Abstract
Human cytomegalovirus US8 is a type I membrane protein that partially colocalizes with cellular endosomal and lysosomal proteins. Although US8 does not have discernible effects on the processing and cell surface distribution of major histocompatibility complex (MHC) class I products, we have demonstrated that US8 binds to MHC class I heavy chains in the endoplasmic reticulum.
Herpesviruses encode a seemingly redundant repertoire of genes that interfere with the major histocompatibility (MHC) class I antigen presentation pathway. In particular, the genomes of the homologous betaherpesviruses mouse cytomegalovirus (MCMV) and human cytomegalovirus (HCMV) both contain a substantial number of genes that regulate MHC class I antigen presentation (termed immunoevasins) to prevent cytotoxic T-cell (CTL) recognition and lysis of infected cells (1, 2, 8, 10-12, 14-16, 18, 24-26). It is unclear why HCMV has multiple genes encoding proteins with the same function, but a plausible explanation is that none of the gene products noted above can accomplish full attenuation of MHC class I antigen expression on its own, as has been recently demonstrated by Kavanagh et al. for MCMV gp34 and gp40 (13). Alternatively, each immunoevasin might be required for specific cell types or might block discrete steps in MHC class I-restricted antigen presentation. The unique short (US) region of the HCMV genome contains several blocks of genes that share homology with one another (termed gene families) (3), four of which (US2, US3, US6, and US11) interfere with the biogenesis of MHC class I antigens. This prompted us to investigate whether another member of this family, US8, also interacts with MHC class I products.
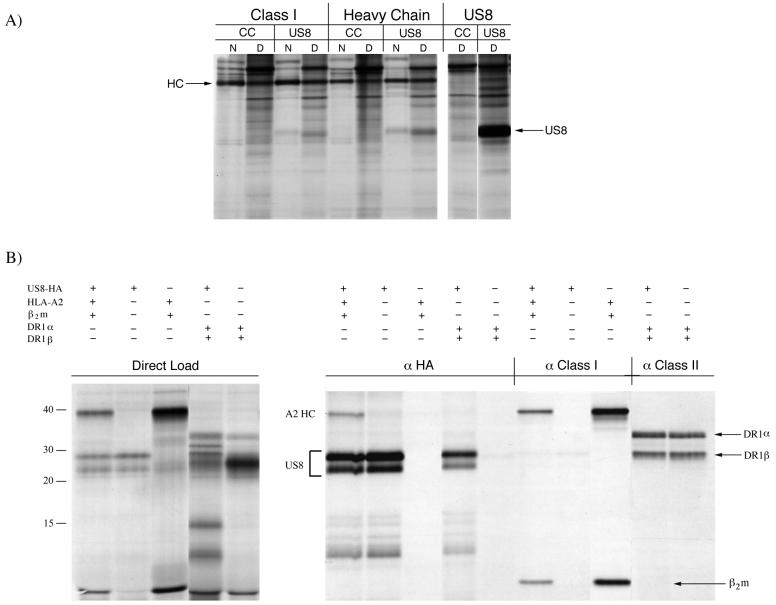
The US8 gene was amplified from HCMV AD169 DNA by PCR using primers that either incorporated the influenza virus hemagglutinin (HA) epitope tag at the C terminus of the protein (US8-HA) or amplified US8 without the addition of the epitope tag (US8) and was cloned into pCDNA3.1 (Invitrogen). Sequencing of the cloned inserts confirmed the identity of the US8 gene and the proper incorporation of the HA tag. To test whether US8 could interact with MHC class I antigens in vivo, U373 astrocytoma cells or U373 cells stably expressing US8-HA were metabolically labeled with Expre35S35S protein labeling mix (NEN) for 20 min and were lysed in either NP-40 lysis buffer (150 mM NaCl, 2 mM CaCl2, 50 mM Tris-HCl [pH 7.4], 0.5% NP-40, 1 mM phenylmethylsulfonyl fluoride, and 2-μg/ml aprotinin) or digitonin lysis buffer (125 mM HEPES [pH 7.7], 750 mM potassium acetate, 1% digitonin, 1 mM phenylmethylsulfonyl fluoride, and 2-μg/ml aprotinin) (4, 24). When carrying out immunoprecipitations of MHC class I products, we observed recovery of a 26-kDa polypeptide, along with class I heavy chains, in US8-HA-expressing cells but not in control cells (Fig. 1A, lanes 3 and 4 and lanes 7 and 8). More of the 26-kDa polypeptide was recovered when antiserum specific for free heavy chains was used and when cells were lysed in the digitonin lysis buffer. Antiserum raised against the bacterially expressed luminal domain of US8 immunoprecipitated a 26-kDa protein from US8-HA-containing cells, as well as a 40-kDa protein (lane 10).
