Abstract
The neutralizing activities of polyclonal antibodies and monoclonal antibodies (MAbs) obtained by immunization of mice with L1 virus-like particles (VLPs) were investigated by using pseudovirion infectivity assays for human papillomavirus type 16 (HPV-16), HPV-31, HPV-33, HPV-45, HPV-58, and HPV-59 to obtain a better definition of cross-neutralization between high-risk HPVs. In this study, we confirmed and extended previous studies indicating that most genital HPV genotypes represent separate serotypes, and the results suggest that the classification of serotypes is similar to that of genotypes. In addition, three cross-neutralizing MAbs were identified (HPV-16.J4, HPV-16.I23, and HPV-33.E12). MAb HPV-16.J4 recognized a conserved linear epitope located within the FG loop of the L1 protein, and HPV-16.I23 recognized another located within the DE loop. The results suggested that reactivity of MAb HPV-16.I23 to L1 protein is lost when leucine 152 of the HPV-16 L1 protein is replaced by phenylalanine. This confirmed the existence of linear epitopes within the L1 protein that induce neutralizing antibodies, and this is the first evidence that such linear epitopes induce cross-neutralization. However, the cross-neutralization induced by L1 VLPs represents less than 1% of the neutralizing activity induced by the dominant conformational epitopes, and it is questionable whether this is sufficient to offer cross-protection in vivo.
Human papillomaviruses (HPVs) have a nonenveloped icosahedral capsid of 50 to 55 nm composed of the major L1 protein and the minor L2 protein. The capsid contains 72 pentamers of L1, centered on the vertices of a T=7 icosahedral lattice (1, 48). The number of L2 molecules per capsid has been estimated to be 12 (48). The major capsid protein L1 of HPV can self-assemble into virus-like particles (VLPs) which have the size, shape, and conformational epitopes of virion capsids (25, 26, 29, 38, 41). Progress has recently been made concerning the structure of papillomavirus capsids (9), and significant progress has been made in the study of neutralizing antibodies, but limited information is available concerning the nature of L1 sequences corresponding to neutralizing epitopes.
Ninety-two HPVs have been identified to date. They induce benign epidermal and mucosal papillomas, and the development of cervical cancer is strongly associated with genital infection by specific types, such as HPV type 16 (HPV-16), HPV-18, HPV-31, HPV-33, HPV-35, HPV-39, HPV-45, HPV-52, HPV-58, and HPV-59 (33). Numerous serologic studies have demonstrated that infection with genital HPVs is followed by a serologic immune response to the major viral capsid protein, L1. This immune response persists for many years and is largely HPV type specific and directed against conformational epitopes (4, 5, 15, 27, 50). Moreover, both linear and conformational epitopes have been identified on the surface of HPV L1 VLPs (11, 13, 35, 51, 52).
Studies using canine papillomavirus and cottontail papillomavirus have shown that immunization with L1 VLPs can protect animals from subsequent challenge with infectious virus (3, 43). Moreover, protection can also be obtained by passive transfer of serum antibodies from vaccinated or naturally infected animals to naive animals, suggesting that the protection is mediated by neutralizing antibodies (3, 19, 43). In addition, immunization of mice with HPV VLPs (but not unassembled L1) generates predominantly type-specific neutralizing antibodies (11, 25, 34, 36, 49).
The first tests developed to determine neutralizing antibodies were based on the mouse xenograft system (2, 28, 32). However, the number of HPV types that have been successfully grown in this model is very limited, and the technique is time-consuming. The second means to measure neutralizing antibodies is to generate pseudovirions in vitro and to measure the inhibition of focus formation or gene expression due to the pseudovirions. Several procedures have been developed to produce pseudovirions. It has been shown that HPV VLPs composed of L1 or L1/L2 have the ability to package the bovine papillomavirus genome or irrelevant plasmid DNA in cellular (36, 40, 49) and acellular (23, 45) systems. The pseudovirions obtained have the ability to transfer the plasmid DNA into cells where the reporter gene is expressed. Moreover, it has been shown that the presence of L2 in HPV VLPs dramatically increases their gene transfer efficiency (23, 49, 53).
It has been demonstrated that neutralization epitopes are present in the L1 major capsid protein (11, 25, 30, 36, 39, 42, 45) and in the L2 minor capsid protein (24, 37). Both linear and conformational epitopes have been identified on the surface of HPV-16 L1 VLPs, and at least three L1 regions, i.e., amino acids 111 to 130, 174 to 185, and 261 to 280, contain linear epitopes (13, 50). The results suggest that conformational B-cell epitopes of HPV virions or VLPs induce neutralizing antibodies (10-14, 20, 35, 39, 51). In contrast, cross-reactive epitopes are linear epitopes and mostly nonneutralizing (12). It has been suggested that such linear epitopes are not surface exposed (14).
The L1 protein sequences of certain genital HPVs share strong homology (8), but the majority of anti-VLP antibodies are not cross-neutralizing (20, 36, 51). Using in vitro infectivity assays, some cross-neutralization between HPV-31 and -33 and between HPV-18 and -45 has been observed (20, 51). Such cross-neutralization is in agreement with the cross-reactivity observed by Roden et al. (35) using hemagglutination assays.
Recombinant HPV VLPs particles are promising vaccine candidates for controlling anogenital HPV disease and are now being evaluated in human subjects (18, 21). It is thus important to determine how many HPV types might be needed in a vaccine intended to protect against most genital HPV-associated cancers. This is related to the types associated with cancers (33), their relative frequency, and the degree of cross-protection that could be induced by one particular type.
The aim of this study was to investigate the neutralizing activity of mouse monoclonal and polyclonal HPV L1 antibodies by using the pseudoinfection system for seven high-risk HPVs in order to obtain better definition of cross-neutralization between genital HPVs. Cross-neutralization between HPV-16, -18, -31, -33, -45, -58, and -59 was investigated, and we also characterized the neutralizing properties of 16 HPV L1 monoclonal antibodies (MAbs).
