Abstract
The KT tumor is a transplantable strain of a human Epstein-Barr virus (EBV)-associated gastric carcinoma (EBVaGC), established in severe combined immunodeficiency (SCID) mice, with which the cytokine expression of EBVaGC can be investigated without interference from the infiltrating lymphocytes. As a part of a high-density oligonucleotide array (GeneChip) analysis of EBVaGC, the interleukin-1β (IL-1β) gene was the only cytokine gene that showed markedly higher expression in the KT tumor cells than in two tumor strains of EBV-negative GC. The results were confirmed by Northern blotting, Western blotting, and enzyme-linked immunosorbent assay. Furthermore, we demonstrated a positive signal for IL-1β mRNA in the carcinoma cells of a surgically resected EBVaGC, but not in EBV-negative GC, by in situ hybridization. In vitro, IL-1β increased the cell growth of a GC cell line, TMK1. Thus, IL-1β may act as an autocrine growth factor in EBVaGC.
Cytokines may play a role in the development and maintenance of human neoplasms, which are associated with inflammation (1, 14), including viral infection (28, 31). Epstein-Barr virus (EBV) is a human oncogenic virus, and various EBV-associated malignancies such as Hodgkin's disease, pyothorax-associated lymphoma, and nasopharyngeal carcinoma (NPC) have also been demonstrated to produce cytokines (4, 13, 19, 21). However, cytokine production has not been fully studied for EBV-associated gastric carcinoma (EBVaGC), the most common malignancy among the various lethal malignancies associated with EBV (10).
EBV is closely related to the genesis of EBVaGC. EBV-encoded small RNA 1 (EBER-1) is present in nearly all of the carcinoma cells even in the intramucosal stage, and EBV in EBVaGC is monoclonal by Southern blot hybridization analysis with probes adjacent to the unique terminal repeat of EBV DNA (11). Furthermore, EBVaGC has unique morphological, genetic, and phenotypic features, compared to EBV-negative GC. EBVaGC is accompanied by dense infiltration of lymphocytes. The genetic pathway differs considerably from that of EBV-negative GC (7). Variant forms of CD44, a cell surface glycoprotein that acts as an adhesion molecule, are specifically expressed in EBVaGC (6).
In vitro cell culture and in vivo transplantation of neoplastic cells that retain the characteristics of the original tumor are indispensable tools for exploring the cell biology and molecular biology of a specific tumor. In particular, analyses of cytokine expression of neoplastic cells must be performed with pure tumor tissues to avoid the influences of the stromal cells and infiltrating inflammatory cells. We recently established a transplantable strain of human EBVaGC in severe combined immunodeficiency (SCID) mice (17). This strain, designated KT, retains the original EBV with the same latency gene expression, thereby enabling us to evaluate cytokine expression of EBVaGC. We now report that interleukin-1β (IL-1β) is the only cytokine highly expressed in KT tumor cells and that this selective up-regulation may play a critical role in this unique type of EBVaGC.
Transplanted strains in SCID or nude mice and cell lines.
The establishment of the EBVaGC strain (KT tumor) has been reported previously (17). Two transplantable strains of EBV-negative GC, one provided by the Tokyo Metropolitan Komagome Hospital (KN-91) and the other by the Jichi Medical School (JS-1), were similarly established in SCID mice. The original tumors were poorly differentiated (KT) and well-differentiated (KN-91 and JS-1) adenocarcinomas (Table 1). Five strains of EBV-negative GC (KR1 to -5), transplanted into nude mice, were kindly provided by Y. Maeda of the Tokyo Metropolitan Komagome Hospital. Seven cell lines derived from human GCs were also used for this investigation (Table 1). MKN-1, MKN-7, MKN-28, MKN-45, and MKN-74 were provided by Riken Cell Bank (Tsukuba, Japan), and NUGC-4 was provided by Health Science Research Resources Bank (Osaka, Japan). TMK1 was a gift from W. Yasui (Hiroshima University). The status of EBV infection in the transplanted strains was evaluated by EBER-1 in situ hybridization (ISH) using an oligonucleotide probe (11) and by nested PCR using the primers of the BamHI-W region, as reported previously (18).
TABLE 1.
Transplanted GC strains in SCID mice and GC cell lines
| Strain or cell line | Histological type |
|---|---|
| Strains | |
| EBVaGC; KT | Poorly differentiated adenocarcinoma |
| EBV-negative GC | |
| KN-91 | Well-differentiated adenocarcinoma |
| JS-1 | Well-differentiated adenocarcinoma |
| KR1 | Poorly differentiated adenocarcinoma |
| KR2 | Poorly differentiated adenocarcinoma |
| KR3 | Poorly differentiated adenocarcinoma |
| KR4 | Moderately differentiated adenocarcinoma |
| KR5 | Moderately differentiated adenocarcinoma |
| GC cell lines | |
| TMK1 | Poorly differentiated adenocarcinoma |
| MKN-1 | Adenosquamous cell carcinoma |
| MKN-7 | Well-differentiated adenocarcinoma |
| MKN-28 | Moderately differentiated adenocarcinoma |
| MKN-45 | Poorly differentiated adenocarcinoma |
| MKN-74 | Moderately differentiated adenocarcinoma |
| NUGC-4 | Poorly differentiated adenocarcinoma |
Total RNA was extracted from eight GC strains and seven GC cell lines by the acid guanidium thiocyanate-phenol-chloroform extraction method, as reported previously (5). mRNA was purified with an Oligotex dT-30 Super kit (Roche Diagnostics, Tokyo, Japan).
