Abstract
Ebola virus is a highly lethal pathogen responsible for several outbreaks of hemorrhagic fever. Here we show that the primate lentiviral binding C-type lectins DC-SIGN and L-SIGN act as cofactors for cellular entry by Ebola virus. Furthermore, DC-SIGN on the surface of dendritic cells is able to function as a trans receptor, binding Ebola virus-pseudotyped lentiviral particles and transmitting infection to susceptible cells. Our data underscore a role for DC-SIGN and L-SIGN in the infective process and pathogenicity of Ebola virus infection.
Ebola virus is responsible for several major outbreaks of hemorrhagic fever, the exceedingly high mortality of which has raised great public concern. Ebola virus research has been hampered by the strict biosafety containment procedures required for handling the infectious agent. However, the structural similarity of Ebola virus glycoprotein (GP) to retroviral envelopes (6) has recently allowed the generation of pseudotyped recombinant retroviral particles that have been used to explore important aspects of the Ebola virus biology (16, 18). Ebola virus cell entry is presumably mediated by the interaction of a cellular receptor with the GP1 subunit of the viral envelope (12). A cofactor for cellular entry of Ebola virus and Marburg filoviruses in certain cell types has been recently identified as the folate receptor α (FRα) (3). This molecule is a glycophosphatidylinositol-linked protein highly conserved in mammalian species and expressed in epithelial and parenchymal cells of a number of organs, but not abundantly in liver or endothelial cells (15).
DC-SIGN (dendritic cell [DC]-specific ICAM-3 grabbing non-integrin, CD209) is a type II membrane protein with a C-type lectin extracellular domain, the expression of which is restricted to immature DC. DC-SIGN appears to play a key role in the initial stages of immune response and in the migratory behavior of DC, because it mediates DC interactions with T lymphocytes and endothelial cells through recognition of ICAM-3 (9) and ICAM-2 (7). DC-SIGN, originally cloned as a human immunodeficiency virus (HIV) gp120-binding protein (5), does not act as a receptor for cellular entry of HIV; instead, it confers to DC the ability to facilitate infection in trans of susceptible cells (8). Recently, DC-SIGN and the newly described DC-SIGN homologue L-SIGN have been shown to bind most lentiviruses of primates: HIV-1 (both R5 and X4 strains), HIV-2, and simian immunodeficiency virus (SIV) (13). Unlike DC-SIGN, L-SIGN is not expressed by DC, but is expressed on the surface of endothelial cells in the liver, lymph node sinuses, and placental villi (2). The affinity of these membrane receptors for retroviral GP and their tissue distribution pattern prompted us to study their potential role as binding and entry cofactors for Ebola virus.
To investigate the participation of DC-SIGN in Ebola virus infection, we have utilized lentiviral particles pseudotyped with Ebola virus GP according to a transient transfection protocol previously described (17). The lentiviral vector pNL4-3.Luc.R−E−10 was used for production of vesicular stomatitis virus G (VSV-G) and Ebola virus Zaire and Reston GP pseudotypes. Expression plasmids for the GP of the Zaire and Reston strains of Ebola virus were kindly provided by A. Sanchez, Centers for Disease Control and Prevention (18). Supernatants were obtained 48 h after transfection, filtered (0.45-μm pore size), and stored frozen at −80°C. Infectious titers were estimated by serial dilution on HeLa cells and were typically in the range of 107 infectious units/ml for VSV-G and 105 infectious units/ml for Ebola virus GP pseudotypes. The following reagents were obtained through the NIH AIDS Research and Reference Reagent Program, Division of AIDS, National Institute for Allergy and Infectious Diseases: DC-SIGN and L-SIGN monoclonal antibody DC28 (0.8 mg/ml as ascitic fluid) from F. Baribaud, S. Pöhlmann, J. A. Hoxie, and R. W. Doms (1); pcDNA3-L-SIGN6 from Mary Carrington; and pNL4-3.Luc.R−E− from Nathaniel Landau (10).
