Abstract
Plus-strand RNA viruses characteristically replicate their genome in association with altered cellular membranes. In the present study, the capacity of hepatitis C virus (HCV) proteins to elicit intracellular membrane alterations was investigated by expressing, in tetracycline-regulated cell lines, a comprehensive panel of HCV proteins individually as well as in the context of the entire HCV polyprotein. As visualized by electron microscopy (EM), expression of the combined structural proteins core-E1-E2-p7, the NS3-4A complex, and protein NS4B induced distinct membrane alterations. By immunogold EM (IEM), the membrane-altering proteins were always found to localize to the respective altered membranes. NS4B, a protein of hitherto unknown function, induced a tight structure, designated membranous web, consisting of vesicles in a membranous matrix. Expression of the entire HCV polyprotein gave rise to membrane budding into rough endoplasmic reticulum vacuoles, to the membranous web, and to tightly associated vesicles often surrounding the membranous web. By IEM, all HCV proteins were found to be associated with the NS4B-induced membranous web, forming a membrane-associated multiprotein complex. A similar web-like structure in livers of HCV-infected chimpanzees was previously described (Pfeifer et al., Virchows Arch. B., 33:233-243, 1980). In view of this finding and the observation that all HCV proteins accumulate on the membranous web, we propose that the membranous web forms the viral replication complex in HCV-infected cells.
Hepatitis C virus (HCV) is a major cause of liver disease, including chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma. Despite the identification in 1989 of HCV as the most common etiologic agent of posttransfusion and sporadic non-A, non-B hepatitis (15, 32), its replication cycle and pathogenesis are incompletely understood. Investigations have been hampered by the lack of a permissive cell culture system which would allow to investigate the full intracellular replication cycle of HCV, including the formation of virus progeny. Progress has been made towards understanding the viral replication cycle by using heterologous expression systems, functional cDNA clones, and selectable subgenomic replicons (30, 34; reviewed in reference 3).
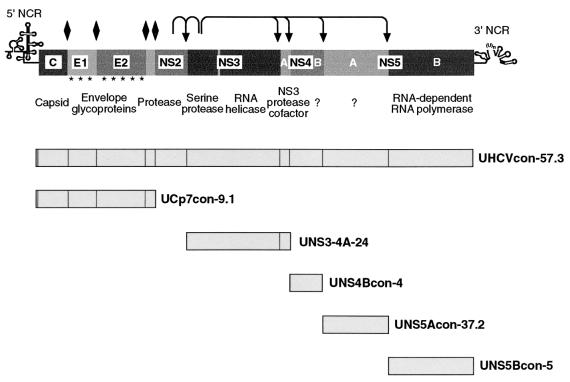
HCV contains a single-stranded RNA genome of positive polarity and of approximately 9,600 nucleotides in length (Fig. 1). The 5′ noncoding region contains an internal ribosomal entry site for cap-independent translation initiation (49, 50). The primary translation product of about 3,000 amino acids is co- and posttranslationally processed by cellular and viral proteases to yield the mature structural and nonstructural proteins. The structural proteins include the core protein, which forms the viral capsid, and the envelope glycoproteins E1 and E2. The nonstructural proteins include an autoprotease (NS2-3), a serine protease and a helicase (NS3), as well as a serine protease cofactor (NS4A) (25, 26). The function of NS4B is unknown. The NS5A protein was suggested to be involved in interferon resistance (21, 47). The NS5B protein is an integral membrane protein (44) and functions, in concert with other nonstructural proteins and as yet unidentified host cell components, as an RNA-dependent RNA polymerase (RdRp) (4) replicating the viral genome in a presumably membrane-bound RNA replication complex.
FIG. 1.
Tetracycline-regulated cell lines. The genetic organization and polyprotein processing of HCV are depicted at the top. Asterisks in the E1 and E2 regions indicate glycosylation of the envelope proteins. Diamonds indicate cleavages of the HCV polyprotein precursor by the ER signal peptidase, and arrows indicate cleavages by HCV NS2-3 and NS3 proteases. The well-characterized and representative clones UHCVcon-57.3, UCp7con-9.1, UNS3-4A-24, UNS4Bcon-4, UNS5Acon-37.2, and UNS5Bcon-5 were used in this study.
Virtually all plus-strand RNA viruses investigated, i.e., picornavirus (6, 10), nidovirus (23, 37), Kunjin virus (51), and certain plant viruses (13), induce distinct membrane alterations which build up a viral RNA replication complex providing the necessary structural scaffold for RNA replication. The formation of membrane-bound replication complexes leads to more or less profound structural alterations of the infected cell. The altered cellular architecture presents as cytopathology which may culminate in cell destruction. For HCV, it is generally believed that the immune response is central to the pathogenesis of liver cell damage, but it is a matter of discussion whether there is, in addition, a direct contribution of the virus through changes in the cellular architecture (reviewed in references 12 and 28). Thus, the analysis of HCV-induced membrane alterations could help to elucidate the pathogenetic mechanisms of HCV-induced liver cell injury.
All HCV proteins interact with host cell membranes, either directly as membrane-binding proteins or, in the case of NS3, via interaction with the membrane-anchoring protein NS4A (52). The core protein has been shown to be targeted to the rough endoplasmic reticulum (rER) membrane by a signal recognition particle-dependent mechanism (42). The envelope glycoproteins and the NS2 protein contain transmembrane domains (17, 43), as does NS4B, which colocalizes with all other nonstructural proteins on rER-derived membranes (29). NS5A was found to be an integral membrane protein, inserted into the membrane via an N-terminal amphipathic alpha-helix (11). NS5B, the HCV RdRp, was recently identified as a new member of the tail-anchored protein family, a class of integral membrane proteins that are membrane targeted posttranslationally via a carboxyterminal membrane insertion sequence (44). However, it is unknown whether HCV proteins have the capability to induce membrane alterations related to a putative replication complex and to virus-induced cytopathology.
In this study, we assessed the propensity of HCV proteins to induce changes in membrane architecture by expressing a comprehensive panel of HCV proteins individually as well as in the context of the entire open reading frame (ORF) in tetracycline-regulated cell lines. We have shown previously that the viral structural and nonstructural proteins are faithfully processed and posttranslationally modified in these cell lines (19, 29, 36, 44, 52). Membrane alterations were analyzed by electron microscopy (EM), and viral proteins were localized by immunogold EM (IEM). The interactions of the nonstructural proteins with one another and the formation of a multiprotein complex on rER-derived membranes (29) necessitate the expression of single proteins in order to assess their individual roles in the induction of membrane alterations.
It was found that distinct patterns of intracellular membrane changes were induced by the structural proteins (core, E1, E2, p7) and the nonstructural proteins NS3-4A and NS4B. Expression of all structural and nonstructural proteins in the context of the entire HCV polyprotein induced similar membrane changes, importantly a tight structure, designated membranous web, consisting of small vesicles embedded in a membranous matrix. By IEM, all structural and nonstructural HCV proteins were found to be associated with this structure. Formation of the membranous web was triggered by the NS4B protein expressed in the absence of any other HCV protein. Interestingly, a morphologically similar structure, termed sponge-like inclusion (38), has been found to be characteristic for HCV-infected livers of chimpanzees. Our findings lead us to propose that the membranous web might comprise the HCV replication complex in infected cells.
