Abstract
Apoptosis of peripheral blood T cells plays an important role in the pathogenesis of human immunodeficiency virus (HIV) infection. In this study, we found that HIV type 1 (HIV-1) primes CD4+ T cells from healthy donors for apoptosis, which occurs after CD95 ligation or CD3-T-cell receptor (TCR) stimulation. CD95-mediated death did not depend on CD4 T-cell infection, since it occurred in the presence of the reverse transcriptase inhibitor didanosine (ddI). In contrast, apoptosis induced by productive infection (CD3-TCR stimulation) is prevented by both CD95 decoy receptor and ddI. Our data suggest that HIV-1 triggers at least two distinct death pathways: a CD95-dependent pathway that does not require viral replication and a viral replication-mediated cell death independent of the CD95 pathway. Further experiments indicated that saquinavir, a protease inhibitor, at a 0.2 μM concentration, decreased HIV-mediated CD95 expression and thus cell death, which is independent of its role in inhibiting viral replication. However, treatment of peripheral blood mononuclear cells from healthy donors with a higher concentration (10 μM) of an HIV protease inhibitor, saquinavir or indinavir, induced both a loss in mitochondrial membrane potential (ΔΨm) and cell death. Thus, protease inhibitors have the potential for both beneficial and detrimental effects on CD4+ T cells independent of their antiretroviral effects.
The depletion of CD4+ T cells is a major determinant of pathogenicity in human immunodeficiency virus type 1 (HIV-1) infection. In primate models of HIV and simian immunodeficiency virus (SIV) infection there is a correlation between enhanced T-cell apoptosis and pathogenesis (1, 13, 14, 21). Both spontaneous and activation-induced apoptosis occur in T cells obtained from HIV-1-infected individuals (1, 14, 18, 31, 40, 43, 55). The magnitude of apoptosis correlates with the stage of HIV disease (33, 44, 47, 48, 59). However, the fact that the viral load established soon after infection correlates with the rate of CD4 T-cell loss and the development of AIDS (higher viral load set point means higher CD4 T-cell loss and faster AIDS progression) (37) supports the idea that active HIV-1 replication directly contributes to the depletion of CD4+ T cells. This depletion may be related in part to apoptosis, as in vitro studies have shown that HIV-1 replication induces apoptosis in T-cell lines and proliferating primary CD4+ T cells stimulated with phytohemagglutinin-interleukin-2 (IL-2) (19, 20, 30, 39, 56). Among potential mechanisms involved in CD4 T-cell depletion during HIV infection, CD95 and its counterpart CD95L have been proposed to play a major role. T cells from HIV-1-infected persons show enhanced cell surface expression of CD95 and exhibit increased susceptibility in vitro to CD95-mediated death, which can be induced either by an agonistic anti-CD95 antibody or by a soluble CD95 ligand (CD95L) (7, 8, 15, 17, 27, 28, 49, 52, 53, 60). However, HIV-mediated death of productively infected CD4+ T cells in vitro has been found to be independent of CD95-CD95L interactions (19, 20, 39, 41, 42).
Highly active antiretroviral therapy produces significant immune system reconstitution with sustained increases in circulating CD4+ T cells after a rapid drop in plasma viral RNA (12, 22, 23) followed by a decrease of apoptotic cells (4-6, 11, 26, 29). However, it has been reported that HIV antiretroviral drugs, in addition to exerting antiviral effects, may have a direct effect on immune cells. The HIV protease inhibitor ritonavir, in addition to modulating proteasome activity and major histocompatibility complex class I-restricted presentation (3), prevented apoptosis and caspase 1 expression in cultures of CD4+ T cells from both healthy controls and HIV-infected individuals (45, 50, 51).
We report that incubation of T cells from healthy donors with HIV-1, in the absence of any T-cell stimulation, is sufficient to induce CD95 expression and prime the cells for CD95- or CD3-mediated cell death. Didanosine (ddI) had no effect on CD95-mediated CD4 T-cell death but did decrease activation-induced T-cell death (AICD) parallel with viral inhibition. In the presence of 0.2 μM saquinavir (SQV), we observed a reduction in T-cell death induced by CD95 ligation partly through the decrease of CD95 surface expression, but in the presence of a higher concentration (10 μM), there was a loss of mitochondrial membrane potential and subsequent toxicity to monocytes and CD4+ T cells. Our data indicate that antiretroviral drugs exert potent effects on HIV-mediated T-cell death dependent and independent of T-cell infection.
MATERIALS AND METHODS
Reagents.
Murine anti-human CD3 (UCHT1) and CD95 (CH11 and 7C11) were from Coulter Corporation, Miami, Fla.; CD14, CD19, CD56, and CD8 antibodies were from Pharmingen, San Diego, Calif. Soluble CD95 receptor decoy (human CD95-Fc immunoglobulin [Ig] fusion protein) was a gift from C. Ware (La Jolla Institute for Allergy and Immunology, La Jolla, Calif.). Fluorescein isothiocyanate (FITC)-labeled CD95 monoclonal antibody (MAb; UB2, IgG1 isotype) and PC5-labeled CD4 MAb (13B8.2) were from Coulter Corporation, and PerCP-labeled CD8 MAb (Leu 2a) was from Becton Dickinson, Mountain View, Calif.; recombinant human IL-2 was kindly provided by Chiron Corporation (Emeryville, Calif.). As a peptide competitive inhibitor of the caspases, zVAD-fmk, an irreversible broad caspase inhibitor (Bachem), was utilized. Other reagents were annexin V-FITC (Boehringer Mannheim, Indianapolis, Ind.), DiOC6 (Molecular Probes, Eugene, Oreg.); ddI, a reverse transcriptase inhibitor (Sigma, St. Quentin, France); and SQV and indinavir (IDV), two HIV protease inhibitors.
Cells and culture conditions.
Heparinized venous peripheral blood was obtained from HIV-seronegative healthy donors. Peripheral blood mononuclear cells (PBMC) were isolated from these samples by Ficoll-Hypaque density gradient centrifugation and then cultured in RPMI 1640 (Gibco/BRL, Gaithersburg, Md.). They were supplemented with 10% heat-inactivated fetal bovine serum (Summit Biotechnology, Greeley, Colo.), 2 mM l-glutamine, 1 mM sodium pyruvate (Gibco), and penicillin-streptomycin (Gibco). When indicated, purified CD4+ T cells were obtained by depleting PBMC of B cells, NK cells, and CD8+ T cells by using CD19, CD56, and CD8 MAbs and magnetic beads coated with anti-mouse IgG (Dynal, Lake Success, N.Y.). PBMC were incubated in the absence or presence of HIV at the indicated multiplicity of infection (MOI) for 2 h at 37°C, washed, and then cultured for 4 days in the absence or presence of HIV drugs (ddI, SQV, and IDV). Where indicated, cells were then incubated with either the agonistic CD95 MAb or the anti-CD3 MAb.
