneider) and was used for the analysis of cell-free DNA and antibody reactivity. These CSF specimens were obtained from newly diagnosed patients with definitive MS (untreated) and from other neurologic diseases (control specimens), including Alzheimer's disease, myasthenia gravis, amyotrophic lateral sclerosis, ischemic stroke, Parkinson's disease, and polymyositis. Both serum and CSF specimens were centrifuged at 9,000 × g for 5 min and stored immediately at −80°C for 1 month (serum specimens) or 8 to 12 months (CSF specimens).
Reagents and peptides.
Media used for cell culture were AIM-V serum-free medium (Gibco BRL, Grand Island, N.Y.) and RPMI 1640 supplemented with l-glutamine, sodium pyruvate, nonessential amino acids, 10 mM HEPES buffer (Gibco), 10% (vol/vol) fetal bovine serum (FBS) (Gibco), and 1% penicillin-streptomycin (Gibco). Recombinant human IL-2 was purchased from Boehringer Mannheim (Indianapolis, Ind.). Hen egg lysozyme was obtained from Sigma (St. Louis, Mo.) and served as a control antigen for the T-cell analysis.
Recombinant 101K protein and truncated fragments of HHV-6.
The following procedure was used to prepare the recombinant viral protein and the fragments by using recombinant DNA technology. pH 6Z-3001, a pUC19 vector with an insert containing the complete HHV-6 101K open reading frame (ORF) (nucleotides 352 to 2925), was provided by the Centers for Disease Control and Prevention (courtesy of P. E. Pellet) (27). DNA of the HHV-6 101K ORF was amplified from the pH 6Z-3001 vector by PCR with a pair of specific primers (sense, 5′-GAAGACAGCAGCGAGATAGAA; antisense, 5′-CGACGCGATCACTGACTTGTCTTTGGC) which were 462 bases downstream of the start codon and terminated adjacent to the stop codon. The PCR conditions were as follows: 25 cycles of 94°C for 50 s, 56°C for 50 s, 72°C for 2 min, and one final extension for 10 min in a PE9700 thermal cycler (Perkin Elmer, Foster City, Calif.). The PCR products were cloned into pCR2.1 TA cloning vector (Invitrogen, Carlsbad, Calif.) and sequenced completely. The four HHV-6 fragments corresponding to the HHV-6 101K protein were prepared as described above. Primers used for each fragment were F1 (sense, 5′-GAAGACAGCAGCGAGATAGAA; antisense, 5′-ATCCGACTCTGGAAATTTATG), F2 (sense, 5′-CAGAGGCGACATAAATTTCC; antisense, 5′-AGAAACGTTATGGGGCGACA), F3 (sense, 5′-ACCGGAGTGTCGCCCCATAA; antisense, 5′-TCGCTGCCCAAGACCCGCT), and F4 (sense, 5′-GCCGGAGAGGAACAGTATGTTCAAGCG; antisense, 5′-CGACGCGATCACTGACTTGTCTTTGGC). PCR was carried out under universal PCR conditions for a total of 25 cycles for each amplification. The expression plasmid was constructed as follows. DNA of the 101K ORF and F1 through F4 fragments were digested with a pCR2.1 TA cloning vector with XhoI and HindIII restriction endonucleases and were cloned into a pRSET expression vector (Invitrogen) in frame, which carried the T7 promoter sequence and a sequence encoding an N-terminal histidine fusion tag. Segments of the 101K ORF encompassing residues 155 to 858, F1 (residues 155 to 350), F2 (residues 341 to 526), F3 (residues 518 to 667), and F4 (residues 654 to 858) were expressed in Escherichia coli strain BL21 (DE3) by optimal induction with 1 mM isopropyl-β-d-thiogalactopyranoside and subsequently purified by using nickel-chelating affinity binding to the histidine tag. The purified protein and the fragments were passed through a Detoxi-Gel endotoxin removing affinity gel (Pierce, Rockford, Ill.) three times to remove trace endotoxins.
Detection of cell-free viral DNA in sera and CSF by nested PCR and Southern hybridization.
Cell-free DNA was extracted from sera and CSF specimens by using the QIAmp DNA purification method (QIAGEN) according to the manufacturer's instructions. To achieve the highest sensitivity for detection of viral DNA, the extracted DNA was amplified by nested PCR with a set of nested primers followed by Southern hybridization. The outer primer was a 101K ORF primer, the inner primers were a sense primer for the F2 fragment and an antisense primer for F3. The sensitivity of the nested PCR was estimated with a quantitative PCR amplification and hybridization of an absolute certainty of pH 6Z-3001 vector with a series of dilutions, which allowed the detection of 5 copies of pH 6Z-3001. For all experiments, 5 μl of extracted DNA was subjected to a first-stage PCR with a total reaction volume of 20 μl for 35 cycles. The second-stage PCR was performed by adding 2 μl of the PCR products from the first-stage PCR as template to a total reaction volume of 20 μl for 30 cycles. Ten microliters of the second-stage PCR products was subjected to 1% agarose gel electrophoresis and vacuum-blotted to a positively charged nylon membrane (Amersham, Piscatway, N.J.). F2 antisense primer was labeled with [γ-32P]ATP (ICN, Irvine, Calif.) at the 5′ terminus, with T4 polynucleotide kinase (Biolabs, Beverly, Mass.) as the probe for Southern hybridization. All CSF specimens or serum specimens were evaluated within one set of experiments, respectively, to minimize variations between individual specimens.
Immunoblot analysis of anti-HHV-6 antibodies in CSF.
Recombinant 101K protein of HHV-6 was electrophoresed on a sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis gel. After blotting, nitrocellulose membrane was cut into strips and then blocked with 5% low-fat milk in Tris-buffered saline (TBS) containing 0.1% Tween 20 (5% TBS-T). The strips were then incubated with undiluted CSF in mini-incubation trays for 1 h at room temperature. A goat anti-human immunoglobulin (Ig) conjugated with horseradish peroxidase was used as a secondary antibody (100 ng/ml in 5% TBS-T) and incubated for 1 h at room temperature. The strips were washed five times, followed by ECL visualization of proteins on membrane (Amersham, Arlington Hights, Ill.).
The precursor frequency analysis of specific T cells.