Comparative analysis of cytokine mRNA expression in the EBVaGC strain by a high-density oligonucleotide array analysis.
To clarify the cellular and molecular abnormalities in EBVaGC, we applied the high-density oligonucleotide array analysis, a rapid method to scan the differential expression all at once. KT, KN-91, and JS-1 cells were subjected to high-density oligonucleotide array analysis using a GeneChip HuGeneFL probe array set. All the procedures were performed exactly according to the instructions provided with the GeneChip sysytems (Affymetrix, Inc., Santa Clara, Calif.). Briefly, 2 μg of mRNA was reverse transcribed using an oligo(dT) primer linked to a T7 promoter. Double-stranded cDNA was synthesized and used for an in vitro transcription reaction in the presence of biotin-UTP and CTP. The amplified cRNA was fragmented by incubation at 94°C for 30 min in the presence of 40 mM Tris-acetate (pH 8.1), 100 mM potassium acetate, and 30 mM magnesium acetate. The fragmented cRNA (approximately 20 μg) was hybridized to the probe arrays overnight at 45°C in a hybidization oven with constant rotation. Scanning of probe arrays and data analysis were performed as described previously (12).
The Affymetrix HuGene FL probe array contains probe sets of 5,600 unique genes. Although nearly 30 genes were highly expressed in EBVaGC at levels comparable to those of IL-1β (data not shown), we focused on the cytokine gene expression in the present paper (Table 2). We took this approach not only because the data were too large to use for an investigation of the significance of each molecule individually, but also because cytokines are important for the development of virus-associated malignancies as well as gastrointestinal tumors (15, 16). To allow reliable data comparison across multiple probe arrays, the expression levels of all samples listed have been globally normalized for all probe sets and scaled to a target intensity of 100, using GeneChip Analysis Suite 3.1. The genes falling into this category are identified in Table 2 with a “greater than” symbol (>) preceding the fold change value.
TABLE 2.
Cytokine expression of human GC with or without EBV infection in SCID mouse by GeneChip assay
| Cytokine gene description | GenBank accession no. | Strain
|
||||
|---|---|---|---|---|---|---|
| KT | KN-91
|
JS-1
|
||||
| Expression levela | Expression level | Fold increase | Expression level | Fold increase | ||
| proIL-1β | X04500 | 411 | 12 | >11.6 | 22 | >11.3 |
| IL-8 | M28130 | 56 | 2 | >3.1 | −7 | >3.1 |
| IL-4 | M13982 | −44 | −131 | >2.3 | −64 | >1.2 |
| IL-2 | X00695 | 11 | −12 | >1.8 | 0 | >1.4 |
| TNFα | X02910 | 5 | −16 | >1.7 | 1 | >1.1 |
| IL-1α | M28983 | 51 | 22 | >1.6 | 12 | >1.9 |
| IL-3 | M20167 | 29 | 33 | 1.6 | 7 | >1.7 |
| IL 15 | U14407 | 15 | −5 | >1.6 | 5 | >1.3 |
| IL-6 | Y00081 | −1 | −18 | >1.5 | 16 | <−1.5 |
| TGFβ | M60314 | 12 | 2 | >1.3 | 7 | >1.2 |
| IFNγ | J00219 | 3 | −7 | >1.3 | 0 | >1.3 |
| TGFβ | M60315 | −8 | −19 | <−1.2 | −17 | <−1.4 |
| IL-7 | J04156 | −21 | −1 | <−1.4 | −7 | <−1.5 |
| IL-10 | U16720 | 9 | 36 | <−1.4 | 30 | <−1.3 |
| IL-17 | U32659 | −14 | 4 | <−1.6 | 17 | <−1.7 |
| IL-13 precursor | U31120 | 32 | 59 | −1.9 | 26 | >1.2 |
| Putative IL-16 precursor | M90391 | 4 | 25 | <−2.2 | −7 | <−1.2 |
| ICE | M87507 | −10 | −4 | <−1.2 | 2 | <−1.4 |
| IL-1 receptor type I | M27492 | 75 | 7 | >3.3 | 12 | >1.9 |
| IL-1 receptor type II | X59770 | 93 | 344 | −6.7 | 61 | 1.5 |
Expression level indicates average difference by the GeneChip assay.
Among the cytokine related genes, only the precursor molecule of IL-1β (proIL-1β) showed a more than fivefold increase in KT intensity compared to that of KN-91 or JS-1. The increase of proIL-1β was more than 11-fold. The expressions of the IL-8 and IL-1α genes were observed with the KT tumor cells and showed slightly higher values than in the other EBV-negative tumor cells. Otherwise, the expression levels of the IL-1β-converting enzyme (ICE) and other cytokine-related genes were not distinctly different between the KT tumor cells and the EBV-negative tumor cells.