MATERIALS AND METHODS
Cell lines inducibly expressing HCV proteins.
Tetracycline-regulated cell lines were established (35, 36) by stable transfection of U-2 OS human osteosarcoma cells (American Type Culture Collection, Rockville, Md.) first with the tetracycline-controlled transactivator (24) and second with different HCV cDNA constructs under the control of a tetracycline-controlled transactivator-dependent promoter. The viral protein expression is tightly regulated and reaches a steady state 24 to 48 h following tetracycline withdrawal (36). The HCV expression cassettes present in the different cell lines used in this study are schematically illustrated in Fig. 1. UHCVcon-57.3 (44), UNS4Bcon-4 (29), UNS5Acon-37.2 (11), and UNS5Bcon-5 (44) cells allow the tightly regulated expression of the entire polyprotein, the NS4B protein, the NS5A protein, and the NS5B RdRp, respectively, derived from a functional HCV H consensus cDNA clone (30). UNS3-4A-24 cells allow the inducible expression of the NS3-4A complex (25), derived from a prototype HCV H cDNA clone. The cell line UCp7con-9.1, allowing the expression of the structural region (core-E1-E2-p7, amino acid positions 1 to 809), will be described in detail elsewhere (D. Moradpour et al., unpublished data). HCV H prototype and consensus cDNA clones were kindly provided by Charles M. Rice, Rockefeller University, New York, N.Y.
Chimpanzees.
For comparison with cell culture data, archival photographs and Epon-embedded liver specimens of chimpanzees were reevaluated. After one or two preinoculation biopsies, the animals were infected with pooled human sera in 1978 and became seropositive for HCV. Liver biopsies were taken at weekly intervals. The experiments were performed at Immuno AG, Vienna, Austria, by Christine and Gerhard Eder. The electron micrographs and the embedded material were kindly supplied by Fred Gudat, Institute for Pathology, University of Basel, Basel, Switzerland.
Abs.
The monoclonal antibodies (MAbs) C7-50 against HCV core protein (35) and 1B6 against NS3 (52) were described previously. A rabbit polyclonal antiserum was raised against recombinant HCV core protein (Moradpour et al., unpublished). MAbs A4 and A11 against E1 and E2, respectively (18), were a kind gift of Jean Dubuisson, Institut Pasteur de Lille, Lille, France, and Harry Greenberg, Stanford University, Stanford, Calif. MAbs 8N and 11H against NS4A and NS5A, respectively, were kindly provided by Jan Albert Hellings, Organon Teknika B.V., Boxtel, The Netherlands. The rabbit polyclonal antisera WU151 against NS4B and WU115 against NS5B (25) were generously provided by Charles M. Rice, Rockefeller University, New York, N.Y. The dilutions of the Abs were determined experimentally.
As secondary Ab, goat antimouse Ab coupled to 10-nm gold or goat antirabbit Ab coupled to 5- or 15-nm gold were used. All gold conjugates were purchased from Amersham, Little Chalfont, United Kingdom.
EM and IEM.
For EM and IEM, cells were induced to express the appropriate protein(s) by tetracycline withdrawal and the cells were harvested 48 h later. For conventional EM, cells were trypsinized and fixed in 2.5% glutaraldehyde followed by 2% osmium tetroxide, dehydrated, prestained with 2% uranyl acetate in acetone, and embedded in Poly/Bed 812 (Polysciences, Warrington, Pa.) according to standard protocols. For IEM, cells were fixed in 2% paraformaldehyde, treated with uranyl carbonate, and embedded in LRGold (London Resin Co., London, United Kingdom) as described previously (6).
