Abstract
Membrane-proximal cysteines 259 and 260 in the cytoplasmic tail of the coxsackievirus and adenovirus receptor (CAR) are known to be essential for the tumor suppression activity of CAR. We demonstrate that these residues provide an S-acylation motif for modification of CAR with the fatty acid palmitate. Substitution of alanine for cysteines 259 and 260 results in the additional localization of CAR in perinuclear compartments with no effect on the efficiency of adenovirus infection. The results indicate that palmitylation is important for stable plasma membrane expression and biological activity of CAR but is not critical for adenovirus receptor performance.
Adenovirus (Ad) infection is initiated by high-affinity binding of Ad fiber to the coxsackievirus-Ad receptor (CAR), which contains an N-terminal exoplasmic region, a single transmembrane domain, and a C-terminal cytoplasmic tail (2, 3, 29). A number of studies have shown that the transmembrane and cytoplasmic domains of CAR are not essential for Ad vector infection of cells and tissues (22, 27, 31, 35-37). In contrast, it was recently demonstrated that the transmembrane and cytoplasmic domains are required for the biological activity of CAR in mediating cellular adhesion and growth suppression of human bladder and prostate tumor cells (20, 21). Strikingly, the suppression of tumor cell growth showed dependence on the presence of cysteines 259 and 260 (21). These first two amino acids in the membrane-proximal region of the cytoplasmic tail of CAR provide a putative signal for S-acylation (23, 25), the covalent posttranslational attachment of long-chain fatty acids to cysteine residues by thioester linkage (24).
In mammalian cells, the saturated 16-carbon fatty acid palmitate is commonly involved in S-acylation of membrane-spanning proteins, an event that can occur at different locations inside cells (24). Newly synthesized membrane proteins can incorporate palmitate in the endoplasmic reticulum (ER) or the cis-Golgi complex (7, 33). S-acylation also takes place at the plasma membrane, where it regulates internalization and recycling of surface receptors and controls stable plasma membrane expression of various surface proteins (1, 6, 14, 17). Palmitylation, a dynamic event driven by cellular palmityl transferase and thioesterase activities, plays an important role in the regulation of signaling activity of a variety of signal transduction molecules at the cell surface (10, 11, 13, 24, 39). In the present study, we evaluated whether the tandem cysteines at positions 259 and 260 in the cytoplasmic tail of CAR, which have recently been implicated in the regulation of CAR-facilitated signaling events (21), constitute a functional fatty acid acylation signal.
Palmitylation of CAR.
Different mutant forms of CAR were used to evaluate the function of cysteines 259 and 260 in S-acylation (Fig. 1). Construction of tailless CAR was done as reported before (37). Constructs encoding C259A, C260A-CAR, and C259Stop-CAR were generated by site-directed mutagenesis. A sensitive method for detection of protein fatty acylation is provided by radiolabeling of cells with synthetic fatty acid analogs that are modified with 125I at the ω-carbon group (4, 32). After labeling of CHO-CAR cells with 125I-IC16 and the relevant synthetic analog for palmitate (4) and immunoprecipitation with anti-CAR monoclonal antibody (RmcB), efficient 125I labeling of a single protein migrating at 46 kDa was observed (Fig. 2A, lanes 3 and 4, top). This 125I-IC16 labeling was not present in control CHO cells (Fig. 2A, lanes 1 and 2, top), which express very little endogenous CAR (2). Labeling with [35S]methionine, performed in parallel to provide an internal control for expression of the protein backbone, confirmed that significant levels of CAR protein were expressed in CHO-CAR cells but not in control CHO cells (Fig. 2A, bottom). Efficient 125I-IC16 labeling of CAR was also observed in human lung epithelial A549 cells, which express large amounts of endogenous CAR (data not shown). In both cases, the CAR-associated 125I-IC16 label was sensitive to alkaline treatment (data not shown), indicating that fatty acid was attached by a thioester linkage characteristic of protein palmitylation (4, 32).
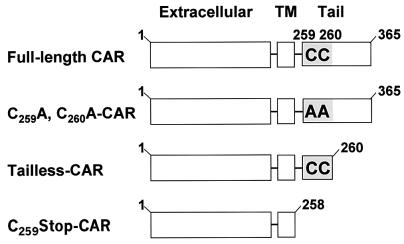
FIG. 1.
