Abstract
Nef enhances the serine phosphorylation of the human immunodeficiency virus type 1 matrix (MA) protein, which suggests that MA may be a functional target of Nef. Using mutants that remain infectious despite the absence of most or all of MA, we show in the present study that the ability of Nef to enhance virus infectivity is not compromised even if MA is entirely replaced by a heterologous lipid anchor.
Nef is an accessory gene product of primate lentiviruses that has been shown to be necessary for efficient virus replication and pathogenesis in vivo (12, 23, 24). Although Nef has only a modest effect on the ability of human immunodeficiency virus type 1 (HIV-1) to spread in T-cell lines, Nef significantly enhances virus replication in primary target cells that are exposed to HIV-1 prior to stimulation (30, 46). In cell culture, Nef has been shown to downregulate the CD4 receptor and major histocompatibility class I molecules from the cell surface (2, 11, 19, 21, 25, 38, 44), to modulate the cellular activation state (4, 5, 14, 16, 42, 48, 50), and to enhance the infectivity of progeny virions (3, 9, 10, 20, 31, 39, 43, 54).
Nef-deficient HIV-1 can be complemented by providing Nef in trans in producer cells but not by expressing Nef in the target cells, indicating that Nef modifies a virion component or alters the molecular makeup of the virion (3, 31). It is unlikely that the viral envelope (Env) glycoproteins are a direct functional target of Nef, because an enhancement of virion infectivity is observed even after pseudotyping of Env-deficient HIV-1 virions with amphotropic murine leukemia virus Env (3, 27, 31). In contrast, Nef has no effect on the infectivity of virions pseudotyped with the glycoprotein of vesicular stomatitis virus, which alters the route of entry (1, 7, 27). Researchers have shown that small amounts of Nef gain access into progeny virions, which raises the possibility that Nef acts as a component of the virion (6, 36, 52). Virion-associated HIV-1 Nef is cleaved by the viral protease, but this processing event does not appear to be necessary for the ability of Nef to augment virion infectivity (8, 29, 35, 51). Nef associates with cellular protein kinases and enhances the serine phosphorylation of the viral matrix (MA) protein (39, 40, 47), but whether this modification is functionally relevant remains unknown.
A recent study shows that Nef can function as an entry factor by enhancing the delivery of viral particles into the cytosol (41). The virus used in that study was produced in 293T cells, demonstrating that the enhancement of viral entry by Nef does not require the presence of CD4 during virus production. Based on these observations, it was proposed that Nef indirectly affects the function of Env by modifying MA (41). It appeared to us that this model could be rigorously tested, because MA is not absolutely required for the completion of the HIV-1 replication cycle (37, 49). Although certain mutations in MA substantially inhibit virus production (15, 18), for example by redirecting assembly to intracellular membranes, we found that large deletions often actually enhance particle production over wild-type (WT) levels (37). The integrity of MA is critical for the incorporation of HIV-1 Env glycoprotein spikes into nascent viral particles (13, 55), but Env incorporation can be completely restored if the long cytoplasmic tail of the HIV-1 transmembrane glycoprotein is removed (17, 28). Indeed, in the absence of the Env cytoplasmic tail, the entire HIV-1 MA domain becomes dispensable for Env incorporation in transiently transfected cells (37). We have also shown that after pseudotyping with C-terminally truncated HIV-1 Env, even a large deletion which removes the globular core of MA has only a modest effect on HIV-1 infectivity (37).
To determine whether the presence of MA is necessary for the CD4-independent effect of Nef on HIV-1 infectivity, we made use of a previously described set of mutant HIV-1 proviruses that lack most or all of MA but are nevertheless able to replicate efficiently in MT4 T lymphoid cells (37). The Δ8-87 mutant lacks most of the globular core of MA, which is formed by 4 α-helices and a C-terminal helix that projects away from the core domain (22). This globular domain contains a basic surface patch thought to interact with acidic phospholipids and represents the portion of MA that is structurally conserved among distantly related retroviruses. The Δ8-126 mutant additionally lacks the relatively variable region that connects the single globular domain of MA with the capsid (CA) in the context of the Gag precursor. The only MA sequences retained in the Δ8-126 mutant are those of six N-terminal residues which provide an attachment site for myristic acid after removal of the initiating methionine, as well as six C-terminal residues to preserve the proteolytic cleavage site at the MA-CA boundary. Lastly, the ΔMA/R3 mutant lacks all MA residues and instead harbors a 15-amino-acid peptide that provides a heterologous myristyl anchor for membrane attachment and a processing site for HIV-1 protease. The ΔMA/R3 mutant also harbors compensatory mutations in the CA and nucleocapsid domains that allow efficient virus replication in MT4 cells (37).