FIG. 1.
HCMV US8 interacts with MHC class I products. (A) U373 (CC) or U373+US8-HA (US8) cells were labeled for 20 min with [35S]methionine-cysteine prior to lysis in either 0.5% NP-40 (N) or 1% digitonin (D). Immunoprecipitations were performed with either monoclonal antibody W6/32, which recognizes folded MHC class I molecules (Class I); a rabbit polyclonal antiserum specific for free heavy chains (Heavy Chain); or a rabbit polyclonal antiserum that recognizes US8 (US8). (B) The indicated in vitro-transcribed mRNAs were translated in vitro in the presence of canine pancreatic microsomes and [35S]methionine. Three microliters of each reaction mixture was analyzed directly by SDS-PAGE (direct load), while the remainder of the reaction mixture was divided into two samples, lysed in 0.5% NP-40, immunoprecipitated with the indicated antibodies (anti-US8-HA [αHA], antibodies specific for the folded MHC class I molecules [α Class I] and antibodies specific for the folded MHC class II products [α Class II]), and analyzed by SDS-PAGE. The apparent positions of molecular mass markers are indicated on the left (in kilodaltons).
To examine whether the interaction between US8 and MHC class I products was specific, we translated US8 mRNA in the presence of microsomes together with either MHC class I or class II mRNAs in vitro (20). A portion of each reaction mixture was either loaded directly onto a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel or lysed in NP-40 lysis buffer prior to being subjected to immunoprecipitations (4, 24). We used antibodies specific for US8-HA (12CA5), the folded MHC class I molecules (W6/32), or the folded MHC class II products (TU 36) (17, 19) (Fig. 1B). The HA antibody immunoprecipitated two US8-HA-specific polypeptides from reaction mixtures containing US8-HA mRNA (lanes 1, 2, and 4). In addition, a 40-kDa protein was also immunoprecipitated from reaction mixtures containing MHC class I heavy chains, HLA-A2, and β2-microglobulin (lane 1), but no additional polypeptides were observed in reaction mixtures containing the MHC class II subunits, DR1α and DR1β (lane 5), even though assembly of the class II product was readily demonstrable (Fig. 1, lanes 9 and 10) (5). However, we did not observe any polypeptides corresponding to US8 protein in these lanes. Furthermore, we did not observe coimmunoprecipitation of CD4 with US8-HA when using translation mixtures containing US8 and CD4 (data not shown). The results, therefore, show that the class I heavy chain can be coimmunoprecipitated with US8 and that this interaction is specific and preferentially involves free class I heavy chains. Neither the structurally related class II molecules nor another member of the immunoglobulin superfamily (CD4) was found to interact with US8. We conclude that a specific interaction between US8 and MHC class I antigens is discernible in live cells as well as in vitro and that this interaction is preserved better in the presence of the mild detergent digitonin.