MATERIALS AND METHODS
Generation of HPV VLPs.
HPV VLPs were produced in Sf21 insect cells by using recombinant baculoviruses encoding the L1 genes of HPV-16, -31, -33, -45, -58, and -59 according to previously described procedures (17, 29, 46). Briefly, the HPV L1 genes were first amplified from an HPV DNA-positive biopsy by using primers containing BglII sites. The amplified product was then inserted into pFastBacI to generate HPV L1 VLPs.
As gene transfer is fairly low when using some of these VLPs (15), L1-L2 VLPs were produced for HPV-33 and -58 in order to increase the level of gene transfer and thus the sensitivity of neutralization tests by using a lower quantity of pseudovirions in the neutralization assay. For this purpose, the HPV-33 and -58 L1 genes were also cloned into pFastBacDual plasmid (Invitrogen, Cergy-Pontoise, France) at the BamHI site for expression under the control of the polyhedrin promoter. The HPV-33 and -58 L2 genes were then amplified from HPV DNA-positive cervical cancer biopsies with primers containing XhoI sites and cloned into pFastBacDual L1 at the XhoI site for expression under the control of the p10 promoter. Recombinant baculoviruses encoding the pFastBacDual L1-L2 were then generated according to the recommendations of the manufacturer (Invitrogen).
VLPs were produced according to a procedure used for HPV-16 VLPs (17, 46). Sf21 cells, maintained in Grace's insect medium supplemented with 10% fetal calf serum (FCS), were infected with the different recombinant baculoviruses at a multiplicity of infection of 10 and were incubated for 72 h at 27°C. Cells were harvested by centrifugation, resuspended in phosphate-buffered saline (PBS) containing 0.5% NP-40, and allowed to stand at room temperature for 30 min. Cell lysates were then centrifuged at 14,000 × g for 15 min at 4°C. The nuclear fractions were further resuspended in ice-cold PBS and sonicated by three 15-s bursts at 60% maximal power (Vibra Cell; Bioblock Scientific, Strasbourg, France). Fractions were then loaded on the top of a preformed CsCl gradient and centrifuged at equilibrium in a Beckman SW28 rotor (20 h, 27,000 rpm, 4°C). Gradient fractions were analyzed for density by refractometry and were tested for the presence of L1 protein by enzyme-linked immunosorbent assay. Immunoreactive fractions were finally pooled and pelleted by ultracentrifugation in a Beckman SW 28 rotor (3 h, 28,000 rpm, 4°C). VLPs were resuspended in PBS, and the protein content was evaluated using a microBCA kit (Pierce, Touzart et Matignon, France). Each preparation was tested for the presence of VLPs by electron microscopy. For this purpose, VLP preparations were applied to 400-mesh carbon-coated grids, negatively stained with 1.5% uranyl acetate, and then examined at a nominal magnification of ×50,000 with a Jeol 1010 electron microscope.
A sample of each VLP preparation was analyzed in by sodium dodecyl sulfate-10% polyacrylamide gel electrophoresis and stained with Coomassie blue reagent, and the purity was analyzed by densitometry using Molecular Analyst software (Bio-Rad, Evry/Seine, France). The L1 protein content ranged from 63% for the HPV-18 VLP preparation to 85% for the HPV-33 VLP preparation.
Generation of pseudovirions.
A 7.1-kbp plasmid (pCMV-Luc; Clontech, Ozyme, Montigny le Bretonneux, France) coding for luciferase was used. The plasmid was linearized by restriction enzyme digestion. Pseudovirions were generated by direct interaction between linearized DNA and VLPs as previously described (15, 47). In this method, 10 μg of VLPs and 0.8 to 1 μg of DNA were mixed in 15 μl of 50 mM NaCl (pH 5) and incubated for 30 min at room temperature. Mixtures of DNA encoding the reporter gene with HBcΔ VLPs or bovine serum albumin (Sigma) were used as negative controls, and a mixture of DNA with Lipofectamine (Invitrogen) was used as a positive control.
HPV neutralization assay.
COS-7 cells grown in monolayers in Dulbecco modified Eagle medium (DMEM)-Glutamax (Invitrogen) supplemented with 10% FCS, 100 IU of penicillin per ml, and 100 μg of streptomycin per ml were seeded in 96-well plates (TPP; ATGC, Marne-la-Vallée, France). Neutralization of the pseudovirions with anti-VLP antibodies was investigated by inhibition of gene transfer. Polyclonal antibodies or MAbs were serially diluted by twofold dilution from 1/25 to 1/25,600 in 50 μl of DMEM-Glutamax and added to pseudovirions in a final volume of 100 μl. The quantity of pseudovirions was adjusted to give a luciferase signal of around 1,000 cps and not outside the range of 500 to 2,000 cps. After incubation at 37°C for 30 min, the different dilutions were added to COS-7 cells. After 1 h at 37°C, the medium was removed and 50 μl of DMEM-Glutamax supplemented with 10% FCS was added. Cells were then incubated for 48 h at 37°C. Luciferase activity was measured by luminescence assay (luciferase reporter gene assay with constant light signal; Roche Molecular Biochemical, Meylan, France). Luminescence was integrated over 10 s (Victor2; Wallac, Perkin-Elmer, Courtaboeuf, France), and the results were expressed as counts per second per well.
Neutralizing activity was expressed as the reciprocal of the maximum dilution of serum or ascites fluids that reduced the luciferase activity, expressed in counts per second, to more than 80% of that observed with pseudovirions. One hundred percent inhibition corresponded to the luciferase activity obtained with DNA alone, and 0% inhibition corresponded to the luciferase activity obtained with each of the pseudovirions.
