Abstract
Oligodeoxynucleotides (ODN) containing unmethylated CpG motifs exert powerful adjuvant activity in vivo and in vitro. Administered with antigen they induce a population of antigen-specific CD8+ T cells. In this study we immunized C57BL/6 mice with bioactive CpG ODN combined with an immunodominant epitope derived from herpes simplex virus (HSV) glycoprotein B (amino acids 498 to 505; SSIEFARL) and analyzed the magnitude and durability of the peptide-specific response. The effectiveness of the CD8+ T-cell response as measured by peptide-specific tetramers, peptide-induced intracellular gamma interferon expression, and resistance to systemic and mucosal challenge during the acute and memory phases was compared with the response induced by immunization with recombinant vaccinia virus encoding SSIEFARL as a minigene (VvgB498-505). Confirming the reports of others, our results demonstrate that the CpG ODN-peptide approach generates an antigen-specific CD8+ T-cell population, but the frequency of CD8+ T cells is lower than that induced by VvgB498-505. Nevertheless, the protection level was comparable when mice were systemically and mucosally challenged during the acute phase. However, such responses by both groups waned with time and were functionally less effective. Still, our results indicate that the CpG ODN-peptide immunization system holds promise as a means of selectively inducing a CD8+ T-cell response against HSV.
Several prevalent viral pathogens still lack effective vaccines: human immunodeficiency virus and most human herpesviruses represent examples. In such infections, T-cell-mediated immunity, particularly that mediated by CD8+ cells, appears as the dominant means of defense (24, 29, 30). Given the background of disappointment with several conventional vaccines evaluated against herpes simplex virus (HSV), novel approaches are needed. The current focus is on DNA vaccines, since such vaccines may act as powerful inducers of T-cell immune mechanisms judged crucial for infection control (8). The immunogenicity of DNA vaccines appears due in part to their intrinsic adjuvant activity (8) caused by their content of unmethylated CpG in the context of particular flanking sequences found in the bacterial DNA that encodes the immunogen (8). Several groups have prepared synthetic CpG motifs containing oligodeoxynucleotides (ODN) and shown them to act as powerful stimulants of leukocyte function in vitro and in vivo (1, 9, 12, 13, 21, 22, 30). Most interestingly, such ODN-containing CpG motifs exert powerful adjuvant activity when used along with protein antigens, with responses sometimes exceeding those achieved by Freund's complete adjuvant (5, 17, 33). Furthermore, it appears that CpG ODN administration alone enhances innate immunity such that animals may resist several infections at least for a 2- to 3-week period (6, 16, 38). Moreover, one study has shown that preadministration of CpG ODN may result in cytotoxic T lymphocyte (CTL) responses to free peptide antigens given at the same site several weeks later (19). Although published studies are few, it would seem that immunization with CpG peptide complexes represents a valuable means of inducing immunity to individual epitopes recognized by CD4+ or CD8+ T cells.
With HSV infections, it remains unclear why some, but not all, latently infected persons suffer periodic clinically evident recurrences. However, from observations of the frequency and severity of HSV lesions in human immunodeficiency virus-positive patients, it appears that a robust CD8+ T-cell response best correlates with minimal lesions (26). Accordingly, boosting CD8+ T-cell responses represents a promising approach for the management of HSV infections. Since CpG-peptide complexes induce notable CD8+ responses in some systems (17, 25, 32, 37), this approach merits evaluation. In the mouse system, one immunodominant CD8+ peptide epitope is well recognized (36). This is the glycoprotein B (gB)498-505 peptide that is the target for around 90% of the CTL response generated by C57BL/6 (B6) mice following infection by HSV type 1 (HSV1) or HSV2 (4, 36). In addition, immunity to only the gB498-505 peptide is sufficient to protect mice against HSV challenge by either the systemic or mucosal routes. Previously, studies showed that CD8+-mediated immunity was induced against gB498-505 either by live virus infection, gB protein and adjuvant immunization, or by the use of a recombinant vaccinia virus vector that expressed the gB498-505 minigene (4, 7, 36). The latter approach induced CD8+ T-cell responses that were quantitatively and qualitatively comparable to those induced by virus infection (4). In the present report, we have immunized mice with bioactive CpG 1826 plus SSIEFARL and compared the magnitude and durability of the peptide epitope-specific response at systemic and mucosal sites with those of VvgB498-505 immunization. Our results show a good systemic CD8+ T-cell response to CpG-gB498-505 immunization and resistance to vaginal HSV challenge when measured 1 to 2 weeks after immunization. However, when mice were infected several weeks after immunization, animals challenged systemically and vaginally succumbed to infection after a delay of clinical signs. Our results are discussed in terms of additional procedures worth pursuing to improve the duration of immunity from CpG-peptide immunization.
MATERIALS AND METHODS
Mice.
Female C57BL/6 mice (6 weeks old) were purchased from Harlan Sprague Dawley (Indianapolis, Ind.). In conducting the research described in this work, the investigators adhered to the Guide for the Care and Use of Laboratory Animals, as proposed by The Committee on Care of Laboratory Animal Resources, Commission on Life Sciences, National Research Council. The facilities are fully accredited by the American Association for Accreditation of Laboratory Animal Care.
Reagents.
The CpG oligonucleotides bioactive CpG 1826 (5′-TCCATGACGTTCCTGACGTT-3′ [immunostimulatory motifs are in boldface and underlined]) and control CpG 1982 (5′-TCCAGGACTTCTCTCAGGTT-3′) were provided by Coley Pharmaceutical Group (Wellesley, Mass.) and had undetectable endotoxin levels. The peptide HSVgB (amino acids [aa] 498 to 505), peptide SSIEFARL, and chicken egg ovalbumin (aa 257 to 264) were synthesized and supplied by Genemed Synthesis, Inc. (South San Francisco, Calif.). The viruses HSV-1 strain KOS, HSV-1 strain 17, and HSV-1 strain McKrae were grown on Vero cell monolayers. The recombinant vaccinia virus encoding the minigene SSIEFARL (VvgB498-505) was grown on CV1 monolayers. This virus was provided to us by S. S. Tevethia (4). All viruses were titrated and stored in aliquots at −80°C until used. The following monoclonal antibodies (MAb) either conjugated with fluorescein isothiocyanate or phycoerythrin or biotinylated were purchased from BD Pharmingen (San Diego, Calif.) were used for fluorescence-activated cell sorter (FACS) staining: CD4, CD8a, CD11b, CD11c, Gr1, CD22, NK1.1, and gamma interferon (IFN-γ). For the third color development, streptavidin conjugated with peridinin chlorophyll protein was used. Major histocompatibility complex (MHC) class I (H2b) tetramers (to measure SSIEFARL-specific T cells) were provided to us by the National Institute of Allergy and Infectious Diseases Tetramer Facility (Rockville, Md.).
