Abstract
The amino acid at position 55 of the E2 glycoprotein (E255) of Sindbis virus (SV) is a critical determinant of SV neurovirulence in mice. Recombinant virus strain TE (E255 = histidine) differs only at this position from virus strain 633 (E255= glutamine), yet TE is considerably more neurovirulent than 633. TE replicates better than 633 in a neuroblastoma cell line (N18), but similarly in BHK cells. Immunofluorescence staining showed that most N18 cells were infected by TE at a multiplicity of infection (MOI) of 50 to 500 and by 633 only at an MOI of 5,000, while both viruses infected essentially 100% of BHK cells at an MOI of 5. When exposed to pH 5, TE and 633 viruses fused to similar extents with liposomes derived from BHK or N18 cell lipids, but fusion with N18-derived liposomes was less extensive (15 to 20%) than fusion with BHK-derived liposomes (∼50%). Binding of TE and 633 to N18, but not BHK, cells was dependent on the medium used for virus binding. Differences between TE and 633 binding to N18 cells were evident in Dulbecco's modified Eagle medium (DMEM), but not in RPMI. In DMEM, the binding efficiency of 633 decreased significantly as the pH was raised from 6.5 to 8.0, while that of TE did not change. The same pattern was observed with RPMI when the ionic strength of RPMI was increased to that of DMEM. TE bound better to heparin-Sepharose than 633, but this difference was not pH dependent. Growth of N18 and BHK cells in sodium chlorate to eliminate all sulfation decreased virus-cell binding, suggesting the involvement of sulfated molecules on the cell surface. Taken together, the presence of glutamine at E255 impairs SV binding to neural cells under conditions characteristic of interstitial fluid. We conclude that mutation to histidine participates in or stabilizes the interaction between the virus and the surface of neural cells, contributing to greater neurovirulence.
Sindbis virus (SV), the prototype member of the genus Alphavirus in the family Togaviridae, causes age-dependent mortality in mice (23). In newborn mice, the AR339 strain of SV causes acute fatal encephalomyeliitis while in older mice the disease is mild and nonfatal. A neuroadapted strain of SV isolated after serial intracerebral passages of SV causes high mortality in older mice associated with severe encephalomyelitis resulting in kyphoscoliosis and hindlimb paralysis (24). Fatal infection correlates with the induction of neuronal death, the primary target cells of SV infection in the nervous system (34).
SV is a plus-strand RNA virus with a cell-derived lipid bilayer membrane containing heterodimers of the two viral envelope glycoproteins, E1 and E2 (51). Entry of SV into host cells involves association of the virus with the cell surface, interaction with specific receptors, clathrin-mediated endocytosis, and acid-induced fusion of SV with the endosomal membrane to release the nucleocapsid into the cytoplasm (29). Coordinated association and dissociation of the E1 and E2 glycoproteins during interaction with the cellular membrane and exposure to acidic pH are required for alphaviruses to deliver genomic RNA into the cytoplasm (19). The E2 glycoprotein is the major mediator of attachment while the E1 glycoprotein carries membrane fusion activity (5, 46, 51, 57). Many studies suggest that the attachment of SV to the cell surface is mediated by a cell surface protein (51). Tissue culture-adapted SV strains become highly dependent on the proteoglycan heparan sulfate (HS) for initial virus attachment (7, 32) and it is likely that multiple cellular and viral factors influence SV attachment and entry into cells in vivo and in vitro.
RNA viruses often adapt quickly to new environments due to the generation and selection of mutants, facilitated by the lack of proofreading of the viral RNA-dependent RNA polymerase. Alterations at the viral entry steps, which include receptor recognition, virus attachment and/or penetration, and membrane fusion, could influence the rate of virus growth and tissue tropism, two major determinants in viral pathogenesis. Studies of SV, Venezuelan equine encephalitis, Semliki Forest virus (SFV), and Ross River virus have demonstrated that mutations in the envelope proteins are important determinants of virus virulence (12, 21, 55, 56), and the mechanisms of virulence have begun to be elucidated. For instance, rapid penetration of BHK cells is negatively correlated with virus virulence for SV and Venezuelan equine encephalitis (11, 12, 39), and binding to HS contributes to the attenuation of SV infection in mice (8, 32). Major determinants of the neurovirulence of neuroadapted strain of Sindbis virus have been mapped to the glycoprotein regions of the genome; in particular, the change from glutamine to histidine at the amino acid at position 55 of the E2 glycoprotein (E255) is rapidly acquired during replication in the nervous system and plays a critical role in the acquisition of neurovirulence (35, 54). Recombinant virus strains TE and 633 were constructed on the same genomic background and differ only at the E255 position, where TE has a histidine and 633 has a glutamine. In 2-week-old mice inoculated intracerebrally, TE causes close to 100% mortality, whereas 633 causes no mortality (54). Neuronal death occurs more often in TE-infected than in 633-infected mice (34). In tissue culture, both TE and 633 replicate equally well in nonneural cell lines, such as BHK cells. However, in mouse brain and in N18 mouse neuroblastoma cells, replication is markedly different (14). TE replicates more rapidly and grows to a higher titer than 633. Therefore, replication of SV in N18 cells may reflect the molecular and cellular basis of neurovirulence in vivo. Results of infectious-center assays indicate that more N18 cells are infected with TE than with 633 at the same multiplicity of infection (MOI) (14), but differences in the initial interactions between these viruses and the N18 cell surface did not appear to account for the profound differences in infection (53).
Knowledge of the interactions between the viral envelope and the cellular receptor(s) could substantially facilitate the understanding of the process of cell entry. In this study, we have extended the studies of infection of N18 cells to identify determinants of the differential infection by TE and 633. We show that the lipids of N18 cells are much less conducive to SV fusion than the lipids of BHK cells and that the ionic strength of the virus diluent alters binding of 633 to the N18 cell surface in a pH-dependent fashion, while binding of TE is unaffected.
MATERIALS AND METHODS
Cell culture, virus stocks, and plaque assays.
BHK-21 cells and the N18 clone of the C1300 neuroblastoma cell line (1) were maintained in Dulbecco's modified Eagle medium (DMEM) (Gibco BRL, Gaithersburg, Md.) supplemented with 10% fetal bovine serum (FBS), 50-μg/ml gentamicin, and 2 mM glutamine (Gibco BRL). The number of PFU on BHK-21 monolayers were determined, and MOIs were calculated based on these data.
To prepare 35S-labeled TE and 633, BHK-21 cells were grown to confluence and infected at an MOI of 1. At 5 h after infection, the medium was replaced with methionine- and cysteine-free DMEM containing 2% FBS and 33-μCi/ml TRANS35S-LABEL (∼1,000 Ci/mmol; ICN, Irvine, Calif.). After overnight incubation, the culture supernatant fluid was clarified and virus was concentrated by polyethylene glycol precipitation, purified by centrifugation through a 15 to 45% (wt/vol) sucrose gradient, and concentrated through a 15% sucrose cushion (15). The final pellet was resuspended in DMEM containing 1% FBS and stored in aliquots at −80°C.
To prepare virus stocks for infection, BHK-21 cells were infected at an MOI of 0.1 and incubated overnight. The supernatant fluid was clarified and virus was concentrated either by polyethylene glycol precipitation or by centrifugation at 32,000 rpm with a Beckman SW-41 rotor at 4°C for 90 min. Pellets were resuspended in DMEM containing 1% FBS and stored in aliquots at −80°C.
