Abstract
The major cause of mortality in measles is generalized suppression of cell-mediated immunity that persists following virus clearance and results in secondary infections. The mechanisms contributing to this long-term immunosuppression are not clear. Herein we present evidence that measles virus (MV) disrupts hematopoiesis by infecting human CD34+ cells and human bone marrow stroma. MV infection does not affect the hematopoietic capability of hematopoietic stem cells (HSCs) directly; rather, the infection impairs the ability of stroma to support development of HSCs. These results suggest that MV-mediated defects in hematopoiesis contribute to the long-term immunosuppression seen in measles.
Measles is a leading cause of infant death in developing countries, resulting in approximately one million fatalities out of the 30 to 40 million cases reported each year (12). Measles virus (MV) is transmitted by the respiratory route and induces fever, cough, conjunctivitis, and a characteristic skin rash. The rash coincides with the induction of potent antiviral cell-mediated immunity (CMI)-promoting virus clearance. At the same time, however, a generalized suppression of CMI occurs which lasts for weeks to months following virus clearance and is the major cause of secondary infections and mortality (12).
A variety of mechanisms have been proposed for MV-induced immunosuppression, including lymphocyte apoptosis or anergy, disruption in efficient antigen presentation, and cytokine imbalances (19, 35). Recent evidence, however, suggests that targeting of bone marrow (BM) cells and suppression of hematopoiesis by MV may play an important role in immunosuppression. One hallmark of MV immunosuppression is profound lymphopenia (including that involving CD4+ T cells, CD8+ T cells, and B cells), monocytopenia, and neutropenia that persists following virus clearance (2, 20, 33). Interestingly, young children with the highest level of BM output (24, 28) recover most quickly from MV-induced lymphopenia and immunosuppression. Older children and adults with a less robust BM reconstitution capacity require longer periods of time for recovery from MV immunosuppression (33). Additional evidence supporting BM damage by MV is suggested by the abnormally (40-fold) increased mobilization of CD34+ hematopoietic progenitor cells from the BM to the peripheral circulation following MV infection (33). Together, the findings of these studies suggest that viral suppression of mature lymphocytes, in combination with a delay in reconstitution from a damaged BM compartment, could account for the full range of immunosuppression seen in measles.
Given the potential for MV to suppress hematopoietic development, we investigated the susceptibility of hematopoietic cells to wild-type MV infection. We evaluated whether umbilical cord blood (UCB) CD34+ cells, a population which includes hematopoietic stem cells (HSCs) and myeloid, erythroid, and lymphoid progenitors, expressed MV receptors and could be infected with MV. In addition, we evaluated the susceptibility of BM stromal cells to MV. Here we demonstrate that MV infection of both cell types occurred and that inhibition of stromal cell function by MV led to disruption in normal hematopoiesis of CD34+ cells.
MATERIALS AND METHODS
CD34+ cell isolation.
UCB cells were obtained with Institutional Review Board approval from placentas obtained from normal donors after delivery of the infants and were collected in 50-ml centrifuge tubes containing 20 U of heparin/ml. Mononuclear cells were isolated from UCB by using Ficoll-Paque Plus (Amersham Pharmacia Biotech AB, Uppsala, Sweden) density centrifugation. The CD34+ mononuclear cell fraction was isolated with superparamagnetic microbead selection using a VarioMACS magnet (Miltenyi Biotech, Gladbach, Germany). The efficiency of the purification was verified by flow cytometry counterstaining with a phycoerythrin (PE)-conjugated CD34 antibody (HPCA-2; Becton Dickinson, San Jose, Calif.), and purity was routinely greater than 95%. The purified CD34+ UCB cells were frozen in Iscove's modified Dulbecco's medium containing 50% fetal bovine serum (HyClone Laboratories, Logan, Utah) and 10% dimethyl sulfoxide, which was later thawed for the infection experiments. The CD34+ cells were thawed in MyeloCult H5100 medium (Stem Cell Technologies, Vancouver, Canada) containing 100 ng of recombinant human (rh) stem cell factor/ml, 50 ng of rh interleukin-3 (rh-IL-3)/ml, and 50 U of rh-IL-6/ml, all purchased from R&D systems (Minneapolis, Minn.). Cells were cultured for 48 h at 37°C in 5% CO2 in a 6-ml round-bottomed polypropylene tube (Becton Dickinson Labware, Lincoln Park, N.J.), with the cap loosely placed to allow for gas exchange.
MV infection of CD34+ cells.
CD34+ cells were infected with MV strain JW (MV-JW) at an multiplicity of infection (MOI) of 2 for 5 h. Control cells received an equivalent volume of either UV-inactivated MV or virus-free medium. At the time of infection, the phenotype of the cells was determined by three-color flow cytometry using a FACSCalibur apparatus (Becton Dickinson). The cells were stained with CD34-PE, CD38-allophycocyanin (APC), CD33-PE, mIgG1-PE, mIgG1-APC (all purchased from Becton Dickinson), and CD46-FITC (Biodesign, Inc., Saco, Maine). After infection, the cells were transferred to tissue culture plates and maintained in the cytokine-containing medium. The infected cells were analyzed at various time intervals after infection for MV hemagglutinin (HA) expression by flow cytometry using a purified mouse monoclonal antibody (MAb) to HA (25) followed by a secondary donkey anti-mouse antibody conjugated to PE (Jackson ImmunoResearch Laboratory, West Grove, Pa.). MV-JW, a wild-type MV strain that was isolated from peripheral blood of a measles patient in Irvine, California, was grown in primary human peripheral blood mononuclear cells (PBMC) as described previously (9, 25). Virus stocks were filtered through a 0.45-μm-pore-diameter filter and then pelleted at 100,000 × g. To determine virus titer by 50% tissue culture infectious dose (TCID50) assay, serial 10-fold dilutions of the purified virus were overlaid on PBMC or B95-8 B lymphocytes in a 96-well plate in triplicate. Titers were calculated by the Karber method in TCID50/ml. For UV-inactivated MV-JW, virus was plated in a shallow petri dish on ice and exposed to a hand-held short-wave UV source (254 nm) for 1 h.