Synthetic peptides.
Two peptides corresponding to linear epitopes identified on the HPV-16 L1 protein were used. Peptide 16 L1a (VGENVPDDLYIKGSG) spans amino acids 267 to 281 of the L1 protein of HPV-16 (6) and contains epitopes recognized by MAb HPV-16.J4, and peptide 16 L1b (GLKAKPKFTLGKRKATPTT) spans amino acids 473 to 492 of HPV-16 (7). For neutralization experiments, increasing amounts of peptides (1 to 16 μg) were mixed with antibodies diluted 1/25 in DMEM-Glutamax (Invitrogen). After 30 min of incubation at 37°C, the samples were tested for neutralizing antibodies against HPV-16 pseudovirions as described below.
Anti-L1 antibodies.
Five 10-week-old BALB/c mice were immunized subcutaneously with 10 μg of purified VLPs of HPV-16, -31, -33, -39, -45, -58, and -59, with AlOH3 as an adjuvant. The immunization schedule was one injection on days 0, 7, and 14 and a booster dose 14 days later. Blood samples were obtained 2 weeks after the last injection, and sera were pooled and then stored at −20°C until tested. The methods used for the production and characterization of MAbs included in this study have been described elsewhere (13). All MAbs have previously been described (13), with the exception of MAbs H45.E30 (immunoglobulin G2a [IgG2a]), H45.H11 (IgG2a), H45.L10 (IgM), and H45.N5 (IgG1), which have been generated (N. D. Christensen, unpublished data) using HPV-45 VLPs prepared from recombinant vaccinia viruses (13). MAb CamVir-1 was purchased from Pharmingen (BD Biosciences, Le Pont-de-Claix, France).
RESULTS
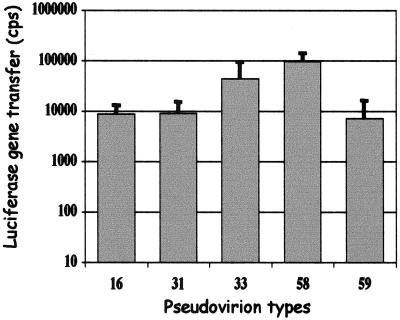
The neutralization activities of polyclonal antibodies and MAbs obtained by immunization of mice with L1 VLPs was investigated with pseudovirion infectivity assays using HPV-16, -31, -33, -45, -58, and -59 pseudovirions. Pseudovirions were generated by direct interaction of a plasmid coding for luciferase and HPV VLPs at a ratio of two VLPs to one DNA molecule. Luciferase gene transfers obtained with 10 μg of VLPs/well were 8,735, 9,128, and 7,208 cps for L1 VLPs corresponding to HPV-16, -31, and -59, respectively, and 43,722 and 95,656 cps for L1-L2 VLPs corresponding to HPV-33 and -58, respectively (Fig. 1).
FIG. 1.
Gene transfer of HPV L1 pseudovirion types 16, 31, and 59 and of HPV L1-L2 pseudovirion types 33 and 58. Data were obtained using 10 μg of VLPs per well.
For the determination of neutralizing antibodies, the quantity of pseudovirions was adjusted to obtain luciferase activity of between 500 and 2,000 cps, and the pseudovirions were incubated with serial dilutions of VLP antisera or MAbs and then added to COS-7 cells. Quantification of luciferase activity after 2 days served as a measurement of infectivity.
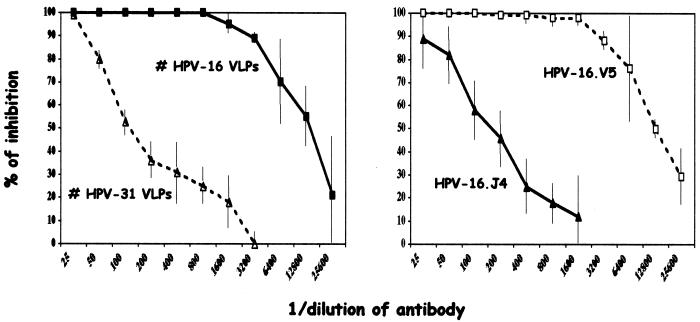
Figure 2 shows the results of titration of HPV-16 neutralizing antibodies for HPV-16 and HPV-31 polyclonal antibodies and for two HPV-16 MAbs (HPV-16.V5 and HPV-16.J4); the data shown are the means from four titrations. Inhibition of gene transfer for these four antibodies increased with antibody concentration. Anti-HPV-16 VLPs and MAb HPV-16.V5 had similar inhibition curves, and about 50% neutralization was still observed for an antibody dilution of 12,800. Much lower inhibition was observed with anti-HPV-31 VLPs and MAb HPV-16.J4. For these two antibodies, 50% neutralization was observed at antibody dilutions of 1/100.
FIG. 2.
Gene transfer inhibition assay. Gene transfer inhibition was done with two HPV-16 MAbs (V5 and J4) and with mouse antisera obtained by immunization with HPV-16 or HPV-31 VLPs.
Cross-neutralization among genital oncogenic HPV types.