Pyrene-labeled TE and 633 viruses were produced on BHK-21 cells, essentially as described before for SFV and SV (5, 38, 46, 57). Briefly, BHK-21 cells were cultured in medium containing 15-μg/ml 16-(1-pyrenyl)-hexadecanoic acid (Molecular Probes, Eugene, Oreg.). At subconfluence, viral infection was initiated with an MOI of 4. At 24 h postinfection, the pyrene-labeled TE or 633 viruses were harvested from the medium by ultracentrifugation with a Beckman type 19 rotor for 2.5 h at 100,000 × g and 4°C and further purified on a sucrose density gradient (20 to 50% wt/vol) by ultracentrifugation with a Beckman SW41 rotor for 16 h at 100,000 × g and 4°C. The phospholipid content of the viruses was determined after lipid extraction according to Bligh and Dyer (3) by phosphate analysis (4).
Immunofluorescence staining and microscopy.
BHK-21 and N18 cells were seeded at 3 × 104 to 4 × 104 cells/well in eight-well Permanox chamber slides (Nalge Nunc, Naperville, Ill.) and allowed to grow for 24 h. Cells were washed once in the specified binding medium and infected with 200 μl of virus diluted in the same binding medium. After 1 h at 37°C, the virus inoculum was replaced with 200 μl of DMEM-2% FBS; 2 h later, 200 μl of DMEM-2% FBS containing 20 mM ammonium chloride was added to each well. After an additional 3 h of incubation, the cells were fixed in 4% paraformaldehyde in phosphate-buffered saline (PBS) for 5 min, washed, and incubated at room temperature overnight with mouse anti-E2c monoclonal antibody (MAb) 209 (36, 49) diluted 1/2,000 in PBS-2% FBS. After three washes in PBS, Alexa 488-conjugated goat anti-mouse immunoglobulin G (Molecular Probes) diluted 1/200 in PBS-2% FBS was added for 30 min, followed by three washes in PBS. To stain the nuclei, 1-μg/ml propidium iodide (Sigma, St. Louis, Mo.) in PBS containing 0.1% Triton X-100 was added to the cells for 1 min. After five washes with PBS and one wash with H2O, the slides were mounted in PermaFluor aqueous mounting medium (Immunon, Pittsburgh, Pa.) and examined with a Nikon Eclipse E800 microscope.
RNA synthesis.
N18 cells were seeded in 12-well plates at 3 × 105 cells per well and cultured overnight. TE and 633 were diluted either in DMEM-1% FBS or in RPMI containing 20 mM HEPES (pH 7.55) and 0.5% bovine serum albumin (BSA). After 1 h of incubation at 37°C in the presence of 5% CO2, the virus inoculum was aspirated and the cells were overlaid with DMEM-2% FBS containing 1-μg/ml actinomycin D (Calbiochem, San Diego, Calif.) and incubated at 37°C. To label newly synthesized viral RNA, DMEM-2% FBS with 20-μg/ml actinomycin D and 150-μCi/ml [3H]uridine (50 Ci/mmol; ICN, Boston, Mass.) was added to the cells and incubated for 1 h. Cell lysates were prepared in 1% sodium dodecyl sulfate (SDS) with 200-μg/ml proteinase K and precipitated in 10% trichloroacetic acid (TCA) at 4°C. The precipitates were harvested onto GF/C glass fiber filters (Whatmann, Maidstone, Kent, United Kingdom) which were washed twice with 5% TCA (4°C) and once in cold 95% ethanol. The filters were counted with BetaMax (ICN, Costa Mesa, Calif.) with a Beckman LS 6500 scintillation counter.
Virus-cell binding assays.
A variety of binding buffers were used and were based on either DMEM, RPMI-1640, or Dulbecco's PBS (8.5 mM Na2HPO4, 1.8 mM KH2 PO4, 2.7 mM KCl, 137 mM NaCl [pH 7.5]) as the basic salt solution. DMEM with sodium bicarbonate (3.7 mg/ml) (DMEM + SB), DMEM without sodium bicarbonate (DMEM − SB), and DMEM + SB without glucose (Gibco BRL) were supplemented with combinations of 1% FBS and 20 mM 4-(2-hydroxyethyl)-1-piperazine ethanesulfonic acid (pH 7.55) (HEPES; Gibco BRL). RPMI without sodium bicarbonate (RPMI − SB) (Gibco BRL) was supplemented with 0.5% BSA and 20 mM HEPES (20). PBS was supplemented with 1 mM MgCl2, 1 mM CaCl2, and 0.5% BSA. To make binding buffers of different pH values, 20 mM phosphate buffers (diluted from 1 M sodium phosphate made with appropriate ratios of 1 M NaH2PO4 and 1 M Na2HPO4) with different pH values were used to replace HEPES. For the compositions of binding buffers used for specific experiments, see legends for Fig. 4 and 5.
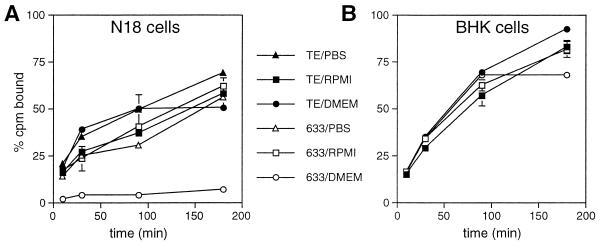
FIG. 4.
Effects of binding buffer on binding of 633 and TE to N18 and BHK cells. N18 (A) and BHK (B) cells were incubated with 35S-labeled TE or 633 diluted in DMEM + SB supplemented with 20 mM HEPES (pH 7.3) and 1% FBS (circle); RPMI−SB supplemented with 20 mM HEPES (pH 7.3) and 0.5% BSA (square); or PBS (pH 7.3) containing 1 mM MgCl2, 1 mM CaCl2, and 0.5% FBS (triangle) at 4°C. Virus binding was assessed after the virus-cell mixture was rocked for 10, 30, 90, and 180 min at 4°C. Binding of 633 to N18 cells was significantly lower than that of TE when viruses were diluted in PBS (P = 0.0036, 0.005, 0.004, and 0.025 at 10, 30, 90, and 180 min, respectively) or DMEM (P < 0.0005 at all time points).
FIG. 5.
Binding of 633 in DMEM to N18, but not BHK, cells is more sensitive to pH changes than binding of TE. (A) BHK and N18 cells were mixed with 35S-labeled TE and 633 in DMEM−SB supplemented with 1% FBS and 20 mM phosphate buffer at pH 6.5, 6.95, 7.4, 7.85, and 8.5 for 90 min at 4°C. Binding of 633 was lower than binding of TE to N18 cells at pHs of 6.95 (P = 0.0013) and above (pHs 7.4 and 8.5, P < 0.0001; pH 7.85, P = 0.0037). (B) The NaCl concentration of RPMI−SB supplemented with 0.5% BSA and 20 mM phosphate (pH 7.5) was raised by 0, 5, 10, 15, 17.5, 25, and 35 mM (final conductivities were 12,500, 12,970, 13,380, 13,730, 14,550, and 15,340 μS, respectively) and binding of TE and 633 was assessed as above. (C) The glucose concentration of DMEM without glucose was adjusted to 11 and 25 mM. N18 cells were mixed with 35S-labeled TE and 633 for 120 min at 4°C. (D) The ionic strength of RPMI-SB containing 0.5% BSA (conductivity = 10,800 μS) was adjusted to that of DMEM-SB containing 1% FBS (conductivity = 11,970 μS) by raising the NaCl concentration approximately 14 mM. Phosphate (20 mM) was used to adjust the pH to 6.6, 7.15, 7.5, 7.85, and 8.10. N18 and BHK cells were incubated with 35S-labeled TE and 633 for 150 min at 4°C.