Bulk-culture LTC-IC assays.
Bulk-culture long term culture-initiating cell (LTC-IC) assays were established by plating 10,000 infected or control CD34+ cells per well of human BM stromal cells (irradiated as feeder cells with 1,500 R) and cultured in MyeloCult H5100. Cells were cultured for 5 weeks, and 50% of the medium was replaced weekly and frozen for viral titer on PBMC. For colony-forming cell (CFC) assays, the entire well was then harvested and clonogenic assays were performed by plating 20,000 infected or control CD34+ cells per milliliter of methyl cellulose containing 50 ng of rh-stem cell factor/ml, 10 ng of rh-GM-CSF/ml, 10 ng of rh-IL-3/ml, and 3 U of rh-erythropoietin (StemCell Technologies)/ml. Colonies derived from CFU granulocyte macrophage (CFU-GM) and burst-forming unit erythroid (BFU-E) progenitors were scored visually 14 days later using an inverted microscope as described previously (29). Statistical analysis was performed using Student's t test and Microsoft Excel software.
BM stroma preparation.
Human BM was obtained, with Institutional Review Board approval, by aspiration from the posterior iliac crest of fully informed, hematologically normal donors. Stromal cells were isolated by culturing human BM cells in MyeloCult H5100 medium (StemCell Technologies) with the addition of freshly prepared hydrocortisone (Sigma, St. Louis, Mo.) to yield a final concentration of 10−6 M. After 3 days, the suspension cells were removed and the adherent stromal cells were grown to confluency. These cells were removed with 0.05% trypsin-0.53 mM EDTA (Gibco BRL, Grand Island, N.Y.) and passaged three times to expand the cells and remove macrophages. The cells were frozen as described above and later thawed for LTC-IC assays and infection experiments.
MV infection of stromal cells.
Human BM stromal cells were thawed in MyeloCult H5100 medium and plated onto glass coverslips. The next day, the cells were infected with MV at an MOI of 0.1. Coverslips were fixed at various times postinfection in 50% ether-50% ethanol for 10 min followed by 100% ethanol for 20 min. Coverslips were stained for MV antigens using a human polyclonal SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) serum followed by a goat anti-human F(ab′)2 antibody conjugated to fluorescein isothiocyanate. Infected cells were visualized using an Olympus fluorescence microscope.
Inhibition of MV infection by antireceptor antibodies.
Antibodies against CD46 (E4.3 [26]), MCI20.6 ([32]) and signaling lymphocyte activation marker (SLAM) (Advanced Immunochemical Inc. [40]) or isotype control antibodies from Pharmingen were used. Five micrograms of antibody was incubated with 105 CD34+ cells or stromal cells for 30 min on ice. Following two washes in phosphate-buffered saline, the cells were incubated with 0.1 TCID50/cell of MV-JW for 1 h. After an additional two washes in phosphate-buffered saline, the cells were plated in a single well of a 24-well plate for 4 days, and then the supernatants were harvested and the virus titer in the supernatant was measured by TCID50 assay (25). Statistical analysis was performed using Student's t test.
RT-PCR.
Colonies were dissolved in Tri-Reagent (Molecular Research Center, Cincinnati, Ohio), and total RNA was prepared according to the manufacturer's instructions. cDNA was generated from 500 ng of total RNA using Moloney murine leukemia virus reverse transcriptase (RT) (Promega, Madison, Wis.), oligo(dT) primer (Pharmacia, Piscataway, N.J.), and 40 U of RNasin (Promega)-0.625 mM deoxynucleoside triphosphate-0.6 mM MgCl2. Amplification of the cDNA was performed with Taq DNA polymerase (Boehringer Mannheim Biochemicals). Primers used to detect the MV nucleoprotein gene were N1 (5′-ATCCGCAGGACAGTCGAAGGT-3′) and N2 (5′-AGGGTAGGCGGATGTTGTTCT-3′) or control primers β-actin 1 (5′-CCTCCTGGGCATGGAGTCC-3′) and β-actin 2 (5′-CGCTCAGGAGGAGCAATGAT-3′). The MgCl2 concentration was increased to 3.1 mM per reaction. PCR conditions for MV nucleocapsid gene (MV-N) consisted of a 1-min denaturing step at 95°C, 1 min of annealing at 55°C, and 2.5 min of extension at 72°C for a total of 40 cycles. PCR conditions for β-actin consisted of a 1-min denaturing step at 95°C, 1 min of annealing at 58°C, and 1 min of extension at 72°C for a total of 40 cycles. If PCR products were not directly visible by ethidium bromide staining, samples were transferred to Nytran membranes and probed with a [γ-32P]ATP-labeled internal N-specific oligonucleotide (5′-GCCATGGCAGGAATCTCGGAAGAACAAGGCTCAGA-3′) (16).
