Abstract
Hantaviruses are rodent-borne agents that cause hemorrhagic fever with renal syndrome or hantavirus pulmonary syndrome in humans. The nucleocapsid protein (N) is relatively conserved among hantaviruses and highly immunogenic in both laboratory animals and humans, and it has been shown to induce efficient protective immunity in animal models. To investigate the ability of recombinant N (rN) from different hantaviruses to elicit cross-protection, we immunized bank voles with rN from Puumala (PUUV), Topografov (TOPV), Andes (ANDV), and Dobrava (DOBV) viruses and subsequently challenged them with PUUV. All animals immunized with PUUV and TOPV rN were completely protected. In the group immunized with DOBV rN, 7 of 10 animals were protected, while only 3 of 8 animals were protected in the group immunized with ANDV rN, which is more closely related to PUUV rN than DOBV rN. Humoral and cellular immune responses after rN immunization were also investigated. The highest cross-reactive humoral responses against PUUV antigen were detected in sera from ANDV rN-immunized animals, followed by those from TOPV rN-immunized animals, and only very low antibody cross-reactivity was observed in sera from DOBV rN-immunized animals. In proliferation assays, T lymphocytes from animals immunized with all heterologous rNs were as efficiently recalled in vitro by PUUV rN as were T lymphocytes from animals immunized with homologous protein. In summary, this study has shown that hantavirus N can elicit cross-protective immune responses against PUUV, and the results suggest a more important role for the cellular arm of the immune response than for the humoral arm in cross-protection elicited by rN.
Viruses belonging to the genus Hantavirus, family Bunyaviridae, are a diverse group of rodent-borne viruses that cause hemorrhagic fever with renal syndrome (HFRS) or hantavirus pulmonary syndrome (HPS) in humans (17). Hantaviruses are present worldwide, but the majority of the approximately 200,000 HFRS cases that are reported annually occur in Asia, where the prototype hantavirus, Hantaan virus (HTNV) causes a severe form of the disease. Seoul virus (SEOV) causes a moderate form of HFRS, mainly in Asia. In Europe, HFRS is caused by Dobrava (DOBV) and Puumala (PUUV) viruses (reviewed in reference 31). The latter is associated with a milder form of HFRS, nephropathia epidemica. In addition, the DOBV-like Saaremaa virus, representing a unique hantavirus serotype, has recently been shown to cause HFRS in Europe (3, 5, 35; Å. Lundkvist, unpublished data). HPS is caused by several viruses in the Americas, with Sin Nombre virus and Andes virus (ANDV) being the most well known in North and South America, respectively. Topografov virus (TOPV) was discovered in Siberia but has not yet been associated with any disease (39). Hantaviruses are associated with specific rodent species, which act as hosts for the virus and in which they establish a persistent or prolonged infection (28, 32).
Hantaviruses are enveloped and have a three-segment negative-strand RNA genome packed in helical nucleocapsids. The three genome segments, i.e., large (L), medium (M), and small (S), encode four structural proteins: the RNA-dependent RNA polymerase, the two envelope glycoproteins G1 and G2, and the nucleocapsid protein (N), respectively (18).
As hantaviruses cause diseases with high morbidity and mortality and as to date there is no specific treatment for hantavirus infections, several strategies have been employed to develop hantavirus vaccines (reviewed in references 15 and 21). These involve inactivated virus vaccines (15, 21), recombinant proteins (26, 33, 42), recombinant vaccinia viruses (7, 27, 33, 34, 41), chimeric and mosaic hepatitis B virus core antigens (20, 36, 37), packaged alphavirus replicons (19), and naked DNA (6, 13, 14, 19).
The N protein, which encapsidates the viral RNA, has been shown to be highly immunogenic in both animal models and humans. We have previously shown that PUUV N completely protects bank voles (Clethrionomys glareolus), the natural host of PUUV, from infection with wild-type PUUV (26). Other studies have also shown that the N proteins from various hantaviruses protect animals from infection (6, 19, 20, 33, 37, 41, 42).
The protective immune responses elicited by the N protein are thought to be mainly cellular, as N-specific antibodies are not neutralizing. However, N-specific antibodies have been shown to induce partial protection in passive immunization experiments with monoclonal antibodies (MAbs) (Å. Lundkvist unpublished data), indicating that N-specific antibodies can contribute to inactivation of hantaviruses through mechanisms other than neutralization.
As hantaviruses are a group of relatively closely related viruses, with several conserved regions within their N proteins, the characteristics of cross-reactive cellular immune responses are most interesting, but these have been investigated in only a small number of studies (1, 2, 11, 38). In addition, a few groups have also reported on cross-protection after vaccination with the N and G1 or G2 proteins (7, 13, 19, 41). However, additional studies concerning cross-reactive immune responses and cross-protection among hantaviruses that are pathogenic for humans are important for the development of preventive vaccines. In this pursuit, knowledge concerning which proteins and which regions within them are essential for induction of cross-protective humoral and cellular immune responses is needed.
To investigate cross-reactive immune responses and potential cross-protection in bank voles, we produced recombinant N (rN) proteins from four different hantaviruses, PUUV, TOPV, ANDV, and DOBV, three of which are known to cause disease in humans. Cross-reactive humoral and cellular immune responses were analyzed after immunization with the rN proteins, and cross-protection was assessed after challenge with PUUV.
MATERIALS AND METHODS
rN proteins.
N proteins from PUUV, TOPV, ANDV, and DOBV, carrying a polyhistidine tag to facilitate protein purification, were produced in Escherichia coli cells. The open reading frames (ORF) of the PUUV/Kazan (GenBank accession number Z84204), TOPV/Ls136V5/94 (AJ011646), DOBV/Slovenia (L41916), and ANDV (AF004660) N protein-encoding genes have been cloned and sequenced previously (4, 22, 24, 39). The primers used for PCR amplification from cDNA were as follows: for PUUV, SPUUV/PQF (5′-TTG CAT GCT TAT GAG TGA CTT GAC AGA CAT CCA A-3′) (forward) and SPUUV/PQR (5′-TTG TCG ACT TAA TCA TAT CTT TAA GGG CTC CTG-3′) (reverse); for TOPV, STOP/PQF (5′-TAT AAG CAT GCT GAG CAA CCT CAA AGA CAT-3′) (forward) and STOP/PQR (5′-TAT AAG TCG ACC TAT ATT TTG AGT GGC TCTT-3′) (reverse); and for DOBV, SDOB/PQF (5′-TAT AAG CAT GCT GGC AAC ACT AGA GGA ACT-3′) (forward) and SDOB/PQR (5′-TAT AAG TCG ACT TAA AGC TTG AGC GGC TCCT-3′) (reverse). All forward and reverse primers contained an SphI and a SalI restriction site, respectively. The N ORF of ANDV was amplified with primers SANDV/PRSETF (5′-GCT ACT ACG ACT AAA GCT GGA ATG AG-3′) (forward) and SANDV/PRSETR (5′-TAT AGA CTA ACC CAC CTC CC-3′) (reverse) as described previously (29). The N ORF of PUUV, TOPV, and DOBV were cloned into the pQE-32 vector (Qiagen, Hilden, Germany), while the N ORF of ANDV was cloned into pRSET (R&D Systems Europe, Oxford, United Kingdom). An irrelevant protein, mouse dihydrofolate reductase (DHFR) (Qiagen), provided by the manufacturer and expressed in the same system, was used as a negative control protein. All constructs were expressed and purified as described earlier (9, 29). The sequences and orientations of the cloned S genes were confirmed by nucleotide sequence analysis with sequencing primers provided by the vector supplier (Qiagen). Cycle sequencing was carried out on plasmid DNA as described previously (8).