The Δ8-87, Δ8-126, and ΔMA/R3 proviruses encode a C-terminally truncated Env protein, which permits multiple rounds of virus transmission to occur in certain cell types (37). To obtain viral genomes that are dependent on complementation by Env expressed in trans, the mutant gag genes were transferred into HXB/Env−/Nef+ and HXB/Env−/Nef− in place of the wild-type gag sequence. The Env-deficient HXB/Env−/Nef+ provirus is a variant of HXB/Nef+ (6) that has the env initiation codon replaced by ACG and also harbors a frameshift mutation at a KpnI site (nucleotide 6346) in the 5′ portion of the env gene. HXB/Env−/Nef+ harbors the intact nef gene of HIV-1LAI, whereas HXB/Env−/Nef− carries only the first 35 residues of Nef because of a 4-bp insertion at a unique XhoI site (nucleotide 8900) (6).
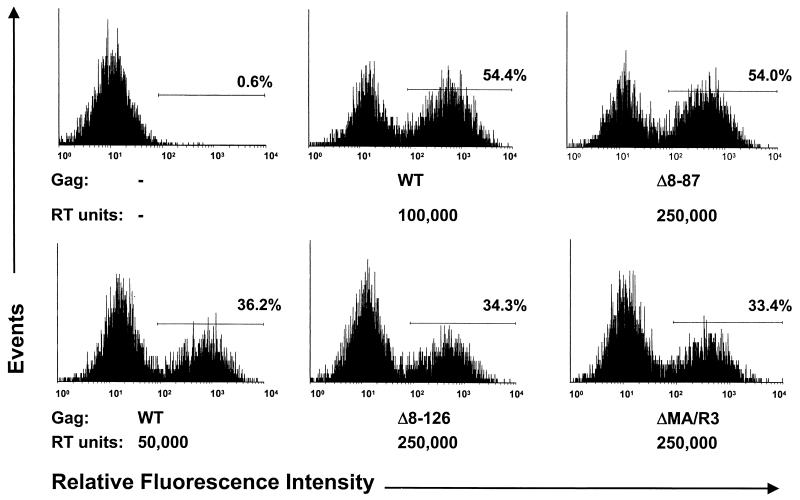
We previously showed that the Δ8-87 deletion has little effect on HIV-1 infectivity in a variety of cell lines, provided that viral particles are produced in the presence of C-terminally truncated HIV-1 Env (37). To compare the effects on virus infectivity of the MA deletions introduced into HXB/Env−/Nef+, the parental and mutant proviruses were transfected into 293T cells together with pEnvHXBΔCT, which expresses an HIV-1 Env precursor that lacks the 144 C-terminal amino acids of the cytoplasmic domain (37). To obtain viral stocks, supernatants were collected 2 days posttransfection, clarified by low-speed centrifugation, and passaged through 0.45-μm-pore-size filters. Equivalent amounts of 32P-reverse transcriptase (RT) activity, measured as described previously (53), were then used to inoculate 7 × 105 GHOST-CXCR4 cells in 80-cm2 tissue culture flasks. To facilitate a comparison of relative infectivities, parallel cultures were inoculated with various dilutions of the parental virus stock. GHOST-CXCR4 cells are human osteosarcoma cells that are stably transfected with human CD4 and CXCR4 and that express green fluorescent protein (GFP) under the control of the HIV-2 long terminal repeat (32). In this setting, infectious HIV-1 particles were expected to transduce the tat gene and thus to induce the synthesis of GFP in the target cells. Quantitation of GFP-positive cells by flow cytometry at 3 days postinfection showed that about 100,000 RT units of the parental virus stock was required to obtain the same number of infected cells as with 250,000 RT units of the Δ8-87 mutant (Fig. 1). In contrast, 50,000 RT units of the parental virus stock was sufficient to infect a percentage of cells similar to that infected with 250,000 RT units of the Δ8-126 and ΔMA/R3 mutants (Fig. 1). The induction of GFP expression was blocked by a 1-amino-acid substitution in integrase (D116A), indicating that viral integration into the host genome was required to obtain a positive signal (data not shown).
FIG. 1.
Relative infectivities of MA deletion mutants. GHOST-CXCR4 cells were infected overnight with the parental (WT) or MA deletion mutant versions of HXB/Env−/Nef+, complemented in trans with C-terminally truncated HIV-1 Env. The relative amounts of recombinant virus used are indicated in RT units. Three days after infection, GFP-positive cells were quantitated by flow cytometry. The percentage of cells within the area defined by the horizontal line in each panel is indicated.
