Abstract
Most human immunodeficiency virus type 1 (HIV-1) viruses in the brain use CCR5 as the principal coreceptor for entry into a cell. However, additional phenotypic characteristics are necessary for HIV-1 neurotropism. Furthermore, neurotropic strains are not necessarily neurovirulent. To better understand the determinants of HIV-1 neurovirulence, we isolated viruses from brain tissue samples from three AIDS patients with dementia and HIV-1 encephalitis and analyzed their ability to induce syncytia in monocyte-derived macrophages (MDM) and neuronal apoptosis in primary brain cultures. Two R5X4 viruses (MACS1-br and MACS1-spln) were highly fusogenic in MDM and induced neuronal apoptosis. The R5 viruses UK1-br and MACS2-br are both neurotropic. However, only UK1-br induced high levels of fusion in MDM and neuronal apoptosis. Full-length Env clones from UK1-br required lower CCR5 and CD4 levels than Env clones from MACS2-br to function efficiently in cell-to-cell fusion and single-round infection assays. UK1-br Envs also had a greater affinity for CCR5 than MACS2-br Envs in binding assays. Relatively high levels of UK1-br and MACS2-br Envs bound to CCR5 in the absence of soluble CD4. However, these Envs could not mediate CD4-independent infection, and MACS2-br Envs were unable to mediate fusion or infection in cells expressing low levels of CD4. The UK1-br virus was more resistant than MACS2-br to inhibition by the CCR5-targeted inhibitors TAK-779 and Sch-C. UK1-br was more sensitive than MACS2-br to neutralization by monoclonal antibodies (2F5 and immunoglobulin G1b12 [IgG1b12]) and CD4-IgG2. These results predict the presence of HIV-1 variants with increased CCR5 affinity and reduced dependence on CCR5 and CD4 in the brains of some AIDS patients with central nervous system disease and suggest that R5 variants with increased CCR5 affinity may represent a pathogenic viral phenotype contributing to the neurodegenerative manifestations of AIDS.
Human immunodeficiency virus type 1 (HIV-1) infects macrophages and microglia in the central nervous system (CNS) and frequently causes dementia and other neurological disorders in patients with AIDS (49, 68). CNS infection with HIV-1 can cause an encephalitis, characterized by reactive astrocytes, myelin pallor, microglial nodules, perivascular inflammation, multinucleated giant cells (MNGC), and neuronal cell loss. HIV-1 enters the CNS in the early stages of infection by trafficking across the blood-brain barrier within infected monocytes and possibly lymphocytes (68). However, CNS infection with HIV-1 is latent and typically does not cause dementia or encephalitis until after clinical progression to AIDS. The genetic evolution of HIV-1 within the brain is distinct from that in lymphoid tissues and other organs (12, 18, 26, 32, 38, 44, 69, 85, 90). Specific sequences within the viral envelope glycoprotein (Env), particularly the gp120 V3 region, have been associated with brain infection (38, 44, 66, 67, 85, 88). The genetic compartmentalization of viral variants in the CNS suggests that adaptive changes may occur in response to unique constraints of the CNS microenvironment, such as different target cell populations and immune selection pressures.
The tropism of HIV-1 is predominantly determined by the sequential interaction of Env with CD4 and a particular coreceptor. Macrophage-tropic HIV-1 primarily uses CCR5 (R5) as a coreceptor (3, 13, 16, 19, 20), whereas T-cell line-tropic (T-tropic) viruses use CXCR4 (X4). Dual-tropic viruses (R5X4) can use both coreceptors. In some patients, HIV-1 disease progression is associated with a general broadening of virus tropism by expansion of coreceptor usage and the emergence of X4 or R5X4 variants (9, 15). However, usage of coreceptors other than CCR5 and CXCR4 by primary viruses in vitro is rare (95), and infection of primary cells occurs, with few exceptions (37, 46), exclusively via CCR5 or CXCR4 (94, 96).
CCR5 is the major coreceptor for HIV-1 infection of macrophages and microglia (2, 29, 34, 72; reviewed in reference 24). Furthermore, CCR5 is the principal coreceptor used by HIV-1 viruses isolated from the brain (2, 34, 48, 53, 72, 77). However, CCR5 usage by primary brain-derived HIV-1 isolates is neither necessary nor sufficient for neurotropism (defined as the ability of viruses to replicate in microglia) (32). Most laboratory-adapted X4 viruses, such as IIIB, MN, and SF-2, do not replicate efficiently in macrophages and microglia (17, 28, 34, 45, 62, 70, 78, 93), but macrophages and microglia can support efficient replication by a subset of primary X4 viruses isolated from blood (32, 36, 62, 75, 76, 86) or brain tissue (32). We previously demonstrated that macrophage (M) tropism predicts HIV-1 neurotropism independent of coreceptor specificity (32). Consistent with this model, a chimeric simian-human immunodeficiency virus that is neurotropic in rhesus macaques contains the env gene from the T-tropic HIV-1 IIIB strain. This virus (SHIVKU-2) was adapted for growth in monocyte-derived macrophages (MDM) and uses only CXCR4 for entry (50).
Infection of the CNS by HIV-1 or simian immunodeficiency virus (SIV) is not sufficient to cause dementia or encephalitis (39, 44, 51, 66), suggesting that neurovirulence is likely to be determined by genetic and biological characteristics that are distinct from those that underlie neurotropism. Apoptosis of neurons and astrocytes is induced by infection with HIV-1 in vitro (62, 71) and has been observed in autopsy brain tissues from AIDS patients (1, 5, 27, 64, 71, 84; reviewed in references 25 and 33). However, only a subset of R5, X4, and R5X4 viruses induce neuronal apoptosis in primary brain cultures (62, 67). Collectively, these findings raise the possibility that dementia may be associated with particular strains of HIV-1 that replicate efficiently in macrophages and microglia, in addition to having the capacity to induce neuronal dysfunction or cell death.
The viral envelope glycoproteins are an important determinant of neurodegenerative mechanisms associated with HIV-1 infection in vitro (62, 67). A major pathway for neuronal injury is thought to involve the release of the gp120 glycoprotein, in soluble or virion-associated form, from infected macrophages and microglia; this may induce cellular dysfunction or apoptosis via interactions with CXCR4 and possibly other chemokine receptors expressed on neurons and other cells in the CNS (35, 41, 54, 97, 98; reviewed in references 40 and 49). Other soluble neurotoxic factors produced by activated or infected macrophages and microglia, including the HIV-1 Tat protein, glutamate, quinolinic acid, l-cysteine, platelet-activating factor, and some as yet unknown, may also contribute to mechanisms of neuronal injury (reviewed in references 25, 31, 40, and 49).
We and others have described primary R5 HIV-1 isolates that replicate to high levels in microglia and have a reduced dependence on CD4 (32, 73) and CCR5 levels (32) for virus entry. Shieh et al. (73) generated a highly fusogenic variant with a reduced dependence on CD4 levels (52, 73) by adapting a blood-derived isolate via serial passage in cultured microglia (BORI-15 isolate). We recently described a naturally occurring variant isolated from the brain of an intravenous drug user that also has a reduced dependence on both CD4 and CCR5 levels (UK1-br isolate) (32). The levels of CD4 and CCR5 expression are lower in microglia in the CNS than in CD4+ T-cells and monocytes in peripheral blood (47, 63, 87). Furthermore, the low level of CD4 expression on rhesus macrophages was shown to restrict infection by M-tropic HIV-1 or T-tropic SIV strains (8, 59). We therefore hypothesized that a reduced dependence on CD4 and/or CCR5 levels by HIV-1 in the CNS may be a means by which R5 HIV-1 strains might acquire neurovirulence.
In this study, we characterized neurotropic R5 and R5X4 viruses isolated from autopsy brain and lymphoid tissues of AIDS patients with dementia and HIV-1 encephalitis. We found that the ability of HIV-1 isolates to induce neuronal apoptosis was closely associated with high levels of fusion in MDM but independent of coreceptor specificity. Although UK1-br and MACS2-br R5 viruses are both neurotropic, neuronal apoptosis was induced only by UK1-br. Env clones from UK1-br had a reduced dependence on CCR5 and CD4 levels in cell-to-cell fusion and single-round infection assays than did Env clones from MACS2-br. Furthermore, the affinity of UK1-br Envs for CCR5 was increased. The UK1-br virus was more resistant to inhibition by the CCR5 inhibitors TAK-779 and Sch-C but more sensitive to neutralizing antibodies and CD4-immunoglobulin G2 (CD4-IgG2) than the MACS2-br virus. These results suggest that neurotropic R5 viruses that have an increased affinity for CCR5 may represent a pathogenic viral phenotype in the CNS.
MATERIALS AND METHODS
Virus isolates.
Primary viruses MACS1-br, MACS1-spln, MACS2-br, and UK1-br were isolated from autopsy brain (MACS1-br, MACS2-br, and UK1-br) and spleen (MACS1-spln) tissue samples from AIDS patients with dementia and HIV-1 encephalitis by coculture with CD8-depleted peripheral blood mononuclear cells (PBMC) as described previously (32) (Table 1). Patients MACS1 and MACS2 were participants in the Chicago Component of the Multicenter AIDS Cohort Study. Tissue samples from patient UK1 were obtained from the Edinburgh Brain Bank. HIV-1 ADA was obtained from the National Institutes of Health AIDS Research and Reference Reagent Program. Virus stocks were prepared from supernatants of infected PBMC as described previously (62). HIV-1 89.6 virus stocks were produced by transfection of 293T cells with proviral plasmid DNA by the calcium phosphate method (34).
TABLE 1.
Characteristics of study subjects and virus isolatesa
| Patient | Clinical dementia | HIV-1 encephalitis | Giant cells | Tissue isolates | Coreceptor usage | Replication kinetics
|
||
|---|---|---|---|---|---|---|---|---|
| PBMC | MDM | Microglia | ||||||
| MACS1 | Yes | Severe | +++ | Brain | CXCR4, CCR5 | +++ | ++ | + |
| Spleen | CXCR4, CCR5, Apj | +++ | +++ | +++ | ||||
| MACS2 | Yes | Moderate | + | Brain | CCR5 | + | +++ | ++ |
| UK1 | Yes | Moderate | ++ | Brain | CCR5 | + | ++ | +++ |
The data shown in this table are published in the work of Gorry et al. (32). The presence of clinical dementia and HIV-1 encephalitis in patients MACS1, MACS2, and UK1 was determined by the clinical history and neuropathological examination at the time of autopsy. The frequency of multinucleated giant cells observed in autopsy brain tissue sections was scored as + (occasional), ++ (moderate), or +++ (high). The coreceptors used by viruses isolated from brain or spleen tissue were determined by infection of transfected Cf2-Luc cells. For replication in PBMC, MDM, and microglia, virus levels were scored as +++, ++, or + based on levels of RT activity in culture supernatants as previously described (32).
Cells.
MDM were purified from PBMC by plastic adherence and cultured for 5 days in RPMI 1640 medium supplemented with 10% (vol/vol) human AB+ serum (Nabi, Boca Raton, Fla.), 100 μg of penicillin and streptomycin/ml, and 12.5 ng of macrophage colony-stimulating factor (R&D Systems, Minneapolis, Minn.)/ml. Primary human fetal brain cultures which contained a mixture of astrocytes, neurons, and microglia were prepared and cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% (vol/vol) bovine calf serum (HyClone, Logan, Utah), 100 μg of penicillin and streptomycin/ml, 2 mM l-glutamine, 1 mM sodium pyruvate, and 5 ng of macrophage colony-stimulating factor/ml as previously described (32). The protocol for tissue procurement was approved by the institutional review board at the Dana-Farber Cancer Institute and was in compliance with federal regulations. Cf2-Luc cells (32), derived from the Cf2th canine thymocyte cell line (13), stably express the luciferase gene under the control of the HIV-1 long terminal repeat. Cf2-Luc cells were cultured in DMEM medium supplemented with 10% (vol/vol) fetal bovine serum (FBS), 100 μg of penicillin and streptomycin/ml, and 0.7 mg of G418 (Gibco BRL, Gaithersburg, Md.)/ml. Cf2th-synCCR5 cells express a codon-optimized version of human CCR5 containing a C-terminal nonapeptide (TETSQVAPA) tag derived from bovine rhodopsin (56) and were cultured in DMEM medium supplemented with 10% (vol/vol) FBS, 100 μg of penicillin and streptomycin/ml, and 0.4 mg of G418 (Gibco BRL)/ml. Cf2th-CCR5 cells stably express low levels of CCR5 and were cultured in DMEM medium supplemented with 10% (vol/vol) FBS, 100 μg of penicillin and streptomycin/ml, and 0.5 mg of G418 (Gibco BRL)/ml.
HIV-1 replication kinetics.
