Abstract
Interferon regulatory factor 1 (IRF-1), IRF-3, and IRF-7 have been tested as genetic adjuvants for influenza virus hemagglutinin (HA) and nucleoprotein vaccine DNAs. Cotransfection of HA with IRF-3 and IRF-7 increased CD4 T-cell responses by 2- to 4-fold and CD8 T-cell responses by more than 10-fold. Following intramuscular deliveries of DNA, both CD4 and CD8 T cells were biased towards type 1 immune responses and the production of gamma interferon. Following gene gun bombardments of DNA, both were biased towards type 2 immune responses and the production of interleukin-4. The biases of the T-cell responses towards type 1 or type 2 were stronger for immunizations with IRF-3 as an adjuvant than for immunizations with IRF-7 as an adjuvant. Moderate adjuvant effects for antibody were observed. The isotypes of the antibody responses reflected the method of DNA delivery; intramuscular deliveries of DNA predominantly raised immunoglobulin G2a (IgG2a), whereas gene gun deliveries of DNA predominantly raised IgG1. These biases were enhanced by the codelivered IRFs. Overall, under the conditions of our experiments, IRF-3 had good activity for T cells, IRF-7 had good activity for both antibody and T cells, and IRF-1 had good activity for antibody.
A central challenge for DNA-based immunization is producing sufficient antigen (Ag) and the correct inflammatory signals to achieve strong immune responses. DNA-based immunizations are like viral infections in producing immunizing proteins within the cells of the host. Unmethylated CpG motifs in plasmids provide adjuvant activity for the expressed Ags by stimulating the Toll 9 receptor (11). Codelivered genes for lymphokines, chemokines, and costimulatory molecules can provide additional adjuvant activity for DNA vaccines (for reviews, see references 4 and 31). Most genetic adjuvants have had only two- to threefold effects. In this study, we examine the adjuvant activity of codelivered interferon regulatory factor (IRF) genes for DNA vaccines. IRFs are transcription factors that serve as mediators for the activation of early inflammatory responses to viral infections. The hypothesis for the study was that DNA vaccines would raise stronger immune responses if transfected cells expressed the early inflammatory factors expressed by virus-infected cells.
IRFs play a critical role in the activation of alpha interferon (IFN-α) and IFN-β as well as interferon-stimulated genes and some chemokines. IFN-α and IFN-β, in turn, stimulate both innate and acquired immune responses. IFN-α promotes differentiation of dendritic cells (17), enhances humoral immunity (7), induces the polarization of CD4 cells to T helper 1 (Th1) effector cells (7), and confers protection to CD8 cells from Ag-induced cell death (19). To date, nine cellular IRFs (24, 35) and three viral IRFs (3, 16, 22) have been identified. Two cellular IRFs, IRF-3 and IRF-7, serve as direct transducers of virus-mediated signaling pathways (1, 2, 36). In infected cells, IRF-3 and IRF-7 are phosphorylated at carboxy-terminal serines and are retained in the nucleus where IRF-3 interacts with the transcription coactivator CBP/p300 (14, 40) and IRF-7 interacts with p300 CBP-associated-factor (unpublished results). The expression of IRF-3 is sufficient for the induction of IFN-β in infected cells (12). The expression of IRF-7 is critical for the induction of IFN-α (18, 34, 39). Down regulation or null mutations in IRF-3 inhibit IFN-α/β genes while defects in both IRF-3 and IRF-7 completely abolish IFN-α/β expression. IRF-1 also activates IFN-α and IFN-β in infected cells (10, 21). However, IFN-α/β induction can be IRF-1 independent (20, 29).
In this study, we test whether IRF-1, IRF-3, and IRF-7 serve as genetic adjuvants for influenza virus hemagglutinin (HA) or nucleoprotein (NP) genes. Vaccines were administered by intramuscular (i.m.) saline injections of DNA that raise Th1-biased responses and by gene gun (g.g.) inoculations that raise Th2-biased responses to DNA-expressed HA and NP (6, 27). The IRF genetic adjuvants had different effects on immune responses. IRF-1 primarily increased antibody (Ab) responses, IRF-3 primarily increased T-cell responses, and IRF-7 increased both Ab and T-cell responses. For both i.m. and g.g. deliveries, the T helper bias conferred by the method of delivery determined the T helper bias of the IRF adjuvant.
MATERIALS AND METHODS
Vaccine plasmids.
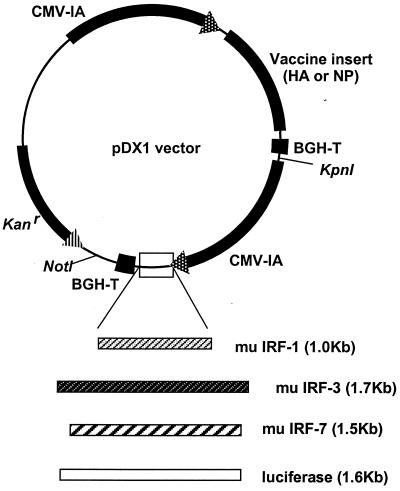
All immunogen and adjuvant inserts were amplified from parental plasmids by PCR and subcloned into the pGA vector (32) or the related pDX1 dual-expression vector (33) by using unique restriction endonuclease sites (Fig. 1). Influenza virus A/PR/8/34 (H1N1) HA and NP were amplified from pJW4303/H1 (30) and pCMV/NP (27). Murine IRF-1 was obtained from pCDM8/mIRF-1 provided by T. Taniguchi (University of Tokyo, Tokyo, Japan). Murine IRF-3 (pBPSRT1/muIRF-3) and IRF-7 (pcDNA3/muIRF-7) (18) were provided by D. Levy (New York University, New York, N.Y.). Plasmids were gown in Escherichia coli DH5α and purified by using Qiagen columns (Qiagen, Valencia, Calif.) according to the manufacturer's instructions.