Animal immunizations and PUUV challenge.
To asses the immunogenicity and protective capacity of the expressed rN proteins, 4- to 10-week-old bank voles, derived from a PUUV-free colony established with animals captured in Sweden (26), were immunized with purified PUUV, TOPV, ANDV, or DOBV rN or DHFR control protein. Bank voles were immunized three times with 50 μg of protein at intervals of 3 weeks. The animals were injected with protein emulsified in Freund's complete adjuvant, incomplete Freund's adjuvant, and phosphate-buffered saline (PBS), respectively. Bank voles were challenged subcutaneously 2 weeks after the last immunization with approximately 20 50% infective doses of wild-type PUUV (strain Kazan). Animals were sacrificed at 21 days postchallenge, and lungs were removed and examined for presence of PUUV RNA by reverse transcription-PCR (RT-PCR) and for the presence of PUUV antigen by enzyme-linked immunosorbent assay (ELISA). Sera were examined for PUUV G2-specific antibodies by ELISA. The animals were maintained in an approved facility under conditions that met all requirements for animal use.
PUUV S-segment RT-PCR.
A nested RT-PCR was used to detect PUUV S-segment RNA in bank vole lung samples essentially as described before (30). Briefly, RNA was extracted from bank vole lung tissue with the Ultraspec RNA isolation system (Biotecx Laboratory, Houston, Tex.) according to the manufacturer's instructions. The following primers were used: Sa31 (5′-TCA TTT GA(A/G) GA(G/T) ATC AAT GGC AT-3′) and PUU5 (5′-CCC ATT CCA ACA TAA ACA GTA GG-3′) for RT and outer PCR and PUU2 (5′-CCA GGC ACA CCA GCA CAG GA-3′) and Sa5 (5′-GCT GTG CC[A/C] ACA GTC TTA GAT GCC-3′) for inner PCR.
ELISA.
For all assays, the following reagents were used. Antigens or antibodies were diluted in 50 mM sodium carbonate buffer (pH 9.6) and applied to Maxisorp plates (Nunc, Roskilde, Denmark) overnight at 4°C. Coated plates were blocked with 3% bovine serum albumin in PBS. All reagents were diluted in dilution buffer (0.5% bovine serum albumin and 0.05% Tween 20 in PBS), and the plates were washed six times with 0.05% Tween 20 in PBS between each step. All incubations were carried out at 37°C for 1 h unless otherwise stated. Assays were developed with either 3,3′,5,5′-tetramethylbenzidine (TMB) (Sigma, St. Louis, Mo.) or p-nitrophenyl phosphate (pNPP) (Sigma) in diethanolamine buffer, and absorbances were measured at 450 or 405 nm, respectively.
(i) rN ELISA.
The antigenicities of the generated rN proteins were evaluated with eight N-specific MAbs raised against PUUV (3H9, 5E1, 5B5, 3G5, 5F4, 1C12, 2E12, 4C3) (24a) and five N-specific MAbs raised against Tula virus (TULV) (3D3, 6A6, 7A4, 3C11, and 1C8) (26a) by ELISA. In addition, antibody reactivities against rN proteins were tested in sera from rN-immunized bank voles. rN proteins or DHFR control protein (1 μg/ml) was applied to Maxisorp plates. After blocking, MAbs (2 μg/ml) or serial fourfold dilutions of bank vole serum (starting at 1:400) were added. Specific antibody binding was detected with horseradish peroxidase-conjugated rabbit anti-mouse immunoglobulin G (IgG) antibodies diluted 1:1,000 (Dako, Glostrup, Denmark), followed by TMB substrate or alkaline phosphatase (ALP)-conjugated goat anti-mouse IgG antibodies diluted 1:1,000 (Jackson Immunoresearch, West Grove, Pa.), followed by pNPP substrate. Samples were regarded as positive when the optical density (OD) with rN protein minus the OD with control protein exceeded 0.100.
(ii) PUUV native N ELISA.
Total IgG responses to PUUV were measured by PUUV native N ELISA, essentially as described previously (16). Rabbit anti-PUUV serum diluted 1:400 was adsorbed to Maxisorp plates overnight at 4°C. After blocking, native PUUV antigen or diluent buffer only was added to the plates, followed by serial fourfold dilutions of bank vole serum (starting at 1:400). Specific antibody binding was detected with ALP-conjugated goat anti-mouse IgG antibodies diluted 1:1,000 (Jackson Immunoresearch), followed by pNPP substrate. Samples were regarded as positive when the OD with antigen minus the OD with diluent buffer exceeded 0.050.
(iii) PUUV G2 ELISA.
Bank vole sera collected after PUUV challenge were analyzed for the presence of antibodies reactive with the PUUV G2 protein by ELISA essentially as described earlier (26). The human MAb 1C9 was applied to Maxisorp plates at 10 μg/ml. The plates were blocked, and native PUUV antigen or diluent buffer only was added to the plates, followed by bank vole serum diluted 1:200. Specific antibody binding was detected with ALP-conjugated donkey anti-mouse IgG antibodies diluted 1:1,000 (Jackson Immunoresearch), followed by pNPP substrate. Samples were regarded as positive when the OD with antigen minus the OD with diluent buffer exceeded 0.100.
(iv) PUUV antigen ELISA.
Detection of PUUV antigen in bank vole lungs was performed essentially as described earlier (25). Briefly, bank vole lungs were mortar ground in 1 ml of Eagle's minimal essential medium supplemented with 2% fetal calf serum and antibiotics, diluted 1:2 in radioimmunoprecipitation assay buffer (0.01 M Tris-HCl [pH 7.8], 2% Triton X-100, 0.15 M NaCl, 0.6 M KCl, 5 mM EDTA, 1% aprotinin, and 1 mM phenylmethylsulfonyl fluoride), and centrifuged (1,500 rpm, 5 min, Sorvall RT6000B centrifuge), followed by dilution 1:2 in dilution buffer before being assayed. Maxisorp plates were coated with MAbs 5E1 and 2E12 (each at 5 μg/ml). The plates were blocked and samples, positive control antigens, and negative control antigens were added to the plates, followed by biotin-labeled MAbs 1C12 and 3G5 (each at 0.1 μg/ml). Streptavidin-peroxidase (Sigma) diluted 1:5,000 was incubated for 45 min at 37°C, followed by addition of TMB substrate. The reaction was stopped with 1 M H2SO4 after 10 to 15 min. Samples were regarded as positive when the mean OD exceeded the mean OD plus three standard deviations obtained with negative control samples.
Epitope mapping (PEPSCAN).