The method for precursor frequency analysis of specific T cells was described previously (51, 52). Briefly, peripheral blood mononuclear cells (PBMC) were prepared from fresh heparinized venous blood by Ficoll gradient separation. PBMC were plated out at 200,000 cells/well in the presence of the 101K protein (35 μg/ml), the truncated fragments (30 μg/ml), and a control antigen (hen egg lysozyme, 40 μg/ml), respectively. The cell number per well had been predetermined as an optimal cell density to detect specific T cells in this system. Seven days later, each culture was examined for specific proliferation to the corresponding antigen in proliferation assays. Briefly, each well was split into four aliquots (approximately 104 cells per aliquot) and cultured in duplicate with irradiated (6,000 rads) autologous PBMC (105 cells per well) in the presence or absence of the corresponding antigens. Cultures were kept for three days and were pulsed with [methyl-3H]thymidine (Amersham) at 1 μCi per well during the last 16 h of culture. Cells were then harvested with an automated cell harvester (Tomtec, Orange, Conn.), and [methyl-3H]thymidine incorporation was measured in a Microbeta Trilux counter (Wallac, Turku, Finland). A well or culture was defined as specific for the corresponding antigen when the counts per minute were greater than 1,500 and exceeded the reference counts per minute (in the absence of the antigen) by at least three times. The frequency of specific T cells was then estimated by dividing the number of specific wells by the total number of PBMC seeded in the initial culture (51, 52). The same method of calculation was used consistently to compare the changes of the T-cell frequency throughout the study.
Cytokine measurement.
The cytokine profile of the resulting HHV-6-specific T-cell lines was determined quantitatively by using enzyme-linked immunosorbent assay (ELISA) kits (PharMingen, San Diego, Calif.) according to the manufacturer's instructions. Culture supernatants were collected 48 h after stimulation of the T-cell lines with the corresponding peptide. Microtiter plates (96 wells; Maxisorp; NUNC, Roskilde, Denmark) were coated overnight at 4°C with 1-μg/ml concentrations of a purified mouse capturing monoclonal antibody to human tumor necrosis factor alpha (TNF-α), IFN-γ, IL-4, and IL-10 in 100 μl of a carbonate buffer (100 mM, pH 9.5). Plates were washed 5 times with phosphate-buffered saline solution (PBS; pH 7.0) containing 0.05% Tween 20 (PBS-T). Nonspecific binding sites were saturated with 10% (wt/vol) FBS in PBS (FBS-PBS) for 1 h and washed subsequently with PBS-T. Supernatants and cytokine standards were added in duplicate wells. Plates were incubated at 4°C overnight and subsequently washed 5 times with PBS-T. One hundred microliters of the matched biotinylated detecting antibody (0.5 μg/ml for IL-4 and IL-10 and 1 μg/ml for IFN-γ and TNF-α; PharMingen) were added to each well and incubated at room temperature for 2 h. Plates were subsequently washed and incubated with a streptavidin-conjugated horseradish peroxidase (1:5,000 dilution) for 1 h. Plates were then washed and 3,3′,5,5′-tetramethylbenzidine (TMB; Sigma) was used as a substrate. The reaction was stopped by adding 1 N HCl. Optical density was measured at 450 nm with an ELISA reader (Bio-Rad Laboratories, Hercules, Calif.), and cytokine concentrations were quantitated by Microplate computer software (Bio-Rad) using a double eight-point standard. The detection limits for these cytokine measurements were said to range from 15 pg/ml to 25 pg/ml.
Flow cytometry.
To analyze the surface expression of CD4 and CD8, 105 cells of each T-cell line were washed in PBS containing 1% FBS and 0.1% sodium azide (FBS-PBS) and resuspended in 100 μl FBS-PBS containing a 1:100 dilution of fluorochrome-labeled antibody (Simultest CD4-FITC/CD8-PE, Leu-3a/2a; Becton Dickinson Immunocytometry Systems, San Jose, Calif.) or appropriate Ig isotype controls (γ2a-FITC/γ1-PE; Becton Dickinson Immunocytometry Systems). After incubation for 30 min on ice, the cells were washed three times in FBS-PBS and fixed in 2% formaldehyde for flow cytometry assay. Stained cell populations were analyzed with a Coulter Epics instrument (Coulter Co., Miami, Fla.) with band filters for phycoethrin (575 nm) and fluorescein isothiocyanate(525 nm).
Detection of specific IgG and IgM titers for the HHV-6 antigens.
All serum specimens were tested for titers of IgG and IgM specific for the recombinant 101K protein by ELISA. Recombinant hepatitis B vaccine (HBV) (Engerix-B; SmithKline Beecham Biologicals, Rixensart, Belgium) was used as control antigen for serum antibody detection. Briefly, microtiter plates were coated with the 101K protein and HBV (1 μg/ml), respectively, and incubated at 4°C overnight. Plates were washed 5 times with PBS-T. Nonspecific binding sites were saturated with FBS-PBS for 1 h and washed again. Each serum specimen was diluted at 1:50, 1:100, 1:500, 1:1,000, and 1:2,000 with FBS-PBS and was added in duplicate wells. Plates were incubated at 4°C overnight and subsequently washed 5 times with PBS-T. One hundred microliters of biotinylated detecting antibody to human IgG or IgM (Sigma) was added to each well and incubated at room temperature for 2 h. After being washed, avidin-conjugated horseradish peroxidase (1:5,000 dilution) was added and plates were incubated for 1 h. Plates were then washed and TMB (Sigma) was used as a substrate for color development. Optical density was measured at 450 nm by using an ELISA reader (Bio-Rad Laboratories), and antibody titers were determined by Microplate computer software (Bio-Rad).
Statistical analysis.
A Student's t test for normally distributed variables and the Mann-Whitney rank-sum test for abnormally distributed variables was used for data analysis. A P value of <0.05 was considered statistically significant.
RESULTS
The detection of cell-free viral DNA of HHV-6 in sera and CSF specimens obtained from MS patients and control subjects.