RESULTS
Infection and MV receptor expression on CD34+ cells.
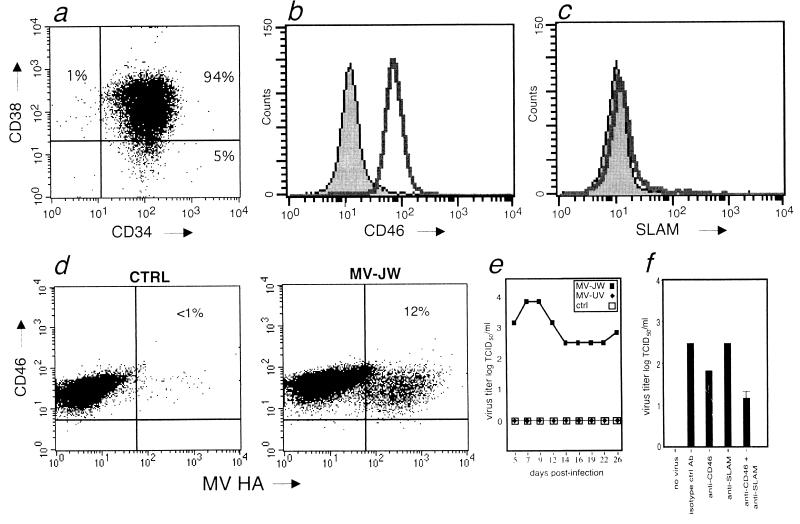
CD34+ cells were examined using MAbs against CD34 and CD38, CD46, or SLAM by using multicolor flow cytometry. As previously reported, UCB-derived CD34+ cells were found to be composed of approximately 94% CD34+ CD38+ cells (a subpopulation containing various committed progenitors of myeloid, lymphoid, and erythroid lineages), and 5% were CD34+ CD38− cells containing pluripotent HSCs (13) (Fig. 1a). Two cellular receptors for MV have been identified, membrane cofactor protein (CD46) and, recently, SLAM (CDw150). CD46 is a complement regulatory protein that is ubiquitously expressed in general but has not previously been demonstrated on CD34+ cells (23). SLAM is a member of the CD2 subset of the immunoglobulin superfamily and is a receptor involved in the activation of T cells and NK cells (39). SLAM has thus far been found on activated T and B lymphocytes, B-cell lines, immature thymocytes, dendritic cells, NK cells, and activated monocytes (6, 38-40). Attenuated laboratory and vaccine strains of MV efficiently use CD46 as a cellular receptor (7, 26, 31). Wild-type isolates of MV can also use CD46 as a cellular receptor, although their affinity for CD46 is markedly reduced (25). Both laboratory and wild-type MV isolates utilize SLAM for entry (40). Although ≥98% of the CD34+ cells were positive for CD46 (Fig. 1b), less than 2% of the cells expressed SLAM (Fig. 1c). CD34+ cells cultured in a cytokine mixture promoting myeloid development, as well as mature CD14+ monocytes, maintained expression of CD46. Under the same conditions, the level of SLAM expression remained ≤2% (data not shown). CD34+ cells were infected with the purified wild-type isolate MV-JW. It was previously shown that wild-type MV-JW can utilize CD46 for entry into human PBMC (25), and it has also been shown that MV-JW can utilize SLAM as a receptor (40). For comparison, an equal volume of MV-JW that had been rendered noninfectious by UV irradiation (MV-UV), or mock-infected cells treated with medium alone, served as control. In the first series of experiments, cells were maintained in culture and the proportion of MV-infected cells was determined by flow cytometry by detecting expression of the MV HA glycoprotein on the cell surface. Typically, the percentage of MV-JW-infected cells rose to a maximum of 12% by 5 days postinfection (Fig. 1d). During this period, CD46 expression levels remained constant. The production of infectious MV from the cells in culture was measured, and as seen in Fig. 1e, significant levels of infectious virus could be recovered. These results demonstrate that the UCB CD34+ cells can be productively infected by wild-type MV and maintain expression of CD46 during myeloid development, with little to no SLAM expression during this period. To determine which of the known MV receptors, CD46 or SLAM, was utilized to enter MV-susceptible cells, inhibition studies using MAbs against these receptors were performed (Fig. 1f). CD34+ cells were incubated with an antireceptor MAb previously shown to inhibit MV infection, specifically, either the MCI20.6 anti-CD46 antibody which recognizes CD46 SCR-1 (32), or the IPO-3 MAb which recognizes SLAM (40). Interestingly, neither antibody efficiently inhibited MV infection, with a less than 10-fold reduction in infection seen with the anti-CD46 MAb (P = 0.4) and no reduction in virus titers seen with the anti-SLAM MAb (Fig. 1f). These results suggest that a novel, as yet unidentified, additional receptor is present and utilized for infection of CD34+ cells by MV.
FIG. 1.
