Abstract
Cell surface glycosaminoglycans (GAGs), in particular heparan sulfate (HS), have been proposed to mediate the attachment of human immunodeficiency virus type 1 (HIV-1) to target cells prior to virus entry, and both the viral gp120 envelope protein and virion-associated cyclophilin A (CypA) have been shown to directly interact with HS and its analogues. To determine the role of GAGs in HIV attachment and infection, we generated HIV-susceptible derivatives of CHO cell lines that either express high levels of GAGs (CHO-K1) or lack GAGs (pgsA745). Using a panel of HIV-1 envelopes, we found that cell surface GAG-mediated effects on virion attachment and infection vary in an envelope strain-dependent but coreceptor-independent manner. In fact, cell surface GAG-mediated enhancement of infection is confined to isolates that contain a highly positively charged V3-loop sequence, while infection by most strains is apparently inhibited by the presence of GAGs. Moreover, the enhancing and inhibitory effects of polycations and polyanions on HIV-1 infection are largely dependent on the presence of cell surface GAGs. These observations are consistent with a model in which GAGs influence in vitro HIV-1 infection primarily by modifying the charge characteristics of the target cell surface. Finally, the effects of GAGs on HIV-1 infection are observed to an equivalent extent whether CypA is present in or absent from virions. Overall, these data exclude a major role for GAGs in mediating the attachment of many HIV-1 strains to target cells via interactions with virion-associated gp120 or CypA.
Heparan sulfate (HS) and chondroitin sulfate (CS) are widely expressed polymeric carbohydrates, termed glycosaminoglycans (GAGs), that exist on the surfaces of cells, covalently attached to membrane-associated core proteins. Of these, HS in particular has been shown to be an important attachment factor for a number of protozoan, bacterial, and viral pathogens (reviewed in reference 42). In contrast, the role of HS in the attachment of human immunodeficiency virus (HIV-1) to the surface of target cells has been somewhat controversial. While it is clear that the expression of CD4 and an appropriate chemokine receptor is necessary for efficient HIV-1 infection, it is less evident whether other cell surface molecules, including HS, might constitute initial viral attachment sites on the target cell (41). Such interactions could, in effect, serve to concentrate virions on the target cell surface prior to specific gp120/CD4/coreceptor engagement and thereby facilitate virus entry. Indeed, the attachment of virions to the target cell surface appears to be the rate-limiting step of HIV-1 entry (26). Evidence that HS plays an important role in attachment and infection by HIV-1 includes the observation that heparin (an analogue of HS) and a number of other sulfated polysaccharides potently inhibit HIV-1 infection (1, 16, 19, 23, 40). In addition, enzymatic removal of HS from the surface of either HeLa-CD4 or T-cell lines can dramatically attenuate both HIV-1 attachment and replication (18, 24, 27, 29, 32). This latter effect appears to be at least somewhat specific, in that enzymatic removal of CS from the target cell surface does not affect HIV-1 infection. These findings are consistent with the hypothesis that the initial contact between an HIV-1 virion and its target cell is mediated by HS. Various components of the virion, including the third hypervariable region of gp120 (V3 loop) as well as a conserved basic coreceptor interaction domain, and elements of the gp41 transmembrane envelope glycoprotein have each been reported to serve as sites of interaction with heparin, HS, and/or other sulfated polysaccharides (6, 7, 8, 15, 22, 25, 31). Intriguingly, one recent study indicated a role for cyclophilin A (CypA) in mediating HIV-1 attachment (32). It is well established that CypA is incorporated into HIV-1 particles during assembly via a specific interaction with the CA domain of Pr55Gag (3, 5, 12, 21, 37). Moreover, genetic or pharmacological attenuation of this interaction abolishes CypA incorporation and reduces the infectivity of progeny virions at a step prior to reverse transcription (4, 5, 12, 37). A potential mechanism that could account for the positive effect of CypA on HIV-1 infectivity was recently proposed. Specifically, a proportion of HIV-1 particle-associated CypA is apparently exposed on the surface of virions (32, 33, 36) and was shown to mediate envelope-independent interactions with HS on the surfaces of target cells via basic amino acid residues situated at the CypA C terminus (32).
In contrast to the aforementioned studies that provided clear evidence in favor of a positive role for cell surface HS in HIV-1 attachment and infection, other investigators have shown that enzymatic removal of HS from the surfaces of primary lymphocytes had no effect on their ability to support HIV-1 replication (18). In addition, some studies have demonstrated marked strain-dependent differences in the degree to which HIV-1 envelopes can bind to HS and other sulfated polysaccharides and to which envelope-dependent virion attachment to target cells exhibits a requirement for cell surface HS (24, 27), although the number of isolates examined thus far is limited.
To resolve these issues, we exploited a derivative of the CHO-K1 cell line, termed pgsA745, that was selected on the basis of the absence of GAG synthesis, following chemical mutagenesis (10, 11). This cell line was found to be deficient in xylosyltransferase, an activity that is essential for the addition of HS and CS polymers to syndecan and glypican core proteins. Thus, pgsA745 cells lack detectable cell surface HS and CS GAGs and, as such, constitute an ideal reagent for testing the role of these molecules in HIV-1 entry. Among a panel of HIV-1 envelopes, we have found marked strain-dependent differences in the preference or otherwise for the presence of cell surface GAGs during HIV-1 attachment and infection. In fact, GAG-dependent enhancement of HIV-1 infection appears to be unusual, with most strains exhibiting higher infectivity in the absence of target cell surface GAGs. These phenotypes are likely determined by envelope charge, since GAG-dependent enhancement of infection is observed only in HIV-1 strains that encode a highly basic gp120 V3-loop sequence. These opposing, strain-dependent effects of cell surface GAGs on HIV-1 infection are independent of which coreceptor is utilized and are observed in both the presence and absence of CypA within the virion particle.
MATERIALS AND METHODS
Plasmid construction.
The infectious proviral DNA clone pNL/HXB has been described previously (2). Similar plasmids were constructed by replacing the HXB envelope with that of ADA, JRFL, YU2, 89.6, 91US005.11, 92MW965.26, 92RW020.5, 92BR020.4, 92UG021.6, 92UG024.2, or 92HT599.24 by substitution of either SalI-BamHI or KpnI-BamHI envelope-containing fragments from proviral or envelope expression plasmids. A further proviral plasmid, pNL/V3(ADA/JRFL), that contains a chimeric envelope gene derived from pIIIB/V3/JRFL (17) was similarly derived. This env gene is derived from HXB, but the V3-loop sequence is replaced by that of JRFL (which is identical in amino acid sequence to that of ADA). A proviral plasmid lacking a functional env gene, termed pNL/δenv, was obtained by removal of an AflIII fragment from pNL/HXB. HIV-1 reporter proviral plasmids that express green fluorescent protein (GFP) in infected cells in place of Nef were derived from R7/3/GFP (a gift from Mark Muesing). In this case, the existing envelope gene was replaced by replacing a SalI-BamHI fragment with those derived from pNL/HXB or pNL/ADA to generate pR7/HXB/GFP and pR7/ADA/GFP, respectively. A similar simian immunodeficiency virus strain mac239 (SIVmac239)-derived GFP reporter virus was constructed as follows: the GFP coding sequence from pEGFP-C1 (Clontech) was amplified with a 5′ oligonucleotide that incorporated sequences from the extreme 3′ end of the SIV env gene, including a unique SacI restriction site, and a 3′ oligonucleotide that included an XhoI site. In addition, the SIVmac239 3′ polypurine tract and 3′ long terminal repeat were amplified with oligonucleotides that incorporated XhoI and EcoRI restriction sites at the 5′ and 3′ ends of the amplicon, respectively. These two PCR products were digested with SacI/XhoI and XhoI/EcoRI, respectively, and inserted into SacI/EcoRI-digested p239SpE3′. The resulting plasmid contains the 3′ half of the SIVmac239 genome with GFP-encoding sequences fused in frame with a vestigial Nef open reading frame. A full-length infectious clone, termed pSIV/GFP, was derived by digesting this plasmid with SphI and inserting an SphI fragment of p239SpSp5′ that encodes the 5′ half of the SIVmac239 genome.