T-cell proliferation.
CD4+ T cells were cultured in 96-well culture plates (Becton Dickinson) at 5 × 104/ml for T-cell proliferation. Antibodies (anti-CD28, 1 μg/ml; anti-CD3, 1 μg/ml) were used in solution. Cells were cultured for 3 days, pulsed overnight with [3H]thymidine (0.5 μCi; Amersham), and harvested before scintillation counting.
Virus preparation.
High-titered stocks of HIV-1LAI (106 50% tissue culture infective doses/ml) were prepared by inoculating CEM at an MOI of 0.001 followed by culture for 10 days. Ten milliliters of this culture was added to 400 ml of uninfected CEM (5 × 105 cells/ml) and grown for 5 to 7 days until abundant syncytia were present. The cells were pelleted (300 × g for 10 min) and then resuspended in 1/100 of the initial volume for 8 h. The supernatant was clarified by centrifugation (800 × g for 10 min). HIV p24 antigen was measured by an enzyme immunoassay as described by the manufacturer (Abbott Laboratories, North Chicago, Ill.).
Measurement of cell death.
Live cells were counted in duplicate by light microscopy using trypan blue dye exclusion. Phosphatidylserine exposure of dying cells was identified by using FITC-conjugated annexin V (R&D Systems, Abingdon, United Kingdom) and two-color flow cytometry (16). Briefly, cells were first stained by incubating them with labeled antibodies, washed with phosphate-buffered saline, and then incubated again in binding buffer with FITC-annexin V (20 min, 4°C), according to the manufacturer's instructions. The percentage of dying CD4+ T cells was calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. The percentage of dying cells was also assessed by flow cytometry using DiOC6, which measures loss in mitochondrial membrane potential (ΔΨm).
RNase protection assay.
The CD95L RNase protection assay was performed as described by the manufacturer (Ambion, Austin, Tex.). CD95L cDNA was kindly provided by S. Nagata (Osaka Bioscience Institute, Osaka, Japan). β-Actin was used as a control. Twenty micrograms of total RNA was hybridized with radiolabeled antisense RNA transcripts, prior to digestion with RNase T1. The samples were separated by urea-sodium dodecyl sulfate-polyacrylamide gel electrophoresis, and then the gels were exposed to X-ray film.
Cell surface staining.
Two-color flow cytofluorometry was performed by costaining cells with directly labeled MAbs (including isotype controls). Lymphocytes were gated under forward and side scatter light parameters.
Statistical analysis.
Statistical significance was calculated by Student's t test.
RESULTS
HIV-1 primes quiescent healthy donor CD4+ T cells for death in response to CD95 and CD3-T-cell receptor (TCR) ligation.
Since HIV-1 replication is promoted by T-cell activation and proliferation, HIV-1-mediated T-cell death has been mainly explored in productively infected cultures of proliferating T cells. In HIV-1-infected persons viral replication is continuous, but the vast majority of T cells are in a noncycling state, and while they are continuously exposed to high concentrations of viral particles, only a small proportion of the particles are infectious (46). Thus, contact and/or penetration of noninfectious particles (without replication) could be sufficient to induce dysregulation in cell death programs, leading thereafter to CD4 T-cell apoptosis.
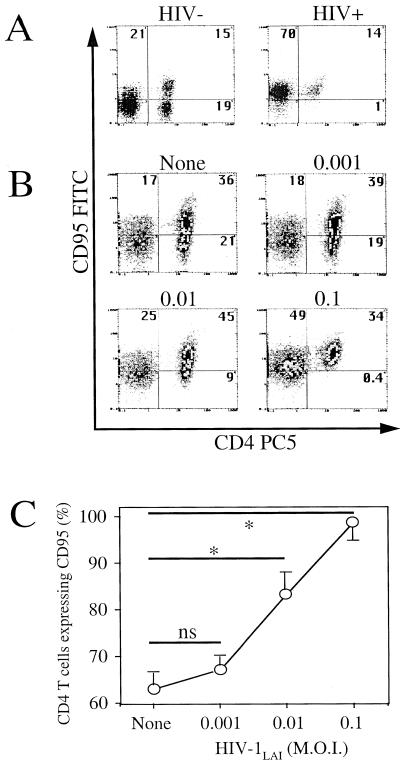
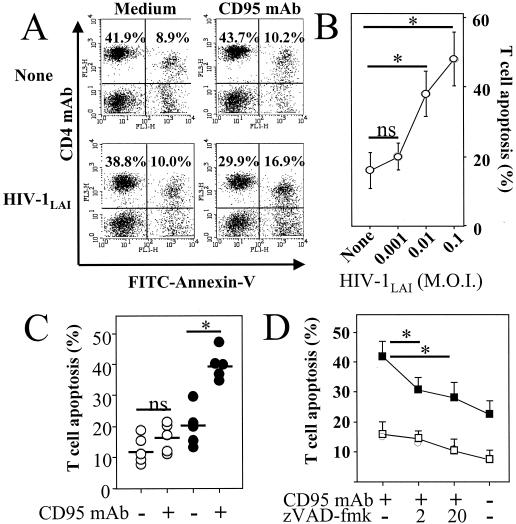
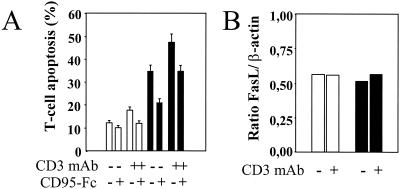
To investigate if T cells can be primed for death by HIV, we incubated quiescent healthy donor PBMC in vitro with HIV-1LAI for 2 h, followed by washing and further incubation for 4 days in medium alone, in the absence of any additional T-cell stimulus. Since HIV-infected individuals have an increased proportion of CD4+ T cells expressing the CD95 receptor (7, 15, 17, 28) (Fig. 1A), which show an enhanced in vitro sensitivity to death induced either by CD95 ligation or by CD3-TCR stimulation, we first assessed CD95 expression in our model. We showed an increase in the proportion of CD4+ T cells expressing CD95 determined by flow cytofluorimetric analysis. This increase was proportional to the HIV-1 inoculum used (Fig. 1B and C). Whereas healthy adult donor PBMC have around 60% of CD4+ T cells expressing CD95, PBMC in our experiment increased their CD95 expression to nearly 100% when they were incubated with HIV-1LAI at an MOI of 0.1 (Fig. 1C). In addition, the percentage of these CD4+ T cells becoming sensitive to CD95 antibody-induced death increased proportionally to the viral inoculum used (MOI, 0.001 to 0.1) (Fig. 2A and B). In order to exclude the possibility that priming of CD4+ T cells in the cultures resulted from monocytes and CD8+ T cells, we purified CD4+ T cells by negative selection prior to incubation with HIV-1LAI. Purified CD4+ T cells incubated with HIV-1LAI (MOI, 0.01) for 2 h also became sensitized to anti-CD95 treatment (Fig. 2C). CD4 T-cell death in response to CD95 ligation was dependent on caspase activation (24), since 1 h of preincubation with the broad caspase inhibitor zVAD-fmk, prior to CD95 antibody treatment, significantly reduced CD95-mediated CD4 T-cell death (Fig. 2D). Two hours of incubation with HIV-1LAI also primed CD4+ T cells for death following CD3 stimulation (Fig. 3A). RNase protection assay of CD95L expression indicated that neither the 4-day incubation with HIV-1LAI nor the subsequent CD3 stimulation (at least during the first 6 h) increased the baseline mRNA level of expressed CD95L (Fig. 3B). However, CD3-induced CD4 T-cell death was reduced by pretreatment with a CD95 decoy receptor (Fig. 3A), suggesting that CD95 engagement by CD95 ligand was involved in activation-induced cell death. Since the cellular localization of CD95L is mainly cytosolic (9), these data suggest a possible relocalization of CD95L protein from cytosolic to membrane fractions following T-cell activation, favoring CD4 T-cell death.