For immunocytochemical labeling, unspecific binding sites on the sections were blocked by goat serum and bovine serum albumin. The sections were then incubated on primary Abs at appropriate dilutions, followed by incubation on gold-tagged secondary Abs before being stained with uranyl acetate and lead hydroxide as described elsewhere (5). For the simultaneous detection of two HCV proteins, the sections were incubated on the appropriate two primary Abs raised in different species, followed by two corresponding antispecies Abs, tagged with two different gold grain sizes (5). As negative controls, UHCVcon-57.3 cells, repressed by tetracycline, were assayed with the anti-HCV Ab listed. UHCVcon-57.3 cells, expressing the entire polyprotein of HCV after tetracycline withdrawal, were tested with an unrelated anti-poliovirus protein 2B MAb. Specimens were viewed and photographed in a Philips CM100 EM.
RESULTS
Expression of the HCV polyprotein induces distinct membrane alterations.
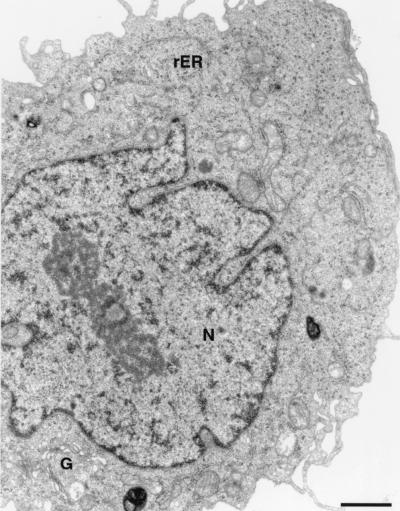
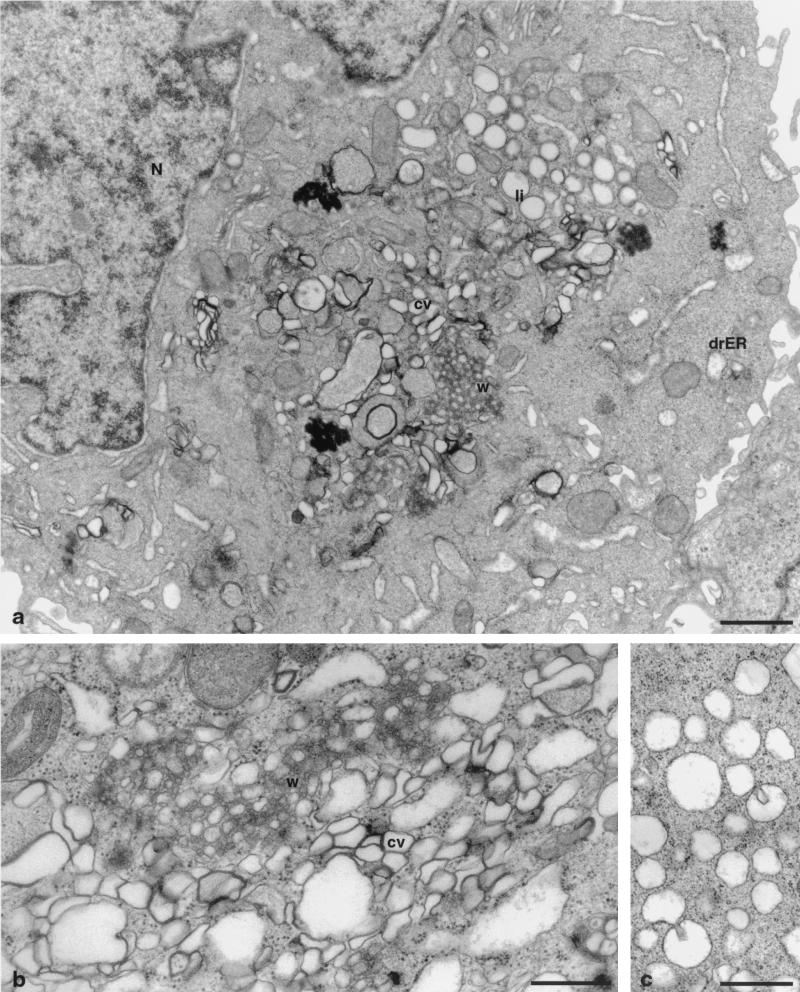
EM analyses of UHCVcon-57.3 cells, which inducibly express the entire ORF derived from a functional genotype 1a HCV cDNA clone (Fig. 1), were performed. UHCVcon-57.3 cells in which HCV protein expression was repressed by tetracycline showed no membrane changes (Fig. 2). Figure 3a demonstrates the appearance of distinct membrane alterations in the cytoplasm of UHCVcon-57.3 cells expressing the HCV polyprotein. The extent and the relative amount of the individual alterations varied considerably from cell to cell. Their aspect, however, was consistent and reproducible, allowing for a schematic classification. One alteration consisted of vesicles embedded in a membranous matrix of circular or very tightly undulating membranes which formed a rather compact structure. This alteration was designated membranous web. A second alteration consisted of tightly associated vesicles which often surrounded the web. Lipid droplets, known to occur as a consequence of HCV core protein expression (2, 27, 35), were observed rather frequently. Figure 3b shows two of the membrane alterations at higher magnification. The membranous web consisted of vesicles with a diameter of approximately 85 nm. The shape of the second type of vesicles was irregular, and their membranes were in such close contact that two lipid bilayers often were fused into one trilayer, hence the proposed name of contiguous vesicles. Interestingly, their aspect resembled closely that of vesicles arising during poliovirus infection (6, 16, 48). Figure 3c shows a further type of vesicles with a ribosome-carrying membrane and thus representing dilated rER. They were of various sizes; in some of the vesicles, intrusions, i.e., budding of the rER membrane into the lumen, may be seen. Occasionally, smooth-surfaced single vesicles (not shown) similar to those depicted in Fig. 4a were found.
FIG. 2.
UHCVcon-57.3 cell from a culture grown in the presence of tetracycline. The expression of the HCV polyprotein is repressed, and the cell shows no pathological findings. G, Golgi; rER, rough endoplasmic reticulum; N, nucleus. Bar, 1 μm.
FIG. 3.
UHCVcon-57.3 cells expressing the entire HCV polyprotein 48 h after tetracycline withdrawal. (a) Low-power overview showing the alterations induced. w, membranous web; li, lipid droplets; cv, contiguous vesicles; drER, dilated rER forming vacuoles; N, nucleus. Bar, 1 μm. (b) Higher magnification of the membranous web (w), surrounded by clusters of contiguous vesicles (cv). Bar, 0.5 μm. (c) Vacuoles of dilated rER, carrying ribosomes on their surfaces. Bar, 0.5 μm.
FIG. 4.
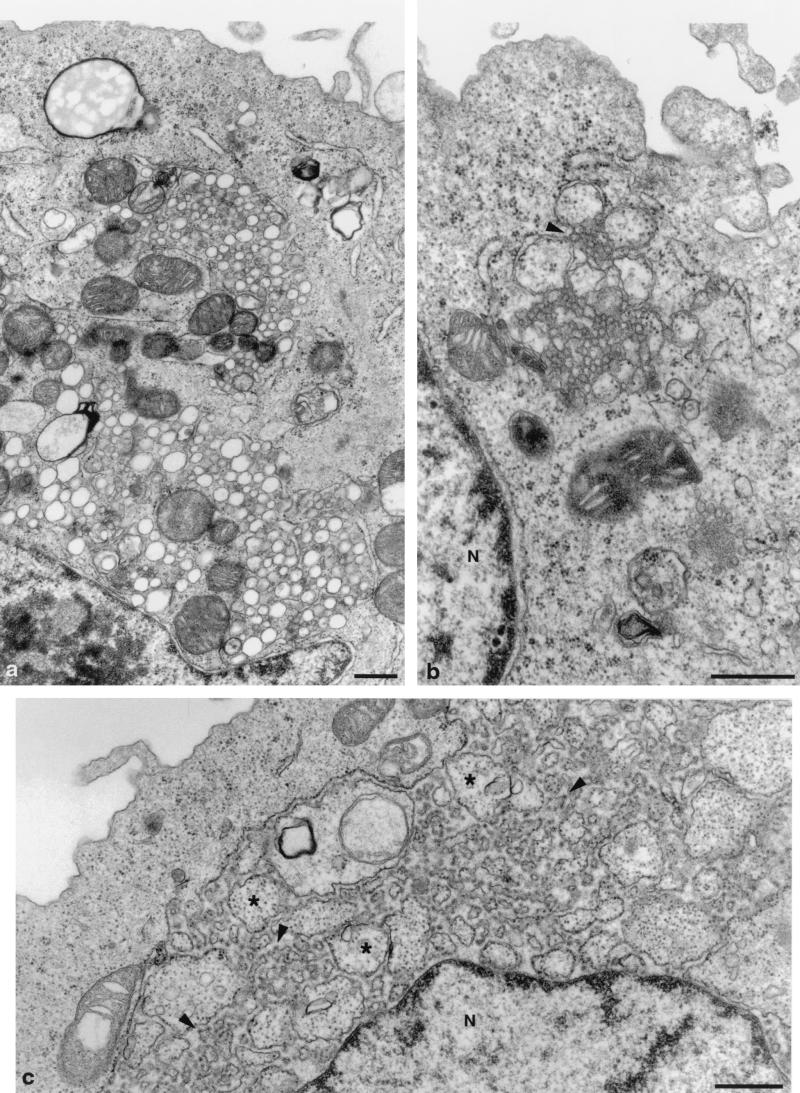
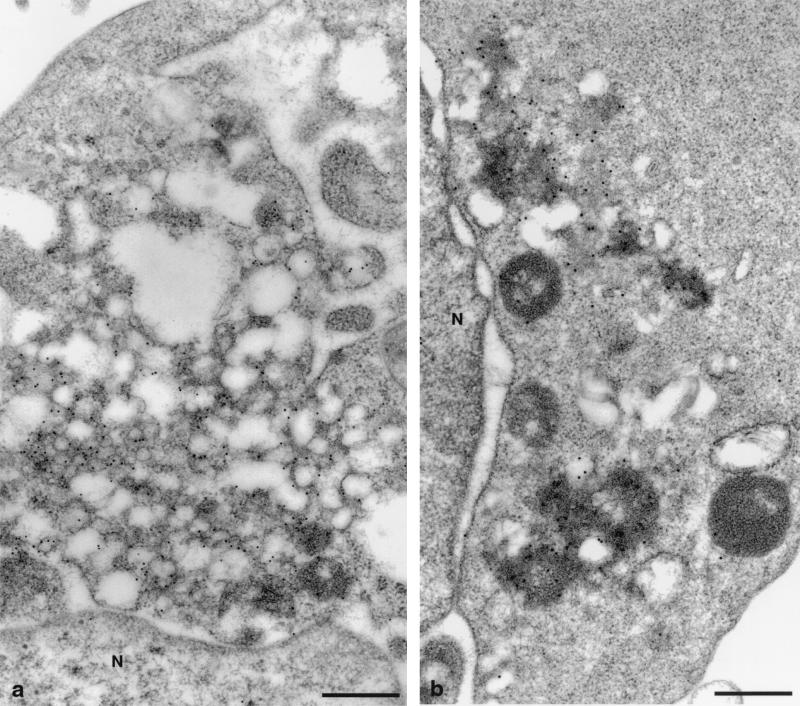
Cells expressing individual HCV proteins. (a) UNS3-4A-24 cells, expressing the proteins NS3 and NS4A, show smooth-surfaced vesicles of varying size. The vesicles are distributed in clusters in the cytoplasm. (b) Protein NS4B expressed by UNS4Bcon-4 cells induces a membranous web. Note the association of the web with remnants of the rER (arrowhead). (c) UCp7con-9.1 cells express the structural proteins core, E1, E2, and p7. Highly dilated rER vacuoles contain numerous invaginations of the rER, budding into the lumen of the large vacuole. Arrowheads, small buds; asterisks, large buds; N, nucleus. Bars, 0.5 μm.