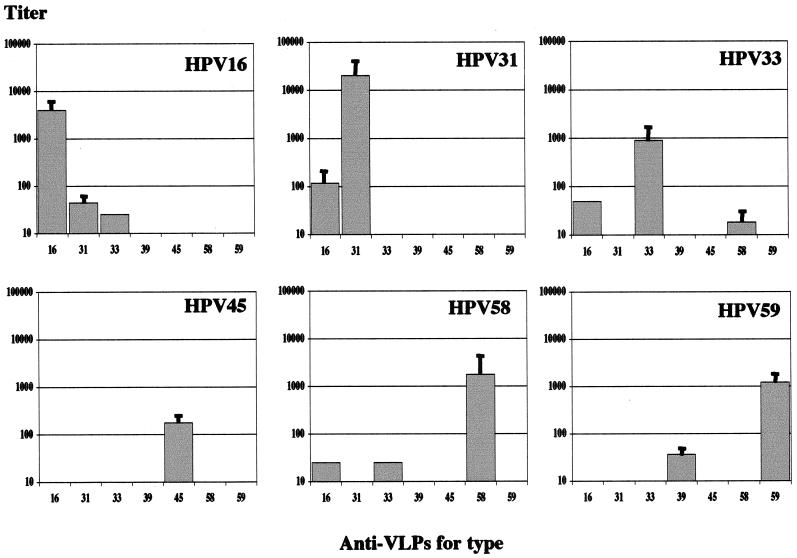
Cross-neutralization between high-risk HPVs was analyzed by investigating the neutralization of HPV-16, -31, -33, -45, -58, and -59 pseudoinfection by polyclonal antibodies obtained by immunization of mice with the corresponding VLPs. Anti-VLP titers of 10,400, 12,160, 9,600, 21,400, 3,333, 2,500, 8,400, and 22,400 were observed for mice immunized with HPV-16, -18, -31, -33, -39, -45, -58, and -59, respectively. Antibodies to HPV-16 VLPs neutralized HPV-16 pseudoinfection as well as HPV-31 and -33 pseudoinfection (Fig. 3). In confirmation of these results, anti-HPV-31 and anti-HPV-33 were shown to neutralize HPV-16. The results also indicated that HPV-33 and HPV-58 were cross-neutralizing and that anti-HPV-16 antibodies neutralized HPV-58 pseudoinfection. No cross-neutralization was observed between HPV-45 and HPV-59 or with the other types investigated. However, antibodies to HPV-39 VLPs neutralized HPV-59 pseudoinfection.
FIG. 3.
In vitro neutralization of HPV pseudovirion types 16, 31, 33, 45, 58, and 59 with antisera to HPV VLPs.
Identification of neutralizing and cross-neutralizing MAbs.
Next, we assayed the cross-neutralization by MAbs. As shown in Table 1, homologous neutralization was detected with six of nine HPV-16 MAbs, one of five HPV-45 MAbs, and each of the MAbs investigated for HPV-31 and HPV-33. However, three MAbs (HPV16.J4, HPV16.I23, and HPV33.E12) evidenced cross-neutralizing activity. MAb HPV16.J4 neutralized all HPV pseudovirions with the exception of HPV-59, with neutralizing titers ranging from 25 to 800. HPV16.I23 neutralized HPV-45 and -59 in addition to HPV16 pseudovirus. HPV-33.E12 neutralized HPV-33, -45, -58, and -59 pseudovirions, with neutralizing titers of 800, 200, 400, and 25, respectively. It should be noted that two MAbs with neutralizing activity (HPV16.L4 and HPV16.J4) were previously shown to recognize linear epitopes.
TABLE 1.
In vitro neutralization of HPV pseudovirions by 16 MAbs reactive with HPV L1 VLPs
| HPV type | MAb
|
Antibody titer | Neutralizing antibody titerb of MAb against HPV pseudovirion type:
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| Clone | Epitope recognizeda | Group A9
|
Group A7
|
||||||
| 16 | 31 | 33 | 58 | 45 | 59 | ||||
| HPV-16 | B20 | L | 4,800 | ||||||
| D9 | L | 100 | |||||||
| E70 | C | 204,800 | 800 | ||||||
| I23 | L | 3,200 | 200 | 50 | 100 | ||||
| J4 | L | 800 | 100 | 50 | 25 | 400 | 800 | ||
| L4 | L | 6,400 | 25 | ||||||
| V5 | C | 204,800 | 6,400 | ||||||
| U4 | C | 51,200 | 400 | ||||||
| CamVir 1 | L | 1,600 | |||||||
| HPV-31 | A6 | C | 3,200 | 100 | |||||
| HPV-33 | E12 | C | 51,200 | 800 | 400 | 200 | 25 | ||
| HPV-45 | C1 | C | 100 | ||||||
| E30 | C | 51,200 | 25 | ||||||
| H11 | C | 3,200 | |||||||
| L10 | C | <100 | |||||||
| N5 | C | <100 | |||||||
C, conformational; L, linear.
Absence of value indicates neutralizing activity <25.
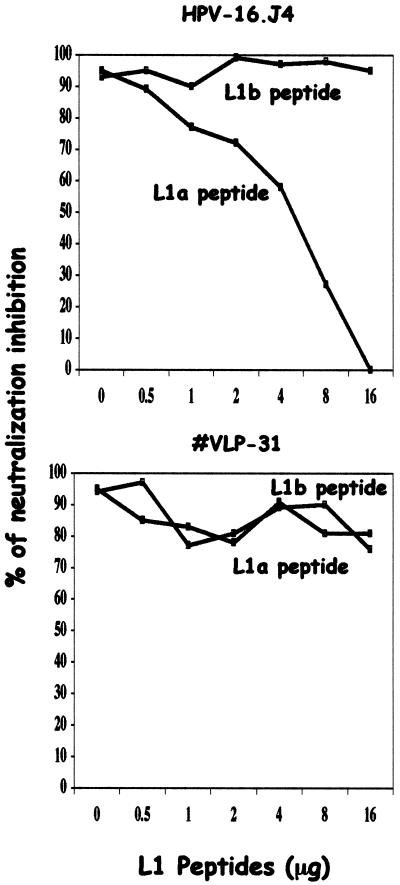
In order to achieve better characterization of the neutralizing activity detected for MAb HPV-16.J4, neutralization was inhibited by preincubation of the neutralizing antibody with increasing concentrations of the synthetic peptide 16L1a (amino acids 267 to 268). As shown in Fig. 4, the neutralizing activity of MAb HPV16.J4 was inhibited by preincubation with the synthetic peptide 16L1a. No inhibition was observed with preincubation of anti-HPV-31 VLP antibody with the same peptide when tested against HPV-16 pseudovirions. No significant inhibition was observed in either case when peptide 16 L1b (amino acids 473 to 492) was used in place of peptide 16L1a.
