Abstract
Theiler's virus infection of the central nervous system (CNS) induces an immune-mediated demyelinating disease in susceptible mouse strains, such as SJL/J, and serves as a relevant infectious model for human multiple sclerosis. It has been previously suggested that susceptible SJL/J mice do not mount an efficient cytotoxic T-lymphocyte (CTL) response to the virus. In addition, genetic studies have shown that resistance to Theiler's virus-induced demyelinating disease is linked to the H-2D major histocompatibility complex class I locus, suggesting that a compromised CTL response may contribute to the susceptibility of SJL/J mice. Here we show that SJL/J mice do, in fact, generate a CD8+ T-cell response in the CNS that is directed against one dominant (VP3159-166) and two subdominant (VP111-20 and VP3173-181) capsid protein epitopes. These virus-specific CD8+ T cells produce gamma interferon (IFN-γ) and lyse target cells in the presence of the epitope peptides, indicating that these CNS-infiltrating CD8+ T cells are fully functional effector cells. Intracellular IFN-γ staining analysis indicates that greater than 50% of CNS-infiltrating CD8+ T cells are specific for these viral epitopes at 7 days postinfection. Therefore, the susceptibility of SJL/J mice is not due to the lack of an early functional Theiler's murine encephalomyelitis virus-specific CTL response. Interestingly, T-cell responses to all three epitopes are restricted by the H-2Ks molecule, and this skewed class I restriction may be associated with susceptibility to demyelinating disease.
Theiler's murine encephalomyelitis virus (TMEV) is a common enteric mouse picornavirus (48). Members of the Theiler's original subgroup of TMEV, such as the BeAn and DA strains, induce a biphasic disease when injected into the CNS of susceptible strains of mice (27). In contrast to the DA strain, the BeAn strain induces an early mild encephalitogenic phase that is clinically undetectable. However, the chronic, progressive demyelinating disease which follows is clinically and histopathologically similar to human multiple sclerosis (MS) (28). In addition, BeAn infection of demyelination-susceptible mice leads to eventual autoimmune responses against myelin autoantigens (33). Furthermore, the various immunological and genetic factors associated with TMEV-induced demyelinating disease (TMEV-IDD) parallel those of human MS (21). Taken together with a suspected viral etiology for MS (1, 11, 46), these similarities render TMEV-IDD a relevant infectious model for the study of human MS.
Demyelinating disease induced in the highly susceptible SJL/J (H-2s) mouse strain following TMEV infection is associated with viral persistence in the CNS (7, 29, 44) and inflammatory Th1-type responses to viral epitopes (15, 50, 51). The predominant CD4+ Th cell epitopes have been mapped to the VP1, VP2, and VP3 capsid proteins of the virus (14, 49, 50). While similar mapping studies have been done for the antibody response to TMEV (17, 19), the role of antibodies in demyelination is unclear. In addition, the role of CD8+ T cells in the pathogenesis of demyelination remains a controversial issue. Resistance to demyelination has been closely associated with the major histocompatibility complex (MHC) class I genetic locus (30, 42), suggesting that class I-restricted CD8+ T cells may be important mediators of protection and/or pathogenesis. Class I-restricted CTL appear to confer protection from TMEV-induced demyelination in resistant strains, as β2-microglobulin-deficient (13, 38, 43) and perforin-deficient (36, 37, 45) mice on a resistant H-2b background fail to clear TMEV from the CNS and develop demyelinating lesions similar to those of susceptible strains. However, the lack of clinical signs (i.e., waddling gait and eventual paralysis) in these mice observed by one group of investigators has led to the hypothesis that CTL may also mediate the CNS pathology responsible for clinical manifestation of disease (36, 41). On the other hand, we and others have shown that such CD8+ T-cell-deficient mice develop full-blown clinical symptoms, especially following immunization with UV-inactivated virus (13, 37, 38). In addition, susceptible SJL/J mice lacking β2-microglobulin exhibit higher levels of demyelination, increased inflammatory Th responses, and earlier onset of clinical disease (5). These results indicate that class I-restricted CD8+ T cells are not necessary for the development of demyelination or clinical symptoms and instead contribute to protection from disease. Such protection by CD8+ T cells may reflect direct down-regulation of the pathogenic Th cell response by regulatory CD8+ T cells (which may or may not be virus specific) or CTL-mediated elimination of a source of stimulation (virus antigen) for these pathogenic Th cells (20). However, the possibility remains that CD8+ T cells may also contribute to immunopathology either by destroying virus-infected cells or through the secretion of proinflammatory cytokines in response to viral antigen. Thus, it is necessary to thoroughly characterize the CTL response in susceptible mouse strains, such as SJL/J, in order to gain a better understanding of the role of these cells in the pathogenesis of demyelinating disease.
The TMEV-specific CTL response in resistant C57BL/6 mice has been fairly well characterized. TMEV infection induces H-2Db-restricted virus-specific CTL responses toward a single predominant epitope (6, 10, 18) and two subdominant epitopes (31), all of which reside on the VP2 and VP3 capsid proteins. However, little is known about the specificity or function of CTL in susceptible SJL/J mice. In fact, no Theiler's virus CTL epitopes have been identified in mouse strains other than C57BL/6. The few existing reports regarding CTL activity in SJL/J mice come to different conclusions as to whether or not this strain has the capability of mounting a virus-specific CD8+ T-cell response. An earlier study suggested that SJL/J mice generate a TMEV-specific CTL response that is detectable throughout the course of infection (26). However, a more recent study from this same group of investigators concluded that CTL infiltrating the CNS of virus-infected SJL/J mice are not TMEV specific (24). Another group has suggested that TMEV infection induces a low but detectable level of virus-specific CTL in SJL/J mice compared to a high level of response in resistant C57BL/6 mice (9). However, these studies analyzed CTL activity in the spleens of mice that were infected intraperitoneally and thus did not address the level of CTL in the target organ, the CNS.
To define the role of these T cells in pathogenesis or protection, assessment of the level and function of virus-specific CD8+ T cells infiltrating the CNS, if they exist, is critically important. By using a library of overlapping 20-mer peptides encompassing the entire P1 capsid protein region of the BeAn strain of TMEV, we report here the identification of one dominant and two subdominant epitopes recognized by CNS-infiltrating CTL from SJL/J mice infected with TMEV. The minimal CTL epitope regions as well as the MHC restriction element of these epitopes have been defined, and the magnitude of the CNS-infiltrating CD8+ T-cell response has been determined. Our results indicate that the majority of CD8+ T cells in the CNS of TMEV-infected SJL/J mice early after infection are virus-specific and fully functional, as assessed by cytokine production and cytolytic activity.
MATERIALS AND METHODS
Animals.
Female SJL/J and C57BL/6 mice were purchased from the Charles River Laboratories (Charles River, Mass.) through the National Cancer Institute (Frederick, Md.). All mice were housed at the Animal Care Facility of Northwestern University.
Virus and cell lines.