Schematic of human CAR constructs. Full-length human CAR (amino acids 1 to 365) contains an N-terminal exoplasmic region, a single transmembrane (TM) domain, and a C-terminal cytoplasmic tail that includes tandem cysteines at positions 259 and 260 (shaded area) next to the transmembrane domain. C259A, C260A-CAR is a full-length CAR construct in which cysteines 259 and 260 are replaced with alanines. In tailless-CAR (amino acids 1 to 260), the cytoplasmic tail is deleted, with the exception of cysteines 259 and 260, whereas in the C259Stop-CAR construct (amino acids 1 to 258), the entire cytoplasmic tail is removed.
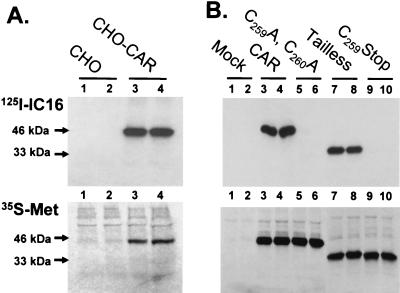
FIG. 2.
Palmitylation of human CAR at cysteines 259 and 260. (A) Control CHO cells (lanes 1 and 2) and CHO-CAR cells (lanes 3 and 4) were starved for 1 h at 37°C in Dulbecco modified Eagle medium containing 2% dialyzed fetal bovine serum and radiolabeled for 4 h at 37°C with 125I-IC16, an iodinated palmitate analog, at 20 μCi/ml (top). In parallel, cells were incubated for 1 h at 37°C in Dulbecco modified Eagle medium minus methionine and cysteine containing 2% dialyzed fetal bovine serum and radiolabeled for 4 h at 37°C with Expre35S35S protein labeling mix at 25 μCi/ml (35S-Met; bottom). Lysates from duplicate radiolabeled dishes were subjected to immunoprecipitation with anti-CAR monoclonal antibody (RmcB) and then subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and autoradiography. Radiolabeled CAR migrated with an apparent molecular mass of 46 kDa, as indicated by the arrow. (B) COS-1 cells were transfected with Lipofectamine (Invitrogen, Carlsbad, Calif.) and empty pcDNA3.1 vector (Mock; lanes 1 and 2) or plasmids containing full-length CAR (lanes 3 and 4), C259A, C260A- CAR (lanes 5 and 6), tailless-CAR (lanes 7 and 8), or C259Stop-CAR (lanes 9 and 10) and radiolabeled with 125I-IC16 (top) and Expre35S35S (35S-Met; bottom). The tailless-CAR and C259Stop-CAR constructs migrated at 33 kDa, as indicated by the arrow.
To analyze the exact site of palmitate linkage to CAR, radiolabeling was performed with transfected COS-1 cells that express either full-length CAR, tailless-CAR containing cysteines 259 and 260, or mutant CAR constructs in which cysteines 259 and 260 were replaced (C259A, C260A-CAR) or deleted (C259Stop-CAR; Fig. 1). Labeling with [35S]methionine showed that all of the constructs were expressed at similar levels and had the correct molecular masses, i.e., 46 kDa for CAR and C259A, C260A-CAR and 33 kDa for tailless-CAR and C259Stop-CAR (Fig. 2B, lanes 3 to 10, bottom). Significantly different labeling profiles were observed after 125I-IC16 incubation. In agreement with the previous results (Fig. 2A), high levels of 125I-IC16 incorporation were observed for full-length CAR (Fig. 2B, lanes 3 and 4, top). Strikingly, tailless-CAR, which contains cysteines 259 and 260 but lacks the remainder of the cytoplasmic tail (Fig. 1), also efficiently incorporated 125I-IC16, to levels similar to those incorporated by full-length CAR (Fig. 2B, compare lanes 7 and 8 with lanes 3 and 4, top). In contrast, no fatty acid incorporation was detected for the C259A, C260A-CAR and C259Stop-CAR constructs (Fig. 2B, lanes 5 and 6 and 9 and 10, top). These results show that the tandem cysteine motif at positions 259 and 260 in the cytoplasmic tail of CAR constitutes a signal for S-acylation with the fatty acid palmitate. The lack of 125I-IC16 incorporation in the C259A, C260A-CAR and C259Stop-CAR constructs (Fig. 2B) demonstrates that palmitylation is restricted to the cysteines at positions 259 and 260. Since both cysteines are equally fitted for palmitate attachment, each cellular CAR molecule can, in principle, be modified with two palmitate groups. However, it is difficult to demonstrate the palmitylation status of each individual cysteine residue unequivocally, as the removal of one cysteine can artificially induce or increase palmitate attachment to the remaining cysteine, complicating the interpretations of labeling experiments. Importantly, the observed palmitylation of the tailless CAR construct (Fig. 2B) shows that this cysteine motif, by itself, is sufficient for S-acylation, with no requirement for the remainder of the cytoplasmic tail. This observation suggests that the biological activity of tailless CAR in the suppression of tumor cell growth (21) is based on the palmitylation status of this construct. This hypothesis is also supported by the absence of this growth suppression activity in a CAR construct that lacks cysteines 259 and 260 (tailless-m; reference 21) and is not palmitylated (Fig. 2B).
