Abstract
Wild-type measles virus (MV) strains use human signaling lymphocyte activation molecule (SLAM) as a cellular receptor, while vaccine strains such as the Edmonston strain can use both SLAM and CD46 as receptors. Although the expression of SLAM is restricted to cells of the immune system (lymphocytes, dendritic cells, and monocytes), histopathological studies with humans and experimentally infected monkeys have shown that MV also infects SLAM-negative cells, including epithelial, endothelial, and neuronal cells. In an attempt to explain these findings, we produced the enhanced green fluorescent protein (EGFP)-expressing recombinant MV (IC323-EGFP) based on the wild-type IC-B strain. IC323-EGFP showed almost the same growth kinetics as the parental recombinant MV and produced large syncytia exhibiting green autofluorescence in SLAM-positive cells. Interestingly, all SLAM-negative cell lines examined also showed green autofluorescence after infection with IC323-EGFP, although the virus hardly spread from the originally infected individual cells and thus did not induce syncytia. When the number of EGFP-expressing cells after infection was taken as an indicator, the infectivities of IC323-EGFP for SLAM-negative cells were 2 to 3 logs lower than those for SLAM-positive cells. Anti-MV hemagglutinin antibody or fusion block peptide, but not anti-CD46 antibody, blocked IC323-EGFP infection of SLAM-negative cells. This infection occurred under conditions in which entry via endocytosis was inhibited. These results indicate that MV can infect a variety of cells, albeit with a low efficiency, by using an as yet unidentified receptor(s) other than SLAM or CD46, in part explaining the observed MV infection of SLAM-negative cells in vivo.
Measles virus (MV) is an enveloped virus of the Morbillivirus genus in the Paramyxoviridae family and has a linear, nonsegmented, negative-strand RNA genome with two envelope glycoproteins, the hemagglutinin (H) and fusion (F) proteins (12). Despite the development of effective live vaccines, measles remains a significant cause of infant mortality worldwide, mainly due to secondary infections caused by MV-induced immunosuppression (12).
Vaccine strains of MV such as the Edmonston strain use human CD46 as a cellular receptor (9, 25). Since CD46 is expressed on all nucleated human cells (19), vaccine strains of MV can infect almost any human cell line. In contrast, wild-type strains of MV isolated in the marmoset B-cell line B95a or human B-cell lines are usually unable to use CD46 as a receptor (6, 13, 17, 18, 36, 37, 46, 47). Recently, we have demonstrated that signaling lymphocyte activation molecule (SLAM; also known as CD150) acts as a cellular receptor for both vaccine and wild-type strains of MV (48). SLAM is a costimulatory molecule in lymphocyte activation (7), and its expression is restricted to activated T and B lymphocytes, immature thymocytes (7, 41), mature dendritic cells (26), and activated monocytes (23), nicely explaining the tropism of MV as well as the lymphopenia and immunosuppression observed in MV infection. We have also reported that viruses obtained from clinical specimens (throat swabs of measles patients) use SLAM but not CD46 as a receptor (28). Previous histopathological studies in vivo, however, have revealed that in addition to infecting SLAM-positive cells of the immune system, MV also infects endothelial (11, 15, 16, 21, 24), epithelial (21, 24, 44), and neuronal cells (3, 24, 40), none of which have been shown to express SLAM (7, 41). Thus, the in vivo receptor usage of MV remains to be determined.
Reverse genetics technology has enabled us to study a number of important problems concerning virus replication and pathogenesis. As for MV, the rescue of the Edmonston strain from cloned DNA was developed in 1995 (32), providing us with many insights into MV biology (10, 30, 31, 35, 49, 50). However, since the vaccine strain does not exhibit pathogenicity in experimentally infected monkeys (1, 17), results obtained with it may not be applicable to clinical problems in vivo. Recently, Takeda et al. have successfully developed the rescue system of a wild-type MV strain that could reproduce the natural course of MV pathology in monkeys, opening the way to molecularly dissecting the pathogenesis of MV infection at the level of viral genomes (45).
In this study, we examined MV entry into SLAM-negative cells. To facilitate the analysis, we recovered a wild-type strain of MV expressing the enhanced green fluorescent protein (EGFP) by using reverse genetics technology and determined its infectivities for various cell lines. The results indicate that wild-type MV can infect SLAM-negative cells, albeit with a low efficiency, via a novel pathway independent of known MV receptors such as SLAM and CD46.
MATERIALS AND METHODS
Cells.
Derivations of cell lines used in this study have previously been described elsewhere (17, 28, 47, 48). B95a cells were maintained in Dulbecco's modified Eagle's medium (DMEM), and MT-2, Jurkat, and EL4 cells were maintained in RPMI 1640 medium; each cell line was supplemented with 10% heat-inactivated fetal bovine serum (FBS) and 50 μg of gentamicin per ml. Vero/Neo and Vero/SLAM cells were maintained in DMEM supplemented with 10% heat-inactivated FBS and 0.5 mg of G418 per ml. CHO/Neo and CHO/SLAM cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 0.5 mg of G418 per ml. CHO/CD46 cells were maintained in RPMI 1640 medium supplemented with 10% heat-inactivated FBS and 0.7 mg of hygromycin B per ml. The 293-3-46 helper cell line (32) stably expressing the MV N and P proteins and T7 RNA polymerase (a kind gift of M. A. Billeter) was maintained in DMEM supplemented with 10% heat-inactivated FBS and 1.2 mg of G418 per ml.
Plasmid constructions.