Cell cultures and culture media.
Vero (African green monkey kidney cell line), CV1 (African green monkey kidney cell line), MC38 (H2b adenocarcinoma), and EMT-6 (H2d mammary adenocarcinoma) cell lines were cultured in Dulbecco's modified Eagle's medium (MediaTech Cellgro) supplemented with 10% (vol/vol) heat-inactivated fetal bovine serum (FBS; GIBCO) and antibiotics (100 IU of penicillin/ml and 100 IU of streptomycin/ml). Freshly isolated cells from spleens and draining lymph nodes (DLN) were cultured in RPMI 1640 (Sigma) supplemented with 10% (vol/vol) heat-inactivated FBS, 1.5 mM l-glutamine, 50 μM 2-mercaptoethanol, and antibiotics (100 IU of penicillin/ml and 100 IU of streptomycin/ml).
Immunization.
In all experiments, C57BL/6 mice were immunized in the hind footpads (fp) with 100 μg of SSIEFARL combined with either 30 μg of bioactive CpG 1826 or control CpG 1982 or with 106 PFU of VvgB498-505. Cells were harvested for further experiments from DLN (popliteal and inguinal) and from spleens. For generation and establishment of the kinetics of immunodominant CTL responses in DLN and spleens, mice were immunized once in the hind fp and cells were isolated on days 3, 5, 7, 9, 12, and 14 postimmunization (p.i.). For priming of cytotoxic peptide-specific CD8+ T cells in the spleen and DLN, mice were immunized twice in the hind fp on days 0 and 7, and 7 days (acute phase) or 60 days (memory phase) after the second immunization the DLN and spleens were aseptically removed. Mice immunized with UV-inactivated HSV-1 strain KOS (UV-HSV-1) served as a positive control, while mice immunized with SSIEFARL peptide only were used as a negative control.
Preparation of cell suspensions. (i) Responders.
Spleens and popliteal and inguinal LN were pressed through a metal sieve and washed in RPMI 1640. Erythrocytes from the spleen were removed by lysis with NH4Cl-Tris buffer and washed twice in RPMI 1640. After the final washing, cells were counted and the viability was assessed by trypan blue exclusion.
(ii) Stimulators.
For in vitro restimulation of primed lymphocytes, spleen cells from naïve C57BL/6 mice were prepared and pulsed with SSIEFARL (5 μg/2 × 106cells) for 3 h at 37°C and then irradiated with 3,000 rads.
(iii) In vitro expansion for CTLs.
Cells isolated from the DLN and spleens were mixed with cells pulsed with peptide in a 2:1 ratio, 10 U of recombinant interleukin-2 (rIL-2)/ml was added, and they were cultured in 24-well tissue culture plates for 5 days at 37°C with 5% CO2. Later, a 51Cr release assay was performed.
(iv) Isolation of vaginal lymphocytes.
The vaginal tracts (VT) from infected mice were isolated and washed three times in Hanks balanced salt solution (HBSS) supplemented with 0.5 mM EDTA (final concentration) and once in RPMI 1640. VT were cut into small pieces and suspended in a solution of collagenase D (Sigma) in RPMI 1640 at a concentration of 1 mg/ml and incubated in an orbital shaker for 1 h at 37°C. After incubation, VT were meshed through the metal sieve and the cells were washed once in RPMI 1640 mixed with HBSS-EDTA in a 1:1 ratio. Finally, they were passed through a nylon-wool column.
ELISA assay.
For in vitro cytokine measurements, supernatant was collected at 24-h increments from incubated cells. Ninety-six-well EIA/RIA plates (Costar) were coated with 2 μg of purified anti-IFN-γ and IL-4 (Pharmingen) MAb per ml in 0.1 M NaH2PO4 and incubated overnight at 4°C. Then, capture antibody solutions were removed, and plates were blocked with 3% nonfat dry milk in phosphate-buffered saline (PBS) for 1 to 2 h in 37°C and washed with PBS supplemented with 10% FBS and 0.05% Tween 20 (PBS-FBS-Tween) (Sigma). After washing, culture supernatants and standards were serially diluted in PBS-FBS-Tween and incubated for 2 to 4 h at room temperature or overnight at 4°C. Plates were washed with PBS-FBS-Tween and overlaid with 1 μg of either biotinylated anti-IFN-γ or IL-4 MAb per ml for 1 h at room temperature and then treated with a 1:1,000 dilution of streptavidin-conjugated horseradish peroxidase for 30 min at room temperature. After the final wash the cytokines were detected by addition of ABTS substrate solution [11 mg of 2,2′-azino-bis(3-ethylbenzthiazoline-6-sulfonic acid) in 20 ml of 0.1 M citric acid, 30 ml of 0.1 M sodium phosphate, and 10 μl of hydroxyperoxide] (Sigma). The concentration of cytokine was calculated with an automated enzyme-linked immunosorbent assay (ELISA) reader (SpectraMAX 340; Molecular Devices, Sunnyvale, Calif.).
Flow cytometry.
A total of 106 cells from all groups was fixed with a 1% solution of paraformaldehyde and preincubated on ice for 5 min with anti-CD16, anti-CD32 MAb to block Fc receptors. Then, they were washed once with staining buffer (PBS containing 3% FBS and 0.1% sodium azide) and incubated with the indicated MAb conjugates for 45 min at 4°C in a total volume of 100 μl of staining buffer. After staining, cells were washed twice with staining buffer, and double- or three-color fluorescence analysis was performed using a FACScan flow cytometer and CellQuest software.
ICG.
To enumerate the number of IFN-γ-producing cells, intracellular cytokine staining was performed. Freshly expanded cells from DLN and spleens were cultured in U-bottom 96-well plates. Cells were left untreated or stimulated with 1 μg of SSIEFARL/106 cells in the presence of 1 μg of brefeldin A (to facilitate intracellular accumulation) and 50 U of rIL-2 for 5 h at 37°C with 5% CO2. Surface staining was then performed using anti-CD8 MAb followed by intracellular IFN-γ (ICG) staining, with a Cytofix/Cytoperm kit (BD Pharmingen, San Diego, Calif.) in accordance with the manufacturer's instructions. Double- or three-color fluorescence analysis was performed using a FACScan flow cytometer and CellQuest software.
Tetramer staining.
A total of 106 cells was suspended in FACS buffer and stained for surface markers using anti-CD8 MAb and tetramers. They were incubated for 45 min to 1 h at 4°C and washed, and double- or three-color fluorescence analysis was performed using a FACScan flow cytometer and CellQuest software.
51Cr release assay.