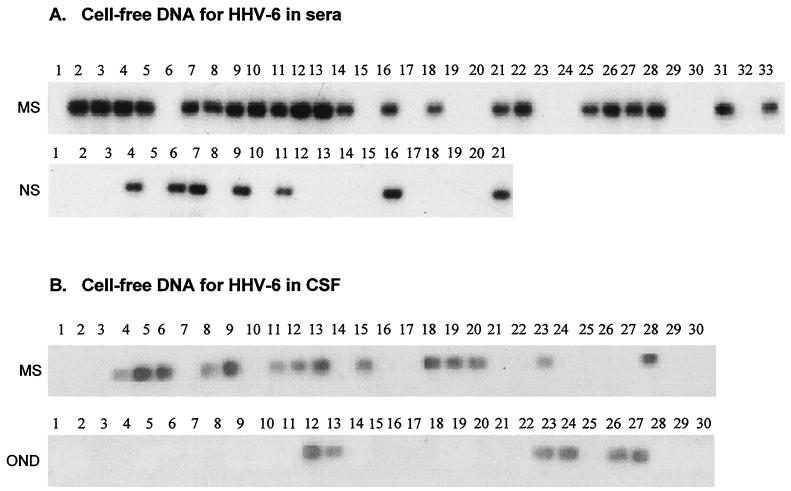
The clinical characteristics of MS patients and control subjects included in the study are shown in Table 1. Serum cell-free DNA was analyzed in a semiquantitative nested PCR with specific primers corresponding to the 101K region of HHV-6. As shown in Fig. 1A, serum cell-free DNA for HHV-6 was detected in 22 out of 33 MS patients (66%) examined; serum cell-free DNA for HHV-6 was detected in 7 out of 21 (33%) control subjects. The analysis was then extended under the same experimental conditions to CSF specimens derived from a separate group of MS patients (n = 30) and a control group of patients with other neurologic diseases (n = 30), which were obtained from a CSF specimen bank (see Materials and Methods). Cell-free DNA for HHV-6 was detected in 14 out of 30 (46%) CSF specimens obtained from MS patients, while only 6 out of 30 (20%) control CSF specimens were found positive for cell-free DNA for HHV-6 (Fig. 1B). There was no significant difference in the percentage of positive cell-free DNA between relapsing-remitting MS patients and secondary progressive MS patients. The findings prompted us to further characterize specific immune responses to HHV-6 in patients with MS and in control subjects.
TABLE 1.
Clinical characteristics of MS patients and control subjects
| Subject | Type of specimen | Analysis | n | Avg age | Sex (male, female) | MS status (relapsing-remitting, secondary progressive) | Mean disease duration (yr) | Mean EDSSf |
|---|---|---|---|---|---|---|---|---|
| MS | Blood | Cell-free DNA, antibody titers | 33 | 46 | 11, 22 | 27, 6 | 8.1 | 4.1 |
| T-cell frequencya | 17 | 48 | 5, 12 | 5, 12 | 14.8 | 5.2 | ||
| CSFb | Cell-free DNA | 30 | 38 | 9, 21 | 26, 4 | 6.2 | 3.4 | |
| Control | Bloodc | Cell-free DNA, antibody titers | 21 | 34 | 5, 16 | |||
| T-cell frequencyd | 18 | 38 | 7, 11 | |||||
| CSFe | Cell-free DNA | 30 | 42 | 12, 18 |
The frequency analysis of T cells was examined in 17 of 33 MS patients in the initial MS group.
Thirty CSF specimens obtained from a CSF specimen bank were derived from a separate group of MS patients.
Healthy individuals.
Twelve of 21 healthy individuals and 6 individuals with other inflammatory neurologic diseases (4 with polymyositis and 2 with myathenia gravis) were examined for T-cell frequency.
CSF specimens obtained from a CSF specimen bank were derived from a group of patients with other neurologic diseases (see Materials and Methods).
EDSS, expanded disability status score.
FIG. 1.
Detection of cell-free viral DNA for HHV-6 in sera and CSF obtained from MS patients and control subjects. Cell-free DNA for HHV-6 was detected in sera and CSF specimens by nested PCR with specific primers corresponding to the 101K region. The amplified PCR products were then hybridized with a digoxigenin-labeled oligonucleotide probe specific for HHV-6. The sensitivity of the detection was estimated to be 5 DNA copies/μl, as described in Material and Methods. (A) Detection of cell-free DNA for HHV-6 in serum specimens of 33 MS patients (MS) and 21 control subjects (Controls). (B) Detection of cell-free DNA for HHV-6 in CSF specimens of a separated group of 30 MS patients (MS) and 30 patients with other neurologic diseases (OND).
T-cell responses to HHV-6 in MS patients and control subjects.
A recombinant HHV-6 101K virion protein and four truncated fragments encompassing residues 155 to 350 (F1), residues 341 to 526 (F2), residues 518 to 667 (F3), and residues 654 to 858 (F4) of the 101K region (Table 2) were prepared and used to examine both the T-cell and antibody responses. The N-terminal residues (1 to 155) were excluded for their extensive sequence homology with the immunoreactive region of human CMV (32).
TABLE 2.
Amino acid sequence of the HHV-6 101K virion protein fragments
| Fragment (residues) | Sequencea |
|---|---|
| F1 (155-350) | 155 EDSSEIENNLQDAEKNMLWYTVYNINDPWDENGYLVTSINKLVYLGKLFVTLNQSWSKLE KVAMSQIVTTQNHLSGHLRKNENFNAVYSQRVLQTPLTGQRVESFLKIITSDYEIIKSSLESYS ASKAFSVPENGPHSLMDFASLDGRMPSDLSLPSISIDTKRPSADLARLKISQPKSLDALPLKT QRRHKFPESD 350 |
| F2 (341-526) | 341 QRRHKFPESDSVDNAGGKILIKKETLGGRDVRATTPVSSVSLMSGVEPLSSLTSTNLDLRDKS HGNYRIGPSGILDFGVKLPAEAQSNTGDVDLLQDKTSIRSPSSGITDVVNGLANLNLRQNKSDV SRPWSKNTAANADVFDPVHRLVSEQTGTPFVLNNSDVAGSEAKLTTHSTETGVSPHNVS 526 |
| F3 (518-667) | 518 TGVSPHNVSLIKDLRDKDGFRKQKKLDLLGSWTKEKNDKAIVHSREVTGDSGDATETVTARD SPVLRKTKHANAIFAGLNKKYARDVSRGGKGNSRDLYSGGNAEKKETSGKFNVDKEMTQNEQ EPLPNLMEAARNAGEEQYVQAGLGQR 667 |
| F4 (654-858) | 654 AGEEQYVQAGLGQRVNKILAEFTNLISLGEKGIQDILHNQSGTELKLPTENKLGRESEEANVER ILEVSDPQNLFKNFKLQNDLDSVQSPFRLPNADLSRDLDSVSFKDALDVKLPGNGEREIDLALQK VKAGERETSDFKVGQDETLIPTQLMKVETPEEKDDVIEKVLRIRQDGETDEETVPGPGVAESLG IAAKDKSVIAS 858 |
Overlapping sequences are underlined.