FIG. 1.
HIV-1LAI-induced CD95 expression. (A) CD95 expression in CD4+ T cells of an uninfected individual (HIV−) and an HIV-infected (HIV+) individual assessed by two-color flow cytometry using CD4- and CD95-labeled antibodies. Numbers indicate percentages in each quadrant. (B) PBMC from a healthy donor were incubated for 2 h in the absence (None) or presence of HIV-1LAI at different MOIs (0.001, 0.01, or 0.1) and then washed and further incubated for 4 days in medium alone. CD95 expression was assessed in CD4+ T cells by two-color flow cytometry. Numbers indicate percentages in each quadrant. (C) The proportion of CD4+ T cells expressing CD95 was calculated as follows: [CD4+ CD95+/(CD4+ CD95+ + CD4+ CD95−)] × 100. Results are the means ± standard deviations of four independent experiments. Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significant statistical difference).
FIG. 2.
HIV-1LAI-mediated priming of CD4+ T cells for CD95-induced death. (A) PBMC were incubated for 2 h in the absence (None) or presence of HIV-1LAI (MOI of 0.01), and cells were washed and incubated for 4 days in medium alone (Medium) or in the presence of an agonistic CD95 MAb (1 μg/ml) (CD95 MAb). Numbers indicate percentages in each quadrant. Dying CD4+ T cells were assessed by two-color flow cytometry, with PC5-labeled CD4 MAb and FITC-labeled annexin V. (B) The percentage of dying CD4+ T cells was calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. Results are the means ± standard deviations of three independent experiments. Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significant statistical difference). (C) Purified CD4+ T cells were incubated for 2 h in the absence (○) or presence (•) of HIV-1LAI (MOI of 0.01) and then washed and incubated for 4 days in medium alone (−) or in the presence (+) of an agonistic CD95 IgM MAb (1 μg/ml). Percentages of dying CD4+ T cells were assessed by two-color flow cytometry, with CD4 MAb and annexin V, and were calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. Each symbol represents one individual donor, and bars represent mean values in each group. Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significant statistical difference). (D) Caspase inhibitor decreased CD95-mediated T-cell death. CD4+ T cells were incubated for 2 h in the absence (□) or presence (▪) of HIV-1LAI (MOI of 0.01) and then washed and further incubated in medium alone for 4 days. CD4+ T cells were then further incubated for 18 h in the absence (−) or presence (+) of 1 μg of agonistic anti-CD95 (CD95 MAb) per ml and in the absence (−) or presence (+) of a 2 μM (“2”) or a 20 μM (“20”) concentration of the broad caspase inhibitor zVAD-fmk. Percentages of dying CD4+ T cells were assessed by flow cytometry with annexin V. The percentage of dying CD4+ T cells was calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. Results are the means ± standard deviations of three independent experiments. Statistical significance was assessed by Student's t test (∗, P < 0.05).
FIG. 3.
HIV-1LAI-mediated priming of CD4+ T cells for CD3-induced death. (A) HIV-1-mediated priming of CD4+ T cells for CD3-induced T-cell death. PBMC were incubated for 2 h in the absence (open bars) or presence (solid bars) of HIV-1LAI (at an MOI of 0.01) and then washed and further incubated in medium alone for 4 days. CD4+ T cells were then purified, by negative selection, and then cultured for 18 h in the absence (−) or presence (+) of CD3 MAb (1 μg/ml) and in the absence (−) or presence (+) of a soluble CD95 decoy receptor (human CD95-Fc Ig fusion protein) (10 μg/ml). Percentages of dying CD4+ T cells were assessed by flow cytometry with annexin V and were calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. The results for one typical experiment out of three performed in duplicate (means ± standard deviations) are shown. (B) Expression of CD95L mRNA was assessed by an RNase protection assay in purified CD4+ T cells (107 cells) isolated from PBMC incubated for 4 days earlier in the absence (open bars) or presence (solid bars) of HIV-1LAI. CD4+ T cells were then further incubated for 6 h in the absence (−) or presence (+) of CD3 MAb (1 μg/ml).
HIV protease inhibitors induce cell death of PBMC from healthy donors.
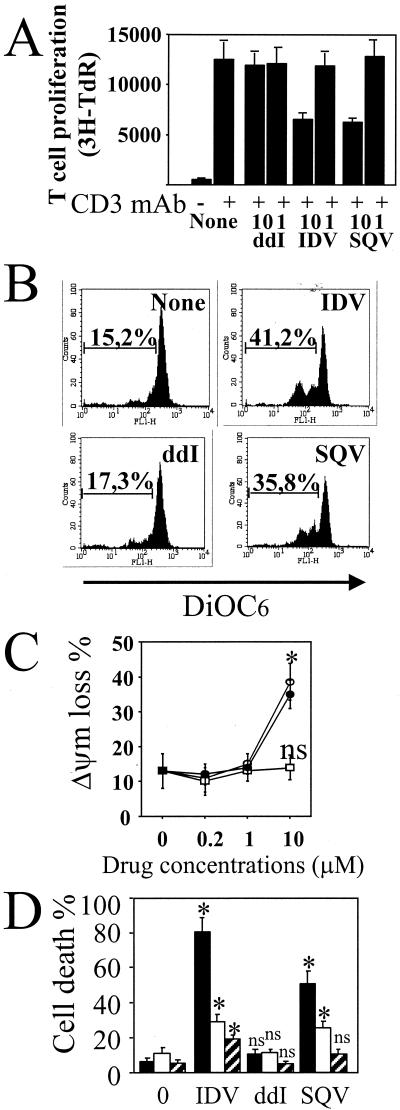
HIV protease inhibitors act during the late stage of the HIV-1 viral cycle by inactivating the HIV-1-encoded aspartyl protease and preventing the cleavage of Gag and Gag-Pol proteins, thereby inhibiting the production of mature infectious HIV-1 virions (35, 36). Treatment with ritonavir in vitro has been reported to markedly decrease apoptosis of both HIV-infected and uninfected T lymphocytes and polymorphonuclear leukocytes, suggesting that HIV protease inhibitors may improve immune function by reducing induction of apoptosis (34, 45, 50, 51). We treated PBMC from HIV-seronegative healthy donors with increasing concentrations of IDV, SQV, or ddI for 3 days and monitored T-cell proliferation and cell death. Both IDV and SQV decreased T-cell proliferation mediated by CD3 MAb in three independent experiments performed with healthy donor cells with a mean decrease for SQV of 53% ± 15% and a mean decrease for IDV of 48% ± 12% (Fig. 4A). Moreover, in the absence of T-cell activation, we observed that 10 μM IDV and SQV induced a loss in membrane mitochondrial potential (ΔΨm) as assessed by flow cytometry using DiOC6 (Fig. 4B and C). There was no effect detected at 1 or 0.2 μM (Fig. 4C). The phenotype of dying cells without T-cell activation was confirmed by flow cytometry using FITC-conjugated annexin V (Fig. 4D). In contrast to ddI, treatment with protease inhibitors (IDV and SQV) at 10 μM induced monocyte and CD4 T-cell death with no major effect on CD8+ T-cell viability (Fig. 4D).