The 51Cr release assay was performed as follows. MC38 and EMT-6 target cells were pulsed with the appropriate peptide (SSIEFARL and SIINFEKL, respectively) at 5 μg/2 × 106 cells and incubated at 37°C for 1 h. In the last 30 min, cells were labeled with 100 μCi of 51Cr (ICN Biomedicals, Inc.). Peptide-untreated cells and cells pulsed with SIINFEKL served as specificity controls, and EMT-6 cells pulsed with SSIEFARL peptide served as an MHC-mismatched control. After pulsing the target with peptides and loading with 51Cr, the cells were washed and added to effector cells at an E/T ratio of 100:1. Plates were incubated for 4 h at 37°C with 5% CO2. 51Cr released from the lysed cells was measured in supernatant fluid, and the percent specific lysis was determined by the following equation: [(effector release − spontaneous release)/(total release − spontaneous release)] × 100%. The spontaneous (medium control) release from the target cells ranged from 9 to 15% of the total release.
Protection assay for HSV-1 infection. (i) Mucosal model.
C57BL/6 mice were immunized twice in the hind fp with VvgB498-505, SSIEFARL combined with bioactive CpG 1826 or control CpG 1982, UV-HSV-1, CpG alone, or SSIEFARL alone or were unvaccinated. After 7 days (acute phase) or 60 days (memory phase), mice were intravaginally challenged with 106 PFU of HSV-1 strain McKrae/mouse. Five days before challenge, to synchronize the estrous cycle, mice were injected subcutaneously (s.c.) with Depo-Provera (Upjohn Co., Kalamazoo, Mich.) at a concentration of 2 mg/mouse in 50 μl of distilled water (dH2O). Mice were examined daily for vaginal inflammation, neurological illness, and death. Clinical severity was graded as follows: 0, no apparent infection; 1, mild inflammation of external vagina; 2, redness and moderate swelling of external vagina; 3, severe redness and inflammation; 4, genital ulceration, severe inflammation, hair loss of genital and surrounding tissue, paresis; and 5, death.
(ii) Zosteriform model.
Zoster challenge experiments were performed as described by Manican et al. (20). Briefly, prior to challenge, the left flank area of the animal was shaved. The animals were anesthetized with avertin (2,2,2,-tribromoethanol, 2-methyl-2-buthanol; Sigma) and a total of 20 scarifications were made on an approximately 4-cm2 area. To such scarification, 10 μl containing 106 PFU of HSV-1 strain 17 were added and gently massaged. Animals were inspected daily for the development of zosteriform ipsilateral lesions, general behavior changes, encephalitis, and mortality. The severity of the lesions was scored as follows: 1, vesicle formation; 2, local erosion and ulceration of the local lesion; 3, mild to moderate ulceration with spreading; 4, severe ulceration, hind limb paralysis, and encephalitis; 5, ultimate death (mice that were moribund and hence euthanized).
Statistical analysis.
The data were analyzed with Student's t test.
RESULTS
Profile of in vitro immune response after vaccination.
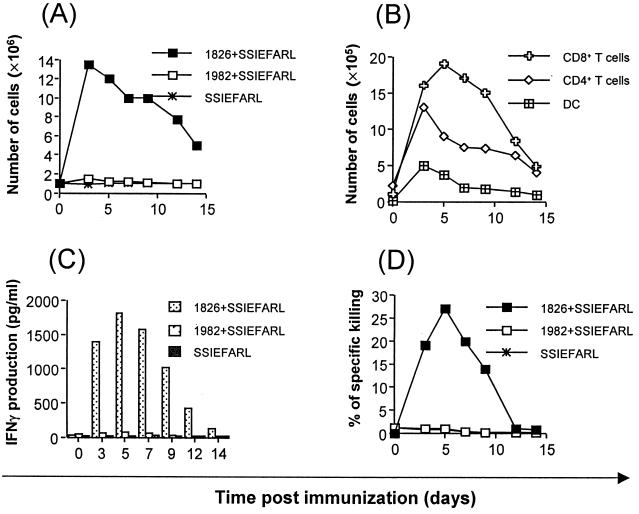
Groups of mice were immunized in the rear fp with a single dose of SSIEFARL peptide or with peptide plus 1826 ODN (bioactive) or plus 1982 ODN (nonbioactive). Sample mice were killed at intervals to quantify both cell numbers and types present in LN and spleens as well as for evidence of peptide-specific CD8+ T-cell responses. The results shown in Fig. 1A indicate that immunization with CpG 1826 plus SSIEFARL caused a rapid increase in LN (but not spleen) cellularity, with the peak effect evident at 3 days p.i. Increased cellularity persisted until at least 14 days (latest time tested). The increase in LN cellularity involved numerous cell types. Early on, dendritic cell and CD4+ T-cell counts were most elevated over controls (peptide recipients alone and control CpG 1982 plus SSIAFARL). The peak CD8+ T-cell increase was evident at 5 days p.i. (Fig. 1B). Other cell types accounting for the increase of cellularity of LN included macrophages, neutrophils, and B cells (data not shown).
FIG. 1.
Analysis of cellularity, cell types, IFN-γ production, and cytotoxic ability in relation to time. C57BL/6 female mice were immunized with a single dose of SSIEFARL or with peptide plus bioactive CpG 1826 or control CpG 1982. Cells from DLN and spleens were isolated at indicated times after immunization. Harvested cells were counted, stained for surface markers, and analyzed in a flow cytometer. The rest of the cells were restimulated as described in Materials and Methods and cultured in vitro for an additional 5 days, and then lytic activity against MC38 cells pulsed with SSIEFARL was scored in standard 51Cr release assays. The data show mean specific lysis at a 50:1 effector/target ratio (n = four mice/time point). Controls that were included and but are not shown consisted of MHC-mismatched irrelevant peptide targets. After 72 h of in vitro culture, supernatants from each time point were collected for detection of IFN-γ. Cytokine levels were determined by standard ELISA. The figure shows results from DLN. (A) The kinetics of cellularity in DLN after single-dose immunization. (B) The increase of specific cell types in DLN of mice immunized. (C) In vitro production of IFN-γ by CD8+ T cells isolated from DLN of mice immunized. (D) The kinetics of SSIEFARL-specific CTL precursors after immunization.
Nonquantitative assays were used to measure the presence of peptide-specific CD8+ T cells in both DLN and spleens. Such responses were only detectable in mice immunized with CpG 1826 plus SSIEFARL, and then only in DLN. Moreover, the strongest responses as measured by the 51Cr release assay and by peptide-induced IFN-γ production from a known number of cells were evident at the time of peak DLN CD8+ cellularity (Fig. 1C and D). No IL-4 production was observed, indicating Th1 immune response polarization. By day 12 these responses were markedly reduced, perhaps indicating that single-dose immunization with CpG plus SSIEFARL induced only marginal CD8+ T-cell responses that were minimally persistent.