Palmitylation and cellular localization of CAR.
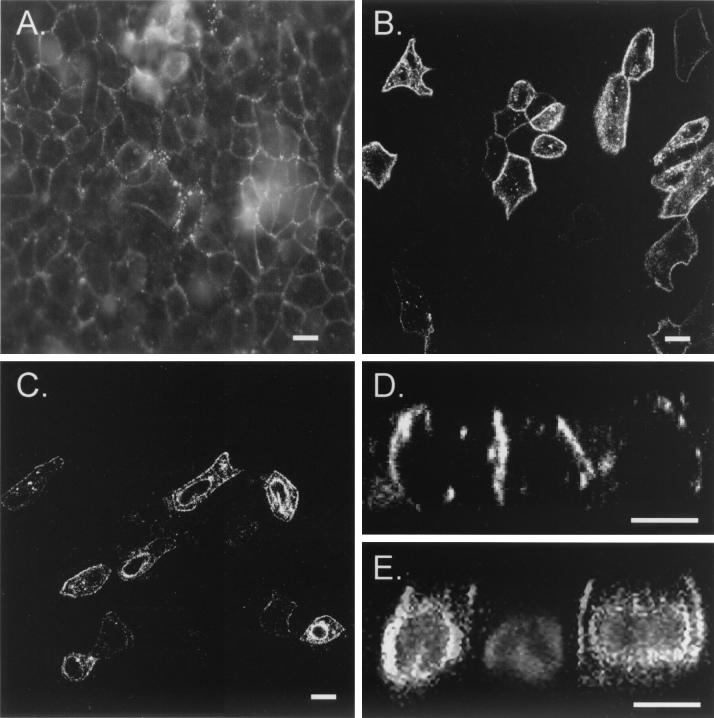
Protein palmitylation occurs at different locations inside the cell and has been associated with regulation of protein transport and cell surface localization of membrane receptors and membrane-associated cytoplasmic proteins (1, 6, 10, 13, 14, 17, 24, 39). Since CAR is abundantly expressed in many epithelial cells in adult tissues (9, 22, 28, 29, 34), we evaluated the role of S-acylation in the intracellular membrane distribution of human CAR in different epithelial cell types by confocal laser scanning fluorescence microscopy. Normal human bronchial epithelial (NHBE) cells, fixed with paraformaldehyde, lysed with Triton X-100, and then stained with anti-CAR monoclonal antibody(RmcB) and fluorescein isothiocyanate-conjugated goat anti-mouse antibody, displayed a uniform distribution of endogenous CAR in the lateral plasma membrane domain (Fig. 3A) in honeycomb patterns typically observed in airway epithelial cells (22, 34). Interestingly, CAR was concentrated in smaller clusters that typically accumulate at sites of contact between two or more cells (Fig. 3A), indicating localization of CAR at specific sublocations in the plasma membrane (22). To evaluate the role of the palmitylation motif in the intracellular distribution of CAR, Madin-Darby canine kidney (MDCK) epithelial cells were infected with an Ad vector encoding human CAR (AdCAR) or nonpalmitylated C259A, C260A-CAR (AdC259A, C260A-CAR). Two days after infection, exogenously expressed human CAR was specifically detected in subsets of MDCK cells infected by the AdCAR vector (Fig. 3B). Western blotting experiments demonstrated that the anti-CAR antibody did not cross-react with endogenous CAR in MDCK cells (data not shown). In agreement with earlier reports (22), human CAR expressed in MDCK cells also displayed lateral plasma membrane staining with punctate patterns, similar to that of endogenous CAR in NHBE cells (Fig. 3, compare panels A and B). In contrast, MDCK cells infected with AdC259A, C260A-CAR showed altered distribution of C259A, C260A-CAR, with prominent localization in perinuclear compartments in addition to the plasma membrane (Fig. 3C). This difference was also shown by analysis of optical sections taken in the vertical, x-z, direction by confocal laser scanning microscopy (Fig. 3D and E). In comparison to the basolateral membrane staining of CAR (Fig. 3D) (22, 34), nonpalmitylated CAR displayed a broader distribution, also appearing in a perinuclear compartment for which the identity is unknown. These findings indicate that, as has been observed for other palmitylated cell surface receptors (1, 6,14, 17, 24), S-acylation is essential for uniform plasma membrane expression of CAR.