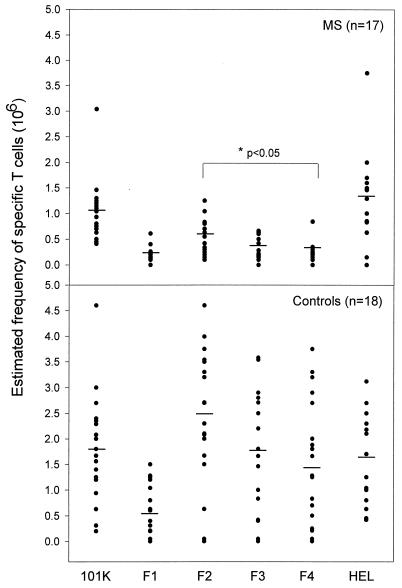
The T-cell responses to HHV-6 were examined in the blood specimens obtained from MS patients (n = 17) and control subjects (n = 18), which included healthy controls and patients with other inflammatory neurologic conditions. As shown in Fig. 2, T cells recognizing the 101K protein could be detected in the majority of the control subjects as well as in MS patients examined. However, they occurred at a substantially lower precursor frequency in patients with MS than in the control group (P < 0.05), while the frequency of T cells specific for hen egg lysozyme, a control antigen, did not differ between the two groups. The T-cell reactivity to HHV-6 in controls was preferentially directed at the 341 to 526 region and, to a lesser extent, to the 518 to 667 and the 654 to 858 regions, as evidenced by the higher precursor frequency of specific T cells recognizing these immunodominant regions in the control subjects (Fig. 2). The 155 to 350 region was the least immunoreactive, as minimal T-cell responses to that region could be detected in both MS and controls. In contrast, there was an overall decrease in the T-cell responses to the 101K protein and the truncated fragments in patients with MS. Although the 341 to 526 region was recognized more frequently, the pattern of hierarchy in recognition of the viral fragments was not apparent in patients with MS. The results suggest decreased T-cell responses to HHV-6 in patients with MS. A panel of 110 resulting T-cell lines derived from MS patients as well as from control subjects were examined by flow cytometry and were found to exclusively express the CD4 phenotype (data not shown).
FIG. 2.
T-cell responses to the 101K protein and the truncated fragments of HHV-6 in MS patients and control subjects. The precursor frequencies of T cells recognizing the 101K protein and the truncated fragments of HHV-6 were examined for 17 MS patients and 18 control subjects (12 healthy individuals and 6 individuals with other inflammatory neurologic conditions). The circles indicate the estimated individual T-cell frequency, and the bar represents the mean precursor frequency of specific T cells within the group.
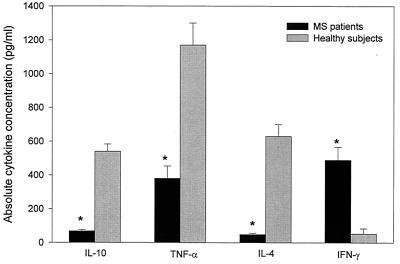
We then addressed whether MS-derived T cells recognizing HHV-6 differed in cytokine profile from those derived from control subjects. Sixty-two T-cell lines obtained from 13 MS patients (3 secondary progressive MS patients and 10 relapsing-remitting MS patients) and 34 T-cell lines derived from 12 control subjects were characterized for the production of IL-4, IL-10, TNF-α, and IFN-γ after challenge with the recombinant 101K protein. The results revealed significant qualitative and quantitative differences in the cytokine profile of the HHV-6-reactive T-cell lines derived from the two groups. As illustrated in Fig. 3, the T-cell lines derived from control subjects produced large amounts of IL-4, IL-10, and TNF-α but not IFN-γ. In contrast, the MS-derived T-cell lines exhibited a completely different cytokine profile characterized by the production of Th1 cytokines (TNF-α and IFN-γ) but not Th2 cytokines (IL-4 and IL-10) that are important for B-cell differentiation. The differences in the concentrations of Th1 and Th2 cytokines between the two panels of the T-cell lines were highly significant (P < 0.05).
FIG. 3.
The cytokine profile of T-cell lines recognizing HHV-6. Cytokine production of T-cell lines specific for HHV-6 derived from patients with MS (MS) and from control subjects (Controls) was measured by ELISA. The T-cell lines were challenged with the recombinant 101K protein, and the supernatants were tested after 48 h for concentrations of the indicated cytokines. The bars indicate the mean concentration (picograms/milliliter) ± standard errors of the means. Asterisks represent statistical significance (P < 0.05) between the two groups. The detection limit of the assays for all cytokines was less than 25 pg/ml.
The antibody reactivity to the 101K virion protein of HHV-6 in MS patients and control subjects.