The BeAn strain of TMEV was propagated and titrated in BHK cells grown in Dulbecco's modified Eagle medium supplemented with 7.5% donor calf serum. The EL-4 (H-2b) cell line was obtained from the American Type Culture Collection (ATCC; Manassas, Va.). EL-4Ds and EL-4Ks cell lines were generated by electroporation of EL-4 cells with the pcDNA-H2Ds2 + 1 (a kind gift from Steven R. King, Wayne State University) or RSV-Ks-SV40 (a gift from Gregory Plautz, Cleveland Clinic) expression construct. NJ101 was a gift from Nick Ponzio (New Jersey Medical School), and KSSV was obtained from Jackson Laboratory (Bar Harbor, Maine). SJKY was generated in the laboratory by simian virus 40 transformation of SJL/J kidney fibroblasts. All cell lines were maintained in RPMI 1640 supplemented with 10% fetal calf serum, glutamine/pyruvate, and antibiotics (complete RPMI-10).
Synthetic peptides and antibodies.
Synthetic peptides were generated with the RaMPS peptide synthesis system (DuPont Co., Wilmington, Del.). All peptide stocks (2 mM) were dissolved in 8% dimethyl sulfoxide in phosphate-buffered saline. All antibodies used for flow cytometry were purchased from Pharmingen (San Diego, Calif.). The purified anti-CD8 antibody was prepared from culture supernatants of the H35-17.2 hybridoma (16) by quaternary aminoethyl sephadex separation.
Infection of mice with TMEV.
For intracerebral infection, 30 μl (approximately 105 PFU) of TMEV BeAn was injected into the right cerebral hemisphere of 6- to 8-week-old mice anesthetized with methoxyflurane.
Isolation of CNS-IL.
Mice were perfused through the left ventricle with 30 ml of sterile Hank's balanced salt solution (HBSS). Excised brains and spinal cords were forced through wire mesh and were incubated at 37°C for 45 min in 250-μg/ml collagenase type 4 (Worthington Biochemical Corp., Lakewood, N.J.). CNS-infiltrating lymphocytes (CNS-IL) were then enriched by continuous Percoll gradient (Pharmacia, Piscataway, N.J.) centrifugation for 30 min at 27,000 × g. Magnetic separation of CD4+ and CD8+ cells was performed according to the manufacturer's protocol (Miltenyi Biotec, Auburn, Calif.).
CTL assays.
All target cells were incubated for 4 to 6 h with peptides. Target cells were labeled with 51Cr (50 μCi per target) for 2 h, washed three times with RPMI 1640, and then resuspended at 3 × 104 cells/ml in complete RPMI supplemented with 5% serum, nonessential amino acids, and 2-mercaptoethanol. 51Cr-labeled target cells were added to a 96-well round-bottom plate at 100 μl/well. CNS effector cells were isolated as described above and cultured overnight in the presence of 5 U of recombinant interleukin-2 (IL-2)/ml. On the day of the assay, live cells were enriched by Histopaque (Sigma, St. Louis, Mo.) centrifugation, resuspended in complete media, and then added to target cells at different dilutions. Supernatants were harvested after 6 h of incubation at 37°C, and mean radioactivity values were calculated from duplicate wells. Percent specific lysis was calculated according to the standard formula [(experimental 51Cr release − spontaneous 51Cr release)/(maximum 51Cr release with 1% Triton X-100 − spontaneous 51Cr release)] × 100%. Spontaneous release for all experiments was <15% of the maximum release.
Cytokine ELISPOT assays.
Enzyme-linked immunospot (ELISPOT) assay plates (Millipore, Bedford, Mass.) were precoated with 1- to 5-μg/ml concentrations of anti-gamma interferon (IFN-γ) antibody in 0.05 M carbonate-bicarbonate buffer, pH 9.6. Plates were washed and then blocked with sterile phosphate-buffered saline containing 1% bovine serum albumin. Plates were incubated for 18 h with 1 × 104 to 2 × 104 CNS-IL plus 106 irradiated (3,000 rad) syngeneic spleen cells in 200 μl of HL-1 medium (Bio-Whittaker, Walkersville, Md.) at 37°C under 5% CO2 in the presence of 2 μM peptide. After being washed, plates were incubated with biotin-conjugated anti-IFN-γ antibody (Endogen, Boston, Mass.) overnight and then with streptavidin-horseradish peroxidase for 3 h. Spots were developed as previously described by using 3-amino-9-ethyl-carbazole (Sigma) in 0.05 M sodium acetate buffer (47).
Intracellular cytokine staining.
Briefly, freshly isolated CNS-IL were cultured in 96-well round-bottom plates in the presence of relevant or control peptide and Brefeldin A for 6 h at 37°C. Cells were then incubated in 50 μl of 2.4G2 hybridoma supernatant (ATCC) for 20 min at 4°C to block Fc receptors. Fluorescein isothiocyanate-conjugated anti-CD8 (clone 53-6.7) antibody diluted in 50 μl of 2.4G2 supernatant was added, and cells were incubated for an additional 30 min at 4°C. After two washes, intracellular IFN-γ staining was performed according to the manufacturer's instructions provided by Pharmingen. The antibodies used were phycoerythrin-labeled rat monoclonal anti-IFN-γ (XMG1.2) and an isotype control (rat immunoglobulin G1). Cells were analyzed on a Becton Dickinson FACSCalibur flow cytometer. Live cells were gated on the basis of light scatter properties.
RESULTS
Identification of 20-mer peptides capable of stimulating IFN-γ production by CNS-infiltrating CD8+ lymphocytes.
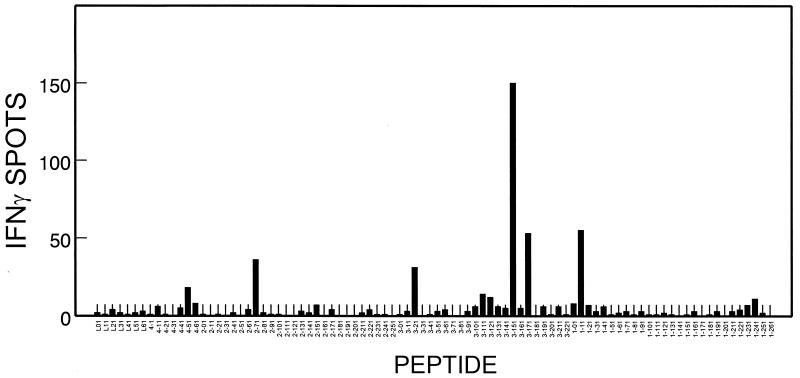
It has previously been shown that the level of IFN-γ-producing CD8+ T cells in response to viral epitopes correlates well with CTL levels measured by cytolytic function and MHC class I peptide tetramer staining (35). To identify potential CTL epitopes within the structural proteins of the BeAn strain of TMEV, CNS-IL from TMEV-infected SJL/J mice were screened with an IFN-γ ELISPOT assay for the presence of virus-specific T cells. CNS-IL were incubated with individual peptides from an overlapping 20-mer peptide library representing all of the TMEV capsid proteins (Leader, VP1, VP2, VP3, and VP4). Figure 1 represents a typical profile of the consistent pattern of IFN-γ production by CNS-IL at an early stage (7 to 10 days) of TMEV infection. Interestingly, the VP3151-170, VP3171-190, and VP111-30 peptides stimulated noticeably higher numbers of IFN-γ-producing cells than the VP271-90, VP321-40, and VP1231-250 peptides, which contain the previously defined Th epitopes (14, 49, 50). This experiment clearly demonstrates the presence of lymphocytes in the CNS of TMEV-infected SJL/J mice that produce IFN-γ in response to three previously unknown capsid protein epitopes. Similar ELISPOT assays were performed to assess the levels of type 2 cytokine (IL-5)-producing cells. However, none of the peptides from the peptide library elicited significant IL-5 production (data not shown).