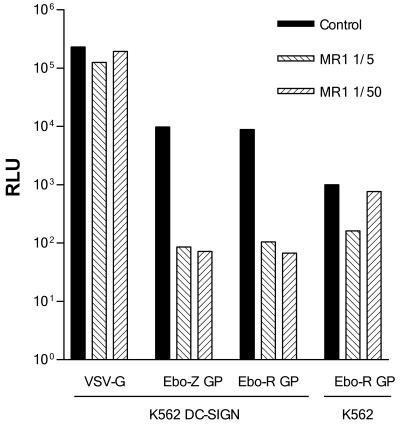
To investigate the role of DC-SIGN in Ebola virus binding and cellular entry, we first used a stable transfectant of DC-SIGN in the erythroleukemic K562 cell line (14). K562 cells were incubated overnight in 24-well plates with supernatants containing Ebola virus GP-pseudotyped lentivirus at a multiplicity of infection (MOI) of 0.1. Infectivity was measured 48 h after infection by luciferase assay with reagents from Promega (Madison, Wis.) in a Berthold Sirius luminometer (Berthold, Munich, Germany) with a dynamic range from 102 to 107 relative light units (RLU). Infectivity of the parental K562 cells with an Ebola virus GP-pseudotyped lentiviral construction was detectable, although relatively low. In contrast, infectivity of the DC-SIGN transfectant cell line was 1 order of magnitude higher, and it was significantly reduced in the presence of the DC-SIGN-specific monoclonal antibody MR-1, thus suggesting that Ebola virus might interact with DC-SIGN and facilitate viral entry into K562-DC-SIGN-transfected cells (Fig. 1). MR-1 was used as tissue culture supernatant (10 μg/ml) and showed no reactivity with HeLa and K562 cells, as well as a panel of myeloid and lymphoid cell lines (14).
FIG. 1.
DC-SIGN-mediated infection of K562-DC-SIGN cells. K562 and K562-DC-SIGN cells were infected with VSV-G-, Ebola virus Zaire (Ebo-Z)-, or Ebola virus Reston (Ebo-R) GP-pseudotyped lentivirus in the absence (control) or presence of the DC-SIGN-specific monoclonal antibody MR-1. Infectivity was measured as luciferase activity 48 h postinfection. One representative experiment out of three is shown.
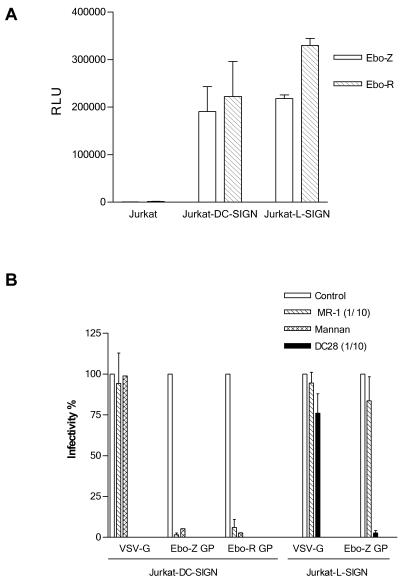
To further characterize the role of DC-SIGN and its close homologue L-SIGN in Ebola virus cell entry, we expressed, by using retroviral vectors, DC-SIGN and L-SIGN in the Jurkat cell line, since these cells are nonpermissive for Ebola virus infection and are considered receptor deficient (16). Recombinant retroviruses were produced as described previously (17) by cotransfection of the plasmids pNGVL-MLV-gag-pol and pCMV-VSV-G and the retroviral vector pLZRs-DC-SIGN-gfp—constructed by subcloning the DC-SIGN coding sequence obtained from placental RNA by reverse transcription-PCR with primers AAA AGG ATC CGC CGC CAC CAT GAG TGA CTC CAA GGA ACC (forward) and AAA AGA ATT CCT ACG CAG GAG GGG GGT TT (reverse), into the bicistronic retroviral vector pLZRs-M10-gfp (17), digested with BamHI and EcoRI—or pLZRs-L-SIGN-gfp constructed in a similar way with the L-SIGN coding sequence obtained from pcDNA3-L-SIGN6. Plasmids pNGVL-MLV-gag-pol, pLZRs-RevM10-gfp, and pCMV-VSV-G were generously provided by G. Nabel, University of Michigan (17). Jurkat cells were transduced with VSV-G-pseudotyped DC-SIGN- or L-SIGN-expressing retroviral vectors by spinoculation for 2 h at 1,500 × g at an MOI of 10. After 48 h, cells were analyzed by fluorescence-activated cell sorting for green fluorescent protein (GFP) and lectin expression (range of positive cells, 10 to 30%) and challenged in 24-well plates with Ebola virus GP pseudotypes or controls: 250,000 cells were resuspended in 250 μl of complete medium (RPMI, 10% fetal bovine serum [FBS]) and incubated overnight with 250 μl of supernatant from transfections. Cells were assayed for luciferase expression 48 h postinfection. For inhibition experiments, cells were preincubated for 10 min at room temperature with the carbohydrate-interaction inhibitor mannan (25 μg/ml; Sigma, St. Louis, Mo.) or lectin-specific antibodies. Jurkat cells expressing DC-SIGN or L-SIGN were clearly infected by Ebola virus Zaire and Reston GP-pseudotyped lentiviral vectors, indicating that expression of either of these two lectins in Jurkat cells is sufficient to confer permissivity (Fig. 2A). The DC-SIGN and L-SIGN dependency of the Jurkat cell infection was confirmed by the clear reduction of infectivity in the presence of mannan and anti-DC-SIGN and anti-L-SIGN antibodies, whereas a VSV-G-pseudotyped control was unaffected (Fig. 2B). Our results clearly indicate that DC-SIGN and L-SIGN are implicated in Ebola virus GP-mediated cell infection; however, the contribution and the specific molecular interactions of DC-SIGN and L-SIGN in Ebola virus cell entry remain to be defined. In this respect, and since many cells known to be susceptible to Ebola virus do not express these lectins, our results, like those recently reported for HIV and SIV (11), support the hypothesis that DC-SIGN and L-SIGN bind and concentrate Ebola virus to the cell membrane, thus facilitating the interaction in cis with cofactors required for cell entry, the low density of which may be limiting for infection of certain cell types.