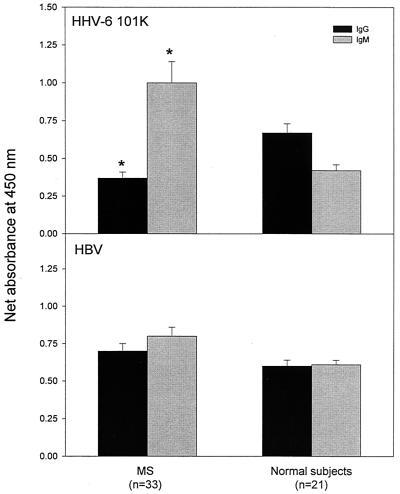
Next we analyzed the occurrence, the reactivity pattern, and the titers of serum IgG and IgM antibodies to the 101K protein by using recombinant HBV as a control viral antigen. Serum IgM antibodies specific for the 101K protein at the serum dilution of 1:100 were detected in a significantly higher proportion of MS patients (27 out of 33, 82%) than in those of control subjects (8 out of 21, 38%; P < 0.05). The HHV-6-specific IgM reactivity measured by optical density at the same serum dilution was consistently elevated in MS patients compared to that in control subjects (P < 0.05) (Fig. 4). The occurrence and the reactivity of serum HHV-6-specific IgM correlated conversely with those of IgG in the same MS patients. IgG specific for HHV-6 101K protein was detected only in 10 out of 33 (30%) MS patients and in 13 out of 21 (62%) control subjects (Fig. 4). In contrast, the antibody reactivity of both IgM and IgG to the control viral antigen (HBV) did not differ significantly between the two groups. Furthermore, antibody titers to the HHV-6 101K protein were determined by serial dilutions of the serum specimens. In control subjects, high IgM titers for the 101K protein were strongly associated with the positive detection of cell-free HHV-6 DNA (mean IgM titer of 1:164 for the viral DNA-positive group versus the mean titer of 1:54 for the viral DNA-negative group). However, in the majority of MS patients, IgM titers for HHV-6 101K protein were elevated regardless of the status of HHV-6 viral DNA (1:200 for the viral DNA-positive group versus 1:177 for viral DNA-negative group), while specific IgG titers were substantially decreased compared to those of control subjects (1:27 for MS patients versus 1:243 for controls). The results consistently indicate a decreased IgG response to HHV-6 in patients with MS, which was associated with an elevated IgM reactivity to the virus.
FIG. 4.
Detection of serum IgG and IgM antibodies specific for the 101K protein in MS patients and control subjects. IgG and IgM antibodies to the 101K protein were detected in serum specimens (dilution 1:100) obtained from 33 MS patients and 21 healthy control subjects by ELISA. The specimens were examined in the same experiments for antibodies to hepatitis B virus (HBV), which was used as a control viral antigen. The net absorbance was calculated as experimental absorbance minus background absorbance in the same plates. The bars represent the mean net absorbance ± standard errors of the means. The asterisk indicates statistical significance (P < 0.05) between the two groups.
Furthermore, we examined the reactivity of serum HHV-6-specific IgG and IgM antibodies to four recombinant fragments encompassing various regions of the 101K protein. To this end, a panel of 22 serum specimens derived from 16 MS patients and 6 control subjects was selected for high IgG and IgM titers and tested for reactivity to the truncated fragments by ELISA. The majority of the selected serum specimens contained both IgG and IgM antibodies for HHV-6 with various reactivities. The experiments showed that HHV-6-specific antibodies of the two classes exhibited distinct antigen recognition patterns. The IgG antibodies reacted selectively to the 341 to 526 region (14 out of 16 specimens for MS and 5 out of 6 specimens for controls) that corresponded to the immunodominant epitope(s) for HHV-6-specific T cells, while the IgM antibodies were directed predominantly at the 155 to 350 region (13 out of 16 specimens for MS and 6 out of 6 specimens for controls). However, the reactivity patterns of specific IgG and IgM antibodies to the 101K fragments did not differ between MS patients and control subjects.
DISCUSSION
This study provides important evidence for the understanding of the potential association of HHV-6 and MS. There are also a number of important issues that emerged from this study. First, the findings support active replication of HHV-6, a normally latent virus, in the peripheral blood as well as in the central nervous compartment of patients with MS. The observation described here is in agreement with an increasing number of reports demonstrating frequent detection of cell-free HHV-6 DNA in serum and CSF specimens by using specific oligonucleotide primers corresponding to selected regions of HHV-6 (1, 2, 4, 22, 29, 36, 37, 46, 50). However, other studies reported conflicting results, as suggested by either infrequent detection of HHV-6 DNA in sera and/or CSF of MS patients or insignificant differences between MS patients and control subjects (6, 14, 24, 25, 28, 41). There are several possibilities that may explain the disparity. For example, different studies employed individually designed primers/probes targeting distinct proteins/regions of the HHV-6 strains, which may account for the discrepancies in the specificity of the various detection systems. Some regions of HHV-6 are known to share sequence homology with other viruses, such as CMV and HHV-7, which may obscure the specificity of the results. Furthermore, the sensitivity of the assay systems used by different studies and quality/storage of patient material may also be attributable to the discrepancies. In this study, the use of the recombinant protein and truncated fragments corresponding to the immunoreactive 101K virion protein of HHV-6 has provided, for the first time, a crucial advantage in discriminating specific immune responses to HHV-6 from cross-reactivity to other related viruses (e.g., CMV and HHV-7). The selected sequence region of the 101K protein has no homology with other known proteins, as determined by a GenBank search. However, although the amino acid sequence of the 101K protein used here is unique to HHV-6, it is indistinguishable between the two strains of HHV-6.
Another important aspect of the present study is related to the characterization of both T-cell responses and antibody reactivity to HHV-6. The results indicate a significantly declined T-cell immunity to HHV-6 seen in patients with MS as evidenced by substantially lower precursor frequency of T cells specific for the HHV-6 viral antigen. The observation that the T-cell responses to all fragments of the 101K protein are decreased in MS patients is suggestive of a functional deficit of HHV-6-specific T cells of polyclonal populations rather than a clonal deficiency in recognition of certain epitopes of the 101K protein. The findings suggest that the T-cell immunity to HHV-6 may be impaired in MS patients. It should be mentioned that the method employed for the T-cell frequency analysis may not be accurate enough to measure the true precursor frequency of antigen-specific T cells. However, it is appropriate, when used consistently, to compare the precursor frequencies of specific T cells between individual patients and groups (31, 42, 49, 50). Furthermore, in this study HHV6-reactive T cells derived from MS patients exhibit a significantly different cytokine profile (Th1 cytokines) compared to those obtained from control subjects (Th2 cytokines). As a result, an ELISPOT assay based either on IFN-γ or other cytokines would significantly skew the results because of the cytokine bias of HHV-6-reactive T cells between MS patients and control subjects. At this time the observed association cannot be explained, as HHV-6 is latent in host T cells as a result of primary infections in childhood in the majority of the general population (13, 50). One possibility is that the impaired T-cell immunity to HHV-6 may be attributable to undefined susceptibility of immune-related genes potentially associated with MS. As a result, HHV-6-reactive T cells are not fully activated by the viral antigens potentially because of poor antigen presentation or lack of adequate costimulation. Alternatively, persistent viral replication and an increased viral load in the blood may alter the functional properties of specific T cells and render them less responsive. The finding bears particular pathological relevance to MS, as the impaired T-cell immunity to HHV-6 may be associated with low-grade infection of HHV-6 that has neurotropic properties and can potentially cause tissue damage in the central nervous system (8). Soldan and colleagues reported recently that while lymphoproliferative response to HHV-6B did not differ between patients with MS and control subjects, a significant increase in the proliferative response to HHV-6A was found in the MS patient cohort. In that study, HHV-6-infected cell lysates were used as the antigen in 5-day proliferation assays (38). There are substantial discrepancies between the two studies, including the viral antigens used (recombinant 101K virion protein versus infected cell lysates) and the sensitivity of the T-cell assay systems (the T-cell frequency analysis versus 5-day proliferation assays). Furthermore, the possibility exists that the immune responses to HHV-6, including T-cell response (38) and antibody reactivity (1, 12), may vary depending upon the selected antigenic components of HHV-6 or the whole virus used in the different assays.