In a second series of experiments, animals were immunized twice with peptide or peptide with bioactive or control CpG ODN (day 0 and day 7). In addition, extra groups of animals were immunized with recombinant vaccinia virus that expressed SSIEFARL as a minigene (VvgB498-505) and with live HSV-1. Furthermore, quantitative assays of peptide-specific CD8+ T-cell responses were used to measure DLN and splenic responses both in the acute phase (7 days post-second immunization) and memory phase (60 days post-second immunization). The results are shown in Fig. 2 and Table 1. The following major points are to be noted.
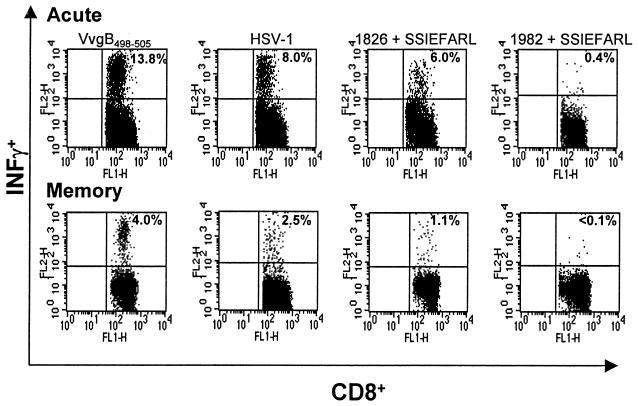
FIG. 2.
Functional analysis of cells isolated from mice during acute and memory phases. C57BL/6 female mice were immunized twice with VvgB498-505 or SSIEFARL plus CpG 1826 or CpG 1982. Mice immunized with live HSV-1 strain KOS served as a positive control, and animals immunized with only SSIEFARL were the negative control (data not shown). Seven days (acute phase) or 60 days (memory phase) postimmunization, cells from DLN and spleens were isolated and stimulated with 1 μg of SSIEFARL/106 cells in the presence of brefeldin A and 50 U of IL-2/100 μl for 5 h. After that time, the production of IFN-γ was determined by intracellular staining. The figure shows the results of analysis of splenocytes gated on CD8+ T cells. The results are representative of at least three independent experiments.
TABLE 1.
Frequency of CD8+/IFNγ+ and CD8+/Tet+ cells in DLN and spleens during the acute and memory phases postimmunization
| Immunogen | Phase | % Cells with marker in:
|
|||
|---|---|---|---|---|---|
| DLN
|
Spleen
|
||||
| CD8+ IFN-γ+ | CD8+ Tet+ | CD8+ IFN-γ+ | CD8+ Tet+ | ||
| CpG 1826 + SSIEFARL | Acute | 4.0 | 3.9 | 6.0 | 5.8 |
| Memory | 0.7 | 0.7 | 1.1 | 1.0 | |
| CpG 1982 + SSIEFARL | Acute | <0.1 | <0.1 | 0.4 | <0.1 |
| Memory | <0.1 | <0.1 | <0.1 | <0.1 | |
| VvgB498-505 | Acute | 1.1 | 1.2 | 13.8 | 13.5 |
| Memory | 0.6 | 0.7 | 4.0 | 4.1 | |
| HSV-1 | Acute | 1.3 | 1.2 | 8.0 | 7.8 |
| Memory | 0.5 | 0.6 | 2.5 | 2.6 | |
| SSIEFARL | Acute | <0.1 | <0.1 | <0.1 | <0.1 |
| Memory | <0.1 | <0.1 | <0.1 | <0.1 | |
Firstly, positive CD8+ T-cell responses (measured by tetramers, peptide-induced intracellular IFN-γ production) were evident only in mice immunized with CpG 1826 plus SSIEFARL, VvgB498-505, and HSV. Secondly, responses to both CpG 1826 plus SSIEFARL and VvgB498-505 were present both in DLN and spleen, with the former having a quantitatively higher response (Table 1). Thirdly, the responses to VvgB498-505 were twofold higher than those to CpG 1826 plus SSIEFARL in the acute phase. Moreover, responses measurable by either tetramers or ICG were similar, indicating that both forms of vaccination yielded functionally equivalent peptide-specific CD8+ responses. Fourthly, markedly different quantitative responses to VvgB498-505 and CpG 1826 plus SSIEFARL were evident in the memory phase. In both groups, responses were significantly reduced compared to those in the acute phase. The response to CpG 1826 plus SSIEFARL decreased 5-fold, whereas in the VvgB498-505-immunized group the decrease was 3.3-fold in the spleen and 2-fold in DLN. Responses measurable both by tetramers and ICG to CpG 1826 plus SSIEFARL and VvgB498-505 at the memory phase were approximately equal, indicating no functional difference in the memory population resulting from CpG 1826-plus-SSIEFARL or VvgB498-505 immunization (Table 1).
Resistance to challenge by immunized mice.
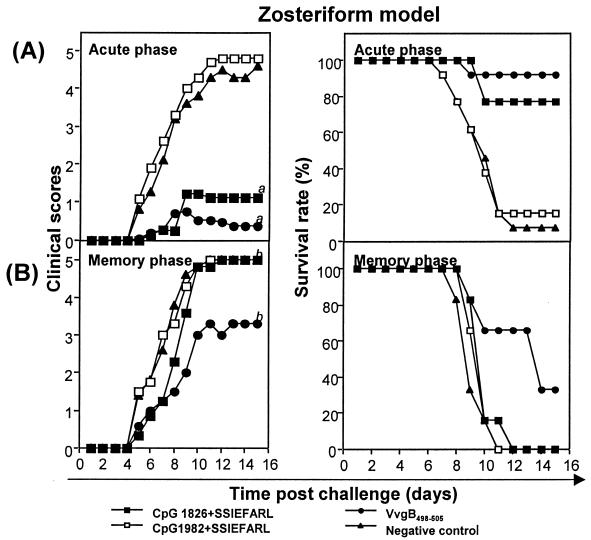
Mice were immunized via the fp on days 0 and 7 with the vaccine preparations described previously. In addition, groups of mice were vaccinated with UV-HSV-1 or ODN 1826 alone without peptide or were unvaccinated. Animals were challenged both in the acute phase (7 days post-second immunization) or memory phase (60 days post-second immunization) by either the systemic or mucosal routes with five and three times the 50% lethal dose, respectively. Following systemic challenge, high levels of resistance were evident in animals immunized with CpG 1826 plus SSIEFARL, VvgB498-505, or UV-HSV-1. None of the animals developed clinically apparent lesions (scores ranged from 0 to <1), and the survival rates were 77% in mice immunized with bioactive CpG 1826 plus SSIEFARL, 90% in animals immunized with VvgB498-505, and 100% in the UV-HSV-1-immunized group (data not shown). Even mice that died of encephalitis did not show any skin lesions, in contrast to control animals. Animals immunized with free peptide, control CpG 1982 plus SSIEFARL, or even CpG 1826 alone without peptide failed to resist challenge (data not shown). All developed skin lesions, and levels of mortality were similar to that in unvaccinated mice (Fig. 3).