IL-1β mRNA expression in the EBVaGC by Northern blot analysis.
To confirm the results obtained from the chip hybridization, Northern blot analyses were performed using the probes of the cytokines that showed more than a fivefold increase in expression in the KT tumor cells. Total RNAs of the GC cell lines and GC strains in SCID or nude mice were subjected to Northern blot hybridization using the probes of a human IL-1β (hIL-1β) cDNA (32) (1,050-bp PstI fragment in a pGEM3Z-based plasmid, provided by I. Nagaoka, Juntendo University) and rat glyceraldehyde 3-phosphate dehydrogenase cDNA (Clontech, Palo Alto, Calif.). Each probe was radiolabeled using the random primer labeling system (Rediprime II; Amersham Pharmacia Biotech, Buckinghamshire, United Kingdom) with 32P-labeled deoxyribonucleotide triphosphates. After being washed at 65°C, the hybridized membranes were autoradiographed.
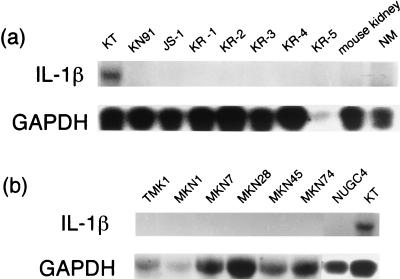
Northern blot analysis showed an intense band corresponding to IL-1β mRNA in the KT tumor cells. The positive control was human peripheral blood monocytes (PBM) which were stimulated with bacterial lipopolysaccharide (LPS), and the specificity was repeatedly confirmed by another experiment (data not shown). No bands were observed in any of the EBV-negative GC strains or cell lines. No IL-1β expression was shown in the normal gastric mucosa. (Fig. 1)
FIG. 1.
Northern blot analysis of the GCs associated or unassociated with EBV. Total RNA extracted from the GC strains in SCID or nude mice (a) and from GC cell lines (b) was subjected to Northern blot analysis using IL-1β and glyceraldehyde 3-phosphate dehydrogenase cDNA probes. Expression of IL-1β was observed only in the KT tumor, a transplantable strain of EBVaGC; the level of expression was high. On the other hand, there was no expression in other strains or in seven GC cell lines, all of which are EBV negative. Normal gastric mucosa does not show any messaging of IL-1β.
IL-1β protein expression in the EBVaGC strain by ELISA and Western blot analysis.
Enzyme-linked immunosorbent assay (ELISA) of IL-1β, an assay that shows no significant cross-reaction with proIL-1β, was performed with the homogenates of KT, KN-91, and JS-1 using an hIL-1β ELISA kit (BioSource Europe, Nivelles, Belgium). Using the homogenate of 500 mg of the tumor tissue in 3 ml of saline, a remarkable elevation of IL-1β was observed (68 pg/ml) with the KT tumor cells, whereas there was no detectable amount in the EBV-negative GC strains (<10 pg/ml).
Immunoprecipitation and Western blot analysis were then carried out against the lysates of KT, KN-91, and JS-1. The lysates were immunoprecipitated with 5 μl of rabbit polyclonal anti-hIL-1β antibody (Genzyme, Cambridge, Mass.). After the addition of 50% (vol/vol) suspension of protein A-Sepharose 4B (Pharmacia, Piscataway, N.J.), the antigen-antibody mixtures were further incubated for 1 h at 4°C, washed three times with lysis buffer, and then dissolved in a 2× concentration of sodium dodecyl sulfate-polyacrylamide gel electrophoresis sample buffer. The mixtures were boiled for 10 min and subjected to sodium dodecyl sulfate-15% polyacrylamide gel electrophoresis. The gel was electrophoretically transferred onto a polyvinylidene difluoride membrane (Millipore, Bedford, Mass.). After nonspecific binding was blocked with 1% (wt/vol) skim milk in Tris-buffered saline, the membrane was serially incubated with goat anti-hIL-1β antibody (2 μg/ml) (R&D Systems, Minneapolis, Minn.), followed by anti-goat immunoglobulin G conjugated with horseradish peroxidase (DAKO A/S, Glostrup, Denmark) as a secondary antibody. The reaction was visualized with the ECL detection system (Amersham Pharmachia Biotech, Buckinghamshire, United Kingdom). Positive controls included the recombinant hIL-1β (DAKO A/S) and the cell lysate and the supernatant of LPS-stimulated human PBM.