Linear B-cell epitopes were mapped by PEPSCAN (12). In total, 86 peptides (10 amino acids [aa] long, with 5-aa overlaps) spanning the N protein sequence of PUUV (strain Sotkamo, aa residues 1 to 433), synthesized on polypropylene pins, were used to locate antibody-reactive peptides. Peptide synthesis and analysis of antibody reactivity have been described earlier (23). Briefly, PEPSCAN antibody reactivities were measured in pools of sera, diluted 1:200, from PUUV, TOPV, ANDV, or DOBV rN protein-immunized or nonimmunized bank voles. Bound antibodies were detected with ALP-conjugated goat anti-mouse IgG diluted 1:1,000 (Jackson Immunoresearch) followed by pNPP substrate. The reactivity detected with sera from nonimmunized animals was subtracted from the reactivity seen with sera from rN-immunized animals.
Proliferation assay.
Bank voles were immunized once with 50 μg of PUUV, TOPV, ANDV, or DOBV rN protein in Freund's complete adjuvant and sacrificed 11 days later. Spleens were removed, and single-cell suspensions were prepared in Click's medium (Sigma) and plated in microtiter plates at 4 × 105 cells per well. Cells from individual animals were restimulated in vitro, in duplicate, with PUUV rN protein or DHFR control protein added at fivefold serial dilutions (starting at 20 μg/ml). Medium alone was used as negative control and 5 μg of concanavalin A per ml was used as a positive control. The cultures were incubated for 72 h, and thereafter 1 μCi of [3H]thymidine (Amersham, Buckinghamshire, United Kingdom) was added and the cultures were incubated for an additional 16 to 20 h. Cells were harvested onto cellulose filters and quenched, and the level of [3H]thymidine incorporation was determined with a liquid scintillation beta-counter. Stimulation indices (SI) of ≥4 were considered to represent significant responses.
Statistical analyses.
The Mann-Whitney U test was used to analyze differences in antibody responses, as determined by PUUV native N ELISA, and differences in proliferative responses against PUUV rN between groups of bank voles immunized with different hantavirus rN antigens. Correlation of antibody responses measured by PUUV native N ELISA and PUUV rN ELISA was determined using Spearman's correlation coefficient (rs).
RESULTS
Expression and antigenicity of recombinant hantavirus N proteins.
The N proteins of TOPV, ANDV, and DOBV were selected for the present study by virtue of their different degrees of amino acid sequence identity with PUUV N, which are 87, 73, and 60%, respectively.
The expressed rN proteins gave bands of the expected size (approximately 50 kDa) by immunoblotting with a pool of N-specific MAbs raised against PUUV (data not shown). Additional bands with lower molecular masses were visualized together with the PUUV, TOPV, ANDV, and DOBV rN proteins, suggesting truncated forms of N. The control protein, DHFR, gave a band of the expected size (approximately 26 kDa) by immunoblotting with a polyhistidine-specific MAb (data not shown). The rN proteins were characterized with a panel of 13 N-specific MAbs raised against PUUV and TULV which recognized three different common epitopes within all rN proteins (MAbs 5E1, 1C12, and 4C3), but also epitopes that were virus specific (Table 1). All of the epitopes earlier detected in E. coli-expressed PUUV rN proteins were detected in the PUUV rN produced in the present study (10, 26, 40). No earlier studies have reported characterization of TOPV, ANDV, or DOBV rN proteins with the panel of MAbs used in the present study.
TABLE 1.
Antigenic characterization of PUUV, TOPV, DOBV, and ANDV rN proteins with a panel of MAbs in ELISA
| Virus and MAb (epitope) | Reactiona with the following antigen: |
||||
|---|---|---|---|---|---|
| Native PUUV | Histidine-tagged: |
||||
| PUUV rN | TOPV rN | ANDV rN | DOBV rN | ||
| PUUV | |||||
| 3H9 (N-a) | + | − | − | − | − |
| 5E1 (N-b) | + | + | + | + | + |
| 5B5 (N-c) | + | + | (+) | + | − |
| 3G5 (N-d) | + | + | − | − | − |
| 5F4 (N-e) | + | − | − | − | − |
| 1C12 (N-f) | + | + | + | + | + |
| 2E12 (N-g) | + | + | + | + | − |
| 4C3 (N-h) | + | + | + | + | + |
| TULV | |||||
| 3D3 (N-a) | − | − | − | − | − |
| 6A6 (N-b) | − | − | − | − | − |
| 7A4 (N-c) | − | − | − | + | − |
| 3C11 (N-d) | + | + | + | + | − |
| 1C8 (N-e) | + | + | − | + | − |
+, positive reaction; (+), weak reaction; −, negative reaction.
Protection of bank voles from infection with PUUV.
PUUV causes a prolonged or persistent infection in bank voles, with no apparent pathogenicity, but viral antigen can be detected in different organs of naturally or experimentally infected animals. To evaluate the capacities of the recombinant PUUV, TOPV, ANDV, and DOBV N proteins to elicit protection, a bank vole challenge model, developed earlier in our laboratory (26), was employed. Animals were immunized three times with 50 μg of rN protein, and 3 weeks after challenge with approximately 20 50% infective doses of wild-type PUUV (strain Kazan), the animals were sacrificed and the lungs were examined for the presence of PUUV S-segment RNA and PUUV N antigen by RT-PCR and ELISA, respectively. After challenge, neither viral RNA nor N antigen could be detected in the lungs of any of the 16 bank voles immunized with PUUV or TOPV rN (Table 2). Viral RNA could be detected in three of eight animals immunized with ANDV rN, while no PUUV N antigen could be detected in any animal. In animals immunized with DOBV rN, viral RNA was detected in 3 of 10 animals, and in one of these animals PUUV N antigen was also detected in the lungs. All six animals immunized with DHFR control protein and all nine nonimmunized animals became PUUV viral RNA and N antigen positive after challenge.
TABLE 2.
PUUV challenge of bank voles immunized with hantavirus rN proteins
| Vaccine | Viral RNA in lungsa | PUUV N antigen in lungsb | PUUV G2 antibodyc | % Pro- tection |
|---|---|---|---|---|
| PUUV rN | 0/9 | 0/9 | 0/9 | 100 |
| TOPV rN | 0/7 | 0/7 | 0/7 | 100 |
| ANDV rN | 3/8 | 0/8 | 5/8 | 37 |
| DOBV rN | 3/10 | 1/10 | 2/10 | 70 |
| DHFR control protein | 6/6 | 6/6 | 6/6 | 0 |
| Nonvaccinated control | 9/9 | 9/9 | 9/9 | 0 |
Number of S-segment RNA-positive animals/number of animals inoculated.
Number of N-antigen-positive animals/number of animals inoculated.
Number of G2-specific-antibody-positive animals/number of animals inoculated.