FIG. 1.
Dual promoter expression vectors. Immunogens are expressed by the first promoter, and IRFs are expressed by the second promoter. The luciferase gene is used as a mock insert. CMV-IA, CMV immediate early promoter including intron A; BGH-T, bovine growth hormone termination sequence; Kanr, kanamycin resistance gene; mu, murine.
The expression of the HA and NP vaccine plasmids was verified by Western blotting. To verify the expression of IRFs, the murine fibroblast cell line L929 was cotransfected with 2 μg of chloramphenicol acetyltransferase (CAT) plasmid driven by the IFN-β promoter, 2 μg of an IRF plasmid, and 0.5 μg of a β-galactosidase-expressing plasmid. The transfected cells were split 14 h later, and 10 h later they were infected with Sendai virus at a multiplicity of infection of 5. Following an additional 16 h, protein extracts were prepared by freezing and thawing and were assayed for CAT activity to assess the effect of the IRFs on the transcriptional activity of the IFN-β promoter (28). The level of expression of the cotransfected β-galactosidase plasmid was used to normalize the differences in transfection efficiency.
DNA inoculations.
Six- to 8-week-old female BALB/c mice (Harlan Sprague-Dawley, Indianapolis, Ind.) were inoculated by i.m. injection or g.g. bombardment as previously described (30). For i.m. injections, various doses of DNA in 50 μl of saline were injected into the quadriceps. Particle bombardment by g.g. was done on freshly shaved abdominal skin (Accel; Geniva, Middleton, Wis.). Each inoculation consisted of two nonoverlapping shots of DNA-coated gold beads (DeGussa-Huls Corp., Ridgefield Park, N.J.) at a helium pressure of 400 lb/in2. Gene gun deliveries of IRF and vaccine DNAs were accomplished by loading a mixture of single-expression vectors on gold beads. All immunizations were done at suboptimal doses of DNA to facilitate the detection of adjuvant effects. The care and use of mice followed institutional guidelines for the handling and care of laboratory animals.
ELISPOT analyses.
For enzyme-linked immunospot (ELISPOT) analyses, splenocytes were harvested 2 weeks after a booster immunization and processed as previously described (26, 33). The capture Abs were anti-mouse IFN-γ and interleukin-4 (IL-4) (R4-6A2 or BVD4-1D11; Pharmingen, San Diego, Calif.), and the detection Abs were biotin-conjugated anti-mouse IFN-γ and IL-4 (XMG1.2 or BVD6-24G2; Pharmingen). For the HA immunogen, an H-2d-restricted HA class I peptide (IYSTVASSL) (5) and a pool of five H-Iad class II peptides (SFERFEIFPKE, HNTNGVTAACSH, CPKYVRSAKLRM, KLKNSYVNKKGK, and NAYVSVVTSNYNRRF) (9) were prepared in RPMI medium and tested in a concentration range from 10−2 to 10−6 M to identify the optimal concentrations for the stimulation of IFN-γ (10−4 M) and IL-4 (10−5 M). For the NP immunogen, an H-2d-restricted NP class I peptide (TYQRTRALV) (5) and a pool of three H-Iad class II peptides (FWRGENGKTRSAYERMCNILKGK, RLIQNSLTIERMVLSAFDERRNK, and AVKGVGTMVMELIRMIKRGINDRN) (8) were tested in a similar way and the peptides were used at a concentration of 10−5 M for both IFN-γ and IL-4 (8). Media containing an irrelevant peptide and phorbol myristate acetate plus ionomycin (50 ng of phorbol myristate acetate/ml and 1 μg of ionomycin/ml) were used as negative and positive controls, respectively. For in vitro stimulations, 1,000,000 cells were incubated in duplicate in the presence of the optimal concentration of peptide and 2 μg of anti-CD28 and anti-CD49d Abs (37.51 and R1-2, respectively; Pharmingen)/ml for 40 h at 37°C in a humidified atmosphere containing 5% CO2.
Intracellular cytokine assays.
Approximately 1 × 106 splenocytes were stimulated in 96-well flat bottom plates with either class I or class II peptide, each at a concentration of 10−4 M, for 2 h at 37°C in a volume of 200 μl of RPMI medium containing 10% fetal calf serum and 1 μg of anti-mouse CD28 and anti-mouse CD49d costimulatory Abs (Pharmingen, Inc.) per ml. Then monensin (10 μg/ml) was added, and the cells were cultured for an additional 4 h at 37°C under 5% CO2. Cells were surface stained with anti-CD8 antibodies conjugated to PerCP (clone 53-6.7; Pharmingen) and with anti-CD4 antibodies conjugated to allophycocyanin (clone RM4-5; Pharmingen) at 8 to 10°C for 30 min, washed twice with cold phosphate-buffered saline (PBS) containing 2% fetal bovine serum, and fixed and permeabilized with Cytofix-Cytoperm solution (Pharmingen). Cells were then incubated with antibodies to mouse IFN-γ (clone XMG1.2; Pharmingen) conjugated to fluorescein isothiocyanate in Perm wash solution (Pharmingen) for 30 min at 4°C. Cells were washed twice with Perm wash and once with plain PBS and resuspended in 1% paraformaldehyde in PBS. Approximately 200,000 events were acquired on the FACSCalibur and analyzed with FloJo software (Tree Star, San Carlos, Calif.).
ELISA and HA inhibition.