Membrane alterations induced by the expressions of individual HCV proteins.
To test which of the HCV proteins induces the membrane alterations observed after the expression of the entire HCV ORF, cell lines expressing individual proteins or combinations of proteins (Fig. 1) were examined. Among the nonstructural proteins, the NS3-4A complex and the NS4B protein each induced a distinct membrane alteration. The proteins NS3 and NS4A, which form a stable complex (52), induced the formation of large amounts of smooth single vesicles (Fig. 4a). NS4B gave raise to the membranous web, at the same time reducing the amount of rER. The remaining rER was found associated, often in continuity, with the web (Fig. 4b). This finding, together with the notion that NS4B colocalizes with the rER marker protein disulfide isomerase (29), suggests that the web is an rER-derived structure. The two most C-terminal proteins, NS5A and NS5B, did not induce distinct membrane alterations, although both proteins are known to interact with membranes (11, 44). Only in some NS5B-expressing UNS5Bcon-5 cells could vacuolated rER be detected (not shown).
The combined structural proteins expressed in UCp7con-9.1 cells (Fig. 1) induced lipid droplets (not shown, but similar to those depicted in Fig. 3a) and, in addition, large dilatations of the rER (Fig. 4c). These dilatations were much bigger than the corresponding structures found during expression of the entire HCV polyprotein (not shown). They contained large amounts of budding membrane protrusions, thus giving the impression of inverse vacuolization, i.e., of vesicles with internalized ribosomes. Some of the budding protrusions were very small, with extremely narrow membrane curvature, but still distinct from virus or virus-like particles.
Of the distinct membrane alterations observed in cells expressing the entire HCV ORF, the contiguous vesicles were not found to be induced by any of the proteins expressed individually but were seen only in cells expressing the entire HCV polyprotein.
Identification of viral proteins associated with membrane alterations.
IEM analyses with mono- and polyclonal Abs against HCV structural and nonstructural proteins were performed with cells expressing single proteins, protein combinations, or the entire polyprotein of HCV. After expression of single proteins or protein combinations, these proteins were found associated with the membrane alterations which they induced. The negative controls, as indicated in Materials and Methods, were consistently found to be negative. In cells expressing the NS3-4A complex, both proteins were located on the smooth single vesicles as shown in Fig. 5a for NS3 (immunolabeling for NS4A not shown). In UNS4Bcon-4 cells, NS4B was also located exclusively on the structure which it induces, i.e., the membranous web (Fig. 5b).
FIG. 5.
Immunogold detection of HCV nonstructural proteins expressed individually. The viral proteins are associated with the membrane alterations which they induce. (a) UNS3-4A-24 cells labeled with anti-NS3 Ab. NS3 is found on smooth small vesicles. (b) UNS4Bcon-4 cells labeled with anti-NS4B Ab. NS4B is found exclusively on the membranous web. N, nucleus. Bars, 0.5 μm.
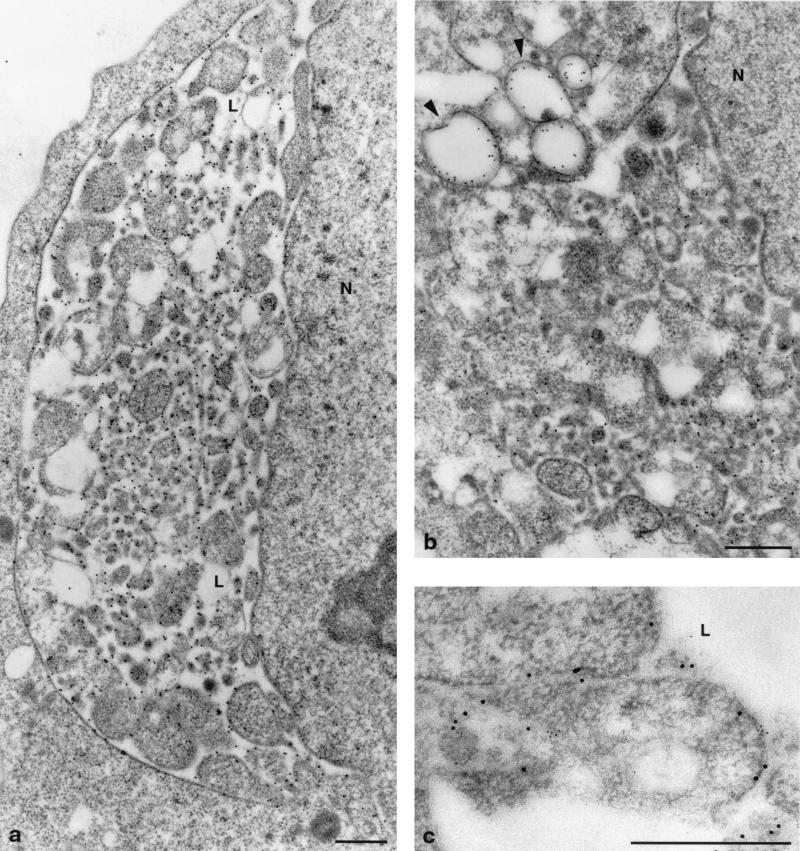
The proteins expressed in UCp7con-9.1 cells were distributed over the membranes budding into the lumen of the rER as shown in Fig. 6a for the E1 protein and in Fig. 6b for the core protein (E2 not shown). In addition, and in accordance with earlier reports (2, 35), core protein was found on lipid droplets (Fig. 6b) and occasionally over the nucleoli (not shown). In double-immunolabeling experiments, E1 and core proteins were both found on the same budding membranes, but still somewhat separated from each other (Fig. 6c). No virus-like particles or (empty) viruses could be seen.