FIG. 4.
Inhibition of J4 neutralizing MAbs and anti-HPV-31 VLP antibody with L1 peptides (amino acids 267 to 281 and 473 to 492).
DISCUSSION
Analysis of the neutralizing activity of polyclonal antibodies obtained by immunization of mice with HPV L1 VLPs indicates the existence of cross-neutralization between types. Cross-reactivity was observed mainly between HPV-16, -31, -33, and -58. The association of these four types is correlated with the existence of high levels of cross-reactivity between these types (16, 20) and the fact that they were allocated to the same group (A9) by genotyping (8). No cross-neutralization was observed between these four types and two genotypes of group A7, namely, types 45 and 59. In addition, neutralization was not observed between types 45 and 59. These results confirmed that neutralizing antibodies obtained after immunization with L1 VLPs are mainly type specific and also that low levels of cross-protection could be obtained between types in group A9.
In this study, we characterized the neutralizing activities of 16 MAbs which reacted with intact L1 capsids of HPV-16, -31, -33, and -45. Neutralizing activity against HPV pseudovirion types 16, 31, 33, 45, 58, and 59 was detected in only about half of them (n = 9). We confirmed that the two HPV16 MAbs (E70 and V5) which are known to be directed against conformational epitopes are neutralizing. In contrast to the findings of White et al. (52), we found that MAb HPV16.U4 neutralized HPV-16 L1 pseudovirions. This could be due to the higher sensitivity of our test. In agreement with previous studies showing that many type-specific conformational MAbs identify virus-neutralizing epitopes (10, 12, 34), these three MAbs evidenced high neutralization titers and were type specific.
We also identified three other type-specific neutralizing antibodies for HPV-16 (L4), HPV-31 (A6), and HPV-45 (E30). MAbs HPV31.A6 and HPV45.E30 recognized conformational epitopes, and MAb HPV16.L4 Ab recognized a linear epitope at the N terminus of the L1 protein (13). In addition, we identified three cross-neutralizing antibodies (HPV16.I23, HPV16.J4, and HPV33.E12). Our results suggested that MAb HPV-16.J4 reacted with epitope ENVPDDLYIKGSGS from amino acid 268 to 281, similar to the epitope defined by Cason et al. (6). We previously observed that immunization with L1 VLPs induced a cross-reactive immune response to this peptide (44). The reactivity of peptides containing this conserved epitope with human sera after natural infection or with mice sera after immunization with VLPs (7, 22, 44) also suggests that this region is accessible on the virion surface. MAb HPV16.J4 did not neutralize HPV-59 pseudovirions. The epitope recognized by this antibody is conserved in HPV-59, and the lack of reactivity suggested that the HPV-59 neutralization test is less sensitive than others.
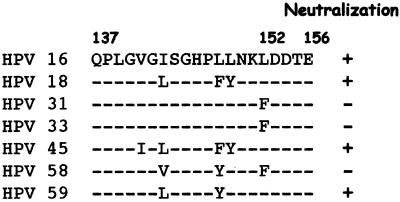
MAb HPV16.I23 recognized a linear epitope, 111QPLGVGISGHPLLNKLDDTE130 (13). This epitope is also a cross-reactive epitope present on HPV-16, -18, -31, -33, -35, and -45. The linear epitope has also been suggested to be present on the surface of the capsid (13), and it is close to an important neutralization domain common to many HPV types and identified on HPV-11 (31). Moreover, from the alignment of different L1 protein sequences corresponding to the epitope recognized by MAb HPV16.I23 (Fig. 5), it could be suggested that the lack of reactivity of this MAb is associated with the presence of a phenylalanine in place of a leucine at position 152 of the HPV-16 L1 protein.
FIG. 5.
Alignment of amino acid sequences corresponding to the HPV-16 linear epitope recognized by MAb HPV16.I23.
The findings described above suggest that, as observed for the L2 protein (24, 37), L1 contains common neutralization epitopes. This raises the question of whether HPV L1 VLPs may serve as a broad-spectrum vaccine against different genital HPVs. Vaccination with all homologous types will not be required, and vaccination with the most frequent types, such as 16 and 18, will have a greater impact than that expected from their frequency.
Our results indicate that the L1 protein contains common linear neutralization epitopes and suggest that some degree of cross-protection could occur. It is tempting to hypothesize that an HPV vaccine composed of the most frequent types (HPV-16 and -18) could also protect against related but less frequent types (HPV-31, -33, -45, and -58). However, low levels of cross-neutralizing antibody were observed after immunization of mice with L1 VLPs, which represented less than 1% of the homologous neutralizing activity. It was not possible to demonstrate that the neutralizing activity detected after heterologous immunization was due to an immune response to the common neutralizing epitope (L1a). Moreover, there is neither virological nor epidemiological evidence of natural cross-protection between related HPV types. It is thus speculation to predict that an HPV L1 VLP vaccine will protect humans against heterologous types for years after immunization. Only clinical immunization trials and investigation of neutralizing antibodies in sera of humans vaccinated with HPV-16 or with HPV-16 and HPV-18 will provide a response to this question in the near future.
In conclusion, we confirmed and extended previous studies indicating that most genital HPV genotypes represent separate serotypes. From the cross-neutralization observed between high-risk HPVs, it has been suggested that classification of serotypes is similar to that of genotypes. In addition, we detected three cross-neutralizing MAbs (HPV16.J4, HPV16.I23, and HPV33.E12). Christensen et al. (14) have previously reported the existence of linear epitopes inducing neutralizing antibodies against HPV-11. We confirmed the existence of neutralizing linear epitopes by using the HPV-16 L1 protein. Moreover, we observed that one of these linear epitopes induced cross-neutralizing antibodies. However, the cross-neutralization induced by L1 VLPs represents less than 1% of the neutralizing activity induced by the dominant conformational epitopes, and it is questionable whether this is sufficient to offer cross-protection in vivo.
