Abstract
Human herpesvirus 6 (HHV-6) is a lymphotropic betaherpesvirus that productively infects T cells and monocytes. HHV-6 isolates can be differentiated into two groups, variants A and B (HHV-6A and HHV-6B). Here, we show a functional difference between HHV-6A and -6B in that HHV-6A induced syncytium formation of diverse human cells but HHV-6B did not. The syncytium formation induced by HHV-6A was observed 2 h after infection; moreover, it was found in the presence of cycloheximide, indicating that HHV-6A induced fusion from without (FFWO) in the target cells. Furthermore, the fusion event was dependent on the expression of the HHV-6 entry receptor, CD46, on the target cell membrane. In addition, we determined that short consensus repeat 2 (SCR2), -3, and -4 of the CD46 ectodomain were essential for the formation of the virus-induced syncytia. Monoclonal antibodies against glycoproteins B and H of HHV-6A inhibited the fusion event, indicating that the syncytium formation induced by HHV-6A required glycoproteins H and B. These findings suggest that FFWO, which HHV-6A induced in a variety of cell lines, may play an important role in the pathogenesis of HHV-6A, not only in lymphocytes but also in various tissues, because CD46 is expressed ubiquitously in human tissues.
Human herpesvirus 6 (HHV-6) was first isolated from the peripheral blood of patients with AIDS and lymphoproliferative disorders (49). HHV-6 isolates can be classified into at least two groups, variant A (HHV-6A) and variant B (HHV-6B). HHV-6B is the causal agent of exanthem subitum (62). The two variants can be differentiated on the basis of genomic polymorphism, antigenicity, and host cell tropism (1, 3, 7, 61).
Seroepidemiological evidence and viral isolation have associated HHV-6 infection with several diseases, which include fulminant hepatitis, mononucleosis-like diseases, immune disorders, lymphoproliferative diseases, and chronic fatigue syndrome, and with patients receiving liver, cardiac, bone marrow, or renal transplants (39)
The main target of HHV-6 is lymphocytes of the T-cell lineage. In vitro, HHV-6 infects and replicates at its highest titers in peripheral blood mononuclear cells and umbilical cord blood mononuclear cells (CBMCs). Outside of the primary T lymphocytes, the two viral variants display different host ranges. Typically, variant A viruses replicate in HSB-2 cells, whereas variant B viruses grow in the less-differentiated Molt-3 T-cell lines (8). HHV-6A has also been reported to infect some glial cell lines, as well as primary astrocytes (19) and endothelial cells (46, 60), and is known to form giant syncytia with multiple nuclei, whereas HHV-6B does not replicate and form syncytia in these cells very well. In addition, in susceptible human T lymphocytes, the virus induces cell-cell fusion that results in the formation of large polykaryocytes. It has been suggested that HHV-6 induces cell-cell fusion as a cytopathic effect that results from viral replication in the T cells and other cell types.
It is not yet known how HHV-6 forms large polykaryocytes in the target cells. Viruses are known to induce cell-cell fusion by two different processes. One is fusion from without (FFWO), and the other is fusion from within (FFWI). FFWO is the induction of cell fusion by viruses in the absence of viral protein synthesis. It requires a high multiplicity of infection (MOI) and is mediated by proteins present in the infecting virions. The best-studied FFWO-inducing viruses are members of the Paramyxoviridae (4). These viruses penetrate the cell by pH-independent fusion of the virion envelope with the plasma membrane. The cell fusion mediated by these viruses is thought to occur by a process similar to viral entry (48). Infections with wild-type strains of herpes simplex virus type 1 (HSV-1) cause rounding-up of the infected cells. However, numerous HSV-1 mutants causing syncytium formation (FFWO and FFWI) have been isolated. These mutants have been called syncytial (Syn) mutants (48).