FIG. 6.
Immunogold detection of structural proteins expressed in UCp7con-9.1 cells. (a) E1 protein is found on the budding membrane invaginations in the lumen of the rER. (b) Core protein is also found on membranes in the lumen of the rER and, in addition, on lipid droplets (arrowheads). (c) Double immunogold staining shows proteins E1 (larger gold grains) and core (smaller gold grains) on the same membrane structures, albeit not closely associated with each other. L, lumen of rER; N, nucleus. Bars, 0.5 μm.
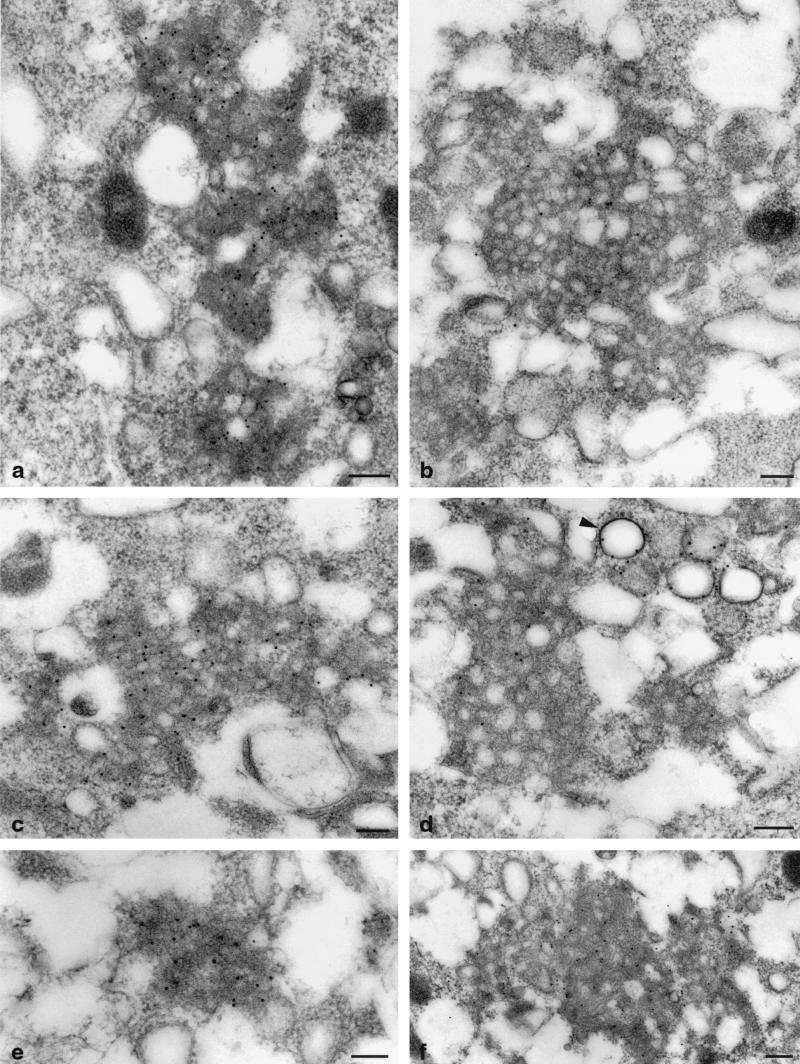
Immunocytochemical preparations of UHCVcon-57.3 cells expressing the entire HCV polyprotein showed strong labeling reactions on the membranous web. The web was labeled with Abs recognizing the nonstructural proteins NS3, NS4A, NS4B, NS5A, and the structural proteins core and E1 (Fig. 7a to f), as well as E2 and NS5B (not shown). The location of the proteins p7 and NS2 could not be investigated because of the lack of Abs suitable for IEM analyses. No label was observed on other membrane alterations, with the exception of rER and dilated rER, which were sometimes weakly labeled for structural proteins, whereas lipid droplets were intensely labeled with anticore Ab (Fig. 7d). It is noteworthy that the contiguous vesicles, representing the most prominent membrane alteration, were not labeled.
FIG. 7.
Immunogold detection of structural and nonstructural proteins in UHCVcon-57.3 cells expressing the entire HCV polyprotein. The cells were labeled with Abs against proteins NS3 (a), NS4B (b), NS5A (c), core (d), NS4A (e), and E1 (f). All proteins tested are found on the membranous web (a to f); the core protein is additionally found on lipid droplets (arrowhead) (d). Bars, 0.2 μm.
Thus, our IEM data indicate that all HCV proteins tested were associated with and retained in the membranous web.
Formation of membrane alterations in chimpanzee liver.
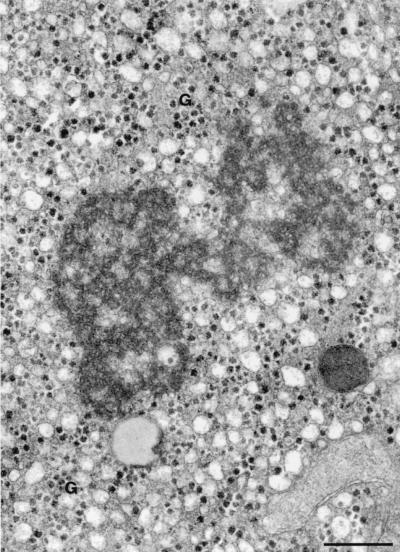
Since HCV replication and HCV-induced liver pathology in chimpanzees were extensively studied, it was of interest to compare membrane alterations in chimpanzee hepatocytes to the membrane alterations observed in our HCV protein-expressing cell cultures. The materials available to us consisted of conventionally Epon-embedded blocks and thus precluded IEM investigation. An analysis of newly made sections of the blocks and of archival EM photographs (obtained from Fred Gudat, Basel, Switzerland) revealed, in addition to dense glycogen granules, structures compatible with the membranous web in cultured cells (Fig. 8). The web-like structure was found in livers of six of seven animals, investigated by weekly biopsies over several months postinfection. In preinoculation biopsies, no web-like structures have been detected. Web-like structures in livers of infected chimpanzees have previously been described as sponge-like inclusions (38).
FIG. 8.
The liver biopsy of an HCV-infected chimpanzee shows a web-like inclusion whose size and structure are comparable to those found in UHCVcon-57.3 cells. G, glycogen granules. Bar, 0.5 μm.