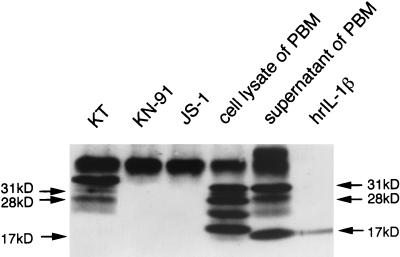
Western blot analysis using IL-1β antibodies showed the band corresponding to proIL-1β at 31 kDa, some extra bands at a lower molecular weight, including 28 kDa (3, 33), and mature secreted IL-1β at 17 kDa in the supernatant of PBM (Fig. 2). In the KT tumor cells, there were 31- and 28-kDa forms of proIL-1β as seen in the cell lysate of PBM, but the major form of proIL-1β showed a higher molecular weight. These bands were not observed in the EBV-negative strains, KN-91 and JS-1. The human proIL-1β gene is rapidly transcribed, and the IL-1β precursor protein is converted to the mature form by ICE at the cell membrane. The predominant form in the cytosol is proIL-1β (8), and the mature form of 17-kDa IL-1β was only faintly observed in the cell lysate of PBM. These facts may explain the absence of the mature form of IL-1β in KT tumor cells in the present study. Furthermore, the difference between intermediate forms of IL-1β of KT and PBM suggests different processing of IL-1β in both cell types.
FIG. 2.
Western blot analysis of the GCs associated or unassociated with EBV. The lysate of the GC strains in SCID mice were subjected to Western blot analysis using anti-hIL-1β antibody. There are three forms of proIL-1β protein in the KT tumor cells, including 31- and 28-kDa proteins, which are similarly observed in the cell lysate and the supernatant of LPS-stimulated human PBM. There are additional extra bands at a lower molecular weight and a mature form of IL-1β at 17 kDa in the cell lysate and the supernatant of LPS-stimulated PBM. There is no band in the EBV-negative GC strains (KN-91 and JS-1), except the bands at the higher molecular weight, which correspond to the immunoglobulin heavy chain.
IL-1β expression in EBVaGC in vivo by ISH.
To evaluate the expression of IL-1β in EBVaGC in vivo, ISH was performed using tumor tissues of surgical specimens. Biotin-labeled antisense and sense single-strand RNA probes specific for IL-1β were prepared with minor modifications of the protocol of Komminoth (23, 24). The plasmid with a 1,050-bp PstI fragment of IL-1β cDNA was linearized by either HindIII for antisense or EcoRI for sense riboprobe synthesis. The linearized plasmid was purified by phenol-chloroform extraction, followed by ethanol precipitation. Then, biotin-labeled RNA probe was generated in either the antisense or sense direction by in vitro transcription with the T7 and SP6 RNA polymerases, respectively (Roche Diagnostics GmbH, Mannheim, Germany).
A total of 16 specimens of GC tissue were obtained from 16 patients at Jichi Medical School Hospital from 1997 to 2000. The EBV infection status of these specimens was examined by EBER-1 ISH. The 16 specimens included 6 surgical specimens of EBVaGC and 10 specimens of EBV-negative GC, almost matched for patient age, patient gender, tumor size, and histological type, as shown in Table 3. For the ISH, 3-μm formaldehyde-fixed and paraffin-embedded sections of KT, KN-91, and human GCs with or without EBV infection were placed on silane-coated slides. After the removal of paraffin, the sections were permeabilized with 200 μg of proteinase K/ml for 15 min at room temperature and rinsed in Tris-buffered saline; the endogenous peroxidase was blocked by using H2O2 for 2 h, and the samples were treated in 100% ethanol for 10 min. Next, 30 to 50 μl of hybridization buffer containing 0.5-ng/ml antisense or sense probe and RNase inhibitor was added to the samples, and the sections were incubated at 37°C overnight. The immunohistological detection was performed using an amplification system based on peroxidase-catalyzed deposition of biotinylated tyramine, with the GenPoint system (DAKO, Carpinteria, Calif.) according to the manufacturer's protocol. The result of ISH was evaluated by two observers who were unaware of the diagnosis, and the positive signal of the cytoplasms was scored in each section as follows: grade 0, lack of positive signal; grade 1, positive signal on fewer than 25% of epithelial cells; grade 2, positive signal on 25 to 50% of epithelial cells; grade 3, positive signal on 50 to 75% of epithelial cells; and grade 4, staining of more than 75% of epithelial cells (Table 3).
TABLE 3.
Characteristics of GCs by ISHa
| Malignancy | Strain and case no. | Patient age | Patient genderb | Locationc | Tumor size (cm) | Depthd | Histological typee | Grading of ISHf |
|---|---|---|---|---|---|---|---|---|
| EBVaGC | KT | 4 | ||||||
| J064 | 62 | M | M | 5 | mp | tub1 | 2 | |
| J080 | 60 | M | M | 10 | se | por1 | 3 | |
| J085 | 74 | M | M | 2 | sm | tub2 | 1 | |
| J197 | 39 | M | A | 4 | mp | por1 | 2 | |
| J230 | 52 | F | A | 4 | ss | por1 | 2 | |
| J260 | 67 | F | A | 8 | se | tub2 | 3 | |
| EBV-negative GC | KN-91 | 0 | ||||||
| J028 | 70 | M | C | 5 | mp | tub1 | 0 | |
| J042 | 71 | M | A | 4 | mp | tub1 | 0 | |
| J072 | 56 | M | M | 8 | se | tub2 | 0 | |
| J076 | 70 | F | M | 1 | sm | tub2 | 0 | |
| J110 | 62 | M | A | 7.5 | se | por1 | 0 | |
| J113 | 50 | F | M | 2.5 | sm | tub2 | 0 | |
| J172 | 62 | M | C | 4 | ss | por1 | 0 | |
| J217 | 75 | M | C | 6.5 | se | tub1 | 0 | |
| J263 | 52 | F | M | 4 | se | por1 | 0 | |
| J266 | 52 | F | A | 8 | se | tub2 | 0 |
Location, depth, and histological type were based on the General Rules of the Japanese Research Society for Gastric Cancer.