Plasmid p(+)MV323, which carries the antigenome of the wild-type IC (Ichinose)-B strain of MV (45), was used as a starting material for plasmid constructions. The BstEII/ClaI fragment (nucleotides 4807 to 14937) was removed from p(+)MV323 by digestion with BstEII and ClaI, and the gap was filled with a small linker to obtain Δp(+)MV323. To generate unique multicloning sites (MCS) of 32 nucleotides in the noncoding region of the N gene in order to introduce a foreign gene, two PCR fragments were amplified from p(+)MV323. PCR fragment no. 1 was amplified with the following primers: 5′-CGCCAAGCGCGCCAGTGAATTGTAATAC-3′ (the BssHII recognition site is underlined) and 5′-TTGACGTCTGATCAGGCGCGCCCTCGGATATCCCTAATCCTG-3′ (the AatII recognition site is underlined; the AscI recognition site is in boldface; the portion of MCS is in italics). PCR fragment no. 2 was amplified with the following primers: 5′-TTGACGTCTACGTATTCGAACGAGATGGCCACACTTTTGAG-3′ (the AatII recognition site is underlined; the BstBI recognition site is in boldface; the portion of MCS is in italics) and 5′-ATCCCTGACCGCGGATGCGAG-3′ (the SacII recognition site is underlined). After fragment no. 1 was digested with BssHII (partial digestion was performed because the newly created MCS included the AscI recognition site, which was also cleaved by BssHII) and AatII and fragment no. 2 was digested with AatII and SacII, these two fragments were ligated to Δp(+)MV323, which had been predigested with BssHII and SacII, to obtain Δp(+)MV323-MCS (the three fragments were ligated together in the same tube). PCR amplification was then performed to clone untranscribed sequences (nucleotides 1690 to 1803) located at the 5′ side of the N gene, which consists of gene termination sequences, intergenic trinucleotides, and gene start sequences (EIS). The primer sequences were as follows: 5′-TTGACGTCGAGAGGCCGAGGACCAGAACAACATC-3′ (the AatII recognition site is underlined) and 5′-TACGTATTCGAACTCCAGTCGTGGGAGTGGATGGTTG-3′ (the BstBI recognition site is underlined). After digestion with AatII and BstBI, the PCR fragment was inserted into the AatII/BstBI sites of Δp(+)MV323-MCS to obtain Δp(+)MV323-EIS. The open reading frame of the EGFP gene was amplified from pEGFP-N1 (Clontech) with the primers 5′-TTGGCGCGCCATGGTGAGCAAGGGCGAGGAG-3′ (the AscI recognition site is underlined) and 5′-TTGACGTCTTACTTGTACAGCTCGTCCATGCC-3′ (the AatII recognition site is underlined). After digestion with AscI and AatII, the EGFP fragment was inserted into the AscI/AatII sites of Δp(+)MV323-EIS to obtain Δp(+)MV323-EGFP. Finally, the small linker was removed from Δp(+)MV323-EGFP by digestion with BstEII and ClaI, and the BstEII/ClaI fragment (nucleotides 4807 to 14937) of p(+)MV323 was inserted back into Δp(+)MV323-EGFP. The final product was designated p(+)MV323-EGFP. To generate p(+)MV323/EdH-EGFP, the PacI/SpeI fragment containing the H gene of the Edmonston B strain was excised from p(+)MV2A (a kind gift of M. A. Billeter) and used to replace the H gene of p(+)MV323-EGFP. Entire sequences of the inserted regions were confirmed by DNA sequencing. All PCR amplifications were carried out with KOD PLUS DNA polymerase with high-fidelity polymerase activity (TOYOBO Biochemicals). The primers were designed so that the final RNA sequences conformed to the rule of six.
Rescue of recombinant viruses.
Recombinant viruses were rescued with the 293-3-46 helper cell line and B95a cells as described previously (32, 45), with minor modifications. In brief, 293-3-46 cells cultured overnight in a 35-mm-diameter dish were transfected with 5 μg of either p(+)MV323, p(+)MV323-EGFP, or p(+)MV323/EdH-EGFP together with 10 ng of pEMC-La (a kind gift of M. A. Billeter), which encodes the MV polymerase L protein under the control of the T7 promoter, by using the calcium phosphate transfection procedure. At 48 h after transfection, 293-3-46 cells were transferred to a 60-mm-diameter dish. When the cells reached ∼80% confluence, B95a cells were overlaid onto the 293-3-46 cell monolayers. After 3 or 4 days, syncytia in overlaid B95a cells were individually picked up and then transferred to B95a cell monolayers in a 35-mm-diameter dish which had reached 50 to 60% confluence. To expand recombinant viruses, passages in B95a cells were carried out, and virus stocks were obtained. The rescued viruses were designated IC323, IC323-EGFP, and IC323/EdH-EGFP, respectively. Titers of these recombinant viruses were determined by calculating the 50% tissue culture infectious dose (TCID50) on B95a cells unless otherwise indicated.
Viral growth.
B95a cells (5 × 105 cells) were infected with IC323, IC323-EGFP, or IC323/EdH-EGFP at a multiplicity of infection (MOI) of 0.01. After 1 h of incubation at 37°C, the cells were washed three times with the complete medium and then incubated in six-well plates at 37°C. Infected cells and medium were harvested at 12, 24, 48, 72, and 96 h after infection, and virus titers were determined on B95a cells by calculating the TCID50.
Fluorescence microscopy.
Adherent cells (2 × 105 cells) and cells in suspension (5 × 105 cells) cultured in six-well plates were infected with rescued viruses at an MOI of 0.001 to 0.1. At 24 h after infection, the cells were examined for EGFP expression under a fluorescence microscope. Imaging analysis was performed with AxioVision software (Carl Zeiss).
Titration of recombinant viruses on various cell lines.