In addition, postchallenge sera were analyzed for G2-specific antibodies by PUUV G2 ELISA, as protected animals should only have antibodies to the N protein with which they were immunized, while infected animals also develop antibodies to the viral glycoproteins. All animals immunized with the PUUV or TOPV rN were found to be negative for G2-specific antibodies after challenge (Table 2). G2-specific antibodies could be detected in five of eight animals immunized with ANDV rN, two of which were negative for both viral RNA and PUUV N antigen in their lungs, suggesting partial protection. Two of 10 animals immunized with DOBV rN had detectable G2 antibodies, and these two animals were also positive for viral RNA in their lungs. In contrast, nonimmunized animals or animals immunized with DHFR control protein all had detectable levels of G2-specifc antibodies after challenge.
Humoral immune responses to recombinant hantavirus N proteins.
To analyze the immunogenicities of the rN antigens used for immunization, antibody titers in sera taken from bank voles after the third rN immunization were examined. Individual sera from each group of bank voles immunized with either PUUV, TOPV, ANDV, or DOBV rN or DHFR control protein were titrated against homologous and heterologous proteins in ELISA (Table 3). Geometric mean antibody titers (GMT) were calculated, from individual titers, for each immunization group.
TABLE 3.
IgG reactivities of bank vole sera from animals immunized with hantavirus rN proteins against hantavirus rN proteins
| Seruma (n) | GMT (range of end point titers) with the following ELISA antigend: |
|||
|---|---|---|---|---|
| PUUV rN | TOPV rN | ANDV rN | DOBV rN | |
| PUUV N (9) | 409,600 (102,400-1,638,400) | 162,550 (102,400-409,600) | 351,127 (102,400-1,638,400) | 13,825 (1,600-102,400) |
| TOPV N (7) | 56,529 (25,600-409,600) | 84,002 (25,600-102,400) | 38,041 (25,600-409,600) | 38,041 (25,600-102,400) |
| ANDV N (8) | 144,815 (102,400-409,600) | 144,815 (102,400-409,600) | 974,198 (409,600-1,638,400) | 1,345 (<400-25,600) |
| DOBV N (10) | 22,286 (6,400-102,400) | 38,802 (6,400-409,600) | 19,401 (1,600-102,400) | 58,813 (25,600-409,600) |
| PUUVb (9) | 34,836 (6,400-102,400) | 2,963 (<400-25,600) | 25,600 (6,400-102,400) | 117 (<400-400) |
| DHFRc (5) | <400 | <400 | <400 | <400 |
Individual sera from bank voles immunized with the indicated antigens.
Individual sera from nonimmunized bank voles drawn 3 weeks after experimental infection with PUUV (strain Kazan).
Individual sera from bank voles immunized with DHFR control protein.
Homologous GMT are in boldface.
The highest GMT for the four groups immunized with rN proteins (409,600, 84,002, 974,198, and 58,813, respectively) were detected against homologous rN protein. The animals in the groups immunized with PUUV rN or infected with wild-type PUUV (strain Kazan) showed the highest degree of cross-reactivity against ANDV rN, while the group with ANDV rN-immunized animals exhibited the highest cross-reactivity, with the same GMT, 144,815, against both PUUV and TOPV rN proteins. Sera from DOBV rN-immunized animals showed the lowest degree of cross-reactivity to heterologous proteins, with GMT ranging between 19,401 and 38,802 (Table 3).
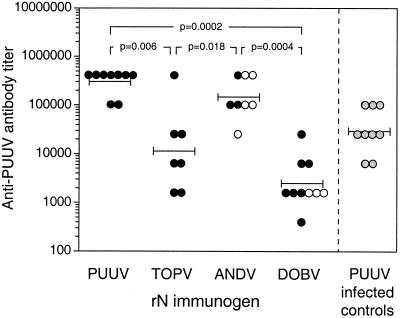
In addition, individual sera from the different immunization groups of animals were titrated against PUUV native N antigen in ELISA. The highest GMT, 301,002, was detected in the group immunized with PUUV rN, with titers ranging from 102,400 to 409,600, followed by the groups immunized with ANDV rN, TOPV rN, and DOBV rN, with GMT of 144,815 (range, 25,600 to 409,600), 11,593 (range, 1,600 to 409,600), and 2,425 (range, 400 to 25,600), respectively (Fig. 1). The GMT in a group of animals infected with PUUV was 29,863 (range, 6,400 to 102,400). Significant differences in mean antibody responses against PUUV native N antigen were seen between the group immunized with PUUV rN and the groups immunized with TOPV (P = 0.006) and DOBV rNs (P = 0.0002) but not with the group immunized with ANDV rN (P = 0.14) when analyzed by the Mann-Whitney U test. In addition, there was a significant difference in mean antibody response between the groups immunized with ANDV and DOBV rNs (P = 0.0004) and with ANDV and TOPV rNs (P = 0.018) but not between groups immunized with DOBV and TOPV rNs (P = 0.08) (Fig. 1). Furthermore, there were no significant differences in antibody titers between the protected and unprotected animals in the ANDV and DOBV rN-immunized groups. The geometric mean antibody response in the immunized animals as measured by the PUUV native N antigen ELISA correlated with the titers of the serum pools analyzed by rN ELISA (rs = 0.943; P = 0.035).
FIG. 1.
Humoral responses in hantavirus rN protein-immunized bank voles. Animals were immunized three times with PUUV, TOPV, ANDV, or DOBV rN protein, and individual sera from each group were titrated against PUUV native N antigen in ELISA. Prechallenge titers of protected (•) and unprotected (○) animals are indicated. Sera from PUUV infected bank voles were included as controls ( ). Bars represent GMT. The Mann-Whitney U test was used for statistical analysis. The P values are shown where significant differences between groups were observed.
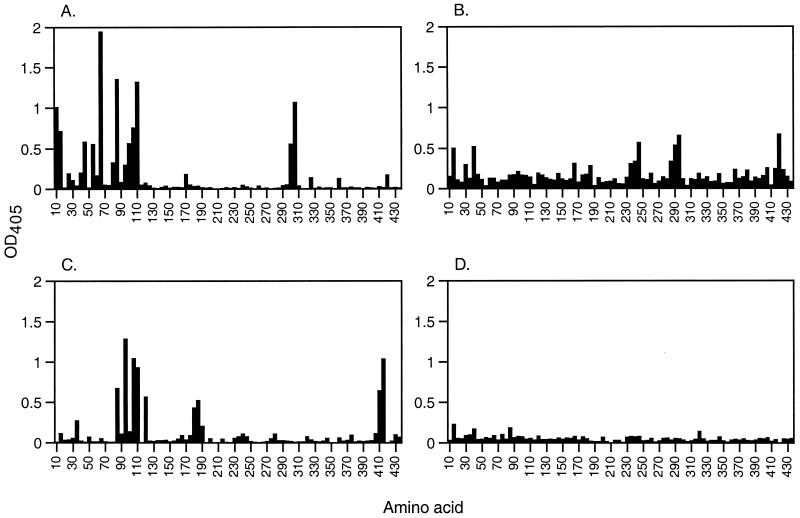
Pools of sera from the different immunization groups were also analyzed for reactivities against antigenic regions within the PUUV N protein. Mapping was performed with PEPSCAN against 86 overlapping decapeptides corresponding to the whole PUUV N sequence. The four serum pools displayed different reactivity patterns against the peptides (Fig. 2). The most abundant peptide reactivity was seen with the serum pool from the PUUV rN-immunized animals, followed by the ANDV and TOPV rN serum pools. Only very low peptide reactivity was seen with the serum pool from bank voles immunized with DOBV rN, and no specific antigenic regions were detected. Several antigenic regions were detected with the three other serum pools. The highest reactivity was seen against aa 1 to 120, in the amino-terminal part of the protein, detected with the serum pool from the PUUV rN-immunized bank voles. Some reactivity against the amino-terminal part was also seen with the TOPV (aa 16 to 45) and ANDV rN (aa 86 to 130) serum pools. The PUUV, TOPV, and ANDV serum pools also reacted with peptides representing the central parts of the protein, i.e., aa 301 to 315, 286 to 305, and 181 to 195, respectively. In addition, the TOPV and ANDV rN serum pools showed some reactivity against the carboxy-terminal part of the protein, i.e., aa 421 to 430 and 411 to 425, respectively.