Infection of CD34+ cells by wild-type MV. (a) Flow cytometric analysis of CD34+ cells from human UCB with CD34+ and CD38+ fluorochrome-conjugated antibodies. (b) Expression of CD46+ on CD34+ cells from human UCB (unfilled histogram) compared to that from an isotype control (shaded histogram). (c) Expression of SLAM on CD34+ cells from human UCB (unfilled histogram) compared to that from an isotype control (shaded histogram). (d) Infection of CD34+ cord blood cells by MV-JW. Cell surface expression of the MV HA glycoprotein versus that of CD46+ is shown. Left panel: mock-infected control (CTRL); right panel: MV-JW infected. The percentage of double-positive (CD46+/MV-HA+) cells in each sample is noted in the upper-right quadrant of each panel. (e) Production of MV from infected CD34+ cells over a 24-day time course measured by a TCID50 assay. (f) Inhibition of MV infection of CD34+ cells by antibodies against CD46 or SLAM. Inhibition of viral entry by using an isotype control antibody (isotype ctrl Ab), anti-CD46 MAb MCI20.6 (P = 0.4 compared to control), anti-SLAM antibody IPO-3, or both antibodies together (P < 0.2 compared to control) is shown. Virus production in the cell supernatant at 4 days postinfection was measured by TCID50 assay and is expressed as the log TCID50 per milliliter of supernatant. Assays were performed in triplicate and are expressed as means ± standard errors.
MV infection in pluripotent HSCs.
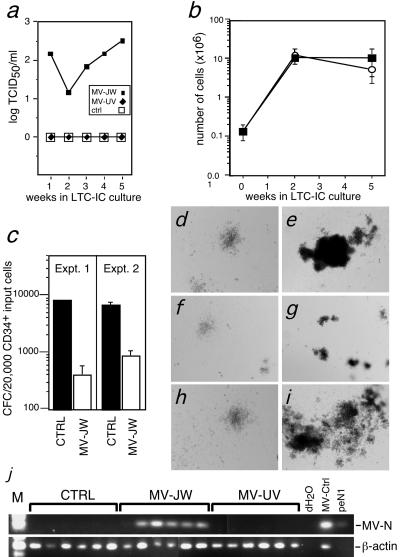
We next assessed whether pluripotent CD34+ HSCs were being targeted by MV. For this purpose, LTC-IC bulk cultures were performed. The LTC-IC culture system allows derivation of myeloid, erythroid, and lymphoid progenitors from pluripotent CD34+ HSCs. Expansion of committed lineages occurs during coculture with irradiated BM stromal cells (8). Stromal cells are a mixture of endothelial and mesenchymal cells and provide growth factors and adhesion molecules required for supporting developing HSCs (11). Typically, cells removed from these cocultures after 2 and 5 weeks are counted to assess committed cell expansion and evaluated in CFC assays to determine progenitor content originating from the HSCs (8). For our studies, MV-infected and UV-MV-treated or mock-infected CD34+ cells were cocultivated with irradiated human BM stromal cells for 5 weeks. Infectious MV production was detected throughout the 5-week time course (Fig. 2a). Infection did not alter committed cell survival and expansion in the cultures, as cell numbers were similar between infected, UV-inactivated virus-treated, and mock-infected control cultures (Fig. 2b). Interestingly, although wild-type MV-JW readily causes cytopathic effects (CPE), primarily syncytium formation in primary human T-cells, no CPE were noted in the long-term cultures during the 5-week time course.
FIG. 2.
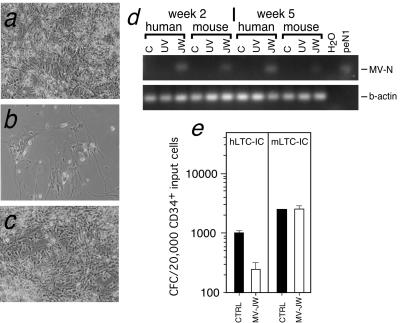
Suppression of hematopoiesis in bulk culture LTC-IC assay. (a) Titers of MV from supernatants of MV-JW as measured by TCID50 assay. Results are representative of 2 independent experiments. (b) Cell numbers in bulk culture LTC-ICs during 5-week cocultivation with human BM stromal cells. Results of two separate experiments are shown. Filled squares, MV-JW cultures; unfilled circles, control cultures. (c) Quantification of total CFCs (including CFU-GM and BFU-E) harvested from week 5 of bulk culture LTC-IC. CFCs from LTC-IC control and MV-JW are shown. Bars indicate means of triplicate culture results ± 1 standard deviation, P < 0.002. (d to i) Light micrographs of colonies formed from CFCs harvested from bulk LTC-IC cultures. Panel d, control and CFU-GM cells; panel e, control and BFU-E; panel f, MV-JW and CFU-GM; panel g, MV-JW and BFU-E; panel h, UV-MV and CFU-GM; panel I, UV-MV and BFU-E. (j) RT-PCR of colonies from control or MV-JW- or MV-UV-infected LTC-IC culture, detecting either the MV-N message (top) or, as a positive control, human β-actin message (bottom). dH2O, no template for PCR; MV-ctrl, RNA template prepared from MV-JW infected human PBMC; peN1, control plasmid DNA template carrying MV-N gene.
To measure the effect of MV infection on the differentiation of individual HSCs, cells from LTC-IC culture were quantitated in a CFC assay. The CFC assay measures the formation of BFU-E, CFU-GM, CFU-granulocyte erythroid macrophage megakaryocyte, and high proliferative potential-CFC colonies (29). CFC resulting from CD34+ cells plated at the start or from week 2 of the long-term cultures showed no differences in the number or size of colonies of mock-infected or MV-UV-treated controls compared with MV-JW-infected cells (data not shown). In contrast, cells removed from the 5-week long-term cultures demonstrated an 8- to 20-fold reduction in the total number of CFCs determined from the MV-infected samples compared to those from the MV-UV (data not shown) or mock-infected controls (Fig. 2c). In addition, the majority of colonies derived from the 5-week MV-JW-infected bulk LTC-IC cultures were markedly reduced in size compared to those from the MV-UV or mock-infected controls (Fig. 2d to i). Similar results were also seen in two additional, independent experiments. To determine the extent of MV infection in the CFC derived from 5-week-culture progenitors, individual colonies were randomly isolated and assessed for the presence of MV-N mRNA by RT-PCR. Pools of four colonies were assessed, and all of the colony pools were MV-N positive (Fig. 2j), indicating that a minimum of 25% of the individual colonies had been infected by MV. CFU-GM and BFU-E colonies were equally infected (data not shown).