All adherent cell lines except for B95a (2 × 104 cells in 50 μl) were cultured in 96-well plates overnight. Cells were infected with 50 μl of serially diluted solutions of the stock containing IC323-EGFP or IC323/EdH-EGFP. After 1 h of incubation at 37°C, 100 μl of the complete medium containing the fusion block peptide (Z-D-Phe-Phe-Gly) (33) was added to each well to keep cell fusion from interfering with the exact determination of virus infectivities. The final concentration of the fusion block peptide was 50 μg per ml. At 24 h after infection, infectious units of the recombinant viruses were determined by counting the number of EGFP-expressing cells under a fluorescence microscope (43). Cells in suspension and B95a cells (5 × 104 cells in 50 μl) were examined in the same fashion.
Blocking virus entry with antibodies.
CHO/Neo, CHO/CD46, and CHO/SLAM cells (2 × 104 cells) were grown in 96-well plates overnight. They were then incubated at 37°C with medium containing M75 (anti-human CD46 monoclonal antibody [MAb]; ascites dilution, 1:50) (38), IPO-3 (anti-human SLAM MAb, 10 μg per ml; Kamiya Biomedical) (41), or control mouse immunoglobulin G (10 μg per ml). One hour after treatment with MAb, cells were infected with 104 TCID50s of IC323-EGFP or IC323/EdH-EGFP. At 1 h after infection, the complete medium containing the fusion block peptide was added to each well as described above. At 24 h after infection, the number of EGFP-expressing cells was counted under a fluorescence microscope. Vero/Neo cells (2 × 104 cells) were cultured in 96-well plates overnight and then infected with 100 infectious units (titrated on Vero/Neo cells) of IC323-EGFP or VSVΔG*-G (the recombinant vesicular stomatitis virus [VSV] expressing green fluorescent protein [GFP]; a kind gift of M. A. Whitt) (43) in the presence of serially diluted ascites containing MAb, anti-MV H protein (C-1) (22), or anti-human cytomegalovirus (HCMV) glycoprotein B (gB) (27-156; a kind gift of W. Britt) (42). Pretreatment of Vero/Neo cells with MAbs was not performed. At 24 h after infection, the number of GFP-expressing cells was counted under a fluorescence microscope.
Inhibition of virus entry via endocytosis.
CHO/Neo and Vero/Neo cells (2 × 104 cells per well) were grown in 96-well plates overnight and then treated with medium containing 50 μM chloroquine (Sigma) for 1 h at 37°C prior to infection. Treated cells were infected with 100 infectious units (titrated on CHO/Neo and Vero/Neo cells) of VSVΔG*-G, IC323-EGFP, or HV5.111 (the recombinant HCMV expressing EGFP; a kind gift of J. Vieira) (14) for 1 h at 37°C in the presence of 50 μM chloroquine. After washing, the medium containing 50 μM chloroquine was added to each well. At 24 h after infection, the number of GFP-expressing cells was counted under a fluorescence microscope.
RESULTS
Production and characterization of recombinant viruses.
The rescue of the wild-type MV IC-B strain (IC323) from cloned cDNA has been reported previously (45). In order to produce the recombinant MV expressing EGFP (IC323-EGFP), we constructed the plasmid containing the full-length cDNAs of the IC-B strain and the EGFP gene (Fig. 1). The EGFP gene was inserted into newly created unique sites located at the 3′ end of the viral genome. We also constructed another plasmid in which the IC-B H gene was replaced with the H gene of the Edmonston strain to obtain IC323/EdH-EGFP. A system involving the helper cell line was then used to rescue recombinant viruses. A comparison of growth kinetics on B95a cells showed no significant differences among the rescued viruses, IC323, IC323-EGFP, and IC323/EdH-EGFP (Fig. 2), indicating that neither the insertion of a foreign gene nor the replacement of the H gene significantly affected viral replication.
FIG. 1.
Schematic diagram of the plasmid inserts encoding recombinant viruses. The six MV genes (N, P, M, F, H, and L) and the EGFP gene are indicated. The shaded areas indicate untranslated regions, and the vertical lines within untranslated regions indicate the positions of intergenic trinucleotides. Also shown in the figure are the T7 promoter that directs the viral RNA genome synthesis, the restriction sites used to replace the H gene, the ribozyme sequence (δ), the terminator sequence (TΦ), and the newly created MCS into which the EGFP gene and EIS were inserted. The parental p(+)MV323 was previously described elsewhere (45).
FIG. 2.
Growth kinetics of recombinant viruses. B95a cells were infected at an MOI of 0.01 with IC323, IC323-EGFP, or IC323/EdH-EGFP. Viruses were harvested at the indicated time points, and the TCID50s were determined on B95a cells. The averages and standard deviations of the results of three experiments are shown.