Mutations in the HIV-1 p24 CA protein that were previously reported to eliminate the incorporation of CypA into virions (5, 32), namely, G89V and P90A/A92E, were generated by overlap PCR methods. Thereafter, BssHII-ApaI-digested PCR products containing the mutations were each introduced into pNL/HXB and pNL/ADA proviral clones.
The retroviral vectors LXSN/mCycT1(Y261C), LXSH/CD4, pBABE/CXCR4, and pBABE/CCR5 have been previously described (2). These encode a single amino acid point mutant (Y261C) of a murine cyclin T1 cDNA, human CD4, CXCR4, and CCR5, respectively.
A plasmid that expresses a GFP-Vpr fusion protein was generated by PCR amplification of the vpr gene from pNL4-3, which was then inserted into the HindIII and SmaI sites of pEGFP-C1 (Clontech).
Viruses and cells.
For infectivity assays, HIV-1 and SIV stocks were generated by transfection of 293T cells with each of the aforementioned proviral plasmids. Supernatants were collected 48 h after transfection and passed through a 0.2-μm filter. In some experiments, cyclosporine (CsA) dissolved in ethanol (or an equal volume of ethanol carrier alone) was added to the transfected 293T cultures to give a final CsA concentration of 10 μM. Alternatively, viral stocks were generated in Jurkat cypA+/+ or Jurkat cypA−/− cells. In this case, NL/HXB or NL/ADA virions, pseudotyped with the vesicular stomatitis virus envelope glycoprotein (VSV-G), were generated by cotransfection of 293T cells with a proviral and VSV-G expression plasmid. Thereafter, Jurkat cypA+/+ or Jurkat cypA−/− cells (5) were infected with the pseudotyped virus and washed extensively, and progeny NL/HXB or NL/ADA virus stocks were harvested 48 h later. To generate fluorescent virions for attachment assays, 293T cells were cotransfected with pEGFP/Vpr and either pNL/HXB or pNL/ADA. Transfected cells were maintained in medium containing 2% fetal calf serum, and supernatants were harvested and filtered as described above. Virions were concentrated by centrifugal filtration with an Ultrafree-15 filter device (Biomax-100; Millipore).
The cell line CHO-K1 and its derivative pgsA745 were obtained from the American Type Culture Collection. Each of these cell lines was sequentially transduced with LXSN- and LXSH-derived retroviral vectors encoding mCycT1(Y261C) and human CD4, respectively, with selection in G418 and hygromycin as described previously (2). Thereafter, the CHO-K1/CycT1/CD4+ and pgsA745/CycT1/CD4+ cell lines were transduced with either CXCR4- or CCR5-expressing pBABE-puro derived vectors followed by selection in puromycin. Stable cell lines that expressed similar levels of each cell surface receptor were sorted by fluorescence-activated cell sorting (FACS) and are referred to herein as K1/X4, K1/R5, 745/X4, and 745/R5, respectively
Infectivity assays.
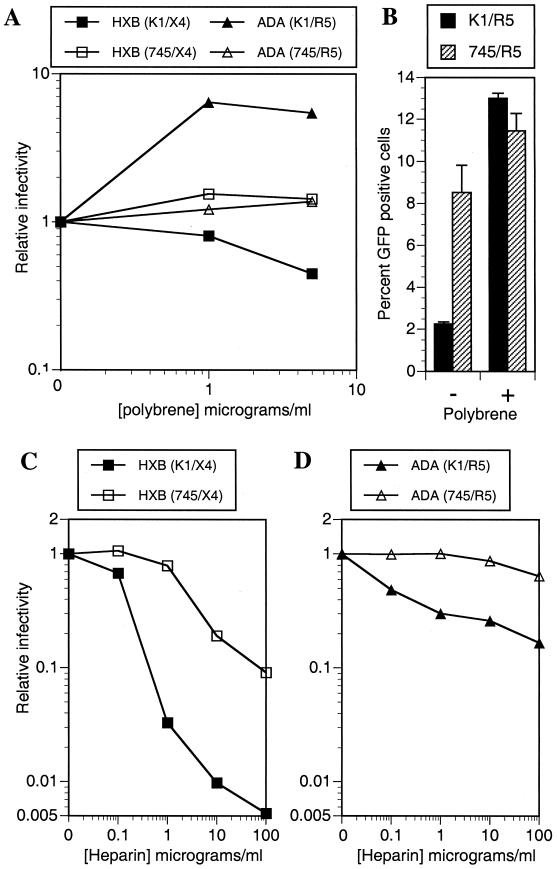
Target cells (K1/X4, K1/R5, 745/X4, and 745/R5) were seeded at 104 cells/well in 48-well plates. The following day, cells were inoculated with serial dilutions of viral stocks, derived from transfected 293T cells. Forty-eight hours after infection, cells were fixed and stained with a murine anti-p24 monoclonal antibody followed by anti-mouse immunoglobulin G (IgG)-fluorescein isothiocyanate, and foci of infection were enumerated, as described previously (2). Alternatively, 105 target cells were seeded in 24-well plates and infected with GFP reporter viruses, and the number of infected cells was enumerated by FACS, 48 h after infection. In some experiments, GFP reporter virus stocks were preincubated with various concentrations of heparin for 2 h at 37°C prior to inoculation of target cells (see Fig. 5). Alternatively, target cells were preincubated with various concentrations of Polybrene in growth medium for 1.5 h at 37°C prior to the addition of virus.
FIG. 5.
Target cell surface GAG-dependent effects of polyanions and polycations on HIV-1 infectivity. (A) CHO-K1- and pgsA745-derived target cells were pretreated with the indicated concentrations of Polybrene prior to infection with R7/HXB/GFP or R7/ADA/GFP. (B) Percent GFP positive K1/R5 and 745/R5 target cells that were untreated or treated with 5 μg of Polybrene per ml prior to infection with R7/ADA/GFP. Alternatively, R7/HXB/GFP (C) and R7/ADA/GFP (D) were incubated with the indicated concentrations of heparin prior to infection of CHO-K1- and pgsA745-derived target cells. For each experiment, the number of infected cells was determined by FACS analysis of GFP expression. Relative infectivity values are numbers of infected cells relative to that obtained with untreated cells and viruses.
Virion attachment assays.
CHO-K1 and pgsA745 cells were plated on gelatin-coated glass coverslips in 24-well plates (2.5 × 104 cells per well) and cultured for 48 h. The cells were subsequently incubated with prechilled medium, supplemented with 20 mM HEPES (pH 7.2), at 4°C for 20 min. Thereafter, the medium was replaced with fresh HEPES-supplemented medium (300 μl/well) containing GFP-Vpr-labeled NL/HXB, NL/ADA, or NL/δenv virions (approximately 20 ng of p24 per well). After incubation at 4°C for 1 h, unbound virions were removed and the cells were washed three times with cold phosphate-buffered saline. Cells and bound virions were then fixed with 4% paraformaldehyde and stained with 1-μg/ml-rhodamine-conjugated concanavalin A (Molecular Probes) and 25 ng of Hoechst 33258 per ml, and the coverslips were mounted on glass slides. Cells and bound virions were observed with a Deltavision deconvolution microscope. Specifically, the entire thickness of the cell monolayer was visualized by collecting 18 images of each field at 200-nm intervals in the z plane. The acquired images were deconvolved and projected onto a single plane to give a two-dimensional representation of the cell monolayer and attached fluorescent virions.
RESULTS
HIV-1 susceptible, GAG-positive and GAG-negative cell lines.