FIG. 1.
Assessment of IFN-γ production by CNS-IL from TMEV-infected SJL/J mice in response to a capsid protein 20-mer peptide library. CNS-IL were isolated from susceptible SJL/J mice at early stages (7 to 10 days) of TMEV infection. IFN-γ-producing cells from 2.5 × 104 CNS-IL were enumerated by ELISPOT assay after 18 h of incubation with 106 syngeneic spleen cells (3,000 rad) and peptides. A representative result (9 days postinfection) from three separate experiments is shown here. Background for phosphate-buffered saline controls was 2 ± 1 spots.
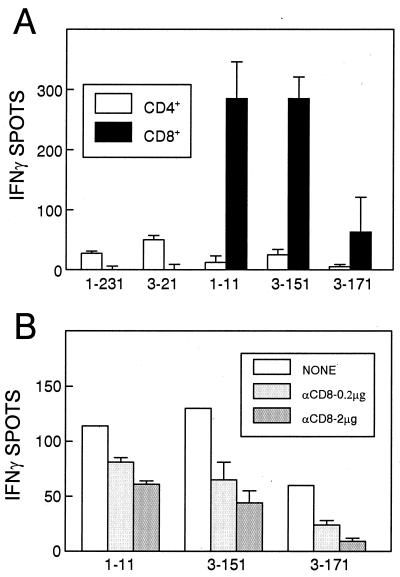
To identify whether any of these three new epitopes represent potential class I-restricted CTL epitopes, CNS-IL from mice infected with TMEV for 7 days were positively selected with anti-CD8 or anti-CD4 antibody-coated magnetic beads, and the separated populations were analyzed by ELISPOT (Fig. 2A). The results clearly indicate that the CD8+ T-cell fraction recognizes the VP111-30, VP3151-170, and VP3171-190 peptides. As expected, the CD4+ T-cell fraction produced IFN-γ in response to known Th epitope-containing peptides, VP1231-250 and VP321-40, but not in response to the three new epitopes. In addition, treatment with an antibody specific for CD8 inhibited IFN-γ production in a dose-dependent manner against these three peptides (Fig. 2B), while no such inhibition was seen for the CD4+ T-cell epitope peptides (data not shown). Therefore, all three of the new epitopes identified here represent CD8+ T-cell epitopes.
FIG. 2.
Identification of three new epitope regions (VP111-30, VP3151-170, and VP3171-190) recognized by CNS-infiltrating CD8+ T cells. (A) Either CD4+ or CD8+ T cells were selected by using anti-CD4 or anti-CD8 antibody-coated magnetic beads and were assessed by IFN-γ ELISPOT assays. Two previously defined Th epitopes (VP1231-250 and VP321-40) were used as controls for separation. An identical number (2 × 104) of cells for individual peptides were used for ELISPOT assays. Background levels were 8 ± 1 spots for CD4+ T cells and 17 ± 6 spots for CD8+ T cells. (B) IFN-γ production in response to these peptides was inhibited in the presence of anti-CD8 antibody. Unseparated CNS-IL (2 × 104) were treated with two different concentrations (0.2 and 2 μg) of antibody during the ELISPOT assay. Background was 1 ± 1 spot. Both experiments were performed at 7 days after TMEV infection.
Definition of class I-restricted minimal epitopes by using truncated peptides.
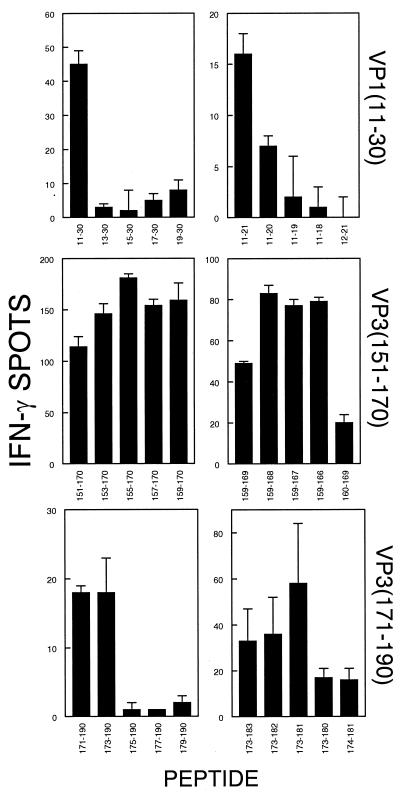
To determine the minimal epitopes within the 20-mer peptides used above, we generated truncated forms of the 20-mer peptides (VP111-30, VP3151-170, and VP3171-190) and assessed which regions are required for T-cell stimulation. Initially, we used peptides truncated by 2 amino acids each to define the N terminus (Fig. 3, left panel). Once the N terminus was defined, serial truncations of the C terminus led to the identification of the minimal epitopes (Fig. 3, right panel). The results indicate that the minimal regions required for activation of CNS-infiltrating CD8+ T cells to produce IFN-γ are VP111-20, VP3159-166, and VP3173-181. The amino acid sequences between VP3159-169 (FNFTAPFI) and VP3173-181 (QTSYTSPTI) are similar to each other and also to other known class I binding motifs (Table 1). However, the amino acid composition and sequence of these epitopes are quite different from that of VP111-20 (SNDDASVDFV). Nevertheless, a common feature of all three epitopes is the hydrophobic C-terminal residue thought to be involved in peptide-MHC contact. We believe that these are the first CD8+ T-cell epitopes identified for the H-2s haplotype.
FIG. 3.
Determination of the minimal epitope regions within VP111-30, VP3151-170, and VP3171-190. The left panel represents IFN-γ-producing cell numbers from the CNS in response to the 20-mer peptides truncated by 2 residues from the N termini. The N-terminal residues required for the maximal response were determined, and these residues were fixed as the N termini for additional peptides truncated by single residues from the C terminus (right panel). The values for control 20-mer peptides (right panel) were the following: VP111-30, 22 ± 6; VP3151-170, 58 ± 3; VP3171-190, 24 ± 3. Data are representative of four separate experiments. Background for all individual assays represented in the figure was <10% of the number of spots seen for the peptide giving maximal stimulation.
TABLE 1.
Sequence comparison of H-2Ks-restricted CTL epitopes within Theiler's virus capsid proteins
| Capsid region | Amino acid sequence |
|---|---|
| VP111-20 | SNDDASVDFV |
| VP3159-166 | FNFTAPFI |
| VP3173-181 | QTSYTSPTI |
| Kb consensusa | YFI |
| YL | |
| M | |
| V | |
| Kd consensusa | YI |
| FL | |
| V |
Sequences are from Rammensee et al. (40).
Lytic activity of CD8+ T cells against existing H-2s target cells and H-2s MHC class I transfectants.