We next investigated whether Nef affected the infectivity of the parental and MA deletion mutant viruses in this assay system. To this end, GHOST-CXCR4 cells were incubated with 250,000 RT units of the Nef+ and Nef− versions of each MA mutant complemented with C-terminally truncated HIV-1 Env. To adjust for the effects of the MA deletions on virus infectivity, parallel cultures were incubated with either 50,000 or 100,000 RT units of the parental Nef+ and Nef− virus stocks, as appropriate. At day 3 postinfection, the cultures were metabolically labeled for 12 h with 50 μCi of [35S]cysteine/ml. Viral proteins in the transfected cells were then immunoprecipitated with serum from a patient infected with HIV-1 and analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE). In parallel, viral particles released during the labeling period were pelleted through 20% sucrose and their protein composition was directly analyzed by SDS-PAGE.
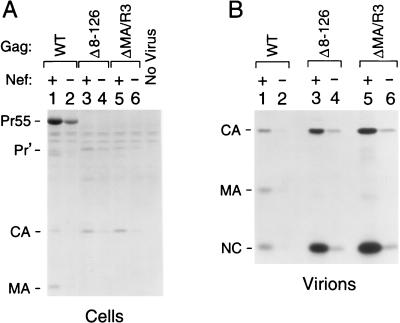
In cells infected with particles that contained WT MA, most of the detectable Gag protein was in the form of the unprocessed Gag precursor (Fig. 2A). The presence of Nef in the viral genome caused about a fivefold increase in the intensity of the Gag precursor band, which indicated an enhancement of infectivity comparable to what has previously been observed in single-cycle replication assays (3, 10, 30, 43). The Δ8-87, Δ8-126, and ΔMA/R3 mutations markedly reduced the levels of unprocessed Gag in the infected cells relative to the levels of fully processed CA, which were slightly increased compared to those obtained with WT particles (Fig. 2A and data not shown). For all three MA mutants, Nef increased the levels of cell-associated Gag to a lesser extent than those reached in the presence of WT MA. However, when levels of viral particle production were compared, it became apparent that Nef had a pronounced effect in both the presence and absence of MA. Quantitative PhosphorImager analysis of the SDS-PAGE data shown in Fig. 2B (see also Fig. 3A) indicated that cultures infected with the Nef+ recombinant virus containing WT MA produced from seven- to ninefold more extracellular particles than cultures infected with the corresponding Nef− virus. Although this effect of Nef was somewhat smaller in the absence of MA residues 8 through 87 (Fig. 3A), an enhancement of similar magnitude to that seen in the presence of WT MA was observed if most (Δ8-126) or all (ΔMA/R3) of MA was deleted (Fig. 2B).
FIG. 2.
Effect of Nef on a single round of virus transmission in the absence of MA. GHOST-CXCR4 cells were infected with 293T cell-derived recombinant viruses that differed in gag and nef as indicated. To ensure that only a single round of infection occurred, C-terminally truncated HIV-1 Env was provided in trans during virus production. The target cells were incubated overnight with 50,000 RT units of the parental Nef+ and Nef− virus stocks and with 250,000 RT units of the MA mutants to compensate for the approximately fivefold reduction in infectivity caused by the Δ8-126 and ΔMA/R3 deletions. (A) Cell-associated Gag proteins detected by immunoprecipitation with patient serum after metabolic labeling of the infected cultures with [35S]cysteine. (B) Virions released during the labeling period were pelleted through sucrose and directly analyzed by SDS-PAGE. Pr55, full-length Gag precursor; Pr', shortened Gag precursors produced by the Δ8-126 and ΔMA/R3 mutants. NC, nucleocapsid.
FIG. 3.
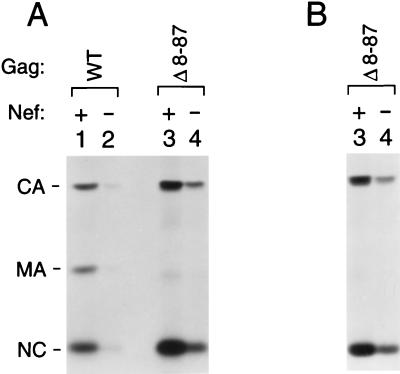
Nef provided in trans during virus production has an effect on the transmission of a MA deletion mutant similar to that of Nef encoded by the viral genome. (A) Virus production by GHOST-CXCR4 cells infected with 293T cell-derived recombinant viruses that differed in gag as indicated and that harbored either an intact or a disrupted nef gene. Env was provided in trans to limit virus transmission to a single cycle. To compensate for the moderate infectivity defect caused by the Δ8-87 mutation, the target cells were incubated with 100,000 RT units of the parental Nef+ and Nef− virus stocks and with 250,000 RT units of the Nef+ or the Nef− Δ8-87 mutant. (B) Virus production by GHOST-CXCR4 cells infected with recombinant viruses obtained by cotransfection of the Env− and Nef− Δ8-87 mutant with expression vectors for C-terminally truncated Env and either intact or frameshifted Nef. To examine the efficiency of a single round of virus transmission, the infected cultures were labeled with [35S]cysteine and virions released into the medium were pelleted through sucrose and directly analyzed by SDS-PAGE. NC, nucleocapsid.