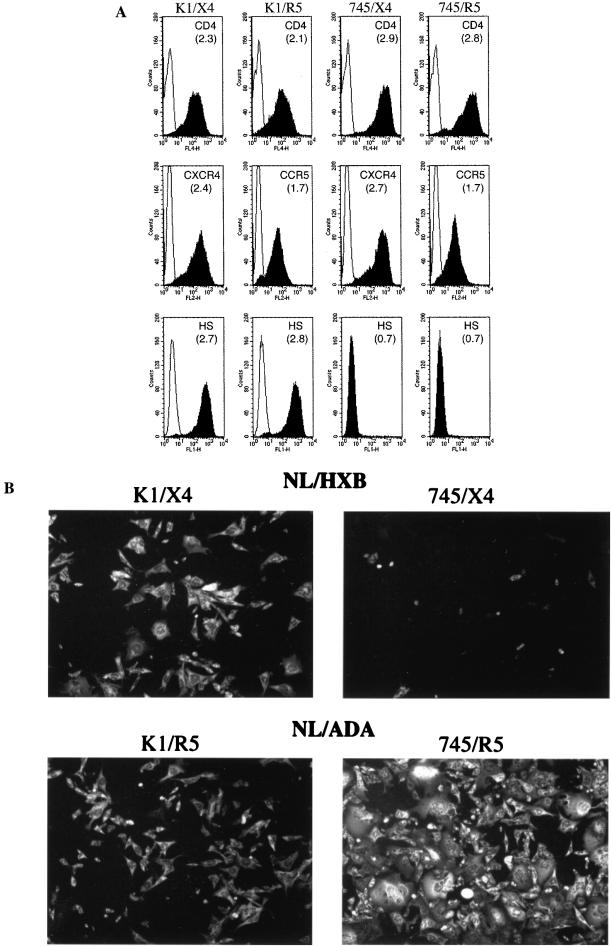
To examine the role of GAGs in HIV-1 attachment and infection, HIV-1-susceptible derivatives of the cell lines CHO-K1 (which express high levels of GAGs) and a derivative, pgsA745 (that entirely lacks GAGs), were constructed. This was accomplished by stable expression of cDNAs encoding human CD4 and a coreceptor (either CXCR4 or CCR5), as well as a mutant form of murine CycT1 (Tyr261Cys) that is able to support primate lentiviral Tat function (2). The expression level of CD4 and coreceptors on cell populations isolated by FACS is shown in Fig. 1A. This analysis showed that the steady-state levels of CD4 and each coreceptor are similar on CHO-K1-derived (K1/X4 and K1/R5) and pgsA745-derived (745/X4 and 745/R5) cell lines, although CD4 expression levels are slightly higher on the pgsA745 derivatives. In addition, we verified that the CHO-K1- and pgsA745-derived cells maintained the GAG-positive and GAG-negative phenotypes of the parental cell lines by FACS analysis with an anti-HS antibody (Fig. 1A).
FIG. 1.
HIV-1-susceptible CHO-K1- and pgsA745-derived cell lines. CHO-K1 and pgsA745 cells were sequentially transduced with retroviral vectors encoding mCycT1(Y261C), human CD4, and either human CXCR4 or human CCR5. Cell populations that express approximately equivalent levels of receptors were sorted by FACS. (A) FACS analysis of receptor and HS expression on CHO-K1 (K1/X4 and K1/R5)- and pgsA745 (745/X4 and 745/R5)-derived cell lines. Receptor and HS expression (filled histograms) was measured using allophycocyanin-conjugated anti-CD4 and phycoerythrin-conjugated anti-CXCR4 or anti-CCR5 antibodies. Alternatively, HS expression was measured using an anti-HS IgM monoclonal antibody followed by fluorescein isothiocyanate-conjugated anti-IgM. The log of the mean fluorescent intensity for each cell line is in parentheses. Background staining with isotype control reagents is shown by the open histograms. (B) The same cell lines were inoculated with NL4-3-derived virus stocks. NL/HXB was used for K1/X4 and 745/X4, and NL/ADA was used for K1/R5 and 745/R5. Forty-eight hours postinoculation, infected cells were visualized by immunofluorescent staining using an anti-p24 monoclonal antibody.
To determine whether, and to what extent, CHO-K1- and pgsA745-derived cell lines were sensitive to HIV-1 infection, K1/X4 and 745/X4 cell lines were inoculated with NL/HXB (X4-tropic). Similarly, K1/R5 and 745/R5 cell lines were inoculated with NL/ADA (R5-tropic). Infection was monitored 48 h postinoculation by immunofluorescence using an anti-p24 monoclonal antibody. As can be seen in Fig. 1B, each of the cell lines was successfully infected by HIV-1 strains bearing an appropriate coreceptor-specific envelope. As has been previously reported, the presence of cell surface GAGs apparently enhanced the level of HXB-enveloped HIV-1 infection (18, 24, 27, 29, 32). Surprisingly, however, the ADA-enveloped virus appeared to be markedly more infectious on the 745/R5 cell line, which lacks GAGs, than the GAG-expressing cell line K1/R5.
Envelope-dependent, opposing effects of target cell surface GAGs on HIV-1 infection.
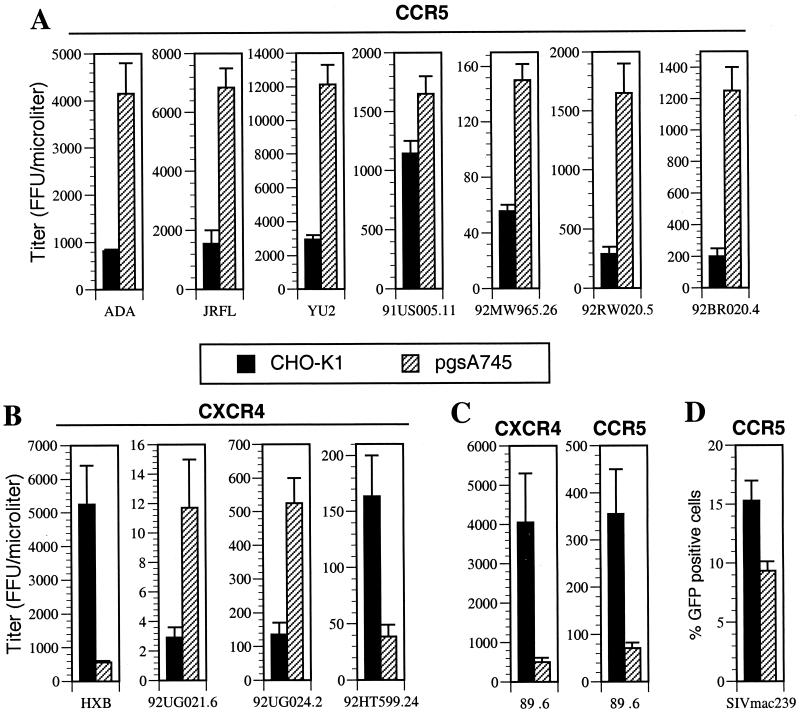
HIV-1 virions containing HXB and ADA HIV-1 envelopes exhibited marked and opposing differences in infectivity in the presence or absence of target-cell-surface GAGs. Therefore, we next examined this phenotype in a more quantitative manner using a panel of pNL4-3-derived infectious HIV-1 molecular clones in which the envelope gene had been replaced with each of those encoded by a selection of isolates. This panel included primary R5-tropic (ADA, JRFL, YU2, 91US005.11, 92MW965.26, 92RW020.5, and 92BR020.4) and primary X4-tropic (92UG021.6, 92UG024.2, and 92HT599.24) envelopes, as well as the T-cell-line-adapted envelope HXB and the dualtropic envelope 89.6. The infectivity of each viral stock, harvested directly from proviral-plasmid-transfected 293T cells, was measured by using both CHO-K1- and pgsA745-derived target cells bearing the appropriate coreceptor. As can be seen in Fig. 2A, each of the primary R5 enveloped virus stocks exhibited higher infectivity on the 745/R5 (GAG-negative) cell line than on the K1/R5 (GAG-positive) cell line. The magnitude of this effect was modest (sixfold or less) but clearly reproducible. Conversely, and as shown in Fig. 2B, two of the X4 viruses (NL/HXB and NL/92HT599.24) exhibited significantly higher infectivity on the GAG-positive cell line K1/X4 than on its GAG-negative counterpart, 745/X4. In the case of HXB, this difference was as much as 10-fold. However, a preference for the presence of cell surface GAGs during virus infection was not a universal property of X4-tropic viruses. Indeed, two other X4 viruses, NL/92UG021.6 and NL/92UG024.2, were clearly more infectious (approximately fourfold) on GAG-negative 745/X4 target cells than on K1/X4 cells (Fig. 2B). Therefore, the preference of a given HIV-1 strain for the presence of GAGs on the target cell surface is envelope dependent but not determined by which coreceptor is used. This conclusion was supported by data obtained with a virus bearing the dualtropic HIV-1 envelope 89.6. As is shown in Fig. 2C, NL/89.6 was clearly more infectious on GAG-positive target cells than on GAG-negative counterparts. Importantly, this phenotype was observed irrespective of which coreceptor, CXCR4 or CCR5, was used during infection. In addition, the R5-tropic SIV isolate mac239 was modestly more infectious on GAG-positive than on GAG-negative target cells (Fig. 2D). Overall, examples of both CXCR4- and CCR5-utilizing primate lentiviruses that exhibited opposing preferences for the presence or absence of target cell surface GAGs were obtained in these experiments. However, the more prevalent phenotype among primary isolate HIV-1 envelopes, albeit with a small (but diverse) sample of isolates, appears to be modestly increased infectivity in the absence of cell surface GAGs.