FIG. 3.
The induction of protective immunity against zoster challenge with HSV following systemic immunization. C57BL/6 female mice were immunized twice (days 0 and 7) in the hind fp with either VvgB498-505, SSIEFARL combined with bioactive CpG 1826 or control CpG 1982, UV-HSV-1, CpG 1826 alone, or SSIEFARL alone or unimmunized (negative control). Seven days (acute phase) or 60 days (memory phase) after boosting, zoster challenge experiments were performed. Animals were inspected daily for the development of zosteriform ipsilateral lesions, general behavior changes, encephalitis, and mortality. The severity of the lesions was scored as follows: 1, vesicle formation; 2, local erosion and ulceration of the local lesion; 3, mild to moderate ulceration with spreading; 4, severe ulceration, hind limb paralysis, and encephalitis; 5, ultimate death (mice that were moribund and hence euthanized). The figure shows the results for mice immunized with VvgB498-505, CpG 1826 plus SSIEFARL, or CpG 1982 plus SSIEFARL and nonimmunized mice. (A) Clinical scores and survival rate of C57BL/6 mice systemically challenged during the acute phase. a = significantly different from values obtained for mice immunized with control CpG 1982 plus SSIEFARL and unimmunized mice (negative control) (P < 0.01). (B) Clinical scores and survival rates of C57BL/6 mice systemically challenged during the memory phase. b = not significantly different from values obtained for mice immunized with control CpG 1982 plus SSIEFARL and unimmunized mice (negative control) (P > 0.05).
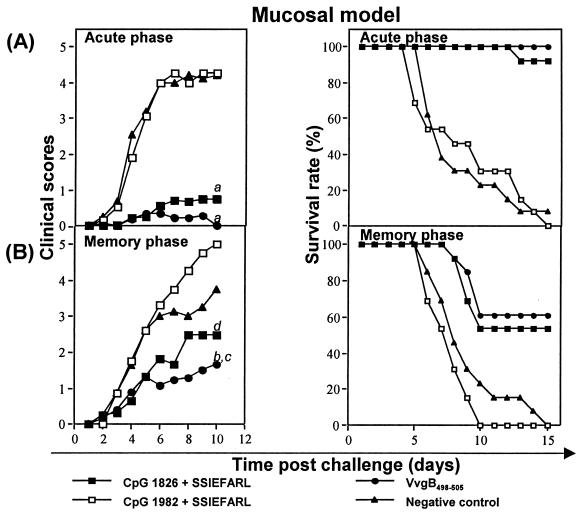
Impressive levels of immunity to vaginal challenge in the acute phase were also evident after immunization with CpG 1826 plus SSIEFARL, VvgB498-505 (Fig. 4), or UV-HSV-1 (data not shown). In these experiments, the level of immunity induced by immunization with CpG 1826 plus SSIEFARL appeared comparable to that engendered by VvgB498-505 immunization. Accordingly, in contrast to unimmunized or peptide-immunized animals, vaginal challenge of animals immunized with bioactive CpG 1826 plus SSIEFARL or VvgB498-505 resulted in no clinical lesions, and 100 and 92% of animals survived challenge, respectively. In such experiments, control animals, including those immunized with CpG 1826 but without peptide (data not shown), all developed severe vaginal lesions following challenge and >90% died after developing encephalitis (Fig. 4).
FIG. 4.
The induction of protective immunity against vaginal challenge with HSV following systemic immunization. C57BL/6 female mice were immunized twice (days 0 and 7) in the hind fp with either VvgB498-505, SSIEFARL combined with bioactive CpG 1826 or control CpG 1982, UV-HSV-1, CpG 1826 alone, or SSIEFARL alone or were unimmunized (negative control). Seven days (acute phase) or 60 days (memory phase) after boosting, mice were intravaginally challenged with 106 PFU of HSV-1 strain McKrae/mouse. Depo-Provera (2 mg/mouse in 50 μl of dH2O) was injected s.c. 5 days before challenge to synchronize the estrous cycle. Mice were examined daily for vaginal inflammation, neurologic illness, and death. Clinical severity was graded as follows: 0, no apparent infection; 1, mild inflammation of external vagina; 2, redness and moderate swelling of external vagina; 3, severe redness and inflammation; 4, genital ulceration, severe inflammation, hair loss of genital and surrounding tissue, paresis; 5, death. The figure shows the results from mice immunized with VvgB498-505, CpG 1826 plus SSIEFARL, or CpG 1982 plus SSIEFARL and nonimmunized mice. (A) Clinical scores and survival rate of C57BL/6 mice intravaginally challenged during acute phase. a = significantly different from values obtained for mice immunized with control CpG 1982 plus SSIEFARL and unimmunized mice (negative control) P < 0.01. (B) Clinical scores and survival rate of C57BL/6 mice intravaginally challenged during memory phase. b = significantly different from values obtained for mice immunized with control CpG 1982 plus SSIEFARL (P < 0.05); c = not significantly different from values obtained for unimmunized mice (negative control) (P > 0.05); d = not significantly different from values obtained for mice immunized with control CpG 1982 plus SSIEFARL and unimmunized mice (negative control) (P > 0.05).
Whereas immunization with CpG 1826 plus SSIEFARL provided a potent protection against systemic or mucosal challenge in the acute phase, in the memory phase animals were less resistant to challenge (Fig. 3 and 4). Moreover, immunity was more persistent in the VvgB498-505-immunized mice than in those immunized with CpG 1826 plus SSIEFARL. For instance, following systemic challenge of the CpG 1826 plus SSIEFARL-immunized animals at 60 days p.i., 100% succumbed to encephalitis. The average day of death was delayed by 1 day compared with that in control groups. For comparison, 60% of the VvgB498-505-immunized animals developed encephalitis and the average day of death was delayed by 3 days.
In the mucosal model, groups of mice infected during the memory phase succumbed to the infection, but the disease in the VvgB498-505 and bioactive CpG 1826 plus SSIEFARL groups was mild compared to that in control mice (clinical scores, 1.5 and 2.5, respectively), with survival rates of 62 and 54%, respectively. The onset of death in both groups was delayed compared to that in control groups, in which disease developed rapidly and with full clinical signs. Such results indicate that, whereas the CpG 1826 plus SSIEFARL immunization approach does induce a protective CD8+ T-cell response that functions both systemically and mucosally, the duration of protective immunity is brief.