FIG. 4.
Effects of HIV drugs on PBMC from healthy donors. (A) PBMC were incubated in the absence (None) or presence of ddI, SQV, and IDV, at different concentrations (10 and 1 μM). After 1 h of in vitro treatment, PBMC were stimulated with CD3 MAb (1 μg/ml). T-cell proliferation was assessed after 3 days. Data represent one typical experiment out of three performed in triplicate (means ± standard deviations). TdR, thymidine. (B) Loss in mitochondrial membrane potential (ΔΨm) in dying cells. ΔΨm was assessed by flow cytometry with DiOC6 at day 3 from unstimulated PBMC. Numbers indicate percentages of ΔΨm loss. PBMC were incubated in the absence (None) or presence of ddI (10 μM), SQV (10 μM), and IDV (10 μM). (C) PBMC were incubated in the absence (0 μM) or presence of ddI (□), SQV (•), and IDV (○), at different concentrations (10, 1, and 0.2 μM). Results are the means of three independent experiments (means ± standard deviations). Statistical significance was calculated by comparing the percentage of ΔΨm loss in treated cells with the percentage in the untreated cells by Student's t test (∗, P < 0.05; ns, no significant statistical difference). (D) PBMC were incubated either in the absence (0) or in the presence of ddI, SQV, and IDV at 10 μM. Specific monocyte (solid bars), CD4 (open bars), and CD8 (hatched bars) T-cell death was assessed after 3 days by two-color flow cytometry with annexin V. Results are the means of three independent experiments (means ± standard deviations). Statistical significance was calculated by comparing the percentage of annexin V-positive cells in treated culture of a particular phenotype with the percentage in the untreated culture by Student's t test (∗, P < 0.05; ns, no significant statistical difference).
HIV protease inhibitor-mediated effect on CD4 T-cell death induced by CD95 and CD3-TCR ligation.
To further examine the role of these drugs in HIV-1-mediated dysregulation of programmed cell death, resting PBMC from healthy donors were incubated for 2 h with HIV-1 (MOI of 0.01) and incubated for 4 days in the absence or presence of ddI or SQV (Fig. 5A). Treatment with SQV decreased CD95-induced CD4+ T-cell death, while ddI had no effect (Fig. 5B and D). Analysis of CD95 expression indicated that the proportion of CD4+ T cells expressing CD95 was decreased in the cells exposed to SQV compared to in vitro treatment with ddI or medium alone (Fig. 5C). Altogether, these data suggest that SQV decreased CD95-mediated cell death in primary CD4+ T cells incubated with HIV, possibly via the inhibition of CD95 expression.
FIG. 5.
HIV drugs decreased CD95 and CD3-mediated cell death. (A) Schedule of experiment. (B) PBMC were incubated for 2 h in the absence (open bars) or presence (solid bars) of HIV-1LAI (MOI of 0.01), and cells were washed and incubated in the absence (None) or presence of ddI (5 μM) and SQV (0.2 μM). CD4+ T cells were purified and then cultured for 18 h in the absence (Medium) or presence of CD95 MAb (1 μg/ml). Results are the means of three independent experiments (means ± standard deviations). Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significance). (C) CD95 expression of CD4+ T cells from PBMC of healthy donors incubated for 2 h in the absence (open bars) or presence (solid bars) of HIV-1LAI (MOI of 0.01), for 4 days in medium alone (None) or in the presence of drugs (ddI and SQV). CD95 expression was assessed in CD4+ T cells by two-color flow cytometry. The proportion of CD4+ T cells expressing CD95 was calculated as follows: [CD4+ CD95+/(CD4+ CD95+ + CD4+ CD95−)] × 100. Results are the means of three independent experiments (means ± standard deviations). Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significance). (D) CD4+ T cells were isolated as described for panel B and further cultured for 18 h in the presence of CD95 MAb (1 μg/ml) or CD3 MAb (1 μg/ml) in the absence (CD3 and CD95) or presence of IL-2 (CD3 IL-2 and CD95 IL-2) (20 ng/ml). Cell death was assessed by flow cytometry with annexin V. The percentage of dying CD4+ T cells was calculated as follows: [CD4+ annexin+/(CD4+ annexin+ + CD4+ annexin−)] × 100. Results are the means of two independent experiments (means ± standard deviations).
IL-2, a TH1 cytokine, has been previously reported to modulate CD95-mediated CD4 T-cell death in both HIV-infected individuals and SIV-infected macaques (15-17). In vitro treatment with IL-2 decreased CD95-mediated T-cell death, and this effect was enhanced by the addition of SQV or ddI. CD3-induced T-cell death was enhanced with the addition of IL-2, and this enhancement was also diminished in the presence of ddI or SQV. T-cell activation with CD3 MAb in combination with CD28 MAb also caused an increase in cell death concomitant with an increase in viral production. HIV-infected CD4+ T cells exposed to ddI allowed higher T-cell proliferation in the absence of viral production, suggesting that in this context viral replication mediated cell death. This was assessed by thymidine incorporation (Fig. 6A ), CD4 T-cell counts (Fig. 6B), and p24 antigen production (Fig. 6C). However, when activated CD4+ T cells were pretreated with ddI in the presence of a CD95 decoy receptor, the level of T-cell proliferation was similar to that in T cells from healthy donors (Fig. 6D). These data suggest that both a CD95-dependent pathway and viral replication-mediated cell death (independent of CD95) are operating in HIV-mediated CD4 T-cell depletion.