It is also noteworthy that all three MA deletions significantly increased the amount of particulate Gag that was released into the culture medium, whereas the amount of total cell-associated Gag was clearly decreased. This effect was seen in both the presence and absence of Nef and was most pronounced for the ΔMA/R3 deletion, which caused an 8- to 10-fold increase in viral particle production per infected cell. Researchers have previously shown that mutations in MA can significantly enhance Gag membrane binding and accelerate the kinetics of Gag release from transfected cells (33, 34, 37, 45). Taken together, these observations suggested that Gag membrane binding is regulated by a mechanism that controls the availability of the N-terminal myristyl moiety for membrane insertion. In this model, MA would sequester the myristyl group until a conformational change leads to its exposure, perhaps to ensure the specific targeting of Gag to the plasma membrane. One would then expect that the myristyl group would become constitutively exposed if MA were deleted, which could in turn explain why particle production is increased under those conditions. Independent of the contribution of the myristyl group to Gag membrane binding, a conformational switch may be required to bring MA into a state that allows efficient Gag polymerization, and it is conceivable that this event is rate limiting for assembly.
Previous observations suggest that the very small amounts of cell-associated Gag in cultures infected with the MA mutants reflect an accelerated release of Gag in the form of extracellular particles, which prevents the intracellular accumulation of significant amounts of Gag (34). This may explain why Nef had a considerably larger effect on particle yields than on the levels of cell-associated Gag. However, an alternative explanation for this discrepancy is that Nef expression in the infected cells specifically stimulated particle production. Consistent with this possibility, Lu et al. observed that Nef increased the release of HIV-1 Gag into the supernatant of transiently transfected COS cells (26). To distinguish between these possibilities, we performed an experiment in which Nef was expressed in trans in the producer cells, which has been shown to augment the infectivity of WT HIV-1, albeit somewhat less efficiently than when Nef is provided in cis (31). 293T cells were transfected with the Δ8-87 mutant of HXB/Env−/Nef− together with pEnvHXBΔCT and either an expression vector for Nef (pSRαNefLAI) or a negative control vector (pSRα Nef−). The pSRαNefLAI vector was obtained by inserting a PCR-generated DNA encoding a Kozak sequence and HIV-1LAI Nef into the XhoI and EcoRI sites of the mammalian expression vector pBJ5. The pSRα Nef− negative control vector was created by inserting 4 bp at the unique XhoI site in the HIV-1LAI nef gene with the Klenow fragment of DNA polymerase I. Equivalent amounts of virus were used to infect GHOST-CXCR4 cells, and virus production by the infected cells was then examined after metabolic labeling as described above. In this experiment, the results of which are shown in Fig. 3B, the effect of Nef on virus production after a single round of virus transmission was comparable to that obtained when Nef was encoded by the viral genome rather than provided in trans (Fig. 3A). Thus, the expression of Nef in the infected cells was not required for the observed effects on particle yields, indicating that these were due to differences in the infectivities of the input viruses.