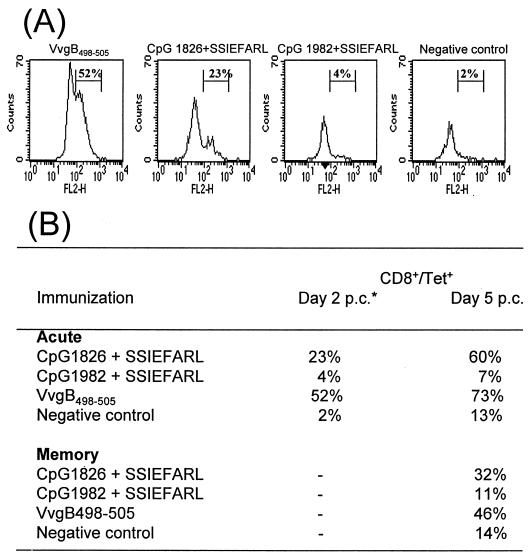
Since it was somewhat surprising to observe mucosal immunity after systemic immunization with bioactive CpG 1826 plus SSIEFARL, vaginal tissues were isolated in both the acute and memory phase to determine if antigen-specific CD8+ cells were demonstrable in tissue prior to or soon after challenge. Using tetramer staining, the epitope specificity of the infiltrating CD8+ T cells was determined. Analysis of tissue revealed no such cells prior to challenge during both the acute and memory phases. However, by 2 days postchallenge in the acute phase, peptide-specific CD8+ T cells were present. Such cells were more abundant in VvgB498-505-immunized animals than in animals immunized with CpG 1826 plus SSIEFARL. By 5 days, cell numbers had increased in both groups of animals and such cells were also present in low numbers in control animals. In the memory phase, peptide-specific CD8+ cells could not be demonstrated at 2 days in animals immunized with either VvgB498-505 or CpG 1826 plus SSIEFARL. Once again, however, cells were present at 5 days, although they were less numerous than at the acute phase. These results are expressed in Fig. 5.
FIG. 5.
Infiltration of antigen-specific cells to the VT following intravaginal challenge with HSV. C57BL/6 female mice were twice immunized (days 0 and 7) in the hind fp with VvgB498-505 or SSIEFARL combined with bioactive CpG 1826 or control CpG 1982 or were unimmunized (negative control). Seven days (acute phase) or 60 days (memory phase) after boosting, mice were intravaginally challenged with 106 PFU of HSV-1 strain McKrae/mouse. Depo-Provera (2 mg/mouse in 50 μl of dH2O) was injected s.c. 5 days before challenge to synchronize the estrous cycle. At days 2 and 5 postchallenge, lymphocytes were isolated from VTs as described in Materials and Methods. Isolated cells were stained with tetramer to determine antigen specificity. (A) Infiltration of antigen-specific cells to the VT 2 days postchallenge during the acute phase. The figure shows analysis of an enriched population of lymphocytes gated on CD8+ T cells. (B) Infiltration of antigen-specific cells to the VT 2 days and 5 days postchallenge (p.c.) during the acute and memory phases.
DISCUSSION
The present study assesses the efficacy of systemic immunization of mice with immunostimulatory CpG ODN mixed with the peptide (gB498-505) that represents the epitope recognized by C57BL/6 mice exposed to HSV. The CpG-peptide immunogen was compared to a recombinant vaccinia virus vector that encoded gB498-505 as a minigene as well as to other control immunogens. Whereas a single intradermal immunization led to only a fleeting CD8+ T-cell response confined to the DLN, two immunizations 1 week apart induced notable specific CD8+ T-cell responses detectable in both local and distal lymphoid tissue. As measured by tetramers, as well as ICG, the CpG-peptide-induced responses were around 50% of those occurring after immunization with VvgB498-505. However, the CpG peptide-induced responses were sufficient to protect against systemic and mucosal HSV challenge at least in the acute phase. In contrast, whereas VvgB498-505-immunized mice still showed significant resistance to mucosal challenge 60 days p.i., most CpG-immunized mice succumbed to such a challenge at this time. Accordingly, our results confirm the observations of others (25, 35) that CpG-peptide mixtures can induce CD8+ T-cell immunity, but at least in the HSV system levels of functional immunity lack the potency of immunization by the VvgB498-505 system.
Many observations have shown that CpG ODN act as excellent adjuvants in mice for numerous protein antigens (17, 18, 23, 28, 31). In addition, slightly different formulations of CpG ODN also appear as promising adjuvants in primate systems (3, 9), making it likely that these adjuvants, which have minimal toxicity, will be acceptable for human use (10). In the present report, we elected to investigate the value of CpG ODN along with a peptide that represents the immunodominant epitope recognized by B6 mice after infection with HSV (4, 36). The approach was chosen since studies in humans indicate that the CD8+ T-cell aspect of immunity may be principally involved in limiting the severity of recurrent HSV lesions (15, 26, 27). Accordingly, vaccines that can selectively enhance functionally relevant CD8+ T-cell responses hold promise as therapeutic vaccines. This concept can only be tested in animal models, however; although CD8+ peptide epitope targets in humans are beginning to be identified (14), immunization with such peptides is not under consideration at present.
Our results demonstrate that CpG-peptide immunization does induce CD8+ T-cell responses against an immunodominant epitope, supporting the work of two previous groups that used different systems (7, 35). The previous reports did not compare the effectiveness of CpG-peptide immunization with other approaches, nor in fact was the duration of the immune responses addressed. Both issues were addressed in this report. Our findings show that the CpG-peptide approach, as currently used, lacked the potency of a VvgB498-505 immunization system. This applies to the magnitude of the CD8+ T-cell response induced and especially to the level of protection against mucosal challenge in the memory phase. The suboptimal immunogenic activity of the CpG-peptide combination could have several explanations. In part, it may relate to the need for both the CpG adjuvant and the cognate peptide to coengage the inducing antigen-presenting cells and responder T cells in lymphoid tissue. Thus, in some systems, incorporating CpG antigen mixtures in alum gels (10) or even covalently linking antigens to CpG ODN results in superior immunogenicity (34). The influence on immunity of physical association within gels of CpG and the gB498-505 peptide is currently being evaluated in our laboratory.