No MV-induced CPE were observed in the bulk LTC-IC culture during the 5 weeks of culture. However, in bulk LTC-IC cultures that were further maintained for 7 to 8 weeks, characteristic MV-associated CPE, including syncytium formation and cell lysis, became apparent in the irradiated stromal cell monolayer compared to those in the controls (Fig. 3a to c), and the stromal monolayer was eventually destroyed. The susceptibility of human stroma to wild-type MV was confirmed by infecting nonirradiated human BM stromal cells at an MOI of 0.1 and screening for the presence of MV antigens by indirect immunofluorescence at days 3, 5, and 7 postinfection. Infected cells and syncytium formation were observed by immunofluorescence staining, and infectious virus was produced (data not shown). In addition, while CD46 expression was observed on >95% of the human stromal cells, less than 1% of the stromal cells expressed SLAM (data not shown). Blockade using antibodies against CD46 or SLAM showed complete inhibition with anti-CD46 antibodies (>3 logs), whereas anti-SLAM antibodies gave 2 logs of inhibition. These results would suggest that in contrast to CD34+ cells, CD46 is primarily utilized on stromal cells while SLAM may function as a coreceptor on these cells. These results indicate differential receptor usage by a wild-type MV strain depending on the target cell. Interestingly, usage of CD46 appears to be associated with CPE, as increased cytopathicity and syncytium formation is found on stromal cells compared to CD34+ cells. This effect may be related to the choice of receptor or to the affinity of viral glycoproteins for the cell surface. Our results demonstrate that BM stromal cells support wild-type MV replication and that in contrast to our findings regarding CD34+ committed progenitor cells, wild-type MV produces cytopathicity in stromal cells. These results suggest that MV-mediated loss of stromal support plays a major role in disrupting the development of HSCs.
FIG. 3.
Suppression of hematopoiesis requires MV infection of BM stromal cells. (a to c) Light micrographs depicting CPE observed in bulk LTC-IC cultures at week 8. Panel a, control; panel b, MV-JW infected; panel c, MV-UV infected. (d) RT-PCR detecting MV-N message in bulk culture LTC-IC cocultivated with either human or mouse stroma at week 2 or week 5 postinfection. Top: RT-PCR of MV-N message from mock-infected control (C), MV-UV-infected (UV), and MV-JW-infected (JW) cells. Bottom: positive control PCR for human β-actin. H2O, no PCR template; peN1, plasmid DNA template carrying MV-N gene. (e) CFC assay demonstrating loss of CFC progenitor cells derived from 5-week bulk culture LTC-IC cocultivated with human stroma (hLTC-IC; P < 0.002) compared to control or mouse stroma (mLTC-IC). Bars indicate means of triplicate cultures ± 1 standard deviation.
Role of stromal infection in MV-induced hematopoietic suppression.
To ascertain whether impairment of HSC development occurred directly as a result of infection or indirectly due to loss of the stromal support, we performed a bulk LTC-IC culture using a mouse stromal cell line (M2-10B4) that supports human CD34+ cell development in long-term culture (15). In contrast to human cells, mouse cells are resistant to MV infection, and this resistance was confirmed following MV inoculation of M2-10B4 cells by TCID50 assay and indirect immunofluorescence staining for MV (data not shown). CD34+ cells were infected with MV and plated on either irradiated human stroma (bulk hLTC-IC culture) or mouse M2-10B4 cells (bulk mLTC-IC culture). Cells obtained from the human and mouse bulk LTC-IC culture at 2 and 5 weeks were positive for MV-N mRNA, demonstrating MV infection in both cultures (Fig. 3d). Cells obtained at 5 weeks from bulk hLTC-IC cultures showed the expected eightfold decrease in their colony-forming potential (Fig. 3e). CFC from hLTC-IC were also found to be positive for MV RNA (Table 1), and MV-induced stromal cell CPE was also noted in the bulk hLTC-IC cultures by week 7 (data not shown). In contrast, cells obtained from the bulk mLTC-IC cultures showed no reduction in CFC numbers compared to controls at 5 weeks (Fig. 3e) or at 7 weeks and no stromal CPE was noted (data not shown). Interestingly, a gradual increase in the proportion of MV-positive colonies arising from the mLTC-IC was observed, with 1 MV-positive colony out of a total of 6 colonies, 1 out of 3, and 2 out of 3 found at 2, 5, and 7 weeks, respectively (Table 1).
TABLE 1.
MV RNA in colonies derived from bulk culture LTC-IC
| Week | Source | No. of MV-positive colonies/total no. of colonies |
|---|---|---|
| 2 | Human | 6/6 |
| 2 | Mouse | 1/6 |
| 5 | Human | 3/3 |
| 5 | Mouse | 1/3 |
| 7 | Human | NDb |
| 7 | Mouse | 2/3 |
Determined by RT PCR of N message and Southern blotting of PCR products.
ND, not determined.