FIG. 2.
(A) Jurkat cells expressing DC-SIGN and L-SIGN are permissive for Ebola virus infection. Control Jurkat cells or Jurkat cells expressing DC-SIGN or L-SIGN by transduction with a retroviral vector were infected with Ebola virus Zaire (Ebo-Z) or Reston (Ebo-R) GP-pseudotyped lentivirus (mean ± standard error, n = 3). (B) Specificity of DC-SIGN- and L-SIGN-mediated infectivity of Jurkat cells. DC-SIGN- and L-SIGN-mediated infectivity of Jurkat cells transduced with the retroviral vectors mentioned above was assessed by preincubation with mannan and specific monoclonal antibodies: MR-1 is DC-SIGN specific, and DC28 exhibits specificity for both DC-SIGN and L-SIGN. Results are shown as the percentage of luciferase activity compared to that of the untreated cells (mean ± standard error, n = 3). Mannan was tested once on Jurkat-DC-SIGN. DC28 was used only for Jurkat L-SIGN.
Finally, the role of DC-SIGN-Ebola virus GP interaction on DC was explored by using monocyte-derived DC (MDDC). MDDC were obtained from blood monocytes according to a standardized protocol (14). Cells were cultured for 5 to 7 days in the presence of granulocyte-macrophage colony-stimulating factor and interleukin-4 to obtain a population of immature MDDC. DC were infected with the lentiviruses pseudotyped with VSV-G and Ebola virus GP (MOI of 10 and 0.1, respectively). Forty-eight to 72 h postinfection, cells were assayed for luciferase expression as described before. Infection of MDDC, although at a low level, was demonstrated by using a VSV-G-pseudotyped control. However, under the conditions used in our experiments and in spite of the high DC-SIGN expression of MDDC, we were unable to readily detect luciferase expression upon infection with Ebola virus GP-pseudotyped lentiviral vectors (data not shown). In this respect, and taking into account the evidence of Ebola virus infection of DC in vitro and in vivo (4), it is possible that limitations of the lentivirus pseudotyping approach, such as low titers or the requirement of additional viral products for entry into DC, might account for this negative result. We next tested whether DC-SIGN on the surface of DC could bind Ebola virus GP-pseudotyped viral particles and facilitate subsequent infection of susceptible cells (Fig. 3). DC were preincubated (150,000 cells in 100 μl) for 20 min at room temperature in the presence or absence of the DC-SIGN-specific antibody MR-1. Supernatants (300 μl) containing Ebola virus GP- or VSV-G-pseudotyped lentiviral particles were then added, and cells were maintained in rotation at room temperature for 2 h. Cells were washed four times in phosphate-buffered saline (PBS)-2% FBS, resuspended in 300 μl of fresh medium, and added to HeLa cells plated in 24-well plates. The same amount of supernatant maintained at room temperature without DC was used as control of infectivity. After 48 h of cocultivation, wells were washed twice with PBS, and HeLa cells were assayed for luciferase activity as described above. The infectivity achieved by cocultivation of HeLa and MDDC, incubated with a high-titer VSV-G-pseudotyped lentiviral supernatant and extensively washed, was more than 2 orders of magnitude lower than that of the initial non-cell-incubated supernatant. The remaining infectivity was unaffected by preincubation of MDDC with a DC-SIGN-specific antibody suggesting that it was most likely due to unspecific binding. In contrast, MDDC incubated with infectious supernatants of Ebola virus GP-pseudotyped viruses retained a higher proportion of the infectivity of the supernatant after extensive washing. This effect was significantly reduced by preincubating MDDC with a DC-SIGN-specific antibody, indicating that MDDC are capable, through DC-SIGN interactions, of binding Ebola virus GP-pseudotyped viruses, maintaining infectivity, and achieving efficient infection in trans of susceptible cells in a way similar to that described for lentiviruses (8).