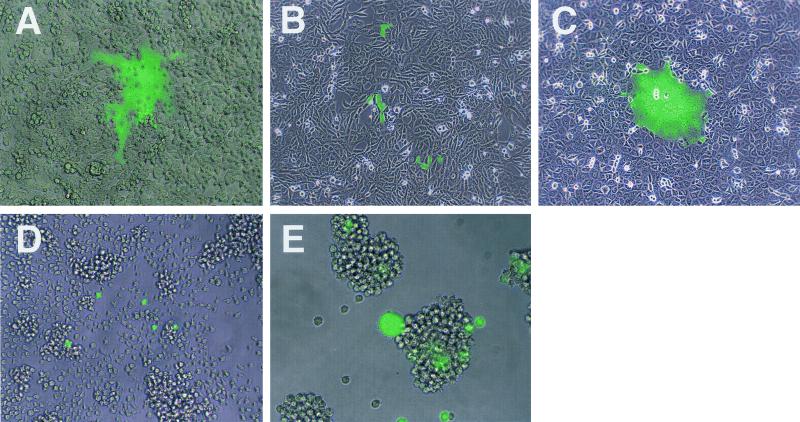
Various cell lines were infected with the EGFP-expressing recombinant viruses and examined for virus spread at 24 h after infection. As expected from the receptor usages of the viruses from which the respective H proteins were derived (the IC-B strain uses SLAM as a receptor, whereas the Edmonston strain can use both SLAM and CD46 as receptors), IC323-EGFP caused cytopathic effects (CPE) in SLAM-positive cells, such as B95a, Vero/SLAM, MT-2, and CHO/SLAM cells (Fig. 3A, C, and E and data not shown), whereas IC323/EdH-EGFP induced CPE in all cells expressing SLAM and/or CD46 (data not shown). Under a fluorescence microscope, syncytia induced by EGFP-expressing viruses showed diffuse green autofluorescence throughout the nucleus and cytoplasm (Fig. 3A, C, and E). Interestingly, the infection of SLAM-negative cells, such as Vero/Neo and Jurkat cells, with IC323-EGFP also resulted in some individual cells exhibiting green autofluorescence, although no syncytium formation was observed (Fig. 3B and D); syncytia were not found even at 5 days after infection (data not shown). Clusters of a few single cells expressing EGFP were often observed in SLAM-negative cells after IC323-EGFP infection (Fig. 3B). Since these clusters of EGFP-positive cells were developed even in the presence of the fusion block peptide added after infection, the division of single infected cells, rather than cell-to-cell virus spread, may be responsible for them. Individual cells exhibiting green autofluorescence were also observed in rodent cells, such as CHO/Neo, CHO/CD46, and EL4 cells, after IC323-EGFP infection (data not shown). These results prompted us to further investigate MV entry into SLAM-negative cells.
FIG. 3.
EGFP autofluorescence in cells infected with IC323-EGFP. B95a (A), Vero/SLAM (C), and MT-2 (E) cells were infected with IC323-EGFP at an MOI of 0.001, and Vero/Neo (B) and Jurkat (D) cells were infected at an MOI of 0.1. At 24 h after infection, the development of CPE was observed under a light microscope, and EGFP autofluorescence was observed under a fluorescence microscope. The images in those observations were combined by using AxioVision software.
Titration of EGFP-expressing viruses on various cell lines.
To quantitate the susceptibility of various cell lines to MV, infectious titers were determined by counting the number of EGFP-positive cells after infection with IC323-EGFP or IC323/EdH-EGFP (Fig. 4). We serially diluted virus stocks, inoculated cell lines with the aliquots, and determined infectious titers at 24 h after infection. Since syncytium formation interferes with the precise determination of the number of infected cells, the fusion block peptide was added to the medium following infection. IC323-EGFP showed much lower infectious titers on SLAM-negative cell lines (Vero/Neo, CHO/Neo, CHO/CD46, Jurkat, and EL4 cells) than on SLAM-positive cell lines (B95a, Vero/SLAM, CHO/SLAM, and MT-2 cells). For example, its infectious titers on Vero/Neo and CHO/Neo cells were 550- and 270-fold lower than those on Vero/SLAM and CHO/SLAM cells, respectively. Nevertheless, its infectious titers on all SLAM-negative cells examined were significant and reached as much as 102.7 to 103.8 infectious units per ml. Collectively, the susceptibilities of SLAM-negative cells to IC323-EGFP were 2 to 3 logs lower than those of SLAM-positive cells. Similarly, IC323/EdH-EGFP showed high infectious titers on SLAM- and/or CD46-positive cell lines (105 to 106 infectious units per ml) and low but significant titers on rodent cell lines not expressing primate SLAM or CD46 (CHO/Neo and EL4 cells).
FIG. 4.
Infectious titers of IC323-EGFP (filled bars) and IC323/EdH-EGFP (open bars) on various cell lines. Cell lines were infected with serially diluted virus solutions, and at 24 h after infection, infectious units of recombinant viruses were determined by counting the number of EGFP-expressing cells under a fluorescence microscope. The fusion block peptide was added to the medium after infection.
MV entry independent of SLAM and CD46.
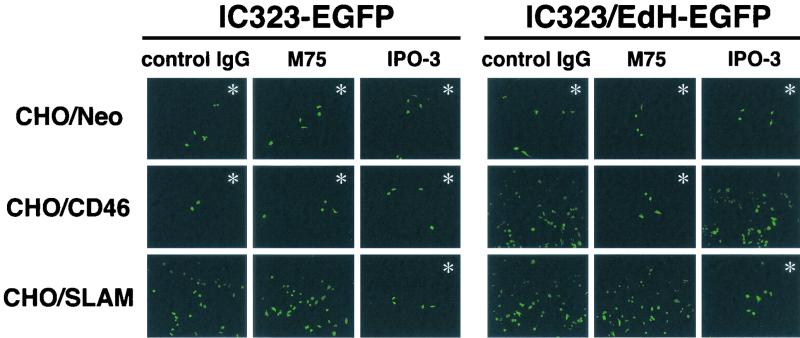
Since IC323-EGFP showed readily detectable infectivities for CHO/Neo cells expressing neither SLAM nor CD46 (Fig. 4), wild-type MV appeared to be able to infect cells independently of SLAM and CD46. To further exclude the involvement of SLAM and CD46 in wild-type MV infection of SLAM-negative cells, we examined how an MAb specific for SLAM (IPO-3) or CD46 (M75) affects IC323-EGFP entry into CHO cell transfectants (Fig. 5). Neither IPO-3 nor M75 inhibited the infection of CHO/Neo cells with IC323-EGFP or IC323/EdH-EGFP, confirming that CHO/Neo cells do not express on their surfaces molecules recognized by these MAbs. IPO-3 strongly suppressed the infection of CHO/SLAM cells with IC323-EGFP or IC323/EdH-EGFP, and M75 almost blocked the infection of CHO/CD46 cells with IC323/EdH-EGFP. In contrast, M75 did not affect the infectivity of IC323-EGFP for CHO/CD46 cells. Furthermore, some EGFP-positive infected cells always remained among the CHO/SLAM and CHO/CD46 cells after treatment with IPO-3 and M75, respectively. Thus, MV can still infect, albeit with a low efficiency, CHO cell transfectants independently of SLAM and CD46, suggesting the presence of another receptor(s) on CHO cells and probably many other types of cells.