DISCUSSION
Our results show that CD34+ committed myeloid progenitors can be directly infected by MV and that developmental suppression of HSCs occurs only in the presence of MV-infected stroma. One possible mechanism for suppression is that disruption of hematopoiesis occurs only when HSCs are infected at a particular differentiation stage that is selected for in the long-term human stromal cell cultures (via expression of receptors or other cellular factors required for replication). The enhanced MV infection of such cells might be due to efficient cell-to-cell contact between infected stroma and HSCs or to a higher viral titer provided by the infected stroma. Our results, demonstrating a high titer from MV-infected human stroma (Fig. 2a) and undetectable MV from the supernatants of 5-week long-term mouse stromal cultures (data not shown) as well as higher numbers of MV-positive CFC from long-term human stromal cultures (Table 1), are consistent with this interpretation.
A second possibility is that MV infection does not directly affect the ability of HSCs to undergo normal hematopoiesis but that the suppression of HSC development is attributable to either a lack of support or active suppression by MV-infected stroma. The absence of HSC suppression by CD34+ cells grown on mouse stroma (Fig. 3e), together with the gradual increase in the proportion of MV-positive colonies in the absence of suppression (Table 1), is consistent with this interpretation. MV infection and disruption of stromal function have been observed in primary human thymic organ cultures, where the destruction of stromal cells by MV leads to apoptosis of immature thymocytes, although the thymocytes themselves are not infected (3). Other immunosuppressive viruses, such as human cytomegalovirus, dengue virus, murine leukemia virus and human immunodeficiency virus type 1, have been reported to target BM stroma and inhibit hematopoiesis by reducing expression of supportive cytokines such as stem cell factor and/or increasing expression of suppressive cytokines such as transforming growth factor β (1, 4, 21, 22, 27, 30, 41). Thus, stromal cell targeting may be a common mechanism used by immunosuppressive viruses to induce hematopoietic suppression. Depletion of MV-infected stromal cells and CD34+ cells from the BM could also be mediated by virus-specific cytotoxic T lymphocytes induced during acute measles infection (17). The combination of a suppressive cytokine milieu in the BM with depletion of both stromal and CD34+ populations may significantly impair repopulation of lymphoid precursors following MV-induced lymphopenia. Once MV was cleared, reconstitution of a functional BM microenvironment and HSC populations would require a 1- to 2-month timeframe, consistent with the length of time typically required to resolve lymphopenia and immunosuppression following acute measles (33).
Our results also indicate that cellular receptors for MV may play an important role in differential targeting of MV to specific hematopoietic lineages in vivo and may help to explain disease pathology. SLAM has been proposed to be the primary MV receptor for wild-type viruses (40); however, the lack of SLAM expression for early hematopoietic and myeloid lineage cells shown to be important targets for wild-type MV both in vitro and in vivo suggests otherwise. Our results strongly suggest that MV utilizes neither CD46 nor SLAM to gain entry into CD34+ cells, suggesting the presence of an alternative, as-yet unidentified third receptor. In contrast, stromal cells appear to be infected via the CD46 receptor, with SLAM possibly functioning as a coreceptor. MV targeting of myeloid progenitors in BM may allow an insidious route of immunosuppression, i.e., the propagation of MV, with minimal CPE, throughout myeloid cells during development. MV has been shown to infect monocyte progenitors (14), mature monocytes, and myeloid-derived antigen-presenting cells such as dendritic cells and Langerhans cells in vitro (10). The loss or dysfunction of macrophage and dendritic cell antigen-presenting cells most likely contributes to the generalized impairment of T-cell-mediated immunity by impairing efficient T activation and inducing widespread T-cell apoptosis or anergy (36, 37). Infection of monocytes and macrophages has also been associated with suppression of IL-12 production. This loss of normal IL-12 regulation leads to the skewing of T-cell responses toward T-helper-2 (18), which has been associated with suppression of CMI in other viral models (5). In contrast to myeloid cells, activated T and B cells express both CD46 and SLAM (39, 40). MV may preferentially utilize SLAM to infect and induce apoptosis in activated lymphocytes, resulting in lymphopenia. To further define the precise mechanisms in vivo by which differential MV receptor expression patterns influence measles targeting, replication, and resulting immunosuppression is of critical importance.
Finally, the ability of MV to directly target primitive HSCs indicates that MV may be a useful candidate vector for delivery of foreign genes to these cells. MV can be stably engineered to express foreign genes (34). Our findings suggest that as long as functional stromal support is present, measles efficiently infects primitive hematopoietic cells with no apparent deleterious effects on hematopoiesis. MV-based vectors are likely to be as safe as or safer than other viral gene delivery vectors, since attenuated vaccine strains of MV have routinely been administered to millions of people worldwide. Thus, it will be interesting to determine whether recombinant MV or MV glycoproteins can promote efficient and stable targeting of foreign genes and/or vectors to HSCs.
Acknowledgments
We thank M. Estrada for excellent technical assistance and D. Naniche, M. Oldstone, D. Mosier, and I. Schulman for helpful discussions and critical readings of the manuscript.
This work was supported by Public Health Service grants AI41514 and AI46441 (M.M.) and DK49886 and DK54938 (B.E.T.).
Footnotes
This is manuscript number 13548-NP from The Scripps Research Institute.