FIG. 3.
MDDC bind Ebola virus GP-pseudotyped particles and transmit infectivity to susceptible cells. MDDC were incubated with infectious supernatants containing VSV-G-, Ebola virus Zaire (Ebo-Z)-, or Ebola virus Reston (Ebo-R) GP-pseudotyped lentiviruses after a brief preincubation in the absence or presence of MR-1 DC-SIGN-specific monoclonal antibody. Cells were extensively washed thereafter and plated onto HeLa cells. The same amount of infectious supernatant (Sup) without incubation with DC was directly added to the HeLa cells as a control of the original infectivity. Cells were assayed for luciferase 48 h after infection. The experiment was performed with cells from two independent donors, and a representative result is shown.
We have found that expression of DC-SIGN and its homologue L-SIGN enhances infectivity of Ebola virus-susceptible cells and is sufficient to confer permissivity for Ebola virus GP-mediated infection to a nonsusceptible cell line. Also, DC-SIGN on the surface of DC appears to act as a trans receptor capable of binding Ebola virus GP-pseudotyped viruses and efficiently transmitting the infection to susceptible cells. DC-SIGN and L-SIGN appear to be universal binding factors for primate lentiviruses. Our data indicate that these molecules have extended participation in other viral infections. The role of these C-type lectins in Ebola virus primary infection and dissemination deserves further investigation.
Acknowledgments
We thank Keith Martin for review of the manuscript.
This work has been partially supported by grants CAM 08.3/0026.1/2000 and FIS 01/0063-01 to A.L.C. and FIS 99/0514 and 01/1430 to R.D.
REFERENCES
- 1.Baribaud, F., S. Pohlmann, T. Sparwasser, M. T. Y. Kimata, Y.-K. Choi, B. S. Haggarty, N. Ahmad, T. Macfarlan, T. G. Edwards, G. J. Leslie, J. Arnason, T. A. Reinhart, J. T. Kimata, D. R. Littman, J. A. Hoxie, and R. W. Doms. 2001. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 75:10281-10289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bashirova, A. A., T. B. H. Geijtenbeek, G. C. F. van Duijnhoven, S. J. van Vliet, J. B. G. Eilering, M. P. Martin, L. Wu, T. D. Martin, N. Viebig, P. A. Knolle, V. N. KewalRamani, Y. van Kooyk, and M. Carrington. 2001. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 193:671-678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan, S. Y., C. J. Empig, F. J. Welte, R. F. Speck, A. Schmaljohn, J. F. Kreisberg, and M. A. Goldsmith. 2001. Folate receptor-alpha is a cofactor for cellular entry by Marburg and Ebola viruses. Cell 106:117-126. [DOI] [PubMed] [Google Scholar]
- 4.Connolly, B. M., K. E. Steele, K. J. Davis, T. W. Geisbert, W. M. Kell, N. K. Jaax, and P. B. Jahrling. 1999. Pathogenesis of experimental Ebola virus infection in guinea pigs. J. Infect. Dis. 179(Suppl. 1):S203-S217. [DOI] [PubMed] [Google Scholar]
- 5.Curtis, B. M., S. Scharnowske, and A. J. Watson. 1992. Sequence and expression of a membrane-associated C-type lectin that exhibits CD4-independent binding of human immunodeficiency virus envelope glycoprotein gp120. Proc. Natl. Acad. Sci. USA 89:8356-8360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gallaher, W. R. 1996. Similar structural models of the transmembrane proteins of Ebola and avian sarcoma viruses. Cell 85:477-478. [DOI] [PubMed] [Google Scholar]