FIG. 6.
T-cell proliferation is restored in the presence of ddI and CD95 decoy receptor. CD4+ T cells were incubated with HIV-1LAI and purified as described for Fig. 5B. Cells were incubated in the absence (open bars) or presence (solid bars) of 5 μM ddI added at day 0 and day 2. CD4+ T cells were further stimulated in the absence (Med) or presence of CD3 MAb (1 μg/ml) or CD3 with CD28 MAbs (CD3 CD28) (1 μg/ml each). At day 3, T-cell proliferation (A), T-cell counts (B), and p24 antigen production (C) were assessed. (A) T-cell proliferation was determined by [3H]thymidine (TdR) incorporation. Results are the means of three independent experiments (means ± standard deviations). (B) T-cell counts were assessed by light microscopy. Results are the means of three independent experiments (means ± standard deviations). (C) Enzyme-linked immunosorbent assay specific for p24 antigen was used to assess viral production. Results are the means of three independent experiments (means ± standard deviations). Statistical significance was assessed by Student's t test (∗, P < 0.05; ns, no significant statistical difference). (D) PBMC were incubated for 2 h in the absence (open bars) or presence (filled bars) of HIV-1LAI (at an MOI of 0.01) and then washed and further incubated in medium alone (None) or in the presence of ddI for 4 days. CD4+ T cells then were purified, by negative selection, and cultured for 18 h inthe presence of either CD3 MAb (1 μg/ml) or CD3 and CD28 MAbs (1 μg/ml each). CD4+ T cells treated with ddI were also incubated in the presence of a soluble CD95 decoy receptor (ddI/CD95-Fc) (10 μg/ml). T-cell counts were assessed by light microscopy at day 4. Results are the means of two independent experiments (means ± standard deviations).
DISCUSSION
In this study, we have shown that incubation of CD4+ T cells with HIV-1 induces CD95 expression and sensitizes the cells to undergo apoptosis in response to CD95 ligation or CD3 activation. Our data also indicate that AICD was reduced by a CD95 decoy receptor and ddI. Thus, at least two processes of AICD in CD4+ T cells incubated with HIV appeared to be operating. Early following T-cell activation, CD4+ T cells die through a CD95-dependent process, which does not require viral replication. This process is followed by an infection-mediated T-cell death. Both of these cell death processes may drive the loss of CD4+ T lymphocytes seen clinically during HIV infection.
Protease inhibitors cause substantial increases in CD4+ T-cell counts (both naive and memory cells) in HIV-infected patients. These inhibitors are presumed to exert their positive effect on CD4+ T-cell numbers and immune function by inhibiting viral replication (10, 12, 22, 23, 32). Recent studies have reported that the susceptibility of peripheral blood T cells to apoptosis decreased in HIV-1-infected adults and children treated with highly active antiretroviral therapy (4-6, 11, 26, 29). This decrease is rapid and is seen as early as 4 days after initiation of protease inhibitor therapy (6). It has been suggested that protease inhibitors may have clinical and immunological benefits, even in the absence of sustained viral suppression, and may have antiapoptotic properties. Apoptosis of in vitro mitogen-stimulated T cells has been reported elsewhere to be modulated by ritonavir, an HIV protease inhibitor, through at least two different pathways, decreased expression of caspase 1 and CD95L (45, 50). We observed that 0.2 μM SQV, another HIV protease inhibitor, prevented HIV-1-induced CD95 expression and decreased CD95 ligation-induced cell death. Analysis of PBMC subsets from HIV-1-infected individuals demonstrated that CD95 expression was significantly reduced on CD45RA+ CD62L+ naive T cells after the start of protease inhibitor therapy (2). Thus, one potential consequence could be the modulation of CD95 expression, which could then explain the direct down-regulation of apoptosis by SQV. However, the mechanism(s) by which SQV decreases CD95 expression remains unknown. Furthermore, treatment of HIV-1-infected PBMC with SQV, ddI, and IL-2 in vitro markedly decreased cell death mediated by CD95 ligation. This phenomenon may explain the increase in CD4+ T-cell counts in HIV-1-infected individuals given IL-2 immunotherapy, but of course this remains to be evaluated clinically.
The concentrations of IDV and SQV in plasma of HIV-infected individuals have been reported to range from 0.2 to 5 μM and from 0.1 to 4 μM, respectively (54, 58), but these concentrations are increased by the concomitant use of ritonavir, which inhibits the metabolism of SQV (25, 38, 57), where concentrations of 7 μM or more can be attained. Mitochondria are pivotal in controlling cell life and death. Thus, mitochondria represent highly sensitive stress sensors for a large variety of stimuli. We found that in vitro treatment of PBMC from healthy donors with either IDV or SQV is associated with a loss in mitochondrial membrane potential. However, the mechanisms by which SQV and IDV induced mitochondrial damage remain to be clarified. We also noted that in vitro treatment of healthy donor PBMC with the combination of IDV (5 μM; cell death, 15.7%) and SQV (5 μM; cell death, 13.9%) is additive and induced cell death in 36.8% of the cells, which was similar to that observed with 10 μM drugs used individually. Thus, the concentrations used in vitro to assess toxicity in this study reflect pharmacologic concentrations. Therefore, our observations reveal new mechanisms for drug protection and toxicity for CD4+ T cells during in vitro HIV infection.
Acknowledgments
J.E. and J.-D.L. contributed equally to the work.
This work was supported by INSERM, Paris 7 University; grants from ANRS, ECS, FRM, and Paris 7 Valorisation (J.C.A.); a doctoral fellowship from ANRS (J.-D.L.) and from ECS (F.P.); the National Institute of Allergy and Infectious Diseases (AI46237); the Center for AIDS Research Genomics Core Laboratory (AI36214); the Universitywide AIDS Research Program and the San Diego Veterans Medical Research Foundation (J.C.); NIH grants AI27670, AI38858, AI43638, and AI29164 (D.D.R.); and the San Diego Veterans Affairs Healthcare System. J.E. was sponsored by a Human Science Frontier Program fellowship.
We thank Shigekazu Nagata for the generous gift of CD95L cDNA, Sara Albanil for technical assistance, Judy Norberg and Michele Lutz for flow cytometric analyses, and David Looney (CFAR core director of molecular biology), G. Peytavin (Bichat-Claude Bernard Hospital), and Davey Smith for critical review of the manuscript.