An alternative explanation for the suboptimal immunogenicity of CpG-peptide may be that the CD8+ T-cell response to the gB498-505 peptide is helper cell dependent. Such helper cell effects might be supplied in part by the vaccinia virus vector system, since a CD4+ T-cell response to multiple vaccinia proteins will be occurring simultaneously in lymphoid tissue. This could explain the greater magnitude of the VvgB498-505 response. Bioactive CpG ODN are well known to induce antigen-presenting dendritic cells to become mature and express molecules deemed essential for superior antigen presentation (2, 11, 12, 30, 31). Indeed, in at least some situations the CpG adjuvant is advocated to replace the need for help mediated, for example, by the CD40-CD40L interaction (32, 37). Curiously, the only previous study that investigated CpG as an adjuvant in any herpesvirus system used the gB protein of HSV given along with CpG (7). In this instance, the CTL response, measured by a standard 51Cr release assay, was induced very moderately and was even below the level of detection in some animals. Using CpG along with gB protein, they also induced production of neutralizing antibody and generation of CD4+ T cells, and so it was not clear which component was responsible for protection. In our studies, all acquired immunity would seem to be attributed to CD8+ T-cell function. We clearly show that the antigen-specific CD8+ T-cell induction with functional ability in spleen and DLN followed immunization with VvgB498-505 and bioactive CpG 1826 along with SSIEFARL. This immunization induced both primary and memory CTL responses directed against this epitope. The difference between our results and those of Rosenthal's group can be explained by using different methods of immunization. Thus, immunization with CpG alone, which in some situations nonspecifically protects animals against a variety of pathogens (6, 16, 38), failed to protect against mucosal challenge by HSV. Following systemic immunization with VvgB498-505 and bioactive CpG along with the CD8 epitope, we demonstrated a significant reduction of disease severity following mucosal HSV challenge in both the acute and memory phases. Although mortality occurred during the memory phase, the onset and deaths were delayed. Furthermore, CD8+ T cells that produced IFN-γ upon peptide stimulation were directly demonstrable in vaginal tissue in the acute phase soon after challenge with HSV. Our results suggest the possibility that the epitope-specific CTLs limit HSV replication at the initial site of infection and thus prevent viral dissemination. These findings indicate the importance of CD8+ T cells in the control of HSV infection.
In conclusion, our results indicate that the CpG-peptide immunization system holds promise as a means of selectively inducing a CD8+ T-cell response against HSV. Further manipulations are required to make the approach achieve maximal efficacy. Such studies are currently under way in our laboratory.
Acknowledgments
This work was supported by National Institutes of Health grant RO1 AI 4646 201.
REFERENCES
- 1.Ballas, Z. K., W. L. Rasmussen, and A. M. Krieg. 1996. Induction of NK activity in murine and human cells by CpG motifs in oligodeoxynucleotides and bacterial DNA. J. Immunol. 157:1840-1845. [PubMed] [Google Scholar]
- 2.Bauer, M., V. Redecke, J. W. Ellwart, B. Scherer, J. P. Kremer, H. Wagner, and G. B. Lipford. 2001. Bacterial CpG-DNA triggers activation and maturation of human CD11c−, CD123+ dendritic cells. J. Immunol. 166:5000-5007. [DOI] [PubMed] [Google Scholar]
- 3.Bauer, S., C. J. Kirschning, H. Hacker, V. Redecke, S. Hausmann, S. Akira, H. Wagner, and G. B. Lipford. 2001. Human TLR9 confers responsiveness to bacterial DNA via species-specific CpG motif recognition. Proc. Natl. Acad. Sci. USA 98:9237-9242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blaney, J. E., Jr., E. Nobusawa, M. A. Brehm, R. H. Bonneau, L. M. Mylin, T. M. Fu, Y. Kawaoka, and S. S. Tevethia. 1998. Immunization with a single major histocompatibility complex class I-restricted cytotoxic T-lymphocyte recognition epitope of herpes simplex virus type 2 confers protective immunity. J. Virol. 72:9567-9574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chu, R. S., O. S. Targoni, A. M. Krieg, P. V. Lehman, and C. V. Harding. 1997. CpG oligodeoxynucleotides act as adjuvants that switch on T helper 1 (Th1) immunity. J. Exp. Med. 186:1623-1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elkins, K. L., T. R. Rhinehart-Jones, S. Stibitz, J. S. Conover, and D. M. Klinman. 1999. Bacterial DNA containing CpG motifs stimulates lymphocyte-dependent protection of mice against lethal infection with intracellular bacteria. J. Immunol. 162:2291-2298. [PubMed] [Google Scholar]
- 7.Gallichan, W. S., R. N. Woolstencroft, T. Guarasci, M. J. McCluskie, H. L. Davis, and K. L. Rosenthal. 2001. Intranasal immunization with CpG oligodeoxynucleotides as an adjuvant dramatically increases IgA and protection against herpes simplex virus-2 in the genital tract. J. Immunol. 166:3451-3457. [DOI] [PubMed] [Google Scholar]
- 8.Gurunathan, S., D. M. Klinman, and R. A. Seder. 2000. DNA vaccines: immunology, application, and optimization. Annu. Rev. Immunol. 18:927-974. [DOI] [PubMed] [Google Scholar]
- 9.Hartmann, G., and A. M. Krieg. 2000. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 164:944-953. [DOI] [PubMed] [Google Scholar]
- 10.Hartmann, G., R. D. Weeratna, Z. K. Ballas, P. Payette, S. Blackwell, I. Suparto, W. L. Rasmussen, M. Waldschmidt, D. Sajuthi, R. H. Purcell, H. L. Davis, and A. M. Krieg. 2000. Delineation of a CpG phosphorothioate oligodeoxynucleotide for activating primate immune responses in vitro and in vivo. J. Immunol. 164:1617-1624. [DOI] [PubMed] [Google Scholar]
- 11.Hartmann, G., G. J. Weiner, and A. M. Krieg. 1999. CpG DNA: a potent signal for growth, activation, and maturation of human dendritic cells. Proc. Natl. Acad. Sci. USA 96:9305-9310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakob, T., P. S. Walker, A. M. Krieg, M. C. Udey, and J. C. Vogel. 1998. Activation of cutaneous dendritic cells by CpG-containing oligodeoxynucleotides: a role for dendritic cells in the augmentation of Th1 responses by immunostimulatory DNA. J. Immunol. 161:3042-3049. [PubMed] [Google Scholar]