M, male; F, female.
C, cardia; M, body; A, antrum.
sm, submucosa; mp, muscularis propria; ss, subserosa; se, serosa exposed; si, serosa infiltrating.
tubl1, well-differentiated adenocarcinoma; tub2, moderately differentiated adenocarcinoma; por1, poorly differentiated adenocarcinoma, solid type.
The grading of ISH-positive signals is explained in the text.
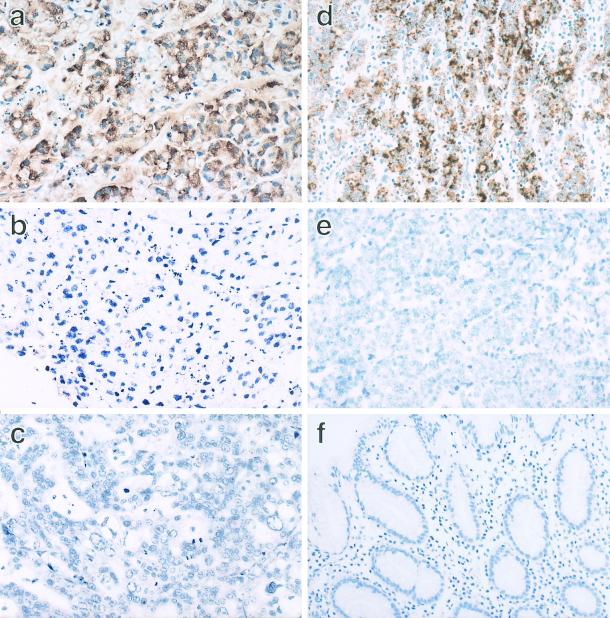
ISH of IL-1β clearly demonstrated a positive signal for IL-1β mRNA in the tumor cells of the KT strain as grade 4 (Fig. 3a) and all six cases of EBVaGC (grade 1 to 3) (Fig. 3d). No positive signal was shown in the EBV-negative strains or the EBV-negative GC cases (Fig. 3c and e). These results indicate that EBVaGC specifically produces IL-1β among the various cytokines. Some reports have examined the relationship between EBV infection and cytokine expression. Matsushima et al. showed the production of IL-1 and the presence of a specific IL-1 receptor in human EBV-transformed B-lymphocytes and the capacity of IL-1 to enhance B-lymphocyte transformation (26, 27). Busson et al. (4) and later Huang et al. (13) demonstrated the presence of transcripts of IL-1α and both subtypes in NPC cells.
FIG. 3.
IL-1β ISH of the GCs associated or unassociated with EBV. IL-1β ISH was applied to the formalin-fixed and paraffin-embedded tissue sections of the tumor strains and the surgically resected carcinomas. With an IL-1β antisense probe, a positive signal of IL-1β mRNA was observed in the cytoplasm of the KT tumor cells (a), whereas no signal was observed in KN-91 (c). There was no signal in the KT tumor cells when the ISH probe was replaced with the sense probe (b). Similarly, a positive signal was specifically observed in the cytoplasm of the surgically resected carcinoma, which is associated with EBV (d). On the other hand, there was no signal in the surgically resected carcinoma, which was EBV negative (e). The normal gastric mucosa was negative for IL-1β (f). Magnification, ×66.
Latent membrane protein 1 (LMP1), one of the latent membrane proteins of EBV, has been known to up-regulate the expression of cytokine genes such as IL-6 and -10 in vitro in lymphoma cells, and both are frequently expressed in Hodgkin and Reed-Sternberg cells. However, in NPC cells, no correlation was observed between the expression of LMP1 and IL-6 or IL-10 (2). Since EBVaGC exhibits latency I, one of the representative forms of latency of EBV (EBNA2 negative, LMP1 negative), up-regulation of IL-1β may be due to EBER, which was recently demonstrated to up-regulate IL-10 expression in a Burkitt cell line, Akata (20). Further studies are necessary to clarify the mechanism of the interaction between EBV and IL-1β expression in EBVaGC.
Significance of IL-1β production in EBVaGC.
Cellular and viral IL-10, which Hodgkin and Reed-Sternberg cells produce, has been implicated to enable these cells to escape the immune response (2, 30). EBVaGC adopts other strategies, for example, the production of IL-1β to induce the migration of large numbers of nonspecific lymphocytes. Such a dense infiltration may physically prevent the direct contact of EBV-specific cytotoxic T cells with the tumor cells.