REFERENCES
- 1.Ameisen, J. C., J. Estaquier, and T. Idziorek. 1994. From AIDS to parasite infection: pathogen-mediated subversion of programmed cell death as a mechanism for immune dysregulation. Immunol. Rev. 142:9-51. [DOI] [PubMed] [Google Scholar]
- 2.Amendola, A., F. Poccia, F. Martini, C. Gioia, V. Galati, M. Pierdominici, M. Marziali, F. Pandolfi, V. Colizzi, M. Piacentini, E. Girardi, G. D'Offizi, et al. 2000. Decreased CD95 expression on naive T cells from HIV-infected persons undergoing highly active anti-retroviral therapy (HAART) and the influence of IL-2 low dose administration. Clin. Exp. Immunol. 120:324-332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andre, P., M. Groettrup, P. Klenerman, R. de Giuli, B. L. Booth, Jr., V. Cerundolo, M. Bonneville, F. Jotereau, R. M. Zinkernagel, and V. Lotteau. 1998. An inhibitor of HIV-1 protease modulates proteasome activity, antigen presentation, and T cell responses. Proc. Natl. Acad. Sci. USA 95:13120-13124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aries, S. P., K. Weyrich, B. Schaaf, F. Hansen, R. H. Dennin, and K. Dalhoff. 1998. Early T-cell apoptosis and Fas expression during antiretroviral therapy in individuals infected with human immunodeficiency virus-1. Scand. J. Immunol. 48:86-91. [DOI] [PubMed] [Google Scholar]
- 5.Badley, A. D., D. H. Dockrell, A. Algeciras, S. Ziesmer, A. Landay, M. M. Lederman, E. Connick, H. Kessler, D. Kuritzkes, D. H. Lynch, P. Roche, H. Yagita, and C. V. Paya. 1998. In vivo analysis of Fas/FasL interactions in HIV-infected patients. J. Clin. Investig. 102:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Badley, A. D., K. Parato, D. W. Cameron, S. Kravcik, B. N. Phenix, D. Ashby, A. Kumar, D. H. Lynch, J. Tschopp, and J. B. Angel. 1999. Dynamic correlation of apoptosis and immune activation during treatment of HIV infection. Cell Death Differ. 6:420-432. [DOI] [PubMed] [Google Scholar]
- 7.Baumler, C. B., T. Bohler, I. Herr, A. Benner, P. H. Krammer, and K. M. Debatin. 1996. Activation of the CD95 (APO-1/Fas) system in T cells from human immunodeficiency virus type-1-infected children. Blood 88:1741-1746. [PubMed] [Google Scholar]
- 8.Bohler, T., C. Baumler, I. Herr, A. Groll, M. Kurz, and K. M. Debatin. 1997. Activation of the CD95 system increases with disease progression in human immunodeficiency virus type 1-infected children and adolescents. Pediatr. Infect. Dis. J. 16:754-759. [DOI] [PubMed] [Google Scholar]
- 9.Bossi, G., and G. M. Griffiths. 1999. Degranulation plays an essential part in regulating cell surface expression of Fas ligand in T cells and natural killer cells. Nat. Med. 5:90-96. [DOI] [PubMed] [Google Scholar]
- 10.Cavert, W., D. W. Notermans, K. Staskus, S. W. Wietgrefe, M. Zupancic, K. Gebhard, K. Henry, Z. Q. Zhang, R. Mills, H. McDade, C. M. Schuwirth, J. Goudsmit, S. A. Danner, and A. T. Haase. 1997. Kinetics of response in lymphoid tissues to antiretroviral therapy of HIV-1 infection. Science 276:960-964. [DOI] [PubMed] [Google Scholar]
- 11.Chavan, S. J., S. L. Tamma, M. Kaplan, M. Gersten, and S. G. Pahwa. 1999. Reduction in T cell apoptosis in patients with HIV disease following antiretroviral therapy. Clin. Immunol. 93:24-33. [DOI] [PubMed] [Google Scholar]
- 12.Collier, A. C., R. W. Coombs, D. A. Schoenfeld, R. L. Bassett, J. Timpone, A. Baruch, M. Jones, K. Facey, C. Whitacre, V. J. McAuliffe, H. M. Friedman, T. C. Merigan, R. C. Reichman, C. Hooper, L. Corey, et al. 1996. Treatment of human immunodeficiency virus infection with saquinavir, zidovudine, and zalcitabine. N. Engl. J. Med. 334:1011-1017. [DOI] [PubMed] [Google Scholar]
- 13.Davis, I. C., M. Girard, and P. N. Fultz. 1998. Loss of CD4+ T cells in human immunodeficiency virus type 1-infected chimpanzees is associated with increased lymphocyte apoptosis. J. Virol. 72:4623-4632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Estaquier, J., T. Idziorek, F. de Bels, F. Barre-Sinoussi, B. Hurtrel, A. M. Aubertin, A. Venet, M. Mehtali, E. Muchmore, P. Michel, et al. 1994. Programmed cell death and AIDS: significance of T-cell apoptosis in pathogenic and nonpathogenic primate lentiviral infections. Proc. Natl. Acad. Sci. USA 91:9431-9435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Estaquier, J., T. Idziorek, W. Zou, D. Emilie, C. M. Farber, J. M. Bourez, and J. C. Ameisen. 1995. T helper type 1/T helper type 2 cytokines and T cell death: preventive effect of interleukin 12 on activation-induced and CD95 (FAS/APO-1)-mediated apoptosis of CD4+ T cells from human immunodeficiency virus-infected persons. J. Exp. Med. 182:1759-1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Estaquier, J., V. Monceaux, M. C. Cumont, A. M. Aubertin, B. Hurtrel, and J. C. Ameisen. 2000. Early changes in peripheral blood T cells during primary infection of rhesus macaques with a pathogenic SIV. J. Med. Primatol. 29:127-135. [DOI] [PubMed] [Google Scholar]
- 17.Estaquier, J., M. Tanaka, T. Suda, S. Nagata, P. Golstein, and J. C. Ameisen. 1996. Fas-mediated apoptosis of CD4+ and CD8+ T cells from human immunodeficiency virus-infected persons: differential in vitro preventive effect of cytokines and protease antagonists. Blood 87:4959-4966. [PubMed] [Google Scholar]
- 18.Finkel, T. H., G. Tudor-Williams, N. K. Banda, M. F. Cotton, T. Curiel, C. Monks, T. W. Baba, R. M. Ruprecht, and A. Kupfer. 1995. Apoptosis occurs predominantly in bystander cells and not in productively infected cells of HIV- and SIV-infected lymph nodes. Nat. Med. 1:129-134. [DOI] [PubMed] [Google Scholar]