Sera were assayed by enzyme-linked immunosorbent assays (ELISAs) to measure specific immunoglobulin G (IgG), IgG1, and IgG2a as previously described (30). The ELISA used pooled sera with known concentrations of anti-influenza virus IgG, IgG1, and IgG2a as standards. The HA inhibition test was performed with turkey red blood cells (25).
RESULTS
Expression of IRFs and Ags.
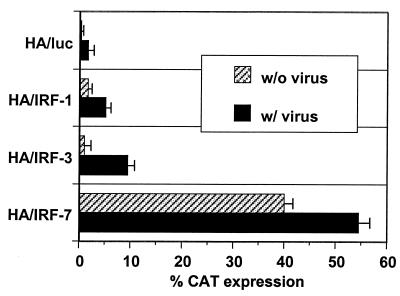
IRF-1, IRF-3, and IRF-7 were coexpressed with HA and NP immunogens by using the pDX1 dual-expression vector (Fig. 1) or expressed alone with the pGA expression vector (32). The expression of the IRFs was verified in transient cotransfections of IRF plasmids with a reporter plasmid containing the CAT gene under the regulation of the IFN-β promoter region. Cotransfected cells were or were not subsequently infected with Sendai virus. IRF-1 and IRF-3 activated the IFN-β promoter only in the presence of viral infection. Consistent with previous findings, IRF-7 activated the promoter both in the presence and in the absence of Sendai virus infection (Fig. 2) (39). In this in vitro transfection assay, the relative activities of the cotransfected IRFs for activation of the IFN-β reporter in the presence of a viral infection were IRF-7 > IRF-3 > IRF-1.
FIG. 2.
Transactivation of the IFN-β promoter by pDX1/HA/IRF constructs. L929 cells were transfected with IFN-β promoter-CAT reporter plasmids and HA-IRF dual-expression constructs as indicated. At 24 h posttransfection, cells were infected with Sendai virus, and CAT activity was measured in the cell lysates at 40 h posttransfection. All transfections were normalized to a constant level of β-galactosidase used as an internal control. Error bars represent standard errors of the means.
Humoral immune responses.
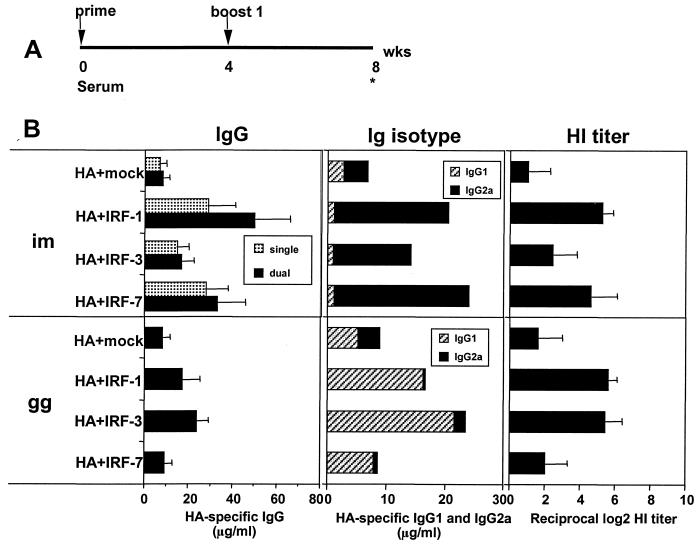
Both the method of DNA delivery and the codelivered IRF influenced the adjuvant activity for Ab to HA (Fig. 3). Mice were inoculated at 0 and 4 weeks by i.m. saline inoculations with 10 μg of HA DNA mixed with 10 μg of an IRF DNA for single-expression plasmids and with 20 μg of the pDX1/HA/IRF dual-expression constructs. Gene gun inoculations used gold beads coated with 0.1 μg of HA DNA, 1.0 μg of an IRF DNA, and 0.9 μg of mock DNA per shot. Sera were collected 4 weeks after the booster immunization (Fig. 3A). Suboptimal levels of vaccine plasmids were used in the experiments to enhance the ability to detect adjuvant effects. Following i.m. deliveries of DNA, IRF-1 and IRF-7 increased the titers of Ab 4- to 6-fold, whereas <2-fold increases occurred with IRF-3 (Fig. 3B, upper left panel). Following g.g. inoculations, IRF-1 and IRF-3 caused two- to threefold increases in the titers of Ab, whereas IRF-7 had no effect. HA inhibition titers measured at the endpoint dilution of serum which inhibited influenza virus-mediated hemagglutination, exhibited a similar trend as the IgG ELISAs (Fig. 3B, right hand panels). The Ig isotype biases for the IRF adjuvant responses were the same as for the non-IRF adjuvant responses; saline injections predominantly raised IgG2a and g.g. inoculations predominantly raised IgG1. However, for both i.m. and g.g. inoculations, the cotransfected IRFs enhanced the isotype biases, skewing responses from 1- to 2-fold to 10- to 20-fold preferences for IgG2a or IgG1, respectively.
FIG. 3.
Adjuvant effects of codelivered IRFs for Ab responses. (A) Immunization schedule. (B) HA-specific IgG response, isotype profile, and HA inhibition (HI) titer. For i.m. immunizations, single designates groups injected with 10 μg of HA-expressing DNA (pJW4303/H1) and 10 μg of an IRF-expressing DNA (pGA/IRF). The dual groups were injected with 20 μg of pDX1/HA/IRF constructs. For g.g. immunization, gold beads delivered 0.1 μg of pJW4303/H1, 1.0 μg of pGA/IRF, and 0.9 μg of mock DNA per shot. Data are expressed as the geometric means of 4 to 5 individual mice ± standard deviations (error bars). Similar results were obtained in two experiments. In a control experiment, no specific Ab was raised by the delivery of 20 μg of the IRF DNAs in the absence of cotransfected HA DNA.