- 7.Geijtenbeek, T. B., D. J. Krooshoop, D. A. Bleijs, S. J. van Vliet, G. C. van Duijnhoven, V. Grabovsky, R. Alon, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 1:353-357. [DOI] [PubMed] [Google Scholar]
- 8.Geijtenbeek, T. B., D. S. Kwon, R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, J. Middel, I. L. Cornelissen, H. S. Nottet, V. N. KewalRamani, D. R. Littman, C. G. Figdor, and Y. van Kooyk. 2000. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell 100:587-597. [DOI] [PubMed] [Google Scholar]
- 9.Geijtenbeek, T. B., R. Torensma, S. J. van Vliet, G. C. van Duijnhoven, G. J. Adema, Y. van Kooyk, and C. G. Figdor. 2000. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 100:575-585. [DOI] [PubMed] [Google Scholar]
- 10.He, J., S. Choe, R. Walker, P. Di Marzio, D. O. Morgan, and N. R. Landau. 1995. Human immunodeficiency virus type 1 viral protein R (Vpr) arrests cells in the G2 phase of the cell cycle by inhibiting p34cdc2 activity. J. Virol. 69:6705-6711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee, B., G. Leslie, E. Soilleux, U. O'Doherty, S. Baik, E. Levroney, K. Flummerfelt, W. Swiggard, N. Coleman, M. Malim, and R. W. Doms. 2001. cis expression of DC-SIGN allows for more efficient entry of human and simian immunodeficiency viruses via CD4 and a coreceptor. J. Virol. 75:12028-12038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Peters, C. J., A. Sanchez, P. E. Rollin, T. G. Ksiazek, and F. A. Murphy. 1996. Filoviridae: Marburg and Ebola viruses, p. 1161-1176. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Field's virology, 3rd ed. Lippincott-Raven, Philadelphia, Pa.
- 13.Pöhlmann, S., F. Baribaud, B. Lee, G. J. Leslie, M. D. Sanchez, K. Hiebenthal-Millow, J. Münch, F. Kirchhoff, and R. W. Doms. 2001. DC-SIGN interactions with human immunodeficiency virus type 1 and 2 and simian immunodeficiency virus. J. Virol. 75:4664-4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Relloso, M., A. Puig-Kröger, O. Muñiz, J. L. Rodríguez-Fernández, G. de la Rosa, N. Longo, J. Navarro, M. A. Muñoz-Fernández, P. Sánchez-Mateos, and A. Corbí. 2002. DC-SIGN (CD209) expression is IL-4 dependent and is negatively regulated by IFN, TGF-β, and anti-inflammatory agents. J. Immunol. 168:2634-2643. [DOI] [PubMed] [Google Scholar]
- 15.Weitman, S. D., R. H. Lark, L. R. Coney, D. W. Fort, V. Frasca, V. R. Zurawski, Jr., and B. A. Kamen. 1992. Distribution of the folate receptor GP38 in normal and malignant cell lines and tissues. Cancer Res. 52:3396-3401. [PubMed] [Google Scholar]
- 16.Wool-Lewis, R. J., and P. Bates. 1998. Characterization of Ebola virus entry by using pseudotyped viruses: identification of receptor-deficient cell lines. J. Virol. 72:3155-3160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang, S., R. Delgado, S. R. King, C. Woffendin, C. S. Barker, Z. Y. Yang, L. Xu, G. P. Nolan, and G. J. Nabel. 1999. Generation of retroviral vector for clinical studies using transient transfection. Hum. Gene Ther. 10:123-132. [DOI] [PubMed] [Google Scholar]
- 18.Yang, Z., R. Delgado, L. Xu, R. F. Todd, E. G. Nabel, A. Sanchez, and G. J. Nabel. 1998. Distinct cellular interactions of secreted and transmembrane Ebola virus glycoproteins. Science 279:1034-1037. [DOI] [PubMed] [Google Scholar]