The virus-cell binding assays were carried out essentially as previously described (7, 52). For chlorate treatment, the cells were dissociated in trypsin and incubated in F-12 HAM medium (Sigma) supplemented with 10 mM sodium chlorate, 2% dialyzed FBS, 50-μg/ml glutamine, and 10,000 U of penicillin per ml for 1 additional day. Cells were washed once and detached by incubation for 15 min in PBS containing 1 mM EDTA. Cells were washed in the specified binding buffer (4°C) and resuspended at 4 × 105 to 7 × 105 cells per ml. Subsequently, 290 μl of the cell suspension was mixed with 10 μl of diluted virus (10,000 cpm) in a 1.7-ml microfuge tube. The tubes were rocked at 4°C for 30 to 180 min and centrifuged in a Microfuge for 5 min at 4,500 rpm at 4°C. The cells were washed twice in 600 μl of the binding buffer and lysed in 100 μl of 1% SDS. The tubes were rinsed once with 100 μl of water. The lysate and the rinse were combined, mixed with Liquiscint (National Diagnostics, Atlanta, Ga.), and counted.
Binding of virus to BHK-21 cells was carried out under similar conditions with cell monolayers in 12-well tissue culture plates. At 95 to 100% confluence, cells were washed twice with 300 μl of the binding buffer (4°C). 35S-labeled TE or 633 was diluted to 10,000 cpm in 300 μl of the appropriate binding buffer and added to each well. The plates were rocked at 4°C for 30 to 180 min, washed twice with 600 μl of binding buffer, and lysed in 300 μl of 1% SDS. Cell lysates were transferred to scintillation vials together with 300 μl of H2O used to rinse the well and counted. All of the binding assays were carried out in triplicate. Three separate aliquots of the viruses used were counted to determine total counts per minute and to calculate the percentage bound.
Binding to HS was determined by heparin-Sepharose chromatography as previously described (7).
Preparation of liposomes.
Phosphatidylcholine (PC) from egg yolk, phosphatidylethanolamine (PE) prepared by transphosphatidylation of egg PC, and egg sphingomyelin (SPM) were obtained from Avanti Polar Lipids (Alabaster, Ala.). High-grade cholesterol (CHOL) was from Solvay Pharmaceuticals (Weesp, The Netherlands). Alternatively, lipids were extracted from N18 and BHK-21 cells according to Bligh and Dyer (3). Large unilamellar vesicles were prepared by a freeze-thaw extrusion procedure. Briefly, lipid mixtures were dried from a chloroform-methanol (2:1) solution under a stream of nitrogen and further dried under vacuum for at least 1 h. The lipids were hydrated with HNE (5 mM HEPES, 150 mM NaC1, and 0.1 mM EDTA [pH 7.4]) and subjected to five cycles of freezing and thawing. The vesicles were then extruded through a Unipore polycarbonate filter with a pore size of 0.2 μm (Nuclepore, Pleasanton, Calif.) with a LiposoFast miniextruder (Avestin, Ottawa, Canada). Liposomes consisted of N18 cell lipids, BHK-21 cell lipids, or a PC-PE-SPM-CHOL mixture (molar ratio, 1:1:1:1.5). The phospholipid content of the liposomes was determined by phosphate analysis (4).
Fusion assay.
Fusion of pyrene-labeled TE or 633 viruses with liposomes was monitored continuously as a decrease of pyrene excimer fluorescence intensity, due to dilution of pyrene-labeled lipids from the viral envelope into the target liposomes (5, 38, 46, 57). Briefly, pyrene-labeled virus (0.5 μM viral phospholipid) and liposomes (200 μM phospholipid) were mixed in the cuvette of an AB2 fluorometer (SLM/Aminco, Urbana, Ill.) in a volume of 0.665 ml of HNE. The content of the cuvette was magnetically stirred and maintained at 37°C. Fusion was initiated by injection of 35 μl of 0.1 M 2-(N-morpholino)ethanesulfonic acid and 0.2 M acetic acid, pretitrated with NaOH to achieve the final desired pH. Pyrene excimer fluorescence intensity was monitored at excitation and emission wavelengths of 345 and 480 nm, respectively. The fusion scale was calibrated such that 0% fusion corresponded to the initial excimer fluorescence intensity and 100% fusion corresponded to the fluorescence intensity at infinite probe dilution obtained by the addition of 35 μl of 0.2 M octaethylene glycol monododecyl ether (C12E8; Fluka Chemie AG, Buchs, Switzerland).
RESULTS
Susceptibility of N18 cells to infection by TE and 633.
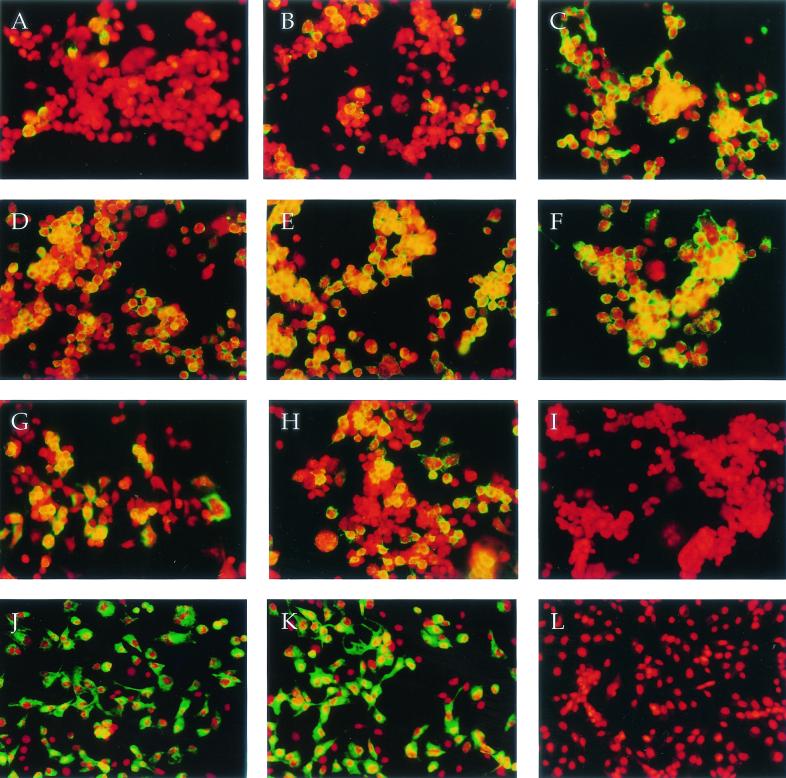
As demonstrated by infectious center assays, TE infects more N18 cells than does 633 at an MOI of 10 to 50 (14). There are at least two possible explanations for this observation. A subpopulation of N18 cells (such as cells at a particular stage of the cell cycle) may be resistant to infection by 633 but not TE, or all of the N18 cells may be susceptible to infection by both viruses, but infection with 633 is much less efficient than infection with TE. To test these hypotheses, N18 cells were infected with TE and 633 at an MOI of 50, 500, or 5,000 and incubated for 2 h to allow virus entry before ammonium chloride was added to block secondary virus infection. Cells were fixed 5 h after infection and stained with an anti-E2 MAb to detect the expression of E2 on the cell surface. Individual cells were identified by propidium iodide staining of the nuclei (Fig. 1). At an MOI of 50, TE infected more N18 cells than did 633 (70 versus 7%) (Fig. 1A and D). At an MOI of 500, 80% of the N18 cells were infected with TE, but only 39% were infected with 633 (Fig. 1B and E). However, at an MOI of 5,000, almost all N18 cells were infected with 633 (91%) as well as with TE (97%) (Fig. 1C and F). Therefore, the whole population of N18 cells was susceptible to infection by 633, but 633 was less efficient at establishing infection than TE. However, N18 cells were much less susceptible than BHK cells to infection with either virus. At an MOI of 5, essentially 100% of BHK cells were infected by either TE or 633 (Fig. 1J and K).