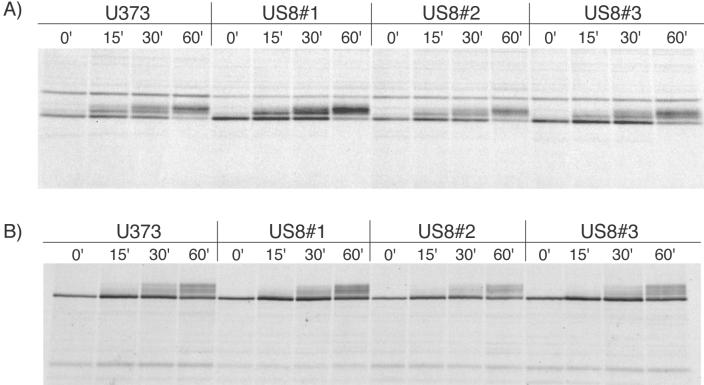
Using flow cytometry, we found no difference between US8-HA-expressing cells and control cells in terms of the amount of MHC class I protein at the surface (data not shown). To test whether US8 had an effect on the maturation of the MHC class I products, we performed pulse-chase analyses followed by endoglycosidase H (endo H) digestions of three clonal cell lines expressing untagged US8 protein. Immunoprecipitated samples were either treated with EndoHf (New England Biolabs) or left untreated (data not shown) prior to analysis by SDS-PAGE (Fig. 2A). As a control, we performed an immunoprecipitation with an antibody that recognizes the human transferrin receptor (Fig. 2B) (23). In US8-expressing cells, the MHC class I heavy chain and the transferrin receptor acquired endo H resistance with kinetics indistinguishable from that of cells not expressing US8, despite the presence of an abundant amount of US8 protein (data not shown). These results demonstrate that although US8 binds class I heavy-chain molecules, it does not affect the maturation of the class I complex.
FIG. 2.
US8 does not affect transport of MHC class I products. U373 or U373+US8 cells were labeled with [35S]methionine-cysteine for 5 min and chased with nonradioactive medium for the indicated time periods prior to lysis in 0.5% NP-40. Immunoprecipitations were performed with either monoclonal antiserum W6/32, which recognizes folded MHC class I molecules (A), or a monoclonal antiserum that recognizes the transferrin receptor (B). The samples were digested with EndoHf prior to analysis by SDS-PAGE.
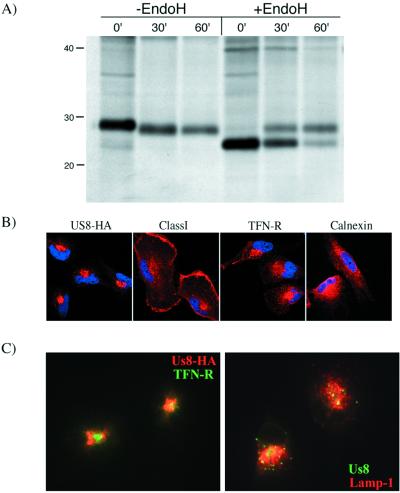
Digestion of US8 immunoprecipitates with endo H revealed that US8 also acquired endo H resistance (Fig. 3A). This is in contrast to the observations for US2, US3, US6, and US11, all of which remain endo H sensitive and are localized to the endoplasmic reticulum (ER). Similar results were observed when US8 was immunoprecipitated from HCMV-infected fibroblasts (data not shown). We determined the intracellular localization of US8 by immunofluorescence spectroscopy (21). Confocal images taken through the center of the cells revealed that US8-HA localized to a distinct perinuclear patch on one side of the nucleus (Fig. 3B). This staining pattern was very similar to that of the transferrin receptor and was distinctly different from that of the ER-resident protein calnexin. US8 localized to fewer compartments than MHC class I proteins, which had a much broader distribution. We also compared the localization of US8-HA, internalized transferrin (Molecular Probes), and the lysosomal protein Lamp-1 (Molecular Probes) by standard microscopy. The dual-color overlay of the merged images is shown in Fig. 3C. Internalized transferrin was found in a bright perinuclear patch and in scattered punctate patches, many of which colocalized with US8-HA. We also observed partial colocalization of US8-HA with Lamp-1. We conclude that US8-HA is targeted to an endosomal-lysosomal location.