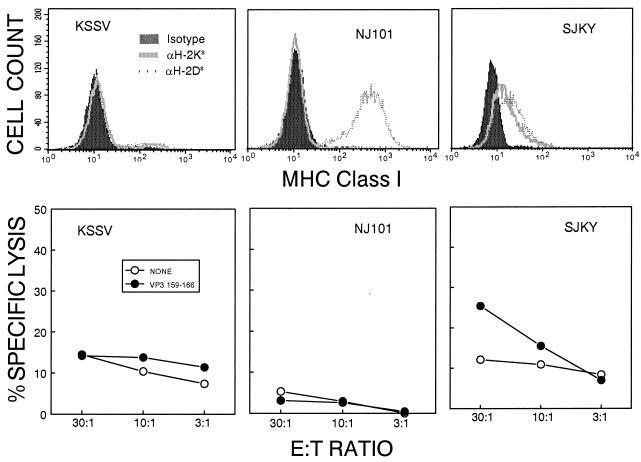
As SJL/J mice have previously been thought to be deficient in CTL activity, we were somewhat surprised by the relatively high number of IFN-γ-producing CD8+ T cells compared to the number of IFN-γ-producing CD4+ Th cells in the CNS of TMEV-infected mice (Fig. 1). One possible explanation for low or undetected CTL activity in previous studies is that SJL/J CTL may produce IFN-γ but may be inefficient at cytolysis of target cells. In order to assess the cytolytic potential of CNS-IL from TMEV-infected mice, three different cell lines were used as target cells in standard 51Cr release assays. These cell lines include KSSV, used to detect CTL levels in previous reports (9, 24), NJ101, a reticulum cell sarcoma line derived from SJL/J mice (23), and SJKY, generated in our laboratory by simian virus 40 transformation of SJL/J kidney fibroblasts (Fig. 4). Interestingly, no significant specific CTL activity was detectable against peptide-loaded KSSV or NJ101 cells, while low levels of peptide-specific lysis were observed against SJKY cells at a higher effector:target ratio (30:1). These levels of cytolytic activity correlated well with the expression level of the H-2Ks molecule on the target cell lines (Fig. 4). The lytic activity against the SJKY cell line combined with the fact that splenic T-cell blasts are also susceptible to low levels of peptide-specific lysis (data not shown) suggested that CTL in the SJL/J strain have lytic capability. However, the low level of MHC expression on SJKY cells led us to generate a more suitable target cell line for our cytolysis assays.
FIG. 4.
Assessment of H-2Ks and H-2Ds expression on the surface of various existing target cell lines and correlation with the levels of cytolysis of the target cells loaded with the VP3159-166 peptide. Epitope-specific cytolytic activity of infiltrating T cells from the CNS of BeAn-infected SJL/J mice was measured at 8 days postinfection in a standard 51Cr release assay. The spontaneous 51Cr release was less than 10%.
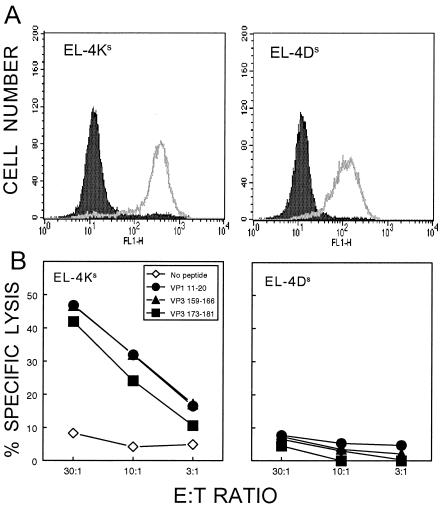
To generate reliable target cells for assessing H-2Ks- and H-2Ds-restricted CTL function, EL-4, a commonly used H-2b CTL target cell line, was transfected with expression constructs for H-2Ds and H-2Ks. The resulting stably transfected clones show levels of expression (Fig. 5A) that are similar to MHC expression levels on SJL/J splenocytes (data not shown). Using these as target cells, the levels of specific cytolysis in the presence of the above putative CTL epitope peptides were assessed. All three peptides yielded significant levels of cytolysis, even at a relatively low effector:target cell ratio (10:1), with EL-4Ks but not with EL-4Ds targets (Fig. 5B). In addition, the minimal epitope regions determined above by ELISPOT assays were confirmed here by target cell lysis assays (data not shown). These results clearly indicate that CTL specific for all three epitopes are lytic and are restricted by the H-2Ks class I molecule.
FIG. 5.
Determination of H-2K/D restriction of epitope peptides for cytolytic function with newly generated target cell lines. The EL-4 (H-2b) cell line was transfected with either H-2Ks or H-2Ds expression constructs. (A) Expression of H-2Ks and H-2Ds molecules on stably transfected EL-4 lines (EL-4Ks and EL-4Ds) was assessed by flow cytometric analysis. (B) Cytolytic function of CNS-infiltrating T cells from TMEV-infected mice (8 days) was assessed to determine the MHC class I (Ks or Ds) restriction for the three new epitopes by using EL-4Ks and EL-4Ds target cells. The spontaneous 51Cr release was less than 10%.
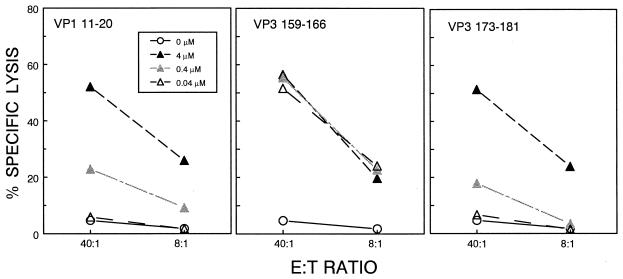
Differential requirements of peptide concentration for maximal CTL function toward different epitopes.
The frequency (based on ELISPOT) of VP3159-166-specific CTL in the CNS of TMEV-infected mice is usually observed to be higher than the frequency of CTL specific for VP111-20 or VP3173-181 (Fig. 1 and 7). This suggests that VP3159-166 may represent the immunodominant CTL epitope among the three. To further determine the hierarchy of epitopes for CTL responses in the CNS, the relative peptide concentration required to yield maximal cytolysis by CNS-IL was measured (Fig. 6). At a high peptide concentration (4 μM), all the epitopes induced relatively high levels of peptide-specific lysis as measured at 40:1 and 8:1 effector:target ratios. However, at lower peptide concentrations (0.4 and 0.04 μM), cytolysis induced by the VP111-20 and VP3173-181 peptides was reduced in a dose-dependent manner. In contrast, the VP3159-166 epitope sensitized target cells for CTL lysis equally well at all peptide concentrations, which is consistent with the dominance of this epitope over the other two TMEV epitopes.
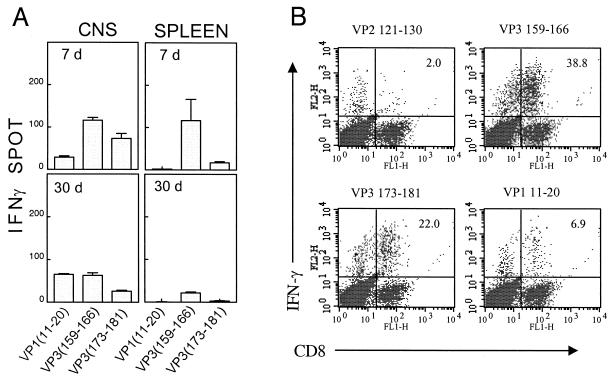
FIG. 7.