FIG. 1.
Susceptibility of N18 (A to I) and BHK (J to L) cells to TE and 633 infection. N18 and BHK cells were infected with TE or 633 at a BHK MOI of 5, 50, 500, or 5,000. The virus inoculum was diluted in either DMEM + SB supplemented with 1% FBS or RPMI−SB supplemented with 20 mM HEPES and 0.5% BSA. At 2.5 h after infection, 20 mM ammonium chloride was added and incubation continued for an additional 3 h. Cells were fixed in paraformaldehyde and stained with an E2-specific MAb (209). Alexa 488-conjugated goat anti-mouse antibody (green) was used as the secondary antibody. Visualization of individual cells was facilitated by a propidium iodine nuclear stain (red). Cells, viruses, medium, and MOI are as follows: N18 plus 633 in DMEM at MOI = 50 (A), 500 (B), or 5,000 (C); N18 plus TE in DMEM at MOI = 50 (D), 500 (E), or 5,000 (F); N18 plus 633 (G) or TE (H) in RPMI at MOI = 50; N18 plus TE in DMEM, control without primary Ab (I); BHK plus TE (J) or 633 (K) in DMEM at MOI = 5; BHK plus TE in DMEM, control without primary Ab (L).
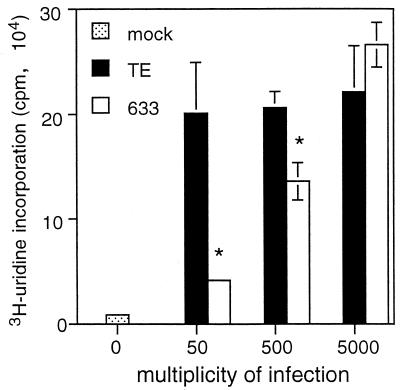
To be sure that the SV glycoprotein on the N18 cell surface reflected virus replication at these high MOIs, viral RNA synthesis was examined (Fig. 2). At an MOI of 5,000, similar levels of [3H]uridine were incorporated into a TCA-precipitable form in TE- and 633-infected N18 cells at 5 to 6 h after infection. There was less viral RNA synthesis in 633- than in TE-infected N18 cells at MOIs of 50 (P = 0.03) and 500 (P = 0.02). These results imply that as long as 633 and TE can enter the N18 cells to initiate virus infection, viral RNA synthesis does not differ.
FIG. 2.
Viral RNA synthesis in N18 cells infected with TE and 633. N18 cells were incubated for 1 h at 37°C in the presence of 5% CO2 with TE and 633 diluted in DMEM + SB-1% FBS to contain BHK MOIs of 0, 50, 500, or 5,000. The cells were maintained in DMEM + SB-2% FBS for 5 h before the addition of 150 mCi of [3H]uridine/ml and 20 mg of actinomycin D/ml. After 1 h, TCA-precipitable counts in the cells were determined. ∗, P < 0.05, Student's t test.
Fusion of 633 and TE to N18 and BHK cell-derived liposomes.
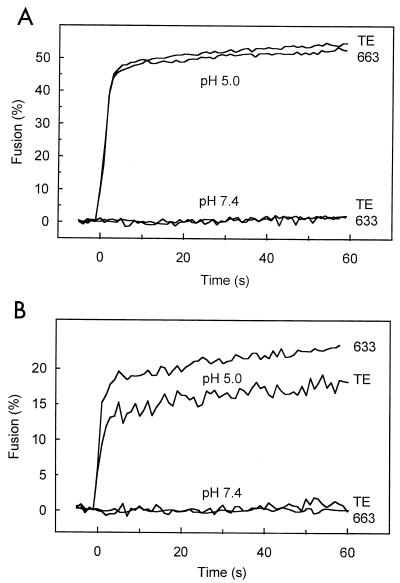
Since a substitution of threonine for isoleucine at E212 of SFV, a related alphavirus, leads to decreased virus-cell fusion by lowering the pH required for the initial E1-E2 dimer dissociation (22), the effects of the mutation at E255 on fusion were examined. Liposomes were prepared using lipid extracts from N18 or BHK cells and used as target membranes for fusion of pyrene-labeled TE and 633 (Fig. 3). At pH 7.4, there was no fusion between the viruses and liposomes. On the other hand, at pH 5.0, TE and 633 fused with BHK- or N18-derived liposomes with similar kinetics and to similar levels. Also, the detailed pH dependencies of the fusion of TE and 633 were very similar (results not shown). Although TE and 633 did not differ in fusion, both viruses fused more extensively with BHK-derived liposomes (∼50% fusion) than with N18-derived liposomes (17 to 20% fusion). Fusion of the viruses with BHK-derived liposomes was very similar to fusion with PC-PE-SPM-CHOL liposomes in terms of both kinetics and final extent (results not shown).
FIG. 3.
Fusion of TE and 633 with liposomes prepared from N18 or BHK cell lipids. Lipids were extracted from N18 (A) and BHK (B) cells and used to prepare liposomes. Pyrene-labeled TE or 633 viruses were mixed with liposomes at 37°C. At 0 s, the buffer pH was adjusted to 5.0 or maintained at 7.4, after which the change in pyrene excimer fluorescence intensity at 480 nm, due to dilution of the pyrene-labeled viral lipids into the liposome membrane, was monitored continuously.
Effect of buffer composition on binding of TE and 633 to N18 cells.
TE and 633 were previously demonstrated to bind to N18 cells to a similar extent. However, the conditions for testing binding were different (RPMI−SB-0.5% BSA-20 mM HEPES at 4°C without CO2) than those used routinely for infection (DMEM + SB-1% FBS at 37°C in 5% CO2) (7, 53). Therefore, binding of 35S-labeled TE and 633 to N18 cells at 4°C was compared with viruses diluted in RPMI−SB, PBS or DMEM + SB (Fig. 4A). TE and 633 bound to N18 cells with similar kinetics and to similar extents when diluted in RPMI−SB-based buffer. TE showed somewhat improved binding in PBS and DMEM compared to RPMI, while 633 showed lower binding than TE when diluted in PBS (P < 0.03 at all time points) and almost no binding when diluted in DMEM + SB (P < 0.0005 at all time points). Under the same conditions, no consistent diluent-dependent differences were detected in TE and 633 binding to BHK cells (Fig. 4B). Consistent with previous observations (53), both viruses were able to bind to BHK cells more efficiently than to N18 cells when diluted either in RPMI (P = 0.0004 for TE; P = 0.0054 for 633) or in DMEM (P < 0.0001 for TE and 633).