- 19.Gandhi, R. T., B. K. Chen, S. E. Straus, J. K. Dale, M. J. Lenardo, and D. Baltimore. 1998. HIV-1 directly kills CD4+ T cells by a Fas-independent mechanism. J. Exp. Med. 187:1113-1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Glynn, J. M., D. L. McElligott, and D. E. Mosier. 1996. Apoptosis induced by HIV infection in H9 T cells is blocked by ICE-family protease inhibition but not by a Fas(CD95) antagonist. J. Immunol. 157:2754-2758. [PubMed] [Google Scholar]
- 21.Gougeon, M. L., S. Garcia, J. Heeney, R. Tschopp, H. Lecoeur, D. Guetard, V. Rame, C. Dauguet, and L. Montagnier. 1993. Programmed cell death in AIDS-related HIV and SIV infections. AIDS Res. Hum. Retrovir. 9:553-563. [DOI] [PubMed] [Google Scholar]
- 22.Gulick, R. M., J. W. Mellors, D. Havlir, J. J. Eron, C. Gonzalez, D. McMahon, D. D. Richman, F. T. Valentine, L. Jonas, A. Meibohm, E. A. Emini, and J. A. Chodakewitz. 1997. Treatment with indinavir, zidovudine, and lamivudine in adults with human immunodeficiency virus infection and prior antiretroviral therapy. N. Engl. J. Med. 337:734-739. [DOI] [PubMed] [Google Scholar]
- 23.Hammer, S. M., K. E. Squires, M. D. Hughes, J. M. Grimes, L. M. Demeter, J. S. Currier, J. J. Eron, Jr., J. E. Feinberg, H. H. Balfour, Jr., L. R. Deyton, J. A. Chodakewitz, M. A. Fischl, et al. 1997. A controlled trial of two nucleoside analogues plus indinavir in persons with human immunodeficiency virus infection and CD4 cell counts of 200 per cubic millimeter or less. N. Engl. J. Med. 337:725-733. [DOI] [PubMed] [Google Scholar]
- 24.Hengartner, M. O. 2000. The biochemistry of apoptosis. Nature 407:770-776. [DOI] [PubMed] [Google Scholar]
- 25.Hsu, A., G. R. Granneman, G. Cao, L. Carothers, A. Japour, T. El-Shourbagy, S. Dennis, J. Berg, K. Erdman, J. M. Leonard, and E. Sun. 1998. Pharmacokinetic interaction between ritonavir and indinavir in healthy volunteers. Antimicrob. Agents Chemother. 42:2784-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson, N., and J. M. Parkin. 1998. Anti-retroviral therapy reverses HIV-associated abnormalities in lymphocyte apoptosis. Clin. Exp Immunol. 113:229-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Katsikis, P. D., M. E. Garcia-Ojeda, J. F. Torres-Roca, I. M. Tijoe, C. A. Smith, and L. A. Herzenberg. 1997. Interleukin-1 beta converting enzyme-like protease involvement in Fas-induced and activation-induced peripheral blood T cell apoptosis in HIV infection. TNF-related apoptosis-inducing ligand can mediate activation-induced T cell death in HIV infection. J. Exp. Med. 186:1365-1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Katsikis, P. D., E. S. Wunderlich, C. A. Smith, and L. A. Herzenberg. 1995. Fas antigen stimulation induces marked apoptosis of T lymphocytes in human immunodeficiency virus-infected individuals. J. Exp. Med. 181:2029-2036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kotler, D. P., T. Shimada, G. Snow, G. Winson, W. Chen, M. Zhao, Y. Inada, and F. Clayton. 1998. Effect of combination antiretroviral therapy upon rectal mucosal HIV RNA burden and mononuclear cell apoptosis. AIDS 12:597-604. [DOI] [PubMed] [Google Scholar]
- 30.Laurent-Crawford, A. G., B. Krust, S. Muller, Y. Riviere, M. A. Rey-Cuille, J. M. Bechet, L. Montagnier, and A. G. Hovanessian. 1991. The cytopathic effect of HIV is associated with apoptosis. Virology 185:829-839. [DOI] [PubMed] [Google Scholar]
- 31.Lewis, D. E., D. S. Tang, A. Adu-Oppong, W. Schober, and J. R. Rodgers. 1994. Anergy and apoptosis in CD8+ T cells from HIV-infected persons. J. Immunol. 153:412-420. [PubMed] [Google Scholar]
- 32.Li, T. S., R. Tubiana, C. Katlama, V. Calvez, H. Ait Mohand, and B. Autran. 1998. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet 351:1682-1686. [DOI] [PubMed] [Google Scholar]
- 33.Liegler, T. J., W. Yonemoto, T. Elbeik, E. Vittinghoff, S. P. Buchbinder, and W. C. Greene. 1998. Diminished spontaneous apoptosis in lymphocytes from human immunodeficiency virus-infected long-term nonprogressors. J. Infect. Dis. 178:669-679. [DOI] [PubMed] [Google Scholar]
- 34.Mastroianni, C. M., F. Mengoni, M. Lichtner, C. D'Agostino, G. d'Ettorre, G. Forcina, M. Marzi, G. Russo, A. P. Massetti, and V. Vullo. 2000. Ex vivo and in vitro effect of human immunodeficiency virus protease inhibitors on neutrophil apoptosis. J. Infect. Dis. 182:1536-1539. [DOI] [PubMed] [Google Scholar]
- 35.McQuade, T. J., A. G. Tomasselli, L. Liu, V. Karacostas, B. Moss, T. K. Sawyer, R. L. Heinrikson, and W. G. Tarpley. 1990. A synthetic HIV-1 protease inhibitor with antiviral activity arrests HIV-like particle maturation. Science 247:454-456. [DOI] [PubMed] [Google Scholar]
- 36.Meek, T. D., D. M. Lambert, G. B. Dreyer, T. J. Carr, T. A. Tomaszek, Jr., M. L. Moore, J. E. Strickler, C. Debouck, L. J. Hyland, T. J. Matthews, et al. 1990. Inhibition of HIV-1 protease in infected T-lymphocytes by synthetic peptide analogues. Nature 343:90-92. [DOI] [PubMed] [Google Scholar]
- 37.Mellors, J. W., C. R. Rinaldo, Jr., P. Gupta, R. M. White, J. A. Todd, and L. A. Kingsley. 1996. Prognosis in HIV-1 infection predicted by the quantity of virus in plasma. Science 272:1167-1170. [DOI] [PubMed] [Google Scholar]
- 38.Merry, C., M. G. Barry, F. Mulcahy, M. Ryan, J. Heavey, J. F. Tjia, S. E. Gibbons, A. M. Breckenridge, and D. J. Back. 1997. Saquinavir pharmacokinetics alone and in combination with ritonavir in HIV-infected patients. AIDS 11:F29-F33. [DOI] [PubMed] [Google Scholar]
- 39.Moutouh, L., J. Estaquier, D. D. Richman, and J. Corbeil. 1998. Molecular and cellular analysis of human immunodeficiency virus-induced apoptosis in lymphoblastoid T-cell-line-expressing wild-type and mutated CD4 receptors. J. Virol. 72:8061-8072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muro-Cacho, C. A., G. Pantaleo, and A. S. Fauci. 1995. Analysis of apoptosis in lymph nodes of HIV-infected persons. Intensity of apoptosis correlates with the general state of activation of the lymphoid tissue and not with stage of disease or viral burden. J. Immunol. 154:5555-5566. [PubMed] [Google Scholar]