In the present study, the observed impaired T-cell immunity to HHV-6 may be associated with the aberrant antibody responses to HHV-6 in MS patients and the increased detection of HHV-6 viral DNA in MS patients. First, there is a significant overall decrease in serum HHV-6-specific IgG titers in MS patients compared to that of control subjects. It is likely that the deficient IgG responses seen in MS patients may be caused by both insufficient T-cell responses and a highly skewed cytokine profile that lacks the production of IL-4 and IL-10. Consequently, B cells expressing surface IgM to HHV-6 may undergo an altered differentiation process as a result of inadequate T-cell help. Some IgM-producing B cells may fail to complete the process of class switching. It is known that Th2 cytokines, such as IL-4, IL-10, and IL-13, are required for functional differentiation and maturation of B cells (5). Second, the IgM titers for HHV-6 are as low as 1:54 in some control subjects in whom serum viral DNA is undetectable and occurred at a mean antibody titer of 1:164 in serum specimens derived from other control subjects positive for HHV-6 viral DNA, suggesting a correlation between the two. The finding may reflect frequent exposure and sensitization of specific B cells producing IgM antibodies as a result of viral infection or active viral replication in a small proportion of the healthy population. However, there is an overall elevation of HHV-6-specific IgM antibodies in patients with MS which is not associated with the status of serum viral DNA for HHV-6. The observation raises the possibility that there may be frequent and transient release of HHV-6 viral particles into the blood circulation as a result of active viral replication in MS patients and viral infection is slowly cleared. Therefore, although at some time points cell-free viral DNA is undetectable in some MS patients, elevated serum IgM titers for HHV-6 may result from residual B cells sensitized by previous exposure to HHV-6 and may persist over time.
It can be speculated that the specific IgM antibodies alone are not effective in clearing the virus, contributing to an increased viral replication of HHV-6 in the blood as well as in the central nervous compartment in patients with MS. The limited ability of HHV-6-specific IgM to neutralize the virus may be related to their general property of being low-affinity antibodies. Furthermore, IgM antibodies have poor ability to penetrate through the blood-brain barrier. Therefore, in the case of MS, active replication of HHV-6 in both the blood and central nervous compartment may be associated with ineffective clearance by the immune system as a result of impaired T-cell responses and insufficient HHV-6 IgG antibody production.
Acknowledgments
We thank Stanley Appel and Larry Schneider for providing the CSF specimens used in this study and Sufang Li and Jeanene De La Rosa for technical assistance.
The work was supported by a research grant from the National Multiple Sclerosis Society and the Richardson Foundation.
REFERENCES
- 1.Ablashi, D. V., H. B. Eastman, C. B. Owen, M. M. Roman, J. Friedman, J. B. Zabriskie, D. L. Peterson, G. R. Pearson, and J. E. Whitman. 2000. Frequent HHV-6 reactivation in multiple sclerosis (MS) and chronic fatigue syndrome (CFS) patients. J. Clin. Virol. 16:179-191. [DOI] [PubMed] [Google Scholar]
- 2.Ablashi, D. V., W. Lapps, M. Kaplan, J. E. Whitman, J. R. Richert, and G. R. Pearson. 1998. Human herpesvirus-6 (HHV-6) infection in multiple sclerosis: a preliminary report. Mult. Scler. 4:490-496. [DOI] [PubMed] [Google Scholar]
- 3.Ablashi, D. V., P. Lusso, C.-L. Hung, S. Z. Salahuddin, S. F. Josephs, T. Llana, B. Kramarsky, P. Biberfeld, P. D. Markham, and R. C. Gallo. 1988. Utilization of human hematopoietic cell lines for the propagation and characterization of HBLV (human herpesvirus 6). Int. J. Cancer 42:787-791. [DOI] [PubMed] [Google Scholar]
- 4.Akhyani, N., R. Berti, M. B. Brennan, S. S. Soldan, J. M. Eaton, H. F. McFarland, and S. Jacobson. 2000. Tissue distribution and variant characterization of human herpesvirus (HHV)-6: increased prevalence of HHV-6A in patients with multiple sclerosis. J. Infect. Dis. 182:1321-1325. [DOI] [PubMed] [Google Scholar]
- 5.Banchereau, J., F. Briere, Y. J. Liu, and F. Rousset. 1994. Molecular control of B lymphocyte growth and differentiation. Stem Cells 12:278-288. [DOI] [PubMed] [Google Scholar]
- 6.Blumberg, B. M., D. J. Mock, J. M. Powers, M. Ito, J. G. Assouline, J. V. Baker, B. Chen, and A. D. Goodman. 2000. The HHV6 paradox: ubiquitous commensal or insidious pathogen? A two-step in situ PCR approach. J. Clin. Virol. 16:159-178. [DOI] [PubMed] [Google Scholar]
- 7.Braun, D. K., G. Dominguez, and P. E. Pellett. 1997. Human herpesvirus 6. Clin. Microbiol. Rev. 10:521-567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carrigan, D. R., D. Harrington, and K. K. Knox. 1996. Subacute leukoencephalitis caused by CNA infection with human herpesvirus-6 manifesting as acute multiple sclerosis. Neurology 47:145-148. [DOI] [PubMed] [Google Scholar]