REFERENCES
- 1.Almeida, G., C. D. Porada, S. St. Jeor, and J. L. Ascensao. 1994. Human cytomegalovirus alters interleukin-6 production by endothelial cells. Blood 83:370-376. [PubMed] [Google Scholar]
- 2.Arneborn, P., and G. Biberfeld. 1983. T-lymphocyte subpopulations in relation to immunosuppression in measles and varicella. Infect. Immun. 39:29-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auwaerter, P., H. Kaneshima, J. M. McCune, G. Wiegand, and D. E. Griffin. 1996. Measles infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahner, I., K. Kearns, S. Coutinho, E. H. Leonard, and D. B. Kohn. 1997. Infection of human marrow stroma by human immunodeficiency virus-1 (HIV-1) is both required and sufficient for HIV-1 induced hematopoietic suppression in vitro: demonstration by gene modification of primary human stroma. Blood 90:1787-1798. [PubMed] [Google Scholar]
- 5.Biron, C. A. 1994. Cytokines in the generation of immune responses to, and resolution of, virus infection. Curr. Opin. Immunol. 6:530-538. [DOI] [PubMed] [Google Scholar]
- 6.Cocks, B. G., C. C. Chang, J. M. Carballido, H. Yssel, J. E. de Vries, and G. Aversa. 1995. A novel receptor involved in T-cell activation. Nature 376:260-263. [DOI] [PubMed] [Google Scholar]
- 7.Dorig, R., A. Marcel, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 8.Eaves, C. J., J. D. Cashman, H. J. Sutherland, T. Otsuka, R. K. Humphries, D. E. Hogge, P. L. Lansdorp, and A. C. Eaves. 1991. Molecular analysis of primitive hematopoietic cell proliferation control mechanisms. Ann. N. Y. Acad. Sci. 628:298-306. [DOI] [PubMed] [Google Scholar]
- 9.Forthal, D. N., S. Aarnaes, J. Blanding, L. de la Maza, and J. G. Tilles. 1992. Degree and length of viremia in adults with measles. J. Infect. Dis. 166: 421-424. [DOI] [PubMed] [Google Scholar]
- 10.Fugier-Vivier, I., C. Servet-Delprat, P. Rivailler, M. Rissoan, Y. Liu, and C. Rabourdin-Combe. 1997. Measles virus suppresses cell-mediated immunity by interfering with the survival and functions of dendritic and T cells. J. Exp. Med. 186:813-823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Greenberger, J. S. 1991. The hematopoietic microenvironment. Crit. Rev. Oncol. Hematol. 11:65-84. [DOI] [PubMed] [Google Scholar]
- 12.Griffin, D. E., and W. J. Bellini. 1996. Measles virus, p. 1267-1312. In B. N. Fields, D. M. Knipe, and P. M. Howley (ed.), Fields virology, vol. 3. Lippincott-Raven, Philadelphia, Pa. [Google Scholar]
- 13.Hao, Q. L., A. J. Shah, F. T. Thiemann, E. M. Smogorzewska, and G. M. Crooks. 1995. A functional comparison of CD34+ CD38− cells in cord blood and bone marrow. Blood 86:3745-3753. [PubMed] [Google Scholar]
- 14.Helin, A., A. Salmi, A. Vanharanta, and R. Vainionpaa. 1999. Measles virus replication in cells of myelomonocytic lineage is dependent on cellular differentiation stage. Virology 253:35-42. [DOI] [PubMed] [Google Scholar]
- 15.Hogge, D. E., P. M. Lansdorp, D. Reid, B. Gerhard, and C. J. Eaves. 1996. Enhanced detection, maintenance, and differentiation of primitive human hematopoietic cells in cultures containing murine fibroblasts engineered to produce human steel factor, interleukin-3, and granulocyte colony-stimulating factor. Blood 88:3765-3773. [PubMed] [Google Scholar]
- 16.Horvat, B., P. Rivailler, G. Varior-Krishnan, A. Cardoso, D. Gerlier, and C. Rabourdin-Combe. 1996. Transgenic mice expressing human measles virus (MV) receptor provide cells exhibiting different permissivities to MV infection. J. Virol. 70:6673-6681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaye, A., A. F. Magnusen, A. D. Sadiq, T. Corrah, and H. C. Whittle. 1998. Ex vivo analysis of cytotoxic T lymphocytes to measles antigens during infection and after vaccination in Gambian children. J. Clin. Investig. 102:1969-1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Karp, C., M. Wysocka, L. Wahl, J. Ahearn, P. Cuomo, B. Sherry, G. Trinchieri, and D. Griffin. 1996. Mechanism of suppression of cell-mediated immunity by measles virus. Science 273:228-231. [DOI] [PubMed] [Google Scholar]
- 19.Karp, C. L. 1999. Measles: immunosuppression, interleukin-12, and complement receptors. Immunol. Rev. 168:91-101. [DOI] [PubMed] [Google Scholar]
- 20.Kim, Y. J., S.-Y. Kim, Y.-Y. Kim, J.-W. Kim, J. H. Lee, K. Han, and W. Lee. 2002. Quantities of receptor molecules for colony stimulating factors on leukocytes in measles. Yonsei Med. J. 43:43-47. [DOI] [PubMed] [Google Scholar]
- 21.Kulkosky, J., M. Bouhamdan, A. Geist, G. Nunnari, D. Phinney, and R. Pomerantz. 2000. Pathogenesis of HIV-1 infection within bone marrow cells. Leuk. Lymphoma 37:497-515. [DOI] [PubMed] [Google Scholar]