DISCUSSION
Expression of individual proteins of several virus species has allowed to elucidate functions of viral gene products. For example, the proteins responsible for triggering the formation of membrane structures involved in viral RNA replication, i.e., the viral replication complex, have been pinpointed in such experiments for poliovirus, hepatitis A virus, and members of the nidoviruses (1, 14, 22, 45, 46). For HCV, the viral replication complex is not yet characterized. In the present work we show that several structural and nonstructural HCV proteins have the propensity to modify the cellular membrane framework in a characteristic way, some of the alterations being possibly related to the formation of a replication complex. An advantage of protein expression systems is that they presumably do not accumulate adaptive mutations, which are necessary for efficient in vitro propagation of replicons (9, 33). Thus, the proteins studied are identical to those synthesized during an HCV infection in humans.
The expression of the entire HCV polyprotein induced membrane alterations similar to those found after expression of individual proteins, although there were differences in the extent of membrane transformation. As an exception, the so-called contiguous vesicles were observed only after expression of the entire ORF. Thus, their formation may depend on a concerted action of several viral proteins. The effect of the proteins, however, might be indirect, since by IEM, little if any viral protein was found to be associated with these vesicles. Formally, we cannot rule out that protein NS2, which was not visualized by IEM in the present work, can induce the contiguous vesicles. However, more recently, this type of vesicles was also found in HuH-7 cells harboring a subgenomic replicon lacking NS2 (R. Gosert et al., unpublished data).
The contiguous vesicles have a striking resemblance to the vesicles arising during poliovirus infection. Since the formation of the poliovirus vesicles involves COPII proteins and membrane structures of an altered anterograde membrane pathway (41), it might well be that the HCV contiguous vesicles also reflect alterations in the exocytotic membrane traffic, brought about by the HCV protein(s).
In cells expressing the NS4B protein alone, the characteristic membrane alteration was a membranous web. In UHCVcon-57.3 cells expressing the entire HCV polyprotein, as well as in liver cells of HCV-infected chimpanzees, this membrane alteration was detected. In UHCVcon-57.3 cells, the web harbored all HCV structural and nonstructural viral proteins tested. Since the actual intracellular site of HCV RNA replication is not yet determined, the significance of the observed membrane alterations induced by HCV proteins cannot at present be assessed for their roles in virus replication. The observations that all HCV proteins associate with the membranous web and that the same structure is found during HCV replication in chimpanzee liver make the web a good candidate for the replication complex. The contiguous vesicles, always found in close proximity to the web, are less likely to represent the replication complex, since there were only few if any viral proteins found to be associated with this structure.
As inferred from the successful construction of subgenomic replicons (34), the nonstructural proteins NS3 to NS5B are sufficient to induce the putative membrane alterations competent to support viral replication. Since we found only NS3-4A and NS4B, but not NS5A and NS5B, to induce membrane alterations, the NS3-4A-induced vesicles (Fig. 4a and 5a) and/or the NS4B-induced web (Fig. 4b and 5b) is likely to be the structure(s) equivalent to the HCV replication complex. At present, we favor the view that both alterations might contribute to the replication complex, since the web carries NS3-4A as well as NS4B protein. In addition, our EM pictures are compatible with an association of NS3-4A- and NS4B-induced structures forming a (higher-order) complex by incorporation of the NS3-4A-induced loose vesicles into a NS4B-induced basic web structure. Such a process would also explain the slight difference in the aspect of the web induced by NS4B alone (Fig. 4b) compared to the web in cells expressing the entire HCV polyprotein (Fig. 3a and b). The two proteins NS5A and NS5B, found not to alter membranes but still to be present on the web, can be posttranslationally targeted to membranes (44). This might indicate that these two proteins, of which at least the RdRp NS5B is necessary for RNA replication, are attracted and incorporated into a nascent or already completed web to form the final functional replication complex.
Very recently, the NS4B protein of the HCV-related pestiviruses was reported to play a role in cytopathogenicity of the virus (39). For HCV, no function for NS4B was described so far. Our finding that NS4B induces a distinct membrane structure, possibly related to virus replication, is the first function of HCV NS4B thus far described. Since in other plus-strand RNA viruses, particularly poliovirus, the formation of a replication complex and the induction of cytopathology are coupled (7, 8, 41), one could speculate that also for HCV, NS4B might be involved in virus-mediated cell injury under certain conditions. Interestingly, adaptive mutations in NS4B have recently been found to greatly enhance replication of HCV replicons in HuH-7 cells (33).
At present, it is open whether the membrane structures, particularly the web, observed in the absence of RNA replication will be the same when RNA synthesis occurs. In the poliovirus system, expression of viral gene products in the absence of RNA replication induced structures morphologically indistinguishable from the membrane alterations harboring the viral replication complex found in poliovirus-infected or replicon-transfected cells. Only the location of replicating RNA was different from that of nonreplicating RNA, in that only replicating RNA was associated with the membranous vesicles of the replication complex (20, 48).
The mechanism by which mature, infectious HCV progeny is formed is still enigmatic. By analogy to other flaviviruses, formation of virus particles is thought to occur by budding of protein E1- and/or E2-containing membranes, together with core (capsid) protein, into the lumen of the rER (for a review, see references 3 and 40). The E1 and E2 proteins contain ER retention signals (19), which is compatible with a role of these proteins in the budding process.
In UCp7con-9.1 cells expressing the HCV structural proteins, we were able to visualize the budding process predicted by several authors. The budding was quite extensive, leading to giant rER vacuoles containing large but also very small membrane buds. In UHCVcon-57.3 cells expressing the structural proteins in the context of the entire HCV polyprotein, budding was much less extensive. This may be explained by an overall lower expression level of structural proteins in UHCVcon-57.3 cells than that observed with UCp7con-9.1 cells, and/or retention of the structural proteins in the membranous web, leading to a lower amount of structural proteins available for the budding process. No (empty) virus particles could be found in either UCp7con-9.1 or UHCVcon-57.3 cells. This was expected, since it was found that particle formation requires structured RNA (31). In addition, it is conceivable that not only virion formation but also release of structural proteins from the web and subsequent migration to the rER has to take place in an RNP complex; thus, it can occur only in a system with ongoing viral RNA replication. In any case, the budding process per se at the rER does not require RNP but can be effected by the structural proteins alone.
Based on the fact that most HCV proteins are membrane-binding proteins, several authors (see reference 3 and references therein) have postulated that at least two replication steps of HCV, i.e., RNA replication and virion formation are (ER) membrane associated. The present paper demonstrates that HCV proteins have the propensity to induce distinct alterations of ER membranes, which may represent, in productively infected cells, the scaffolds necessary for virus multiplication.
Acknowledgments
We thank Elke Bieck for excellent technical assistance, Andrea Glaser for superb photographic work, Jean Dubuisson and Harry Greenberg for MAbs A4 and A11, Jan Albert Hellings for MAbs 8N and 11H, and Charles M. Rice for the HCV H prototype and consensus cDNAs and antisera WU151 and WU115. We are grateful to Fred Gudat and Christine and Gerhard Eder for permission to use the chimpanzee material.