The levels of IFN-γ-producing CD8+ T cells in the CNS of TMEV-infected SJL mice. (A) IFN-γ-producing cell numbers in response to the predominant and subdominant CTL epitopes in the CNS and periphery are shown. An identical viral stock was used to infect SJL/J mice at two different time points (30 and 7 days before assay), and then IFN-γ ELISPOT production was analyzed for both groups at the same time to minimize experimental variation. The mononuclear cell numbers recovered from the CNS at 7 and 30 days postinfection were 1.1 × 106 and 0.5 × 106, respectively. Background was 1 ± 1 spot for both CNS and spleen at both time points. Data are representative of three separate experiments. (B) Enumeration of epitope-responsive CD8+ T cells in the CNS of virus-infected mice by flow cytometric analysis following intracellular staining for IFN-γ. Infiltrating mononuclear cells from TMEV-infected SJL/J mice (8 days) were incubated with individual epitope peptides or a control peptide for 6 h in the presence of Brefeldin A and then were analyzed after being stained with FITC-labeled anti-CD8 and PE-conjugated anti-IFN-γ antibodies. A representative experiment from four separate experiments is shown here. Data from the other three experiments are as follows: VP3159-166 (31, 37, and 23%), VP3173-181 (30, 16, and 15%), VP111-20 (13, 7, and 6%).
FIG. 6.
Differential requirements of peptide concentration for maximal CTL function against the three CTL epitopes. Three different concentrations (0.04, 0.4, and 4 μM) of the minimal epitope peptides were used to sensitize target cells (EL-4Ks) for lysis by CNS-infiltrating CTL. A representative result of three separate experiments is shown here. The spontaneous release was less than 14%.
Predominance of viral epitope-specific CD8+ T-cell population among the CNS-infiltrating CD8+ T cells from TMEV-infected mice.
As others have suggested that CTL may be partly responsible for immune-mediated pathogenesis leading to neurologic deficits, we were interested to know if virus-specific CTL were present in the CNS around the time of disease onset. IFN-γ-producing cells specific for the three epitopes defined here were enumerated by ELISPOT at an early time point (7 days), representing the peak of CNS-infiltrating CD8+ T-cell response, and at a late time point (30 days), corresponding to the time just prior to disease onset in SJL/J mice. The virus levels at 7 to 10 days postinfection in the CNS corresponds to the time of the peak CD8+ T-cell responses (39). The results, shown in Fig. 7A, indicate that the relative level of CNS-infiltrating CTL specific for the three epitopes is as follows: VP3159-166 > VP3173-181 > VP111-20. This predominant VP3159-166-specific CD8+ T-cell response is even more pronounced in the periphery (spleen). At 30 days after viral infection, TMEV-specific CTL are still present in the CNS, but the overall numbers are reduced to 30 to 65% of the peak response (Table 2). The dominance hierarchy of CD8+ T-cell response to these epitopes is generally maintained throughout the course of viral infection, although some fluctuation, particularly in the level of T cells specific for VP111-20, is observed at 30 days postinfection (Fig. 7 and Table 2). The assessment of cytolytic function of infiltrating CD8+ T cells at these same time points (data not shown) indicates that CNS-infiltrating CD8+ T cells specific for all three viral epitopes are functional throughout the course of infection with respect to both cytokine production and lytic activity.
TABLE 2.
Dominance hierarchy of IFN-γ-producing, epitope-specific CD8+ T cells infiltrating the CNS during viral infection
| Assay and epitope | No. of IFN-γ-producing cells per 104 cells (per CNS) postinfection at:
|
|||
|---|---|---|---|---|
| 6-8 days | 29-35 days | |||
| ELISPOTa | ||||
| VP111-20 | 13 ± 3 | (1,838) | 49 ± 30 | (2,401) |
| VP3159-166 | 74 ± 27 | (10,805) | 79 ± 33 | (3,871) |
| VP3173-181 | 40 ± 8 | (5,807) | 27 ± 10 | (1,299) |
| Flow cytometryb | ||||
| VP111-20 | 105 | (15,840) | 47 | (4,651) |
| VP3159-166 | 409 | (61,380) | 275 | (27,499) |
| VP3173-181 | 239 | (35,838) | 139 | (13,872) |
Three separate ELISPOT experiments were combined in this table. The averages of CNS-infiltrating mononuclear cells were 1.47 × 106 ± 0.6 × 106 and 0.49 × 106 ± 0.01 × 106 cells at 6 to 8 and 29 to 35 days, respectively. The results at 4 to 5 weeks after viral infection were not consistent in different experiments.
Enumeration based on intracellular IFN-γ staining was approximately 5- to 10-fold greater than that assessed by ELISPOT assays. The total cell numbers recovered from a CNS in this experiment were 1.5 × 106 and 1 × 106 at 8 and 30 days postinfection, respectively.
To determine what proportion of CD8+ T cells within the CNS-infiltrating population are TMEV specific, epitope-specific T cells were enumerated by intracellular IFN-γ staining (Fig. 7B). The results indicate that approximately 37% of infiltrating CD8+ T cells are reactive to the VP3159-166 peptide, 20% to VP3173-181, and 5% to VP111-20 (after subtraction of 2% background seen with a control peptide), accounting for 62% of the total CD8+ population in the CNS. Results from four separate experiments showed this same trend (45 to 75% of CNS CD8+ cells specific for these epitopes) and are consistent with the dominance hierarchy (VP3159-166 > VP3173-181 > VP111-20) seen in the ELISPOT and CTL assays (Fig. 6 and 7). In addition, stimulation with different combinations of these peptides led to respective increases in the number of IFN-γ-producing CD8+ T cells over that seen with any single peptide alone (data not shown). This additive effect suggests that all three peptides represent real CTL epitopes rather than cross-reactive mimotopes (22), and CTL populations specific for each epitope thus represent distinct effector cell populations. The assessment with intracellular IFN-γ staining appears to be approximately 5- to 10-fold more sensitive compared to the values obtained by ELISPOT assays in detecting epitope responding CD8+ T cells (Table 2). Again, the hierarchy of epitope dominance appears to be maintained even at a later time point (30 days postinfection) on the basis of the intracellular IFN-γ staining, although the overall level is reduced compared to that seen at 7 to 8 days postinfection. Thus, in contrast to a previous report (24), these data clearly indicate that the majority of the infiltrating CD8+ T cells in the CNS of TMEV-infected susceptible SJL/J mice are virus specific.