Acknowledgments
This study was supported by grants from the Association pour la Recherche sur le Cancer (no. 5836) and from the Association “Vaincre la Mucoviscidose.” Alba-Lucia Combita and Latifa Bousarghin were supported by a fellowship from COLCIENCIAS and the Région Centre, respectively.
REFERENCES
- 1.Baker, T. S., W. W. Newcomb, N. H. Olson, L. M. Cowsert, C. Olson, and J. C. Brown. 1991. Structures of bovine and human papillomaviruses. Analysis by cryoelectron microscopy and three-dimensional image reconstruction. Biophys. J. 60:1445-1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnez, W., C. DaRin, C. Borkhuis, K. de Mesy Jansen, R. C. Reichman, and R. C. Rose. 1998. Isolation and propagation of human papillomavirus type 16 in human xenografts implanted in the severe combined immunodeficiency mouse. J. Virol. 72:5256-5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breitburd, F., R. Kirnbauer, N. L. Hubbert, B. Nonnenmacher, C. Trin-Dinh-Desmaret, G. Orth, J. T. Schiller, and D. R. Lowy. 1995. Immunization with virus-like particles from cottontail rabbit papillomavirus (CRPV) can protect against experimental CRPV infection. J. Virol. 69:3959-3963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carter, J. J., L. A. Koutsky, G. C. Wipf, N. D. Christensen, S. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 1996. The natural history of human papillomavirus type 16 capsid antibodies among a cohort of university women. J. Infect. Dis. 174:927-936. [DOI] [PubMed] [Google Scholar]
- 5.Carter, J. J., L. A. Koutsky, J. P. Hughes, S. K. Lee, J. Kuypers, N. Kiviat, and D. A. Galloway. 2000. Comparison of human papillomavirus type 16, 18, and 6 capsid antibody responses following incident infection. J. Infect. Dis. 181:1911-1919. [DOI] [PubMed] [Google Scholar]
- 6.Cason, J., D. Patel, J. Naylor, D. Lunney, P. S. Shepherd, J. M. Best, and D. J. McCance. 1989. Identification of immunogenic regions of the major coat protein of human papillomavirus type 16 that contain type-restricted epitopes. J. Gen. Virol. 70:2973-2987. [DOI] [PubMed] [Google Scholar]
- 7.Cason, J., P. K. Kambo, J. M. Best, and D. J. McCance. 1992. Detection of antibodies to a linear epitope on the major coat protein (L1) of human papillomavirus 16 (HPV16) in sera from patients with cervical intraepithelial neoplasia and in children. Int. J. Cancer 50:349-355. [DOI] [PubMed] [Google Scholar]
- 8.Chan, S. Y., H. Delius, A. L. Halpern, and H. U. Bernard. 1995. Analysis of genomic sequences of 95 papillomavirus types: uniting typing, phylogeny and taxonomy. J. Virol. 69:3074-3083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen, X. S., R. L. Garcea, I. Goldberg, G. Casini, and S. C. Harrison. 2000. Structure of small virus-like particles assembled from the L1 protein of human papillomavirus 16. Mol. Cell 5:557-567. [DOI] [PubMed] [Google Scholar]
- 10.Christensen, N. D., J. W. Kreider, N. M. Cladel, S. D. Patrick, and P. A. Welsh. 1990. Monoclonal antibody-mediated neutralization of infectious human papillomavirus type 11. J. Virol. 64:5678-5681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Christensen, N. D., R. Hopfl, S. L. DiAngelo, N. M. Cladel, S. D. Patrick, P. A. Welsh, L. R. Budgeon, C. A. Reed, and J. W. Kreider. 1994. Assembled baculovirus-expressed human papillomavirus type 11 L1 capsid protein virus-like particles are recognized by neutralizing monoclonal antibodies and induce high titres of neutralizing antibodies. J. Gen. Virol. 75:2271-2276. [DOI] [PubMed] [Google Scholar]
- 12.Christensen, N. D., R. Kirnbauer, J. T. Schiller, S. J. Ghim, R. Schlegel, A. B. Jenson, and J. W. Kreider. 1994. Human papillomavirus types 6 and 11 have antigenically distinct strongly immunogenic conformationally dependent neutralizing epitopes. Virology 205:329-335. [DOI] [PubMed] [Google Scholar]
- 13.Christensen, N. D., J. Dillner, C. Eklund, J. J. Carter, G. C. Wipf, C. A. Reed, N. M. Cladel, and D. A. Galloway. 1996. Surface conformational and linear epitopes on HPV-16 and HPV-18 L1 virus-like particles as defined by monoclonal antibodies. Virology 223:174-184. [DOI] [PubMed] [Google Scholar]
- 14.Christensen, N. D., C. A. Reed, N. M. Cladel, K. Hall, and G. S. Leiserowitz. 1996. Monoclonal antibodies to HPV-6 L1 virus-like particles identify conformational and linear neutralizing epitopes on HPV-11 in addition to type-specific epitopes on HPV-6. Virology 224:477-486. [DOI] [PubMed] [Google Scholar]
- 15.Combita, A.-L., A. Touzé, L. Bousarghin, P.-Y. Sizaret, N. Muñoz, and P. Coursaget. 2001. Gene transfer using human papillomavirus pseudovirions varies according to genotype and required cell surface heparan sulfate. FEMS Microbiol. Lett. 204:183-188. [DOI] [PubMed] [Google Scholar]
- 16.Combita, A.-L., M.-M. Bravo, A. Touzé, O. Orozco, and P. Coursaget. 2002. Serologic response to human oncogenic papillomavirus types 16, 18, 31, 33, 39, 58 and 59 virus-like particles in Colombian women with invasive cervical cancer. Int. J. Cancer 97:796-804. [DOI] [PubMed] [Google Scholar]
