Abstract
Fas/Fas ligand (FasL) interactions regulate disease outcome in coxsackievirus B3 (CVB3)-induced myocarditis. MRL+/+ mice infected with CVB3 develop severe myocarditis, a dominant CD4+ Th1 (gamma interferon [IFN-γ+]) response to the virus, and a predominance of γδ T cells in the myocardial infiltrates. MRL lpr/lpr and MRL gld/gld mice, which lack normal expression of Fas and express a mutated FasL, respectively, have minimal myocarditis and show a dominant CD4+ Th2 (interleukin-4 [IL-4+]) phenotype to CVB3. Spleen cells from virus-infected wild-type, lpr, and gld animals proliferate equally to virus in vitro. Adoptive transfer of γδ T cells from hearts of CVB3-infected MRL+/+ mice (FasL+) into infected MRL gld/gld recipients (FasL−/Fas+) restores both disease susceptibility and Th1 cell phenotype. However, transfer of these cells into MRL lpr/lpr recipients (FasL+/Fas−) did not promote myocarditis and the viral response remained Th2 biased. This paralleled the expression of very high surface levels of FasL by myocardial γδ T cells, as well as their propensity to selectively lyse Th2 virus-specific CD4+ T cells. These results demonstrate that Fas/FasL interactions conferred by γδ Τ cells on lymphocyte subpopulations may regulate the cytokine response to CVB3 infection and pathogenicity.
Coxsackieviruses are closely associated with myocarditis and dilated cardiomyopathy in humans. Serum levels of soluble Fas (CD95) and Fas ligand (FasL) predict disease course in patients with acute myocarditis, with the highest levels of these factors occurring in patients with a fatal outcome (11). Fas-dependent apoptosis of cardiac myocytes is observed in some cases of clinical and experimental myocarditis (10, 18, 54). In other instances, apoptosis may be restricted to the myocardial inflammatory cell infiltrate and occurs primarily in CD4+ T cells (7, 18, 27, 59). In the case of direct Fas-dependent apoptosis of cardiac myocytes, the mechanism by which Fas contributes to pathogenesis in myocarditis is self-evident. It is less clear whether Fas-dependent apoptosis affects pathogenicity when only the infiltrating inflammatory cells die.
We have previously reported that coxsackievirus B3 (CVB3)-induced myocarditis in BALB/c mice depends upon generation of CD4+ Th1 (gamma interferon [IFN-γ+]) cell responses and that CD4+ Th2 cell responses protect against CVB3-induced disease (24). T cells expressing the γδ T-cell receptor (TCR) regulate the CD4+ Th phenotype in vivo. Although cytokines produced by γδ T cells may partially contribute to their modulation of the CD4+ cell response (20), direct cell-cell contact between the γδ+ and CD4+ cells is additionally important in vitro (19). In this study, we provide evidence in vivo that γδ T-cell modulation of the CD4+ cytokine phenotype requires Fas-FasL interaction to promote a pathogenic Th1 response.
MATERIALS AND METHODS
Mice.
BALB/c, MRL+/+, MRL lpr/lpr (lpr), and MRL gld/gld (gld) mice were originally purchased from Jackson Laboratories (Bar Harbor, Maine). Breeding colonies were maintained at the University of Vermont. Male mice 5 to 8 weeks of age were used in all experiments.
Infection.
Animals received injections of 105 PFU of CVB3 (variant H3-49) intraperitoneally in 0.5 ml of phosphate-buffered saline (PBS) (24). Animals were euthanatized 7 days after infection by intraperitoneal injection of 120 mg of sodium pentobarbital/kg of body weight in 0.5 ml of PBS.
Histology.
Hearts were removed and divided in half, and the apex portion of the heart was fixed in 10% buffered formalin. Tissue was paraffin embedded, sectioned, stained with hematoxylin and eosin, and evaluated for inflammation by image analysis using the procedure reported by Knowlton et al. (29). Briefly, heart sections were viewed in transmitted light mode with an Olympus BX50 compound light microscope, and true-color digital images were captured with a Sony DXC-960MD/LLP video camera connected to a video frame grabber on a SUN SPARCstation5. Image processing and analysis were done using IMIX software (Princeton Gamma Tech Inc., Princeton, N.J.).
Virus titer.
The remaining cardiac tissue not used for histology was homogenized in RPMI 1640 medium containing 5% fetal bovine serum (FBS), centrifuged at 300 × g to remove cellular debris, serially titrated using 10-fold dilutions, and added to HeLa cell monolayers in the plaque-forming assay described previously (29).
Isolation of lymphocytes.
To purify myocardial γδ T cells, hearts were perfused with sterile PBS, minced finely, and digested three times with 0.4% collagenase II. Cells were washed, and lymphocytes were isolated by centrifugation on Histopaque (Sigma, St. Louis, Mo.). Cells were incubated at 4°C for 30 min on a rocker platform in 60-mm-diameter plastic plates previously coated with 10 μg of monoclonal anti-γδ TCR antibody (Pharmingen, San Diego, Calif.)/ml. Nonadherent cells were removed by vigorous washing of the plates. Adherent (γδ+) cells were retrieved by scraping the plate with a sterile rubber policeman, followed by washing the cells three times with medium. The proportion of γδ+ cells was determined by flow cytometry. For adoptive transfer, cells were washed twice with PBS, counted by trypan blue exclusion, and adjusted to 5 × 103 γδ+ cells/0.2 ml of PBS. Recipient mice were injected intravenously through the tail vein with 0.2 ml of the cell suspension 4 to 6 h after virus infection.
CD4+ T lymphocytes were isolated from spleens that were pressed through fine nylon mesh screens, centrifuged on Histopaque, and incubated on washed nylon wool. The nonadherent population was retrieved by washing the nylon wool, incubating with a 1:100 dilution of FcBlock (anti-FcγIII/II antibody), anti-I-Ak antibody, and anti-CD8α antibody (all from Pharmingen) for 30 min at 4°C, washing once, and then incubating with 1 ml each of magnetic particles conjugated with goat-anti-mouse immunoglobulin G (IgG) and goat anti-rat IgG (Polysciences, Inc., Warrington, Pa.) for 30 min on a rocker platform at 4°C. The magnetic particles and adherent cells were removed using two passes over a BioMag magnetic support. Remaining cells were counted by trypan blue exclusion and evaluated for purity by using flow cytometry. Approximately 105 cells were incubated with a 1:100 dilution of FcBlock and a 1:100 dilution of fluorescein isothiocyanate (FITC)-rat anti-CD4 antibody (Pharmingen) for 20 min on ice in PBS-1% bovine serum albumin (BSA), washed once in PBS-BSA, and then resuspended in 2% paraformaldehyde. Isotype controls consisted of lymphocytes incubated with FcBlock and a 1:100 dilution of FITC-rat IgG (Pharmingen). Cells were evaluated using a Coulter Epics Elite flow cytometer.