In the present study, we show that HHV-6A, but not HHV-6B, mediates FFWO in a variety of cells expressing human CD46. In other words, HHV-6A induced cell-cell fusion in target cells without viral protein synthesis through human CD46. In addition, we determined that short consensus repeat 2 (SCR2), SCR3, and SCR4 of the CD46 ectodomain were required for virus-induced multinucleated polykaryocyte formation.
MATERIALS AND METHODS
Cells, viruses, and antibodies.
Vero (African green monkey kidney), 293T (a human kidney cell line), HEL (a primary human embryonic lung cell line), U373 (a glioblastoma cell line), BALB/c 3T3 (a mouse cell line), and OMK (an owl monkey kidney cell line) cells were grown in Dulbecco's modified Eagle's medium, supplemented with 10% fetal calf serum (FCS). Chinese hamster ovary (CHO) cells and CHO cells stably expressing CD46 isoforms (24) or CD46 deletion mutants (25) were grown in Ham's F-12 medium with 10% FCS. CHO cells stably expressing CD46 were prepared as described previously (25). Briefly, eukaryotic expression vectors containing CD46 cDNA species were transfected into CHO cells. Transfected CHO cells were maintained in Ham's F-12 medium with 10% FCS supplemented with 0.5 mg of G-418 or hygromycin B/ml. Colonies resistant to G-418 or hygromycin B were isolated. Expression of CD46 was monitored by flow cytometry with monoclonal antibodies (MAbs) as described below. Several T-cell lines, MT4, Sup-T1, HSB-2, Jurkat, and Molt-3 cells, and CBMCs were cultured in RPMI 1640 with 10% FCS. HHV-6A strains U1102 and GS, HHV-6B strain HST, and clinical isolates KYM and NYM were propagated on CBMCs, and titers of the HHV-6A and HHV-6B viruses were estimated by using Sup-T1 and MT4 cells, respectively (33).
HHV-6 cell-free virus was prepared as described previously (32). When HHV-6-infected CBMCs showed evidence of more than 80% infection by immunofluorescence antibody assay (IFA), the cells were lysed by freezing and thawing them twice and spun at 1,500 × g for 10 min. The supernatant was used as cell-free virus.
MAbs against human CD46, M75, M160, M177, and E4.3 were reported previously (25). J4-48 was purchased from Beckman Coulter. An MAb against HHV-6A glycoprotein H (gH), 2E4, was generously provided by G. Campadelli-Fiume (University of Bologna, Bologna, Italy) (16). A MAb against HHV-6A gB, 87-y-13 (56), a MAb against HHV-6B REP/U94, which is expressed in HHV-6-infected cells and which is a nonstructural protein, REP A1 (10), and a MAb against HHV-6 early protein, OHV-2 (32), were described previously. Rabbit antisera specific for HHV-6 immediate-early protein IE1 (12, 18, 23) were generated from rabbits injected with the glutathione S-transferase (GST)-IE1 fusion protein. A DNA fragment comprising the full-length IE1 gene open reading frame was amplified by PCR and inserted in frame into the pGEX-4T-1 bacterial expression vector (Amersham Pharmacia). The resultant GST-IE1 fusion protein was expressed in Escherichia coli DH5α and was affinity purified and used to raise antibodies (Abs) in rabbits.
Virus infection.
To monitor HHV-6 infection, Vero, 293T, HEL, U373, BALB/c 3T3, OMK, CHO, and CHO-CD46 (STC/CYT2; human CHO cells stably expressing CD46) (25) cells (5 × 105 per well) were seeded on glass coverslips, grown to 80% confluence, and overlaid with 105 HHV-6-infected CBMCs or Sup-T1 cells (nearly 80% positive for HHV-6 late antigen by IFA) or infected with cell-free HHV-6 at 103 to 105 50% tissue culture infective doses (TCID50) at 37°C for 60 min by centrifugation (1,500 × g) and grown on glass coverslips in a six-well plate. On days 1 to 3 after inoculation, the cell monolayers were washed three times with phosphate-buffered saline (PBS), fixed with acetone, and probed with an MAb specific for HHV-6 early antigen (OHV-2) and rabbit serum against HHV-6 IE1.