Monocyte-derived macrophages were allowed to mature for 5 days prior to being seeded in six-well tissue culture plates at approximately 90% confluence. An amount of virus equivalent to 50,000 3H cpm reverse transcriptase (RT) units in a volume of 2 ml was allowed to adsorb to the cell monolayers for 3 h at 37°C. Virus was then removed, and the cells were rinsed three times with phosphate-buffered saline (PBS) prior to addition of 2 ml of culture medium. Fifty-percent media changes were performed twice weekly for 28 days, and supernatants were tested for HIV-1 replication by RT assays. Mixed fetal brain cells were seeded into 48-well tissue culture plates coated with poly-l-lysine at a density of 1.6 × 105/well and cultured for 5 days. Microglia were then added at a density of 0.2 × 105/well and cultured further for 2 days. Brain cell cultures were infected by overnight incubation with 10,000 3H cpm RT units of HIV-1 in a volume of 0.5 ml. Virus was removed, and the cells were rinsed three times with warm culture medium prior to addition of 0.25 ml of mixed brain cell-conditioned media. After culturing for 4 days, 0.25 ml of fresh culture media was added and cells were incubated for an additional 24 days. Fifty-percent media changes were performed weekly, and supernatants were tested for HIV-1 replication by p24 antigen enzyme-linked immunosorbent assay (NEN, Boston, Mass.) using the manufacturer's protocol.
Apoptosis assays.
Apoptosis in primary brain cultures was measured 28 days postinfection. Cells were collected in trypsin, added to wells of 96-well v-bottom plates (Costar, Corning, N.Y.), and washed twice with PBS prior to resuspension in 10 μl of propidium iodide (50 μg/ml) (Pharmingen, San Diego, Calif.) and incubation for 10 min at room temperature. The cells were then washed three times with PBS, resuspended in 100 μl of cytofix-cytoperm solution (Pharmingen), and incubated for 20 min at 4°C. After washing three times with perm/wash buffer (Pharmingen), the cells were incubated in 50 μl of fluorescein isothiocyanate-labeled terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) solution (Boehringer Mannheim) for 1 h at 37°C, washed twice with perm/wash buffer (Pharmingen), and resuspended in 400 μl of 1% (wt/vol) paraformaldehyde. Two-color flow-cytometric analysis was used to measure apoptosis of cells identified as neurons by forward scatter-side scatter profile. This population contains >95% of cells with positive intracellular staining for the neuronal marker MAP-2 using a fluorescein isothiocyanate-conjugated monoclonal antibody and negative intracellular staining for glial fibrillary acidic protein using a phycoerythrin-conjugated monoclonal antibody (Sigma, St. Louis, Mo.) (J. Wang and D. Gabuzda, unpublished data). TUNEL-positive, propidium iodide-negative neurons were considered to be apoptotic.
PCR amplification, HIV-1 Env cloning, and sequence analysis.
Genomic DNA was extracted from PBMC infected with UK1-br and MACS2-br primary isolates using a DNeasy DNA extraction kit (Qiagen) according to the manufacturer's protocol. Full-length Env genes were amplified from genomic DNA with RTth XL polymerase and nested primers using a hot start with AmpliWax PCR Gem 50 (Applied Biosystems). The outer primers were 5717F (5′-ACTTGGGCAGG AGTGGAAGC-3′) and 9100R (5′-TAGCCCTTCCAGTCCCCCCTTTTCTTTT-3). The inner primers were 5800F (5′-AGAATTGGGTGTCGACATAGCAGAATAGGC-3′) and 8821R (5′-TTTTGACCACTTGCCACCCAT-3′). The primer numbers refer to nucleotide positions in pNL4-3. PCR was performed in a model 9600 thermocycler (Perkin-Elmer) programmed at 80°C for 5 min, 94°C for 1 min, 40 cycles of 95°C for 15 s, 62°C for 8 min, and a final extension of 72°C for 10 min. PCR product DNA was gel purified and cloned into pCR3.1-Uni (Invitrogen). Functional full-length Env clones were identified by Western blot analysis of gp120/gp160 in transfected 293T cells and by fusion assays. Env clones were sequenced by Big Dye terminator sequencing (Applied Biosystems) and analyzed using a model 3100 Genetic Analyzer (Applied Biosystems).
Western blot analysis.
For analysis of Env expression, 293T cells were transfected with 2.5 μg of different pCR3.1env clones. At 72 h after transfection, cells were rinsed twice in PBS and resuspended in 400 μl of ice-cold lysis buffer (0.5% [vol/vol] NP-40; 0.5% [wt/vol] sodium deoxycholate; 50 mM NaCl; 25 mM Tris-HCl [pH 8.0]; 10 mM EDTA; 5 mM benzamidine HCl; 10 mM phenylmethylsulfonyl fluoride) for 20 min, followed by centrifugation at 15,300 × g for 10 min to remove cellular debris. Cell lysates were separated in 8.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gels and analyzed by Western blotting using rabbit anti-gp120 polyclonal antisera (American Biotechnologies Inc.). Env proteins were visualized using horseradish peroxidase-conjugated anti-rabbit immunoglobulin G antibodies and enhanced chemiluminescence (NEN).
Site-directed mutagenesis.
Mutant Env plasmids containing a restored potential N-linked glycosylation site in the V1V2 stem region (D208N; corresponding to position 197 in HXB2 Env) of UK1-br Env clones were created by PCR-based mutagenesis (Stratagene, La Jolla, Calif.). Primers 208-F (5′-GGTTGATATCTTGTAACACCTCAACCG-3′) and 208-R (5′-C GGTTGAGGTGTTACAAGATATCAACC-3′) introduced a silent EcoRV restriction site and an asparagine residue in place of aspartic acid at position 208. 5′ and 3′ PCR fragments were created and used as the template to create full-length mutant Env clones by splice overlap PCR. The final product was subcloned back into pCR3.1 using HindIII and EcoRI restriction sites. Successful mutagenesis was demonstrated by EcoRV digestion. The amino acid changes were verified by DNA sequencing of the entire gp160 region.
Fusion assays.
293T cells (105) cotransfected with 15 μg of Env-expressing plasmid and 2 μg of pLTR-Tat were mixed with Cf2-Luc cells (106) that had been cotransfected with different amounts of pcDNA3-CD4 and pcDNA3-CCR5 as indicated and then incubated at 37°C in 0.75 ml of culture medium. Eight to twelve hours later, cells were harvested and assayed for luciferase activity (Promega, Madison, Wisc.) according to the manufacturer's protocol. 293T cells cotransfected with a nonfunctional Env (pSVIII-ΔKSenv) and pLTR-Tat were used as negative controls to determine the background level of luciferase activity.
Single-round infection assays.
An Env complementation assay was used to quantitate HIV-1 entry as described previously (13). Briefly, recombinant HIV-1 luciferase reporter viruses were generated by cotransfection of 293T cells with 16 μg of pNL4-3env−Luc, which contains an HIV-1 provirus with a deletion in the env gene and a replacement of the nef gene with a luciferase gene, and 6 μg of pCR3.1Env or pSVIIIEnv plasmid. The calcium phosphate method was used. Cf2th cells intended for use as target cells were cotransfected with different amounts of pcDNA3-CD4 and pcDNA3-CCR5 as indicated. Approximately 48 h after transfection, these cells were infected by incubation with 20,000 3H cpm RT units of recombinant luciferase reporter viruses. Reporter viruses pseudotyped with a nonfunctional Env (pSVIII-ΔKSenv) were used as negative controls. Sixty hours later, cells were harvested and assayed for luciferase activity.
Radiolabeling of Env glycoproteins.
293T cells were transfected with 15 μg of pCR3.1Env plasmid or cotransfected with 15 μg of pSVIIIEnv plasmid and 2 μg of pLTR-Tat using the GenePORTER 2 transfection system (Gene Therapy Systems, San Diego, Calif.). One day after transfection, the cells were labeled for 48 h with [35S]cysteine (100 μCi/ml) and [35S]methionine (100 μCi/ml). The supernatants were harvested, cleared by centrifugation at 200 × g for 5 min at 4°C, and stored at 4°C. The amount of radiolabeled gp120 glycoprotein in the supernatants was determined by immunoprecipitation with AIDS patient sera and protein A-Sepharose beads, and densitometry of autoradiographed SDS-10% PAGE gels. For CCR5 binding assays, the concentrations of radiolabeled gp120 in supernatants were normalized by dilution with culture medium.
Env-CCR5 binding assay.
Cf2th cells (2 × 106 to 4 × 106) expressing high (Cf2th-synCCR5) or low (Cf2th-CCR5) levels of CCR5 were washed with PBS, added to microcentrifuge tubes, and incubated with 500 μl of radiolabeled gp120 and 0.2% sodium azide for 2 h at 37°C with gentle agitation. In some experiments, soluble CD4 (sCD4; ImmunoDiagnostics, Inc., Woburn, Mass.) (6 μg/ml) was incubated with the gp120 solution for 1 h at room temperature prior to addition to the cells. The supernatants were then removed, and the cells were washed four times with cold DMEM medium prior to lysis in 0.5 ml of ice-cold IP buffer (50 mM Tris-HCl [pH 7.5], 150 mM NaCl, 1% [vol/vol] Triton X-100, and a cocktail of protease inhibitors) and incubation for 25 min at 4°C with gentle agitation. The lysates were cleared by centrifugation at 14,000 × g for 30 min at 4°C. The gp120 glycoproteins in the cleared lysates were then immunoprecipitated with AIDS patient sera and protein A-Sepharose beads and visualized by autoradiography of SDS-10% PAGE gels.
Virus inhibition studies.
The effects of the CCR5 inhibitors TAK-779 (7) and Sch-C (79) on virus replication in PBMC were assayed as described elsewhere (81). Briefly, PBMC were incubated for 30 min with a range of concentrations of each inhibitor (0.01 to 100 μM) prior to infection with the UK1-br and MACS2-br primary isolates. Virus replication was measured by production of HIV-1 p24 antigen in culture supernatants for 14 days (82). The production of p24 antigen in the presence of an inhibitor was expressed as a percentage of the amount produced in control cultures containing no inhibitor.
Neutralization assays.
Human monoclonal antibodies (MAb) against HIV-1 gp120 (IgG1b12 and 2G12) and gp41 (2F5), and the tetrameric CD4-immunoglobulin (CD4-IgG2) molecule have been described previously (4, 10, 11, 60, 82, 83). Neutralization of virus replication in PBMC was assessed as described previously (82). Briefly, virus was incubated for 30 min with a range of concentrations of each MAb or CD4-IgG2 (0.01 to 100 μg/ml) prior to infection with the UK1-br and MACS2-br isolates. Virus replication was measured and percent neutralization was calculated as described above.
Nucleotide sequence accession numbers.
The gp160 nucleotide sequences reported here have been assigned GenBank accession numbers AF491737 to AF491742.
RESULTS
Primary neurotropic HIV-1 isolates.
We previously described four neurotropic R5 and R5X4 viruses isolated from brain and spleen tissues of AIDS patients with dementia and HIV-1 encephalitis (32). The characteristics of the patients and virus isolates are summarized in Table 1. MACS2-br and UK1-br use only CCR5, whereas MACS1-br and MACS1-spln can use either CCR5 or CXCR4. Both R5X4 viruses were shown to enter microglia primarily via CXCR4 (32). Of eight alternative coreceptors tested (CCR2b, CCR3, CCR8, CX3CR1, Gpr1, Gpr15, Strl33, and Apj), MACS1-spln could use Apj in addition to CCR5 and CXCR4 for entry in transfected cells, but the other isolates could use none of these alternative coreceptors (32). All four viruses replicated in MDM and microglia (Table 1). Thus, all four virus isolates are M-tropic and neurotropic.
Syncytium formation in macrophages and microglia.
Multinucleated giant cells in the brain, caused by fusion of HIV-1-infected microglia and macrophages, are a neuropathologic hallmark of AIDS dementia. MDM were infected with the neurotropic HIV-1 viruses and examined for syncytia formation (Fig. 1A). The R5 ADA virus, and the primary X4 virus CB1-br, which does not replicate in MDM (32), were included as positive and negative controls, respectively. The R5X4 isolates MACS1-spln and MACS1-br induced syncytia in >90% of cells by day 7 or 10 postinfection, respectively. The R5X4 virus 89.6 and X4 virus SG3 (30), previously shown to replicate efficiently in MDM and microglia and to induce neuronal apoptosis in primary brain cultures (62, 71), also induced extensive syncytia (P. Gorry and D. Gabuzda, unpublished observations). Infection by the R5 virus UK1-br and the ADA control virus induced syncytia in >75% of MDM by day 7. In contrast, the R5 isolate MACS2-br induced syncytia in <5% of cells at day 7 despite high levels of virus replication (Table 1). Thus, high levels of fusion in MDM were induced by infection with neurotropic R5X4 isolates and a subset of neurotropic R5 isolates.