Since in a previous study (14) and in the above experiments (Fig. 1 and 2) comparing TE and 633 entry and replication in N18 cells, virus was diluted in DMEM + SB, it was important to determine whether TE and 633 would replicate similarly in N18 cells if virus was diluted in RPMI rather than DMEM. N18 cells were infected with TE or 633 diluted in RPMI−SB at an MOI of 50. Infection of N18 cells with TE and 633 in RPMI led to similarly high proportions (47 and 68%) of infected cells at 5 h (Fig. 1G and H).
Sensitivity to pH of TE and 633 and binding to N18 cells in DMEM.
To investigate whether binding of TE and 633 diluted in DMEM to N18 or BHK cells is sensitive to pH, DMEM−SB supplemented with 1% FBS was adjusted to pHs between 6.5 and 8.5 with 20 mM phosphate (Fig. 5A). Binding to BHK cells did not change over this pH range, while binding of 633 to N18 cells decreased at pHs above 7.0. Binding of TE to N18 cells decreased only above pH 8.0. Binding of 633 was significantly lower than that of TE at pHs of 6.95 and above (P < 0.005). pH-dependent binding differences to N18 cells were not observed when these viruses were diluted in RPMI (results not shown).
Effects of glucose concentration and ionic strength on binding of TE and 633 to N18 cells.
The composition of RPMI and DMEM differs in a number of ways, including glucose concentration (11 and 25 mM), ionic strength (10,800 μS and 11,970 μS) and Ca2+ concentration (0.4 and 1.8 mM). To determine the relevance of these differences, each parameter was adjusted independently and binding was assessed. DMEM + SB without glucose was supplemented with 11 or 25 mM glucose and 1% FBS. TE bound to N18 cells better than 633 at both concentrations of glucose (Fig. 5C), so lowering the glucose concentration of DMEM to that of RPMI did not improve 633 binding. The ionic strength of RPMI (pH 7.5) was systematically increased by the addition of NaCl (Fig. 5B), resulting in a steady decline in virus binding to N18 cells that was greater for 633 than TE. Binding to BHK cells was not affected. The conductivity of RPMI was adjusted to that of DMEM, requiring the addition of about 14 mM NaCl, and binding was assessed at different pHs (Fig. 5D). Binding to N18 cells, but not to BHK cells, of TE and 633 diluted in RPMI of increased ionic strength differed significantly in a pH-dependent manner (Fig. 5D), and the pattern was similar to that observed for virus diluted in DMEM (Fig. 5A). Lastly, increasing the Ca2+ concentration of RPMI to that of DMEM did not decrease binding of either virus to N18 cells but did enhance the difference between TE and 633, possibly due to the concomitant increase in ionic strength. There was no effect on binding to BHK cells (results not shown).
The role of sulfated molecules in TE and 633 binding to N18 and BHK cells.
HS, a highly negatively charged molecule, is a determinant of SV binding to the cell surface. HS binding increases the efficiency of infection of CHO and BHK cells (7, 32). To determine if the differences in binding of TE and 633 to N18 cells and the effect of pH on that binding could be explained by the relative strength of binding to HS, we examined binding to heparin-Sepharose and to cells differing in the levels of sulfated molecules on the cell surface. Each virus was bound to a heparin-Sepharose column at a NaCl concentration of 50 mM and then eluted with a 50 to 500 mM NaCl gradient prepared at either pH 6.5 or 7.5 (5 mM phosphate) The results show that 633 bound less tightly to HS than TE at both pH values: at pH values of 6.5 and 7.5, TE eluted at 403 and 320 mM NaCl, respectively; 633 eluted at 346 and 302 mM NaCl, respectively.
To investigate whether HS or other sulfated molecules participated in the SV-N18 cell binding that is ionic strength- and pH-sensitive, N18 and BHK cells were grown in low-sulfate medium in the presence of 10 mM sodium chlorate, a potent inhibitor of ATP sulfurylase, the first enzyme in the biosynthesis of the cosubstrate for sulfation (2, 28). Binding assays were carried out in DMEM−SB containing 1% FBS and 20 mM phosphate, pH 6.5 or 7.5, conditions at which TE and 633 showed the least and the most significant differences in binding to N18 cells (Fig. 5A). At pH 6.5, binding of TE and 633 to N18 cells was decreased similarly by the chlorate treatment, indicating that binding of both viruses to N18 cells is affected by the desulfation of the cells (Fig. 6A). At pH 7.5, TE reacted well with sulfated molecules on the surface of N18 cells, but 633 did not. Binding of TE (P = 0.0045) and 633 (P = 0.0025) to the chlorate-treated N18 cells was lower than to untreated N18 cells (Fig. 6A), but differences between TE and 633 were still observed. Chlorate treatment decreased binding of TE and 633 to BHK cells at pH 7.5 from >70% to approximately 10% (Fig. 6B), similar to the binding of both viruses to CHO cells lacking HS (results not shown).
FIG. 6.
Sulfated molecules appear to be involved in the ionic strength- and pH-sensitive binding of TE and 633 to N18 cells. N18 (A) and BHK (B) cells were grown in Ham's medium containing a low concentration of sulfate with (+) or without (−) 10 mM sodium chlorate overnight. Binding was performed in DMEM−SB containing 1% FBS and 20 mM phosphate at pH 6.5 or 7.5.
DISCUSSION
The substitution of the amino acid histidine for glutamine at residue 55 of the E2 glycoprotein has a profound effect on the neurovirulence of SV. This is reflected in the relative abilities of recombinant viruses containing either histidine (TE) or glutamine (633) to replicate in mouse brain and in N18 neuroblastoma cells (34, 54). In the present studies, we have shown that all N18 cells can be infected by both viruses, but infection is 10- to 100-fold less efficient for 633 than for TE. Both viruses infect N18 cells less efficiently than BHK cells, presumably as a result of decreased binding to the cell surface combined with a limited ability of the lipids in the plasma membrane of N18 cells to support SV fusion. Both TE and 633 bound to HS, a major determinant of binding to BHK and CHO cells (32). Binding of the viruses to N18 cells differed in that 633, but not TE, was exquisitely sensitive to the composition of the diluent used in the binding assay and decreased substantially as ionic strength and pH increased to physiologic levels. Treatment of N18 cells with sodium chlorate to inhibit sulfation decreased the binding of TE at pH 7.5 to approximately the binding of 633 to N18 cells not treated with chlorate. The limited binding of 633 was further decreased by chlorate treatment. The data suggest that BHK cells have a sulfated molecule to which TE and 633 bind efficiently to initiate infection and that this molecule is either not present on N18 cells or present in an altered form. The interaction of SV with the primary receptor on N18 cells is affected by a glutamine at E255 in a way that impairs binding at physiologic conditions of salt concentration and pH.