IL-1 modulates the cell growth of the renal cell carcinoma cells in vitro (22). Some GC cell lines express IL-1α, a cytokine which acts as an autocrine growth stimulator (15). Since IL-1α and IL-1β share many biological effects (35), we can speculate that the IL-1β produced by EBV-infected epithelial cells contributes to tumor growth. The expression of type I IL-1β receptor was also increased in KT cells, but that of type II, which cannot mediate any biological signal, was similar in the strains with or without association of EBV (Table 2). Thus, the possibility that IL-1β serves as an autocrine growth stimulator for GC cells was further evaluated, as follows.
Effect of IL-1β on cell growth of GC cell lines by cell growth assay.
The growth of three GC cell lines, TMK-1, MKN-1, and MKN-7, was compared with and without exogenous IL-1β. These cells did not produce IL-1β as shown in the Northern blot (Fig. 1) but may be useful to test the potential growth effect of IL-1β. TMK-1 has been shown to respond to IL-1α and to possess a type I IL-1 receptor (15). The cells were seeded in six-well dishes (Costar, Cambridge, Mass.) at 104 cells per well and cultured in RPMI 1640 containing 0.5% fetal bovine serum in the presence or absence of IL-1β (10 U/ml). Recombinant hIL-1β (DAKO A/S) was added 1 day after seeding. The number of cells was counted using a Neubauer-type counting chamber on the seventh day after seeding.
There was no distinct effect on MKN-1 or MKN-7 cells, but a significant increase in the number of TMK-1 cells was observed with 10 U of IL-1β/ml on the seventh day (P < 0.01) (Table 4). The different effect of exogenous IL-1β on the EBV negative gastric tumor cells might be due to the difference in histological types of the carcinoma or expresson level of the type I IL-1 receptor. Nevertheless, IL-1β augmented the cell growth of a GC cell line, TMK-1, suggesting that IL-1β is an autocrine growth factor in EBVaGC.
TABLE 4.
Effect of IL-1β on cell growth of GC cell linesa
| Cell line | IL-1β (U/ml) | No. of cells (104/well) |
|---|---|---|
| TMK-1b | 10 | 26.90 ± 1.45 |
| 0 | 13.60 ± 1.61 | |
| MKN-1 | 10 | 6.73 ± 0.37 |
| 0 | 7.40 ± 0.36 | |
| MKN-7 | 10 | 4.37 ± 0.18 |
| 0 | 5.26 ± 0.20 |
The cells were cultured with or without IL-1β antigen for 6 days, and the cell numbers were counted. The data represent the average ± standard error of three independent experiments.
The culture of TMK-1 was examined in three separate experiments. The values were significantly different from that of the control (P < 0.01 after 7 days by the Student t test).
As for the underlying mechanism of cell growth in EBVaGC, IL-1β may act as an inhibitor of apoptosis, just as it has been shown to act in certain cell lines (9) and in the gut epithelial cells of IL-1 receptor knockout mice (34). EBVaGC in vivo showed a low frequency of apoptosis when examined by the terminal deoxynucleotidyl-mediated dUTP-nick end labeling method (25, 29) and by immunohistochemistry of single-strand DNA (J. Shibahara et al., unpublished data).
In conclusion, we have shown that EBVaGC specifically produces IL-1β among the cytokine-related genes. The selective production of IL-1β may be closely associated with the biological behavior of this particular type of GC associated with EBV.
Acknowledgments
We thank S. Iwasaka, M. Takanashi, M. Sunami, R. Ikeno, and Y. Hayashi for helping with the preparation of the manuscript. We also thank I. Nagaoka (Juntendo University, Tokyo, Japan) for his generous gift of the IL-1β cDNA probe and T. Kasahara (Kyoritsu College of Pharmacy, Tokyo, Japan) for his advice on the method and the discussion.
This work was supported by a Grant-in Aid for Scientific Research on Priority Areas (no. 09253103) from the Ministry of Education, Science, Sports, and Culture of Japan.