FIG. 5.
Effects of anti-SLAM and anti-CD46 MAbs on the infection of CHO cell transfectants with recombinant viruses. CHO/Neo, CHO/CD46, and CHO/SLAM cells were pretreated with either control mouse immunoglobulin G (control IgG), anti-CD46 MAb (M75), or anti-SLAM MAb (IPO-3) and then infected with 104 TCID50s of IC323-EGFP or IC323/EdH-EGFP. The fusion block peptide was added to the medium after infection. At 24 h after infection, EGFP autofluorescence was observed under a fluorescence microscope. Where the numbers of EGFP-positive cells were small, pictures showing those cells were purposefully taken, as many fields did not contain EGFP-positive cells (indicated by ∗). For this reason, numerical comparison between samples may not be accurately done in the figure.
SLAM-independent entry is not mediated by endocytosis.
Endocytosis is one of the strategies used by cells for the uptake of extracellular molecules, including viruses (51). To determine whether SLAM-independent MV entry is mediated by endocytosis, CHO/Neo and Vero/Neo cells were infected with IC323-EGFP in the presence of chloroquine, a weak base which raises endosomal pH (27). The cells were also infected with VSV (VSVΔG*-G) and HCMV (HV5.111). It is known that VSV requires endocytosis and low endosomal pH to enter cells (5) whereas HCMV enters cells without endocytosis (8). The results showed that chloroquine treatment almost completely abolished the infectivity of VSV but did not affect MV entry (Table 1). Thus, MV infection via a SLAM-independent pathway(s) is not mediated by endocytosis. The infectivity of HCMV was slightly increased after chloroquine treatment. Although the reason for this finding with HCMV was not elucidated in this study, a similar observation has been reported previously (8).
TABLE 1.
Viral entry after chloroquine treatmenta
| Cell line | Viral entry (% of control level)
|
||
|---|---|---|---|
| VSV | MV | HCMV | |
| CHO/Neo | 0 | 96.7 | 139.4 |
| Vero/Neo | 1.1 | 92 | 123.4 |
Cells were cultured in medium containing 50 μM chloroquine throughout the experiment. The number of EGFP-positive cells in the chloroquine-treated sample was compared with that in the untreated control.
SLAM-independent entry requires MV envelope proteins.
The H protein of MV has receptor binding activity, and the F protein mediates membrane fusion (12, 29). To investigate whether SLAM-independent MV entry requires these MV envelope glycoproteins, two experiments were performed. First, anti-H protein MAb markedly blocked IC323-EGFP infection of Vero/Neo cells in a dose-dependent manner, while an irrelevant control MAb had a minimal effect on virus entry (Fig. 6A). These MAbs had no inhibitory effects on the infectivity of VSV (Fig. 6B). Second, IC323-EGFP entry was completely abolished in the presence of the fusion block peptide (added prior to infection) (data not shown). These results suggest that MV envelope glycoproteins are directly involved in SLAM-independent MV entry.
FIG. 6.
Effects of anti-H protein MAb on IC323-EGFP infection of Vero/Neo cells. Vero/Neo cells were infected with 100 infectious units (as determined on Vero/Neo cells) of IC323-EGFP (A) or VSVΔG*-G (B) in the presence of serially diluted ascites containing anti-H protein MAb (C-1) or anti-HCMV gB MAb (27-156). At 24 h after infection, the number of GFP-expressing cells was counted under a fluorescence microscope. The averages and standard deviations of the results for three wells are indicated as percentages of the control level obtained in the absence of antibody.
DISCUSSION
In this study, we first described the rescue of a wild-type strain of MV bearing EGFP as a reporter (IC323-EGFP) by using reverse genetics technology. A great advantage of this recombinant virus is that it allows an easy and highly sensitive detection of infected cells thanks to its ability to generate green autofluorescence. In fact, we were able to detect IC323-EGFP infection of those cells which had been considered nonsusceptible to wild-type strains of MV as determined by infection with natural MV or VSV pseudotypes bearing MV envelope proteins (46, 47). The reason why IC323-EGFP was more sensitive in detecting MV entry than were VSV pseudotypes expressing GFP (47) may be that the former has the authentic virion structure whereas the latter may not carry the proper quantity and/or quality of MV envelope proteins because of their nature as a heterologous virus system.
While IC323-EGFP produced large syncytia exhibiting green autofluorescence in SLAM-positive cells, it hardly spread from the originally infected SLAM-negative cells, not inducing syncytia in them. When the number of EGFP-expressing cells after infection was counted, the infectivities of IC323-EGFP for SLAM-negative cells were 2 to 3 logs lower than those for SLAM-positive cells. Anti-CD46 antibody did not block IC323-EGFP infection of CHO/CD46 cells. Furthermore, the infectious titer of IC323-EGFP on CHO/CD46 cells was not higher than that on CHO/Neo cells, confirming that CD46 cannot act as a receptor for IC323-EGFP. On the other hand, both anti-MV H antibody and the fusion block peptide abolished IC323-EGFP infection of SLAM-negative cells. Experiments using chloroquine suggested that MV entry into SLAM-negative cells occurs through the pH-independent fusion at the host cell surface between the MV envelope and the plasma membrane. Taken together, these results indicate that MV can infect, albeit with a reduced efficiency, a variety of cells via an unknown receptor(s) other than SLAM or CD46.