FIG. 2.
Variable, envelope-dependent effects of cell surface GAGs on HIV-1 and SIV infectivity. The CHO-K1- and pgsA745-derived target cells described for Fig. 1 that express either the CCR5 or CXCR4 coreceptor were inoculated with serial dilutions of NL4-3-derived viruses bearing R5 (A), X4 (B), or dually tropic (C) envelope proteins. Infection was quantitated 48 h after inoculation in wells that contained an appropriate number of foci (20 to 100) and expressed as focus-forming units (FFU) per microliter of virus-containing supernatant. (D) K1/R5 and 745/R5 target cells were each inoculated with the SIV/GFP reporter virus and the percent GFP-positive, infected cells was determined by FACS 48 h later. The means and standard deviations of three independent infection experiments are shown.
Envelope-dependent, opposing effects of GAGs on HIV-1 virion attachment.
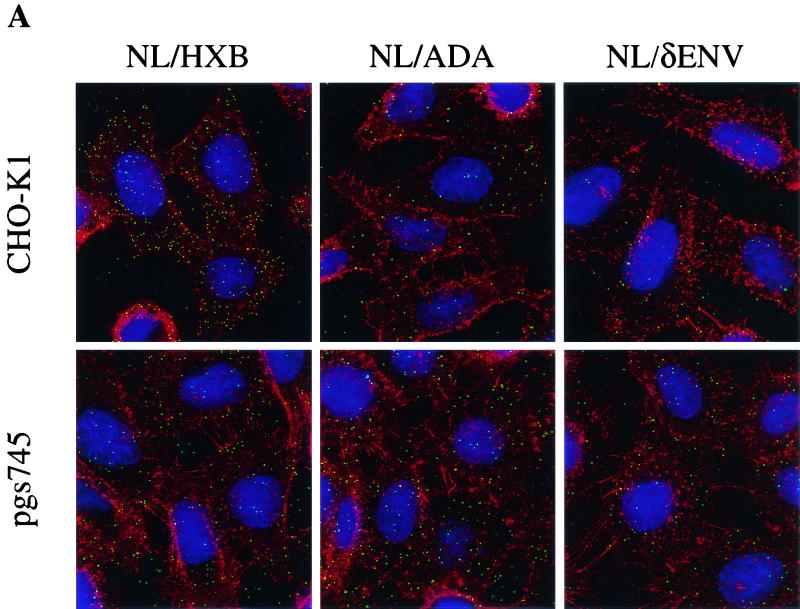
Previous work has suggested an important role for GAGs, in particular HS, in the attachment of HIV-1 to target cells (24, 27, 29). To examine whether HIV-1 virions bearing different envelope proteins display differences in their respective abilities to attach to GAG-positive versus GAG-negative cells, we generated fluorescent virus particles bearing either HXB or ADA envelope proteins. This was done by cotransfection of 293T cells with pNL/HXB or pNL/ADA along with a GFP-Vpr fusion protein expression plasmid. As has been previously described, the incorporation of a GFP-Vpr fusion protein yields virions that can be visualized with a fluorescence microscope (35). To generate control virus-like particles that lack a functional envelope protein, pNL/δenv was substituted for the infectious proviral plasmids.
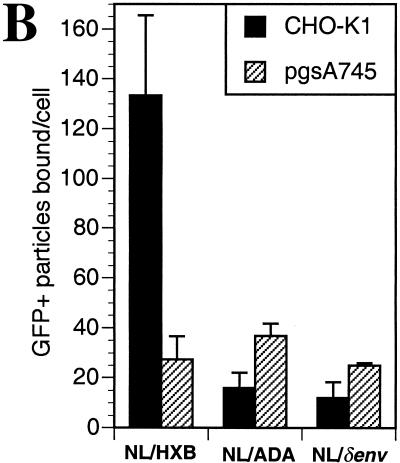
Fluorescent virions were incubated with CHO-K1 or pgsA745 cells, which lack HIV-1 receptors, under conditions (4°C) that permit virus attachment but not entry. Virion attachment was evaluated microscopically after washing, fixation, and staining to reveal cellular architecture. Representative images are presented in Fig. 3A, and enumeration of virions attached to cells is shown in Fig. 3B. Green fluorescent NL/HXB particles attached approximately fivefold more efficiently to CHO-K1 than to pgsA745 cells (Fig. 3). This GAG-dependent enhancement of virion attachment was clearly envelope dependent. Indeed, fluorescent NL/ADA particles did not bind efficiently to CHO-K1 cells and, in fact, attached slightly more efficiently to pgsA745 cells (Fig. 3). The magnitude of this difference was small (approximately 2.5-fold) but reproducible in multiple repetitions of this experiment. Virions lacking an envelope protein attached poorly to both cell lines but, like NL/ADA, exhibited a modest preference for attachment to pgA745 rather than CHO-K1 cells (Fig. 3). The opposing phenotypes of HXB and ADA enveloped virions in the receptor-independent attachment assays are reminiscent of those observed in the (receptor-dependent) infectivity assays (Fig. 2). These observations suggest, particularly in the case of NL/HXB, that cell surface GAG-mediated effects on HIV-1 infectivity are manifested prior to specific receptor engagement and influence virion attachment to target cells.
FIG. 3.
Effects of cell surface GAGs on receptor-independent HIV-1 particle attachment. Fluorescent, GFP-Vpr-labeled virions (20 ng of p24) bearing either HXB, ADA, or no envelope proteins were incubated with CHO-K1 or pgsA745 cells on coverslips at 4°C. After 1 h, the cells were washed, fixed, and stained with rhodamine-conjugated concanavalin A and Hoechst 33258 to reveal cellular architecture. (A) The images are a composite of those collected through the entire thickness of the cell monolayer and projected onto a two-dimensional plane. (B) The number of green fluorescent particles attached to at least four individual cells was counted; the means and standard deviations of these counts are shown.
The gp120 V3 loop is a major determinant of target cell surface GAG effects on HIV-1 infection.
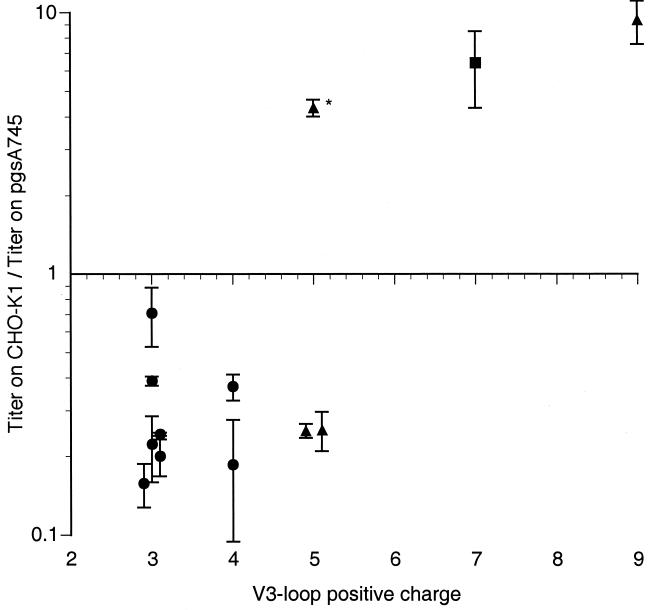
Because the panel of HIV-1 clones used in Fig. 2 displayed markedly different, envelope-dependent preferences for the presence or absence of target cell surface GAGs during infection, we next sought to correlate this phenotype with properties of the viral envelope protein. Since the presence of highly sulfated GAGs could, in principle, markedly affect the overall electrostatic charge in the vicinity of the plasma membrane, it appeared at least possible that charged surfaces on the viral envelope glycoprotein could represent significant viral determinants of the GAG-mediated phenotype. Indeed, it has been previously reported that sulfated polysaccharides can bind to basic surfaces on the gp120 envelope protein of HIV-1 including the V3-loop and CD4 binding-induced epitopes (6, 15, 25, 30, 31). As can be seen in Fig. 4, the extent to which the gp120 V3 loop displays a positive charge correlated with the ability of the envelope protein to differentially mediate infection of GAG-positive versus GAG-negative cells. Indeed, only envelopes that contain a highly basic V3-loop sequence were more infectious on GAG-positive cells than on GAG-negative counterparts. The one exception was the 92HT599.24 envelope, which bears a net positive charge of only +5 yet is clearly more infectious on GAG-positive cells than on GAG-negative cells. However, the V3 loop of this isolate is highly unusual in that it contains four histidine residues (as opposed to one or two in most other isolates, including those used in this study). Histidine is conventionally not included in calculations of V3-loop charge. Nevertheless, because the pKa of histidine is close to 7, it is likely that the simplistic calculations of V3-loop charge used here and elsewhere somewhat underestimate the neutral pH-positive charge on gp120 V3 loops in general and the 92HT599.24 isolate in particular. Despite this minor caveat, the V3-loop charge appears to be predictive of whether a given HIV-1 envelope mediates higher levels of infection on GAG-positive or GAG-negative target cells.