FIG. 3.
US8 partially colocalizes with cellular endosomal-lysosomal proteins. (A) U373+US8-HA cells were pulsed with [35S]methionine-cysteine for 5 min and chased with nonradioactive medium for the time periods indicated. The cells were lysed in 0.5% NP-40 and immunoprecipitated with antiserum specific for the HA epitope. After immunoprecipitation, half of each sample was treated with endo H (+EndoH) while half was left untreated (−EndoH). Apparent positions of molecular mass markers are indicated on the left (in kilodaltons). (B) Fixed, permeabilized U373+US8-HA cells were incubated with either a primary monoclonal antibody specific for the HA epitope (US8-HA); monoclonal antibody W6/32, which recognizes folded MHC class I molecules (Class I); a monoclonal antibody that recognizes the human transferrin receptor (TFN-R); or a monoclonal antibody specific for the human ER chaperone, calnexin. The cells were then incubated with an Alexa-568-conjugated secondary antibody (red fluorescence) and the DNA-binding dye DAPI (4′,6′-diamidino-2-phenylindole; blue fluorescence) (Sigma) prior to analysis with a Bio-Rad MRC1024 laser-scanning confocal microscope. (C) Fixed, permeabilized U373+US8-HA cells were incubated with either a monoclonal antibody that recognizes the HA epitope, a monoclonal antibody specific for the lysosomal protein Lamp-1, or a rabbit polyclonal antiserum generated against the luminal domain of US8. The cells were then incubated with either an Alexa-568-conjugated anti-mouse secondary antibody (red fluorescence) or an Alexa-488-conjugated anti-rabbit secondary antibody (green fluorescence). Some cells were incubated with fluorescently labeled human transferrin at 37°C prior to fixation of the cells (TFN-R; green fluorescence). Images were obtained with a Spot RT digital camera mounted on a TE300 Nikon microscope. Merged two-color overlays demonstrating the colocalization of internalized transferrin, Lamp-1, and US8 are shown.
Here we report on the fifth HCMV-encoded protein from the US region that directly interacts with the MHC class I antigen presentation pathway (for a review, see reference 22). US8 is a type I membrane protein that partially colocalizes with markers of the endosomal-lysosomal pathway and acquires endo H resistance. Other homologous transmembrane proteins encoded by the US region reside strictly within the ER, where they exert their effects (1, 7). Surprisingly, we found that US8 exits the ER. By using two different anti-MHC class I antibodies, we found that US8-HA was coimmunoprecipitated with class I molecules in US8-HA-expressing cells. More US8-HA protein was preferentially recovered in association with free heavy chains, suggesting an interaction of US8 with MHC class I molecules in the ER prior to their association with β2-microglobulin and peptide. This was corroborated by in vitro translation experiments that faithfully recapitulated assembly of multimeric membrane glycoproteins. Although US8 appears to bind to MHC class I molecules in the ER, most of US8 localizes to other cellular compartments. This suggests that US8 interacts only transiently with MHC class I molecules, as has also been suggested for US3 (9). Binding of US8 to MHC class I molecules had little effect on transport of class I antigens from the ER to the Golgi complex. This suggests that US8's behavior may be similar to that of MCMV gp34, which binds mouse MHC class I Kb alleles without necessarily affecting maturation or cell surface distribution of the protein (14). It has been shown that gp34 does indeed abrogate a Kb-restricted CTL response (13). It will be interesting to determine whether US8 has similar effects on HCMV-specific CTLs. A possibility that also requires further experimentation is the occurrence of interactions among the members of the US family. Notwithstanding suggested structural similarities, studies of the functions of the different US products are beginning to reveal valuable differences (1, 6, 7).
Acknowledgments
The AD169 strain of HCMV and human foreskin fibroblast cells were kindly provided by Donald Coen, Harvard Medical School. U373-MG astrocytoma cells (U373) were generously provided by Thomas Jones. We thank all members of the Ploegh lab for continual support and guidance.