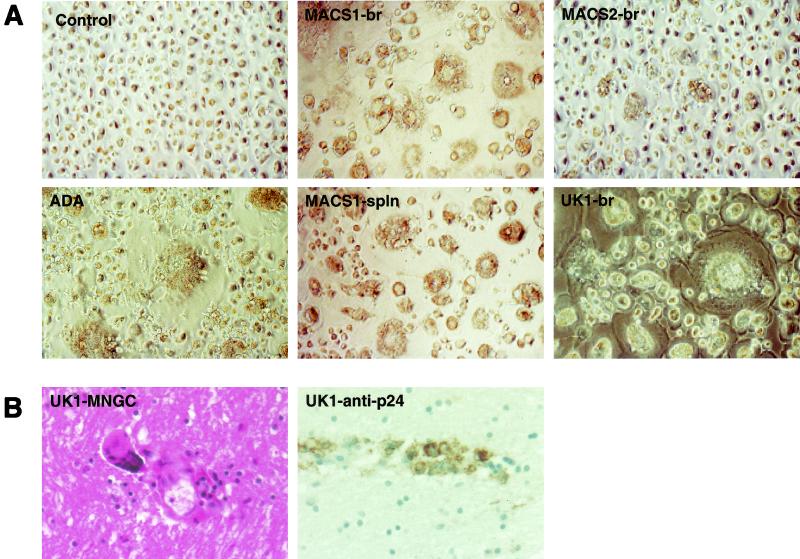
FIG. 1.
Syncytium formation in MDM induced by neurotropic HIV-1 isolates. (A) MDM were infected with equivalent amounts of HIV-1 as described in Materials and Methods. Syncytia formation was observed at day 7 (ADA, MACS1-spln, UK1-br, MACS2-br) or 10 (MACS1-br) postinfection. Control cells were exposed to the X4 primary virus isolate CB1-br, which does not replicate in MDM (32). (B) Multinucleated giant cells in brain tissue sections of patient UK1 stained with hematoxylin and eosin (left panel) and immunocytochemical detection of HIV-1 p24 antigen in brain tissue (right panel). Photographs were taken using an Eclipse TE 300 microscope (Nikon, Osaka, Japan) at a magnification of ×400.
To determine whether the ability of R5 viruses isolated from the brain to induce syncytium formation in MDM was associated with the presence of MNGC in the brain in vivo, tissue sections were examined for the presence of MNGC and HIV-1 antigens. Histopathological examination of brain tissue from patient UK1 revealed many MNGC staining positive for HIV-1 p24 antigen (Fig. 1B), whereas few MNGC were observed in sections from patient MACS2 (data not shown). Many MNGC were observed in brain tissue sections from patient MACS1 (data not shown). These results suggest that MNGC formation may result from infection of the CNS by highly fusogenic R5X4 or R5 HIV-1 variants.
Cytopathicity of HIV-1 viruses in primary brain cultures.
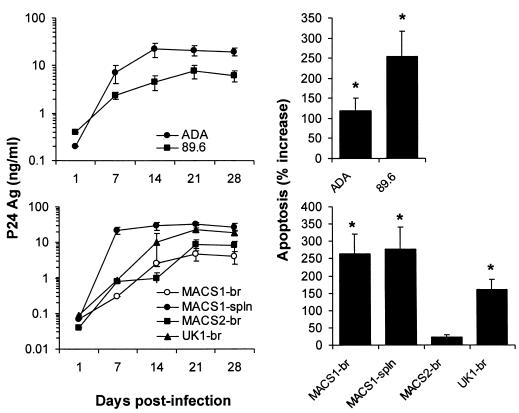
We next analyzed the relationship between fusogenicity in MDM and the ability of HIV-1 viruses to induce neuronal apoptosis in primary brain cultures. The R5 ADA and R5X4 89.6 isolates, which have been previously shown to induce neuronal apoptosis (62, 71), were included as positive controls. The R5X4 MACS1-spln and R5 MACS2-br and UK1-br viruses replicated to high levels in primary brain cultures, whereas the R5X4 MACS1-br virus did so only moderately (Fig. 2). Neuronal apoptosis was quantitated at day 28 postinfection. Apoptosis levels in cultures infected with ADA and 89.6 control viruses increased by 120 and 260 percent, respectively, compared to mock-infected control cultures (P < 0.05). Apoptosis levels in cultures infected with MACS1-br, MACS1-spln, and UK1-br viruses increased by 160 to 280 percent over levels in mock-infected control cultures (P < 0.05) but were not significantly elevated in cultures infected with MACS2-br. Thus, the ability of HIV-1 viruses to induce neuronal apoptosis in primary brain cultures was closely associated with their fusogenicity in MDM (Fig. 1A) but was independent of coreceptor specificity.
FIG. 2.
Apoptosis induced by infection of primary brain cultures with neurotropic HIV-1 isolates. Control viruses (ADA, 89.6) or primary virus isolates (MACS1-br, MACS1-spln, MACS2-br, UK1-br) were used at equivalent amounts to infect microglia in mixed brain cell cultures. Virus replication was measured weekly by HIV-1 p24 production in culture supernatants for 28 days postinfection. Apoptosis of neurons was measured at day 28 as described in Materials and Methods. Data are expressed as the percent change in comparison to control cells treated with culture medium alone, with the control value set at 0%. Data shown are means of duplicate infections, with error bars representing standard deviations. Results are representative of three independent experiments with cells obtained from different donors. Asterisk, P < 0.05 for the difference in apoptosis levels in a comparison with mock-infected cultures by Student's t test.
Characterization of full-length gp160 Env clones.
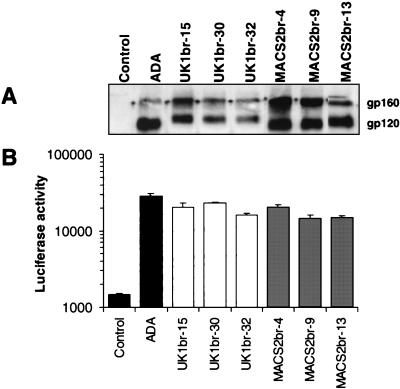
Although the UK1-br and MACS2-br R5 viruses are both neurotropic, neuronal apoptosis was induced only by UK1-br. To identify the viral determinants which underlie differences in cytopathicity between the R5 UK1-br and R5 MACS2-br viruses, the entire gp160 coding region of HIV-1 Env was cloned into the pCR3.1 expression vector. The biological activity of Envs predicted to contain uninterrupted gp160 coding regions was determined by analysis of gp160/gp120 expression in 293T cells transfected with each Env plasmid. Env clones expressing distinct gp160 and gp120 proteins were detected in 3 of 12 clones from MACS2-br and 3 of 9 clones from UK1-br (Fig. 3A). The remaining clones expressed gp160 only, or no detectable Env protein (data not shown). Env function was initially examined using syncytia assays (Fig. 3B). Cf2-Luc target cells were cotransfected with CD4 and CCR5 and then mixed with 293T effector cells expressing Env and Tat. Effector cells expressing the ADA Env or a nonfunctional Env (ΔKS) were used as positive and negative controls, respectively. Three Env clones from each virus mediated fusion with target cells expressing CD4 and CCR5. Thus, three correctly processed and functional Env clones were obtained from each virus.
FIG. 3.
Expression and cell-to-cell fusion activity of full-length Env clones. Gp160 Env genes of virus isolates UK1-br and MACS2-br were cloned into the pCR3.1 expression vector (Invitrogen) from genomic DNA of PBMC infected with each isolate, as described in Materials and Methods. 293T cells were cotransfected with 10 μg (each) of Env plasmid plus 2 μg of pLTR-Tat. (A) Env expression at 72 h posttransfection was measured by Western blot analysis of cell lysates using rabbit anti-gp120 polyclonal antisera. Positions of gp160 and gp120 are indicated on the right. (B) Fusion assays were performed using 293T cells expressing each Env and Cf2-Luc cells expressing CD4 and CCR5 as described in Materials and Methods. Data are expressed as means from duplicate experiments. Error bars represent standard deviations.
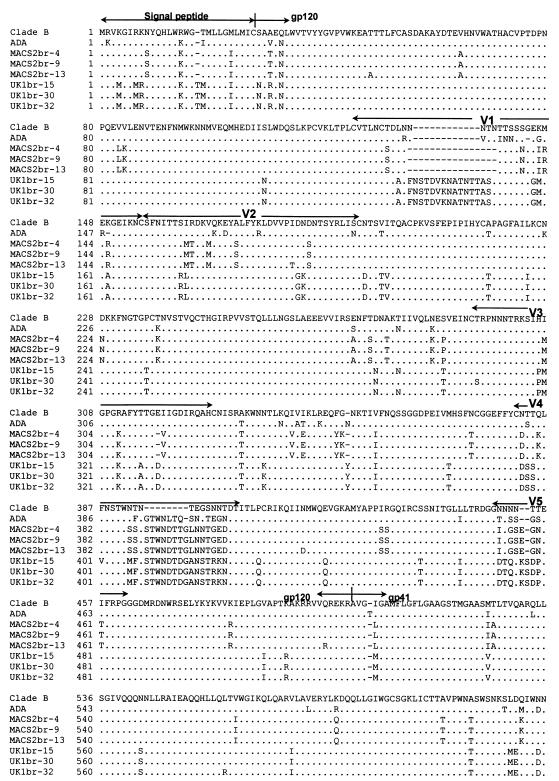
We sequenced the entire gp160-coding region of the six functional Env clones (Fig. 4 ). The gp160 amino acid sequences were uninterrupted in the MACS2br-13 and UK1br-15 and -30 Env clones, but the cytoplasmic regions of gp41 from the MACS2br-9 and -4 and the UK1br-32 Env clones were prematurely truncated. The net charges of the V3 variable loops of MACS2-br and UK1-br Env clones were +4 and +3, respectively. All three Env clones from UK1-br lacked a potential N-linked glycosylation site at asparagine 208 in the V1/V2 stem region, which corresponds to position 197 in HXB2, consistent with our previous sequence analysis of the RT-PCR-amplified HIV-1 V1V2 region (32). The elimination of a glycosylation site at this position is sufficient for CD4-independent infection by HIV-1 ADA, achieved by altering the position of the V1/V2 variable loops and exposing the CCR5-binding site in gp120 (42, 43, 91, 92). The UK1-br Env clones acquired three additional potential glycosylation sites in a 12-amino-acid extension in V1. The net gain of two glycosylation sites is consistent with the altered mobility of gp120 in polyacrylamide gels (Fig. 3A).
FIG. 4.
Env amino acid sequences. Full-length HIV-1 gp160 amino acid sequences were obtained from Env genes cloned into pCR3.1 as described in Materials and Methods. Amino acid alignments are compared to Env from HIV-1 ADA and the clade B consensus sequence. Dots indicate residues identical to the clade B consensus, and dashes indicate gaps. Note the asparagine-to-aspartic acid substitution at position 208 and the 12-amino-acid insertion in V1 for UK1-br Env clones.
Dependence of cytopathic and noncytopathic neurotropic R5 variants on CD4 and CCR5.
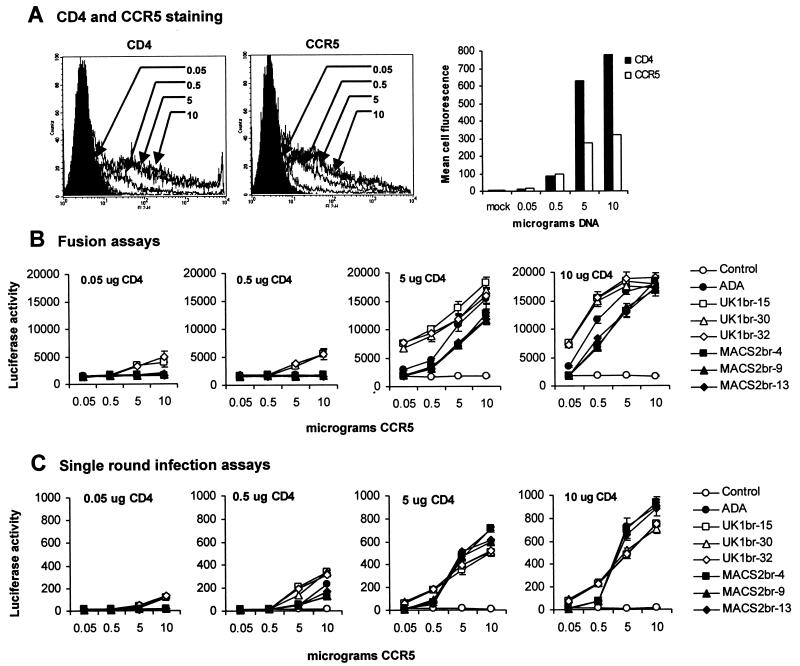
We previously showed that the UK1-br primary isolate has a reduced dependence on CD4 and CCR5 levels for entry into transfected cells (32). To investigate what viral determinants underlie CD4 and CCR5 dependence, fusion and infection assays were performed with three UK1-br Env clones and three MACS2-br Env clones. 293T effector cells expressing each Env were fused with Cf2-luc target cells transfected with different amounts of CD4- and CCR5-expressing plasmids ranging from 0.05 to 10 μg (Fig. 5). Effector cells expressing the ADA Env and a nonfunctional Env (ΔKS) were used as positive and negative controls, respectively. A close relationship was found between the levels of CD4 and CCR5 expressed on the cell surface and the amount of CD4- and CCR5-expressing plasmid used for transfection, respectively (Fig. 5A). Western blot analysis of Env in cell lysates of 293T effector cells confirmed that similar levels of gp160 and gp120 were expressed from each clone (Fig. 3A).