FIG. 4.
Correlation between V3-loop positive charge and GAG effects on HIV-1 infection. For each HIV-1 envelope described for Fig. 2, the overall positive charge of the V3 loop was calculated (some points are slightly offset from the integer value for clarity). The means and standard deviations of the ratio of titers obtained with CHO-K1 and pgsA745 target cells in three experiments are shown. Virus envelopes with distinct coreceptor preferences are depicted by different symbols (circles, R5 strains; triangles, X4 strains; square, dualtropic strain), and a single isolate with an unusually high histidine content within the V3 loop is marked with an asterisk.
To specifically determine the role of the V3 loop in mediating GAG-dependent infection, we examined the properties of NL/V3(ADA/JRFL). This virus contains a chimeric envelope which is derived predominantly from HXB but in which the highly charged V3 loop (+9) has been replaced by that of ADA, which is less charged (+3) and identical in sequence to that of JRFL. The data are presented in Table 1. In fact, the response of NL/V3(ADA/JRFL) to the presence or absence of target cell surface GAGs was similar to that of NL/ADA and NL/JRFL, which are more infectious on GAG-negative target cells, rather than NL/HXB which exhibits the opposite phenotype (Table 1). Thus, while GAG effects on HIV-1 infection are independent of coreceptor selectivity (Fig. 2), the gp120 V3 loop is a major determinant of both properties.
TABLE 1.
The V3-loop is the major viral determinant of cell surface GAG-mediated effects on HIV-1 infection
| Envelope | Infectious titer (FFU/ml)a (ratiob)
|
|||
|---|---|---|---|---|
| HXB | ADA | JRFL | V3(ADA/JRFL)c | |
| CHO-K1 | (5.3 ± 1.1) × 106 | (8.2 ± 0.4) × 105 | (1.6 ± 0.4) × 106 | (2.8 ± 0.6) × 106 |
| pgsA-745 | (5.6 ± 0.5) × 105 (9.5) | (4.2 ± 0.6) × 106 (0.19) | (6.8 ± 0.7) × 106 (0.24) | (7.3 ± 1.7) × 106 (0.38) |
Infectious titers of NL4-3-derived viruses containing the indicated envelope genes were measured by using CHO-K1- and pgsA745-derived target cells, and foci of infection were enumerated after immunofluorescence as described in Materials and Methods. FFU, focus-forming units.
Titer on CHO-K1-derived target cells/titer on pgsA745-derived target cells.
V3(ADA/JRFL) is a chimeric envelope containing V3-loop sequences of ADA/JRFL in in an HXB background.
The effects of polyanions and polycations on HIV-1 infection are modulated by cell surface GAGs.
The aforementioned data implicate the V3 loop, and its charge properties in particular, as an important viral determinant of GAG effects on HIV-1 infection. Of note, a recent study suggested that the V3 loop plays a major role in determining the overall charge of the gp120 surface that is predicted to project away from the virion and is therefore likely to be that which first encounters the target cell (20). This finding is especially pronounced when the V3 loop is highly basic. Therefore, we speculated that the enhancing and inhibitory effects of negatively charged GAGs on HIV-1 infection represent relatively nonspecific, envelope-cell surface interactions between like or opposing electrostatic charges. Such interactions could facilitate infection by HIV-1 isolates bearing highly positively charged V3 loops (such as HXB). Alternatively, negatively charged GAGs on the surface of target cells could impart an electrostatic “barrier” that inhibits infection by virions bearing less positively charged gp120s such as ADA. Polybrene, a polycation, is widely used to facilitate retroviral infection of cultured cells and is thought to mediate its effects by neutralizing or reversing repulsive negative charges on the surfaces of target cells (28). Indeed, HIV-1 primary isolate infection of engineered cell lines is known to be facilitated by Polybrene (39). If our speculation (that GAG-mediated effects on HIV-1 infection are simply the result of cell surface charge modification) was correct, then we would predict that Polybrene would inhibit infection of GAG-positive cells by NL/HXB and enhance infection of GAG-positive cells by NL/ADA. Moreover, these effects should be absent, or less evident, upon infection of cells lacking GAGs.
As is shown in Fig. 5A and B, this prediction proved to be accurate. Indeed, NL/HXB infection of K1/X4 cells was modestly inhibited (twofold) by Polybrene. A similar result was obtained previously with the HIV-1/IIIB isolate and a range of human cell lines (14). Moreover, this inhibition was observed only on GAG-positive cells; NL/HXB infection of 745/X4 cells was unaffected by Polybrene (Fig. 5A). Conversely, NL/ADA infection of K1/R5 cells was significantly enhanced (approximately sixfold) by Polybrene. Again, the effect was GAG dependent, in that little or no enhancement of infection was observed when target cells lacked GAGs (Fig. 5A). In fact, Polybrene treatment of GAG-positive cells increased NL/ADA infection to a level similar to that observed in Polybrene-treated or untreated GAG-negative cells (Fig. 5B).
As is shown in Fig. 5C and D, we also observed marked target cell surface GAG-dependent effects on polyanion-mediated inhibition of HIV-1 infection. For these experiments we used heparin, a highly sulfated analogue of HS. Polyanions, such as heparin, can bind to a conserved basic surface on gp120, as well as the V3 loop (6, 15, 25), that together are thought to represent the major site of interaction for coreceptors. If inhibition of specific viral coreceptor binding is the sole mechanism by which polyanions inhibit HIV-1 infection, then the presence or absence of target cell surface GAGs should have little or no effect on the potency with which polyanions inhibit infection by a given HIV-1 strain. However, while heparin potently inhibited NL/HXB infection of K1/X4 cells, it was between 1 and 2 orders of magnitude less effective at preventing NL/HXB infection of 745/X4 cells (Fig. 5C). Therefore, the majority of the infection-inhibiting activity of heparin is likely to be attributable to either (i) competition for GAG binding sites on NL/HXB virion envelopes or (ii) reduction of the overall positive charge on NL/HXB virions, such that electrostatic attraction between positive charges on the virion and negatively charged GAGs on the target cell is attenuated. The residual weak inhibitory effect of heparin on NL/HXB infection that is observed when 745/X4 cells are used as targets is likely to result from the blockade of more specific envelope-receptor interactions involving positively charged virion envelope surfaces (20, 25).
In the case of NL/ADA, heparin was a poor inhibitor of infection (Fig. 5D). Indeed, NL/ADA infection of 745/R5 cells was almost entirely refractory to inhibition by heparin. However, a modest inhibitory activity of heparin was observed when GAG-positive target cells were used. Since cell surface GAGs themselves impart a negative charge that is apparently inhibitory to NL/ADA attachment and infection (Fig. 2 and 3), it is likely that inefficient binding of negatively charged heparin to the ADA envelope accentuates this repulsive electrostatic effect.
Overall, these results suggest that modifying the charge characteristics of either the target cell or the virion can have significant effects on the efficiency of HIV-1 infection. Furthermore, they strongly suggest that GAGs on the surface of HIV-1 target cells impart a negative charge that can either enhance or inhibit HIV-1 infection, in an envelope-dependent manner. Finally, cell surface GAGs are the predominant cellular determinant that govern the effects of polyanions and polycations on the in vitro infectivity of HIV-1.