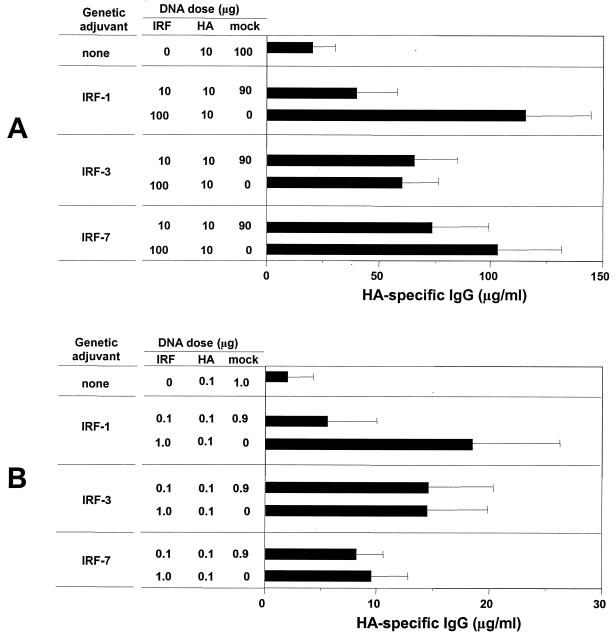
Tests for the dose dependence of the IRF effects on Ab responses revealed that increased doses of the IRF DNAs increased Ab responses for IRF-1 but not for IRF-3 or IRF-7 (Fig. 4). Single-expression vectors for HA, the IRFs, and a mock DNA (vector without insert) were mixed to achieve a 10-fold difference in the amount of the IRF plasmid but a constant dose of HA and total DNA. Mice were immunized at 0 and 4 weeks, and the sera were analyzed for Ab at 8 weeks. The 10-fold increase in the concentration of IRF-1 DNA but not IRF-3 or IRF-7 DNA increased the Ab response by about threefold for both i.m. (Fig. 4A) and g.g. (Fig. 4B) deliveries of DNA. These results indicated the low dose of IRF DNA (10 μg for i.m. inoculations and 0.1 μg for g.g. inoculations) had placed us on the plateau for adjuvant effects for Ab for IRF-3 and IRF-7 but not for IRF-1.
FIG. 4.
Effect of the dose of IRF DNA on raised Ab. (A) For i.m. immunizations, mice were injected with 10 μg of HA-expressing DNA immunogen (pJW4303/H1) and the indicated doses (10 or 100 μg) of an IRF-expressing DNA (pGA/IRF). The low-dose groups were supplemented with 90 μg of mock DNA to adjust the total amount of DNA to 110 μg. (B) For g.g. immunization, gold beads delivered 0.1 μg of pJW4303/H1 and the indicated dose (0.1 or 1.0 μg) of pGA/IRF and the low-dose groups were adjusted with 0.9 μg of mock DNA to a total of 1.1 μg of DNA per shot. Data are expressed as the geometric means of 4 to 5 individual mice ± standard deviations (error bars). The immunization schedule was the same as depicted in Fig. 3A.
Cell-mediated immune responses.
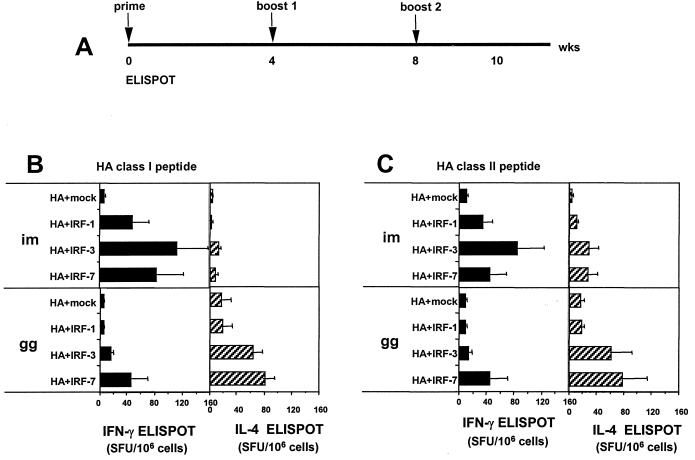
Codelivered IRF-3 and IRF-7 had good adjuvant activities for T-cell responses against HA (Fig. 5). For these studies, three DNA immunizations were given at 0, 4, and 8 weeks and ELISPOT analyses were conducted on peptide-stimulated splenocytes 2 weeks after the third DNA immunization (Fig. 5A). The use of IRF-3 and IRF-7 resulted in >10-fold increases in the number of class I peptide-stimulated IFN-γ-producing CD8 splenocytes (Fig. 5B). Codelivered IRF-1 had a more modest effect on the CD8 cell response. The i.m. deliveries of DNA only raised background levels of IL-4-producing CD8 cells. In contrast, the g.g. deliveries of DNA raised both IL-4- and IFN-γ-producing class I peptide-specific CD8 cells with the frequencies of IL-4-producing cells being about twice that of IFN-γ-producing cells. IRF-3 and IRF-7 had the best adjuvant activities for g.g.-raised CD8 cells, increasing the frequencies of IL-4-producing cells by four- to fivefold.
FIG. 5.