FIG. 5.
Effect of CD4 and CCR5 levels on cell-to-cell fusion and HIV-1 entry. Cf2th cells were transfected with 0.05, 0.5, 5, or 10 μg of CD4- and CCR5-expressing plasmid and analyzed for surface expression of CD4 or CCR5 by flow cytometry (A). Similar results were obtained with transfected Cf2-Luc cells (data not shown). Cf2-Luc (B) or Cf2th (C) cells were cotransfected with 0.05, 0.5, 5, or 10 μg of CD4-expressing plasmid and 0.05, 0.5, 5, or 10 μg of CCR5-expressing plasmid to generate 16 populations of cells expressing different combinations of each receptor. The total amount of DNA in each transfection was adjusted to 20 μg with pcDNA3. 293T cells cotransfected with 10 μg of Env plasmid and 2 μg of pLTR-Tat were used in fusion assays with transfected Cf2-Luc cells as described in Materials and Methods (B). Luciferase reporter viruses pseudotyped with each Env were used to infect transfected Cf2th cells as described in Materials and Methods (C). Data are expressed as means from duplicate experiments. Error bars represent standard deviations. Similar results were obtained in two independent experiments.
At low levels of CD4 expression (0.05 or 0.5 μg of CD4-expressing plasmid), effector cells expressing UK1br-15 and -32 Envs could mediate fusion with target cells expressing high levels of CCR5 (5 or 10 μg of CCR5-expressing plasmid) (Fig. 5B). In contrast, effector cells expressing the MACS2-br Envs and the control ADA Env could not mediate fusion with target cells expressing low levels of CD4. At high levels of CD4 expression (5 or 10 μg of CD4-expressing plasmid), the levels of fusion within effector cell populations expressing UK1-br or MACS2-br Envs were similar. However, effector cells expressing UK1-br Envs could mediate higher levels of fusion than cells expressing MACS2-br Envs or the control ADA Env and could mediate fusion with target cells expressing very low levels of CCR5 (0.05 μg of CCR5-expressing plasmid). At high levels of CD4 expression, effector cells expressing ADA Env displayed an intermediate phenotype, mediating levels of fusion higher than those of cells expressing MACS2-br Envs but lower than those of cells expressing UK1-br Envs. At high levels of CD4 and CCR5 expression (10 μg of each expressing plasmid), similar levels of fusion between effector cells expressing different Envs were observed. These results demonstrate that the UK1br-15 and -32 Envs have a reduced dependence on CD4 levels for cell-to-cell fusion and that all three UK1-br Envs have a reduced dependence on CCR5 levels.
We next performed single-round entry assays with luciferase-encoding viruses pseudotyped with each Env, using Cf2th cells expressing different amounts of CD4 and CCR5 (Fig. 5C). At low levels of CD4 expression (0.05 or 0.5 μg of CD4-expressing plasmid), viruses pseudotyped with UK1-br Envs could enter cells more efficiently than viruses pseudotyped with ADA or MACS2-br Envs when the cells expressed high levels of CCR5 (5 or 10 μg of CCR5-expressing plasmid). At high levels of CD4 expression (5 or 10 μg of CD4-expressing plasmid), viruses pseudotyped with UK1-br Envs could enter cells expressing low levels of CCR5 (0.05 and 0.5 μg of CCR5-expressing plasmid) whereas viruses pseudotyped with ADA and MACS2-br Envs could not. However, at high levels of CD4 and CCR5 expression (5 or 10 μg of CD4- and/or CCR5-expressing plasmid), viruses pseudotyped with MACS2-br and ADA Envs could enter cells more efficiently than viruses pseudotyped with UK1-br Envs. These results demonstrate that viruses pseudotyped with UK1-br Envs have a reduced dependence on CD4 and CCR5 levels for entry. However, viruses pseudotyped with MACS2-br and ADA entered cells expressing high levels of both receptors more efficiently.
Env-CCR5 binding assays.
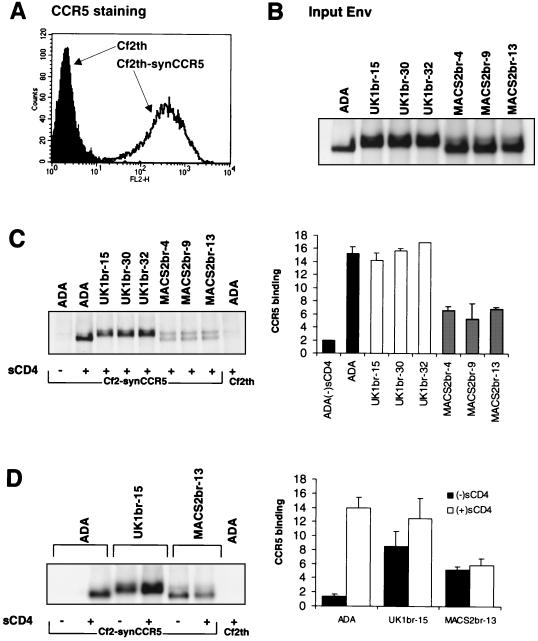
To determine whether the reduced dependence on CCR5 levels by UK1-br Envs was attributable to an increase in their affinity for CCR5, binding assays were performed using radiolabeled Env glycoproteins (Fig. 6). In these assays Cf2th-synCCR5 cells, which express high levels of CCR5 on their surface (Fig. 6A), were used as target cells. Equivalent amounts of radiolabeled Env were used in the assays (Fig. 6B). The binding of ADA Env to Cf2th-synCCR5 cells in the absence of soluble CD4 (sCD4) or to the parental Cf2th cells in the presence of sCD4 served as negative controls. In the presence of sCD4, approximately two- to threefold-higher levels of ADA Env and UK1br-15, -30, and -32 Env glycoproteins bound to Cf2th-synCCR5 cells compared to the binding of MACS2br-4, -9, and -13 Envs (Fig. 6C). This suggests that Env glycoproteins from ADA and UK1br-15, -30, and −32 have greater affinities for CCR5 than Env glycoproteins from MACS2br-4, -9, and -13. To determine whether the reduced dependence on CD4 levels for cell-to-cell fusion and entry by UK1-br Envs was due to CD4-independent binding of CCR5, binding assays using ADA, UK1br-15, and MACS2br-13 Env glycoproteins were performed in the presence or absence of sCD4 (Fig. 6D). Background levels of ADA Env bound to Cf2th-synCCR5 cells in the absence of sCD4, consistent with previous studies (42, 43). Surprisingly, relatively high levels of both UK1br-15 and MACS2br-13 Env glycoproteins bound to CCR5 in the absence of sCD4, suggesting that Envs from both brain-derived viruses were capable of CD4-independent binding to CCR5.
FIG. 6.
CCR5 binding assays. Cf2th-synCCR5 cells were analyzed for surface expression of CCR5 by flow cytometry (A). Equivalent amounts of radiolabeled soluble Env proteins (B) were used in binding assays with Cf2-synCCR5 cells in the presence of sCD4 (C) or in the presence and absence of sCD4 (D). Cells were washed and lysed, and the bound Env glycoproteins were immunoprecipitated using AIDS patient serum and protein A Sepharose beads. Proteins were analyzed using SDS-PAGE (C and D, left) and then quantitated by densitometry (C and D, right). The gels shown in panels C and D are representative of those from two independent experiments. The quantitation of CCR5 binding shown in panels C and D represent means and standard deviations of values obtained in two independent experiments, normalized against background levels of ADA Env bound to the parental Cf2th cell line.
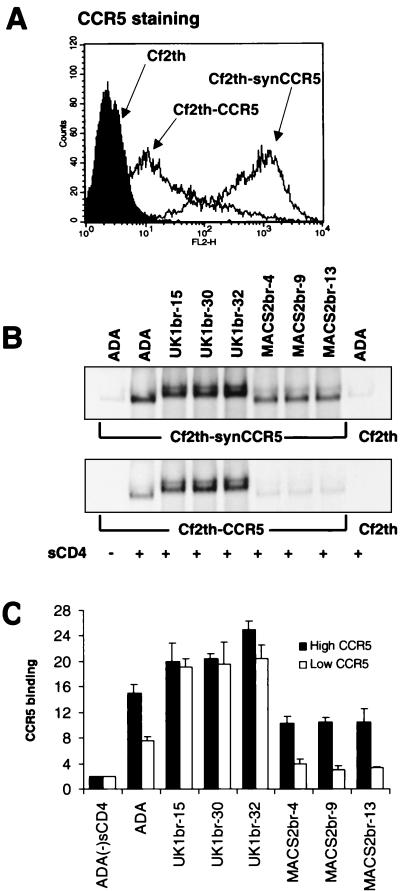
To further investigate differences in CCR5 affinity between UK1-br and MACS2-br Env glycoproteins, we next performed CCR5 binding assays with cells expressing different amounts of CCR5 (Fig. 7). The level of CCR5 expression on Cf2th-CCR5 cells was approximately 2 logs lower than on Cf2th-synCCR5 cells (Fig. 7A). The level of Env glycoproteins bound to CCR5 expressed on Cf2th-synCCR5 cells was similar to that shown in Fig. 6C, in that there was an approximately twofold increase in CCR5 binding by ADA and UK1-br Envs compared to MACS2-br Envs (Fig. 7B and C). However, the level of UK1-br Env glycoproteins bound to CCR5 expressed on Cf2th-CCR5 cells was approximately twofold higher than the binding of ADA Env and approximately fourfold higher than MACS2-br Envs. Together, these results demonstrate that there are high-affinity interactions between CCR5 and UK1-br Envs and show that there is a relationship between the extent of CCR5-mediated Env fusion and the affinity of Env for CCR5. The results also suggest that CD4-independent binding of CCR5 alone is not sufficient to mediate cell-to-cell fusion or infection in cells expressing low levels of CD4.
FIG. 7.
CCR5 binding assays with cells expressing different amounts of CCR5. Cf2th-synCCR5 and Cf2th-CCR5 cells were analyzed for surface expression of CCR5 by flow cytometry (A). Equivalent amounts of radiolabeled soluble Env proteins (see Fig. 6A) were used in binding assays with Cf2th-synCCR5 or Cf2th-CCR5 cells in the presence of sCD4. Cells were washed and lysed, and the bound Env glycoproteins were immunoprecipitated using AIDS patient serum and protein A Sepharose beads. Proteins were analyzed using SDS-PAGE (B) and then quantitated by densitometry (C). The gels shown in panel B are representative of those from two independent experiments. The quantitation of CCR5 binding shown in panel C represents means and standard deviations of values obtained in two independent experiments, normalized against background levels of ADA Env bound to the parental Cf2th cell line.
Effect of N-linked glycosylation in the V1V2 stem region of Env on CCR5 binding and CD4 dependence.
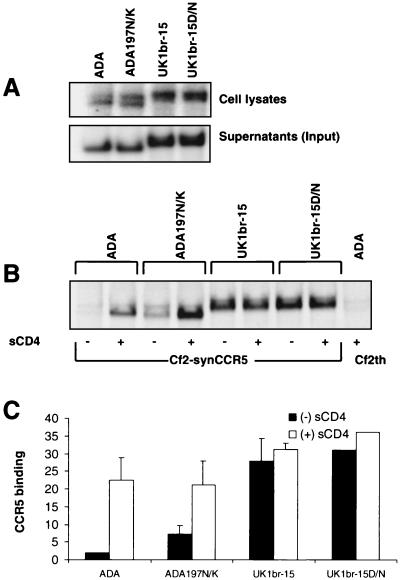
The loss of a single potential N-linked glycosylation site at position 197 in the V1V2 stem region of HIV-1 ADA is sufficient to allow CD4-independent infection (42, 43). Since all the UK1-br Env clones have a naturally occurring aspartic acid substitution at this site (Fig. 4), we investigated whether this change contributed to increased CCR5 affinity and/or CD4-independent CCR5 binding (Fig. 8). Site-directed mutagenesis was used to create an aspartic acid-to-asparagine substitution at position 208 (corresponding to position 197 in HIV-1 HXB2) in the UK1br-15 Env clone, thus restoring the potential glycosylation site (UK1br-15D/N). As an additional control, a mutant ADA Env clone containing an asparagine-to-lysine substitution at position 197 was included (ADA 197N/K). The levels of Env present in cell lysates and supernatants were similar in cells expressing the parental clones and those expressing the mutant ADA and UK1br-15 Env clones (Fig. 8A).
FIG. 8.