DISCUSSION
CD8+ T cells play an important role in clearance of viruses and other intracellular pathogens from infected hosts. Thus, protection from disease caused by these pathogens may rely heavily on this important effector cell population. However, it is also possible that, in some circumstances, CD8+ CTL may mediate unwanted immunopathology in an infected host due to destruction of infected cells in an effort to clear the infectious agent. Two examples of such CTL-mediated immunopathology include lymphocytic choriomeningitis virus infection in mice (4, 12) and some forms of viral hepatitis in humans (32, 34). TMEV-IDD is a virus-induced, immune-mediated disease, but the exact role of CD8+ T cells in pathogenesis is not yet known. While it is evident that class I-restricted responses contribute to viral clearance in both susceptible and resistant strains (5, 13, 38, 43), what remains unclear is whether or not susceptible strains are able to mount anti-viral CTL responses similar to that seen in resistant mice. In the absence of a vigorous antiviral response, chronic infection may lead to the persistent activation and recruitment of pathogenic CD4+ T cells that are specific for virus or for CNS autoantigens (33). On the other hand, virus-specific CTL that persist in the presence of chronic infection in susceptible mice may contribute to immunopathology by destroying virus-infected cells or through the production of proinflammatory cytokines. In order to address these issues, it is critically important to define the specificity and function of these effector cells. In the present study, we have defined the epitope specificity and function of the CTL response to TMEV in the SJL/J mouse strain, the prototypical TMEV-IDD-susceptible strain.
We report here that SJL/J mice mount an H-2Ks-restricted response to one dominant (VP3159-166) and two subdominant (VP111-20 and VP3173-181) capsid protein epitopes. To our knowledge these are the first CTL epitopes defined for the H-2s haplotype. Epitope-specific CD8+ T cells infiltrating the CNS of virus-infected SJL/J mice demonstrate both cytolytic function and IFN-γ production. In fact, the majority of CNS-infiltrating CD8+ T cells of TMEV-infected mice are virus specific. This is similar to what is seen in resistant C57BL/6 mice, where tetramer staining has shown that 50 to 60% of the CD8+ T cells infiltrating the CNS of C57BL/6 mice are specific for a dominant viral epitope (18). Studies by another group of investigators have suggested that SJL/J mice and other H-2s strains cannot mount virus-specific CTL responses to TMEV (24, 25) and that this is due to inhibition of H-2Ks-restricted responses by a viral protein called L* (8), which is translated in an alternate reading frame from the rest of the viral proteins. It is noteworthy that those studies were done with the DA strain of TMEV, whereas the BeAn strain was used here. Therefore, it is conceivable that the level and/or function of L* protein in BeAn-infected cells is different from that of DA-infected cells. However, the open reading frames for the BeAn genome predict the translation of a functional L* protein, although potential differences in L* protein translation or function between DA and BeAn cannot be excluded. In addition, our preliminary data strongly suggest CNS-IL from DA-infected SJL/J mice do, in fact, exhibit Ks-restricted CTL activity against at least one of the epitopes defined here. Thus, the inability to detect CTL activity in these previous studies may be due to the use of target cell lines, such as KSSV (24), which show poor Ks expression and are not susceptible to class I-restricted CTL-mediated lysis in our experiments (Fig. 4).
Our results indicate that the association of disease resistance with the H-2D genetic locus is not due to the fact that only H-2D molecules can present viral epitopes to CTL, since a comparable CTL response restricted by H-2K is found in susceptible SJL/J mice. However, it is interesting to speculate that while H-2K molecules can present viral peptides to CTL and stimulate the initial response, H-2K expression levels in the target organ (i.e., on resident CNS cells) may be too low to efficiently present TMEV epitopes to infiltrating CTL. There was a previous attempt to assess the expression levels of H-2K and H-2D molecules in the CNS of TMEV-infected mice (2). This study, based on staining intensity, has suggested the preferential up-regulation of H-2Dq expression in susceptible B10.Q and B10.RBQ mice. Thus, such a differential expression of H-2Ds versus H-2Ks molecules in the CNS of SJL/J mice is conceivable. A possible effect of this could be inefficient viral clearance resulting in viral persistence and disease development. Thus, strains such as SJL/J that generate a predominantly H-2K-restricted CTL response may be unable to control TMEV replication in the CNS, which could lead to persistent CNS inflammation and development of demyelinating disease.
The CTL response in the CNS of TMEV-infected susceptible SJL/J mice seems to be qualitatively similar to that in resistant C57BL/6 mice with respect to IFN-γ production and cytolytic function. In addition, the overall percentage of infiltrating CD8+ T cells that are TMEV specific in SJL/J mice is similar to that reported for C57BL/6 mice (18). Therefore, the difference in susceptibility to TMEV-IDD cannot be attributed to a lack of virus-specific CTL or CTL effector function in the SJL/J strain. However, it is conceivable that the overall number of virus-specific CNS-infiltrating CD8+ T cells may be higher in resistant C57BL/6 mice compared to that in SJL/J mice. It should also be noted here that the potential CTL response to noncapsid proteins has not yet been analyzed, and CTL specific for unknown epitopes may exist. Nevertheless, the high level (>60% of CNS CD8+ T cells) of response to the three epitopes defined here suggests that they represent the predominant SJL/J CTL epitopes. It is interesting to note that significant numbers of TMEV-specific CTL are maintained in the CNS even at 30 days postinfection, which corresponds to the time of disease onset. While the overall number of virus-specific T cells has decreased by this time, the relative proportion of infiltrating CD8+ cells specific for each epitope remains roughly the same (Table 2). This sustained CTL response within the CNS likely reflects ongoing responses to persisting virus in these mice. In addition to the CTL response, other facets of the immune response may play as-yet undetermined roles in the outcome of TMEV infection. For example, SJL/J mice are known to be deficient in the generation of functional NK cells compared to other mouse strains. Clearly, the lack of an early antiviral NK cell response could contribute to increased viral replication and, ultimately, viral persistence. Also, the CD4+ T-cell responses to viral infection may differ between SJL/J and C57BL/6 mice. For example, high levels of infiltrating T cells may represent myelin autoantigen-reactive CD4+ T cells found in a high precursor frequency in both naïve and TMEV-infected SJL/J mice (3, 33). These CD4+ T cells may contribute in some way to the immunopathology associated with TMEV-IDD. As TMEV-specific CTL are present in the CNS around the time of disease onset (Fig. 7 and Table 2), it is possible that they could also contribute to this same immunopathology. However, their role in protection and/or pathogenesis remains unknown. The fine characterization of the CTL response presented here will provide an important foundation for studies that will help in gaining a better understanding of the exact role of virus-specific CD8+ T cells in TMEV-IDD.
Acknowledgments
Bong Su Kang and Michael Lyman made equal contributions to this study.
This work was supported by grants NS23349, NS28752, and NS33008 from the U.S. Public Health Service and grant RG2956-A-3 from the National Multiple Sclerosis Society.