- 13.Klinman, D. M., A. K. Yi, S. L. Beaucage, J. Conover, and A. M. Krieg. 1996. CpG motifs present in bacteria DNA rapidly induce lymphocytes to secrete interleukin 6, interleukin 12, and interferon gamma. Proc. Natl. Acad. Sci. USA 93:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koelle, D. M., H. B. Chen, M. A. Gavin, A. Wald, W. W. Kwok, and L. Corey. 2001. CD8 CTL from genital herpes simplex lesions: recognition of viral tegument and immediate early proteins and lysis of infected cutaneous cells. J. Immunol. 166:4049-4058. [DOI] [PubMed] [Google Scholar]
- 15.Koelle, D. M., C. M. Posavad, G. R. Barnum, M. L. Johnson, J. M. Frank, and L. Corey. 1998. Clearance of HSV-2 from recurrent genital lesions correlates with infiltration of HSV-specific cytotoxic T lymphocytes. J. Clin. Investig. 101:1500-1508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krieg, A. M., L. Love-Homan, A. K. Yi, and J. T. Harty. 1998. CpG DNA induces sustained IL-12 expression in vivo and resistance to Listeria monocytogenes challenge. J. Immunol. 161:2428-2434. [PubMed] [Google Scholar]
- 17.Lipford, G. B., M. Bauer, C. Blank, R. Reiter, H. Wagner, and K. Heeg. 1997. CpG-containing synthetic oligonucleotides promote B and cytotoxic T cell responses to protein antigen: a new class of vaccine adjuvants. Eur. J. Immunol. 27:2340-2344. [DOI] [PubMed] [Google Scholar]
- 18.Lipford, G. B., T. Sparwasser, M. Bauer, S. Zimmermann, E. S. Koch, K. Heeg, and H. Wagner. 1997. Immunostimulatory DNA: sequence-dependent production of potentially harmful or useful cytokines. Eur. J. Immunol. 27:3420-3426. [DOI] [PubMed] [Google Scholar]
- 19.Lipford, G. B., T. Sparwasser, S. Zimmermann, K. Heeg, and H. Wagner. 2000. CpG-DNA-mediated transient lymphadenopathy is associated with a state of Th1 predisposition to antigen-driven responses. J. Immunol. 165:1228-1235. [DOI] [PubMed] [Google Scholar]
- 20.Manickan, E., and B. T. Rouse. 1995. Roles of different T-cell subsets in control of herpes simplex virus infection determined by using T-cell-deficient mouse models. J. Virol. 69:8178-8179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Martin-Orozco, E., H. Kobayashi, J. Van Uden, M. D. Nguyen, R. S. Kornbluth, and E. Raz. 1999. Enhancement of antigen-presenting cell surface molecules involved in cognate interactions by immunostimulatory DNA sequences. Int. Immunol. 11:1111-1118. [DOI] [PubMed] [Google Scholar]
- 22.Messina, J. P., G. S. Gilkeson, and D. S. Pisetsky. 1993. The influence of DNA structure on the in vitro stimulation of murine lymphocytes by natural and synthetic polynucleotide antigens. Cell. Immunol. 147:148-157. [DOI] [PubMed] [Google Scholar]
- 23.Moldoveanu, Z., L. Love-Homan, W. Q. Huang, and A. M. Krieg. 1998. CpG DNA, a novel immune enhancer for systemic and mucosal immunization with influenza virus. Vaccine 16:1216-1224. [DOI] [PubMed] [Google Scholar]
- 24.Nash, A. A., A. Jayasuriya, J. Phelan, S. P. Cobbold, H. Waldmann, and T. Prospero. 1987. Different roles for L3T4+ and Lyt 2+ T cell subsets in the control of an acute herpes simplex virus infection of the skin and nervous system. J. Gen. Virol. 68:825-833. [DOI] [PubMed] [Google Scholar]
- 25.Oxenius, A., M. M. Martinic, H. Hengartner, and P. Klenerman. 1999. CpG-containing oligonucleotides are efficient adjuvants for induction of protective antiviral immune responses with T-cell peptide vaccines. J. Virol. 73:4120-4126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Posavad, C. M., M. L. Huang, S. Barcy, D. M. Koelle, and L. Corey. 2000. Long term persistence of herpes simplex virus-specific CD8+ CTL in persons with frequently recurring genital herpes. J. Immunol. 165:1146-1152. [DOI] [PubMed] [Google Scholar]
- 27.Posavad, C. M., D. M. Koelle, and L. Corey. 1996. High frequency of CD8+ cytotoxic T-lymphocyte precursors specific for herpes simplex viruses in persons with genital herpes. J. Virol. 70:8165-8168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Roman, M., E. Martin-Orozco, J. S. Goodman, M. D. Nguyen, Y. Sato, A. Ronaghy, R. S. Kornbluth, D. D. Richman, D. A. Carson, and E. Raz. 1997. Immunostimulatory DNA sequences function as T helper-1-promoting adjuvants. Nat. Med. 3:849-854. [DOI] [PubMed] [Google Scholar]
- 29.Schmid, D. S., and B. T. Rouse. 1992. The role of T cell immunity in control of herpes simplex virus. Curr. Top. Microbiol. Immunol. 179:57-74. [DOI] [PubMed] [Google Scholar]
- 30.Simmons, A., and D. C. Tscharke. 1992. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: implications for the fate of virally infected neurons. J. Exp. Med. 175:1337-1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sparwasser, T., E. S. Koch, R. M. Vabulas, K. Heeg, G. B. Lipford, J. W. Ellwart, and H. Wagner. 1998. Bacterial DNA and immunostimulatory CpG oligonucleotides trigger maturation and activation of murine dendritic cells. Eur. J. Immunol. 28:2045-2054. [DOI] [PubMed] [Google Scholar]
- 32.Sparwasser, T., R. M. Vabulas, B. Villmow, G. B. Lipford, and H. Wagner. 2000. Bacterial CpG-DNA activates dendritic cells in vivo: T helper cell-independent cytotoxic T cell responses to soluble proteins. Eur. J. Immunol. 30:3591-3597. [DOI] [PubMed] [Google Scholar]
- 33.Sun, S., H. Kishimoto, and J. Sprent. 1998. DNA as an adjuvant: capacity of insect DNA and synthetic oligodeoxynucleotides to augment T cell responses to specific antigen. J. Exp. Med. 187:1145-1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tighe, H., K. Takabayashi, D. Schwartz, R. Marsden, L. Beck, J. Corbeil, D. D. Richman, J. J. Eiden, Jr., H. L. Spiegelberg, and E. Raz. 2000. Conjugation of protein to immunostimulatory DNA results in a rapid, long-lasting and potent induction of cell-mediated and humoral immunity. Eur. J. Immunol. 30:1939-1947. [DOI] [PubMed] [Google Scholar]
- 35.Vabulas, R. M., H. Pircher, G. B. Lipford, H. Hacker, and H. Wagner. 2000. CpG-DNA activates in vivo T cell epitope presenting dendritic cells to trigger protective antiviral cytotoxic T cell responses. J. Immunol. 164:2372-2378. [DOI] [PubMed] [Google Scholar]
- 36.Wallace, M. E., R. Keating, W. R. Heath, and F. R. Carbone. 1999. The cytotoxic T-cell response to herpes simplex virus type 1 infection of C57BL/6 mice is almost entirely directed against a single immunodominant determinant. J. Virol. 3:7619-7626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wild, J., M. J. Grusby, R. Schirmbeck, and J. Reimann. 1999. Priming MHC-I-restricted cytotoxic T lymphocyte responses to exogenous hepatitis B surface antigen is CD4+ T cell dependent. J. Immunol. 163:1880-1887. [PubMed] [Google Scholar]
- 38.Zimmermann, S., O. Egeter, S. Hausmann, G. B. Lipford, M. Rocken, H. Wagner, and K. Heeg. 1998. CpG oligodeoxynucleotides trigger protective and curative Th1 responses in lethal murine leishmaniasis. J. Immunol. 160:3627-3630. [PubMed] [Google Scholar]