FIG. 3.
Immunolocalization of CAR in epithelial cells. The intracellular membrane distribution of CAR in NHBE cells (A) or of human CAR (B and D) or nonpalmitylated C259A, C260A-CAR (C and E) expressed in MDCK cells by Ad-mediated gene transfer was analyzed by fixation in 4% paraformaldehyde for 30 min at room temperature, lysis for 20 min at room temperature with 1% Triton X-100 in phosphate-buffered saline containing 1 mM CaCl2 and 5 mM MgCl2, and staining with anti-CAR monoclonal antibody (RmcB) in phosphate-buffered saline containing 1 mM CaCl2 and 5 mM MgCl2 containing 0.2% albumin, followed by a fluorescein isothiocyanate-conjugated goat-anti-mouse secondary antibody. The distribution of CAR protein is visualized in white, and the 4′,6′-diamidino-2-phenylindole-stained nuclei in panel E are grey. Cells were monitored by laser scanning confocal microscopy en face (A to C) or in the x-z direction (D and E). Bars, 10 μm.
Since protein palmitylation can occur both in the ER or early Golgi and at the plasma membrane, the lack of palmitylation may reduce the transport of newly synthesized C259A, C260A-CAR through the ER-Golgi pathway or, alternatively, it may cause increased internalization of C259A, C260A-CAR from the plasma membrane, resulting in additional perinuclear localization. The same relative distribution of nonpalmitylated C259A, C260A-CAR between the plasma membrane and the perinuclear region can be seen inside cells expressing higher or lower levels of C259A, C260A-CAR protein (Fig. 3C). This implies that the altered distribution of nonpalmitylated C259A, C260A-CAR is not a consequence of Ad vector-driven overexpression.
Palmitylation of CAR and Ad-mediated gene transfer.
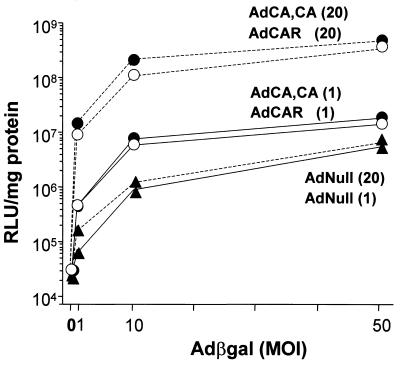
It is known that the transmembrane and cytoplasmic tail domains of CAR are not critical for Ad infection and Ad-mediated gene delivery to different cells and tissues (22, 27, 31, 35-37). Interestingly, the tailless CAR construct used in these studies contained cysteines 259 and 260, which mediate palmitylation (Fig. 1 and 2B). GPI-CAR, containing a glycosyl-phosphatidylinositol-glycolipid membrane anchor replacing the transmembrane and cytoplasmic regions, is another lipidated CAR construct capable of mediating Ad infection (22, 31, 35, 37). Both of these fatty acid and glycolipid modifications direct aggregation or clustering of proteins into membrane subdomains (8, 26, 32, 39). Since the Ad fiber can bind three CAR molecules and immobilization of CAR receptors increases the stability of the interactions between Ad fiber and CAR (5, 18), it was of relevance to reevaluate the significance of S-acylation of full-length CAR for the efficiency of Ad infection. To test this concept, CHO cells were first infected with different amounts of Ad vector encoding full-length CAR, which is palmitylated (Fig. 2), or an Ad vector expressing mutant C259A, C260A-CAR, which is not palmitylated (Fig. 2B). Two days after infection, at which time both CAR constructs were expressed at the same protein level (data not shown), cells were reinfected with increasing amounts of Ad reporter vector expressing the LacZ transgene from Escherichia coli (Adβgal) (19). Transgene expression (β-galactosidase [βgal] activity) was measured 1 day after Adβgal infection. Preinfection of CHO cells with Ad vector encoding CAR resulted in a dose-dependent increase in transgene expression levels over the background, measured in cells preinfected with the control, empty AdNull vector (Fig. 4). Remarkably, the transgene expression in cells expressing vector-derived CAR or nonpalmitylated C259A, C260A-CAR showed very similar profiles (Fig. 4). Likewise, the βgal activity levels in transfected CHO cells expressing either tailless CAR or C259Stop-CAR also displayed no significant differences (data not shown), in agreement with previous observations (21). Together, these results indicate that the possible localization of CAR in membrane lipid domains mediated by protein lipidation has no relevance for the efficiency of Ad infection. Recent structural analysis of coxsackievirus B3 in association with CAR suggests that interactions between cytoplasmic tails and transmembrane domains of separate CAR molecules drive pairwise binding of CAR to coxsackievirus capsids, and it will be of interest to test the role of CAR palmitylation in the clustering of bivalent CAR and its effect on coxsackievirus binding and infection (15).