Different types of cells exhibit different degrees of susceptibility to SV infection. Clearly, both TE and 633 infect N18 cells much less efficiently than BHK cells. An MOI of 50 to 500 (determined with BHK cells) was required to establish synchronous infection in N18 cells with TE, while 633 required an MOI of 5,000 (Fig. 1). Both viruses bind less efficiently to N18 cells than to BHK cells (Fig. 4). Furthermore, the viruses fuse less extensively with N18-derived liposomes than with BHK-derived liposomes (Fig. 3).
It is well established that the lipid composition of the target membrane has a profound effect on the membrane fusion characteristics of enveloped viruses (41, 44, 50). For alphaviruses, studies with both liposomal model systems as well as with cells have shown that membrane interaction and fusion are dependent on CHOL (5, 30, 40, 57, 59). Furthermore, fusion requires the presence of sphingolipid in the target membrane (10, 37, 38). CHOL appears to be primarily involved in the initial low-pH-dependent binding of the virus to the target membrane lipids, while sphingolipids presumably play a catalytic role in the subsequent fusion event itself (38). There is no reason to suspect that in our present study the N18 liposomes were deficient in either CHOL or sphingolipid: the plasma membrane of N18 cells has a high CHOL-to-phospholipid ratio, and SPM is a major component of the phospholipid fraction (9). The detailed fusion characteristics of TE or 633 in the liposomal model system (Fig. 3) indicate that with N18-derived liposomes a smaller fraction of the virus fuses than with BHK-derived liposomes or liposomes consisting of an artificial PC-PE-SPM-CHOL mixture. Yet, the fraction of virus that does fuse exhibits very similar fusion kinetics in either case. In view of the high CHOL content of the liposomes, it is unlikely that the low extent of fusion is due to a low degree of binding. It thus appears that with N18-derived liposomes part of the liposome-bound virus remains unfused. This is suggestive of a degree of heterogeneity at the surface of the liposomes, such that sites or domains that do support efficient fusion coexist with other areas that do not. Although recent experiments have shown that the presence of CHOL-sphingolipid domains in target liposomes is neither required nor inhibitory to alphavirus membrane fusion (B. L. Waarts and J. Wilschut, unpublished observations), the fusion of TE and 633 with N18 liposomes is reminiscent of SV fusion with liposomes containing domains with a relatively high degree of fatty acid saturation, resulting in tight lipid packing. Cultured neuroblastoma cells generally contain low concentrations of polyunsaturated fatty acids, unless specific fatty acid-supplemented medium is used (42). In addition, N18 cell membranes contain N-acylphosphatidylethanolamine and N-acylethanolamine (25), which may contribute to a tighter lipid packing and a decreased susceptibility to SV fusion.
While there was a distinct difference between N18- and BHK-derived liposomes in terms of fusion with TE or 633, paired comparison showed that both viruses fused equally well. Apparently, substitution of His for Gln at E255 has little effect on the viral fusion process, suggesting that an improved interaction of the TE virus with target cell membrane lipids does not account for the increased neurovirulence of TE relative to that of 633. Fusion of alphaviruses is mediated by the E1 glycoprotein, presumably in a homotrimeric configuration (5, 46, 57), while receptor interaction is a function of E2 (47). Therefore, it is likely that amino acid changes in E1 primarily affect fusion with the neuronal cell membrane, while changes in E2 are expected to have their principal effects on virus binding.
Significant differences in binding to N18 cells were observed between TE and 633 diluted in DMEM, but not in RPMI, while binding to BHK cells was similar. These differences were explained primarily by the higher ionic strength of DMEM, which was associated with a loss of 633 binding to N18 cells at physiologic pH. Binding of both TE and 633 to N18 and BHK cells was diminished after treatment with chlorate, suggesting that the binding of SV to N18 cells is mediated primarily by sulfated molecules which are highly negatively charged with ligand interactions that are often sensitive to ionic strength and pH (6).
Binding of 633 to BHK cells was not sensitive to changes in pH and ionic strength, implying that the sulfated groups on the surface of BHK and N18 cells with which SV is interacting are different. The sulfated molecules on BHK cells could provide more stable interactions to the SV glycoproteins than those on N18 cells and therefore would be less sensitive to the interference by high salt concentration. Alternately, the density of sulfated molecules on BHK cells could be more abundant than on N18 cells, providing stronger binding interactions. Therefore, increased efficiency of SV infection of BHK cells compared to N18 cells is likely to represent a combination of better initial binding to sulfated molecules including HS on the cell surface and the fusogenicity of the lipids present in the plasma membrane. Both of these parameters are suboptimal in N18 cells so that selection of viral variants with improved attachment and/or fusion would be predicted to increase neurovirulence.
Binding of proteins to glycosaminoglycans has been considered to be primarily electrostatic, but recent studies indicate that this interaction is both more complicated and more specific than solely charge-determined interactions would imply. Proteoglycan expression is regulated in both developmental and in tissue-specific ways (18, 27, 31). Proteoglycans vary in core proteins; in the types, locations, and numbers of glycosaminoglycan chains attached; and in the degree of sulfation. This variability allows relatively specific binding of a wide range of ligands (27). Of the HS proteoglycans, syndecan-2 is most abundant in liver and kidney and syndecan-3 is highly expressed in neonatal, but not adult, brain (45). Little is known about the proteoglycans expressed on neurons, and the sulfated molecule on N18 cells involved in SV binding to the cell surface has not been identified. Tyrosine sulfation is a relatively common posttranslational modification of proteins (26), and sulfation of CCR5 at an N-terminal tyrosine residue has been implicated as an important factor in cytokine-CCR5 binding and in HIV entry into cells (17).
The region of the E2 glycoprotein we investigated in the present study is important for virulence in other alphaviruses as well. A mutant with a deletion of amino acids 55 to 61 of E2 of Ross River virus generates small plaques and is less virulent in mice (55). Whether E255 is involved in binding to the sulfated molecules directly or indirectly is not known. Glutamine is not charged at neutral pHs, while histidine is titratable with pKR′ at pH 6.0. At higher pH, histidine becomes less protonated, which is predicted to decrease the affinity of E2 for negatively charged sulfate groups. When histidine has been implicated directly in HS binding, this interaction is improved at pH 6.5 (6). However, with DMEM, binding of TE to N18 cells remained unaffected when the pH was raised between 7 and 8, suggesting that E255 is not directly involved in the charge interactions between E2 and the cell surface.
It is possible that additional receptors are present on N18 and BHK cells that are cell specific and that their interactions are facilitated by the virus-sulfate molecule interactions. Other viruses use different cell receptors on different cells. Mouse hepatitis virus uses isoforms of the murine carcinoembryonic antigen gene family (16) and herpes simplex viruses are able to use a number of different receptors alone or in combination to infect various cell types such as epithelial and neuronal cells (48, 58).
From the results of the binding assays, the attachment of 633 virus to N18 cells appears more susceptible to changes in the environment than that of TE. The association between the change from glutamine to histidine at E255 and SV isolates with higher degrees of neurovirulence has been repeatedly detected in our laboratory (33), suggesting that virus with a histidine at E255 has a strong selective advantage in mouse brain. Altered kinetics of virus entry into the cell could affect virus growth rate and virulence by influencing the establishment or spread of the virus. Efficiency of virus spread is a critical determinant for neurovirulence of polytropic murine retroviruses, rabies virus, and mouse hepatitis virus (13, 43). Considering the ionic strength and pH of extracellular fluid, histidine at E255 would facilitate virus spread in mouse brain. Therefore, we hypothesize that TE has an envelope that interacts stably with the neuronal receptor in the microenvironment of the CNS and is able to initiate more rounds of replication before the onset of the immune response, thus increasing virulence.