Precursor frequency analysis.
Limiting dilution analysis was performed as described by Morris et al. (36). Briefly, CD4+ lymphocytes were suspended in RPMI 1640 medium containing 10% FBS, 1 mM nonessential amino acids, 1 mM sodium pyruvate, penicillin, streptomycin, l-glutamine, and 2 × 10−5 M 2-mercaptoethanol. Between 0 and 1,000 purified CD4+ T cells were dispensed into each well of 96-well tissue culture plates together with 5 × 105 irradiated (2,000 R) syngeneic splenocytes as antigen-presenting cells and 10 μg of sucrose-purified CVB3/ml. The wells were cultured for 14 days, washed three times, and restimulated with 0.2 ml of medium containing 1 μg of concanavalin A (Life Technologies)/ml. After 24 h of incubation at 37°C, the supernatants were retrieved and assayed by enzyme-linked immunosorbent assay for IFN-γ or interleukin-4 (IL-4) by using cytokine-specific kits from Pharmingen Inc. (23). Groups consisted of 44 wells for each concentration of CD4+ cells cultured with antigen and 8 wells cultured without antigen. Estimates of precursor frequency were obtained from the maximum-likelihood method described by Good et al. (13).
Lymphocyte proliferation.
For lymphocyte proliferation assays, 106 mesenteric lymph node cells were cultured in 0.2 ml of RPMI 1640 medium as described above to which either 0 or 10 μg of sucrose-purified CVB3/ml was added. After 5 days of culture at 37°C in a humidified 5% CO2 incubator, 1 μCi of [3H]thymidine (ICN Inc., Costa Mesa, Calif.) was added overnight and the cells were harvested onto glass fiber strips. Radioactivity was determined using a Packard Liquid Scintillation counter. Groups consisted of four replicate cultures.
CD4+ T-cell clones.
CD4+ T-cell clones were produced as described previously (26). Briefly, purified CD4+ T cells were isolated from BALB/c mice infected 7 days earlier with 105 PFU of H3 virus and cultured on irradiated (3,000 R) syngeneic splenocytes preincubated for 4 h with 1 μg of sucrose-purified H3 virus/ml in RPMI 1640 medium containing 10% FBS, l-glutamine, 20 U of recombinant IL-2/ml, and either 10 ng of recombinant IL-12/ml and 10 μg of monoclonal anti-IL-4 antibody/ml or 10 ng of recombinant IL-4/ml and 10 μg of anti-IFN-γ antibody/ml (cytokines and antibodies from Pharmingen). Viable cells were recovered at 10 days by centrifugation on Histopaque (Sigma) and restimulated at 0.3 cells/well on fresh virus-loaded, irradiated antigen-presenting cells. After two limiting dilutions, clones were maintained by restimulation at 10-day intervals.
Intracellular cytokine staining.
CD4+ T-cell clones were washed, and 105 cells were incubated for 4 h at 37°C in RPMI 1640 medium with 10% FBS, 50 ng of phorbol myristate acetate/ml, 500 ng of ionomycin/ml, and 10 μg of brefeldin A (Sigma)/ml. Cells were washed and incubated in PBS-1% BSA containing 10 μg of brefeldin A/ml and a 1:100 dilution of both Cy-Chrome rat anti-mouse CD4 (clone GK1.5; Pharmingen) and FcBlock (clone 2.4G2; Pharmingen) for 20 min at 4°C. The cells were washed in PBS-BSA-brefeldin A, fixed for 10 min in 2% paraformaldehyde, and then incubated for 20 min in PBS-BSA-0.05% saponin containing a 1:100 dilution of FcBlock, FITC-rat anti-IFN-γ antibody (clone XMG 1.2; Pharmingen), and PE-rat anti-IL-4 (clone BVD4-1D11; Pharmingen). The cells were washed once in PBS-BSA-saponin and once with PBS-BSA and then resuspended in 2% paraformaldehyde and analyzed using a Coulter Epics Elite instrument with a single excitation wavelength (488 nm) and band filters for PE (575 nm), FITC (525 nm), and Cy-Chrome (670 nm). Each cell population was characterized for cell size (forward scatter) and complexity (side scatter) and then gated on the Cy-Chrome-positive (CD4+) cell population, which comprised greater than 90% of the cells. Results represent the FITC+ or PE+ cells in the Cy-Chrome+ cell population. Isotype controls were run for each fluorochrome by using Cy-Chrome, FITC, and PE rat IgG1 (clone R3-34; Pharmingen) and represented <2% of the cell populations.
Cytotoxicity assay.
CD4+ T-cell clones were washed and labeled with 100 μCi of 51Cr (Na251CrO4; ICN) for 2 h at 37°C. The cells were washed and plated into 96-well tissue culture plates at 103 cells/well. γδ+ cells were isolated from the hearts of donor mice 7 days after infection with H3 virus and were assayed at effector/target cell ratios of 5:1, 10:1, or 30:1 with the labeled CD4+ T-cell clones for 6 h at 37°C. In some wells, recombinant mouse Fas-Fc chimeric protein (R&D Systems, Minneapolis, Minn.) was added at a final concentration of 0.5 μg/ml. Half of the supernatant from each well was removed and counted using a Packard gamma counter (Packard, Downers Grove, Ill.). Maximum releasable radioactivity was determined by lysing targets with 6 N HCl. Percent lysis was calculated as follows: [(cpm of wells with γδ+ cells) − (cpm of medium control wells)]/[(cpm of HCl wells) − (cpm of medium control wells)] × 100%, where cpm is counts per minute.
Staining for surface FasL expression.
0.5 × 106 lymphocytes from spleen or hearts were stained for cell surface expression of FasL using the Enzymatic Amplification Staining Kit (EAS Kit; Flow-Amp Systems, Ltd., Cleveland, Ohio). Cells were washed twice with staining buffer (PBS, pH 7.4; 1% BSA; 1% FBS) and then incubated at 4°C for 20 min with a 6.0-pg/ml concentration of either isotype control hamster IgG-biotin or hamster anti-murine FasL-biotin (Pharmingen). After two washes with staining buffer, all samples were incubated with a 1:50 dilution of streptavidin-horseradish peroxidase secondary reagent (EAS kit) at 4°C for 20 min. Cells were subsequently washed twice with staining buffer then once with PBS, pH 7.4. Cells were reacted with a 1:20 dilution of amplifier solution (EAS Kit) at room temperature for 20 min followed by two washes with staining buffer. Cells were then stained with directly conjugated anti-γδ-FITC antibody (Pharmingen) simultaneously with streptavidin-PE (Caltag Laboratories) and incubated at 4°C for 20 min. Following two washes with staining buffer, cells were fixed in methanol-free 1% formaldehyde/PBS and stored at 4°C until analyzed by flow cytometry.
Statistics.