Effect of IRF genetic adjuvants codelivered with HA DNA on the frequencies of HA-specific CD8 and CD4 T cells. (A) Immunization schedule. (B) HA-specific cytokine production by splenocytes stimulated with an H-2d-restricted HA class I peptide. (C) HA-specific cytokine production by splenocytes stimulated with a pool of H-Iad-restricted HA class II peptides. For i.m. immunizations, mice were injected with 20 μg of the dual-expression constructs; for g.g. immunizations, gold beads delivered 0.2 μg of the dual-expression constructs. Spot forming units (SFU) are the means of 4 to 5 individual mice ± standard deviations (error bars) per million cells. Cells treated with an irrelevant peptide showed <10 SFU/106 cells for both cytokines. In a control experiment, no specific ELISPOTs (<10 SFU/106 splenocytes) were raised by the delivery of 20 μg of the IRF DNAs in the absence of cotransfected HA DNA.
IRF adjuvant effects for CD4 T cells were not as great as those for CD8 T cells (Fig. 5C). CD4 responses were measured following in vitro stimulation of splenocytes with a pool of H-Iad class II peptides. Again, IRF-3 and IRF-7 had the highest adjuvant activities. Following i.m. delivery of DNA, most peptide-specific CD4 cells were IFN-γ producing, whereas following g.g. immunizations, most cells were IL-4 producing. IRF-7 raised more mixed responses than IRF-3 following both i.m. and g.g. deliveries of DNA, with differences in the frequencies of IFN-γ- and IL-4-producing cells being <2-fold.
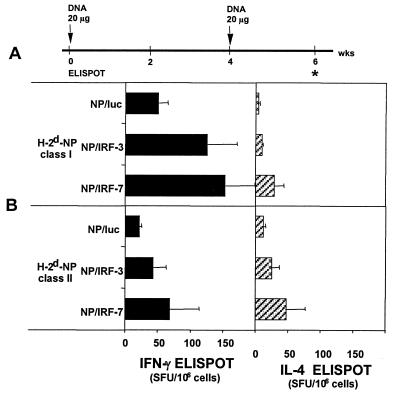
The effects of the codelivered IRF genetic adjuvants were tested for i.m. immunizations with NP that has an immunodominant epitope in BALB/c mice (5). Both IRF-3 and IRF-7 increased the frequency of NP-specific CD8 cells and CD4 cells as measured in the ELISPOT assay (Fig. 6). These enhancement effects were not as great (≤3 times) as had been observed for the codelivered HA gene.
FIG. 6.
Effect of IRF genetic adjuvants codelivered with NP DNA on the frequencies of NP-specific CD8 and CD4 T cells. (A) Immunization schedule. (B) NP-specific cytokine production by splenocytes stimulated with an H-2d-restricted NP class I peptide and a pool of H-Iad-restricted NP class II peptides. Mice were injected with 20 μg of the dual-expression constructs. Spot forming units (SFU) are the means of 4 to 5 individual mice ± standard deviations (error bars) per million cells. Cells treated with an irrelevant peptide showed <10 SFU/106 cells for both cytokines.
Intracellular cytokine assay for IFN-γ responses.
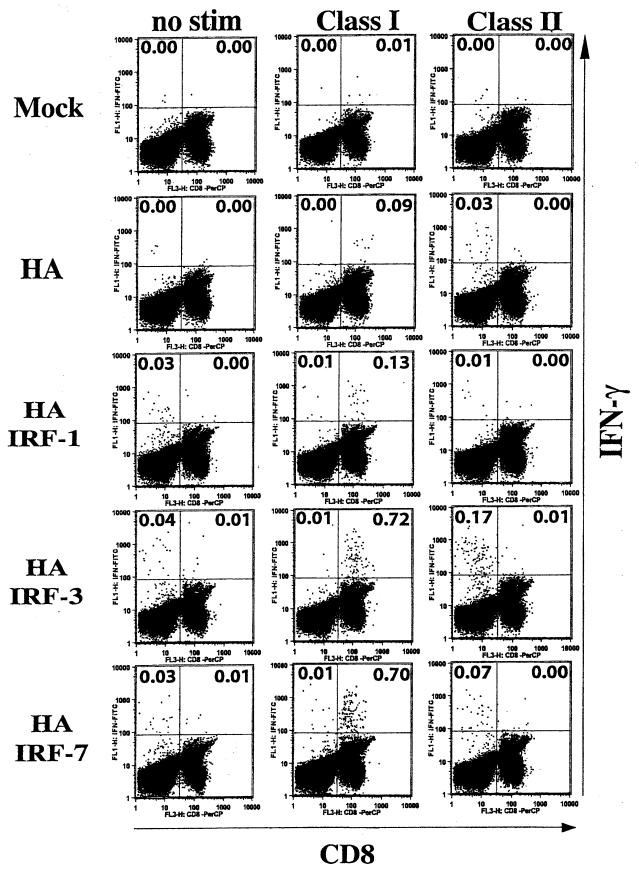
Intracellular cytokine assays for IFN-γ-producing cells also revealed marked increases in the frequencies of HA-specific CD8 cells following i.m. codelivery of HA and IRF-3 or IRF-7 DNA (Fig. 7). Intracellular cytokine assays were conducted on splenocytes stimulated for 6 h in vitro with class I or class II peptides. The results of these assays clearly demonstrated >7-fold increases in the assisted CD8 responses for codelivered IRF-3 and IRF-7. Again, increases in CD4 responses were lower than the increases in CD8 responses and resulted in only two- to fourfold enhancements in the frequency of IFN-γ-producing cells. IRF-1 had smaller effects on the magnitude of the cellular responses.
FIG. 7.