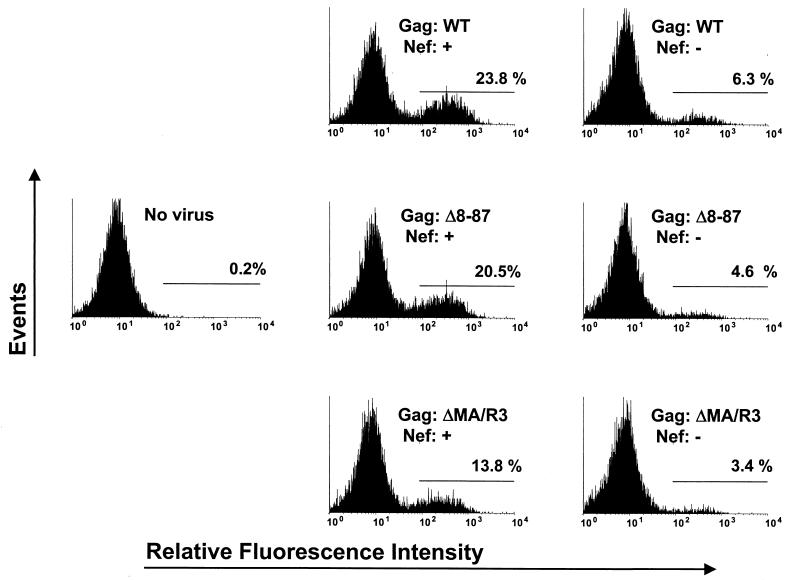
To address the possibility that MA plays a cell type-dependent role in the Nef-mediated enhancement of virus infectivity, we also examined the infectivity of virus stocks produced in a T-cell line. Jurkat T-antigen (Tag) cells were transfected with pEnvHXBΔCT and either the parental HXB/Env−/Nef+ and HXB/Env−/Nef−proviruses or the Δ8-87 and ΔMA/R3 mutant versions. Virus-containing supernatants were harvested 2 days posttransfection, normalized for RT activity, and again used to infect GHOST-CXCR4 cells. In an effort to adjust for the infectivity defects caused by the MA deletions, viruses harboring the Δ8-87 and ΔMA/R3 mutations were used at 2.5- and 5-fold higher concentrations, respectively, than the parental Nef+ and Nef− viruses. Quantitation of GFP-positive cells by flow cytometry at day 4 postinfection showed that Nef increased the number of infected cells by about fourfold in the presence of WT MA and had similar effects on the infectivities of the Δ8-87 and ΔMA/R3 mutants (Fig. 4).
FIG. 4.
Nef enhances the infectivity of virus produced in T cells independent of the presence or absence of MA. GHOST-CXCR4 cells were inoculated with the indicated recombinant viruses produced in Jurkat Tag cells. C-terminally truncated HIV-1 Env was provided in trans in the Jurkat Tag cells to permit only a single round of virus replication. To compensate for the reductions in infectivity caused by the deletions in MA, Δ8-87 and ΔMA/R3 recombinant viruses were used at 2.5- and 5-fold higher concentrations, respectively, than the recombinant viruses containing WT MA. Four days after infection, GFP-positive cells were quantitated by flow cytometry. The percentage of cells within the area defined by the horizontal line in each panel is indicated.
Taken together, our results provide genetic evidence that MA is dispensable for the ability of Nef to enhance the infectivity of HIV-1 virions produced either in CD4-negative adherent cells or in a T-cell line. Apart from its role in the incorporation and organization of the Env glycoprotein spikes, MA interacts with membrane lipids and controls the selective membrane targeting of Gag. In this regard, Zheng et al. recently reported that Nef targets HIV-1 budding to lipid rafts in transiently transfected 293T cells and that this increases the concentration of specific lipids in the viral membrane, thereby making the virus more infectious (56). Since we find that Nef increases the infectivity of virus produced in 293T cells even if MA is totally absent, it would appear that MA is not crucial for the reported Nef-mediated enrichment of Gag in lipid rafts (56). Also, MA deletion mutant viruses are defective with regard to their ability to discriminate between intracellular membranes and the plasma membrane (15, 37), which argues against the possibility that MA deletion viruses retain the ability to bud from specific membrane compartments. Thus, if Nef indeed acts by altering the viral lipid composition, its ability to enhance viral infectivity in the absence of MA might indicate a more general effect on cellular lipid homeostasis.
Acknowledgments
GHOST-CXCR4 cells were obtained through the AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH, from V. N. KewalRamani and D. R. Littman. We also thank G. Crabtree for Jurkat Tag cells.
This work was supported by NIH grant AI42510 and by Center for AIDS Research grant AI28691. We also acknowledge the support of the G. Harold and Leila Y. Mathers Charitable Foundation.