Effect of N-linked glycosylation on CCR5 binding and CD4 dependence. Env glycoproteins were immunoprecipitated from cell lysates or supernatants of radiolabeled 293T cells transfected with wild-type ADA or UK1-br Env plasmids or with mutants containing a loss of a potential glycosylation site at position 197 (ADA197N/K) or a restored glycosylation site at position 208 (UK1br-15D/N) (A). Equivalent amounts of radiolabeled soluble Env proteins were used in binding assays with Cf2th-synCCR5 cells in the presence or absence of sCD4. Cells were washed and lysed, and the bound Env glycoproteins were immunoprecipitated using AIDS patient serum and protein A Sepharose beads. Proteins were analyzed using SDS-PAGE (B) and then quantitated by densitometry (C). The gel shown in panel B is representative of two independent experiments. The quantitation of CCR5 binding shown in panel C represents means and standard deviations of values obtained in two independent experiments, normalized against background levels of ADA Env bound to the parental Cf2th cell line.
CCR5 binding assays using Cf2th-synCCR5 cells were performed in the presence and absence of sCD4 (Fig. 8B and C). Elimination of the N-linked glycosylation site at asparagine 197 of ADA resulted in an approximately twofold increase in CD4-independent CCR5 binding, consistent with previous studies (42, 43). However, restoration of an N-linked glycosylation site at position 208 in UK1br-15 Env had no effect on the levels of CCR5 binding in the presence or absence of sCD4 (Fig. 8B and C). These results suggest that effects of N-linked glycosylation in the V1V2 stem region of HIV-1 Env on CD4-independent CCR5 binding are strain dependent and do not apply to the UK1br-15 clone.
Sensitivity of UK1-br and MACS2-br virus isolates to CCR5 inhibitors.
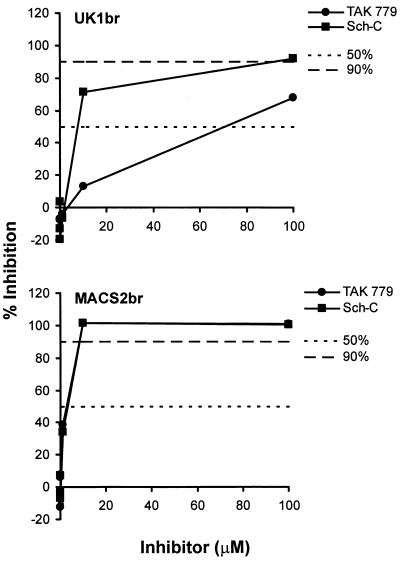
To determine whether the higher CCR5 affinity of the UK1-br primary isolate makes it relatively resistant to inhibitors of entry via CCR5, we performed infection assays in the presence of the small molecule CCR5 antagonists TAK-779 and Sch-C. PBMC were infected with UK1-br and MACS2-br primary isolates in the presence of increasing concentrations of the inhibitors, ranging from 0.01 to 100 μM (Fig. 9). At day 14 postinfection, there was less inhibition of replication by both compounds in cultures infected with UK1-br, compared to cultures infected with MACS2-br. Similar results were obtained at days 7 and 10 postinfection (data not shown). The 50% inhibitory concentrations (IC50s) and IC90s for TAK-779 were approximately 17- and 5-fold higher, respectively, for cultures infected with UK1-br compared to MACS2-br (Table 2). Similarly, IC50s and IC90s for Sch-C were approximately 2- and 4.5-fold higher, respectively, for cultures infected with UK1-br. Of note is that although MACS2-br was approximately equally sensitive to TAK-779 and Sch-C, UK1br was about 10-fold less sensitive to TAK-779 than to Sch-C (Fig. 9). The reason for this difference is as yet unclear, but UK1-br is clearly the more resistant of the two viruses to CCR5 inhibitors. This and the preceding experiments together imply that an increased affinity of Env for CCR5 can reduce the sensitivity of HIV-1 to CCR5 antagonists. This is consistent with the observations that HIV-1 escape from a small molecule inhibitor similar to Sch-C involves, at least in part, an increase in the affinity of the virus for CCR5 (80).
FIG. 9.
Sensitivity of UK1-br and MACS2-br viruses to CCR5 inhibitors. PBMC were treated with the CCR5 antagonists TAK-779 and Sch-C at concentrations increasing by 10-fold from 0.01 to 100 μM and then infected with the UK1-br and MACS2-br primary isolates. Virus production postinfection was assessed at day 14 by measuring the amount of soluble HIV-1 p24 antigen in the culture supernatant (82). The production of p24 antigen in inhibitor-treated cultures was calculated as a percentage of that produced in the absence of an inhibitor (defined as 100%). The mean values obtained from an assay performed in duplicate are shown and are representative of those from two independent experiments. Similar results were obtained at days 7 and 10 postinfection (data not shown).
TABLE 2.
IC50s and IC90s of CCR5 inhibitors, Env MAbs, and CD4-IgG2a
| Isolate | TAK-779
|
Sch-C
|
2F5
|
2G12
|
IgG1b12
|
CD4-IgG2
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | IC50 | IC90 | |
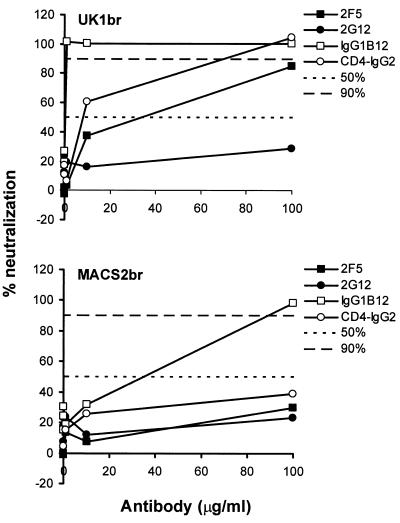
| UK1-br | 70 | >100 | 8 | 90 | 33 | >100 | R | R | <1 | <1 | 8 | 70 |
| MACS2-br | 4 | 20 | 4 | 20 | R | R | R | R | 33 | 89 | R | R |
Sensitivity of UK1-br and MACS2-br virus isolates to antibody neutralization.
We next measured the sensitivity of UK1-br and MACS2-br isolates to neutralization by MAbs and CD4-IgG2. The neutralizing reagents were the human MAbs 2F5 (60, 82), 2G12 (82, 83), IgG1b12 (10, 11), and the CD4-IgG2 molecule (4). PBMC were infected with UK1-br and MACS2-br primary isolates in the presence of increasing concentrations of these reagents ranging from 0.01 to 100 μg/ml (Fig. 10). At day 14 postinfection, greater neutralization by IgG1b12 was observed in cultures infected with UK1-br than with cultures infected with MACS2-br. UK1-br was sensitive to neutralization by 2F5 and CD4-IgG2, whereas MACS2-br was resistant to neutralization by both agents. Both UK1-br and MACS2-br isolates resisted neutralization by 2G12. Similar results were obtained at days 7 and 10 postinfection (data not shown). UK1-br was particularly sensitive to IgG1b12, with both IC50s and IC90s <1 μg/ml compared to values of 33 and 89 μg/ml, respectively, for MACS2-br (Table 2). One interpretation of these results is that structural features of the UK1-br Env that are associated with an increased affinity for CCR5 may render this virus unusually sensitive to neutralization by gp120 and gp41 MAbs and CD4-IgG2.
FIG. 10.
Sensitivity of UK1-br and MACS2-br viruses to neutralization. Each virus was incubated for 30 min with MAbs or CD4-IgG2 at concentrations increasing by 10-fold from 0.01 to 100 μg/ml. The mixtures were then added to PBMC. Virus production in culture supernatants at day 14 postinfection was measured by quantitation of soluble HIV-1 p24 antigen (82). Virus replication and its inhibition were measured and calculated as described in the legend to Fig. 9. The mean values obtained from an assay performed in duplicate are shown.
DISCUSSION
In this study, we used primary R5 and R5X4 HIV-1 viruses isolated from brain tissue from AIDS patients with dementia and HIV-1 encephalitis to investigate phenotypic characteristics that were associated with neurovirulence. We describe a neurovirulent primary R5 virus (UK1-br) that has increased CCR5 affinity and reduced dependence on CCR5 and CD4. The UK1-br R5 virus was highly fusogenic in MDM and induced neuronal apoptosis in primary brain cultures. In contrast, the MACS2-br R5 virus was neurotropic but did not exhibit these cytopathic effects. UK1-br Env clones had an increased affinity for CCR5 but a reduced dependence on CCR5 and CD4 levels, compared to MACS2-br Env clones, in cell-to-cell fusion and infection assays. Together, these results suggest that a subset of neurotropic R5 viruses that are highly fusogenic in MDM, and that have increased affinity for CCR5, may contribute to neurodegenerative manifestations of AIDS. Both R5X4 viruses analyzed in this study (MACS1-br and MACS1-spln) induced high levels of fusion in MDM and neuronal apoptosis. Thus, our studies further suggest that neurotropic R5X4 viruses with highly fusogenic envelope glycoproteins represent another pathogenic viral phenotype in the CNS.
UK1-br Env glycoproteins could mediate cell-to-cell fusion with target cells expressing low levels of CD4 or CCR5, suggesting that high-affinity Env-receptor interactions may underlie the increased fusogenicity of these Envs. These findings, taken together with the high frequency of MNGC in the brain of patient UK1, suggest that R5 viruses with highly fusogenic Env glycoproteins may be cytopathic in the CNS. Since neurons do not express CCR5, neurotoxicity induced by R5 viruses is likely to be indirect, possibly via the production of soluble neurotoxic factors by infected or activated microglia. One possible mechanism of neurotoxicity induced by R5 viruses may be an increased release of neurotoxic factors from macrophages and microglia undergoing cell-to-cell fusion. Another may be increased production of CC-chemokines (14) by microglia following activation by soluble or virion-associated R5 Envs, a phenomenon that appears to be strain specific. Future studies will investigate a larger series of neurotropic R5 viruses to further elucidate the relationship between fusogenicity in macrophages and microglia, affinity for CCR5 and CD4, and HIV-1 cytopathicity in the CNS.
Neuronal apoptosis was induced not only by the R5 virus UK1-br but also by the R5X4 viruses MACS1-br and MACS1-spln. These R5X4 viruses primarily use CXCR4 for entry into microglia (32). Apoptosis levels were higher in brain cultures infected with MACS1-br and MACS1-spln viruses than with the R5 viruses UK1-br and ADA, suggesting increased neurotoxicity or different modes of action by R5X4 viruses compared to R5 viruses. These findings are consistent with our previous studies, which showed that X4 and R5X4 variants induce neuronal apoptosis more frequently than R5 variants (62). Neuropathological examination of brain tissue sections of patient MACS1 revealed many MNGC. Moreover, the MACS1-br and MACS1-spln viruses were highly fusogenic in MDM cultures. These findings, together with those of previous studies (50, 62), suggest that a subset of R5X4 and X4 viruses which replicate efficiently in MDM and microglia are present in the brains of AIDS patients with dementia; they may represent a pathogenic viral phenotype in the CNS. Although neurons do not express CD4 and are not infected by HIV-1, subpopulations express cell surface CXCR4 (24, 25). Soluble or virion-associated gp120 from R5X4 or X4 viruses can bind to CXCR4 expressed on neurons in the absence of CD4 and thereby induce signaling and apoptosis (41, 54, 97, 98; reviewed in references 25 and 40). Thus, direct interactions between R5X4 or X4 Env glycoproteins and CXCR4 expressed on neurons may induce neuronal apoptosis or dysfunction.
Env glycoproteins from both UK1-br and MACS2-br R5 viruses could bind to CCR5 in the absence of CD4. However, these Envs could not mediate CD4-independent infection. Furthermore, this property was not sufficient to mediate fusion or infection in cells expressing low levels of CD4 or CCR5, since relatively high levels of both receptors were required for infection and cell-to-cell fusion by MACS2-br Envs. It is possible that Env glycoprotein trimers could exhibit differences in CCR5 binding assays compared to monomeric gp120. Premature truncations of 60 (MACS2br-9 and UK1br-32) or 10 amino acids (MACS2br-4) in the cytoplasmic region of gp41 did not appear to have adverse effects on fusion or entry mediated by these Envs. Whether Envs with truncated cytoplasmic tails are more frequent in brain tissue than in other tissues remains to be determined. Surprisingly, MACS2-br Envs could mediate higher levels of entry in single-round infection assays than UK1-br Envs at high levels of expression of both CD4 and CCR5. The explanation for this observation is unclear. One possibility is that the UK1-br Env may exist in a “pretriggered” conformation that may be more sensitive to high concentrations of CD4 than the MACS2-br Env.