Intracellular cytokine analyses. Splenocytes from selected mice in the experiment presented in Fig. 5 were stimulated with either class I or class II peptides for 6 h or not stimulated (no stim). Cells were stained with antibodies to CD8 conjugated to PerCP, antibodies to CD4 conjugated to allophycocyanin, and antibodies to mouse IFN-γ conjugated to fluorescein isothiocyanate. Cells were initially gated on lymphocytes and followed by CD4- and CD8-positive cells. Frequencies are for the percent specific CD4 cells of the total CD4 cells (upper left quadrants) and for the percent specific CD8 of the total CD8 cells (upper right quadrants).
DISCUSSION
Our results reveal that IRF-3 and IRF-7 serve as genetic adjuvants for DNA-raised CD8 responses. Following i.m. deliveries of DNA, codelivered IRF-3 and IRF-7 increased the frequencies of IFN-γ-specific CD8 cells for HA by >10-fold (Fig. 5B). Following g.g. deliveries of DNA, the codelivered IRFs were associated with increases in both IFN-γ- and IL-4-secreting CD8 ELISPOTs (Fig. 5B and C). The strong adjuvant effects of IRF-3 and IRF-7 for IFN-γ-producing HA-specific CD8 cells were verified with intracellular cytokine staining (Fig. 7). Attempts to verify the IL-4-producing ELISPOTs by intracellular cytokine assays were not successful due to technical difficulties in detecting IL-4. Codelivery of IRF-3 and IRF-7 with HA resulted in stronger adjuvant effects for CD8 cells than did codelivery of these IRFs with NP (Fig. 6B). This may reflect a higher activity of the IRF adjuvants for the subdominant HA epitope than for the immunodominant NP epitope (5). Codelivery of IRF-1 had weaker effects on T-cell responses. This, however, may be due to the use of the dual-expression vectors in which HA and IRF-1 were equimolar rather than single-expression vectors where higher ratios of IRF-1 to the immunogen could have been tested (Fig. 4).
The effects on raised CD4 cells were lower than those on raised CD8 cells, with most of the effects on CD4 cells being only three- to fivefold (Fig. 5-7). For IRF-3 adjuvant responses, i.m. inoculations raised predominantly IFN-γ-producing T cells whereas g.g. deliveries raised predominantly IL-4-producing T cells. This would be consistent with i.m. IRF-3 adjuvant responses being biased towards type 1 cells that produce IFN-γ but not IL-4 and with g.g. IRF-3 adjuvant responses being biased towards type 2 cells that produce IL-4 but not IFN-γ. IRF-7 adjuvant effects resulted in approximately equal frequencies of IFN-γ- and IL-4-producing CD4 cells. These could have reflected Th0 cells, which produce both IFN-γ and IL-4, or a mixture of Th1 and Th2 cells.
IRF-1 and IRF-7 showed the most consistent adjuvant activities for Ab responses. The magnitude of the adjuvant effect for IRF-1 depended on the amount of codelivered IRF DNA and increased in magnitude when 10 times more of the IRF-1 plasmid than the vaccine plasmid was coadministered (Fig. 4). The Ab responses, in the presence or absence of codelivered IRF genes, followed the biases towards IgG2a (i.m. immunizations) or IgG1 (g.g. immunizations) that are characteristically raised by these two different methods of DNA delivery (Fig. 3) (6). Interestingly, the extent of the skewing of these isotype biases were enhanced by the codelivered IRF DNAs.
Mechanistic studies to determine whether IRF-induced adjuvant activities were mediated by IFN-α and IFN-β did not meet with success. Analysis of the muscle target sites by reverse transcription-PCR at 48 h after DNA delivery failed to detect any transcripts for IFN-α, IFN-β, and the p40 IL-12 genes (data not shown). Performance of i.m. immunizations in the presence of Abs to IFN-α revealed a switch in the apparent T helper type from Th1 to Th2 but did not affect the magnitude of the response (unpublished observations).
During viral infections, IRF-3 and IRF-7 are activated by phosphorylation (14, 18, 39, 40). At this point we do not know whether the IRF adjuvant effects required phosphorylation or were the result of overexpression of IRF-3 and IRF-7. Both IRF-3 and IRF-7 can be detected in the nucleus in the absence of viral infections (39), and overexpression of IRF-3 or IRF-7 in uninfected cells activates the expression of IFN-β or IFN-α, respectively (12, 15). Recently, it has been shown that both IRF-3 and IRF-7 activate expression of the histone 3 gene in uninfected cells, indicating that posttranslational modification by phosphorylation may not be a requirement for transactivation (38). In a transient transfection assay in mouse fibroblasts, IRF-7, but not IRF-3, activated the promoter for the IFN-β gene in the absence of viral infection (Fig. 2). Phosphorylation of IRF-3 can occur in response to double-stranded RNA (37), DNA damaging agents (13), and lipopolysaccharide (23) and could potentially occur as a result of unmethylated CpG motifs interacting with the Toll 9 receptor (11). This latter interpretation would be consistent with the stronger adjuvant effects observed for i.m. than for g.g. deliveries of DNA. Following i.m. DNA immunizations, microgram levels of extracellular DNA are at least transiently available for the stimulation of Toll receptors.
In summary, our results reveal IRF-3 and IRF-7 serving as adjuvants for IFN-γ-producing CD8 cells following i.m. injections of DNA. Our results also reveal IRF-3 serving as a stronger Th1 adjuvant than IRF-7 in i.m. immunizations. And finally, our results reveal IRF-7 and IRF-1 serving as genetic adjuvants for Ab responses. The magnitude of the adjuvant effects depend on the codelivered Ag (Fig. 5 and 6).
Acknowledgments
This research was supported by U.S. Public Health Service research grant R01 AI34946 from the National Institute of Allergy and Infectious Diseases and by base grant support RR00165 from the National Center for Research Resources to the Yerkes National Primate Research Center.