We have previously demonstrated that SLAM is a receptor for both wild-type and vaccine strains of MV (48). Viruses obtained from throat swabs of measles patients were found to use SLAM but not CD46 as a receptor (28). Manchester et al. reported that clinical isolates of MV obtained by using peripheral blood mononuclear cells used CD46 as a cellular receptor (20), but our study showed that these isolates could use both SLAM and CD46 as receptors, with the former acting as a better receptor (48). The known distribution of SLAM (2, 7, 23, 26, 41, 48) coincides with the tropism of MV, and the use of SLAM as a receptor may be able to explain most of MV pathology, including lymphopenia and immunosuppression. However, it is thought that MV enters by the respiratory route, initially infecting epithelial cells in the respiratory tract (12). Furthermore, there have been reports that in addition to infecting cells of the immune system, MV also infects endothelial (11, 15, 16, 21, 24), epithelial (21, 24, 44), and neuronal cells (3, 24, 40) in vivo, none of which have been shown to express SLAM.
One explanation for this paradox is that all of these cells express low levels of SLAM sufficient for MV infection. We are currently investigating this possibility by using immunohistochemistry and flow cytometry. Another explanation would be the emergence of variant viruses that can use CD46 or other molecules efficiently as a receptor. Given that RNA viruses have relatively high mutation rates, variants that show the broader tropism may be easily generated in vivo. In fact, a single amino acid substitution in the H protein at position 481 to tyrosine (4, 13, 18, 39, 52) or at position 546 to glycine (34, 39) enabled wild-type MV strains to use CD46 as a receptor. We, however, did not detect such changes in the H genes of viruses recovered from SLAM-negative Jurkat and Vero cells infected with IC323-EGFP (data not shown). Furthermore, we think it unlikely that variant viruses emerged in our experiments that could interact strongly with a receptor(s) other than SLAM. If this had been the case, those viruses would have easily spread to surrounding cells that used such a receptor, but this did not occur.
Although the two possibilities described above cannot be completely excluded at the moment, the SLAM-independent MV entry we described in this study may well explain the observed infection of SLAM-negative cells in vivo. The molecule involved in this inefficient process is unknown, but its identification may deepen our understanding of MV tropism and pathogenesis. On the other hand, it should be emphasized that this molecule is not comparable to SLAM or CD46 as an MV receptor, because it allows MV entry with much lower efficiency and no syncytium formation after MV infection.
Lastly, it may be worthwhile to mention future applications of these recombinant viruses possessing EGFP as a reporter. With these viruses, it should be possible to monitor viral spread in situ in cultured cells as well as experimentally infected animal models, in which EGFP will be a convenient tool for the identification of infected cells and the recovery of replicating viruses.
Acknowledgments
We thank M. A. Billeter, M. A. Whitt, W. Britt, J. Vieira, and T. Seya for providing reagents.
This work was supported by grants from the Ministry of Education, Science and Culture of Japan and from the Organization for Drug ADR Relief, R&D Promotion and Product Review of Japan.
REFERENCES
- 1.Auwaerter, P. G., P. A. Rota, W. R. Elkins, R. J. Adams, T. DeLozier, Y. Shi, W. J. Bellini, B. R. Murphy, and D. E. Griffin. 1999. Measles virus infection in rhesus macaques: altered immune responses and comparison of the virulence of six different virus strains. J. Infect. Dis. 180:950-958. [DOI] [PubMed] [Google Scholar]
- 2.Aversa, G., C. C. Chang, J. M. Carballido, B. G. Cocks, and J. E. de Vries. 1997. Engagement of the signaling lymphocytic activation molecule (SLAM) on activated T cells results in IL-2-independent, cyclosporin A-sensitive T cell proliferation and IFN-gamma production. J. Immunol. 158:4036-4044. [PubMed] [Google Scholar]
- 3.Baczko, K., J. Lampe, U. G. Liebert, U. Brinckmann, V. ter Meulen, I. Pardowitz, H. Budka, S. L. Cosby, S. Isserte, and B. K. Rima. 1993. Clonal expansion of hypermutated measles virus in a SSPE brain. Virology 197:188-195. [DOI] [PubMed] [Google Scholar]
- 4.Bartz, R., U. Brinckmann, L. M. Dunster, B. Rima, V. Ter Meulen, and J. Schneider-Schaulies. 1996. Mapping amino acids of the measles virus hemagglutinin responsible for receptor (CD46) downregulation. Virology 224:334-337. [DOI] [PubMed] [Google Scholar]
- 5.Blumenthal, R., A. Bali-Puri, A. Walter, D. Covell, and O. Eidelman. 1987. pH-dependent fusion of vesicular stomatitis virus with Vero cells. Measurement by dequenching of octadecyl rhodamine fluorescence. J. Biol. Chem. 262:13614-13619. [PubMed] [Google Scholar]
- 6.Buckland, R., and T. F. Wild. 1997. Is CD46 the cellular receptor for measles virus? Virus Res. 48:1-9. [DOI] [PubMed] [Google Scholar]
- 7.Cocks, B. G., C. C. Chang, J. M. Carballido, H. Yssel, J. E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature 376:260-263. [DOI] [PubMed] [Google Scholar]
- 8.Compton, T., R. R. Nepomuceno, and D. M. Nowlin. 1992. Human cytomegalovirus penetrates host cells by pH-independent fusion at the cell surface. Virology 191:387-395. [DOI] [PubMed] [Google Scholar]
- 9.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 10.Duprex, W. P., S. McQuaid, L. Hangartner, M. A. Billeter, and B. K. Rima. 1999. Observation of measles virus cell-to-cell spread in astrocytoma cells by using a green fluorescent protein-expressing recombinant virus. J. Virol. 73:9568-9575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Esolen, L. M., K. Takahashi, R. T. Johnson, A. Vaisberg, T. R. Moench, S. L. Wesselingh, and D. E. Griffin. 1995. Brain endothelial cell infection in children with acute fatal measles. J. Clin. Investig. 96:2478-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffin, D. E. 2001. Measles virus, p. 1401-1441. In D. M. Knipe, P. M. Howley, D. E. Griffin, R. A. Lamb, M. A. Martin, B. Roizman, and S. E. Straus (ed.), Fields virology, 4th ed. Lippincott-Raven, Philadelphia, Pa.