- 9.Challoner, P. B., K. T. Smith, J. D. Parker, D. L. MacLeod, S. N. Coulter, T. M. Rose, E. R. Schultz, J. L. Bennett, R. L. Garber, and M. Chang. 1995. Plaque-associated expression of human herpesvirus 6 in multiple sclerosis. Proc. Natl. Acad. Sci. USA 92:7440-7444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Coates, A. R., and J. Bell. 1998. HHV-6 and multiple sclerosis. Nat. Med. 4:537-538. [DOI] [PubMed] [Google Scholar]
- 11.Dalgleish, A. G. 1997. Viruses and multiple sclerosis. Acta Neurol. Scand. 95:8-15. [DOI] [PubMed] [Google Scholar]
- 12.Enbom, M., F. Z. Wang, S. Fredrikson, C. Martin, H. Dahl, and A. Linde. 1999. Similar humoral and cellular immunological reactivities to human herpesvirus 6 in patients with multiple sclerosis and controls. Clin. Diagn. Lab. Immunol. 6:545-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farr, T. J., G. B. Harnet, G. R. Pietroboni, and M. R. Bucens. 1990. The distribution of antibodies to HHV-6 compared with other herpesviruses in young children. Epidemiology 105:603-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goldberg, S. H., A. V. Albright, R. P. Lisak, and F. Gonzalez-Scarano. 1999. Polymerase chain reaction analysis of human herpesvirus-6 sequences in the sera and cerebrospinal fluid of patients with multiple sclerosis. J. Neurovirol. 5:134-139. [DOI] [PubMed] [Google Scholar]
- 15.Hafler, D. 1999. The distinction blurs between an autoimmune versus microbial hypothesis in multiple sclerosis. J. Clin. Investig. 104:527-529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hunter, S. F., and D. A. Hafler. 2000. Ubiquitous pathogens. Links between infection and autoimmunity in MS? Neurology 55:164-165. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, R. T. 1994. The virology of demyelinating diseases. Neurology 36(Suppl.):S54-S60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kennedy, P. G. E., and J. Steiner. 1994. On the possible viral aetiology of multiple sclerosis. QJM 87:523-528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kimberlin, D. W., and R. J. Whitley. 1998. Human herpesvirus-6: neurologic implications of a newly-described viral pathogen. J. Neurovirol. 4:474-485. [DOI] [PubMed] [Google Scholar]
- 20.Knox, K. K., J. H. Brewer, J. M. Henry, D. J. Harrington, and D. R. Carrigan. 2000. Human herpesvirus 6 and multiple sclerosis: systemic active infections in patients with early disease. Clin. Infect. Dis. 31:894-903. [DOI] [PubMed] [Google Scholar]
- 21.Liedtke, W., R. Malessa, P. Faustmann, and A. M. Eis-Hubinger. 1995. Human herpesvirus-6 polymerase chain reaction findings in human immunodeficiency virus associated neurological disease and multiple sclerosis. J. Neurovirol. 1:2553-2558. [DOI] [PubMed] [Google Scholar]
- 22.Lopez, C., P. Pellett, J. Stewart, C. Goldsmith, K. Sanderlin, J. Black, D. Warfield, and P. Feorino. 1988. Characteristics of human herpesvirus-6. J. Infect. Dis. 157:1271-1273. [DOI] [PubMed] [Google Scholar]
- 23.Luppi, M., P. Barozzi, A. Maiorana, R. Marasca, and G. Torelli. 1994. Human herpesvirus infection in normal human brain tissue. J. Infect. Dis. 169:943-944. [DOI] [PubMed] [Google Scholar]
- 24.Martin, C., M. Enbom, M. Söderström, S. Fredrikson, H. Dahl, J. Lycke, T. Bergstrom, and A. Linde. 1997. Absence of seven human herpesviruses, including HHV-6, by polymerase chain reaction in CSF and blood from patients with multiple sclerosis and optic neuritis. Acta Neurol. Scand. 95:280-283. [DOI] [PubMed] [Google Scholar]
- 25.Mayne, M., J. Krishnan, L. Metz, A. Nath, A. Auty, B. M. Sahai, and C. Power. 1998. Infrequent detection of human herpesvirus 6 DNA in peripheral blood mononuclear cells from multiple sclerosis patients. Ann. Neurol. 44:391-394. [DOI] [PubMed] [Google Scholar]
- 26.Merelli, E., R. Bedin, P. Sola, P. Barozzi, G. L. Mancardi, G. Ficarra, and G. Franchini. 1997. Human herpesvirus-6 and human herpesvirus-8 DNA sequences in brains of multiple sclerosis patients, normal adults and children. J. Neurol. 7:450-454. [DOI] [PubMed] [Google Scholar]
- 27.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, R. L. Yauch, K. L. Neville, Y. Katz-Levy, A. Carrizosa, and B. S. Kim. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3:1133-1136. [DOI] [PubMed] [Google Scholar]
- 28.Mirandola, P., A. Stefan, E. Brambilla, G. Campadelli-Fiume, and L. M. Grimaldi. 1999. Absence of human herpesvirus 6 and 7 from spinal fluid and serum of multiple sclerosis patients. Neurology 53:1367-1368. [DOI] [PubMed] [Google Scholar]
- 29.Monteyne, P., J.-F. Bureau, and M. Brahic. 1998. Viruses and multiple sclerosis. Curr. Opin. Neurol. 11:287-291. [DOI] [PubMed] [Google Scholar]
- 30.Nielsen, L., A. Larsen, M. Munk, and B. Vestergaard. 1997. Human herpesvirus-6 immunoglobulin G antibodies in patients with multiple sclerosis. Acta Neurol. Scand. 169:76-78. [DOI] [PubMed] [Google Scholar]
- 31.Ota, K., M. Matsui, E. L. Milford, G. A. Mackin, H. L. Weiner, and D. A. Hafler. 1990. T cell recognition of an immunodominant MBP epitope in multiple sclerosis. Nature 346:183-187. [DOI] [PubMed] [Google Scholar]