FIG. 4.
Adβgal infection of CHO cells expressing CAR and nonpalmitylated CAR. CHO cells were infected with an Ad vector carrying no transgene (AdNull [▴]) or an Ad vector expressing wild-type CAR (AdCAR [○]) or C259A, C260A-CAR (AdCA, CA [•]) for 1 h at 37°C at 1 (solid lines) or 20 (broken lines) PFU per cell. After 2 days, triplicate monolayers of infected CHO cells were subsequently exposed to Adβgal for 1 h at 37°C at 0, 1, 10, or 50 PFU per cell and β-galactosidase activity was measured 24 h after Adβgal infection. Results are presented as the mean ± the standard deviation of triplicate monolayers of cells and are representative of two experiments. MOI, multiplicity of infection; RLU, relative light units.
Conclusions.
The cytoplasmic tail of CAR represents a significant part of its total protein structure and displays a high degree of homology (95%) between humans and mice (2, 3, 29), implying an important biological role for this domain. The present study provides direct evidence that the cytoplasmic tail of CAR contains a functional motif for S-acylation with the fatty acid palmitate (Fig. 2), consisting of cysteines 259 and 260, which are conserved in CAR in all of the species studied. The CAR tail also contains a consensus protein tyrosine phosphorylation motif and a signal for interaction with proteins containing PSD95/DLG/ZO-1 domains (2, 3, 12, 29), suggesting the involvement of CAR in intracellular signaling events. The role of protein palmitylation in the regulation of the activity of signaling proteins by mediation of the assembly or clustering of the molecules into specialized subdomains of the plasma membrane, such as caveolae or membrane lipid rafts, has received significant interest (10, 11, 13, 24, 26, 39). This concept supports a model in which palmitylation regulates the association of CAR with membrane subdomains that provide optimal microenvironments for the cellular activity of CAR.
However, CAR is an orphan receptor whose natural cellular function, other than as a receptor for viruses, remains unknown (2, 9, 28). CAR expression patterns show variability among different species and between different tissues and stages of development (9, 28). In rodents, the receptor is highly expressed during embryonic development throughout the nervous system and in epithelial cells, but after birth, nervous expression rapidly decreases and CAR is primarily restricted to epithelial cells (28). CAR levels may be up-regulated again in adult tissue in response to different stimuli, such as tissue regeneration, immune reactions, or cell density changes, indicating that CAR expression is tightly regulated during development and adulthood (9, 28). Interestingly, CAR expression levels differ considerably among human bladder, prostate, and other cancer cell types. Importantly, the CAR levels are low in highly tumorigenic cells and upregulation of CAR suppresses tumor cell growth (20, 21). The extent of CAR-mediated growth inhibition appears to be closely connected to the observed levels of CAR-facilitated intercellular adhesion activity in cancer cells (20, 21). This cellular activity of CAR as a cell adhesion molecule is supported by earlier reports demonstrating the capacity of exodomains of CAR to direct homotypic interactions (16, 30). The CAR-mediated cellular adhesion activity in tumor cells requires the exoplasmic and transmembrane domains of CAR, whereas tumor cell growth suppression also depends on the presence of cysteines 259 and 260 in the cytoplasmic domain (20, 21), which has been shown in the present study to provide a protein palmitylation motif.