FIG. 2.
Epitope scan analysis of antibody reactivity to peptides spanning the entire PUUV N protein (433 aa long). The first bar corresponds to aa 1 to 10, the second bar corresponds to aa 6 to 15, etc. Each bar indicates the antibody reactivity of serum pools from bank voles immunized with PUUV (n = 9) (A), TOPV (n = 8) (B), ANDV (n = 8) (C), and DOBV (n = 10) (D) rN proteins. The reactivity of serum pools from nonimmunized bank voles was subtracted.
T-cell proliferative responses to recombinant hantavirus N proteins.
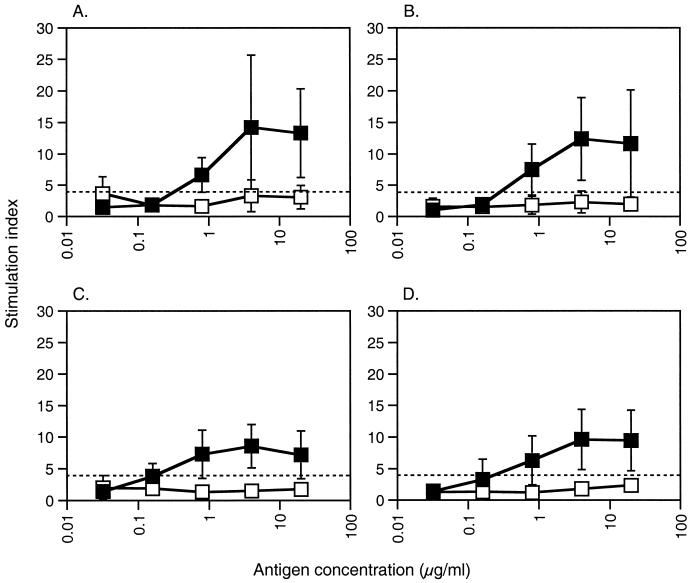
The immunogenicities of the rN proteins were further evaluated at the T-cell level with a proliferation assay in which spleen lymphocytes from individual bank voles primed with PUUV, TOPV, ANDV, and DOBV rNs were restimulated in vitro with PUUV rN or DHFR control protein. Efficient T-lymphocyte priming was achieved in all four groups after a single immunization with 50 μg of rN protein in Freund's complete adjuvant (Fig. 3). The PUUV rN protein recalled specific T-lymphocyte proliferation down to antigen concentrations of 0.8 μg/ml. All four groups responded with equally low proliferation (mean SI, 1.96; range, 1.22 to 3.66) after recall with DHFR control protein, compared to the PUUV rN protein restimulation. In contrast to the humoral responses, where clear differences in antibody responses were seen between the groups, there were minor proliferative differences between the groups when mean SIs were compared (Fig. 4). These differences did resemble the variations observed in protection; however, they were not statistically significant when analyzed with the Mann-Whitney U test.
FIG. 3.
Proliferative responses of lymphocytes from bank voles immunized with hantavirus rN proteins after in vitro recall. Single-cell suspensions of spleen lymphocytes from individual bank voles immunized with PUUV (A), TOPV (B), ANDV (C), or DOBV (D) rN protein were cultured in the presence of PUUV rN (▪) or DHFR control protein (□). Values are given as means ± standard deviations of SI, of individual bank voles in groups of five or six animals. The dashed lines represent the cutoff for a positive response, which was set to SI = 4. The range for spontaneous proliferation (medium alone) was 411 to 18,370, and that for positive control proliferation (5 μg of concanavalin A per ml) was 34,879 to 200,404. The range for proliferation of experimental samples stimulated with titrated rN proteins was 331 to 122,608, and that for experimental samples stimulated with titrated DHFR control protein was 197 to 42,020. The lowest count for an experimental sample above the set cutoff was 3,245 cpm.
FIG. 4.
Comparison of proliferative responses of lymphocytes from bank voles immunized with different hantavirus rN proteins. Ratios between the mean SI from lymphocyte cultures of TOPV, ANDV, or DOBV rN-immunized animals and the mean SI from lymphocyte cultures of PUUV rN-immunized animals at a concentration of 4 μg of recall antigen per ml are shown. Lymphocytes were cultured in the presence of PUUV rN (black bars) or DHFR control protein (white bars).
DISCUSSION
In this study, we have analyzed the cross-protective capacities of rN proteins from TOPV, ANDV, and DOBV against PUUV infection. All rN proteins gave rise to some degree of cross-protection in bank voles, and interestingly, the amino acid sequence identity of the N proteins with the PUUV N protein did not correlate with protection. In addition, the analysis of cross-reactive humoral and cellular immune responses suggested that the cellular responses to the N protein were more important than humoral responses for induction of protection. This is the first demonstration of N protein-evoked cross-protection between PUUV and TOPV, ANDV, or DOBV.
Cross-protection and the characteristics of cross-reactive immune responses have been investigated in several studies. Cross-reactive cytotoxic T lymphocytes (CTLs) from animals and humans infected with various hantaviruses have been reported. CTLs have been shown to target cells infected with (1, 2) or carrying peptides representing (11, 38) N protein sequences from a variety of heterologous hantaviruses. Cross-reactive cellular responses have also been observed in peripheral blood lymphocytes from vaccinees in human clinical trials with a recombinant vaccinia virus vaccine expressing HTNV proteins (27, 34). Furthermore, transfer of CTLs, primed through PUUV or Prospect Hill virus infection, into nude mice has been reported to significantly reduce HTNV levels, and immunization with the same viruses completely protected mice from lethal challenge with HTNV (1). In contrast, immunization with DNA vaccines expressing the M genes of SEOV and HTNV did not confer protection against infection with PUUV, while they did protect against heterologous infection with DOBV, SEOV, and HTNV (13, 19). In addition, cross-neutralizing antibodies were detected in monkeys immunized with the same vaccines (13). Similarly, vaccination with vaccinia virus expressing HTNV or SEOV proteins, gave rise to protection against heterologous virus challenge with SEOV and HTNV but not PUUV (7, 41).