REFERENCES
- 1.Aiken, C. 1997. Pseudotyping human immunodeficiency virus type 1 (HIV-1) by the glycoprotein of vesicular stomatitis virus targets HIV-1 entry to an endocytic pathway and suppresses both the requirement for Nef and the sensitivity to cyclosporin A. J. Virol. 71:5871-5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aiken, C., J. Konner, N. R. Landau, M. E. Lenburg, and D. Trono. 1994. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell 76:853-864. [DOI] [PubMed] [Google Scholar]
- 3.Aiken, C., and D. Trono. 1995. Nef stimulates human immunodeficiency virus type 1 proviral DNA synthesis. J. Virol. 69:5048-5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander, L., Z. Du, M. Rosenzweig, J. U. Jung, and R. C. Desrosiers. 1997. A role for natural simian immunodeficiency virus and human immunodeficiency virus type 1 nef alleles in lymphocyte activation. J. Virol. 71:6094-6099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baur, A. S., E. T. Sawai, P. Dazin, W. J. Fantl, C. Cheng-Mayer, and B. M. Peterlin. 1994. HIV-1 Nef leads to inhibition or activation of T cells depending on its intracellular localization. Immunity 1:373-384. [DOI] [PubMed] [Google Scholar]
- 6.Bukovsky, A. A., T. Dorfman, A. Weimann, and H. G. Göttlinger. 1997. Nef association with human immunodeficiency virus type 1 virions and cleavage by the viral protease. J. Virol. 71:1013-1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chazal, N., G. Singer, C. Aiken, M.-L. Hammarskjöld, and D. Rekosh. 2001. Human immunodeficiency virus type 1 particles pseudotyped with envelope proteins that fuse at low pH no longer require Nef for optimal infectivity. J. Virol. 75:4014-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen, Y. L., D. Trono, and D. Camaur. 1998. The proteolytic cleavage of human immunodeficiency virus type 1 Nef does not correlate with its ability to stimulate virion infectivity. J. Virol. 72:3178-3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chowers, M. Y., M. W. Pandori, C. A. Spina, D. D. Richman, and J. C. Guatelli. 1995. The growth advantage conferred by HIV-1 nef is determined at the level of viral DNA formation and is independent of CD4 downregulation. Virology 212:451-457. [DOI] [PubMed] [Google Scholar]
- 10.Chowers, M. Y., C. A. Spina, T. J. Kwoh, N. J. Fitch, D. D. Richman, and J. C. Guatelli. 1994. Optimal infectivity in vitro of human immunodeficiency virus type 1 requires an intact nef gene. J. Virol. 68:2906-2914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 12.Deacon, N. J., A. Tsykin, A. Solomon, K. Smith, M. Ludford-Menting, D. J. Hooker, D. A. McPhee, A. L. Greenway, A. Ellett, C. Chatfield, et al. 1995. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science 270:988-991. [DOI] [PubMed] [Google Scholar]
- 13.Dorfman, T., F. Mammano, W. A. Haseltine, and H. G. Göttlinger. 1994. Role of the matrix protein in the virion association of the human immunodeficiency virus type 1 envelope glycoprotein. J. Virol. 68:1689-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Du, Z., S. M. Lang, V. G. Sasseville, A. A. Lackner, P. O. Ilyinskii, M. D. Daniel, J. U. Jung, and R. C. Desrosiers. 1995. Identification of a nef allele that causes lymphocyte activation and acute disease in macaque monkeys. Cell 82:665-674. [DOI] [PubMed] [Google Scholar]
- 15.Föcke, M., A. Janetzko, R. L. Shoeman, and H.-G. Kräusslich. 1993. A large deletion in the matrix domain of the human immunodeficiency virus gag gene redirects virus particle assembly from the plasma membrane to the endoplasmic reticulum. J. Virol. 67:4972-4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fackler, O. T., X. Lu, J. A. Frost, M. Geyer, B. Jiang, W. Luo, A. Abo, A. S. Alberts, and, B. M. Peterlin. 2000. p21-activated kinase 1 plays a critical role in cellular activation by Nef. Mol. Cell. Biol. 20:2619-2627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed, E. O., and M. A. Martin. 1995. Virion incorporation of envelope glycoproteins with long but not short cytoplasmic tails is blocked by specific, single amino acid substitutions in the human immunodeficiency virus type 1 matrix. J. Virol. 69:1984-1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Freed, E. O., J. M. Orenstein, A. J. Buckler-White, and M. A. Martin. 1994. Single amino acid changes in the human immunodeficiency virus type 1 matrix protein block virus particle production. J. Virol. 68:5311-5320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Garcia, J. V., and A. D. Miller. 1991. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature 350:508-511. [DOI] [PubMed] [Google Scholar]