Flow cytometry analysis.
The protein levels expressed on the cells (5 × 105) were assessed by flow cytometry as described previously using anti-CD46 (M177, E4.3) (25) and a fluorescein isothiocyanate (FITC)-labeled secondary Ab. Cells were grown for 2 days at 37°C and then were harvested into PBS and reacted with MAbs to CD46 in PBS containing 0.2% bovine serum albumin (BSA) for 1 h on ice. Cells were washed in PBS with 2% BSA, spun down, reacted with FITC-labeled anti-mouse immunoglobulin G in PBS with 2% BSA for 30 min on ice, and washed. Samples containing 5 × 105 cells were analyzed in a FACS analyzer IV (Becton Dickinson, Mountain View, Calif.).
Assay for syncytium formation.
Cells were grown in chamber slides, and then they were then washed with PBS, fixed with Bouin solution (picric acid, formalin, acetic acid [15:5:1]), and stained with hematoxylin and eosin (HE). The total number of nuclei in polykaryocytes with more than five nuclei was counted under the microscope.
FFWO assays.
To determine whether HHV-6A induced FFWO, phosphonoformic acid (PFA), which inhibits viral DNA synthesis, was used. Vero cells (2 × 105 to 5 × 105 per well) were seeded on glass coverslips, grown to 80% confluence, and overlaid with 105 HHV-6-infected CBMCs (nearly 90% positive for HHV-6 late antigen by IFA) or infected with cell-free HHV-6 at 104 TCID50 by centrifugation for 60 min and then cultured with medium supplemented with PFA (300 μg/ml) and fixed 24 h after infection.
Vero or MT4 cells were treated with 50 μg of cycloheximide (CHX)/ml for 1 h and spun with virus (HHV-6A or HHV-6B) stock containing CHX at 37°C for 1 h at 1,500 × g. After incubation with 50 μg of CHX/ml at 37°C for 2 h, the cells were fixed and stained with HE.
Virus stock (500 μl) was placed in a 35-mm tissue culture dish (IWAKI) and irradiated at 2,500 J/m2 with UV light, and Vero, MT4, and Sup-T1 cells were infected with the UV-inactivated virus for 1 h by centrifugation. After incubation at 37°C for 17 h, the cells were fixed and stained with HE. UV-treated virus stocks failed to express detectable HHV-6 immediate-early protein IE1 when examined by immunofluorescence staining 17 h after infection (data not shown).
Neutralization assays of polykaryocyte formation.
Vero cells were infected with strain U1102, which had been preincubated with 10 μl of a 50-fold dilution of a specific HHV-6A MAb against gH (2E4) (16), a specific HHV-6A MAb against gB (87-y-13) (56), or a specific HHV-6 MAb against U94/REP (REP A1) (10) in a final volume of 0.5 ml at 37°C for 1 h. After infection as described above, the cells were rinsed with PBS twice, and the medium was replaced. The cells were fixed and stained with HE 24 h later.
RESULTS
HHV-6A strain U1102 mediates syncytium formation in Vero and HEL cells, but HHV-6B strain HST does not.
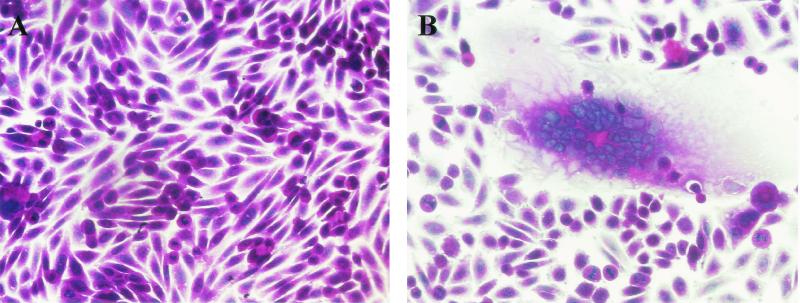
Vero and HEL cells were overlaid with HHV-6A (strain U1102), HHV-6B (strain HST), or mock-infected CBMCs. On day 1 after infection, the Vero and HEL cells were fixed and stained with HE.