The N protein has been used in several protection studies utilizing different animal models. The protective effect of N has varied from no protection at all (14) or partial protection (6, 19, 20, 33, 37, 41, 42) to complete protection (26). The contrasting results on the protective capacities of different hantavirus N proteins could depend on several factors. These include variations in the ability of different vaccination strategies, e.g., recombinant proteins, recombinant viruses, or DNA, to induce protective immune responses, as well as the use of various animal models, challenge viruses, and methods for evaluating protection. Several rodent species can be infected with hantaviruses. In the present study we have employed a PUUV infection model, based on colonized bank voles (the natural host of PUUV), which was developed in our laboratory (26). The results obtained with this model have been found to be highly reproducible, and it has been used in several studies concerning immune responses to and protective efficacy of recombinant proteins, virus-like particles, and MAbs (20, 26, 37; Å. Lundkvist, unpublished data).
Here, we used the N proteins from three hantaviruses known to cause disease in humans, namely, PUUV and DOBV, which cause HFRS in Europe, and ANDV, which causes HPS in South America. We also included the N protein from TOPV, which is closely related to PUUV but has not yet been found to cause any disease in humans. The rN proteins in this study had the expected sizes as observed by immunoblotting with N-specific MAbs raised against PUUV. Furthermore, the proteins were characterized with a panel of 13 N-specific MAbs by ELISA. With this panel, eight epitopes were detected in the PUUV rN, all of which have been found earlier in PUUV rN proteins expressed in E. coli (10, 26, 40). The MAbs revealed a unique binding pattern against each one of the other proteins, with eight, six, and three MAbs reacting with the ANDV, TOPV, and DOBV rNs, respectively. The conformations of these E. coli expressed proteins are not known. However, they most probably differ from their native forms, as they lack epitopes which are present in native viral antigens and proteins expressed in insect cells. Nevertheless, regardless of their conformations and exposed epitopes, the utilized proteins were capable of inducing immune responses giving rise to protection as well as cross-protection against PUUV infection.
The N proteins are relatively conserved among hantaviruses, and the overall identities at the amino acid level when the PUUV N protein is compared to the TOPV, ANDV, and DOBV N proteins are 87, 73, and 60%, respectively. However, the level of identity differs in various parts of the protein, and when the amino acid sequences are compared in a similarity plot, four regions become apparent (Fig. 5). The amino-terminal 150 aa (region I) and a central region (aa 200 to 340; region III) show low identity (range, 44 to 86%), while 50 aa in the central region (aa 150 to 200; region II) and the carboxy-terminal 90 aa (region IV) show higher identity (range, 80 to 97%) (Fig. 5). Several antigenic regions have been identified along the entire N protein molecule, and the main B-cell epitopes are located in the amino-terminal part of the protein (10, 26). Furthermore, peptides have been used for mapping of cellular responses against the N protein in a mouse model (9). In that study, four different regions in N were shown to be important for inducing T-lymphocyte proliferative responses. Two of these regions were located within the amino-terminal 120 aa, which have earlier been shown to confer protection in bank voles (26), while the other two were located in the central part of the molecule (aa 200 to 300). In addition, several CTL epitopes have been identified in the Sin Nombre virus and HTNV N proteins (11, 38).
FIG. 5.
Plots of similarity (generated by Stuart Ray's SimPlot 2.5) between hantavirus N proteins. The curves are comparisons between the query sequence, PUUV/Kazan, and reference sequences TOPV/Ls136V5/94, ANDV, and DOBV/Slovenia. Each point plotted is the percent amino acid identity within a sliding window 60 aa wide, with a step size of 10 aa.
In challenge experiments we observed complete protection against PUUV infection in bank voles immunized with both PUUV rN and TOPV rN, which could perhaps be expected, since these two proteins show a high overall amino acid identity (87%). More surprisingly, DOBV rN, with the lowest degree of aa identity (60%) with PUUV N, gave rise to protection against PUUV infection in as many as 70% of the animals, while ANDV N (73% aa identity with PUUV N) showed a more moderate level of protection (37%). The regions involved in inducing the high degree of protection as well as cross-protection remain to be resolved, but it could be speculated that the highly conserved regions (II and IV) in the central and carboxy-terminal parts of N could be important for cross-protection.
In an attempt to determine the type of immune responses accountable for the high degree of cross-protection, we analyzed both the humoral and cellular immune responses after immunization. Analysis of antibody responses showed that all rN proteins gave rise to antibodies which were cross-reactive with recombinant and native PUUV N protein, although at various degrees, with the highest cross-reactivity elicited by ANDV rN and the lowest elicited by DOBV rN. Additionally, there were no significant differences in antibody titers between protected and unprotected animals in the ANDV and DOBV rN-immunized groups, indicating that antibody responses against the N protein were not the major contributors of protection. Sera from TOPV and ANDV rN-immunized bank voles cross-reacted with peptides representing several regions within the PUUV N protein by PEPSCAN, while no cross-reactivity against PUUV peptides was seen in sera from animals immunized with DOBV rN. For analysis of cellular responses, we developed a proliferation assay in which bank vole spleen lymphocytes were used. In this assay T lymphocytes from animals immunized with TOPV, ANDV, and DOBV rN proteins were as efficiently recalled in vitro by PUUV rN as were T lymphocytes from PUUV rN-immunized animals. Small differences in proliferative responses were seen when the groups were compared; they were, however, not statistically significant.
The overall high cross-proliferative response, the high degree of protection elicited by DOBV rN in the absence of highly cross-reactive antibodies, and the relatively low degree of protection mediated by ANDV rN, despite high cross-reactive antibody titers, suggest a more important role for the cellular arm of the immune response in cross-protection elicited by the N protein. However, as unpublished observations by our group indicated that passive immunization with antibodies against N can have a protective effect in vivo, the influence of N-specific antibodies in protection cannot be ruled out (Å. Lundkvist, unpublished data). As antibody responses elicited by the N protein are not neutralizing, potential effector functions may depend on antibody-dependent cytotoxicity and/or complement-mediated cytolysis dependent on antibodies.
In conclusion, we have observed cross-protection against PUUV infection in bank voles and cross-reactive humoral and cellular immune responses after immunization with different hantavirus N proteins. Since the N proteins of hantaviruses circulating in different continents are able to elicit a high degree of cross-protection in animals, the N protein should be considered as an essential component of broadly reactive vaccines against hantavirus infection.
Acknowledgments
We thank Christel Werner, Ulrika Edbäck, and Kjell Eklund for technical assistance with animal care and Katarina Brus Sjölander for technical assistance. Pär Bierke, Niklas Ahlborg, and Andreas Mörner are acknowledged for valuable discussions.
This work was financially supported by grants from the EC (contract no. BMH4-CT97-2499 and QLK2-CT-1999-01119) (to Å.L.), the Swedish Medical Research Council (project no. 12177 and 12642) (to Å.L.), and the Nordic Foundation for Advanced Studies (to A.P.).