This work was supported by grant Mo 799/1-2 from the Deutsche Forschungsgemeinschaft (D.M. and H.E.B.), grant 31-055397.98 from the Swiss National Science Foundation (K.B.), grant 01 K1 9951 from the Bundesministerium für Bildung und Forschung (D.M. and H.E.B.), and by Jubiläumsstiftung der Schweiz. Rentenanstalt/Swiss Life (R.G.).
REFERENCES
- 1.Aldabe, R., and L. Carrasco. 1995. Induction of membrane proliferation by poliovirus proteins 2C and 2BC. Biochem. Biophys. Res. Commun. 206:64-76. [DOI] [PubMed] [Google Scholar]
- 2.Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Z. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Bréchot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bartenschlager, R., and V. Lohmann. 2000. Replication of hepatitis C virus. J. Gen. Virol. 81:1631-1648. [DOI] [PubMed] [Google Scholar]
- 4.Behrens, S. E., L. Tomei, and R. Defrancesco. 1996. Identification and properties of the RNA-dependent RNA polymerase of hepatitis C virus. EMBO J. 15:12-22. [PMC free article] [PubMed] [Google Scholar]
- 5.Bienz, K., and D. Egger. 1995. Immunocytochemistry and in situ hybridization in the electron microscope: combined application in the study of virus-infected cells. Histochem. Cell Biol. 103:325-338. [DOI] [PubMed] [Google Scholar]
- 6.Bienz, K., D. Egger, and L. Pasamontes. 1987. Association of polioviral proteins of the P2 genomic region with the viral replication complex and virus-induced membrane synthesis as visualized by electron microscopic immunocytochemistry and autoradiography. Virology 160:220-226. [DOI] [PubMed] [Google Scholar]
- 7.Bienz, K., D. Egger, Y. Rasser, and W. Bossart. 1980. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology 100:390-399. [DOI] [PubMed] [Google Scholar]
- 8.Bienz, K., D. Egger, M. Troxler, and L. Pasamontes. 1990. Structural organization of poliovirus RNA replication is mediated by viral proteins of the P2 genomic region. J. Virol. 64:1156-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blight, K. J., A. A. Kolykhalov, and C. M. Rice. 2000. Efficient initiation of HCV RNA replication in cell culture. Science 290:1972-1974. [DOI] [PubMed] [Google Scholar]
- 10.Bolten, R., D. Egger, R. Gosert, G. Schaub, L. Landmann, and K. Bienz. 1998. Intracellular localization of poliovirus plus- and minus-strand RNA visualized by strand-specific fluorescent in situ hybridization. J. Virol. 72:8578-8585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brass, V., E. Bieck, R. Montserret, B. Wölk, J. A. Hellings, H. E. Blum, F. Penin, and D. Moradpour. 2002. An amino-terminal amphipathic alpha-helix mediates membrane association of the hepatitis C virus nonstructural protein 5A. J. Biol. Chem. 277:8130-8139. [DOI] [PubMed] [Google Scholar]
- 12.Cerny, A., and F. V. Chisari. 1999. Pathogenesis of chronic hepatitis C: immunological features of hepatic injury and viral persistence. Hepatology 30:595-601. [DOI] [PubMed] [Google Scholar]
- 13.Chen, J., and P. Ahlquist. 2000. Brome mosaic virus polymerase-like protein 2a is directed to the endoplasmic reticulum by helicase-like viral protein 1a. J. Virol. 74:4310-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho, M. W., N. Teterina, D. Egger, K. Bienz, and E. Ehrenfeld. 1994. Membrane rearrangement and vesicle induction by recombinant poliovirus 2C and 2BC in human cells. Virology 202:129-145. [DOI] [PubMed] [Google Scholar]
- 15.Choo, Q.-L., G. Kuo, A. J. Weiner, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. Isolation of a cDNA clone derived from a blood-borne non-A, non-B viral hepatitis genome. Science 244:359-362. [DOI] [PubMed] [Google Scholar]
- 16.Dales, S., H. J. Eggers, I. Tamm, and G. E. Palade. 1965. Electron microscopic study of the formation of poliovirus. Virology 26:379-389. [DOI] [PubMed] [Google Scholar]
- 17.Dubuisson, J. 2000. Folding, assembly and subcellular localization of hepatitis C virus glycoproteins. Curr. Top. Microbiol. Immunol. 242:135-148. [DOI] [PubMed] [Google Scholar]
- 18.Dubuisson, J., H. H. Hsu, R. C. Cheung, H. B. Greenberg, D. G. Russell, and C. M. Rice. 1994. Formation and intracellular localization of hepatitis C virus envelope glycoprotein complexes expressed by recombinant vaccinia and Sindbis viruses. J. Virol. 68:6147-6160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duvet, S., L. Cocquerel, A. Pillez, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 20.Egger, D., N. Teterina, E. Ehrenfeld, and K. Bienz. 2000. Formation of the poliovirus replication complex requires coupled viral translation, vesicle production, and viral RNA synthesis. J. Virol. 74:6570-6580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gale, M. J., Jr., M. J. Korth, N. M. Tang, S. L. Tan, D. A. Hopkins, T. E. Dever, S. J. Polyak, D. R. Gretch, and M. G. Katze. 1997. Evidence that hepatitis C virus resistance to interferon is mediated through repression of the PKR protein kinase by the nonstructural 5A protein. Virology 230:217-227. [DOI] [PubMed] [Google Scholar]
- 22.Gosert, R., D. Egger, and K. Bienz. 2000. A cytopathic and a cell culture adapted hepatitis-A virus strain differ in cell killing but not in intracellular membrane rearrangements. Virology 266:157-169. [DOI] [PubMed] [Google Scholar]
- 23.Gosert, R., A. Kanjanahaluethai, D. Egger, K. Bienz, and S. C. Baker. 2002. RNA replication of mouse hepatitis virus takes place at double-membrane vesicles. J. Virol. 76:3697-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gossen, M., and H. Bujard. 1992. Tight control of gene expression in mammalian cells by tetracycline-responsive promoters. Proc. Natl. Acad. Sci. USA 89:5547-5551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grakoui, A., C. Wychowski, C. Lin, S. M. Feinstone, and C. M. Rice. 1993. Expression and identification of hepatitis C virus polyprotein cleavage products. J. Virol. 67:1385-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hijikata, M., H. Mizushima, T. Akagi, S. Mori, N. Kakiuchi, N. Kato, T. Tanaka, K. Kimura, and K. Shimotohno. 1993. Two distinct proteinase activities required for the processing of a putative nonstructural precursor protein of hepatitis C virus. J. Virol. 67:4665-4675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hope, R. G., and J. McLauchlan. 2000. Sequence motifs required for lipid droplet association and protein stability are unique to the hepatitis C virus core protein. J. Gen. Virol. 81:1913-1925. [DOI] [PubMed] [Google Scholar]