Statistical analysis was done by Wilcoxon Ranked Score analysis for histology, virus titers, and CD4+ T-cell phenotype. Student's t test was used for the proliferation assay.
RESULTS
Myocarditis and viral Th1 responses are diminished in mice bearing mutations of Fas (lpr) and FasL (gld).
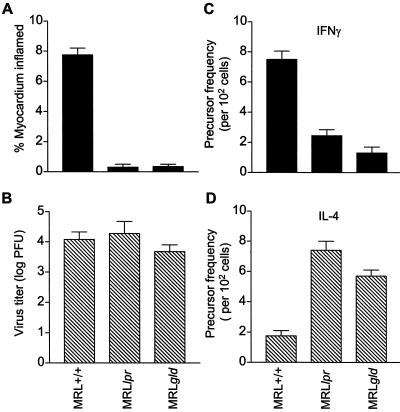
To assess the contribution by Fas in the cytokine response to CVB3 infection, MRL+/+, MRL lpr, and MRL gld mice were infected with virus and euthanatized 7 days later. Hearts were evaluated for inflammation histologically, the infiltrating lymphocytes were phenotyped, and virus concentrations were determined. In parallel with these studies, splenic CD4+ T cells were evaluated by precursor frequency analysis for virus-specific cells that produce either IFN-γ or IL-4, hallmarks, respectively, of either Th1 or Th2 responses. An example of the myocardial histology is shown in Fig. 1 and summarized for all mice in Fig. 2A. MRL+/+ animals developed significant myocardial injury with infiltrating lymphocytes, whereas both lpr and gld mice were resistant to cardiac damage. Cardiac virus titers (Fig. 2B) and antiviral antibody titers (data not shown) in all three animal groups were equivalent. CVB3-specific T-cell proliferative responses to sucrose-purified virus did not significantly differ between MRL lpr and MRL+/+ mice (10,753 ± 1,011 cpm for MRL+/+ lymphocytes stimulated with 10 μg of virus/ml compared to 9,499 ± 753 cpm for MRL lpr lymphocytes). These data confirms that MRL lpr mice respond normally to virus infection in vivo. Finally, precursor frequency analysis of CVB3-immune CD4+ T cells for either IFN-γ (Fig. 2C) or IL-4 (Fig. 2D) indicates a strong CD4+IFN-γ+ (Th1) response in MRL+/+ mice but predominant CD4+ IL-4+ (Th2) response in both MRL lpr and gld animals. This was highly reproducible in three experiments.
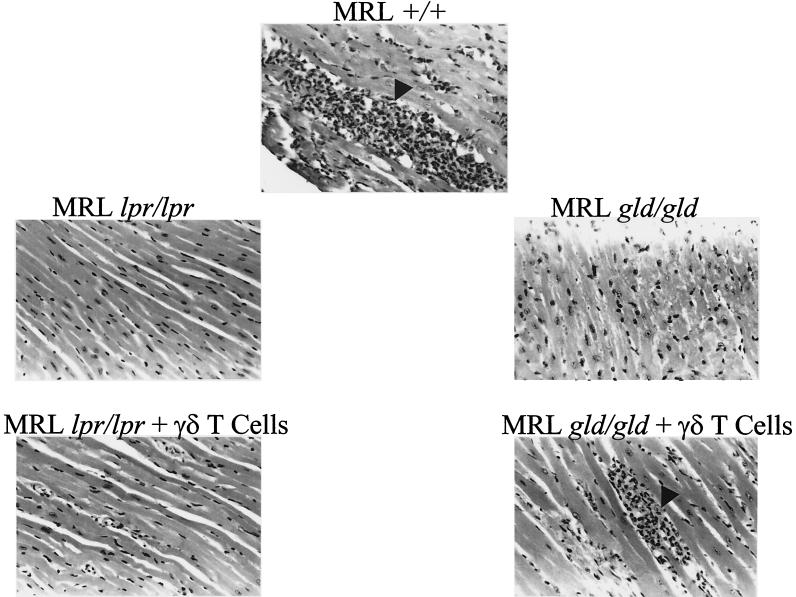
FIG. 1.
Myocarditis resistance in mice lacking Fas or FasL. Shown are representative hematoxylin-and-eosin-stained micrographs of myocardium from MRL+/+, MRL lpr, and MRL gld mice that were infected 7 days previously with CVB3. A second experiment is shown in the bottom panels of MRL lpr and MRL gld mice after CVB3 infection and simultaneous transfer of 5 × 103 syngeneic γδ T cells from spleens of CVB3-infected MRL+/+ mice. Arrowheads indicate mononuclear infiltrates in myocardium. Magnification, ×70.
FIG. 2.
Mice bearing mutations in Fas or FasL fail to develop myocarditis despite viral titers similar to those of wild-type mice. MRL+/+, MRL lpr, and MRL gld mice were infected with CVB3, and hearts were analyzed 7 days later for myocarditis as percent myocardium with infiltrates (A) and virus titer as log PFU (B). CD4+ splenocytes from the CVB3-infected mice were incubated at limiting dilution with irradiated syngeneic splenocytes and CVB3. After 14 days, positive wells were restimulated for 24 h and supernatants were assayed by enzyme-linked immunosorbent assay for IFN-γ (C) and IL-4 (D). Estimates of precursor frequency were determined by the maximum-likelihood method (13).
Adoptively transferred γδ T cells provoke Th1 response to CVB3 infection in gld mice.
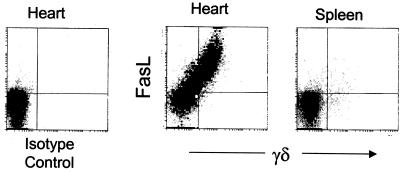
CVB3-infected wild-type MRL mice accumulated large numbers of infiltrating γδ T cells in the heart (Fig. 3) (18). The majority of myocardial γδ cells lacked expression of CD4 and CD8 but strongly expressed FasL as late as 7 days after infection. The high levels of surface FasL was confined to the γδ subset and was not observed on αβ T cells from either the hearts or spleens of the same mice (Fig. 3). We have shown previously that adoptive transfer of γδ T cells provokes a Th1 virus-specific response and myocarditis in mice that would ordinarily manifest a Th2 response and little myocarditis by a nonpathogenic CVB3 variant (24). This shift in myocarditis susceptibility and Th1 cell phenotype in recipient mice is accompanied by apoptosis in the CD4+ T-cell subpopulation. Given the lack of myocarditis in infected lpr or gld mice, combined with the observed high levels of FasL expression by myocardial γδ T cells, we examined the potential contribution of Fas to regulation of the Th1/Th2 balance by γδ T cells in vivo.
FIG. 3.
Myocardial γδ T cells express high levels of surface FasL. Cardiac infiltrating mononuclear cells were isolated from hearts and spleens of CVB3-infected MRL+/+ mice 7 days after infection and stained for expression of surface γδ TCR and FasL. Results were consistent in two separate experiments.