- 17.El Mehdaoui, S., A. Touzé, S. Laurent, P. Y. Sizaret, D. Rasschaert, and P. Coursaget. 2000. Gene transfer using recombinant rabbit hemorrhagic disease virus capsids with genetically modified DNA encapsidation capacity by addition of packaging sequences from L1 or L2 protein of human papillomavirus type 16. J. Virol. 74:10332-10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans, T. G., W. Bonnez, R. C. Rose, S. Koenig, L. Demeter, J. A. Suzich, D. O'Brien, M. Campbell, W. I. White, J. Balsley, and R. C. Reichman. 2001. A phase 1 study of a recombinant viruslike particle vaccine against human papillomavirus type 11 in healthy adult volunteers. J. Infect. Dis. 183:1485-1493. [DOI] [PubMed] [Google Scholar]
- 19.Ghim, S., J. Newsome, J. Bell, J. P. Sundberg, R. Schlegel, and A. B. Jenson. 2000. Spontaneously regressing oral papillomas induce systemic antibodies that neutralize canine oral papillomavirus. Exp. Mol. Pathol. 68:147-151. [DOI] [PubMed] [Google Scholar]
- 20.Giroglou, T., M. Sapp, C. Lane, C. Fligge, N. D. Christensen, R. E. Streeck, and R. C. Rose. 2001. Immunological analyses of human papillomavirus capsids. Vaccine 19:1783-1793. [DOI] [PubMed] [Google Scholar]
- 21.Harro, C. D., Y. Y. Pang, R. B. Roden, A. Hildesheim, Z. Wang, M. J. Reynolds, T. C. Mast, R. Robinson, B. R. Murphy, R. A. Karron, J. Dillner, J. T. Schiller, and D. R. Lowy. 2001. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J. Natl. Cancer Inst. 93:284-292. [DOI] [PubMed] [Google Scholar]
- 22.Heino, P., B. Skyldberg, M. Lehtinen, I. Rantala, B. Hagmar, J. W. Kreider, R. Kirnbauer, and J. Dillner. 1995. Human papillomavirus type 16 capsids expose multiple type-restricted and type-common antigenic epitopes. J. Gen. Virol. 76:1141-1153. [DOI] [PubMed] [Google Scholar]
- 23.Kawana, K. H., Y. Yoshikawa, K. Taketani, K. Yoshiike, and T. Kanda. 1998. In vitro construction of pseudovirions of human papillomavirus type 16: incorporation of plasmid DNA into reassembled L1/L2 capsids. J. Virol. 72:10298-10300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kawana, K. H., Y. Yoshikawa, K. Taketani, K. Yoshiike, and T. Kanda. 1999. Common neutralization epitope in minor capsid protein L2 of human papillomavirus type 16 and 6. J. Virol. 73:6188-6190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kirnbauer, R., F. Booy, N. Chen, D. R. Lowy, and J. T. Schiller. 1992. Papillomavirus L1 major capsid protein self-assembled into virus-like particles that are highly immunogenic. Proc. Natl. Acad. Sci. USA 89:12180-12184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kirnbauer, R., J. Taub, H. Greenstone, R. Roden, M. Durst, L. Gissmann, D. R. Lowy, and J. T. Schiller. 1993. Efficient self assembly of human papillomavirus type 16 L1 and L1-L2 into virus-like particles. J. Virol. 67:6929-6936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kirnbauer, R., N. L. Hubbert, C. M. Wheeler, T. M. Becker, D. R. Lowy, and J. T. Schiller. 1994. A virus-like particle enzyme-linked immunosorbent assay detects serum antibodies in a majority of women infected with human papillomavirus type-16. J. Natl. Cancer Inst. 86:494-499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kreider, J. W., M. K. Howett, A. E. Leure-Dupree, R. J. Zaino, and J. A. Weber. 1987. Laboratory production in vivo of infectious human papillomavirus type 11. J. Virol. 61:590-593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Cann, P., P. Coursaget, S. Iochmann, and A. Touzé. 1994. Self-assembly of human papillomavirus type 16 capsids by expression of the L1 protein in insect cells. FEMS Microbiol. Lett. 117:269-274. [DOI] [PubMed] [Google Scholar]
- 30.Leiserowitz, G. S., K. S. Hall, C. A. Foster, M. E. Hitchcock, N. D. Christensen, K. Heim, and L. H. Smith. 1997. Detection of serologic neutralizing antibodies against HPV-11 in patients with condyloma acuminata and cervical dysplasia using an in vitro assay. Gynecol. Oncol. 66:295-299. [DOI] [PubMed] [Google Scholar]
- 31.Ludmerer, S. W., D. Benincasa, and G. E. Mark III. 1996. Two amino-acid residues confer type specificity to a neutralizing, conformationally dependent epitope on human papillomavirus type 11. J. Virol. 70:4791-4794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ludmerer, S. W., D. Benincasa, G. E. Mark III, and N. D. Christensen. 1997. A neutralizing epitope of human papillomavirus type 11 is principally described by a continuous set of residues which overlap a distinct linear, surface-exposed epitope. J. Virol. 71:3834-3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muñoz, N. 2000. Human papillomavirus and cancer: the epidemiological evidence. J. Clin. Virol. 19:1-5. [DOI] [PubMed] [Google Scholar]