- 13.Hsu, E. C., F. Sarangi, C. Iorio, M. S. Sidhu, S. A. Udem, D. L. Dillehay, W. Xu, P. A. Rota, W. J. Bellini, and C. D. Richardson. 1998. A single amino acid change in the hemagglutinin protein of measles virus determines its ability to bind CD46 and reveals another receptor on marmoset B cells. J. Virol. 72:2905-2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jarvis, M. A., C. E. Wang, H. L. Meyers, P. P. Smith, C. L. Corless, G. J. Henderson, J. Vieira, W. J. Britt, and J. A. Nelson. 1999. Human cytomegalovirus infection of Caco-2 cells occurs at the basolateral membrane and is differentiation state dependent. J. Virol. 73:4552-4560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kimura, A., K. Tosaka, and T. Nakao. 1975. Measles rash. I. Light and electron microscopic study of skin eruptions. Arch. Virol. 47:295-307. [DOI] [PubMed] [Google Scholar]
- 16.Kirk, J., A. L. Zhou, S. McQuaid, S. L. Cosby, and I. V. Allen. 1991. Cerebral endothelial cell infection by measles virus in subacute sclerosing panencephalitis: ultrastructural and in situ hybridization evidence. Neuropathol. Appl. Neurobiol. 17:289-297. [DOI] [PubMed] [Google Scholar]
- 17.Kobune, F., H. Sakata, and A. Sugiura. 1990. Marmoset lymphoblastoid cells as a sensitive host for isolation of measles virus. J. Virol. 64:700-705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lecouturier, V., J. Fayolle, M. Caballero, J. Carabana, M. L. Celma, R. Fernandez-Munoz, T. F. Wild, and R. Buckland. 1996. Identification of two amino acids in the hemagglutinin glycoprotein of measles virus (MV) that govern hemadsorption, HeLa cell fusion, and CD46 downregulation: phenotypic markers that differentiate vaccine and wild-type MV strains. J. Virol. 70:4200-4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 20.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McChesney, M. B., C. J. Miller, P. A. Rota, Y. D. Zhu, L. Antipa, N. W. Lerche, R. Ahmed, and W. J. Bellini. 1997. Experimental measles. I. Pathogenesis in the normal and the immunized host. Virology 233:74-84. [DOI] [PubMed] [Google Scholar]
- 22.McFarlin, D. E., W. J. Bellini, E. S. Mingioli, T. N. Behar, and A. Trudgett. 1980. Monospecific antibody to the haemagglutinin of measles virus. J. Gen. Virol. 48:425-429. [DOI] [PubMed] [Google Scholar]
- 23.Minagawa, H., K. Tanaka, N. Ono, H. Tatsuo, and Y. Yanagi. 2001. Induction of the measles virus receptor SLAM (CD150) on monocytes. J. Gen. Virol. 82:2913-2917. [DOI] [PubMed] [Google Scholar]
- 24.Moench, T. R., D. E. Griffin, C. R. Obriecht, A. J. Vaisberg, and R. T. Johnson. 1988. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J. Infect. Dis. 158:433-442. [DOI] [PubMed] [Google Scholar]
- 25.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ohgimoto, S., K. Ohgimoto, S. Niewiesk, I. M. Klagge, J. Pfeuffer, I. C. Johnston, J. Schneider-Schaulies, A. Weidmann, V. ter Meulen, and S. Schneider-Schaulies. 2001. The haemagglutinin protein is an important determinant of measles virus tropism for dendritic cells in vitro. J. Gen. Virol. 82:1835-1844. [DOI] [PubMed] [Google Scholar]
- 27.Ohkuma, S., and B. Poole. 1978. Fluorescence probe measurement of the intralysosomal pH in living cells and the perturbation of pH by various agents. Proc. Natl. Acad. Sci. USA 75:3327-3331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ono, N., H. Tatsuo, Y. Hidaka, T. Aoki, H. Minagawa, and Y. Yanagi. 2001. Measles viruses on throat swabs from measles patients use signaling lymphocytic activation molecule (CDw150) but not CD46 as a cellular receptor. J. Virol. 75:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ono, N., H. Tatsuo, K. Tanaka, H. Minagawa, and Y. Yanagi. 2001. V domain of human SLAM (CDw150) is essential for its function as a measles virus receptor. J. Virol. 75:1594-1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Patterson, J. B., D. Thomas, H. Lewicki, M. A. Billeter, and M. B. Oldstone. 2000. V and C proteins of measles virus function as virulence factors in vivo. Virology 267:80-89. [DOI] [PubMed] [Google Scholar]
- 31.Radecke, F., and M. A. Billeter. 1996. The nonstructural C protein is not essential for multiplication of Edmonston B strain measles virus in cultured cells. Virology 217:418-421. [DOI] [PubMed] [Google Scholar]
- 32.Radecke, F., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Richardson, C. D., A. Scheid, and P. W. Choppin. 1980. Specific inhibition of paramyxovirus and myxovirus replication by oligopeptides with amino acid sequences similar to those at the N-termini of the F1 or HA2 viral polypeptides. Virology 105:205-222. [DOI] [PubMed] [Google Scholar]