- 20.Goldsmith, M. A., M. T. Warmerdam, R. E. Atchison, M. D. Miller, and W. C. Greene. 1995. Dissociation of the CD4 downregulation and viral infectivity enhancement functions of human immunodeficiency virus type 1 Nef. J. Virol. 69:4112-4121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberg, M. E., A. J. Iafrate, and J. Skowronski. 1998. The SH3 domain-binding surface and an acidic motif in HIV-1 Nef regulate trafficking of class I MHC complexes. EMBO J. 17:2777-2789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hill, C. P., D. Worthylake, D. P. Bancroft, A. M. Christensen, and W. I. Sundquist. 1996. Crystal structures of the trimeric human immunodeficiency virus type 1 matrix protein: implications for membrane association and assembly. Proc. Natl. Acad. Sci. USA 93:3099-3104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jamieson, B. D., G. M. Aldrovandi, V. Planelles, J. B. Jowett, L. Gao, L. M. Bloch, I. S. Chen, and J. A. Zack. 1994. Requirement of human immunodeficiency virus type 1 nef for in vivo replication and pathogenicity. J. Virol. 68:3478-3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kestler, H. W., III, D. J. Ringler, K. Mori, D. L. Panicali, P. K. Sehgal, M. D. Daniel, and R. C. Desrosiers. 1991. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell 65:651-662. [DOI] [PubMed] [Google Scholar]
- 25.Lama, J., A. Mangasarian, and D. Trono. 1999. Cell-surface expression of CD4 reduces HIV-1 infectivity by blocking Env incorporation in a Nef- and Vpu-inhibitable manner. Curr. Biol. 9:622-631. [DOI] [PubMed] [Google Scholar]
- 26.Lu, X., X. Wu, A. Plemenitas, H. Yu, E. T. Sawai, A. Abo, and B. M. Peterlin. 1996. CDC42 and Rac1 are implicated in the activation of the Nef-associated kinase and replication of HIV-1. Curr. Biol. 6:1677-1684. [DOI] [PubMed] [Google Scholar]
- 27.Luo, T., J. L. Douglas, R. L. Livingston, and J. V. Garcia. 1998. Infectivity enhancement by HIV-1 Nef is dependent on the pathway of virus entry: implications for HIV-based gene transfer systems. Virology 241:224-233. [DOI] [PubMed] [Google Scholar]
- 28.Mammano, F., E. Kondo, J. Sodroski, A. Bukovsky, and H. G. Göttlinger. 1995. Rescue of human immunodeficiency virus type 1 matrix protein mutants by envelope glycoproteins with short cytoplasmic domains. J. Virol. 69:3824-3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller, M. D., M. T. Warmerdam, S. S. Ferrell, R. Benitez, and W. C. Greene. 1997. Intravirion generation of the C-terminal core domain of HIV-1 Nef by the HIV-1 protease is insufficient to enhance viral infectivity. Virology 234:215-225. [DOI] [PubMed] [Google Scholar]
- 30.Miller, M. D., M. T. Warmerdam, I. Gaston, W. C. Greene, and M. B. Feinberg. 1994. The human immunodeficiency virus-1 nef gene product: a positive factor for viral infection and replication in primary lymphocytes and macrophages. J. Exp. Med. 179:101-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Miller, M. D., M. T. Warmerdam, K. A. Page, M. B. Feinberg, and W. C. Greene. 1995. Expression of the human immunodeficiency virus type 1 (HIV-1) nef gene during HIV-1 production increases progeny particle infectivity independently of gp160 or viral entry. J. Virol. 69:579-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mörner, A., A. Björndal, J. Albert, V. N. KewalRamani, D. R. Littman, R. Inoue, R. Thorstensson, E. M. Fenyö, and E. Björling. 1999. Primary human immunodeficiency virus type 2 (HIV-2) isolates, like HIV-1 isolates, frequently use CCR5 but show promiscuity in coreceptor usage. J. Virol. 73:2343-2349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ono, A., and E. O. Freed. 1999. Binding of human immunodeficiency virus type 1 Gag to membrane: role of the matrix amino terminus. J. Virol. 73:4136-4144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paillart, J. C., and H. G. Göttlinger. 1999. Opposing effects of human immunodeficiency virus type 1 matrix mutations support a myristyl switch model of Gag membrane targeting. J. Virol. 73:2604-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pandori, M., H. Craig, L. Moutouh, J. Corbeil, and J. Guatelli. 1998. Virological importance of the protease-cleavage site in human immunodeficiency virus type 1 Nef is independent of both intravirion processing and CD4 down-regulation. Virology 251:302-316. [DOI] [PubMed] [Google Scholar]
- 36.Pandori, M. W., N. J. Fitch, H. M. Craig, D. D. Richman, C. A. Spina, and J. C. Guatelli. 1996. Producer-cell modification of human immunodeficiency virus type 1: Nef is a virion protein. J. Virol. 70:4283-4290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Reil, H., A. A. Bukovsky, H. R. Gelderblom, and H. G. Göttlinger. 1998. Efficient HIV-1 replication can occur in the absence of the viral matrix protein. EMBO J. 17:2699-2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ross, T. M., A. E. Oran, and B. R. Cullen. 1999. Inhibition of HIV-1 progeny virion release by cell-surface CD4 is relieved by expression of the viral Nef protein. Curr. Biol. 9:613-621. [DOI] [PubMed] [Google Scholar]