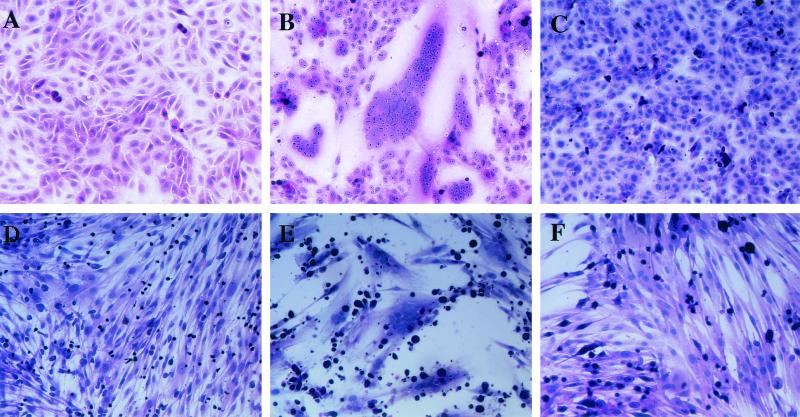
Multinuclear cells were found in the Vero and HEL cultures (Fig. 1B and E) that were overlaid with U1102-infected CBMCs or Sup-T1 cells, but they were not found in Vero or HEL cells overlaid either with HST- or mock-infected CBMCs or MT4 cells (Fig. 1C and F). These results indicate that HHV-6A, but not HHV-6B, can form polykaryocytes in Vero and HEL cells. Consistent with this result, both the HHV-6A GS and U1102 strains induced syncytia in Vero and HEL cells but the other HHV-6B strains, which were two clinical isolates, did not (data not shown).
FIG. 1.
HHV-6A U1102-induced syncytia in Vero cells. Vero cells were overlaid with mock-infected CBMCs (A), HHV-6A U1102-infected CBMCs (B), or HHV-6B HST-infected CBMCs (C). HEL cells were overlaid with mock-infected Sup-T1 cells (D), U1102-infected Sup-T1 cells (E), or HST-infected MT4 cells (F). After 24 h, cells were fixed and stained with HE. Photographs of representative cell monolayers are shown. The results are from one of three experiments performed.
To examine whether cell-free viruses could induce syncytium formation, Vero cells were centrifuged with U1102 and HST at 37°C for 1 h. The multinucleated cells were observed in the Vero cell cultures inoculated with U1102 at an MOI of 0.01 or 0.1 (Fig. 2) but were not seen in the cultures inoculated with HST at an MOI of 0.1 (data not shown).
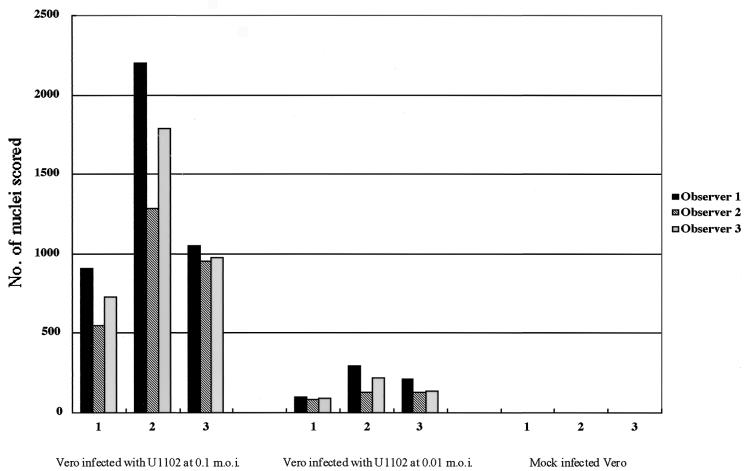
FIG. 2.