The effects of target cell surface GAGs on HIV-1 infection are independent of CypA.
The results presented thus far indicate that the effects of cell surface GAGs on HIV-1 infection are mediated largely, perhaps entirely, by the viral envelope protein. These data are difficult to reconcile with a recently proposed model in which virion encapsidated cyclophilin translocates to the surface of the virion from where it mediates attachment to the target cell via a specific interaction with HS (32, 33). However, to address this issue specifically, we adopted three experimental strategies to deplete virions of CypA and examined the effects of these manipulations on the infectivity of HIV-1 strains that are more infectious in the presence (NL/HXB) or absence (NL/ADA) of target cell surface GAGs. If the role of CypA in HIV-1 infection is to promote virion attachment to HS on the surface of target cells, then the absence of CypA from virions should reduce HIV-1 infectivity on HS expressing cells. However, the presence or absence of virion-associated CypA should have no effect on infectivity when cells that entirely lack HS are used as targets.
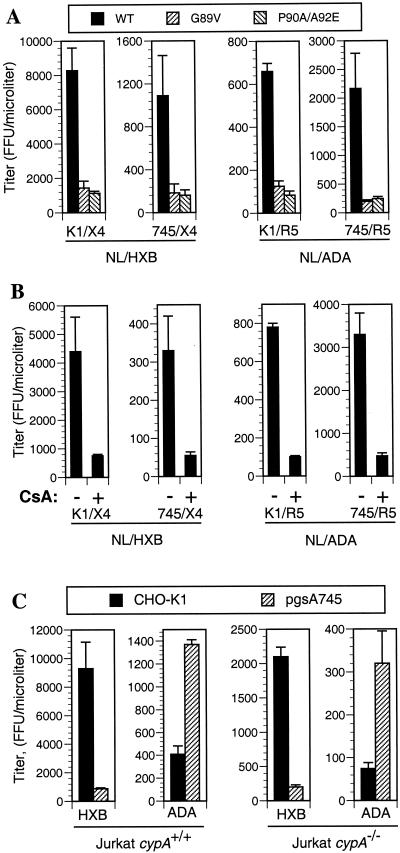
We first constructed proviral plasmids in which residues within the CA protein that are essential for CypA incorporation were mutated (G89V and P90A). The P90A mutation was combined with a further substitution (A92E) that was previously reported to partially restore replication competence to HIV-1 when CypA incorporation is blocked by pharmacological or genetic manipulations (3). As can be seen in Fig. 6A, NL/HXB virions containing these mutant CA proteins were less infectious (five- to sixfold) when K1/X4 cells were used as targets, as expected. However, both wild-type and mutant virions were approximately 10-fold less infectious on 745/X4 cells. Put another way, the effect of the CA mutations that prevent CypA incorporation on NL/HXB infectivity was similar when K1/X4 (HS-positive) and 745/X4 (HS-negative) target cells were used. This result is inconsistent with the notion that CA mutations reduce HIV-1 infectivity by preventing CypA-dependent interactions between the virion and target cell surface HS. Furthermore, a virus (NL/ADA) that is more infectious on cells that lack HS exhibited similar reductions in infectivity upon mutation of the CA protein to abolish CypA incorporation (Fig. 6A). Once again, the reduction in infectivity that results from genetically preventing CypA incorporation into NL/ADA virions was observed on both HS-positive and HS-negative target cells
FIG. 6.
The effects of cell surface GAGs on HIV-1 infectivity are independent of CypA incorporation. (A) The infectivity of wild-type or capsid mutant derivatives (G89V and P90A/A92E) of NL/HXB and NL/ADA was measured by using CHO-K1- and pgsA745-derived target cells. The infectious titer of each virus on each cell line was measured as described for Fig. 2 with normalization for minor variation in the amount of p24 in each viral supernatant. (B) NL/HXB and NL/ADA virus stocks were produced in 293T cells in the presence or absence of CsA, and infectious titers were measured after normalization, as for panel A, by using CHO-K1- and pgsA745-derived target cells. (C) NL/HXB and NL/ADA were each pseudotyped with the VSV-G envelope protein and introduced into the unmodified Jurkat cell line or a derivative lacking both copies of the cypA gene. Progeny virions were harvested, and the infectivity of each was measured with CHO-K1 and pgsA745 target cells. For each experiment, the results presented are the means and standard deviations of three titrations.
The incorporation of CypA into HIV-1 virions is also effectively blocked by treating virus-producing cells with CsA, a drug that prevents CypA-CA interaction by competing for the CA binding site on CypA (3, 37). As a result, virions harvested from CsA-treated cells exhibit reduced infectivity. As is shown in Fig. 6B, virus stocks harvested from CsA-treated, pNL/HXB-transfected 293T cells were indeed less infectious than those derived from untreated cells. The magnitude of the CsA-induced reduction in infectivity (five- to sixfold) was similar to that observed when CypA incorporation was abolished by mutation of residues within the CA protein. Importantly, however, this phenotype was observed whether or not HS was present on the target cells that were used to measure infectivity. Furthermore, CsA treatment of NL/ADA-producing cells also led to equivalent reductions in virion infectivity regardless of whether HS-positive or -negative cells were used for virus titration.
Finally, we made use of a Jurkat-derived cell line in which both copies of the cypA gene had been disrupted by homologous recombination. HIV-1 replication is attenuated in cypA−/− cells because virions derived from them are less infectious (5). Since Jurkat cells lack the CCR5 coreceptor and therefore cannot support entry of NL/ADA, both NL/HXB and NL/ADA genomes were introduced into cypA+/+ and cypA−/− Jurkat cells by pseudotyping with VSV-G. The infectivity of progeny virions was then measured by using HS-positive and -negative cells, as was done previously. It is important to note that while each virus was introduced into the Jurkat cells by using the VSV envelope, their progeny display only the authentic HIV-1 (HXB or ADA) envelope. This was verified by demonstrating that each Jurkat-derived virus was noninfectious on cells that did not express the appropriate HIV-1 envelope-specific coreceptor (data not shown). As is shown in Fig. 6C, Jurkat cypA+/+-derived NL/HXB and NL/ADA virions displayed a phenotype virtually identical to that observed when these viruses were obtained from transfected 293T cells (compare Fig. 6C with Fig. 2), i.e., NL/HXB was more infectious on GAG-positive K1/X4 cells than on GAG-negative 745/X4 cells, whereas NL/ADA was more infectious on 745/R5 than on K1/R5 cells. While the levels of infectious NL/HXB and NL/ADA harvested from Jurkat cypA−/− cells were reduced compared to that obtained from Jurkat cypA+/+ cells, each of these viruses exhibited the same preference for the presence (NL/HXB) or absence (NL/ADA) of GAG expression by target cells. These data clearly show that CypA incorporation into virion particles does not modulate the differential ability of HIV-1 to infect cells that do or do not express HS.
DISCUSSION
In this series of experiments we have shown that the presence of GAGs on the surfaces of target cells can modulate their susceptibility to HIV-1 infection. These findings are in agreement with previously published data which showed that HIV-1 strains derived from the IIIB/LAV/LAI isolate, termed HXB in this study, exhibit significantly higher levels of infectivity (approximately 10-fold) when HS is present on the target cell surface (Fig. 1 and 2) (18, 27, 29, 32). However, we have found that this phenotype is a characteristic of a minority of HIV-1 strains. In fact, most of the envelope proteins used in the experiments presented herein, which included representatives from subtypes A, B, C, and D, confer higher infectivity when target cells entirely lack GAGs (Fig. 2). Only 2 of 12 envelope proteins derived from primary isolates (one X4 and one X4R5) exhibited a clear preference for the presence of target cell surface GAGs during infection.