- 39.Saksela, K., G. Cheng, and D. Baltimore. 1995. Proline-rich (PxxP) motifs in HIV-1 Nef bind to SH3 domains of a subset of Src kinases and are required for the enhanced growth of Nef+ viruses but not for down-regulation of CD4. EMBO J. 14:484-491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sawai, E. T., A. Baur, H. Struble, B. M. Peterlin, J. A. Levy, and C. Cheng-Mayer. 1994. Human immunodeficiency virus type 1 Nef associates with a cellular serine kinase in T lymphocytes. Proc. Natl. Acad. Sci. USA 91:1539-1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schaeffer, E., R. Geleziunas, and W. C. Greene. 2001. Human immunodeficiency virus type 1 Nef functions at the level of virus entry by enhancing cytoplasmic delivery of virions. J. Virol. 75:2993-3000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schrager, J. A., and J. W. Marsh. 1999. HIV-1 Nef increases T cell activation in a stimulus-dependent manner. Proc. Natl. Acad. Sci. USA 96:8167-8172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwartz, O., V. Marechal, O. Danos, and J. M. Heard. 1995. Human immunodeficiency virus type 1 Nef increases the efficiency of reverse transcription in the infected cell. J. Virol. 69:4053-4059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schwartz, O., V. Marechal, S. Le Gall, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 45.Spearman, P., R. Horton, L. Ratner, and I. Kuli-Zade. 1997. Membrane binding of human immunodeficiency virus type 1 matrix protein in vivo supports a conformational myristyl switch mechanism. J. Virol. 71:6582-6592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spina, C. A., T. J. Kwoh, M. Y. Chowers, J. C. Guatelli, and D. D. Richman. 1994. The importance of nef in the induction of human immunodeficiency virus type 1 replication from primary quiescent CD4 lymphocytes. J. Exp. Med. 179:115-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Swingler, S., P. Gallay, D. Camaur, J. Song, A. Abo, and D. Trono. 1997. The Nef protein of human immunodeficiency virus type 1 enhances serine phosphorylation of the viral matrix. J. Virol. 71:4372-4377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swingler, S., A. Mann, J. Jacque, B. Brichacek, V. G. Sasseville, K. Williams, A. A. Lackner, E. N. Janoff, R. Wang, D. Fisher, and M. Stevenson. 1999. HIV-1 Nef mediates lymphocyte chemotaxis and activation by infected macrophages. Nat. Med. 5:997-1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang, C. T., Y. Zhang, J. McDermott, and E. Barklis. 1993. Conditional infectivity of a human immunodeficiency virus matrix domain deletion mutant. J. Virol. 67:7067-7076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, J. K., E. Kiyokawa, E. Verdin, and D. Trono. 2000. The Nef protein of HIV-1 associates with rafts and primes T cells for activation. Proc. Natl. Acad. Sci. USA 97:394-399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welker, R., M. Harris, B. Cardel, and H.-G. Kräusslich. 1998. Virion incorporation of human immunodeficiency virus type 1 Nef is mediated by a bipartite membrane-targeting signal: analysis of its role in enhancement of viral infectivity. J. Virol. 72:8833-8840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Welker, R., H. Kottler, H. R. Kalbitzer, and H. G. Krausslich. 1996. Human immunodeficiency virus type 1 Nef protein is incorporated into virus particles and specifically cleaved by the viral proteinase. Virology 219:228-236. [DOI] [PubMed] [Google Scholar]
- 53.Willey, R. L., D. H. Smith, L. A. Lasky, T. S. Theodore, P. L. Earl, B. Moss, D. J. Capon, and M. A. Martin. 1988. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J. Virol. 62:139-147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiskerchen, M., and C. Cheng-Mayer. 1996. HIV-1 Nef association with cellular serine kinase correlates with enhanced virion infectivity and efficient proviral DNA synthesis. Virology 224:292-301. [DOI] [PubMed] [Google Scholar]
- 55.Yu, X., X. Yuan, Z. Matsuda, T. H. Lee, and M. Essex. 1992. The matrix protein of human immunodeficiency virus type 1 is required for incorporation of viral envelope protein into mature virions. J. Virol. 66:4966-4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng, Y., A. Plemenitas, T. Linnemann, O. T. Fackler, and B. M. Peterlin. 2001. Nef increases infectivity of HIV via lipid rafts. Curr. Biol. 11:875-879. [DOI] [PubMed] [Google Scholar]