Our results predict that adaptive evolution of HIV-1 in the brain may select for viral variants that can infect cells in the CNS in a CD4-dependent fashion at relatively low levels of surface CD4 expression. The expression of CD4 and CCR5 on microglia is lower than on monocytes and CD4+ T-cells in the peripheral blood (47, 63, 87). Moreover, previous studies demonstrated that limiting levels of CD4 expression on rhesus macrophages restrict infection by M-tropic HIV-1 or T-tropic SIV strains (8, 59). By analogy, low levels of CD4 or CCR5 expressed on human microglia may restrict infection by certain HIV-1 strains (65). Consistent with this model, a highly fusogenic R5 isolate with reduced dependence on CD4 levels for virus entry could replicate to high levels in microglia (52, 73). This virus was derived by adaptation of a blood-derived isolate by serial passage in microglia. Our findings imply that CD4-independent binding of CCR5 may be associated with a subset of Envs from brain-derived viruses. This property does not necessarily permit CD4-independent infection, but it may facilitate infection of cells that express relatively low levels of CD4. Analysis of larger numbers of Envs in different regions of the brain (77, 88) will be required to determine the frequency of viral variants in the CNS that can bind to CCR5 independent of CD4 and whether such variants are more frequent in the CNS than in other tissue compartments. Reduced dependence on CD4 and/or CCR5 levels may be a means by which R5 variants acquire neurovirulence. For example, a neurovirulent strain of SIV uses CCR5 for CD4-independent infection of brain capillary endothelial cells (21).
A naturally occurring, potential N-linked glycosylation site at position 208 in the V1V2 stem region has been lost from all the UK1-br Env clones (Fig. 4), a feature observed in only 7 of 220 clade B HIV-1 Env sequences (Table 3). Elimination of a glycosylation site at this position in HIV-1 ADA is sufficient to allow CD4-independent infection (43). However, restoration of a potential N-linked glycosylation site at this position in the UK1br-15 Env clone did not eliminate CD4-independent CCR5 binding, suggesting additional determinants for CD4-independent binding. Interestingly, both UK1-br and MACS2-br Env clones contain loss of a highly conserved N-linked glycosylation site at the base of the V4 loop (position 386 in HXB2) that is partially responsible for the CD4 independence of the 8X variant of HIV-1 IIIB (22). Thus, effects of N-linked glycosylation in the V1V2 stem region on CD4-independent CCR5 binding are highly strain specific.
TABLE 3.
Env sequences lacking a potential glycosylation site at position 197a
| Env strain or clone | Sequence | GenBank accession no. | Reference |
|---|---|---|---|
| Clade B consensus | SYRLISCNTS | ||
| UK1br-15 | SYRLISCDTS | AF491740 | |
| UK1br-30 | SYRLISCDTS | AF491741 | |
| UK1br-32 | SYRLISCDTS | AF491742 | |
| Clone CA5 | SYRLINCDTS | AJ277821 | Unpublished |
| GB8, clone 46R | SYILTKCDAS | AJ271445 | Farrar, 1991 (23) |
| Strain KB-1gp32 | CYRLISCDTS | D12582 | Shimizu, 1992 (74) |
| Clone 320.2a.6 | SYRLIHCDSS | L08661 | Andeweg, 1992 (6) |
| Isolate NY5 | SYTLINCDTS | M38431 | Willey, 1986 (89) |
| Clone WEAU 1.60 | SYTLINCKSS | U21135 | Unpublished |
| JRFL | SYRLISCDTS | U63632 | O'Brien, 1990 (61) |
Seven of 208 Clade B HIV-1 Env sequences screened from the Los Alamos National Laboratory HIV Database contained a loss of a potential N-linked glycosylation site at position 197 (relative to HXB2) in the V1V2 stem region. Boldface indicates amino acid substitutions at that site.
The UK1-br virus was less sensitive than MACS2-br to inhibition by the CCR5-targeted small molecule inhibitors TAK-779 and Sch-C. HIV-1 escape from AD101, a CCR5 antagonist in the same family as Sch-C, occurred in PBMC despite the persistent and exclusive use of CCR5 as a coreceptor. The selection pressure of AD101 did not cause the test virus to acquire the ability to use CXCR4; instead, the initial stage of the escape pathway involved a reduction in the level of CCR5 required for virus entry (80). Together, these two sets of findings suggest that high-affinity Env-CCR5 interactions may increase the resistance of HIV-1 to CCR5-specific entry inhibitors. Whether this will impede effective therapy by CCR5 antagonists remains to be determined by clinical studies (55, 58, 79, 80).
UK1-br was more sensitive than MACS2-br to neutralization by MAbs 2F5 and IgG1b12 and the tetrameric CD4-IgG2 molecule. These results are strikingly similar to those of previous studies demonstrating that a CD4-independent variant of HIV-1 ADA (ADA197N/K) was unusually sensitive to neutralization by 2F5, IgG1b12, and sCD4 but not by the anti-CCR5 MAb 2D7 (42). In the latter study, the increased neutralization sensitivity to MAbs and sCD4 was thought to result from an increase in the exposure of the CCR5 binding domain and its associated antibody epitopes. While N-linked glycosylation in the V1V2 stem region does not appear to be responsible for CD4-independent CCR5 binding of UK1-br Envs, our results suggest that some structural features of UK1-br Env may resemble those in HIV-1 ADA197N/K. HIV-1 variants that have increased sensitivity to antibody neutralization may be able to replicate and persist more efficiently in the CNS compared than in other tissue compartments because neutralizing antibody concentrations are even lower in the CNS than in the peripheral blood. The absence of any selection pressure from antibodies may allow the evolution of higher-affinity CCR5 binding sites that are also more vulnerable to the binding of neutralizing antibodies. This would be consistent with a long-standing concept that the protection of Env from neutralizing antibodies can carry the price of a less efficient interaction with its entry receptors (57).
Our studies predict that HIV-1 variants in the CNS with increased CCR5 affinity and reduced dependence on CCR5/CD4 may be present in some patients with CNS disease. These viruses would be the consequence of adaptive evolution for infection of target cells that express very low levels of CD4. Our studies further suggest that neurotropic R5X4 viruses and a subset of neurotropic R5 viruses with increased CCR5 affinity may contribute to neurodegenerative mechanisms in the CNS. Understanding the role of HIV-1 strain variability and molecular determinants of the HIV-1 envelope glycoprotein in mechanisms leading to CNS injury may advance the development of therapeutics to inhibit CNS infection and prevent neurologic injury in AIDS patients.
Acknowledgments
We thank G. Babcock for assistance with CCR5 binding assays and J. Sodroski and J. Wang for helpful discussions. We are also grateful to J. Sodroski for providing Cf2th, Cf2-Luc, and Cf2th-synCCR5 cell lines; J. Sodroski, R. Doms, and S. Peiper for providing coreceptor plasmids; and the NIH AIDS Research and Reference Reagent Program for providing the ADA and 89.6 isolates and TAK-779. J.P.M. thanks Bahige Baroudy and Stuart McCombie of Schering Plough Research Institute for providing Sch-351125 (Sch-C).
This work was supported by NIH grants NS37277 and NS35734 to D.G. and AI41420 to J.P.M. J.E.B. was supported by NIH DA13127 and UK MCRC G9708080. We also acknowledge support from the Multicenter AIDS Cohort Study (U01 AI35039), an NIDA supplement, and the G. Harold and Leila Mathers Charitable Foundation. Core facilities were supported by Center for AIDS Research grants (AI28691 and CA79458) and the DFCI/Harvard Center for Cancer Research grants. The Edinburgh Brain Bank is supported by UK MCR SPG9708080. A.M. was supported in part by an NSF predoctoral fellowship. D.G. and J.P.M. are Elizabeth Glaser Scientists supported by the Pediatric AIDS Foundation.
REFERENCES
- 1.Adie-Biassette, H., Y. Levy, M. Colombel, F. Poron, S. Natchev, C. Keohane, and F. Gray. 1995. Neuronal apoptosis in HIV infection in adults. Neuropathol. Appl. Neurobiol. 21:218-227. [DOI] [PubMed] [Google Scholar]
- 2.Albright, A. V., J. T. C. Shieh, T. Itoh, B. Lee, D. Pleasure, M. J. O'Connor, R. W. Doms, and F. Gonzalez-Scarano. 1999. Microglia express CCR5, CXCR4, and CCR3, but of these, CCR5 is the principal coreceptor for human immunodeficiency virus type 1 dementia isolates. J. Virol. 73:205-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alkhatib, G., C. Combadiere, C. C. Broder, Y. Feng, P. E. Kennedy, P. M. Murphy, and E. A. Berger. 1996. CC CKR5: A RANTES, MIP-1α, MIP-1β receptor as a fusion cofactor for macrophage-tropic HIV-1. Science 272:1955-1958. [DOI] [PubMed] [Google Scholar]
- 4.Allaway, G. P., K. L. Davis-Bruno, G. A. Beaudry, E. B. Garcia, E. L. Wong, A. M. Ryder, K. W. Hasel, M.-C. Gauduin, R. A. Koup, S. J. McDougal, and P. J. Maddon. 1995. Expression and characterization of CD4IgG2, a novel heterotetramer which neutralizes primary HIV-1 isolates. AIDS Res. Hum. Retrovir. 11:533-540. [DOI] [PubMed] [Google Scholar]
- 5.An, S. F., F. Gray, and F. Scaravalli. 1995. Programmed cell death in brains of HIV-1-positive pre-AIDS patients. Lancet 346:911-912. [DOI] [PubMed] [Google Scholar]
- 6.Andeweg, A. C., M. Groenink, P. Leeflang, R. E. Y. de Goede, A. D. M. E. Osterhaus, M. Tersmette, and M. L. Bosch. 1992. Genetic and functional analysis of a set of HIV-1 envelope genes obtained from biological clones with varying syncytium inducing capacities. AIDS Res. Hum. Retrovir. 8:1803-1813. [DOI] [PubMed] [Google Scholar]
- 7.Baba, M., O. Nishimura, N. Kanzaki, M. Okamoto, H. Sawada, Y. Iizawa, M. Shiraishi, Y. Aramaki, K. Okonogi, Y. Ogawa, K. Meguro, and M. Fujino. 1999. A small molecule, nonpeptide CCR5 antagonist with highly potent and selective anti-HIV-1 activity. Proc. Natl. Acad. Sci. USA 96:5698-5703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bannert, N., D. Schenten, S. Craig, and J. Sodroski. 2000. The level of CD4 expression limits infection of primary rhesus monkey macrophages by a T-tropic simian immunodeficiency virus and macrophagetropic human immunodeficiency viruses. J. Virol. 74:10984-10993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Björndal, Å., H. Deng, M. Jansson, J. R. Fiore, C. Colognesi, A. Karlsson, J. Albert, G. Scarlatti, D. R. Littman, and E. M. Fenyö. 1997. Coreceptor usage of primary human immunodeficiency virus type 1 isolates varies according to biological phenotype. J. Virol. 71:7478-7487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burton, D. R., C. F. Barbas, M. A. A. Persson, S. Koenig, M. Chanock, and R. A. Lerner. 1991. A large array of human monoclonal antibodies to HIV-1 from combinatorial libraries of asymptomatic seropositive individuals. Proc. Natl. Acad. Sci. USA 88:10134-10137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burton, D. R., J. Pyati, R. Koduri, S. J. Sharp, G. B. Thornton, P. W. H. I. Parren, L. S. W. Sawyer, R. M. Hendry, N. Dunlop, P. L. Nara, M. Lamacchia, E. Garratty, E. R. Stiehm, Y. H. Bryson, Y. Cao, J. P. Moore, D. D. Ho, and C. F. Barbas III. 1994. Efficient neutralization of primary isolates of HIV-1 by a recombinant human monoclonal antibody. Science 266:1024-1027. [DOI] [PubMed] [Google Scholar]
- 12.Chang, J., R. Jozwiak, B. Wang, T. Ng, Y. C. Ge, W. Bolton, D. E. Dwyer, C. Randle, R. Osborn, A. C. Cunningham, and N. D. Saksena. 1998. Unique HIV type 1 V3 region sequences derived from six different regions of brain: region-specific evolution within host-determined quasispecies. AIDS Res. Hum. Retrovir. 14:25-30. [DOI] [PubMed] [Google Scholar]
- 13.Choe, H., M. Farzan, Y. Sun, N. Sullivan, B. Rollins, P. D. Ponath, L. Wu, C. R. Mackay, G. LaRosa, W. Newman, N. Gerard, C. Gerard, and J. Sodroski. 1996. The β-chemokine receptors CCR3 and CCR5 facilitate infection by primary HIV-1 isolates. Cell 85:1135-1148. [DOI] [PubMed] [Google Scholar]