- 41.Noraz, N., J. Gozlan, J. Corbeil, T. Brunner, and S. A. Spector. 1997. HIV-induced apoptosis of activated primary CD4+ T lymphocytes is not mediated by Fas-Fas ligand. AIDS 11:1671-1680. [DOI] [PubMed] [Google Scholar]
- 42.Ohnimus, H., M. Heinkelein, and C. Jassoy. 1997. Apoptotic cell death upon contact of CD4+ T lymphocytes with HIV glycoprotein-expressing cells is mediated by caspases but bypasses CD95 (Fas/Apo-1) and TNF receptor 1. J. Immunol. 159:5246-5252. [PubMed] [Google Scholar]
- 43.Oyaizu, N., T. W. McCloskey, M. Coronesi, N. Chirmule, V. S. Kalyanaraman, and S. Pahwa. 1993. Accelerated apoptosis in peripheral blood mononuclear cells (PBMCs) from human immunodeficiency virus type-1 infected patients and in CD4 cross-linked PBMCs from normal individuals. Blood 82:3392-3400. [PubMed] [Google Scholar]
- 44.Patki, A. H., D. L. Georges, and M. M. Lederman. 1997. CD4+-T-cell counts, spontaneous apoptosis, and Fas expression in peripheral blood mononuclear cells obtained from human immunodeficiency virus type 1-infected subjects. Clin. Diagn. Lab. Immunol. 4:736-741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Phenix, B. N., J. B. Angel, F. Mandy, S. Kravcik, K. Parato, K. A. Chambers, K. Gallicano, N. Hawley-Foss, S. Cassol, D. W. Cameron, and A. D. Badley. 2000. Decreased HIV-associated T cell apoptosis by HIV protease inhibitors. AIDS Res. Hum. Retrovir. 16:559-567. [DOI] [PubMed] [Google Scholar]
- 46.Piatak, M., Jr., M. S. Saag, L. C. Yang, S. J. Clark, J. C. Kappes, K. C. Luk, B. H. Hahn, G. M. Shaw, and J. D. Lifson. 1993. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science 259:1749-1754. [DOI] [PubMed] [Google Scholar]
- 47.Prati, E., R. Gorla, F. Malacarne, P. Airo, D. Brugnoni, F. Gargiulo, A. Tebaldi, F. Castelli, G. Carosi, and R. Cattaneo. 1997. Study of spontaneous apoptosis in HIV+ patients: correlation with clinical progression and T cell loss. AIDS Res. Hum. Retrovir. 13:1501-1508. [DOI] [PubMed] [Google Scholar]
- 48.Samuelsson, A., C. Brostrom, N. van Dijk, A. Sonnerborg, and F. Chiodi. 1997. Apoptosis of CD4+ and CD19+ cells during human immunodeficiency virus type 1 infection—correlation with clinical progression, viral load, and loss of humoral immunity. Virology 238:180-188. [DOI] [PubMed] [Google Scholar]
- 49.Silvestris, F., P. Cafforio, M. A. Frassanito, M. Tucci, A. Romito, S. Nagata, and F. Dammacco. 1996. Overexpression of Fas antigen on T cells in advanced HIV-1 infection: differential ligation constantly induces apoptosis. AIDS 10:131-141. [DOI] [PubMed] [Google Scholar]
- 50.Sloand, E. M., P. N. Kumar, S. Kim, A. Chaudhuri, F. F. Weichold, and N. S. Young. 1999. Human immunodeficiency virus type 1 protease inhibitor modulates activation of peripheral blood CD4+ T cells and decreases their susceptibility to apoptosis in vitro and in vivo. Blood 94:1021-1027. [PubMed] [Google Scholar]
- 51.Sloand, E. M., J. Maciejewski, P. Kumar, S. Kim, A. Chaudhuri, and, N. Young. 2000. Protease inhibitors stimulate hematopoiesis and decrease apoptosis and ICE expression in CD34+ cells. Blood 96:2735-2739. [PubMed] [Google Scholar]
- 52.Sloand, E. M., J. P. Maciejewski, T. Sato, J. Bruny, P. Kumar, S. Kim, F. F. Weichold, and N. S. Young. 1998. The role of interleukin-converting enzyme in Fas-mediated apoptosis in HIV-1 infection. J. Clin. Investig. 101:195-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sloand, E. M., N. S. Young, P. Kumar, F. F. Weichold, T. Sato, and J. P. Maciejewski. 1997. Role of Fas ligand and receptor in the mechanism of T-cell depletion in acquired immunodeficiency syndrome: effect on CD4+ lymphocyte depletion and human immunodeficiency virus replication. Blood 89:1357-1363. [PubMed] [Google Scholar]
- 54.Stein, D. S., D. G. Fish, J. A. Bilello, S. L. Preston, G. L. Martineau, and G. L. Drusano. 1996. A 24-week open-label phase I/II evaluation of the HIV protease inhibitor MK-639 (indinavir). AIDS 10:485-492. [DOI] [PubMed] [Google Scholar]
- 55.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 56.Terai, C., R. S. Kornbluth, C. D. Pauza, D. D. Richman, and D. A. Carson. 1991. Apoptosis as a mechanism of cell death in cultured T lymphoblasts acutely infected with HIV-1. J. Clin. Investig. 87:1710-1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.van Heeswijk, R. P., A. I. Veldkamp, R. M. Hoetelmans, J. W. Mulder, G. Schreij, A. Hsu, J. M. Lange, J. H. Beijnen, and P. L. Meenhorst. 1999. The steady-state plasma pharmacokinetics of indinavir alone and in combination with a low dose of ritonavir in twice daily dosing regimens in HIV-1-infected individuals. AIDS 13:F95-F99. [DOI] [PubMed] [Google Scholar]
- 58.van Heeswijk, R. P., A. I. Veldkamp, J. W. Mulder, P. L. Meenhorst, J. M. Lange, J. H. Beijnen, and R. M. Hoetelmans. 2000. Once-daily dosing of saquinavir and low-dose ritonavir in HIV-1-infected individuals: a pharmacokinetic pilot study. AIDS 14:F103-F110. [DOI] [PubMed] [Google Scholar]
- 59.Wasmuth, J. C., K. H. Klein, F. Hackbarth, J. K. Rockstroh, T. Sauerbruch, and U. Spengler. 2000. Prediction of imminent complications in HIV-1-infected patients by markers of lymphocyte apoptosis. J. Acquir. Immune Defic. Syndr. 23:44-51. [DOI] [PubMed] [Google Scholar]
- 60.Yang, Y., Z. H. Liu, C. F. Ware, and J. D. Ashwell. 1997. A cysteine protease inhibitor prevents activation-induced T-cell apoptosis and death of peripheral blood cells from human immunodeficiency virus-infected individuals by inhibiting upregulation of Fas ligand. Blood 89:550-557. [PubMed] [Google Scholar]