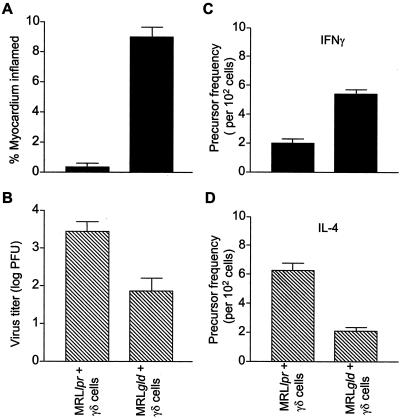
MRL lpr or MRL gld mice were infected intraperitoneally with virus and on the same day given γδ T cells intravenously that had been isolated from the hearts of CVB3-infected MRL+/+ donors. Adoptive transfer of as few as 5 × 103 γδ T cells into gld recipients restored myocarditis intensity to levels observed in wild-type animals, despite a significant (P < 0.05) reduction in cardiac virus titers (Fig. 4B). The increased pathogenicity in gld mice conferred by the γδ T cells was paralleled by a shift in virus-reactive CD4+ T cells to a Th1-like phenotype (Fig. 4C). By contrast, transfer of γδ T cells into lpr recipients only slightly reduced cardiac virus titers and had no effect on either myocardial inflammation (Fig. 4A) or the dominant Th2 phenotype (Fig. 4D). Adoptive transfer of γδ T cells isolated from the spleens of uninfected MRL+/+ mice had no effect on myocarditis, virus titers, or cytokine production (data not shown). Similarly, adoptive transfer of immune γδ T cells to uninfected wild-type mice did not provoke myocarditis (data not shown). Thus, the γδ T cells did not directly induce substantial myocardial inflammation.
FIG. 4.
Adoptive transfer of γδ T cells restores myocarditis to CVB3-infected FasL-deficient gld mice, but not to Fas-deficient lpr mice. γδ T cells were purified from the spleens of CVB-infected MRL+/+ mice and 5 × 103 cells adoptively transferred to lpr or gld mice at the time of infection with CVB3. Shown are the myocarditis scores (A) and virus titers (B) from mice infected 7 days earlier. CD4+ splenocytes were analyzed for frequency of CVB3-specific T cells producing IFN-γ (C) or IL-4 (D).
γδ T cells are selectively lytic toward Th2 CVB3-specific CD4+ T-cell clones.
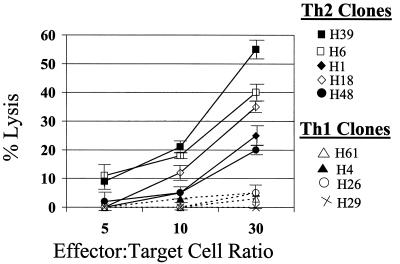
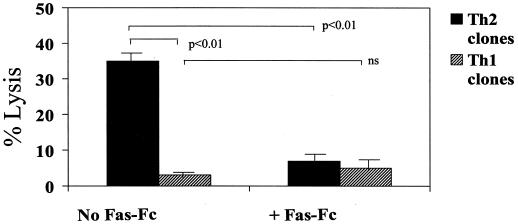
Given the Th1 predominance in CVB-infected MRL+/+ mice and the ability of myocardial γδ T cells to promote a Th1 response at least in part via FasL, we examined whether lysis of CD4+ cells by γδ+ cells might be selective, based on the cytokine profile of the target CD4+ cell. Initially, viral-specific CD4+ Th1 and Th2 clones were established from the infiltrating lymphocytes of hearts from CVB3-infected BALB/c mice by limiting dilution as described previously (26). CD4+ cell clones were stained for CD4 on their cell surface, intracellularly stained for IFN-γ and IL-4, and then analyzed by flow cytometry (Fig. 5, flow diagrams are gated on the CD4+ cell population and show intracellular cytokine expression). Five Th2 clones and four Th1 clones were 51Cr labeled and incubated at effector/target cell ratios of 5:1, 10:1, or 30:1 with enriched γδ T cells isolated from the hearts of CVB3-infected BALB/c mice. Figure 6 shows that the γδ T cells were more cytolytic toward all Th2 clones than Th1 CD4+ clones with maximal cytotoxicity occurring at the 30:1 effector/target cell ratio. To confirm that killing of the CD4+ T cells is Fas dependent, CD4+ Th2 and Th1 clones were cultured at a 30:1 effector/target cell ratio with the γδ T cells in the presence of 0.5 μg of recombinant chimeric Fas-Fc protein/ml (Fig. 7). Fas-Fc inhibited killing of all five of the Th2 cell clones but had no effect on the minimal cytolytic activity against the Th1 cell clones. Consistent with these finings, we have also observed Fas-dependent lysis selectively of CD4+ Th2 cells by human γδ T-cell clones from the synovial fluid of Lyme arthritis patients (R. C. Budd, unpublished observations).
FIG. 5.
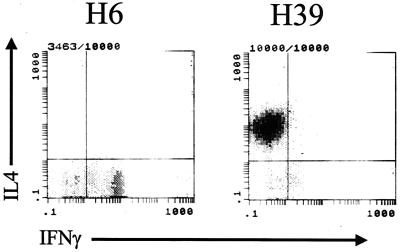
Flow analysis of cytokine production by CD4+ cell clones. CD4+ cell clones were stimulated with phorbol myristate acetate, ionomycin, and brefeldin A for 4 h, stained with Cy-Chrome anti-CD4 antibody, fixed with paraformaldehyde, permeabilized with 0.5% saponin, and stained intracellularly with FITC-anti-IFN-γ antibody and PE-anti-IL-4 antibody. Flow was gated on the CD4+ cell population, which comprised >90% of the population, and evaluated simultaneously for IFN-γ and IL-4 expression. Representative Th1 (H6) and Th2 (H39) clones are shown.
FIG. 6.
Cardiac γδ T cells are cytolytic preferentially toward Th2 targets. γδ+ T cells isolated from the hearts of CVB3-infected BALB/c mice were placed directly on 51Cr-labeled CVB3-specific CD4+ Th1 (dashed lines) or Th2 (solid lines) target cells and cytolysis was measured after 6 h. Results were consistent in two additional experiments. Results are mean percent lysis ± standard error of the mean in five replicate wells for each data point.
FIG. 7.
γδ T cells kill CD4+ Th2 cell clones through Fas-dependent mechanisms. γδ T cells were incubated at a 30:1 effector/target cell ratio on the five CD4+ Th2 and four CD4+ Th1 cell clones (51Cr labeled) represented in Fig. 6 for 6 h in medium alone or in medium containing 0.5 μg of Fas-Fc fusion protein/ml. Results are the mean percent lysis ± standard error of the mean of the Th1 and Th2 clones, with five replicate wells/clone.