- 22.LaRussa, V. F., and B. L. Innis. 1995. Mechanisms of dengue virus-induced bone marrow suppression. Balliere's Clin. Hematol. 8:249-270. [DOI] [PubMed] [Google Scholar]
- 23.Liszewski, M. K., T. W. Post, and J. P. Atkinson. 1991. Membrane cofactor protein (MCP or CD46): newest member of the regulators of complement activation gene cluster. Annu. Rev. Immunol. 9:431-455. [DOI] [PubMed] [Google Scholar]
- 24.Mackall, C. L., F. T. Hakim, and R. E. Gress. 1997. Restoration of T-cell homeostasis after T-cell depletion. Semin. Immunol. 9:339-346. [DOI] [PubMed] [Google Scholar]
- 25.Manchester, M., D. S. Eto, A. Valsamakis, P. B. Liton, R. Fernandez-Munoz, P. A. Rota, W. J. Bellini, D. N. Forthal, and M. B. A. Oldstone. 2000. Clinical isolates of measles virus use CD46 as a cellular receptor. J. Virol. 74:3967-3974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manchester, M., M. K. Liszewski, J. P. Atkinson, and M. B. A. Oldstone. 1994. Multiple isoforms of CD46 (membrane cofactor protein) serve as receptors for measles virus. Proc. Natl. Acad. Sci. USA 91:2161-2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mayer, A., J. Podlech, S. Kurz, H.-P. Steffens, S. Maiberger, K. Thalmeier, P. Angele, L. Dreher, and M. Reddehase. 1997. Bone marrow failure by cytomegalovirus is associated with an in vivo deficiency in the expression of essential stromal hemopoietin genes. J. Virol. 71:4589-4598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McFarland, R. D., D. C. Douek, R. A. Koup, and L. J. Picker. 2000. Identification of a human recent thymic emigrant phenotype. Proc. Natl. Acad. Sci. USA 97:4215-4220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyoshi, H., K. A. Smith, D. E. Mosier, I. M. Verma, and B. E. Torbett. 1999. Transduction of human CD34+ cells that mediate long-term engraftment of NOD/SCID mice by HIV vectors. Science 283:682-686. [DOI] [PubMed] [Google Scholar]
- 30.Moses, A. V., S. Williams, M. L. Heneveld, J. Strussenberg, M. Rarick, M. Loveless, G. Bagby, and J. A. Nelson. 1996. Human immunodeficiency virus infection of bone marrow endothelium reduces induction of stromal hematopoietic growth factors. Blood 87:919-925. [PubMed] [Google Scholar]
- 31.Naniche, D., G. Varior-Krishnan, F. Cervino, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naniche, D., T. F. Wild, C. Rabourdin-Combe, and D. Gerlier. 1993. A monoclonal antibody recognized a human cell surface glycoprotein involved in measles virus binding. J. Gen. Virol. 73:2617-2624. [DOI] [PubMed] [Google Scholar]
- 33.Okada, H., F. Kobune, T. A. Sato, T. Kohama, Y. Takeuchi, T. Abe, N. Takayama, T. Tsuchiya, and M. Tashiro. 2000. Extensive lymphopenia due to apoptosis of uninfected lymphocytes in acute measles patients. Arch. Virol. 145:905-920. [DOI] [PubMed] [Google Scholar]
- 34.Radecke, R., P. Spielhofer, H. Schneider, K. Kaelin, M. Huber, C. Dotsch, G. Christiansen, and M. A. Billeter. 1995. Rescue of measles viruses from cloned DNA. EMBO J. 14:5773-5784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schneider-Schaulies, S., and V. ter Meulen. 1999. Pathogenic aspects of measles virus infections. Arch. Virol. Suppl. 15:139-158. [DOI] [PubMed] [Google Scholar]
- 36.Servet-Delprat, C., P. O. Vidalain, O. Azocar, F. Le Deist, A. Fischer, and C. Rabourdin-Combe. 2000. Consequences of Fas-mediated human dendritic cell apoptosis induced by measles virus. J. Virol. 74:4387-4393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Servet-Delprat, C., P. O. Vidalain, H. Bausinger, S. Manie, F. Le Deist, O. Azocar, D. Hanau, A. Fischer, and C. Rabourdin-Combe. 2000. Measles virus induces abnormal differentiation of CD40 ligand-activated human dendritic cells. J. Immunol. 164:1753-1760. [DOI] [PubMed] [Google Scholar]
- 38.Sidorenko, S. P., and E. A. Clark. 1993. Characterization of a cell surface glycoprotein IPO-3, expressed on activated human B and T lymphocytes. J. Immunol. 151:4614-4624. [PubMed] [Google Scholar]
- 39.Tangye, S. G., J. H. Phillips, and L. L. Lanier. 2000. The CD-2 subset of the Ig superfamily of cell surface molecules: receptor-ligand pairs expressed by NK cells and other immune cells. Semin. Immunol. 12:149-157. [DOI] [PubMed] [Google Scholar]
- 40.Tatsuo, H., N. Ono, K. Tanaka, and Y. Yanagi. 2000. SLAM (CDw150) is a cellular receptor for measles virus. Nature 406:893-897. [DOI] [PubMed] [Google Scholar]
- 41.Tse, K., J. Morrow, N. K. Hughes, and V. S. Gallicchio. 1994. Stromal cell lines derived from LP-BM5 murine leukemia virus-infected long-term bone marrow cultures impair hematopoiesis in vitro. Blood 84:1508-1518. [PubMed] [Google Scholar]