REFERENCES
- 1.Asada, H., K. Balachandra, M. Tamura, K. Kondo, and K. Yamanishi. 1989. Cross-reactive immunity among different serotypes of virus causing haemorrhagic fever with renal syndrome. J. Gen. Virol. 70:819-825. [DOI] [PubMed] [Google Scholar]
- 2.Asada, H., M. Tamura, K. Kondo, Y. Dohi, and K. Yamanishi. 1988. Cell-mediated immunity to virus causing haemorrhagic fever with renal syndrome: generation of cytotoxic T lymphocytes. J. Gen. Virol. 69:2179-2188. [DOI] [PubMed] [Google Scholar]
- 3.Avsic-Zupanc, T., K. Nemirov, M. Petrovec, T. Trilar, M. Poljak, A. Vaheri, and A. Plyusnin. 2000. Genetic analysis of wild-type Dobrava hantavirus in Slovenia: co-existence of two distinct genetic lineages within the same natural focus. J. Gen. Virol. 81:1747-1755. [DOI] [PubMed] [Google Scholar]
- 4.Avsic-Zupanc, T., A. Toney, K. Anderson, Y. K. Chu, and C. Schmaljohn. 1995. Genetic and antigenic properties of Dobrava virus: a unique member of the Hantavirus genus, family Bunyaviridae. J. Gen. Virol. 76:2801-2808. [DOI] [PubMed] [Google Scholar]
- 5.Brus Sjölander, K., I. Golovljova, V. Vasilenko, A. Plyusnin, and Å. Lundkvist. 2002. erological divergence of Dobrava and Saaremaa hantaviruses: evidence for two distinct serotypes. Epidemiol. Infect. 128:99-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucht, G., K. Brus Sjölander, S. Eriksson, L. Lindgren, Å. Lundkvist, and F. Elgh. 2001. Modifying the cellular transport of DNA-based vaccines alters the immune response to hantavirus nucleocapsid protein. Vaccine 19:3820-3829. [DOI] [PubMed] [Google Scholar]
- 7.Chu, Y. K., G. B. Jennings, and C. S. Schmaljohn. 1995. A vaccinia virus-vectored Hantaan virus vaccine protects hamsters from challenge with Hantaan and Seoul viruses but not Puumala virus. J. Virol. 69:6417-6423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.de Carvalho Nicacio, C., Å. Lundkvist, K. Brus Sjölander, A. Plyusnin, E. M. Salonen, and E. Björling. 2000. A neutralizing recombinant human antibody Fab fragment against Puumala hantavirus. J. Med. Virol. 60:446-454. [DOI] [PubMed] [Google Scholar]
- 9.de Carvalho Nicacio, C., M. Sällberg, C. Hultgren, and Å. Lundkvist. 2001. T-helper and humoral responses to Puumala hantavirus nucleocapsid protein: identification of T-helper epitopes in a mouse model. J. Gen. Virol. 82:129-138. [DOI] [PubMed] [Google Scholar]
- 10.Elgh, F., Å. Lundkvist, O. A. Alexeyev, G. Wadell, and P. Juto. 1996. A major antigenic domain for the human humoral response to Puumala virus nucleocapsid protein is located at the amino-terminus. J. Virol. Methods 59:161-172. [DOI] [PubMed] [Google Scholar]
- 11.Ennis, F. A., J. Cruz, C. F. Spiropoulou, D. Waite, C. J. Peters, S. T. Nichol, H. Kariwa, and F. T. Koster. 1997. Hantavirus pulmonary syndrome: CD8+ and CD4+ cytotoxic T lymphocytes to epitopes on Sin Nombre virus nucleocapsid protein isolated during acute illness. Virology 238:380-390. [DOI] [PubMed] [Google Scholar]
- 12.Geysen, H. M., S. J. Rodda, T. J. Mason, G. Tribbick, and P. G. Schoofs. 1987. Strategies for epitope analysis using peptide synthesis. J. Immunol. Methods 102:259-274. [DOI] [PubMed] [Google Scholar]
- 13.Hooper, J. W., D. M. Custer, E. Thompson, and C. S. Schmaljohn. 2001. DNA vaccination with the Hantaan virus M gene protects hamsters against three of four HFRS hantaviruses and elicits a high-titer neutralizing antibody response in rhesus monkeys. J. Virol. 75:8469-8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hooper, J. W., K. I. Kamrud, F. Elgh, D. Custer, and C. S. Schmaljohn. 1999. DNA vaccination with hantavirus M segment elicits neutralizing antibodies and protects against Seoul virus infection. Virology 255:269-278. [DOI] [PubMed] [Google Scholar]
- 15.Hooper, J. W., and D. Li. 2001. Vaccines against hantaviruses. Curr. Top. Microbiol. Immunol. 256:171-191. [DOI] [PubMed] [Google Scholar]
- 16.Hörling, J., Å. Lundkvist, M. Jaarola, A. Plyusnin, H. Tegelström, K. Persson, H. Lehväslaiho, B. Hörnfeldt, A. Vaheri, and B. Niklasson. 1996. Distribution and genetic heterogeneity of Puumala virus in Sweden. J. Gen. Virol. 77:2555-2562. [DOI] [PubMed] [Google Scholar]
- 17.Johnson, K. M. 2001. Hantaviruses: history and overview. Curr. Top. Microbiol. Immunol. 256:1-14. [DOI] [PubMed] [Google Scholar]
- 18.Jonsson, C. B., and C. S. Schmaljohn. 2001. Replication of hantaviruses. Curr. Top. Microbiol. Immunol. 256:15-32. [DOI] [PubMed] [Google Scholar]
- 19.Kamrud, K. I., J. W. Hooper, F. Elgh, and C. S. Schmaljohn. 1999. Comparison of the protective efficacy of naked DNA, DNA-based Sindbis replicon, and packaged Sindbis replicon vectors expressing Hantavirus structural genes in hamsters. Virology 263:209-219. [DOI] [PubMed] [Google Scholar]
- 20.Koletzki, D., Å. Lundkvist, K. Brus Sjölander, H. R. Gelderblom, M. Niedrig, H. Meisel, D. H. Krüger, and R. Ulrich. 2000. Puumala (PUU) hantavirus strain differences and insertion positions in the hepatitis B virus core antigen influence B-cell immunogenicity and protective potential of core-derived particles. Virology 276:364-375. [DOI] [PubMed] [Google Scholar]
- 21.Krüger, D. H., R. Ulrich, and Å. Lundkvist. 2001. Hantavirus infections and their prevention. Microbes Infect. 3:1129-1144. [DOI] [PubMed] [Google Scholar]
- 22.Lopez, N., P. Padula, C. Rossi, S. Miguel, A. Edelstein, E. Ramirez, and M. T. Franze-Fernandez. 1997. Genetic characterization and phylogeny of Andes virus and variants from Argentina and Chile. Virus Res. 50:77-84. [DOI] [PubMed] [Google Scholar]