- 14.Choe, W., D. J. Volsky, and M. Potash. 2001. Induction of rapid and extensive β-chemokine synthesis in macrophages by human immunodeficiency virus type 1 and gp120, independently of their coreceptor phenotype. J. Virol. 75:10738-10745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Connor, R. I., K. E. Sheridan, D. Ceradini, S. Choe, and N. R. Landau. 1997. Change in coreceptor use correlates with disease progression in HIV-1-infected individuals. J. Exp. Med. 185:621-628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng, H. K., R. Liu, W. Ellmeier, S. Choe, D. Unutmaz, M. Burkhart, P. Di Marzio, S. Marmon, R. E. Sutton, C. M. Hill, C. B. Davis, S. C. Peiper, T. J. Schall, D. R. Littman, and N. R. Landau. 1996. Identification of a major co-receptor for primary isolates of HIV-1. Nature 381:661-666. [DOI] [PubMed] [Google Scholar]
- 17.Dimitrov, D. S., D. Norwood, T. S. Stantchev, Y. Feng, X. Xiao, and C. C. Broder. 1999. A mechanism of resistance to HIV-1 entry: inefficient interactions of CXCR4 with CD4 and gp120 in macrophages. Virology 259:1-6. [DOI] [PubMed] [Google Scholar]
- 18.Donaldson, Y. K., J. E. Bell, E. C. Holmes, E. S. Hughes, H. K. Brown, and P. Simmonds. 1994. In vivo distribution and cytopathology of variants of human immunodeficiency virus type 1 showing restricted sequence variability in the V3 loop. J. Virol. 68:5991-6005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Doranz, B. J., J. Rucker, Y. Yi, R. J. Smyth, M. Samson, S. C. Peiper, M. Parmentier, R. G. Collman, and R. W. Doms. 1996. A dual-tropic primary HIV-1 isolate that uses fusin and the β-chemokine receptors CKR-5, CKR-3 and CKR-2b as fusion cofactors. Cell 85:1149-1158. [DOI] [PubMed] [Google Scholar]
- 20.Dragic, T., V. Litwin, G. P. Allaway, S. R. Martin, Y. Huang, K. A. Nagashima, C. Cayanan, P. J. Maddon, R. A. Koup, J. P. Moore, and W. A. Paxton. 1996. HIV-1 entry into CD4+ cells is mediated by the chemokine receptor CC-CKR-5. Nature 381:667-673. [DOI] [PubMed] [Google Scholar]
- 21.Edinger, A. L., J. L. Mankowski, B. J. Doranz, B. J. Margulies, B. Lee, J. Rucker, M. Sharron, T. L. Hoffman, J. F. Berson, M. C. Zink, V. M. Hirsch, J. E. Clements, and R. W. Doms. 1997. CD4-independent, CCR5-dependent infection of brain capillary endothelial cells by a neurovirulent simian immunodeficiency virus strain. Proc. Natl. Acad. Sci. USA 94:14742-14747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edwards, T. G., T. L. Hoffman, F. Baribaud, S. Wyss, C. C. LaBranche, J. Romano, J. Adkinson, M. Sharron, J. A. Hoxie, and R. W. Doms. 2001. Relationships between CD4 independence, neutralization sensitivity, and exposure of a CD4-induced epitope in a human immunodeficiency virus type 1 envelope protein. J. Virol. 75:5230-5239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Farrar, G. H., M. A. Roff, T. Amin, J. Ball, A. M. Parrett, U. Battacharyya, J. Booth, M. H. Wansbrough-Jones, and P. J. Greenaway. 1991. Characterization of a series of human immunodeficiency virus isolates derived sequentially from a single patient. J. Med. Virol. 34:104-113. [DOI] [PubMed] [Google Scholar]
- 24.Gabuzda, D., and J. Wang. 1999. Chemokine receptors and virus entry in the central nervous system. J. Neurovirol. 5:643-658. [DOI] [PubMed] [Google Scholar]
- 25.Gabuzda, D., and J. Wang. 2000. Chemokine receptors and mechanisms of cell death in HIV neuropathogenesis. J. Neurovirol. 6(Suppl. 1):S24-S32. [PubMed] [Google Scholar]
- 26.Gartner, S., R. A. McDonald, E. A. Hunter, F. Bouwman, Y. Liu, and M. Popovic. 1997. Gp120 sequence variation in brain and in T-lymphocyte human immunodeficiency virus type 1 isolates. J. Hum. Virol. 1:3-18. [PubMed] [Google Scholar]
- 27.Gelbard, H. A., H. J. James, L. R. Sharer, S. W. Perry, Y. Saito, A. M. Kazee, B. M. Blumberg, and L. G. Epstein. 1995. Apoptotic neurons in brains from paediatric patients with HIV-1 encephalitis and progressive encephalopathy. Neuropathol. Appl. Neurobiol. 21:208-217. [DOI] [PubMed] [Google Scholar]
- 28.Ghorpade, A., A. Nukuna, M. Che, S. Haggerty, Y. Persidsky, E. Carter, L. Carhart, L. Shafer, and H. E. Gendelman. 1998. Human immunodeficiency virus neurotropism: an analysis of viral replication and cytopathicity for divergent strains in monocytes and microglia. J. Virol. 72:3340-3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ghorpade, A., M. E. Xia, B. T. Hyman, Y. Persidsky, A. Nukuna, P. Bock, M. Che, J. Limoges, H. E. Gendelman, and C. R. Mackay. 1998. Role of the β-chemokine receptors CCR3 and CCR5 in human immunodeficiency virus type 1 infection of monocytes and microglia. J. Virol. 72:3351-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ghosh, S. K., P. N. Fultz, E. Keddie, M. S. Saag, P. M. Sharp, B. H. Hahn, and G. M. Shaw. 1993. A molecular clone of HIV-1 tropic and cytopathic for human and chimpanzee lymphocytes. Virology 194:858-864. [DOI] [PubMed] [Google Scholar]
- 31.Gonzalez-Scarano, F., and G. Baltuch. 1999. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 22:219-240. [DOI] [PubMed] [Google Scholar]
- 32.Gorry, P. R., G. Bristol, J. A. Zack, C. Birch, J. E. Bell, N. Bannert, K. Crawford, D. Schols, E. De Clerq, K. Kunstman, S. M. Wolinsky, and D. Gabuzda. 2001. Macrophage tropism of primary human immunodeficiency virus type 1 isolates from brain and lymphoid tissue predicts neurotropism independent of coreceptor specificity. J. Virol. 75:10073-10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gray, F., H. Adie Biassette, F. Brion, T. Ereau, I. Le Maner, V. Levy, and G. Corcket. 1999. Neuronal apoptosis in human immunodeficiency virus infection. J. Neurovirol. 6(Suppl. 1):S38-S43. [PubMed] [Google Scholar]
- 34.He, J., Y. Chen, M. Farzan, H. Choe, A. Ohagen, S. Gartner, J. Buscigilo, X. Yang, W. Hofmann, W. Newman, C. R. MacKay, J. Sodroski, and D. Gabuzda. 1997. CCR3 and CCR5 are co-receptors for HIV-1 infection of microglia. Nature 385:645-649. [DOI] [PubMed] [Google Scholar]
- 35.Hesselgesser, J., D. Taub, P. Baskar, M. Greenberg, J. Hoxie, D. L. Kolson, and R. Horuk. 1998. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1á is mediated by the chemokine receptor CXCR4. Curr. Biol. 8:595-598. [DOI] [PubMed] [Google Scholar]
- 36.Hibbitts, S., J. D. Reeves, G. Simmons, P. W. Gray, L. G. Epstein, D. Schols, E. De Clercq, T. N. C. Wells, A. E. I. Proudfoot, and P. R. Clapham. 1999. Coreceptor ligand inhibition of fetal brain cell infection by HIV Type 1. AIDS Res. Hum. Retrovir. 15:989-1000. [DOI] [PubMed] [Google Scholar]
- 37.Hoffman, T. L., E. B. Stephens, O. Narayan, and R. W. Doms. 1998. HIV type I envelope determinants for use of the CCR2b, CCR3, STRL33, and APJ coreceptors. Proc. Natl. Acad. Sci. USA 95:11360-11365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hughes, E. S., J. E. Bell, and P. Simmons. 1997. Investigation of the dynamics of the spread of human immunodeficiency virus to brain and other tissues by evolutionary analysis of sequences from the p17gag and env genes. J. Virol. 71:1272-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Joag, S. V., E. B. Stephens, D. Gailbreath, W. Zhu, Z. Li, L. Foresman, L. J. Zhao, D. M. Pinson, and O. Narayan. 1995. Simian immunodeficiency virus SIVmac chimeric virus whose env gene was derived from SIV-encephalitic brain is macrophage-tropic but not neurovirulent. J. Virol. 69:1367-1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kaul, M., G. Garden, and S. A. Lipton. 2001. Pathways to neuronal injury and apoptosis in HIV-associated dementia. Nature 410:988-994. [DOI] [PubMed] [Google Scholar]
- 41.Kaul, M., and S. A. Lipton. 1999. Chemokines and activated macrophages in HIV gp120-induced neuronal apoptosis. Proc. Natl. Acad. Sci. USA 96:8212-8216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kolchinsky, P., E. Kiprilov, and J. Sodroski. 2001. Increased neutralization sensitivity of CD4-independent human immunodeficiency virus variants. J. Virol. 75:2041-2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolchinsky, P., E. Kiprilov, P. Bartley, R. Bubinstein, and J. Sodroski. 2001. Loss of a single N-linked glycan allows CD4-independent human immunodeficiency virus type 1 infection by altering the position of the gp120 V1/V2 variable loops. J. Virol. 75:3435-3443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Korber, B. T. M., K. J. Kunstman, B. K. Patterson, M. Furtado, M. M. McEvilly, R. Levy, and S. M. Wolinsky. 1994. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J. Virol. 68:7467-7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lapham, C. K., M. B. Zaitseva, S. Lee, T. Romanstseva, and H. Golding. 1999. Fusion of monocytes and macrophages with HIV-1 correlates with biochemical properties of CXCR4 and CCR5. Nat. Med. 5:303-308. [DOI] [PubMed] [Google Scholar]
- 46.Lee, S., H. L. Tiffany, L. King, P. M. Murphy, H. Golding, and M. B. Zaitseva. 2000. CCR8 on human thymocytes functions as a human immunodeficiency virus type 1 coreceptor. J. Virol. 74:6946-6952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lewin, S. R., S. Sonza, L. B. Irving, C. F. McDonald, J. Mills, and S. M. Crowe. 1996. Surface CD4 is critical to in vitro HIV infection of human alveolar macrophages. AIDS Res. Hum. Retrovir. 12:877-883. [DOI] [PubMed] [Google Scholar]
- 48.Li, S., J. Juarez, M. Alali, D. Dwyer, R. Collman, A. Cunningham, and H. M. Naif. 1999. Persistent CCR5 utilization and enhanced macrophage tropism by primary blood human immunodeficiency virus type 1 isolates from advanced stages of disease and comparison to tissue-derived isolates. J. Virol. 73:9741-9755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lipton, S. A., and H. E. Gendelman. 1995. Dementia associated with the acquired immunodeficiency syndrome. N. Engl. J. Med. 332:934-940. [DOI] [PubMed] [Google Scholar]
- 50.Liu, Z. Q., S. Muhkerjee, M. Sahni, C. McCormick-Davis, K. Leung, Z. Li, V. H. Gattone, C. Tian, R. W. Doms, T. L. Hoffman, R. Raghavan, O. Narayan, and E. B. Stephens. 1999. Derivation and biological characterization of a molecular clone of SHIVKU-2 that causes AIDS, neurological disease, and renal disease in rhesus macaques. Virology 260:295-307. [DOI] [PubMed] [Google Scholar]
- 51.Mankowski, J. L., M. T. Flaherty, J. P. Spelman, D. A. Hauer, P. J. Didier, A. M. Amedee, M. Murphy-Corb, L. M. Kirstein, A. Munoz, J. E. Clements, and M. C. Zink. 1997. Pathogenesis of simian immunodeficiency virus encephalitis: viral determinants of neurovirulence. J. Virol. 71:6055-6060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin, J., C. C. LaBranche, and F. Gonzalez-Scarano. 2001. Differential CD4/CCR5 utilization, gp120 conformation, and neutralization sensitivity between envelopes from a microglia-adapted human immunodeficiency virus type 1 and its parental isolate. J. Virol. 75:3568-3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.McGavin, C. H., S. A. Land, K. L. Sebire, D. J. Hooker, A. D. Gurusinghe, and C. J. Birch. 1996. Syncytium-inducing phenotype and zidovudine susceptibility of HIV-1 isolated from post-mortem tissue. AIDS 10:47-53. [DOI] [PubMed] [Google Scholar]
- 54.Meucci, O., A. Fatatis, A. A. Simen, T. J. Bushell, P. W. Gray, and R. J. Miller. 1998. Chemokines regulate hippocampal neuronal signaling and gp120 neurotoxicity. Proc. Natl. Acad. Sci. USA 95:14500-14505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michael, N. L., and J. P. Moore. 1999. HIV-1 entry inhibitors: evading the issue. Nat. Med. 5:740-742. [DOI] [PubMed] [Google Scholar]
- 56.Mirzabekov, T., N. Bannert, M. Farzan, W. Hofmann, P. Kolchinsky, L. Wu, R. Wyatt, and J. Sodroski. 1999. Enhanced expression, native purification, and characterization of CCR5, a principal HIV-1 coreceptor. J. Biol. Chem. 274:28745-28750. [DOI] [PubMed] [Google Scholar]
- 57.Moore, J. P., and D. D. Ho. 1995. HIV-1 neutralization: the consequences of viral adaptation to growth on transformed T cells. AIDS 9(Suppl. A):S117-S136. [PubMed] [Google Scholar]
- 58.Moore, J. P., and M. Stevenson. 2000. New targets for inhibitors of HIV-1 replication. Nat. Rev. Mol. Cell Biol. 1:40-49. [DOI] [PubMed] [Google Scholar]