DISCUSSION
These studies demonstrate that myocardium-infiltrating γδ T cells are capable of modulating cytokine profiles of CD4+ cells in vivo toward a Th1 phenotype in a manner that is at least partly Fas dependent. This likely involves the expression of Fas by CD4+ cells coupled with high and sustained levels of FasL expression by γδ T cells that are abundant at sites of myocardial inflammation.
Considerable information exists regarding the initial generation and transcriptional regulation of Th1 and Th2 cells in vitro (38, 43, 44, 52, 61). It is less certain how cytokine biases are maintained during the complex cellular interactions of an in vivo immune response to an infection. Whereas certain types of infection, such as many viruses and bacteria, can promote a Th1 response characterized by IFN-γ (32, 50), other infections such as helminths often evoke a Th2 predominance, typified by IL-4 (6). Cytokine skewing is often observed also in autoimmune syndromes. In many instances, this manifests as a Th1 predominance, as in diabetes, inflammatory arthritis, and multiple sclerosis (15, 46, 48, 60), whereas other disorders, such as systemic lupus erythematosus, may bear a Th2 phenotype (12).
At sites of inflammation in either infectious or autoimmune diseases, the cytokine profile of the infiltrating T lymphocytes is influenced by various regulatory cells. These include the type of antigen-presenting cell as well as the particular costimulatory molecules they express (17, 30, 31, 42). Areas of chronic inflammation also often contain a significant proportion of γδ T cells, even though they comprise only 2 to 5% of peripheral blood T cells (1, 2, 14, 47). The reasons for their accumulation and the nature of their function are both poorly understood. Generally most studies of infectious diseases have reported slightly more rapid clearance of infection in the presence rather than absence of γδ+ T cells (16, 28, 35, 45, 56). γδ T cells are rapidly recruited after vaccinia virus infection and are important in suppressing virus replication (49). In the present study, a decreased viral titer was indeed observed in CVB3-infected gld mice upon adoptive transfer of γδ+ T cells. This was observed to a much lower degree in lpr mice. Recently, we have shown that Vγ4+ T cells, the γδ+ Τ-cell subpopulation primarily associated with promoting CVB3-induced myocarditis in BALB/c mice (20), selectively kill infected myocytes by Fas-dependent mechanisms (unpublished data). Presumably, rapid killing of infected cells should interrupt the virus replication cycle, resulting in lowered progeny virus production. In this case, lpr mice would not show reduced virus titers in the heart since, in the absence of Fas, this form of viral clearance would not occur.
Some models of autoimmunity have reported improvement with depletion of γδ T cells, whereas others were provoked (37, 39, 40). This may reflect in part how each particular autoimmune syndrome is affected by biases toward Th1 versus Th2 cytokine environments. In other situations, different outcomes in the same autoimmune model have been observed depending upon whether the γδ+ cells were removed in vivo by genetic deletion versus antibody depletion, the latter of which would activate γδ+ cells in the process of removing them. Thus, both collagen-induced arthritis in mice (41) and adjuvant arthritis in rats (39) were made worse after the administration of anti-γδ antibody. The Th1 cytokine environment observed in inflamed joint fluid in Lyme arthritis and rheumatoid arthritis (48, 60) might reflect the influence of synovial γδ T cells, which accumulate to significant levels in the synovium in both of these diseases (2, 58). One potential problem with the use of lpr or gld mice is the known accumulation of abnormal T cells in these strains. Both lpr and gld mice accumulate large numbers of CD4−CD8− T cells over time, and these cells manifest defective IL-2 production and proliferation (5, 8, 9). However, total numbers of mature CD4+ and CD8+ T cells are also actually expanded in lpr mice (3), and in contrast to the CD4− CD8− T cells, these mature T cells are potent cytokine producers (4). In this study, we intentionally used young (5 to 8 weeks old) mice, prior to the onset of lymphadenopathy or CD4− CD8− T-cell accumulation. Consequently, the overall T-cell proliferative response and precursor frequency to CVB3 was similar among lpr, gld, and wild-type mice. Whether lpr mice show a bias toward a specific Th phenotype is controversial, with some investigators finding an imbalance towards Th1 cells (53), whereas others find increased IL-4 and Th2 responses in MRL lpr mice compared to MRL+/+ mice (33, 55). This could be influenced by the antigen stimulus. Thus, at least for CVB3 infection, we observed a pronounced Th2 bias in MRL lpr and gld mice.
Both MRL and BALB/c mice were used for these studies. We have previously shown that γδ T cells adoptively transfer susceptibility to myocarditis in BALB/c mice and modulate the CD4+ Th cytokine profile in vivo toward Th1 (20, 22, 24). Thus, the findings obtained with MRL mice are quite similar to those obtained with BALB/c mice and indicate that γδ T cells are commonly involved in myocarditis susceptibility in genetically distinct mouse strains. Current studies cannot determine whether γδ T cells determine myocarditis susceptibility exclusively through Fas/FasL regulation of CD4+ Th cell bias or whether these effectors may also directly kill either infected or uninfected cardiac myocytes by Fas ligation. The in vitro studies showing that BALB/c γδ+ T cells directly kill virus-specific CD4+ Th2 cell clones in vitro cannot exclude the possibility that the important Fas-dependent response in vivo might be the ability of γδ T cells to kill Fas+ cardiac myocytes. Also, while CD4+ T-cell clones are susceptible to γδ T-cell-mediated killing, this does not directly indicate that virus-specific CD4+ cells in vivo are equivalently susceptible to lysis. Long-term tissue culture clones may differ from CD4+ T cells in vivo. The low frequency of virus-specific CD4+ T cells in peripheral lymphoid organs makes it difficult to demonstrate direct γδ T-cell killing of noncloned CD4+ T cells. However, very similar results have been observed in human Lyme arthritis where γδ T cells are increased in the synovial fluid lymphocytes (57). Stimulation of these lymphocytes with the causative spirchochete, Borrelia burgdorferi, leads to activation of the γδ T cells and apoptosis of many of the noncloned synovial CD4+ T cells. This apoptosis is prevented by either removal of the γδ T cells before activation or addition of Fas-Fc.
Three explanations, not mutually exclusive, could account for the Th1 cytokine bias promoted by the γδ T cells used in this study. In the first instance, the γδ T cells may supply a source of high levels of surface FasL that would directly kill Fas-sensitive Th2 cells in vivo, as we observed in vitro. This would also be consistent with the known high expression of FasL mRNA by murine γδ T cells (51). At present, it is not certain why γδ T cells might selectively lyse Th2 cells unless they express a molecule recognized by γδ T cells. A second possibility is that the γδ T cells promote a Th1 cytokine environment through their release of IFN-γ or IL-12. This is a strong possibility since we have shown that the γδ T-cell subpopulation most associated with Th1 cell bias is a potent producer of IFN-γ (20). If cytokine production by the γδ T-cell subpopulation is solely responsible for CD4+ cell bias and myocarditis susceptibility, it is possible that Fas/FasL interactions may be necessary for γδ T-cell migration into the myocardium rather than for direct killing of Th2 cells in vivo. This possibility cannot be ruled out by the present data. A third possibility is that the γδ cells do not directly interact with the CD4+ cells but modulate Th phenotype by eliminating a specific antigen-presenting cell that would normally bias CD4+ cell responses towards Th2 cytokine production. Although we cannot totally eliminate this possibility, it seems unlikely since we have previously shown selective apoptosis only in the CD4+ T-cell population in the presence of γδ cells (19).