REFERENCES
- 1.Allen, I., and B. Brankin. 1993. Pathogenesis of multiple sclerosis—the immune diathesis and the role of viruses. J. Neuropathol. Exp. Neurol. 52:95-105. [DOI] [PubMed] [Google Scholar]
- 2.Altintas, A., Z. Cai, L. R. Pease, and M. Rodriguez. 1993. Differential expression of H-2K and H-2D in the central nervous system of mice infected with Theiler's virus. J. Immunol. 151:2803-2812. [PubMed] [Google Scholar]
- 3.Anderson, A. C., L. B. Nicholson, K. L. Legge, V. Turchin, H. Zaghouani, and V. K. Kuchroo. 2000. High frequency of autoreactive myelin proteolipid protein-specific T cells in the periphery of naive mice: mechanisms of selection of the self-reactive repertoire. J. Exp. Med. 191:761-770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baenziger, J., H. Hengartner, R. Zinkernagel, and G. Cole. 1986. Induction or prevention of immunopathological disease by cloned cytotoxic T cell lines specific for lymphocytic choriomeningitis virus. Eur. J. Immunol. 16:387-393. [DOI] [PubMed] [Google Scholar]
- 5.Begolka, W. S., L. M. Haynes, J. K. Olson, J. Padilla, K. L. Neville, M. D. Canto, J. Palma, B. S. Kim, and S. D. Miller. 2001. CD8-deficient SJL mice display enhanced susceptibility to Theiler's virus infection and increased demyelinating pathology. J. Neurovirol. 7:409-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borson, N. D., C. Paul, X. Lin, W. K. Nevala, M. A. Strausbauch, M. Rodriguez, and P. J. Wettstein. 1997. Brain-infiltrating cytolytic T lymphocytes specific for Theiler's virus recognize H2Db molecules complexed with a viral VP2 peptide lacking a consensus anchor residue. J. Virol. 71:5244-5250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bureau, J. F., X. Montagutelli, F. Bihl, S. Lefebvre, J. L. Guenet, and M. Brahic. 1993. Mapping loci influencing the persistence of Theiler's virus in the murine central nervous system. Nat. Genet. 5:87-91. [DOI] [PubMed] [Google Scholar]
- 8.Chen, H. H., W. P. Kong, L. Zhang, P. L. Ward, and R. P. Roos. 1995. A picornaviral protein synthesized out of frame with the polyprotein plays a key role in a virus-induced immune-mediated demyelinating disease. Nat. Med. 1:927-931. [DOI] [PubMed] [Google Scholar]
- 9.Dethlefs, S., M. Brahic, and E. L. Larsson-Sciard. 1997. An early, abundant cytotoxic T-lymphocyte response against Theiler's virus is critical for preventing viral persistence. J. Virol. 71:8875-8878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dethlefs, S., N. Escriou, M. Brahic, S. van der Werf, and E. L. Larsson-Sciard. 1997. Theiler's virus and Mengo virus induce cross-reactive cytotoxic T lymphocytes restricted to the same immunodominant VP2 epitope in C57BL/6 mice. J. Virol. 71:5361-5365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dhib-Jalbut, S., and D. E. McFarlin. 1990. Immunology of multiple sclerosis. Ann. Allergy 64:433-444. [PubMed] [Google Scholar]
- 12.Doherty, P., J. Allan, F. Lynch, and R. Ceredig. 1990. Dissection of an inflammatory process induced by CD8+ T cells. Immunol. Today 11:55-59. [DOI] [PubMed] [Google Scholar]
- 13.Fiette, L., C. Aubert, M. Brahic, and C. P. Rossi. 1993. Theiler's virus infection of β2-microglobulin-deficient mice. J. Virol. 67:589-592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerety, S. J., W. J. Karpus, A. R. Cubbon, R. G. Goswami, M. K. Rundell, J. D. Peterson, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. V. Mapping of a dominant immunopathologic VP2 T cell epitope in susceptible SJL/J mice. J. Immunol. 152:908-918. [PubMed] [Google Scholar]
- 15.Gerety, S. J., M. K. Rundell, M. C. Dal Canto, and S. D. Miller. 1994. Class II-restricted T cell responses in Theiler's murine encephalomyelitis virus-induced demyelinating disease. VI. Potentiation of demyelination with and characterization of an immunopathologic CD4+ T cell line specific for an immunodominant VP2 epitope. J. Immunol. 152:919-929. [PubMed] [Google Scholar]
- 16.Golstein, P., C. Goridis, A. M. Schmitt-Verhulst, B. Hayot, A. Pierress, A. Van Agthoven, Y. Kaufmann, Z. Eshhar, and M. Pierres. 1982. Lymphoid cell surface interaction structures detected using cytolysis-inhibiting monoclonal antibodies. Immunol. Rev. 68:5-42. [DOI] [PubMed] [Google Scholar]
- 17.Inoue, A., Y. K. Choe, and B. S. Kim. 1994. Analysis of antibody responses to predominant linear epitopes of Theiler's murine encephalomyelitis virus. J. Virol. 68:3324-3333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Johnson, A. J., M. K. Njenga, M. J. Hansen, S. T. Kuhns, L. Chen, M. Rodriguez, and L. R. Pease. 1999. Prevalent class I-restricted T-cell response to the Theiler's virus epitope Db:VP2121-130 in the absence of endogenous CD4 help, tumor necrosis factor alpha, gamma interferon, perforin, or costimulation through CD28. J. Virol. 73:3702-3708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim, B. S., Y. K. Choe, M. A. Crane, and C. R. Jue. 1992. Identification and localization of a limited number of predominant conformation-independent antibody epitopes of Theiler's murine encephalomyelitis virus. Immunol. Lett. 31:199-205. [DOI] [PubMed] [Google Scholar]
- 20.Kim, B. S., M. A. Lyman, B. S. Kang, H. K. Kang, H. G. Lee, M. Mohindru, and J. P. Palma. 2001. Pathogenesis of virus-induced immune-mediated demyelination. Immunol. Res. 24:121-130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim, B. S., J. P. Palma, A. Inoue, and C. S. Koh. 2000. Pathogenic immunity in Theiler's virus-induced demyelinating disease: a viral model for multiple sclerosis. Arch. Immunol. Ther. Exp. 48:373-379. [PubMed] [Google Scholar]
- 22.Lenstra, J. A., J. H. Erkens, J. G. Langeveld, W. P. Posthumus, R. H. Meloen, F. Gebauer, I. Correa, L. Enjuanes, and K. K. Stanley. 1992. Isolation of sequences from a random-sequence expression library that mimic viral epitopes. J. Immunol. Methods 152:149-157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lin, T. Z., H. Fernandes, R. Yauch, N. M. Ponzio, and E. Raveche. 1992. IL-10 production in a CD5+ B cell lymphoma arising in a CD4 monoclonal antibody-treated SJL mouse. Clin. Immunol. Immunopathol. 65:10-22. [DOI] [PubMed] [Google Scholar]
- 24.Lin, X., L. R. Pease, P. D. Murray, and M. Rodriguez. 1998. Theiler's virus infection of genetically susceptible mice induces central nervous system-infiltrating CTLs with no apparent viral or major myelin antigenic specificity. J. Immunol. 160:5661-5668. [PubMed] [Google Scholar]