Acknowledgments
We thank Andrew Byrnes for many helpful discussions and Marcia Lyons for technical assistance.
This work was supported by research grants RO1 NS18596 (D.E.G.) and R01 HL1666 (J.W.) and by training grant T32 AI 07417 (R.K.) from the National Institutes of Health.
REFERENCES
- 1.Amano, T., E. Richelson, and M. Nirenberg. 1972. Neurotransmitter synthesis by neuroblastoma clones. Proc. Natl. Acad. Sci. USA 69:258-263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baeuerle, P. A., and W. B. Huttner. 1986. Chlorate—a potent inhibitor of protein sulfation in intact cells. Biochem. Biophys. Res. Commun. 141:870-877. [DOI] [PubMed] [Google Scholar]
- 3.Bligh, E., and W. Dyer. 1959. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 37:911-917. [DOI] [PubMed] [Google Scholar]
- 4.Böttcher, C. J. F., C. M. van Gent, and C. Fries. 1961. A rapid and sensitive sub-micro phosphorus determination. Anal. Chim. Acta 24:203-204. [Google Scholar]
- 5.Bron, R., J. M. Wahlberg, H. Garoff, and J. Wilschut. 1993. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 12:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brunden, K. R., N. J. Richter-Cook, N. Chaturvedi, and R. C. Frederickson. 1993. pH-dependent binding of synthetic beta-amyloid peptides to glycosaminoglycans. J. Neurochem. 61:2147-2154. [DOI] [PubMed] [Google Scholar]
- 7.Byrnes, A. P., and D. E. Griffin. 1998. Binding of Sindbis virus to cell surface heparan sulfate. J. Virol. 72:7349-7356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Byrnes, A. P., and D. E. Griffin. 2000. Large-plaque mutants of Sindbis virus show reduced binding to heparan sulfate, heightened viremia, and slower clearance from the circulation. J. Virol. 74:644-651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chakravarthy, B. R., M. W. Spence, J. T. Clarke, and H. W. Cook. 1985. Rapid isolation of neuroblastoma plasma membrane on Percoll gradients. Characterization and lipid composition. Biochim. Biophys. Acta 812:223-233. [DOI] [PubMed] [Google Scholar]
- 10.Corver, J., L. Moesby, R. K. Erukulla, K. C. Reddy, R. Bittman, and J. Wilschut. 1995. Sphingolipid-dependent fusion of Semliki Forest virus with cholesterol-containing liposomes requires both the 3-hydroxyl group and the double bond of the sphingolipid backbone. J. Virol. 69:3220-3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis, N. L., F. J. Fuller, W. G. Dougherty, R. A. Olmsted, and R. E. Johnston. 1986. A single nucleotide change in the E2 glycoprotein gene of Sindbis virus affects penetration rate in cell culture and virulence in neonatal mice. Proc. Natl. Acad. Sci. USA 83:6771-6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Davis, N. L., N. Powell, G. F. Greenwald, L. V. Willis, B. J. B. Johnson, J. F. Smith, and R. E. Johnston. 1991. Attenuating mutations in the E2 glycoprotein gene of Venezuelan equine encephalitis virus: construction of single and multiple mutants in a full-length cDNA clone. Virology 183:20-31. [DOI] [PubMed] [Google Scholar]
- 13.Dietzchold, B., T. Wiktor, J. Trojanowski, R. Macfarlan, W. Wunner, M. Torres-Anjel, and H. Koprowski. 1985. Differences in cell-to-cell spread of pathogenic and apathogenic rabies virus in vivo and in vitro. J. Virol. 56:12-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dropulic, L. K., J. M. Hardwick, and D. E. Griffin. 1997. A single amino acid change in the E2 glycoprotein of Sindbis virus confers neurovirulence by altering an early step of virus replication. J. Virol. 71:6100-6105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dubuisson, J., and C. M. Rice. 1993. Sindbis virus attachment: isolation and characterization of mutants with impaired binding to vertebrate cells. J. Virol. 67:3363-3374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dveksler, G., C. Dieffenbach, C. Cardellichio, K. McCuaig, M. Pensiero, G. Jiang, N. Beauchemin, and K. Holmes. 1993. Several members of the mouse carcinoembryonic antigen-related glycoprotein family are functional receptors for the coronavirus mouse hepatitis virus-A59. J. Virol. 67:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Farzan, M., T. Mirabekov, P. Kolchinsky, R. Wyatt, M. Cayabyab, N. Gerard, C. Gerard, J. Sodroski, and H. Choe. 1999. Tyrosine sulfation of the amino terminus of CCR5 facilitates HIV-1 entry. Cell 96:667-676. [DOI] [PubMed] [Google Scholar]
- 18.Feyzi, E., T. Saldeen, E. Larsson, U. Lindahl, and M. Salmivirta. 1998. Age-dependent modulation of heparan sulfate structure and function. J. Biol. Chem. 273:13395-13398. [DOI] [PubMed] [Google Scholar]
- 19.Flynn, D. C., W. J. Meyer, J. M. Mackenzie, Jr., and R. E. Johnston. 1990. A conformational change in Sindbis virus glycoproteins E1 and E2 is detected at the plasma membrane as a consequence of early virus-cell interactions. J. Virol. 64:3643-3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fries, E., and A. Helenius. 1979. Binding of Semliki Forest virus and its spike glycoproteins to cells. Eur. J. Biochem. 97:213-220. [DOI] [PubMed] [Google Scholar]
- 21.Glasgow, G. M., B. J. Sheahan, G. J. Atkins, J. M. Wahlberg, A. Salminen, and P. Liljestrom. 1991. Two mutations in the envelope glycoprotein E2 of Semliki Forest virus affecting the maturation and entry patterns of the virus alter pathogenicity for mice. Virology 185:741-748. [DOI] [PubMed] [Google Scholar]
- 22.Glomb-Reinmund, S., and M. Kielian. 1998. fus-1, a pH shift mutant of Semliki Forest virus, acts by altering spike subunit interactions via a mutation in the E2 subunit. J. Virol. 72:4281-4287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Griffin, D. E. 1986. Alphavirus pathogenesis and immunity, p. 209-249. In S. Schlesinger and M. J. Schlesinger (ed.), The Togaviridae and Flaviviridae. Plenum Publishing Corp., New York, N.Y.