- 28.Houghton, M. 1995. Hepatitis C viruses, p. 1035-1058. In B. N. Fields, D. M. Knipe, P. M. Howley (ed.), Virology, vol. 1. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 29.Hügle, T., F. Fehrmann, E. Bieck, M. Kohara, H. G. Kräusslich, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. Hepatitis C virus nonstructural protein 4B is an integral endoplasmic reticulum membrane protein. Virology 284:70-81. [DOI] [PubMed] [Google Scholar]
- 30.Kolykhalov, A. A., E. V. Agapov, K. J. Blight, K. Mihalik, S. M. Feinstone, and C. M. Rice. 1997. Transmission of hepatitis C by intrahepatic inoculation with transcribed RNA. Science 277:570-574. [DOI] [PubMed] [Google Scholar]
- 31.Kunkel, M., M. Lorinczi, R. Rijnbrand, S. M. Lemon, and S. J. Watowich. 2001. Self-assembly of nucleocapsid-like particles from recombinant hepatitis C virus core protein. J. Virol. 75:2119-2129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kuo, G., Q.-L. Choo, H. J. Alter, G. L. Gitnick, A. G. Redeker, R. H. Purcell, T. Miyamura, J. L. Dienstag, M. J. Alter, C. E. Stevens, G. E. Tegtmeier, F. Bonino, M. Colombo, W.-S. Lee, C. Kuo, K. Berger, J. R. Shuster, L. R. Overby, D. W. Bradley, and M. Houghton. 1989. An assay for circulating antibodies to a major etiologic virus of human non-A, non-B hepatitis. Science 244:362-364. [DOI] [PubMed] [Google Scholar]
- 33.Lohmann, V., F. Körner, A. Dobierzewska, and R. Bartenschlager. 2001. Mutations in hepatitis C virus RNAs conferring cell culture adaptation. J. Virol. 75:1437-1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lohmann, V., F. Körner, J. O. Koch, U. Herian, L. Theilmann, and R. Bartenschlager. 1999. Replication of subgenomic hepatitis C virus RNAs in a hepatoma cell line. Science 285:110-113. [DOI] [PubMed] [Google Scholar]
- 35.Moradpour, D., C. Englert, T. Wakita, and J. R. Wands. 1996. Characterization of cell lines allowing tightly regulated expression of hepatitis C virus core protein. Virology 222:51-63. [DOI] [PubMed] [Google Scholar]
- 36.Moradpour, D., P. Kary, C. M. Rice, and H. E. Blum. 1998. Continuous human cell lines inducibly expressing hepatitis C virus structural and nonstructural proteins. Hepatology 28:192-201. [DOI] [PubMed] [Google Scholar]
- 37.Pedersen, K. W., Y. vanderMeer, N. Roos, and E. J. Snijder. 1999. Open reading frame 1a-encoded subunits of the arterivirus replicase induce endoplasmic reticulum-derived double-membrane vesicles which carry the viral replication complex. J. Virol. 73:2016-2026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pfeifer, U., R. Thomssen, K. Legler, U. Böttcher, W. Gerlich, E. Weinmann, and O. Klinge. 1980. Experimental non-A, non-B hepatitis: four types of cytoplasmic alteration in hepatocytes of infected chimpanzees. Virchows Arch. B Cell Pathol. Incl. Mol. Pathol. 33:233-243. [DOI] [PubMed] [Google Scholar]
- 39.Qu, L., L. K. McMullan, and C. M. Rice. 2001. Isolation and characterization of noncytopathic pestivirus mutants reveals a role for nonstructural protein NS4B in viral cytopathogenicity. J. Virol. 75:10651-10662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Reed, K. E., and C. M. Rice. 2001. Overview of hepatitis C virus genome structure, polyprotein processing, and protein properties. Curr. Top. Microbiol. Immunol. 242:55-84. [DOI] [PubMed] [Google Scholar]
- 41.Rust, R. C., L. Landmann, R. Gosert, B. L. Tang, W. J. Hong, H. P. Hauri, D. Egger, and K. Bienz. 2001. Cellular COPII proteins are involved in production of the vesicles that form the poliovirus replication complex. J. Virol. 75:9808-9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Santolini, E., G. Migliaccio, and N. La Monica. 1994. Biosynthesis and biochemical properties of the hepatitis C virus core protein. J. Virol. 68:3631-3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Santolini, E., L. Pacini, C. Fipaldini, G. Migliaccio, and N. Monica. 1995. The NS2 protein of hepatitis C virus is a transmembrane polypeptide. J. Virol. 69:7461-7471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schmidt-Mende, J., E. Bieck, T. Hügle, F. Penin, C. M. Rice, H. E. Blum, and D. Moradpour. 2001. Determinants for membrane association of the hepatitis C virus RNA-dependent RNA polymerase. J. Biol. Chem. 276:44052-44063. [DOI] [PubMed] [Google Scholar]
- 45.Snijder, E. J., H. vanTol, N. Roos, and K. W. Pedersen. 2001. Non-structural proteins 2 and 3 interact to modify host cell membranes during the formation of the arterivirus replication complex. J. Gen. Virol. 82:985-994. [DOI] [PubMed] [Google Scholar]
- 46.Suhy, D. A., T. H. Giddings, and K. Kirkegaard. 2000. Remodeling the endoplasmic reticulum by poliovirus infection and by individual viral proteins: an autophagy-like origin for virus-induced vesicles. J. Virol. 74:8953-8965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan, S. L., and M. G. Katze. 2001. How hepatitis C virus counteracts the interferon response: the jury is still out on NS5A. Virology 284:1-12. [DOI] [PubMed] [Google Scholar]
- 48.Teterina, N. L., D. Egger, K. Bienz, D. M. Brown, B. L. Semler, and E. Ehrenfeld. 2001. Requirements for assembly of poliovirus replication complexes and negative-strand RNA synthesis. J. Virol. 75:3841-3850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tsukiyama-Kohara, K., N. Iizuka, M. Kohara, and A. Nomoto. 1992. Internal ribosome entry site within hepatitis C virus RNA. J. Virol. 66:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, C., P. Sarnow, and A. Siddiqui. 1993. Translation of human hepatitis C virus RNA in cultured cells is mediated by an internal ribosome-binding mechanism. J. Virol. 67:3338-3344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Westaway, E. G., J. M. MacKenzie, M. T. Kenney, M. K. Jones, and A. A. Khromykh. 1997. Ultrastructure of Kunjin virus-infected cells: colocalization of NS1 and NS3 with double-stranded RNA, and of NS2B with NS3, in virus-induced membrane structures. J. Virol. 71:6650-6661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wölk, B., D. Sansonno, H. G. Kräusslich, F. Dammacco, C. M. Rice, H. E. Blum, and D. Moradpour. 2000. Subcellular localization, stability, and trans-cleavage competence of the hepatitis C virus NS3-NS4A complex expressed in tetracycline-regulated cell lines. J. Virol. 74:2293-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]