- 23.Lundkvist, Å., S. Björsten, B. Niklasson, and N. Ahlborg. 1995. Mapping of B-cell determinants in the nucleocapsid protein of Puumala virus: definition of epitopes specific for acute immunoglobulin G recognition in humans. Clin. Diagn. Lab. Immunol. 2:82-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lundkvist, Å., Y. Cheng, K. Brus Sjölander, B. Niklasson, A. Vaheri, and A. Plyusnin. 1997. Cell culture adaptation of Puumala hantavirus changes the infectivity for its natural reservoir, Clethrionomys glareolus, and leads to accumulation of mutants with altered genomic RNA S segment. J. Virol. 71:9515-9523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Lundkvist, Å., A. Fatouros, and B. Niklasson. 1991. Antigenic variation of European haemorrhagic fever with renal syndrome virus strains characterized using bank vole monoclonal antibodies. J. Gen. Virol. 72:2097-2103. [DOI] [PubMed] [Google Scholar]
- 25.Lundkvist, Å., J. Hörling, S. Björsten, and B. Niklasson. 1995. Sensitive detection of hantaviruses by biotin-streptavidin enhanced immunoassays based on bank vole monoclonal antibodies. J. Virol. Methods 52:75-86. [DOI] [PubMed] [Google Scholar]
- 26.Lundkvist, Å., H. Kallio-Kokko, K. Brus Sjölander, H. Lankinen, B. Niklasson, A. Vaheri, and O. Vapalahti. 1996. Characterization of Puumala virus nucleocapsid protein: identification of B-cell epitopes and domains involved in protective immunity. Virology 216:397-406. [DOI] [PubMed] [Google Scholar]
- 26a.Lundkvist, Å., O. Vapalahti, A. Plyusnin, K. B. Sjölander, B. Niklasson, and A. Vaheri. 1996. Characterization of Tula virus antigenic determinants defined by monoclonal antibodies raised against baculovirus-expressed nucleocapsid protein. Virus Res. 45:29-44. [DOI] [PubMed] [Google Scholar]
- 27.McClain, D. J., P. L. Summers, S. A. Harrison, A. L. Schmaljohn, and C. S. Schmaljohn. 2000. Clinical evaluation of a vaccinia-vectored Hantaan virus vaccine. J. Med. Virol. 60:77-85. [DOI] [PubMed] [Google Scholar]
- 28.Meyer, B. J., and C. S. Schmaljohn. 2000. Persistent hantavirus infections: characteristics and mechanisms. Trends Microbiol. 8:61-67. [DOI] [PubMed] [Google Scholar]
- 29.Padula, P. J., C. M. Rossi, M. O. Della Valle, P. V. Martinez, S. B. Colavecchia, A. Edelstein, S. D. Miguel, R. D. Rabinovich, and E. L. Segura. 2000. Development and evaluation of a solid-phase enzyme immunoassay based on Andes hantavirus recombinant nucleoprotein. J. Med. Microbiol. 49:149-155. [DOI] [PubMed] [Google Scholar]
- 30.Plyusnin, A., J. Hörling, M. Kanerva, J. Mustonen, Y. Cheng, J. Partanen, O. Vapalahti, S. K. Kukkonen, J. Niemimaa, H. Henttonen, B. Niklasson, Å. Lundkvist, and A. Vaheri. 1997. Puumala hantavirus genome in patients with nephropathia epidemica: correlation of PCR positivity with HLA haplotype and link to viral sequences in local rodents. J. Clin. Microbiol. 35:1090-1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Plyusnin, A., D. H. Krüger, and Å. Lundkvist. 2001. Hantavirus infections in Europe. Adv. Virus Res. 57:105-136. [DOI] [PubMed] [Google Scholar]
- 32.Plyusnin, A., and S. P. Morzunov. 2001. Virus evolution and genetic diversity of hantaviruses and their rodent hosts. Curr. Top. Microbiol. Immunol. 256:47-75. [DOI] [PubMed] [Google Scholar]
- 33.Schmaljohn, C. S., Y. K. Chu, A. L. Schmaljohn, and J. M. Dalrymple. 1990. Antigenic subunits of Hantaan virus expressed by baculovirus and vaccinia virus recombinants. J. Virol. 64:3162-3170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schmaljohn, C. S., S. E. Hasty, and J. M. Dalrymple. 1992. Preparation of candidate vaccinia-vectored vaccines for haemorrhagic fever with renal syndrome. Vaccine 10:10-13. [DOI] [PubMed] [Google Scholar]
- 35.Sibold, C., R. Ulrich, M. Labuda, Å. Lundkvist, H. Martens, M. Schutt, P. Gerke, K. Leitmeyer, H. Meisel, and D. H. Krüger. 2001. Dobrava hantavirus causes hemorrhagic fever with renal syndrome in central Europe and is carried by two different Apodemus mice species. J. Med. Virol. 63:158-167. [PubMed] [Google Scholar]
- 36.Ulrich, R., D. Koletzki, S. Lachmann, Å. Lundkvist, A. Zankl, A. Kazaks, A. Kurth, H. R. Gelderblom, G. Borisova, H. Meisel, and D. H. Krüger. 1999. New chimaeric hepatitis B virus core particles carrying hantavirus (serotype Puumala) epitopes: immunogenicity and protection against virus challenge. J. Biotechnol. 73:141-153. [DOI] [PubMed] [Google Scholar]
- 37.Ulrich, R., Å. Lundkvist, H. Meisel, D. Koletzki, K. Brus Sjölander, H. R. Gelderblom, G. Borisova, P. Schnitzler, G. Darai, and D. H. Krüger. 1998. Chimaeric HBV core particles carrying a defined segment of Puumala hantavirus nucleocapsid protein evoke protective immunity in an animal model. Vaccine 16:272-280. [DOI] [PubMed] [Google Scholar]
- 38.Van Epps, H. L., C. S. Schmaljohn, and F. A. Ennis. 1999. Human memory cytotoxic T-lymphocyte (CTL) responses to Hantaan virus infection: identification of virus-specific and cross-reactive CD8+ CTL epitopes on nucleocapsid protein. J. Virol. 73:5301-5308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vapalahti, O., Å. Lundkvist, V. Fedorov, C. J. Conroy, S. Hirvonen, A. Plyusnina, K. Nemirov, K. Fredga, J. A. Cook, J. Niemimaa, A. Kaikusalo, H. Henttonen, A. Vaheri, and A. Plyusnin. 1999. Isolation and characterization of a hantavirus from Lemmus sibiricus: evidence for host switch during hantavirus evolution. J. Virol. 73:5586-5592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vapalahti, O., Å. Lundkvist, H. Kallio-Kokko, K. Paukku, I. Julkunen, H. Lankinen, and A. Vaheri. 1996. Antigenic properties and diagnostic potential of Puumala virus nucleocapsid protein expressed in insect cells. J. Clin. Microbiol. 34:119-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Xu, X., S. L. Ruo, J. B. McCormick, and S. P. Fisher-Hoch. 1992. Immunity to Hantavirus challenge in Meriones unguiculatus induced by vaccinia-vectored viral proteins. Am. J. Trop. Med. Hyg. 47:397-404. [DOI] [PubMed] [Google Scholar]
- 42.Yoshimatsu, K., Y. C. Yoo, R. Yoshida, C. Ishihara, I. Azuma, and J. Arikawa. 1993. Protective immunity of Hantaan virus nucleocapsid and envelope protein studied using baculovirus-expressed proteins. Arch. Virol 130:365-376. [DOI] [PubMed] [Google Scholar]