REFERENCES
- 1.Balkwill, F., and A. Mantovani. 2001. Inflammation and cancer: back to Virchow? Lancet 357:539-545. [DOI] [PubMed] [Google Scholar]
- 2.Beck, A., D. Pazolt, G. G. Grabenbauer, J. M. Nicholls, H. Herbst, L. S. Young, and G. Niedobitek. 2001. Expression of cytokine and chemokine genes in Epstein-Barr virus-associated nasopharyngeal carcinoma: comparison with Hodgkin's disease. J. Pathol. 194:145-151. [DOI] [PubMed] [Google Scholar]
- 3.Bomford, R., E. Abdulla, C. Hughes-Jenkins, D. Simpkin, and J. Schmidt. 1987. Antibodies to interleukin-1 raised with synthetic peptides: identification of external sites and analysis of interleukin-1 synthesis in stimulated human peripheral blood monocytes. Immunology 62:543-549. [PMC free article] [PubMed] [Google Scholar]
- 4.Busson. P., K. Braham, G. Ganem, F. Thomas, D. Grausz, M. Lipinski, H. Wakasugi, and T. Tursz. 1987. Epstein-Barr virus-containing epithelial cells from nasopharyngeal carcinoma produce interleukin 1α. Proc. Natl. Acad. Sci. USA 84:6262-6266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. [DOI] [PubMed] [Google Scholar]
- 6.Chong, J.-M., M. Fukayama, Y. Hayashi, N. Funata, T. Takizawa, M. Koike, M. Muraoka, R. Kikuchi-Yanoshita, M. Miyaki, and S. Mizuno. 1997. Expression of CD44 variants in gastric carcinoma with or without Epstein-Barr virus. Int. J. Cancer 74:450-454. [DOI] [PubMed] [Google Scholar]
- 7.Chong, J.-M., M. Fukayama, Y. Hayashi, T. Takizawa, M. Koike, M. Konishi, R. Kikuchi-Yanoshita, and M. Miyaki. 1994. Microsatellite instability in the progression of gastric carcinoma. Cancer Res. 54:4595-4597. [PubMed] [Google Scholar]
- 8.Dinarello, C. A. 1996. Biologic basis for interleukin-1 in disease. Blood 87:2095-2147. [PubMed] [Google Scholar]
- 9.Friedlander, R. M., V. Gagliardini, R. Rotello, and J. Yuan. 1996. Functional role of interleukin 1β (IL-1β) in IL-1β-converting enzyme-mediated apoptosis. J. Exp. Med. 184:717-724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fukayama, M., J.-M. Chong, and Y. Kaizaki. 1998. Epstein-Barr virus and gastric carcinoma. Gastric Cancer 1:104-114. [DOI] [PubMed] [Google Scholar]
- 11.Fukayama, M., Y. Hayashi, Y. Iwasaki, J.-M. Chong, T. Ooba, T. Takizawa, M. Koike, S. Mizutani, M. Miyaki, and K. Hirai. 1994. Epstein-Barr virus-associated gastric carcinoma and Epstein-Barr virus infection of the stomach. Lab. Investig. 71:73-81. [PubMed] [Google Scholar]
- 12.Hippo, Y., M. Yashiro, M. Ishii, H. Taniguchi, S. Tsutsumi, K. Hirakawa, T. Kodama, and H. Aburatani. 2001. Differential gene expression profile of scirrhous gastric cancer cells with high metastatic potential to peritoneum or lymph nodes. Cancer Res. 61:889-895. [PubMed] [Google Scholar]
- 13.Huang, Y.-T., T.-S. Sheen, C.-L. Chen, J. Lu, Y. Chang, J.-Y. Chen, and C.-H. Tsai. 1999. Profile of cytokine expression in nasopharyngeal carcinomas: a distinct expression of interleukin 1 in tumor and CD4+ T cells. Cancer Res. 59:1599-1605. [PubMed] [Google Scholar]
- 14.Hudson, J. D., M. A. Shoaibi, T. Maestro, A. Carnero, G. J. Hannon, and D. H. Beach. 1999. A proinflammatory cytokine inhibits p53 tumor suppressor activity. J. Exp. Med. 190:1375-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ito, R., Y. Kitadai, E. Kyo, H. Yokozaki, W. Yasui, U. Yamashita, H. Nikai, and E. Tahara. 1993. Interleukin 1α acts as an autocrine growth stimulator for human gastric carcinoma cells. Cancer Res. 53:4102-4106. [PubMed] [Google Scholar]
- 16.Ito, R., W. Yasui, H. Kuniyasu, H. Yokozaki, and E. Tahara. 1997. Expression of interleukin-6 and its effect on the cell growth of gastric carcinoma cell lines. Jpn. J. Cancer Res. 88:953-958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Iwasaki, Y., J.-M. Chong, Y. Hayashi, R. Ikeno, K. Arai, M. Kitamura, M. Koike, K. Hirai, M. Fukayama. 1998. Establishment and characterization of a human Epstein-Barr virus-associated gastric carcinoma in SCID mice. J. Virol. 72:8321-8326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaizaki, Y., S. Sakurai, J.-M. Chong, and M. Fukayama. 1999. Atrophic gastritis, Epstein-Barr virus infection, and Epstein-Barr virus-assoociated gastric carcinoma. Gastric Cancer 2:101-108. [DOI] [PubMed] [Google Scholar]