- 32.Pellett, P. E., D. Sanchez-Martinez, G. Dominguez, J. B. Black, E. Anton, C. Greenamoyer, and T. R. Dambaugh. 1993. A strongly immunoreactive virion protein of human herpesvirus 6 variant B strain Z29: identification and characterization of the gene and mapping of a variant-specific monoclonal antibody reactive epitope. Virology 195:521-531. [DOI] [PubMed] [Google Scholar]
- 33.Reilly, M., D. Mix, and G. M. Winslow. 2000. Detection of viral superantigen-class II MHC interactions at the cell surface. Mol. Immunol. 37:987-993. [DOI] [PubMed] [Google Scholar]
- 34.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, and B. Kramarsky. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 35.Secchiero, P., D. R. Carrigan, Y. Asano, L. Benedetti, R. W. Crowley, A. L. Komaroff, R. C. Gallo, and P. Lusso. 1995. Detection of human herpesvirus 6 in plasma of children with primary infection and immunosuppressed patients by polymerase chain reaction. J. Infect. Dis. 171:273-280. [DOI] [PubMed] [Google Scholar]
- 36.Sola, P., E. Merelli, R. Marasca, M. Poggi, M. Luppi, M. Montorsi, and G. Torelli. 1993. Human herpesvirus 6 and multiple sclerosis: survey of anti-HHV-6 antibodies by immunofluorescence analysis and of viral sequences by polymerase chain reaction. J. Neurol. Neurosurg. Psychiatry 56:917-919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soldan, S. S., R. Berti, N. Salem, P. Secchiero, L. Flamand, P. A. Calabresi, M. B. Brennan, H. W. Maloni, H. F. McFarland, H. C. Lin, M. Patnaik, and S. Jacobson. 1997. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 3:1394-1397. [DOI] [PubMed] [Google Scholar]
- 38.Soldan, S. S., T. P. Leist, K. N. Juhng, H. F. McFarland, and S. Jacobson. 2000. Increased lymphoproliferative response to human herpesvirus type 6A variant in multiple sclerosis patients. Ann. Neurol. 47:306-313. [PubMed] [Google Scholar]
- 39.Steinman, L., and M. B. A. Oldstone. 1997. More mayhem from molecular mimics. Antibodies against human herpesvirus-6 have been found in patients with multiple sclerosis. Could this be another sighting in the hunt for the viral trigger of the disease? Nat. Med. 3:1321-1322. [DOI] [PubMed] [Google Scholar]
- 40.Sutkowski, N., B. Conrad, D. A. Thorley-Lawson, and B. T. Huber. 2001. Epstein-Barr virus transactivates the human endogenous retrovirus HERV-K18 that encodes a superantigen. Immunity 15:579-589. [DOI] [PubMed] [Google Scholar]
- 41.Taus, C., E. Pucci, E. Cartechini, A. Fie, G. Giuliani, M. Clementi, and S. Menzo. 2000. Absence of HHV-6 and HHV-7 in cerebrospinal fluid in relapsing-remitting multiple sclerosis. Acta Neurol. Scand. 101:224-228. [DOI] [PubMed] [Google Scholar]
- 42.Tejada-Simon, M. V., Y. Zang, D. Yang, J. Hong, S. Li, R. Singh, E. Van den Berg-Loonen, J. Killian, V. Rivera, and J. Zhang. 2000. T cell reactivity to myelin antigens and their structural and functional properties at different clinical stages of multiple sclerosis. Int. Immunol. 12:1641-1650. [DOI] [PubMed] [Google Scholar]
- 43.Torres, B. A., S. Kominsky, G. Q. Perrin, A. C. Hobeika, and H. M. Johnson. 2001. Superantigens: the good, the bad, and the ugly. Exp. Biol. Med. 226:164-176. [DOI] [PubMed] [Google Scholar]
- 44.Ueda, K., K. Kusuhare, M. Hirose, K. Okada, C. Miyazaki, K. Tokugawa, M. Nakayama, and K. Yamanishi. 1989. Exanthem subitum and antibody to human herpesvirus-6. J. Infect. Dis. 159:750-752. [DOI] [PubMed] [Google Scholar]
- 45.Wandinger, K.-P., W. Jabs, A. Siekhaus, S. Bubel, P. Trillenberg, H. Wagner, K. Wessel, H. Kirchner, and H. Hennig. 2000. Association between clinical disease activity and Epstein-Barr virus reactivation in MS. Neurology 55:178-184. [DOI] [PubMed] [Google Scholar]
- 46.Wilborn, F., C. A. Schmidt, V. Brinkmann, K. Jendroska, H. Oettle, and W. Siegert. 1994. A potential role for human herpesvirus type 6 in nervous system disease. J. Neuroimmunol. 49:213-214. [DOI] [PubMed] [Google Scholar]
- 47.Wucherpfennig, K. W., and J. L. Strominger. 1995. Molecular mimicry in T cell-mediated autoimmunity: viral peptides activate human T cell clones specific for myelin basic protein. Cell 80:695-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yamamoto, M., J. B. Black, J. A. Stewart, C. Lopez, and P. E. Pellett. 1990. Identification of a nucleocapsid protein as a specific serological marker of human herpesvirus 6 infection. J. Clin. Microbiol. 28:1957-1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yamanishi, K., T. Okino, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed]
- 50.Yoshikawa, T., S. Suga, Y. Asano, T. Yazaki, H. Kodama, and T. Ozaki. 1989. Distribution of antibodies to a causative agent of exanthem subitum (human herpesvirus-6) in healthy individuals. Pediatrics 84:675-677. [PubMed] [Google Scholar]
- 51.Zhang, J., S. Markovic-Plese, B. Lacet, J. Raus, H. L. Weiner, and D. A. Hafler. 1994. Increased frequency of interleukin 2-responsive T cells specific for myelin basic protein and proteolipid protein in peripheral blood and cerebrospinal fluid of patients with multiple sclerosis. J. Exp. Med. 179:973-984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang, J., R. Medaer, P. Stinissen, D. Hafler, and J. Raus. 1993. MHC restricted clonotypic depletion of human myelin basic protein by T cell vaccination. Science 261:1451-1454. [DOI] [PubMed] [Google Scholar]