Different subsets of γδ+ T cells have divergent effects on adaptive immunity. For example, we found that γδ+ cells expressing the Vγ4 TCR make substantial amounts of IFN-γ and promote CD4+ Th1-cell responses in CVB3-infected mice, whereas Vγ1+ T cells promote CD4+ Th2-cell responses even though the Vγ1+ cells make IFN-γ (20, 21). Similar to our findings, nasally induced tolerance to ovalbumin, which provoked a Th2 response, was broken and deviated toward a Th1 phenotype upon the adoptive transfer of as few as 5,000 γδ T cells (34). Conversely, a different study of nasal ovalbumin challenge found that γδ-deficient mice manifested actually decreased specific IgE and IgG1, pulmonary IL-5 release, and decreased eosinophilic infiltration, reflecting a decrease of the Th2 response that is typical of asthma (62). In these two seemingly opposing reports the proposed mechanism of bias by the γδ+ T cells was through their production of either IFN-γ or IL-4, respectively. Our data are consistent with an additional method which involves selective cytolysis of Th2 cells by γδ+ T cells.
Acknowledgments
This work was supported by the National Institutes of Health (grants HL58583, AI45666, and AR43).
REFERENCES
- 1.Balbi, B., D. R. Moller, M. Kirby, K. J. Holroyd, and R. G. Crystal. 1990. Increased numbers of T lymphocytes with gamma delta-positive antigen receptors in a subgroup of individuals with pulmonary sarcoidosis. J. Clin. Investig. 85:1353-1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brennan, F. M., M. Londei, A. M. Jackson, T. Hercend, M. B. Brenner, R. N. Maini, and M. Feldmann. 1988. T cells expressing gamma delta chain receptors in rheumatoid arthritis. J. Autoimmun. 1:319-326. [DOI] [PubMed] [Google Scholar]
- 3.Budd, R. C., J. H. Schumacher, G. Winslow, and T. R. Mosmann. 1991. Elevated production of interferon-gamma and interleukin 4 by mature T cells from autoimmune lpr mice correlates with Pgp-1 (CD44) expression. Eur J. Immunol 21:1081-1084. [DOI] [PubMed] [Google Scholar]
- 4.Budd, R. C., N. Van Houten, J. Clements, and P. F. Mixter. 1994. Parallels in T lymphocyte development between lpr and normal mice. Semin. Immunol. 6:43-48. [DOI] [PubMed] [Google Scholar]
- 5.Clements, J., S. Cooper, and R. Budd. 1995. Abnormal regulation of the IL-2 promoter in lpr CD4−CD8− T lymphocytes results in constitutive expression of a novel nuclear factor of activated T cells-binding factor. J. Immunol. 154:6372-6381. [PubMed] [Google Scholar]
- 6.Coffman, R. L., B. W. Seymour, S. Hudak, J. Jackson, and D. Rennick. 1989. Antibody to interleukin-5 inhibits helminth-induced eosinophilia in mice. Science 245:308-310. [DOI] [PubMed] [Google Scholar]
- 7.Colston, J., B. Chandrasekar, and G. Freeman. 1998. Expression of apoptosis-related proteins in experimental coxsackievirus myocarditis. Cardiovasc. Res. 38:158-168. [DOI] [PubMed] [Google Scholar]
- 8.Davignon, J. L., L. W. Arnold, P. L. Cohen, and R. A. Eisenberg. 1991. CD3 expression, modulation and signaling in T-cell subpopulations from MRL/Mp-lpr/lpr mice. J. Autoimmun. 4:831-844. [DOI] [PubMed] [Google Scholar]
- 9.Duan, J., R. Fagard, and M. Madaio. 1996. Abnormal signal transduction through CD4 leads to altered tyrosine phosphorylation in T cells derived from MRL-lpr/lpr mice. Autoimmunity 23:231-243. [DOI] [PubMed] [Google Scholar]
- 10.Felzen, B., M. Shilkrut, H. Less, I. Sarapov, G. Maor, R. Coleman, R. Robinson, G. Berke, and O. Binah. 1998. Fas (CD95/Apo-1)-mediated damage to ventricular myocytes induced by cytotoxic T lymphocytes from perforin-deficient mice: a major role for inositol 1,4,5-triphosphate. Circ. Res. 82:438-450. [DOI] [PubMed] [Google Scholar]
- 11.Fuse, K., M. Kodama, Y. Okura, M. Ito, S. Hirono, K. Kato, H. Hanawa, and Y. Aizawa. 2000. Predictors of disease course in patients with acute myocarditis. Circulation 102:2829-2835. [DOI] [PubMed] [Google Scholar]
- 12.Fuss, I. J., W. Strober, J. K. Dale, S. Fritz, G. R. Pearlstein, J. M. Puck, M. J. Lenardo, and S. E. Straus. 1997. Characteristic T helper 2 T cell cytokine abnormalities in autoimmune lymphoproliferative syndrome, a syndrome marked by defective apoptosis and humoral autoimmunity. J. Immunol. 158:1912-1918. [PubMed] [Google Scholar]
- 13.Good, M. F., A. W. Boyd, and G. J. Nossal. 1983. Analysis of true anti-hapten cytotoxic clones in limit dilution microcultures after correction for “anti-self” activity: precursor frequencies, Ly-2 and Thy-1 phenotype, specificity, and statistical methods. J. Immunol. 130:2046-2055. [PubMed] [Google Scholar]
- 14.Groh, V., S. Porcelli, M. Fabbi, L. L. Lanier, L. J. Picker, T. Anderson, R. A. Warnke, A. K. Bhan, J. L. Strominger, and M. B. Brenner. 1989. Human lymphocytes bearing T cell receptor γ/δ are phenotypically diverse and evenly distributed throughout the lymphoid system. J. Exp. Med. 169:1277-1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Heath, W. R., J. Allison, M. W. Hoffmann, G. Schonrich, G. Hammerling, B. Arnold, and J. F. A. P. Miller. 1992. Autoimmune diabetes as a consequence of locally produced interleukin-2. Nature 359:547-549. [DOI] [PubMed] [Google Scholar]
- 16.Hiromatsu, K., Y. Yoshikai, S. Matsuzaki, K. Ohga, K. Muramoiri, K. Matsumoto, J. Bluestone, and K. Nomoto. 1992. A protective role of γ/δ T cells in primary infection with Listeria monocytogenes in mice. J. Exp. Med. 175:49-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hseih, C. S., S. E. Macatonia, C. S. Tripp, S. F. Wolf, A. O'Garra, and K. M. Murphy. 1993. Development of Th1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science 260:547-549. [DOI] [PubMed] [Google Scholar]