- 24.Griffin, D. E., and R. T. Johnson. 1977. Role of the immune response in recovery from Sindbis virus encephalitis in mice. J. Immunol. 118:1070-1075. [PubMed] [Google Scholar]
- 25.Gulaya, N. M., A. A. Melnik, D. I. Balkov, G. L. Volkov, M. V. Vysotskiy, and V. E. Vaskovsky. 1993. The effect of long-chain N-acylethanolamines on some membrane-associated functions of neuroblastoma C1300 N18 cells. Biochim. Biophys. Acta 1152:280-288. [DOI] [PubMed] [Google Scholar]
- 26.Huttner, W. 1982. Sulphation of tyrosine residues—a widespread modification of proteins. Nature 299:273-276. [DOI] [PubMed] [Google Scholar]
- 27.Kato, M., H. Wang, M. Bernfield, J. T. Gallagher, and J. E. Turnbull. 1994. Cell surface syndecan-1 on distinct cell types differs in fine structure and ligand binding of its heparan sulfate chains. J. Biol. Chem. 269:18881-18890. [PubMed] [Google Scholar]
- 28.Keller, K. M., P. R. Brauer, and J. M. Keller. 1989. Modulation of cell surface heparan sulfate structure by growth of cells in the presence of chlorate. Biochemistry 28:8100-8107. [DOI] [PubMed] [Google Scholar]
- 29.Kielian, M., and A. Helenius. 1986. Entry of alphaviruses, p. 91-119. In S. Schlesinger and M. J. Schlesinger (ed.), The Togaviridae and Flaviviridae. Plenum Publishing Corp., New York, N.Y.
- 30.Kielian, M. C., and A. Helenius. 1984. Role of cholesterol in fusion of Semliki Forest virus with membranes. J. Virol. 52:281-283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim, C. W., O. A. Goldberger, R. L. Gallo, and M. Bernfield. 1994. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol. Biol. Cell 5:797-805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimstra, W. B., K. D. Ryman, and R. E. Johnston. 1998. Adaptation of Sindbis virus to BHK cells selects for use of heparan sulfate as an attachment receptor. J. Virol. 72:7357-7366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levine, B., and D. E. Griffin. 1993. Molecular analysis of neurovirulent strains of Sindbis virus that evolve during persistent infection of scid mice. J. Virol. 67:6872-6875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis, J., S. L. Wesselingh, D. E. Griffin, and J. M. Hardwick. 1996. Alphavirus-induced apoptosis in mouse brains correlates with neurovirulence. J. Virol. 70:1828-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lustig, S., A. C. Jackson, C. S. Hahn, D. E. Griffin, E. G. Strauss, and J. H. Strauss. 1988. The molecular basis of Sindbis virus neurovirulence in mice. J. Virol. 62:2329-2336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mendoza, Q. P., J. Stanley, and D. E. Griffin. 1988. Monoclonal antibodies to the E1 and E2 glycoproteins of Sindbis virus: definition of epitopes and efficiency of protection from fatal encephalitis. J. Gen. Virol. 69:3015-3022. [DOI] [PubMed] [Google Scholar]
- 37.Moesby, L., J. Corver, R. K. Erukulla, R. Bittman, and J. Wilschut. 1995. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry 34:10319-10324. [DOI] [PubMed] [Google Scholar]
- 38.Nieva, J.-L., R. Bron, J. Corver, and J. Wilschut. 1994. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 13:2797-2804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Olmsted, R. A., R. S. Baric, B. A. Sawyer, and R. E. Johnston. 1984. Sindbis virus mutants selected for rapid growth in cell culture display attenuated virulence in animals. Science 225:424-426. [DOI] [PubMed] [Google Scholar]
- 40.Phalen, T., and M. Kielian. 1991. Cholesterol is required for infection by Semliki forest virus. J. Cell Biol. 112:615-623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puri, A., P. Hug, K. Jernigan, J. Barchi, H.-Y. Kim, J. Hamilton, J. Wiels, G. J. Murray, R. O. Brady, and R. Blumenthal. 1998. The neutral glycosphingolipid globotriaosylceramide promotes fusion mediated by a CD4-dependent CXCR4-utilizing HIV type 1 envelope glycoprotein. Proc. Natl. Acad. Sci. USA 95:14435-14440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Robert, J., G. Rebel, and P. Mandel. 1978. Utilization of polyunsaturated fatty acid supplements by cultured neuroblastoma cells. J. Neurochem. 30:543-548. [DOI] [PubMed] [Google Scholar]
- 43.Robertson, S., K. Hasenkrug, B. Chesebro, and J. Portis. 1997. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglial cells. J. Virol. 71:5287-5294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Roos, D. S., and P. W. Choppin. 1985. Biochemical studies on cell fusion. I. Lipid composition of fusion-resistant cells. J. Cell Biol. 101:1578-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salmivirta, M., and M. Jalkanen. 1995. Syndecan family of cell surface proteoglycans: developmentally regulated receptors for extracellular effector molecules. Experientia 51:863-872. [DOI] [PubMed] [Google Scholar]
- 46.Smit, J. M., R. Bittman, and J. Wilschut. 1999. Low-pH-dependent fusion of Sindbis virus with receptor-free cholesterol and sphingolipid-containing liposomes. J. Virol. 73:8476-8484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith, T. J., R. H. Cheng, N. H. Olson, P. Peterson, E. Chase, R. J. Kuhn, and T. S. Baker. 1995. Putative receptor binding sites on alphaviruses as visualized by cryoelectron microscopy. Proc. Natl. Acad. Sci. USA 92:10648-10652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spear, P., R. Eisenberg, and G. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 49.Stanley, J., S. J. Cooper, and D. E. Griffin. 1985. Alphavirus neurovirulence: monoclonal antibodies discriminating wild-type from neuroadapted Sindbis virus. J. Virol. 56:110-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stegmann, T., S. Nir, and J. Wilschut. 1989. Membrane fusion activity of influenza virus. Effects of gangliosides and negatively charged phospholipids in target liposomes. Biochemistry 28:1698-1704. [DOI] [PubMed] [Google Scholar]
- 51.Strauss, J. H., and E. G. Strauss. 1994. The alphaviruses: gene expression, replication and evolution. Microbiol. Rev. 58:491-562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tucker, P. C., and D. E. Griffin. 1991. The mechanism of altered Sindbis virus neurovirulence associated with a single amino acid change in the E2 glycoprotein. J. Virol. 65:1551-1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tucker, P. C., S. H. Lee, N. Bui, D. Martinie, and D. E. Griffin. 1997. Amino acid changes in the Sindbis virus E2 glycoprotein that increase neurovirulence improve entry into neuroblastoma cells. J. Virol. 71:6106-6112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tucker, P. C., E. G. Strauss, R. J. Kuhn, J. H. Strauss, and D. E. Griffin. 1993. Viral determinants of age-dependent virulence of Sindbis virus in mice. J. Virol. 67:4605-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vrati, S., S. G. Faragher, R. C. Weir, and L. Dalgarno. 1986. Ross River virus mutant with a deletion in the E2 gene: properties of the virion, virus-specific macromolecule synthesis, and attenuation of virulence of mice. Virology 151:222-232. [DOI] [PubMed] [Google Scholar]
- 56.Vrati, S., P. J. Kerr, R. C. Weir, and L. Dalgarno. 1996. Entry kinetics and mouse virulence of Ross River virus mutants altered in neutralization epitopes. J. Virol. 70:1745-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wahlberg, J. M., R. Bron, J. Wilschut, and H. Garoff. 1992. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J. Virol. 66:7309-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Warner, M., R. Geraghty, W. Martinez, R. Montgomery, J. Whitbeck, R. Xu, R. Eisenberg, G. Cohen, and P. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2, and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]
- 59.White, J., and A. Helenius. 1980. A pH dependent fusion between the Semliki Forest virus membrane and liposomes. Proc. Natl. Acad. Sci. USA 77:3273-3277. [DOI] [PMC free article] [PubMed] [Google Scholar]