In conclusion, the observations in the present study support the concept that palmitylation plays an important role in the regulation of the cellular distribution and biological activity of CAR. These findings suggest that manipulation of the palmitylation status of CAR presents an important mechanism for regulation of the tumor cell growth-suppressing potential of CAR. In this context, chemically modified fatty acid analogs that act as inhibitors of protein palmitylation and have the ability to manipulate the membrane distribution and activity of surface proteins should provide interesting tools with which to test this concept (38).
Acknowledgments
We thank M. Resh (Memorial Sloan-Kettering Cancer Center, New York, N.Y.) for the IC16 analog; M. Welsh, J. Zabner, and R. Walters (University of Iowa Medical College, Iowa City) for the AdCAR vector; V. Bonilha for help with microscopy; M. Kadnar for technical support; and N. Mohamed for help in preparing the manuscript.
This study was supported, in part, by the Will Rogers Memorial Fund, Los Angeles, Calif.; the Cystic Fibrosis Foundation, Bethesda, Md.; and GenVec, Inc., Gaithersburg, Md. W. van't Hof, as a Parker B. Francis Fellow in Pulmonary Research, is supported, in part, by the Francis Families Foundation.
REFERENCES
- 1.Alvarez, E., N. Girones, and R. J. Davis. 1990. Inhibition of the receptor-mediated endocytosis of diferric transferrin is associated with the covalent modification of the transferrin receptor with palmitic acid. J. Biol. Chem. 265:16644-16655. [PubMed] [Google Scholar]
- 2.Bergelson, J. M., J. A. Cunningham, G. Droguett, E. A. Kurt-Jones, A. Krithivas, J. S. Hong, M. S. Horwitz, R. L. Crowell, and R. W. Finberg. 1997. Isolation of a common receptor for Coxsackie B viruses and adenoviruses 2 and 5. Science 275:1320-1323. [DOI] [PubMed] [Google Scholar]
- 3.Bergelson, J. M., A. Krithivas, L. Celi, G. Droguett, M. S. Horwitz, T. Wickham, R. L. Crowell, and R. W. Finberg. 1998. The murine CAR homolog is a receptor for coxsackie B viruses and adenoviruses. J. Virol. 72:415-419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berthiaume, L., S. M. Peseckis, and M. D. Resh. 1995. Synthesis and use of iodo-fatty acid analogs. Methods Enzymol. 250:454-466. [DOI] [PubMed] [Google Scholar]
- 5.Bewley, M. C., K. Springer, Y. B. Zhang, P. Freimuth, and J. M. Flanagan. 1999. Structural analysis of the mechanism of adenovirus binding to its human cellular receptor, CAR. Science 286:1579-1583. [DOI] [PubMed] [Google Scholar]
- 6.Blanpain, C., V. Wittamer, J. M. Vanderwinden, A. Boom, B. Renneboog, B. Lee, E. Le Poul, L. El Asmar, C. Govaerts, G. Vassart, R. W. Doms, and M. Parmentier. 2001. Palmitoylation of CCR5 is critical for receptor trafficking and efficient activation of intracellular signaling pathways. J. Biol. Chem. 276:23795-23804. [DOI] [PubMed] [Google Scholar]
- 7.Bonatti, S., G. Migliaccio, and K. Simons. 1989. Palmitylation of viral membrane glycoproteins takes place after exit from the endoplasmic reticulum. J. Biol. Chem. 264:12590-12595. [PubMed] [Google Scholar]
- 8.Brown, D. A., and J. K. Rose. 1992. Sorting of GPI-anchored proteins to glycolipid-enriched membrane subdomains during transport to the apical cell surface. Cell 68:533-544. [DOI] [PubMed] [Google Scholar]
- 9.Carson, S. D. 2001. Receptor for the group B coxsackieviruses and adenoviruses: CAR. Rev. Med. Virol. 11:219-226. [DOI] [PubMed] [Google Scholar]
- 10.Casey, P. J. 1995. Protein lipidation in cell signaling. Science 268:221-225. [DOI] [PubMed] [Google Scholar]