This work was supported by the National Institutes of Health (grants R37-AI33456 and P01-AI42257). R.S.T. is a Novartis Fellow of the Life Sciences Research Foundation.
REFERENCES
- 1.Ahn, K., A. Angulo, P. Ghazal, P. A. Peterson, Y. Yang, and K. Fruh. 1996. Human cytomegalovirus inhibits antigen presentation by a sequential multistep process. Proc. Natl. Acad. Sci. USA 93:10990-10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn, K., A. Gruhler, B. Galocha, T. R. Jones, E. J. Wiertz, H. L. Ploegh, P. A. Peterson, Y. Yang, and K. Fruh. 1997. The ER-luminal domain of the HCMV glycoprotein US6 inhibits peptide translocation by TAP. Immunity 6:613-621. [DOI] [PubMed] [Google Scholar]
- 3.Bankier, A. T., S. Beck, R. Bohni, C. M. Brown, R. Cerny, M. S. Chee, C. A. Hutchison III, T. Kouzarides, J. A. Martignetti, E. Preddie, et al. 1991. The DNA sequence of the human cytomegalovirus genome. DNA Seq. 2:1-12. [DOI] [PubMed] [Google Scholar]
- 4.Beersma, M. F., M. J. Bijlmakers, and H. L. Ploegh. 1993. Human cytomegalovirus down-regulates HLA class I expression by reducing the stability of class I H chains. J. Immunol. 151:4455-4464. [PubMed] [Google Scholar]
- 5.Bijlmakers, M. J., P. Benaroch, and H. L. Ploegh. 1994. Assembly of HLA DR1 molecules translated in vitro: binding of peptide in the endoplasmic reticulum precludes association with invariant chain. EMBO J. 13:2699-2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Furman, M. H., H. L. Ploegh, and D. Tortorella. 2002. Membrane-specific, host-derived factors are required for US2- and US11-mediated degradation of MHC class I molecules. J. Biol. Chem. 277:3258-3267. [DOI] [PubMed] [Google Scholar]
- 7.Gewurz, B. E., R. Gaudet, D. Tortorella, E. W. Wang, H. L. Ploegh, and D. C. Wiley. 2001. Antigen presentation subverted: structure of the human cytomegalovirus protein US2 bound to the class I molecule HLA-A2. Proc. Natl. Acad. Sci. USA 98:6794-6799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gilbert, M. J., S. R. Riddell, B. Plachter, and P. D. Greenberg. 1996. Cytomegalovirus selectively blocks antigen processing and presentation of its immediate-early gene product. Nature 383:720-722. [DOI] [PubMed] [Google Scholar]
- 9.Gruhler, A., P. A. Peterson, and K. Fruh. 2000. Human cytomegalovirus immediate early glycoprotein US3 retains MHC class I molecules by transient association. Traffic 1:318-325. [DOI] [PubMed] [Google Scholar]
- 10.Hengel, H., J. O. Koopmann, T. Flohr, W. Muranyi, E. Goulmy, G. J. Hammerling, U. H. Koszinowski, and F. Momburg. 1997. A viral ER-resident glycoprotein inactivates the MHC-encoded peptide transporter. Immunity 6:623-632. [DOI] [PubMed] [Google Scholar]
- 11.Jones, T. R., and L. Sun. 1997. Human cytomegalovirus US2 destabilizes major histocompatibility complex class I heavy chains. J. Virol. 71:2970-2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jones, T. R., E. J. Wiertz, L. Sun, K. N. Fish, J. A. Nelson, and H. L. Ploegh. 1996. Human cytomegalovirus US3 impairs transport and maturation of major histocompatibility complex class I heavy chains. Proc. Natl. Acad. Sci. USA 93:11327-11333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kavanagh, D. G., M. C. Gold, M. Wagner, U. H. Koszinowski, and A. B. Hill. 2001. The multiple immune-evasion genes of murine cytomegalovirus are not redundant: m4 and m152 inhibit antigen presentation in a complementary and cooperative fashion. J. Exp. Med. 194:967-978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kleijnen, M. F., J. B. Huppa, P. Lucin, S. Mukherjee, H. Farrell, A. E. Campbell, U. H. Koszinowski, A. B. Hill, and H. L. Ploegh. 