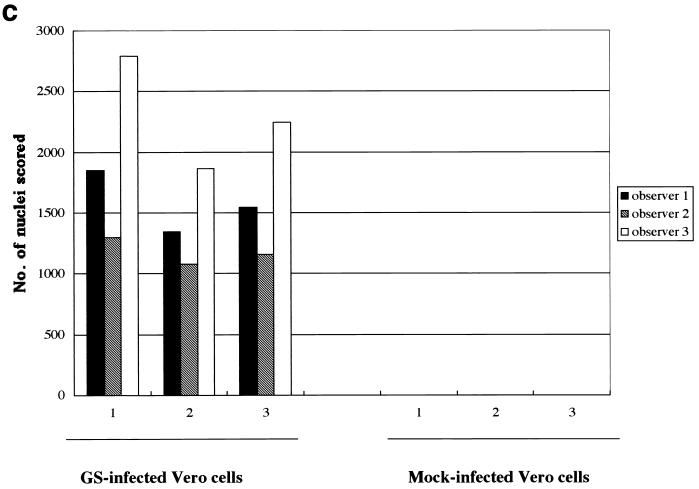
Variation in fusion scored in three experiments. Shown is the total number of nuclei in polykaryocytes with more than five nuclei, scored by each observer, on 28-mm2 areas of Vero cells inoculated with U1102 at MOIs of 0.1 and 0.01 by centrifugation.
To quantify the extent of fusion induced, experimental and control wells were fixed and all the nuclei in polykaryocytes were counted in a blinded fashion by three independent observers. The total number of nuclei in polykaryocytes with more than five aggregated nuclei were scored. Figure 2 shows the results obtained by the three observers for 28-mm2 areas. U1102 inoculation resulted in 500- to 1,500-fold more nuclei involved in fusion events than were seen in control cultures, and the number of nuclei per polykaryocyte depended on the infectious unit titer of the virus.
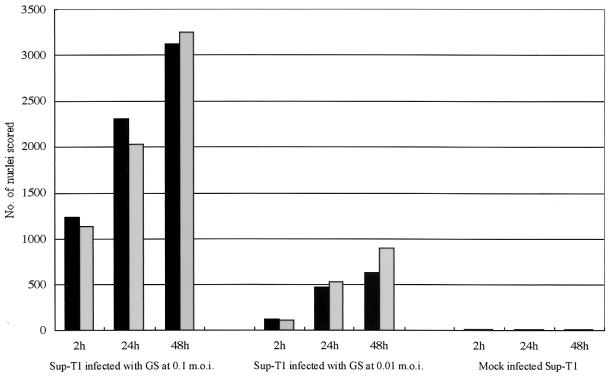
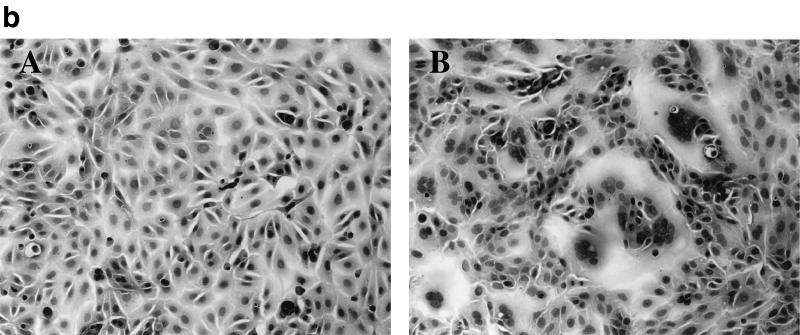
The cells (Vero, HEL, U373, and Sup-T1) were inoculated with U1102 by centrifugation, cultured, and fixed at several time points after infection. As shown in Fig. 3, the total number of nuclei in Sup-T1 cells gradually increased over 48 h, and large syncytia containing a large number of nuclei had increased in size by 48 h postinfection (p.i.).