- 19.Kanno, H., N. Naka, Y. Yasunaga, K. Iuchi, S. Yamauchi, M. Hashimoto, and K. Aozasa. 1997. Production of the immunosuppressive cytokine interleukin-10 by Epstein-Barr-virus-expressing pyothorax-associated lymphoma. Am. J. Pathol. 150:349-357. [PMC free article] [PubMed] [Google Scholar]
- 20.Kitagawa, N., M. Goto, K. Kurozumi, S. Maruo, M. Fukayama, T. Naoe, M. Yasukawa, K. Hino, T. Suzuki, S. Todo, and K. Takada. 2000. Epstein-Barr virus-encoded poly(A)− RNA supports Burkitt's lymphoma growth through interleukin-10 induction. EMBO J. 19:6742-6750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Klein, S. C., D. Kube, H. Abt, V. Diehl, and H. Tesch. 1996. Promotion of IL8, IL10, TNF and TNF production by EBV infection. Leuk. Res. 20:633-636. [DOI] [PubMed] [Google Scholar]
- 22.Koch, I., H. Depenbrock, S. Danhauser-Riedl, J. W. Rastetter, and A.-R. Hanauske. 1995. Interleukin 1 modulates growth of human renal carcinoma cells in vitro. Br. J. Cancer 71:794-800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Komminoth, P. 1992. Digoxigenin as an alternative probe labeling for in-situ hybridization. Diagn. Mol. Pathol. 1:142-150. [PubMed] [Google Scholar]
- 24.Komminoth, P., F. B. Merk, I. Leav, H. J. Wolfe, and J. Roth. 1992. Comparison of 35S- and digoxigenen-labeled RNA and oligonucleotide probes for in situ hybridization. Expression of mRNA of the seminal vesicle secretion protein II and androgen receptor genes in the rat prostate. Histochemistry 98:217-228. [DOI] [PubMed] [Google Scholar]
- 25.Kume, T., K. Oshima, Y. Yamashita, T. Shirakusa, and M. Kikuchi. 1999. Relationship between Fas-ligand expression on carcinoma cell and cytotoxic T-lymphocyte response in lymphoepithelioma-like cancer of the stomach. Int. J. Cancer 84:339-343. [DOI] [PubMed] [Google Scholar]
- 26.Matsushima, K., T. Akahoshi, M. Yamada, Y. Furutani, and J. J. Oppenheim. 1986. Properties of a specific interleukin 1 (IL-1) receptor on human Epstein-Barr virus-transformed B lymphocytes: identity of the receptor for IL 1-α and IL 1-β. J. Immunol. 136:4496-4502. [PubMed] [Google Scholar]
- 27.Matsushima, K., Y. D. Kuang, G. Tosato, S. J. Hopkins, and J. J. Oppenheim. 1985. B-cell-derived interleukin 1 (IL-1)-like factor. Cell. Immunol. 94:406-417. [DOI] [PubMed] [Google Scholar]
- 28.Nakamoto, Y., L. G. Guidotti, C. V. Kuhlen, P. Fowler, and F. V. Chisari. 1998. Immune pathogenesis of hepatocellular carcinoma. J. Exp. Med. 188:341-350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ohfuji, S., M. Osaki, S. Tsujitani, M. Ikeguchi, T. Sairenji, and H. Ito. 1996. Low frequency of apoptosis in Epstein-Barr virus-associated gastric carcinoma with lymphoid stroma. Int. J. Cancer 68:710-715. [DOI] [PubMed] [Google Scholar]
- 30.Ohshima, K., J. Suzumiya, M. Akamatu, M. Takeshita, and M. Kikuchi. 1995. Hunan and viral interleukin-10 in Hodgkin's disease, and its influence on CD4+ and CD8+ T lymphocytes. Int. J. Cancer 62:5-10. [DOI] [PubMed] [Google Scholar]
- 31.Robertson, F. M., M. S. Ross, K. L. Tober, B. W. Long, and T. M. Oberyszym. 1996. Inhibition of pro-inflammatory cytokine gene expression and papilloma growth during murine multistage carcinogenesis by pentoxifylline. Carcinogenesis 17:1719-1728. [DOI] [PubMed] [Google Scholar]
- 32.Tocci, M. J., N. I. Hutchinson, P. M. Cameron, K. E. Kirk, D. J. Norman, J. Chin, E. A. Rupp, G. A. Limjuco, V. M. Bonilla-Argudo, and J. A. Schmidt. 1987. Expression in Escherichia coli of fully active recombinant human IL 1β: comparison with native human IL 1β. J. Immunol. 138:1109-1114. [PubMed] [Google Scholar]
- 33.Wewers, M. D., A. V. Winnard, and H. A. Dare. 1999. Endotoxin-stimulated monocytes release multiple forms of IL 1β, including a proIL 1β form whose detection is affected by export. J. Immunol. 162:4858-4863. [PubMed] [Google Scholar]
- 34.Wolf, S. E., M. A. Debroy, H. Ikeda, M. Feschke, S. Matin, S. Rajaraman, T. C. Ko, E. W. Englander, J. G. Norman, and J. C. Thompson. 2000. Increased small bowel epithelial turnover in interleukin-1 receptor knockout mice. Ann. Surg. 232:42-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wood, D. D., E. K. Bayne, M. B. Goldring, M. Gowen, D. Hamerman, J. L. Fumes, E. J. Ihrie, P. E. Lipsky, and M.-J. Staruch. 1985. The four biochemically distinct species of human interleukin 1 all exhibit similar biological activities. J. Immunol. 134:895-903. [PubMed] [Google Scholar]