We are indebted to Helen Drake-Perrow for expert administrative assistance.
REFERENCES
- 1.Au, W. C., P. A. Moore, D. W. LaFleur, B. Tombal, and P. M. Pitha. 1998. Characterization of the interferon regulatory factor-7 and its potential role in the transcription activation of interferon A genes. J. Biol. Chem. 273:29210-29217. [DOI] [PubMed] [Google Scholar]
- 2.Au, W. C., P. A. Moore, W. Lowther, Y. T. Juang, and P. M. Pitha. 1995. Identification of a member of the interferon regulatory factor family that binds to the interferon-stimulated response element and activates expression of interferon-induced genes. Proc. Natl. Acad. Sci. USA 92:11657-11661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Burysek, L., W. S. Yeow, B. Lubyova, M. Kellum, S. L. Schafer, Y. Q. Huang, and P. M. Pitha. 1999. Functional analysis of human herpesvirus 8-encoded viral interferon regulatory factor 1 and its association with cellular interferon regulatory factors and p300. J. Virol. 73:7334-7342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen, A. D., J. D. Boyer, and D. B. Weiner. 1998. Modulating the immune response to genetic immunization. FASEB J. 12:1611-1626. [PubMed] [Google Scholar]
- 5.Deng, Y., J. W. Yewdell, L. C. Eisenlohr, and J. R. Bennink. 1997. MHC affinity, peptide liberation, T cell repertoire, and immunodominance all contribute to the paucity of MHC class I-restricted peptides recognized by antiviral CTL. J. Immunol. 158:1507-1515. [PubMed] [Google Scholar]
- 6.Feltquate, D. M., S. Heaney, R. G. Webster, and H. L. Robinson. 1997. Different T helper cell types and antibody isotypes generated by saline and gene gun DNA immunization. J. Immunol. 158:2278-2284. [PubMed] [Google Scholar]
- 7.Finkelman, F. D., A. Svetic, I. Gresser, C. Snapper, J. Holmes, P. P. Trotta, I. M. Katona, and W. C. Gause. 1991. Regulation by interferon alpha of immunoglobulin isotype selection and lymphokine production in mice. J. Exp. Med. 174:1179-1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao, X. M., F. Y. Liew, and J. P. Tite. 1989. Identification and characterization of T helper epitopes in the nucleoprotein of influenza A virus. J. Immunol. 143:3007-3014. [PubMed] [Google Scholar]
- 9.Gerhard, W., A. M. Haberman, P. A. Scherle, A. H. Taylor, G. Palladino, and A. J. Caton. 1991. Identification of eight determinants in the hemagglutinin molecule of influenza virus A/PR/8/34 (H1N1) which are recognized by class II-restricted T cells from BALB/c mice. J. Virol. 65:364-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada, H., T. Fujita, M. Miyamoto, Y. Kimura, M. Maruyama, A. Furia, T. Miyata, and T. Taniguchi. 1989. Structurally similar but functionally distinct factors, IRF-1 and IRF-2, bind to the same regulatory elements of IFN and IFN-inducible genes. Cell 58:729-739. [DOI] [PubMed] [Google Scholar]
- 11.Hemmi, H., O. Takeuchi, T. Kawai, T. Kaisho, S. Sato, H. Sanjo, M. Matsumoto, K. Hoshino, H. Wagner, K. Takeda, and S. Akira. 2000. A Toll-like receptor recognizes bacterial DNA. Nature 408:740-745. [DOI] [PubMed] [Google Scholar]
- 12.Juang, Y., W. Lowther, M. Kellum, W. C. Au, R. Lin, J. Hiscott, and P. M. Pitha. 1998. Primary activation of interferon A and interferon B gene transcription by interferon regulatory factor 3. Proc. Natl. Acad. Sci. USA 95:9837-9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim, T., T. Y. Kim, Y. H. Song, I. M. Min, J. Yim, and T. K. Kim. 1999. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 274:30686-30689. [DOI] [PubMed] [Google Scholar]
- 14.Lin, R., C. Heylbroeck, P. M. Pitha, and J. Hiscott. 1998. Virus-dependent phosphorylation of the IRF-3 transcription factor regulates nuclear translocation, transactivation potential, and proteasome-mediated degradation. Mol. Cell. Biol. 18:2986-2996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin, R., Y. Mamane, and J. Hiscott. 2000. Multiple regulatory domains control IRF-7 activity in response to virus infection. J. Biol. Chem. 275:34320-34327. [DOI] [PubMed] [Google Scholar]
- 16.Lubyova, B., and P. M. Pitha. 2000. Characterization of a novel human herpesvirus 8-encoded protein, vIRF-3, that shows homology to viral and cellular interferon regulatory factors. J. Virol. 74:8194-8201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luft, T., K. C. Pang, E. Thomas, P. Hertzog, D. N. Hart, J. Trapani, and J. Cebon. 1998. Type I IFNs enhance the terminal differentiation of dendritic cells. J. Immunol. 161:1947-1953. [PubMed] [Google Scholar]
- 18.Marie, I., J. E. Durbin, and D. E. Levy. 1998. Differential viral induction of distinct interferon-alpha genes by positive feedback through interferon regulatory factor-7. EMBO J. 17:6660-6669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marrack, P., J. Kappler, and T. Mitchell. 1999. Type I interferons keep activated T cells alive. J. Exp. Med. 189:521-530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matsuyama, T., T. Kimura, M. Kitagawa, K. Pfeffer, T. Kawakami, N. Watanabe, T. M. Kundig, R. Amakawa, K. Kishihara, A. Wakeham, et al. 1993. Targeted disruption of IRF-1 or IRF-2 results in abnormal type I IFN gene induction and aberrant lymphocyte development. Cell 75:83-97. [PubMed] [Google Scholar]
- 21.Miyamoto, M., T. Fujita, Y. Kimura, M. Maruyama, H. Harada, Y. Sudo, T. Miyata, and T. Taniguchi. 1988. Regulated expression of a gene encoding a nuclear factor, IRF-1, that specifically binds to IFN-beta gene regulatory elements. Cell 54:903-913. [DOI] [PubMed] [Google Scholar]
- 22.Moore, P. S., C. Boshoff, R. A. Weiss, and Y. Chang. 1996. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science 274:1739-1744. [DOI] [PubMed] [Google Scholar]
- 23.Navarro, L., and M. David. 1999. p38-dependent activation of interferon regulatory factor 3 by lipopolysaccharide. J. Biol. Chem. 274:35535-35538. [DOI] [PubMed] [Google Scholar]
- 24.Nguyen, H., J. Hiscott, and P. M. Pitha. 1997. The growing family of interferon regulatory factors. Cytokine Growth Factor Rev. 8:293-312. [DOI] [PubMed] [Google Scholar]
- 25.Palmer, D. F., M. T. Coleman, W. R. Dowdle, and G. C. Schild. 1975. Advanced laboratory techniques for influenza diagnosis. U.S. Department of Health, Education, and Welfare, Washington, D.C.