- 34.Roden, R. B., E. M. Weissinger, D. W. Henderson, F. Booy, R. Kirnbauer, J. F. Mushinshi, D. R. Lowy, and J. T. Schiller. 1994. Neutralization of bovine papillomavirus by antibodies to L1 and L2 capsid proteins. J. Virol. 68:7570-7574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roden, R. B., N. L. Hubbert, R. Kirnbauer, N. D. Christensen, D. R. Lowy, and J. T. Schiller. 1996. Assessment of the serological relatedness of genital human papillomaviruses by hemagglutination inhibition. J. Virol. 70:3298-3301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roden, R. B., H. L. Greenstone, R. Kirnbauer, F. P. Booy, J. Jessie, D. Lowy, and J. T. Schiller. 1996. In vitro generation and type-specific neutralization of a human papillomavirus type 16 virion pseudotype. J. Virol. 70:5875-5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Roden, R. B. S., W. H. Yutzy IV, R. Fallon, S. Inglis, D. R. Lowy, and J. T. Schiller. 2000. Minor capsid protein of human genital papillomavirus contains subdominant, cross-neutralizing epitopes. Virology 270:254-257. [DOI] [PubMed] [Google Scholar]
- 38.Rose, R. C., W. Bonnez, R. C. Reichman, and R. L. Garcea. 1993. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of virus-like particles. J. Virol. 67:1936-1944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose, R. C., R. C. Reichman, and W. Bonnez. 1994. Human papillomavirus (HPV) type 11 recombinant virus-like particles induce the formation of neutralizing antibodies and detect HPV-specific antibodies in human sera. J. Gen. Virol. 75:2075-2079. [DOI] [PubMed] [Google Scholar]
- 40.Rossi, J. L., L. Gissmann, K. Jansen, and M. Müller. 2000. Assembly of human papillomavirus type 16 in Saccharomyces cerevisiae. Hum. Gene Ther. 20:1165-1176. [DOI] [PubMed] [Google Scholar]
- 41.Sasagawa, T., P. Pushko, G. Steers, S. E. Gscheimeissner, M. Hajibagheri, J. Finch, L. Crawford, and M. Tommasino. 1995. Synthesis and assembly of virus like particles of human papillomavirus type 6 and type 16 in fission yeast Schizosaccharomyces pombe. Virology 206:403-410. [DOI] [PubMed] [Google Scholar]
- 42.Stauffer, Y., K. Raj, K. Masternak, and P. Beard. 1998. Infectious human papillomavirus type 18 pseudovirions. J. Mol. Biol. 283:529-536. [DOI] [PubMed] [Google Scholar]
- 43.Suzich, J. A., S. Ghim, F. J. Palmer-Hill, W. I. White, J. K. Tamura, J. Bell, J. A. Newsome, A. B. Jenson, and R. Schlegel. 1995. Systemic immunization with papillomavirus L1 protein completely prevents the development of viral mucosal papillomavirus. Proc. Natl. Acad. Sci. USA 92:11553-11557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Touzé, A., C. Dupuy, D. Mahé, P.-Y. Sizaret, and P. Coursaget. 1998. Production of recombinant virus-like particles from human papillomavirus type 6 and 11, and study of serological reactivities between HPV 6, 11, 16 and 45 by ELISA: implications for papillomavirus prevention and detection. FEMS Microbiol. Lett. 160:111-118. [DOI] [PubMed] [Google Scholar]
- 45.Touzé, A., and P. Coursaget. 1998. In vitro gene transfer using human papillomavirus-like particles. Nucleic Acids Res. 26:1317-1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Touzé, A., S. El Mehdaoui, P.-Y. Sizaret, C. Mougin, N. Muñoz, and P. Coursaget. 1998. The L1 major capsid protein of human papillomavirus type 16 variants affects the yield of virus-like particle produced in an insect cell expression system. J. Clin. Microbiol. 36:2046-2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Touzé, A., L. Bousarghin, C. Ster, A.-L. Combita, R. Roingeard, and P. Coursaget. 2001. Gene transfer using human polyomavirus BK virus-like particles expressed in insect cells. J. Gen. Virol. 82:3005-3009. [DOI] [PubMed] [Google Scholar]
- 48.Trus, B. L., R. B. Roden, H. L. Greenstone, M. Vrhel, J. T. Schiller, and F. P. Booy. 1997. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat. Struct. Biol. 4:413-420. [DOI] [PubMed] [Google Scholar]
- 49.Unckell, F., R. E. Streeck, and M. Sapp. 1997. Generation and neutralization of pseudovirions of human papillomavirus type 33. J. Virol. 71:2934-2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang, Z., N. D. Christensen, J. T. Schiller, and J. Dillner. 1997. A monoclonal antibody against intact human papillomavirus type 16 capsids blocks the serological reactivity of most human sera. J. Gen. Virol. 78:2209-2215. [DOI] [PubMed] [Google Scholar]
- 51.White, W. I., S. D. Wilson, W. Bonnez, R. C. Rose, S. Koenig, and J. A. Suzich. 1998. In vitro infection and type-restricted antibody-mediated neutralization of authentic human papillomavirus type 16. J. Virol. 72:959-964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.White, W. I., S. D. Wilson, F. J. Palmer-Hill, R. M. Woods, S. J. Ghim, L. A. Hewitt, D. M. Goldman, S. J. Burke, A. B. Jenson, S. Koenig, and J. A. Suzich. 1999. Characterization of a major neutralizing epitope on human papillomavirus type 16 L1. J. Virol. 73:4882-4889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yeager, M. D., M. Aste-Amezaga, D. R. Brown, M. M. Martin, M. J. Shah, J. C. Cook, N. D. Christensen, C. Ackerson, R. S. Lowe, J. F. Smith, P. Keller, and K. U. Jansen. 2000. Neutralization of human papillomavirus (HPV) pseudovirions: a novel and efficient approach to detect and characterize HPV neutralizing antibodies. Virology 278:570-577. [DOI] [PubMed] [Google Scholar]