- 34.Rima, B. K., J. A. Earle, K. Baczko, V. ter Meulen, U. G. Liebert, C. Carstens, J. Carabana, M. Caballero, M. L. Celma, and R. Fernandez-Munoz. 1997. Sequence divergence of measles virus haemagglutinin during natural evolution and adaptation to cell culture. J. Gen. Virol. 78:97-106. [DOI] [PubMed] [Google Scholar]
- 35.Schneider, H., K. Kaelin, and M. A. Billeter. 1997. Recombinant measles viruses defective for RNA editing and V protein synthesis are viable in cultured cells. Virology 227:314-322. [DOI] [PubMed] [Google Scholar]
- 36.Schneider-Schaulies, J., L. M. Dunster, F. Kobune, B. Rima, and V. ter Meulen. 1995. Differential downregulation of CD46 by measles virus strains. J. Virol. 69:7257-7259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schneider-Schaulies, J., J. J. Schnorr, U. Brinckmann, L. M. Dunster, K. Baczko, U. G. Liebert, S. Schneider-Schaulies, and V. ter Meulen. 1995. Receptor usage and differential downregulation of CD46 by measles virus wild-type and vaccine strains. Proc. Natl. Acad. Sci. USA 92:3943-3947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seya, T., T. Hara, M. Matsumoto, and H. Akedo. 1990. Quantitative analysis of membrane cofactor protein (MCP) of complement. High expression of MCP on human leukemia cell lines, which is down-regulated during cell differentiation. J. Immunol. 145:238-245. [PubMed] [Google Scholar]
- 39.Shibahara, K., H. Hotta, Y. Katayama, and M. Homma. 1994. Increased binding activity of measles virus to monkey red blood cells after long-term passage in Vero cell cultures. J. Gen. Virol. 75:3511-3516. [DOI] [PubMed] [Google Scholar]
- 40.Sidhu, M. S., J. Crowley, A. Lowenthal, D. Karcher, J. Menonna, S. Cook, S. Udem, and P. Dowling. 1994. Defective measles virus in human subacute sclerosing panencephalitis brain. Virology 202:631-641. [DOI] [PubMed] [Google Scholar]
- 41.Sidorenko, S. P., and E. A. Clark. 1993. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 151:4614-4624. [PubMed] [Google Scholar]
- 42.Spaete, R. R., R. M. Thayer, W. S. Probert, F. R. Masiarz, S. H. Chamberlain, L. Rasmussen, T. C. Merigan, and C. Pachl. 1988. Human cytomegalovirus strain Towne glycoprotein B is processed by proteolytic cleavage. Virology 167:207-225. [DOI] [PubMed] [Google Scholar]
- 43.Takada, A., C. Robison, H. Goto, A. Sanchez, K. G. Murti, M. A. Whitt, and Y. Kawaoka. 1997. A system for functional analysis of Ebola virus glycoprotein. Proc. Natl. Acad. Sci. USA 94:14764-14769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Takahashi, H., Y. Umino, T. A. Sato, T. Kohama, Y. Ikeda, M. Iijima, and R. Fujisawa. 1996. Detection and comparison of viral antigens in measles and rubella rashes. Clin. Infect. Dis. 22:36-39. [DOI] [PubMed] [Google Scholar]
- 45.Takeda, M., K. Takeuchi, N. Miyajima, F. Kobune, Y. Ami, N. Nagata, Y. Suzaki, Y. Nagai, and M. Tashiro. 2000. Recovery of pathogenic measles virus from cloned cDNA. J. Virol. 74:6643-6647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tanaka, K., M. Xie, and Y. Yanagi. 1998. The hemagglutinin of recent measles virus isolates induces cell fusion in a marmoset cell line, but not in other CD46-positive human and monkey cell lines, when expressed together with the F protein. Arch. Virol. 143:213-225. [DOI] [PubMed] [Google Scholar]
- 47.Tatsuo, H., K. Okuma, K. Tanaka, N. Ono, H. Minagawa, A. Takade, Y. Matsuura, and Y. Yanagi. 2000. Virus entry is a major determinant of cell tropism of Edmonston and wild-type strains of measles virus as revealed by vesicular stomatitis virus pseudotypes bearing their envelope proteins. J. Virol. 74:4139-4145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 49.Tober, C., M. Seufert, H. Schneider, M. A. Billeter, I. C. Johnston, S. Niewiesk, V. ter Meulen, and S. Schneider-Schaulies. 1998. Expression of measles virus V protein is associated with pathogenicity and control of viral RNA synthesis. J. Virol. 72:8124-8132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Valsamakis, A., H. Schneider, P. G. Auwaerter, H. Kaneshima, M. A. Billeter, and D. E. Griffin. 1998. Recombinant measles viruses with mutations in the C, V, or F gene have altered growth phenotypes in vivo. J. Virol. 72:7754-7761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wileman, T., C. Harding, and P. Stahl. 1985. Receptor-mediated endocytosis. Biochem. J. 232:1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Xie, M., K. Tanaka, N. Ono, H. Minagawa, and Y. Yanagi. 1999. Amino acid substitutions at position 481 differently affect the ability of the measles virus hemagglutinin to induce cell fusion in monkey and marmoset cells co-expressing the fusion protein. Arch. Virol. 144:1689-1699. [DOI] [PubMed] [Google Scholar]