- 18.Huber, S. 1997. Coxsackievirus-induced myocarditis is dependent on distinct immunopathogenic responses in different strains of mice. Lab. Invest. 76:691-701. [PubMed] [Google Scholar]
- 19.Huber, S., R. Budd, K. Rossner, and M. Newell. 1999. Apoptosis in coxsackievirus B3-induced myocarditis and dilated cardiomyopathy. N. Y. Acad. Sci. 887:181-190. [DOI] [PubMed] [Google Scholar]
- 20.Huber, S. A., D. Graveline, W. K. Born, and R. L. O'Brien. 2001. Cytokine production by Vγ+-T-cell subsets is an important factor determining CD4+-Th-cell phenotype and susceptibility of BALB/c mice to coxsackievirus B3-induced myocarditis. J. Virol. 75:5860-5868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huber, S., D. Graveline, M. Newell, W. Born, and R. O'Brien. 2000. Vγ1+ T cells suppress and Vγ4+ T cells promote susceptibility to coxsackievirus B3-induced myocarditis in mice. J. Immunol. 165:4174-4181. [DOI] [PubMed] [Google Scholar]
- 22.Huber, S., A. Mortensen, and G. Moulton. 1996. Modulation of cytokine expression by CD4+ T cells during coxsackievirus B3 infections of BALB/c mice initiated by cells expressing the γδ+ T-cell receptor. J. Virol. 70:3039-3045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huber, S., and B. Pfaeffle. 1994. Differential Th1 and Th2 cell responses in male and female BALB/c mice infected with coxsackievirus group B type 3. J. Virol. 68:5126-5132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber, S., J. Polgar, P. Schultheiss, and P. Schwimmbeck. 1994. Augmentation of pathogenesis of coxsackievirus B3 infections in mice by exogenous administration of interleukin-1 and interleukin-2. J. Virol. 68:195-206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huber, S., M. Sakkinen, C. David, M. Newell, and R. Tracy. 2001. T helper cell phenotype regulates atherosclerosis in mice under conditions of mild hypercholesterolemia. Circulation 103:2610-2616. [DOI] [PubMed] [Google Scholar]
- 26.Huber, S. A., J. Kupperman, and M. K. Newell. 1999. Estradiol prevents and testosterone promotes Fas-dependent apoptosis in CD4+ Th2 cells by altering Bcl2 expression. Lupus 8:384-387. [DOI] [PubMed] [Google Scholar]
- 27.Ishiyama, S., M. Hiroe, T. Nishikawa, T. Shimojo, S. Abe, H. Fujisaki, H. Ito, K. Yamakawa, N. Kobayashi, T. Kasajima, and F. Marumo. 1998. The Fas/FasL system is involved in the pathogenesis of autoimmune myocarditis in rats. J. Immunol. 161:4695-4701. [PubMed] [Google Scholar]
- 28.Kaufmann, S. H., and C. H. Ladel. 1994. Role of T cell subsets in immunity against intracellular bacteria: experimental infections of knock-out mice with Listeria monocytogenes and Mycobacterium bovis BCG. Immunobiology 191:509-519. [DOI] [PubMed] [Google Scholar]
- 29.Knowlton, K., E. Jeon, N. Berkley, R. Wessely, and S. Huber. 1996. A mutation in the puff region of VP2 attenuates the myocarditic phenotype of an infectious cDNA of the Woodruff variant of coxsackievirus B3. J. Virol. 70:7811-7818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kuchroo, V. K., M. P. Das, J. A. Brown, A. M. Ranger, S. S. Zamvil, R. A. Sobel, H. L. Weiner, N. Nabavi, and L. H. Glimcher. 1995. B7-1 and B7-2 costimulatory molecules activate differentially the Th1/Th2 developmental pathways: application to autoimmune disease therapy. Cell 80:707-718. [DOI] [PubMed] [Google Scholar]
- 31.Le Gros, G., S. Z. Ben-Sasson, R. Seder, F. D. Finkelman, and W. E. Paul. 1990. Generation of interleukin-4 (IL-4)-producing cells in vivo and in vitro: IL-2 and IL-4 are required for in vitro generation of IL-4-producing CD4+ T cells. J. Exp. Med. 172:921-929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Locksley, R. M., F. P. Heinzel, M. D. Sadick, B. J. Holaday, and K. D. Gardner, Jr. 1987. Murine cutaneous leishmaniasis: susceptibility correlates with differential expansion of helper T-cell subsets. Ann. Inst. Pasteur Immunol. 138:744-749. [DOI] [PubMed] [Google Scholar]
- 33.Magilavy, D., K. Foys, and T. Gajewski. 1992. Liver of MRL/lpr mice contain interleukin-4-producing lymphocytes and accessory cells that support the proliferation of Th2 helper T lymphocyte clones. Eur J. Immunol. 22:2359-2365. [DOI] [PubMed] [Google Scholar]
- 34.McMenamin, C., C. Pimm, M. McKersey, and P. Holt. 1994. Regulation of IgE responses to inhaled antigen in mice by antigen-specific γ-δ+ T cells. Science 265:1869-1873. [DOI] [PubMed] [Google Scholar]
- 35.Mixter, P. F., V. Camerini, B. J. Stone, V. L. Miller, and M. Kronenberg. 1994. Mouse T lymphocytes that express a γδ T-cell antigen receptor contribute to resistance to Salmonella infection in vivo. Infect. Immun. 62:4618-4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morris, L., A. Troutt, E. Handman, and A. Kelso. 1992. Changes in the precursor frequencies of IL-4 and IFN-γ secreting CD4+ cells correlate with resolution of lesions in murine cutaneous leishmaniasis. J. Immunol. 149:2715.. [PubMed] [Google Scholar]
- 37.Mukasa, A., K. Hioromatsu, G. Matsuzaki, R. O'Brien, W. Born, and K. Nomoto. 1995. Bacterial infection of the testis leading to autoaggressive immunity triggers apparently opposed responses of α-β and γ-δ T cells. J. Immunol. 155:2047.. [PubMed] [Google Scholar]
- 38.Paul, W. E., and R. A. Seder. 1994. Lymphocyte response and cytokines. Cell 76:241-251. [DOI] [PubMed] [Google Scholar]
- 39.Pelegri, C., P. Kuhlein, E. Buchner, C. B. Schmidt, A. Franch, M. Castell, T. Hunig, F. Emmrich, and R. W. Kinne. 1996. Depletion of γδ T cells does not prevent or ameliorate, but rather aggravates rat adjuvant arthritis. Arthritis Rheum. 38:204-215. [DOI] [PubMed] [Google Scholar]