Furthermore, these discordant phenotypes appear to be determined by the gp120 V3 loop and correlate with V3-loop charge (Table 1; Fig. 4). In fact, only virus strains bearing a gp120 protein that contain a highly basic V3-loop sequence exhibit a preferential ability to infect GAG-positive cells. This observation, coupled with other data presented here or recently published by other groups (20), suggests that the effect of cell surface GAGs on HIV-1 infection simply reflects electrostatic repulsion or attraction between like or oppositely charged components of the target cell and virion. Specifically, the V3 loop is likely to be a major determinant of the overall charge of the face of gp120 that projects toward the target cell, particularly when V3 itself is highly basic (20). Thus, the target-cell-proximal faces of, for example, HXB and 89.6 gp120, both of which confer higher infectivity on GAG-positive cells, are substantially more positively charged than is that of, for example, JRFL, which exhibits the inverse phenotype (20) (Table 1; Fig. 2). Moreover, the polycation Polybrene and the polyanion heparin had effects on HIV-1 infection that differed according to which envelope protein was present. For instance, infection by NL/ADA, whose V3 loop bears a low positive charge, was significantly enhanced by pretreating target cells with Polybrene. This compound is thought to enhance infection by diverse retroviruses by neutralizing or reversing negative charges on the surface of target cells (28). Consistent with this notion, Polybrene modestly inhibited infection by NL/HXB, whose envelope is highly positively charged. Significantly, both of these effects were dependent on the presence of GAGs on the target cell surface (Fig. 5). The implication of these results is that GAGs impart a negative charge to the target cell surface that inhibits attachment and infection by less positively charged virion particles (e.g., NL/ADA) but promotes attachment and infection by positively charged virions (e.g., NL/HXB) and can be neutralized by Polybrene.
Similarly, the effects of heparin on HIV-1 infection were also dependent on both the viral envelope and on target cell GAG expression. Sulfated polysaccharides have greater affinity for, and higher antiviral activity against, X4 envelopes such as HXB than R5 envelopes such as ADA and JRCSF (Fig. 5) (25). The results presented herein indicate that the ability of heparin to block both NL/HXB and NL/ADA infection is at least partly dependent on the presence of GAGs on the surface of target cells (Fig. 5). When viewed in conjunction with the results discussed above, these findings suggest that the binding of heparin to positively charged components of the virion (which is more efficient in the case of NL/HXB) neutralizes virion-associated positive charge and increases the amount of virion-associated negative charge. The obvious consequence would be that electrostatic attraction to negatively charged target cell surface GAGs would be decreased and electrostatic repulsion by like charges would be accentuated.
The positive and negative effects of target cell surface GAGs and exogenously added heparin on HIV-1 infection appear to be strictly dependent on the envelope sequence. These findings are not consistent with the hypothesis that CypA mediates the initial interaction of the virion with the target cell by binding to cell surface HS. Indeed, pharmacological (CsA treatment) or genetic (CA mutation) elimination of CypA incorporation into NL/HXB or NL/ADA particles reduced virion infectivity in a manner that was independent of whether HS was present on the surface of target cells (Fig. 6). Most compellingly, NL/HXB or NL/ADA virus stocks derived from cypA−/− Jurkat cells, which lack CypA, exhibited a preference for the presence or absence of GAGs on the surface of target cells identical to that of stocks derived from cypA+/+ Jurkat cells or 293T cells. Clearly, the reduced infectivity of HIV-1 virions that results from a lack of CypA cannot be ascribed to elimination of HS-dependent attachment to target cells.
The differential effects on HIV-1 infection that result from the presence or absence of GAGs on the surface of CHO-K1-derived target cells are likely to represent extreme manifestations of these phenotypes. HS expression is particularly high on CHO-K1 cells and completely absent from pgsA745 cells (9, 10) (Fig. 1). Even so, these two cell lines differ rather modestly, 10-fold or less, in their susceptibility to infection by HIV-1 virions bearing each of the envelopes tested. The predominant natural target cells for HIV-1 infection, namely, primary T lymphocytes, express only low levels of HS (9, 34). Thus, the effects, if any, of HS expression on HIV-1 infection of primary cells are likely to be more modest than that documented herein for CHO-derived cells. In fact, significant positive effects of target cell surface GAGs on HIV-1 infection have been obtained predominantly with immortalized cell lines and cell line-adapted virus isolates (18, 24, 27, 29, 32). Indeed, heparinase treatment of primary lymphocytes was found to have negligible effects on HIV-1 infection mediated by either an X4 or R5 envelope (18).
Despite these findings it remains formally possible that cell surface GAGs might play some role in the biology of HIV-1 in vivo. For instance, infection of macrophages could be either positively or negatively influenced by cell surface GAGs, which are more abundant on this cell type (34). In addition, high concentrations of the chemokine RANTES have been shown to dramatically enhance in vitro infection by both HIV-1 and murine leukemia virus virions, in an envelope-independent manner (13). RANTES-dependent infection enhancement is at least partly mediated through increased virion attachment, is dependent on cell surface GAGs, and is most likely the result of virion-cell cross-linking by RANTES oligomers (38). It is unclear at present whether local concentrations of RANTES in vivo might reach the levels required for this effect. Finally, the apparent selective transmission of R5 HIV-1 strains at mucosal surfaces, where GAGs appear more abundant, could in principle be influenced by the phenotypic variation in envelope properties described herein. In this case, it would be necessary to envision an indirect mechanism whereby GAGs selectively sequester, and thereby prevent the transmission of, X4 strains bearing highly positively charged envelopes while allowing the transmission of strains bearing less positively charged envelopes.
Overall, however, the data presented here indicate that, in most cases, the initial encounter between an HIV-1 virion and its target cell is unlikely to be mediated by gp120-GAG or CypA-GAG interactions. Thus, at least for the majority of primary HIV-1 strains, we find no evidence to suggest that low level of GAGs present on circulating T-cells in vivo would play a significant direct role in promoting HIV-1 attachment and replication.
Acknowledgments
We are indebted to Thomas Hope for advice on the generation and microscopic visualization of GFP-Vpr-labeled virions. We also thank Mark Muesing and Linqi Zhang for gifts of reagents. SIVmac239-derived plasmid clones were obtained through the AIDS Research and Reference Reagent Program from Ronald Desrosiers.
This work was supported by The Donald A. Pels Charitable Trust, the Howard Hughes Medical Institute, NIAID grants AI36199 (to J.L.) and AI50111 (to P.D.B.), and the Columbia-Rockefeller Center for AIDS Research (P30 AI42848).