- 11.Chen, C. A., and D. R. Manning. 2001. Regulation of G proteins by covalent modification. Oncogene 20:1643-1652. [DOI] [PubMed] [Google Scholar]
- 12.Cohen, C. J., J. Gaetz, T. Ohman, and J. M. Bergelson. 2001. Multiple regions within the coxsackievirus and adenovirus receptor cytoplasmic domain are required for basolateral sorting. J. Biol. Chem. 276:25392-25398. [DOI] [PubMed] [Google Scholar]
- 13.Dunphy, J. T., and M. E. Linder. 1998. Signalling functions of protein palmitoylation. Biochim. Biophys. Acta 1436:245-261. [DOI] [PubMed] [Google Scholar]
- 14.Fukushima, Y., T. Saitoh, M. Anai, T. Ogihara, K. Inukai, M. Funaki, H. Sakoda, Y. Onishi, H. Ono, M. Fujishiro, T. Ishikawa, K. Takata, R. Nagai, M. Omata, and T. Asano. 2001. Palmitoylation of the canine histamine H2 receptor occurs at Cys(305) and is important for cell surface targeting. Biochim. Biophys. Acta 1539:181-191. [DOI] [PubMed] [Google Scholar]
- 15.He, Y., P. R. Chipman, J. Howitt, C. M. Bator, M. A. Whitt, T. S. Baker, R. J. Kuhn, C. W. Anderson, P. Freimuth, and M. G. Rossmann. 2001. Interaction of coxsackievirus B3 with the full length coxsackievirus-adenovirus receptor. Nat. Struct. Biol. 8:874-878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Honda, T., H. Saitoh, M. Masuko, T. Katagiri-Abe, K. Tominaga, I. Kozakai, K. Kobayashi, T. Kumanishi, Y. G. Watanabe, S. Odani, and R. Kuwano. 2000. The coxsackievirus-adenovirus receptor protein as a cell adhesion molecule in the developing mouse brain. Brain Res. Mol. Brain Res. 77:19-28. [DOI] [PubMed] [Google Scholar]
- 17.Jing, S. Q., and I. S. Trowbridge. 1990. Nonacylated human transferrin receptors are rapidly internalized and mediate iron uptake. J. Biol. Chem. 265:11555-11559. [PubMed] [Google Scholar]
- 18.Lortat-Jacob, H., E. Chouin, S. Cusack, and M. J. van Raaij. 2001. Kinetic analysis of adenovirus fiber binding to its receptor reveals an avidity mechanism for trimeric receptor-ligand interactions. J. Biol. Chem. 276:9009-9015. [DOI] [PubMed] [Google Scholar]
- 19.Mastrangeli, A., C. Danel, M. A. Rosenfeld, L. Stratford-Perricaudet, M. Perricaudet, A. Pavirani, J. P. Lecocq, and R. G. Crystal. 1993. Diversity of airway epithelial cell targets for in vivo recombinant adenovirus-mediated gene transfer. J. Clin. Investig. 91:225-234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Okegawa, T., Y. Li, R. C. Pong, J. M. Bergelson, J. Zhou, and J. T. Hsieh. 2000. The dual impact of coxsackie and adenovirus receptor expression on human prostate cancer gene therapy. Cancer Res. 60:5031-5036. [PubMed] [Google Scholar]
- 21.Okegawa, T., R. C. Pong, Y. Li, J. M. Bergelson, A. I. Sagalowsky, and J. T. Hsieh. 2001. The mechanism of the growth-inhibitory effect of coxsackie and adenovirus receptor (CAR) on human bladder cancer: a functional analysis of CAR protein structure. Cancer Res. 61:6592-6600. [PubMed] [Google Scholar]
- 22.Pickles, R. J., J. A. Fahrner, J. M. Petrella, R. C. Boucher, and J. M. Bergelson. 2000. Retargeting the coxsackievirus and adenovirus receptor to the apical surface of polarized epithelial cells reveals the glycocalyx as a barrier to adenovirus-mediated gene transfer. J. Virol. 74:6050-6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ponimaskin, E., and M. F. Schmidt. 1998. Domain-structure of cytoplasmic border region is main determinant for palmitoylation of influenza virus hemagglutinin (H7). Virology 249:325-335. [DOI] [PubMed] [Google Scholar]
- 24.Resh, M. D. 1999. Fatty acylation of proteins: new insights into membrane targeting of myristoylated and palmitoylated proteins. Biochim. Biophys. Acta 1451:1-16. [DOI] [PubMed] [Google Scholar]