- 40.Peng, S. L., M. P. Madaio, A. C. Hayday, and J. Craft. 1996. Propagation and regulation of systemic autoimmunity by γδ T cells. J. Immunol. 157:5689-5698. [PubMed] [Google Scholar]
- 41.Peterman, G. M., C. Spencer, A. I. Sperling, and J. A. Bluestone. 1993. Role of γδ T cells in murine collagen-induced arthritis. J. Immunol. 151:6546-6558. [PubMed] [Google Scholar]
- 42.Rincon, M., J. Anguita, T. Nakamura, E. Fikrig, and R. A. Flavell. 1997. Interleukin (IL)-6 directs the differentiation of IL-4 producing CD4+ T cells. J. Exp. Med. 185:461-469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rincon, M., B. Derijard, C. W. Chow, R. J. Davis, and R. A. Flavell. 1997. Reprogramming the signaling requirement for AP-1 (activator protein-1) activation during differentiation of precursor CD4+ T-cells into effector Th1 and Th2 cells. Genes Funct. 1:51-68. [DOI] [PubMed] [Google Scholar]
- 44.Rincon, M., and R. A. Flavell. 1997. Transcription mediated by NFAT is highly inducible in effector CD4+ T helper 2 (Th2) cells but not in Th1 cells. Mol. Cell. Biol. 17:1522-1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosat, J. P., H. R. MacDonald, and J. A. Louis. 1993. A role for γδ+ T cells during experimental infection of mice with Leishmania major. J. Immunol. 150:550-555. [PubMed] [Google Scholar]
- 46.Ruddle, N. H., C. M. Bergman, K. M. McGrath, E. G. Lingenheld, M. L. Grunnet, S. J. Padula, and R. B. Clark. 1990. An antibody to lymphotoxin and tumor necrosis factor prevents transfer of experimental allergic encephalomyelitis. J. Exp. Med. 172:1193-1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rust, C., Y. Kooy, S. Pena, M. L. Mearin, P. Kluin, and F. Koning. 1992. Phenotypical and functional characterization of small intestinal TcR γδ+ T cells in coeliac disease. Scand J. Immunol. 35:459-468. [DOI] [PubMed] [Google Scholar]
- 48.Saxne, T., M. A. Palladino, D. Heinegard, N. Talal, and F. A. Wollheim. 1988. Detection of tumor necrosis factor alpha but not tumor necrosis factor beta in rheumatoid arthritis synovial fluid and serum. Arthritis Rheum. 31:1041-1051. [DOI] [PubMed] [Google Scholar]
- 49.Selin, L. K., P. A. Santolucito, A. K. Pinto, E. Szomolanyi-Tsuda, and R. M. Welsh. 2001. Innate immunity to viruses: control of vaccinia virus infection by γδ T cells. J. Immunol. 166:6784-6794. [DOI] [PubMed] [Google Scholar]
- 50.Street, N. E., J. H. Schumacher, T. A. Fong, H. Bass, D. F. Fiorentino, J. A. Leverah, and T. R. Mosmann. 1990. Heterogeneity of mouse helper T cells. Evidence from bulk cultures and limiting dilution cloning for precursors of Th1 and Th2 cells. J. Immunol. 144:1629-1639. [PubMed] [Google Scholar]
- 51.Suda, T., T. Okazaki, Y. Naito, T. Yokota, N. Arai, S. Ozaki, K. Nakao, and S. Nagata. 1995. Expression of the Fas ligand in cells of T cell lineage. J. Immunol. 154:3806-3813. [PubMed] [Google Scholar]
- 52.Szabo, S. J., S. T. Kim, G. L. Costa, X. Zhang, C. G. Fathman, and L. H. Glimcher. 2000. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell 100:655-669. [DOI] [PubMed] [Google Scholar]
- 53.Takahashi, S., L. Fossati, M. Iwamoto, R. Merino, R. Motta, T. Kobayakawa, and S. Izui. 1996. Imbalance towards Th1 predominance is associated with acceleration of lupus-like autoimmune syndrome in MRL mice. J. Clin. Investig. 97:1597-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Toyozaki, T., M. Hiroe, M. Tanaka, S. Nagata, H. Ohwada, and F. Marumo. 1998. Levels of soluble Fas ligand in myocarditis. Am. J. Cardiol. 82:246-248. [DOI] [PubMed] [Google Scholar]
- 55.Tsai, C., T. Wu, S. Huang, K. Sun, S. Hsieh, S. Han, H. Yu, and C. Yu. 1995. Abnormal splenic and thymic IL-4 and TNF-alpha expression in MRL-lpr/lpr mice. Scand J. Immunol. 41:157-163. [DOI] [PubMed] [Google Scholar]
- 56.Tsuji, M., P. Mombaerts, L. Lefrancois, R. S. Nussenzweig, F. Zavala, and S. Tonegawa. 1994. γδ T cells contribute to immunity against the liver stages of malaria in αβ T-cell-deficient mice. Proc. Natl. Acad. Sci. USA 91:345-349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vincent, M., K. Roessner, D. Lynch, S. M. Cooper, L. H. Sigal, and R. C. Budd. 1996. Apoptosis of Fashigh CD4+ synovial T cells by Borrelia-reactive Fas-ligandhigh γδ T cells in Lyme arthritis. J. Exp Med. 184:2109-2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Vincent, M. S., K. Roessner, T. Sellati, C. D. Huston, L. H. Sigal, S. M. Behar, J. D. Radolf, and R. C. Budd. 1998. Lyme arthritis synovial γδ T cells respond to Borrelia burgdorferi lipoproteins and lipidated hexapeptides. J. Immunol. 161:5762-5771. [PubMed] [Google Scholar]
- 59.Yamada, T., A. Matsumori, W. Wang, N. Ohashi, K. Shiota, and S. Sasayama. 1999. Apoptosis in congestive heart failure induced by viral myocarditis in mice. Heart Vessels 14:29-37. [DOI] [PubMed] [Google Scholar]
- 60.Yssel, H., M. C. Shanafelt, C. Soderberg, P. V. Schneider, J. Anzola, and G. Peltz. 1991. Borrelia burgdorferi activates a T helper type 1-like T cell subset in Lyme arthritis. J. Exp. Med. 174:593-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang, D. H., L. Cohn, P. Ray, K. Bottomly, and A. Ray. 1997. Transcription factor GATA-3 is differentially expressed in murine Th1 and Th2 cells and controls Th2-specific expression of the interleukin-5 gene. J. Biol. Chem. 272:21597-21603. [DOI] [PubMed] [Google Scholar]
- 62.Zuany-Amorim, C., C. Ruffie, S. Haile, B. B. Vargaftig, P. Pereira, and M. Pretolani. 1998. Requirement for γδ T cells in allergic airway inflammation. Science 280:1265-1267. [DOI] [PubMed] [Google Scholar]