REFERENCES
- 1.Baba, M., R. Snoeck, R. Pauwels, and E. de Clercq. 1988. Sulfated polysaccharides are potent and selective inhibitors of various enveloped viruses, including herpes simplex virus, cytomegalovirus, vesicular stomatitis virus, and human immunodeficiency virus. Antimicrob. Agents Chemother. 32:1742-1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bieniasz, P. D., and B. R. Cullen. 2000. Multiple blocks to human immunodeficiency virus type 1 replication in rodent cells. J. Virol. 74:9868-9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braaten, D., C. Aberham, E. K. Franke, L. Yin, W. Phares, and J. Luban. 1996. Cyclosporine A-resistant human immunodeficiency virus type 1 mutants demonstrate that Gag encodes the functional target of cyclophilin A. J. Virol. 70:5170-5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braaten, D., E. K. Franke, and J. Luban. 1996. Cyclophilin A is required for an early step in the life cycle of human immunodeficiency virus type 1 before the initiation of reverse transcription. J. Virol. 70:3551-3560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Braaten, D., and J. Luban. 2001. Cyclophilin A regulates HIV-1 infectivity, as demonstrated by gene targeting in human T cells. EMBO J. 20:1300-1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Callahan, L. N., M. Phelan, M. Mallinson, and M. A. Norcross. 1991. Dextran sulfate blocks antibody binding to the principal neutralizing domain of human immunodeficiency virus type 1 without interfering with gp120-CD4 interactions. J. Virol. 65:1543-1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cladera, J., I. Martin, and P. O'Shea. 2001. The fusion domain of HIV gp41 interacts specifically with heparan sulfate on the T-lymphocyte cell surface. EMBO J. 20:19-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Demaria, S., and Y. Bushkin. 1996. Soluble CD4 induces the binding of human immunodeficiency virus type 1 to cells via the V3 loop of glycoprotein 120 and specific sites in glycoprotein 41. AIDS Res. Hum. Retrovir. 12:281-290. [DOI] [PubMed] [Google Scholar]
- 9.de Parseval, A., and J. H. Elder. 2001. Binding of recombinant feline immunodeficiency virus surface glycoprotein to feline cells: role of CXCR4, cell-surface heparans, and an unidentified non-CXCR4 receptor. J. Virol. 75:4528-4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Esko, J. D., K. S. Rostand, and J. L. Weinke. 1988. Tumor formation dependent on proteoglycan biosynthesis. Science 241:1092-1096. [DOI] [PubMed] [Google Scholar]
- 11.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Franke, E. K., H. E. Yuan, and J. Luban. 1994. Specific incorporation of cyclophilin A into HIV-1 virions. Nature 372:359-362. [DOI] [PubMed] [Google Scholar]
- 13.Gordon, C. J., M. A. Muesing, A. E. Proudfoot, C. A. Power, J. P. Moore, and A. Trkola. 1999. Enhancement of human immunodeficiency virus type 1 infection by the CC-chemokine RANTES is independent of the mechanism of virus-cell fusion. J. Virol. 73:684-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haraguchi, Y., Y. Takeuchi, and H. Hoshino. 1997. Inhibition of plating of human T cell leukemia virus type I and syncytium-inducing types of human immunodeficiency virus type 1 by polycations. AIDS Res. Hum. Retrovir. 13:1517-1523. [DOI] [PubMed] [Google Scholar]
- 15.Harrop, H. A., D. R. Coombe, and C. C. Rider. 1994. Heparin specifically inhibits binding of V3 loop antibodies to HIV-1 gp120, an effect potentiated by CD4 binding. AIDS 8:183-192. [DOI] [PubMed] [Google Scholar]
- 16.Herold, B. C., A. Siston, J. Bremer, R. Kirkpatrick, G. Wilbanks, P. Fugedi, C. Peto, and M. Cooper. 1997. Sulfated carbohydrate compounds prevent microbial adherence by sexually transmitted disease pathogens. Antimicrob. Agents Chemother. 41:2776-2780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hwang, S. S., T. J. Boyle, H. K. Lyerly, and B. R. Cullen. 1992. Identification of envelope V3 loop as the major determinant of CD4 neutralization sensitivity of HIV-1. Science 257:535-537. [DOI] [PubMed] [Google Scholar]
- 18.Ibrahim, J., P. Griffin, D. R. Coombe, C. C. Rider, and W. James. 1999. Cell-surface heparan sulfate facilitates human immunodeficiency virus type 1 entry into some cell lines but not primary lymphocytes. Virus Res. 60:159-169. [DOI] [PubMed] [Google Scholar]
- 19.Ito, M., M. Baba, A. Sato, R. Pauwels, E. De Clercq, and S. Shigeta. 1987. Inhibitory effect of dextran sulfate and heparin on the replication of human immunodeficiency virus (HIV) in vitro. Antivir. Res. 7:361-367. [DOI] [PubMed] [Google Scholar]
- 20.Kwong, P. D., R. Wyatt, Q. J. Sattentau, J. Sodroski, and W. A. Hendrickson. 2000. Oligomeric modeling and electrostatic analysis of the gp120 envelope glycoprotein of human immunodeficiency virus. J. Virol. 74:1961-1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Luban, J., K. L. Bossolt, E. K. Franke, G. V. Kalpana, and S. P. Goff. 1993. Human immunodeficiency virus type 1 Gag protein binds to cyclophilins A and B. Cell 73:1067-1078. [DOI] [PubMed] [Google Scholar]
- 22.Mbemba, E., V. Chams, J. C. Gluckman, D. Klatzmann, and L. Gattegno. 1992. Molecular interaction between HIV-1 major envelope glycoprotein and dextran sulfate. Biochim. Biophys. Acta 1138:62-67. [DOI] [PubMed] [Google Scholar]
- 23.McClure, M. O., J. P. Moore, D. F. Blanc, P. Scotting, G. M. Cook, R. J. Keynes, J. N. Weber, D. Davies, and R. A. Weiss. 1992. Investigations into the mechanism by which sulfated polysaccharides inhibit HIV infection in vitro. AIDS Res. Hum. Retrovir. 8:19-26. [DOI] [PubMed] [Google Scholar]
- 24.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moulard, M., H. Lortat-Jacob, I. Mondor, G. Roca, R. Wyatt, J. Sodroski, L. Zhao, W. Olson, P. D. Kwong, and Q. J. Sattentau. 2000. Selective interactions of polyanions with basic surfaces on human immunodeficiency virus type 1 gp120. J. Virol. 74:1948-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O'Doherty, U., W. J. Swiggard, and M. H. Malim. 2000. Human immunodeficiency virus type 1 spinoculation enhances infection through virus binding. J. Virol. 74:10074-10080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohshiro, Y., T. Murakami, K. Matsuda, K. Nishioka, K. Yoshida, and N. Yamamoto. 1996. Role of cell surface glycosaminoglycans of human T cells in human immunodeficiency virus type-1 (HIV-1) infection. Microbiol. Immunol. 40:827-835. [DOI] [PubMed] [Google Scholar]
- 28.Palsson, B., and S. Andreadis. 1997. The physico-chemical factors that govern retrovirus-mediated gene transfer. Exp. Hematol. 25:94-102. [PubMed] [Google Scholar]
- 29.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 30.Rider, C. C., D. R. Coombe, H. A. Harrop, E. F. Hounsell, C. Bauer, J. Feeney, B. Mulloy, N. Mahmood, A. Hay, and C. R. Parish. 1994. Anti-HIV-1 activity of chemically modified heparins: correlation between binding to the V3 loop of gp120 and inhibition of cellular HIV-1 infection in vitro. Biochemistry 33:6974-6980. [DOI] [PubMed] [Google Scholar]
- 31.Roderiquez, G., T. Oravecz, M. Yanagishita, D. C. Bou-Habib, H. Mostowski, and M. A. Norcross. 1995. Mediation of human immunodeficiency virus type 1 binding by interaction of cell surface heparan sulfate proteoglycans with the V3 region of envelope gp120-gp41. J. Virol. 69:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saphire, A. C., M. D. Bobardt, and P. A. Gallay. 1999. Host cyclophilin A mediates HIV-1 attachment to target cells via heparans. EMBO J. 18:6771-6785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saphire, A. C., M. D. Bobardt, and P. A. Gallay. 2000. Human immunodeficiency virus type 1 hijacks host cyclophilin A for its attachment to target cells. Immunol. Res. 21:211-217. [DOI] [PubMed] [Google Scholar]
- 34.Saphire, A. C., M. D. Bobardt, Z. Zhang, G. David, and P. A. Gallay. 2001. Syndecans serve as attachment receptors for human immunodeficiency virus type 1 on macrophages. J. Virol. 75:9187-9200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sherry, B., G. Zybarth, M. Alfano, L. Dubrovsky, R. Mitchell, D. Rich, P. Ulrich, R. Bucala, A. Cerami, and M. Bukrinsky. 1998. Role of cyclophilin A in the uptake of HIV-1 by macrophages and T lymphocytes. Proc. Natl. Acad. Sci. USA 95:1758-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thali, M., A. Bukovsky, E. Kondo, B. Rosenwirth, C. T. Walsh, J. Sodroski, and H. G. Gottlinger. 1994. Functional association of cyclophilin A with HIV-1 virions. Nature 372:363-365. [DOI] [PubMed] [Google Scholar]
- 38.Trkola, A., C. Gordon, J. Matthews, E. Maxwell, T. Ketas, L. Czaplewski, A. E. Proudfoot, and J. P. Moore. 1999. The CC-chemokine RANTES increases the attachment of human immunodeficiency virus type 1 to target cells via glycosaminoglycans and also activates a signal transduction pathway that enhances viral infectivity. J. Virol. 73:6370-6379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Trkola, A., J. Matthews, C. Gordon, T. Ketas, and J. P. Moore. 1999. A cell line-based neutralization assay for primary human immunodeficiency virus type 1 isolates that use either the CCR5 or the CXCR4 coreceptor. J. Virol. 73:8966-8974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ueno, R., and S. Kuno. 1987. Dextran sulphate, a potent anti-HIV agent in vitro having synergism with zidovudine. Lancet i:1379. [DOI] [PubMed]
- 41.Ugolini, S., I. Mondor, and Q. J. Sattentau. 1999. HIV-1 attachment: another look. Trends Microbiol. 7:144-149. [DOI] [PubMed] [Google Scholar]
- 42.Wadstrom, T., and A. Ljungh. 1999. Glycosaminoglycan-binding microbial proteins in tissue adhesion and invasion: key events in microbial pathogenicity. J. Med. Microbiol. 48:223-233. [DOI] [PubMed] [Google Scholar]