- 59.Mori, K., M. Rosenzweig, and R. C. Desrosiers. 2000. Mechanisms for adaptation of simian immunodeficiency virus to replicate in alveolar macrophages. J. Virol. 74:10852-10859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Muster, T., R, Guinea, A. Trkola, M. Purtscher, A. Klima, F. Steindl, P. Palese, and H. Katinger. 1994. Cross-neutralizing antibodies against divergent human immunodeficiency virus type 1 isolates induced by the gp41 sequence ELDKWAS. J. Virol. 68:4031-4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Brien, W. A., Y. Koyanagi, A. Namazie, J. Q. Zhao, A. Diagne, K. Idler, J. A. Zack, and I. S. Chen. 1990. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 348:69-73. [DOI] [PubMed] [Google Scholar]
- 62.Ohagen, A., S. Ghosh, J. He, K. Huang, Y. Chen, M. Yuan, R. Osathanondh, S. Gartner, B. Shi, G. Shaw, and D. Gabuzda. 1999. Apoptosis induced by infection of primary brain cultures with diverse human immunodeficiency virus type 1 isolates: evidence for a role of the envelope. J. Virol. 73:897-906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ometto, L., M. Zanchetta, A. Cabrelle, G. Esposito, M. Mainardi, L. Chieco-Bianchi, and A. De Rossi. 1999. Restriction of HIV type 1 infection in macrophages heterozygous for a deletion in the CC-chemokine receptor 5 gene. AIDS Res. Hum. Retrovir. 15:1441-1452. [DOI] [PubMed] [Google Scholar]
- 64.Petito, C. K., and B. Roberts. 1995. Evidence of apoptotic cell death in HIV encephalitis. Am. J. Pathol. 146:1121-1130. [PMC free article] [PubMed] [Google Scholar]
- 65.Platt, E. J., K. Wehrly, S. E. Kuhmann, B. Chesebro, and D. Kabut. 1998. Effects of CCR5 and CD4 cell surface concentrations on infections by macrophagetropic isolates of human immunodeficiency virus type 1. J. Virol. 72:2855-2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Power, C., J. C. McArthur, R. T. Johnson, D. E. Griffin, J. D. Glass, S. Perryman, and B. Chesebro. 1994. Demented and nondemented patients with AIDS differ in brain derived human immunodeficiency virus type 1 envelope sequences. J. Virol. 68:4643-4649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Power, C., J. C. McArthur, A. Nath, K. Wehrly, M. Mayne, J. Nishio, T. Langelier, R. T. Johnson, and B. Chesebro. 1998. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J. Virol. 72:9045-9053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price, R. W. 1996. Neurological complications of HIV infection. Lancet 348:445-452. [DOI] [PubMed] [Google Scholar]
- 69.Shapshak, P., D. M. Segal, K. A. Crandall, R. K. Fujimura, B. T. Zhang, K. Q. Xin, K. Okuda, C. K. Petito, C. Eisdorfer, and K. Goodkin. 1999. Independent evolution of HIV type 1 in different brain regions. AIDS Res. Hum. Retrovir. 15:811-820. [DOI] [PubMed] [Google Scholar]
- 70.Schmidtmayerova, H., M. Alfano, G. Nuovo, and M. Bukrinsky. 1998. Human immunodeficiency virus type 1 T-lymphotropic strains enter macrophages via a CD4- and CXCR4-mediated pathway: replication is restricted at a postentry level. J. Virol. 72:4633-4642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shi, B., U. De Girolami, J. He, S. Wang, A. Lorenzo, J. Buscigilo, and D. Gabuzda. 1996. Apoptosis induced by HIV-1 infection of the central nervous system. J. Clin. Investig. 98:1979-1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shieh, J. T. C., A. V. Albright, M. Sharron, S. Gartner, J. Strizki, R. W. Doms, and F. Gonzalez-Scarano. 1998. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J. Virol. 72:4243-4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Shieh, J. T. C., J. Martin, G. Baltuch, M. H. Malim, and F. Gonzalez-Scarano. 2000. Determinants of syncytium formation in microglia by human immunodeficiency virus type 1: role of the V1/V2 domains. J. Virol. 74:693-701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Shimizu, H., F. Hasebe, H. Tsuchie, S. Morikawa, H. Ushijima, and T. Kitamura. 1992. Analysis of a human immunodeficiency virus type 1 isolate carrying a truncated transmembrane glycoprotein. Virology 189:534-546. [DOI] [PubMed] [Google Scholar]
- 75.Simmons, G., J. D. Reeves, A. McKnight, N. Dejucq, S. Hibbitts, C. A. Power, E. Aarons, D. Schols, E. De Clercq, A. E. I. Proudfoot, and P. R. Clapham. 1998. CXCR4 as a functional coreceptor for human immunodeficiency virus type 1 infection of primary macrophages. J. Virol. 72:8453-8457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Simmons, G., D. Wilkinson, J. D. Reeves, M. Dittmar, S. Beddows, J. Weber, G. Carnegie, U. Desselberger, P. W. Gray, R. A. Weiss, and P. R. Clapham. 1996. Primary, syncytium-inducing human immunodeficiency virus type 1 isolates are dual-tropic and most can use either LESTR or CCR5 as coreceptors. J. Virol. 70:8355-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Smit, T. K., B. Wang, T. Ng, R. Osborne, B. Brew, and N. K. Saksena. 2001. Varied tropism of HIV-1 isolates derived from different regions of adult brain cortex discriminate between patients with and without AIDS dementia complex (ADC): evidence for neurotropic HIV variants. Virology 279:509-526. [DOI] [PubMed] [Google Scholar]
- 78.Strizki, J. M., A. V. Albright, H. Sheng, M. O'Connor, L. Perrin, and F. Gonzalez-Scarano. 1996. Infection of primary human microglia and monocyte-derived macrophages with human immunodeficiency virus type 1 isolates: evidence of differential tropism. J. Virol. 70:7654-7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Strizki, J. M., S. Xu, N. E. Wagner, L. Wojcik, J. Lu, Y. Hou, M. Endres, A. Palani, S. Shapiro, J. W. Clader, J. Tagat, S. McCombie, K. Cox, A. B. Fawzi, C-C. Chou, C. Pugliese-Sivo, L. Davies, M. E. Moreno, D. D. Ho, A. Trkola, C. A. Stoddart, J. P. Moore, G. R. Reyes, and B. M. Baroudy. 2001. SCH-C (SCH 351125), an orally bioavailable, small molecule antagonist of the chemokine receptor CCR5, is a potent inhibitor of HIV-1 infection in vitro and in vivo. Proc. Natl. Acad. Sci. USA 98:12718-12723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Trkola, A., S. E. Kuhmann, J. M. Strizki, E. Maxwell, T. Ketas, T. Morgan, P. Pugach, S. Xu, L. Wojcik, J. Tagat, A. Palani, S. Shapiro, J. W. Clader, S. McCombie, G. R. Reyes, B. M. Baroudy, and J. P. Moore. 2002. HIV-1 escape from a small molecule, CCR5-specific entry inhibitor does not involve CXCR4 use. Proc. Natl. Acad. Sci. USA 99:395-400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Trkola, A., W. A. Paxton, S. P. Monard, J. A. Hoxie, M. A. Siani, D. A. Thompson, L. Wu, C. R. Mackay, R. Horuk, and J. P. Moore. 1998. Genetic subtype-independent inhibition of human immunodeficiency virus type 1 replication by CC and CXC chemokines. J. Virol. 72:396-404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Trkola, A., A. B. Pomales, H. Yuan, B. Korber, P. J. Maddon, G. P. Allaway, H. Katinger, C. F. Barbas, D. R. Burton, D. D. Ho, and J. P. Moore. 1995. Cross-clade neutralization of primary isolates of human immunodeficiency virus type 1 by human monoclonal antibodies and tetrameric CD4-IgG. J. Virol. 69:6609-6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Trkola, A., M. Purtscher, T. Muster, C. Ballaun, A. Buchacher, N. Sullivan, K. Srinivasan, J. Sodroski, J. P. Moore, and H. Katinger. 1996. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J. Virol. 70:1100-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vallat, A. V., U. De Girolami, J. He, A. Mhashilkar, W. Marasco, B. Shi, F. Gray, J. Bell, C. Keohane, T. W. Smith, and D. Gabuzda. 1998. Localization of HIV-1 coreceptors CCR5 and CXCR4 in the brain of children with AIDS. Am. J. Pathol. 152:167-178. [PMC free article] [PubMed] [Google Scholar]
- 85.van't Wout, A. B., L. J. Ran, C. L. Kuiken, N. A. Kootstra, S. T. Pais, and H. Schuitemaker. 1998. Analysis of the temporal relationship between human immunodeficiency virus type 1 quasispecies in sequential blood samples and various organs obtained at autopsy. J. Virol. 72:488-496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Verani, A., E. Pesenti, S. Polo, E. Tresoldi, G. Scarlatti, P. Lusso, A. G. Siccardi, and D. Vercelli. 1998. CXCR4 is a functional coreceptor for infection of human macrophages by CXCR4-dependent primary HIV-1 isolates. J. Immunol. 161:2084-2088. [PubMed] [Google Scholar]
- 87.Wang, J., K. Crawford, M. Yuan, H. Wang, P. R. Gorry, and D. Gabuzda. 2002. Th2 cytokines regulate CCR5 and CD4 expression and HIV-1 replication in human macrophages and microglia. J. Infect. Dis. 185:885-897. [DOI] [PubMed] [Google Scholar]
- 88.Wang, T. H., Y. K. Donaldson, R. P. Brettle, J. E. Bell, and P. Simmonds. 2001. Identification of shared populations of human immunodeficiency virus type 1 infecting microglia and tissue macrophages outside the central nervous system. J. Virol. 75:11686-11699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Willey, R. L., R. A. Rutledge, S. Dias, T. Folks, T. Theodore, C. E. Buckler, and M. A. Martin. 1986. Identification of conserved and divergent domains within the envelope gene of the acquired immunodeficiency syndrome retrovirus. Proc. Natl. Acad. Sci. USA 83:5038-5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wong, J. K., C. C. Ignacio, F. Torriani, D. Havlir, N. J. S. Fitch, and D. D. Richman. 1997. In vivo compartmentalization of human immunodeficiency virus: evidence from the examination of pol sequences from autopsy tissues. J. Virol. 71:2059-2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wyatt, R., P. D. Kwong, E. Desjardins, R. Sweet, J. Robinson, W. Hendrickson, and J. Sodroski. 1998. The antigenic structure of the human immunodeficiency virus gp120 envelope glycoprotein. Nature 393:705-711. [DOI] [PubMed] [Google Scholar]
- 92.Wyatt, R., N. Sullivan, M. Thali, H. Repke, D. Ho, J. Robinson, M. Posner, and J. Sodroski. 1993. Functional and immunological characterization of human immunodeficiency virus type 1 envelope glycoproteins containing deletions of the major variable regions. J. Virol. 67:4557-4565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yi, Y., S. Rana, J. D. Turner, N. Gaddis, and R. G. Collman. 1998. CXCR-4 is expressed by primary macrophages and supports CCR5-independent infection by dual-tropic but not T-tropic isolates of human immunodeficiency virus type 1. J. Virol. 72:772-777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhang, Y., B. Lou, R. B. Lal, A. Gettie, P. A. Marx, and J. P. Moore. 2000. Use of inhibitors to evaluate coreceptor usage by simian and simian/human immunodeficiency viruses and human immunodeficiency virus type 2 in primary cells. J. Virol. 74:6893-6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhang, Y. J., T. Dragic, Y. Cao, L. Kostrikis, D. S. Kwon, D. R. Littman, V. N. KewalRamani, and J. P. Moore. 1998. Use of coreceptors other than CCR5 by non-syncytium-inducing adult and pediatric isolates of human immunodeficiency virus type 1 is rare in vitro. J. Virol. 72:9337-9344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhang, Y. J., and J. P. Moore. 1999. Will multiple coreceptors need to be targeted by inhibitors of human immunodeficiency virus type 1 entry? J. Virol. 73:3443-3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zheng, J., A. Ghorpade, D. Niemann, R. L. Cotter, M. R. Thylin, L. Epstein, J. M. Swartz, R. B. Shepard, X. Liu, A. Nukuna, and H. E. Gendelman. 1999. Lymphotropic virions affect chemokine receptor-mediated neural signaling and apoptosis: implications for human immunodeficiency virus type 1-associated dementia. J. Virol. 73:8256-8267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zheng, J., M. R. Thylin, A. Ghorpade, H. Xiong, Y. Persidsky, R. Cotter, D. Niemann, M. Che, Y. C. Zeng, H. A. Gelbard, R. B. Shepard, J. M. Swartz, and H. E. Gendelman. 1999. Intracellular CXCR4 signaling, neuronal apoptosis and neuropathogenic mechanisms of HIV-1-associated dementia. J. Neuroimmunol. 98:185-200. [DOI] [PubMed] [Google Scholar]