- 25.Lin, X., R. P. Roos, L. R. Pease, P. Wettstein, and M. Rodriguez. 1999. A Theiler's virus alternatively initiated protein inhibits the generation of H-2K-restricted virus-specific cytotoxicity. J. Immunol. 162:17-24. [PubMed] [Google Scholar]
- 26.Lindsley, M. D., R. Thiemann, and M. Rodriguez. 1991. Cytotoxic T cells isolated from the central nervous systems of mice infected with Theiler's virus. J. Virol. 65:6612-6620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lipton, H. L. 1975. Theiler's virus infection in mice: an unusual biphasic disease process leading to demyelination. Infect. Immun. 11:1147-1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipton, H. L., and M. C. Dal Canto. 1976. Chronic neurologic disease in Theiler's virus infection of SJL/J mice. J. Neurol. Sci. 30:201-207. [DOI] [PubMed] [Google Scholar]
- 29.Lipton, H. L., J. Kratochvil, P. Sethi, and M. C. Dal Canto. 1984. Theiler's virus antigen detected in mouse spinal cord 2 1/2 years after infection. Neurology 34:1117-1119. [DOI] [PubMed] [Google Scholar]
- 30.Lipton, H. L., and R. Melvold. 1984. Genetic analysis of susceptibility to Theiler's virus-induced demyelinating disease in mice. J. Immunol. 132:1821-1825. [PubMed] [Google Scholar]
- 31.Lyman, M. A., H. G. Lee, B. S. Kang, H. K. Kang, and B. S. Kim. 2002. Capsid-specific cytotoxic T lymphocytes recognize three distinct H-2Db-restricted regions of the BeAn strain of Theiler's virus and exhibit different cytokine profiles. J. Virol. 76:3125-3134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maini, M., C. Boni, C. Lee, J. Larrubia, S. Reignat, G. Ogg, A. King, J. Herberg, R. Gilson, A. Alisa, R. Williams, D. Vergani, N. Naoumov, C. Ferrari, and A. Bertoletti. 2000. The role of virus-specific CD8+ cells in liver damage and viral control during persistent hepatitis B virus infection. J. Exp. Med. 191:1269-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, S. D., C. L. Vanderlugt, W. S. Begolka, W. Pao, R. L. Yauch, K. L. Neville, Y. Katz-Levy, A. Carrizosa, and B. S. Kim. 1997. Persistent infection with Theiler's virus leads to CNS autoimmunity via epitope spreading. Nat. Med. 3:1133-1136. [DOI] [PubMed] [Google Scholar]
- 34.Moriyama, T., S. Guilhot, K. Klopchin, B. Moss, C. Pinkert, R. Palmiter, R. Brinster, O. Kanagawa, and F. Chisari. 1990. Immunobiology and pathogenesis of hepatocellular injury in hepatitis B virus transgenic mice. Science 248:361-364. [DOI] [PubMed] [Google Scholar]
- 35.Murali-Krishna, K., J. D. Altman, M. Suresh, D. J. Sourdive, A. J. Zajac, J. D. Miller, J. Slansky, and R. Ahmed. 1998. Counting antigen-specific CD8 T cells: a reevaluation of bystander activation during viral infection. Immunity 8:177-187. [DOI] [PubMed] [Google Scholar]
- 36.Murray, P. D., D. B. McGavern, X. Lin, M. K. Njenga, J. Leibowitz, L. R. Pease, and M. Rodriguez. 1998. Perforin-dependent neurologic injury in a viral model of multiple sclerosis. J. Neurosci. 18:7306-7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Palma, J. P., H.-G. Lee, B.-S. Kang, M. Dal Canto, S. D. Miller, and B. S. Kim. 2001. Enhanced susceptibility to Theiler's virus-induced demyelinating disease in perforin-deficient mice. J. Neuroimmunol. 116:125-135. [DOI] [PubMed] [Google Scholar]
- 38.Pullen, L. C., S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1993. Class I-deficient resistant mice intracerebrally inoculated with Theiler's virus show an increased T cell response to viral antigens and susceptibility to demyelination. Eur. J. Immunol. 23:2287-2293. [DOI] [PubMed] [Google Scholar]
- 39.Pullen, L. C., S. H. Park, S. D. Miller, M. C. Dal Canto, and B. S. Kim. 1995. Treatment with bacterial LPS renders genetically resistant C57BL/6 mice susceptible to Theiler's virus-induced demyelinating disease. J. Immunol. 155:4497-4503. [PubMed] [Google Scholar]
- 40.Rammensee, H. G., T. Friede, and S. Stevanoviic. 1995. MHC ligands and peptide motifs: first listing. Immunogenetics 41:178-228. [DOI] [PubMed] [Google Scholar]
- 41.Rivera-Quinones, C., D. McGavern, J. D. Schmelzer, S. F. Hunter, P. A. Low, and M. Rodriguez. 1998. Absence of neurological deficits following extensive demyelination in a class I-deficient murine model of multiple sclerosis. Nat. Med. 4:187-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rodriguez, M., and C. S. David. 1985. Demyelination induced by Theiler's virus: influence of the H-2 haplotype. J. Immunol. 135:2145-2148. [PubMed] [Google Scholar]
- 43.Rodriguez, M., A. J. Dunkel, R. L. Thiemann, J. Leibowitz, M. Zijlstra, and R. Jaenisch. 1993. Abrogation of resistance to Theiler's virus-induced demyelination in H-2b mice deficient in β2-microglobulin. J. Immunol. 151:266-276. [PubMed] [Google Scholar]
- 44.Rodriguez, M., J. Leibowitz, and P. Lampert. 1983. Persistent infection of oligodendrocytes in Theiler's virus induced encephalomyelitis. Ann. Neurol. 13:426-433. [DOI] [PubMed] [Google Scholar]
- 45.Rossi, C. P., A. McAllister, M. Tanguy, D. Kagi, and M. Brahic. 1998. Theiler's virus infection of perforin-deficient mice. J. Virol. 72:4515-4519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Soldan, S. S., R. Berti, N. Salem, P. Secchiero, L. Flamand, P. A. Calabresi, M. B. Brennan, H. W. Maloni, H. F. McFarland, H. C. Lin, M. Patnaik, and S. Jacobson. 1997. Association of human herpes virus 6 (HHV-6) with multiple sclerosis: increased IgM response to HHV-6 early antigen and detection of serum HHV-6 DNA. Nat. Med. 3:1394-1397. [DOI] [PubMed] [Google Scholar]
- 47.Targoni, O. S., and P. V. Lehmann. 1998. Endogenous myelin basic protein inactivates the high avidity T cell repertoire. J. Exp. Med. 187:2055-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Theiler, M. 1937. Spontaneous encephalomyelitis of mice: a new virus disease. J. Exp. Med. 65:705-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yauch, R. L., K. Kerekes, K. Saujani, and B. S. Kim. 1995. Identification of a major T-cell epitope within VP3 amino acid residues 24 to 37 of Theiler's virus in demyelination-susceptible SJL/J mice. J. Virol. 69:7315-7318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yauch, R. L., and B. S. Kim. 1994. A predominant viral epitope recognized by T cells from the periphery and demyelinating lesions of SJL/J mice infected with Theiler's virus is located within VP1233-244. J. Immunol. 153:4508-4519. [PubMed] [Google Scholar]
- 51.Yauch, R. Y., J. P. Palma, H. Yahikozawa, C.-S. Koh, and B. S. Kim. 1998. Role of individual T-cell epitopes of Theiler's virus in the pathogenesis of demyelination correlates with the ability to induce a Th1 response. J. Virol. 72:6169-6174. [DOI] [PMC free article] [PubMed] [Google Scholar]