- 25.Schweizer, A., J. Rohrer, and S. Kornfeld. 1995. Determination of the structural requirements for palmitoylation of p63. J. Biol. Chem. 270:9638-9644. [DOI] [PubMed] [Google Scholar]
- 26.Simons, K., and E. Ikonen. 1997. Functional rafts in cell membranes. Nature 387:569-572. [DOI] [PubMed] [Google Scholar]
- 27.Tallone, T., S. Malin, A. Samuelsson, J. Wilbertz, M. Miyahara, K. Okamoto, L. Poellinger, L. Philipson, and S. Pettersson. 2001. A mouse model for adenovirus gene delivery. Proc. Natl. Acad. Sci. USA 98:7910-7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomko, R. P., C. B. Johansson, M. Totrov, R. Abagyan, J. Frisen, and L. Philipson. 2000. Expression of the adenovirus receptor and its interaction with the fiber knob. Exp. Cell Res. 255:47-55. [DOI] [PubMed] [Google Scholar]
- 29.Tomko, R. P., R. Xu, and L. Philipson. 1997. HCAR and MCAR: the human and mouse cellular receptors for subgroup C adenoviruses and group B coxsackieviruses. Proc. Natl. Acad. Sci. USA 94:3352-3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van Raaij, M. J., E. Chouin, H. van der Zandt, J. M. Bergelson, and S. Cusack. 2000. Dimeric structure of the coxsackievirus and adenovirus receptor D1 domain at 1.7 A resolution. Struct. Fold Des. 8:1147-1155. [DOI] [PubMed] [Google Scholar]
- 31.van't Hof, W., and R. G. Crystal. 2001. Manipulation of the cytoplasmic and transmembrane domains alters cell surface levels of the coxsackie-adenovirus receptor and changes the efficiency of adenovirus infection. Hum. Gene Ther. 12:25-34. [DOI] [PubMed] [Google Scholar]
- 32.van't Hof, W., and M. D. Resh. 2000. Targeting proteins to plasma membrane and membrane microdomains by N-terminal myristoylation and palmitoylation. Methods Enzymol. 327:317-330. [DOI] [PubMed] [Google Scholar]
- 33.Veit, M., and M. F. Schmidt. 1993. Timing of palmitoylation of influenza virus hemagglutinin. FEBS Lett. 336:243-247. [DOI] [PubMed] [Google Scholar]
- 34.Walters, R. W., T. Grunst, J. M. Bergelson, R. W. Finberg, M. J. Welsh, and J. Zabner. 1999. Basolateral localization of fiber receptors limits adenovirus infection from the apical surface of airway epithelia. J. Biol. Chem. 274:10219-10226. [DOI] [PubMed] [Google Scholar]
- 35.Walters, R. W., W. van't Hof, S. M. Yi, M. K. Schroth, J. Zabner, R. G. Crystal, and M. J. Welsh. 2001. Apical localization of the coxsackie-adenovirus receptor by glycosyl-phosphatidylinositol modification is sufficient for adenovirus-mediated gene transfer through the apical surface of human airway epithelia. J. Virol. 75:7703-7711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wan, Y. Y., R. P. Leon, R. Marks, C. M. Cham, J. Schaack, T. F. Gajewski, and J. DeGregori. 2000. Transgenic expression of the coxsackie/adenovirus receptor enables adenoviral-mediated gene delivery in naive T cells. Proc. Natl. Acad. Sci. USA 97:13784-13789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang, X., and J. M. Bergelson. 1999. Coxsackievirus and adenovirus receptor cytoplasmic and transmembrane domains are not essential for coxsackievirus and adenovirus infection. J. Virol 73:2559-2562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Webb, Y., L. Hermida-Matsumoto, and M. D. Resh. 2000. Inhibition of protein palmitoylation, raft localization, and T cell signaling by 2-bromopalmitate and polyunsaturated fatty acids. J. Biol. Chem. 275:261-270. [DOI] [PubMed] [Google Scholar]
- 39.Zhang, W., R. P. Trible, and L. E. Samelson. 1998. LAT palmitoylation: its essential role in membrane microdomain targeting and tyrosine phosphorylation during T cell activation. Immunity 9:239-246. [DOI] [PubMed] [Google Scholar]