- 26.Pertmer, T. M., A. E. Oran, J. M. Moser, C. A. Madorin, and H. L. Robinson. 2000. DNA vaccines for influenza virus: differential effects of maternal antibody on immune responses to hemagglutinin and nucleoprotein. J. Virol. 74:7787-7793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pertmer, T. M., T. R. Roberts, and J. R. Haynes. 1996. Influenza virus nucleoprotein-specific immunoglobulin G subclass and cytokine responses elicited by DNA vaccination are dependent on the route of vector DNA delivery. J. Virol. 70:6119-6125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raj, N. B., W. C. Au, and P. M. Pitha. 1991. Identification of a novel virus-responsive sequence in the promoter of murine interferon-alpha genes. J. Biol. Chem. 266:11360-11365. [PubMed] [Google Scholar]
- 29.Reis, L. F., H. Ruffner, G. Stark, M. Aguet, and C. Weissmann. 1994. Mice devoid of interferon regulatory factor 1 (IRF-1) show normal expression of type I interferon genes. EMBO J. 13:4798-4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Robinson, H. L., C. A. Boyle, D. M. Feltquate, M. J. Morin, J. C. Santoro, and R. G. Webster. 1997. DNA immunization for influenza virus: studies using hemagglutinin- and nucleoprotein-expressing DNAs. J. Infect. Dis. 176:S50-S55. [DOI] [PubMed] [Google Scholar]
- 31.Robinson, H. L., and T. M. Pertmer. 2000. DNA vaccines for viral infections: basic studies and applications. Adv. Virus Res. 55:1-74. [DOI] [PubMed] [Google Scholar]
- 32.Ross, T. M., Y. Xu, R. Bright, and H. L. Robinson. 2000. C3d enhancement of anti-hemagglutinin antibodies protects DNA vaccinated mice after influenza challenge. Nat. Immunol. 1:127-131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sasaki, S., R. R. Amara, A. E. Oran, J. M. Smith, and H. L. Robinson. 2001. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19:543-547. [DOI] [PubMed] [Google Scholar]
- 34.Sato, M., H. Suemori, N. Hata, M. Asagiri, K. Ogasawara, K. Nakao, T. Nakaya, M. Katsuki, S. Noguchi, N. Tanaka, and T. Taniguchi. 2000. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-alpha/beta gene induction. Immunity 13:539-548. [DOI] [PubMed] [Google Scholar]
- 35.Taniguchi, T., K. Ogasawara, A. Takaoka, and N. Tanaka. 2001. Irf family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19:623-655. [DOI] [PubMed] [Google Scholar]
- 36.Wathelet, M. G., C. H. Lin, B. S. Parekh, L. V. Ronco, P. M. Howley, and T. Maniatis. 1998. Virus infection induces the assembly of coordinately activated transcription factors on the IFN-beta enhancer in vivo. Mol. Cell 1:507-518. [DOI] [PubMed] [Google Scholar]
- 37.Weaver, B. K., K. P. Kumar, and N. C. Reich. 1998. Interferon regulatory factor 3 and CREB-binding protein/p300 are subunits of double-stranded RNA-activated transcription factor DRAF1. Mol. Cell. Biol. 18:1359-1368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xie, R., A. J. van Wijnen, C. van Der Meijden, M. X. Luong, J. L. Stein, and G. S. Stein. 2001. The cell cycle control element of histone H4 gene transcription is maximally responsive to interferon regulatory factor pairs IRF-1/IRF-3 and IRF-1/IRF-7. J. Biol. Chem. 276:18624-18632. [DOI] [PubMed] [Google Scholar]
- 39.Yeow, W. S., W. C. Au, Y. T. Juang, C. D. Fields, C. L. Dent, D. R. Gewert, and P. M. Pitha. 2000. Reconstitution of virus-mediated expression of interferon alpha genes in human fibroblast cells by ectopic interferon regulatory factor-7. J. Biol. Chem. 275:6313-6320. [DOI] [PubMed] [Google Scholar]
- 40.Yoneyama, M., W. Suhara, Y. Fukuhara, M. Fukuda, E. Nishida, and T. Fujita. 1998. Direct triggering of the type I interferon system by virus infection: activation of a transcription factor complex containing IRF-3 and CBP/p300. EMBO J. 17:1087-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]