1997. A mouse cytomegalovirus glycoprotein, gp34, forms a complex with folded class I MHC molecules in the ER which is not retained but is transported to the cell surface. EMBO J. 16:685-694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Krmpotic, A., M. Messerle, I. Crnkovic-Mertens, B. Polic, S. Jonjic, and U. H. Koszinowski. 1999. The immunoevasive function encoded by the mouse cytomegalovirus gene m152 protects the virus against T cell control in vivo. J. Exp. Med. 190:1285-1296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lehner, P. J., J. T. Karttunen, G. W. Wilkinson, and P. Cresswell. 1997. The human cytomegalovirus US6 glycoprotein inhibits transporter associated with antigen processing-dependent peptide translocation. Proc. Natl. Acad. Sci. USA 94:6904-6909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Parham, P., C. J. Barnstable, and W. F. Bodmer. 1979. Use of a monoclonal antibody (W6/32) in structural studies of HLA-A,B,C antigens. J. Immunol. 123:342-349. [PubMed] [Google Scholar]
- 18.Reusch, U., W. Muranyi, P. Lucin, H. G. Burgert, H. Hengel, and U. H. Koszinowski. 1999. A cytomegalovirus glycoprotein re-routes MHC class I complexes to lysosomes for degradation. EMBO J. 18:1081-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shaw, S., A. Ziegler, and R. DeMars. 1985. Specificity of monoclonal antibodies directed against human and murine class II histocompatibility antigens as analyzed by binding to HLA-deletion mutant cell lines. Hum. Immunol. 12:191-211. [DOI] [PubMed] [Google Scholar]
- 20.Story, C. M., M. H. Furman, and H. L. Ploegh. 1999. The cytosolic tail of class I MHC heavy chain is required for its dislocation by the human cytomegalovirus US2 and US11 gene products. Proc. Natl. Acad. Sci. USA 96:8516-8521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tortorella, D., B. E. Gewurz, M. H. Furman, D. J. Schust, and H. L. Ploegh. 2000. Viral subversion of the immune system. Annu. Rev. Immunol. 18:861-926. [DOI] [PubMed] [Google Scholar]
- 23.van de Rijn, M., A. H. Geurts van Kessel, V. Kroezen, A. J. van Agthoven, K. Verstijnen, C. Terhorst, and J. Hilgers. 1983. Localization of a gene controlling the expression of the human transferrin receptor to the region q12 leads to qter of chromosome 3. Cytogenet. Cell Genet. 36:525-531. [DOI] [PubMed] [Google Scholar]
- 24.Wiertz, E. J., T. R. Jones, L. Sun, M. Bogyo, H. J. Geuze, and H. L. Ploegh. 1996. The human cytomegalovirus US11 gene product dislocates MHC class I heavy chains from the endoplasmic reticulum to the cytosol. Cell 84:769-779. [DOI] [PubMed] [Google Scholar]
- 25.Wiertz, E. J., D. Tortorella, M. Bogyo, J. Yu, W. Mothes, T. R. Jones, T. A. Rapoport, and H. L. Ploegh. 1996. Sec61-mediated transfer of a membrane protein from the endoplasmic reticulum to the proteasome for destruction. Nature 384:432-438. [DOI] [PubMed] [Google Scholar]
- 26.Ziegler, H., R. Thale, P. Lucin, W. Muranyi, T. Flohr, H. Hengel, H. Farrell, W. Rawlinson, and U. H. Koszinowski. 1997. A mouse cytomegalovirus glycoprotein retains MHC class I complexes in the ERGIC/cis-Golgi compartments. Immunity 6:57-66. [DOI] [PubMed] [Google Scholar]