FIG. 3.
Time course of syncytium formation induced by HHV-6A strain GS. GS infected Sup-T1 cells were fixed at different times after infection (2, 24, and 48 h p.i) and stained with HE. Total numbers of nuclei in polykaryocytes with more than five nuclei were scored on 20-mm2 areas of Sup-T1 cells inoculated with GS at MOIs of 0.1 and 0.01. The mean scores of three independent observers are plotted. The two types of bars shown for each set of conditions are from duplicate experiments.
HHV-6A induces FFWO in a variety of human cells.
The U1102-mediated syncytium formation of Vero cells was seen 2 h after infection. This result led us to speculate that U1102 could cause FFWO in HEL and Vero cells. To investigate this possibility, syncytium formation in Vero cells overlaid with U1102-infected CBMCs in the presence of inhibitor of viral DNA polymerase PFA (300 μg/ml) was examined (Fig. 4a, panel B). Syncytium formation was observed in Vero cells overlaid with U1102-infected CBMCs but not in Vero cells overlaid with CBMCs (Fig. 4a, panel A). Polykaryocytes were also observed in the Vero cells inoculated with UV-inactivated U1102 (Fig. 4b, panel B). Furthermore, the polykaryocytes were induced by U1102 or GS in Vero cells in the presence of CHX (50 μg/ml). As shown in Fig. 4c, GS inoculation gave rise to significant levels of fusion in Vero cells treated with CHX.
FIG. 4.
U1102-induced FFWO in Vero cells. (a) Vero cells were overlaid with mock-infected CBMCs (A) or U1102-infected CBMCs (B), cultured with medium supplemented with PFA (300 μg/ml), and fixed 24 h p.i. (b) Vero cells were mock infected (A) or infected with UV-inactivated U1102 (B) and fixed at 17 h p.i. The results shown are from one of three experiments. (c) Vero cells were mock infected or infected with GS, cultured with CHX (50 μg/ml), and fixed at 2 h p.i. Fusion was quantified by counting the number of nuclei in polykaryocytes with more than five nuclei. Variations in fusion scored in three experiments are shown.
To determine whether HHV-6A would cause syncytia in other cells, 293T, U373, HeLa, BALB/c 3T3, OMK, CHO, MT4, Molt-3, Jurkat, and Sup-T1 cells were inoculated with cell-free U1102 by centrifugation. Fusion was seen in the 293T, U373, HeLa, MT4, Molt-3, Jurkat, and Sup-T1 cells but not in the OMK, BALB/c 3T3, and CHO cells (data not shown).
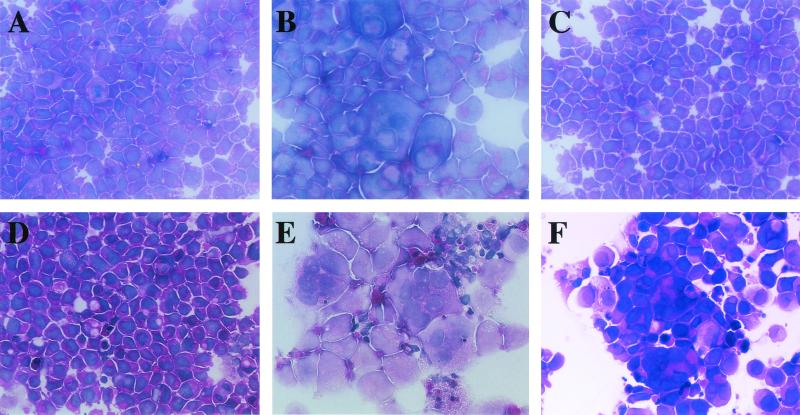
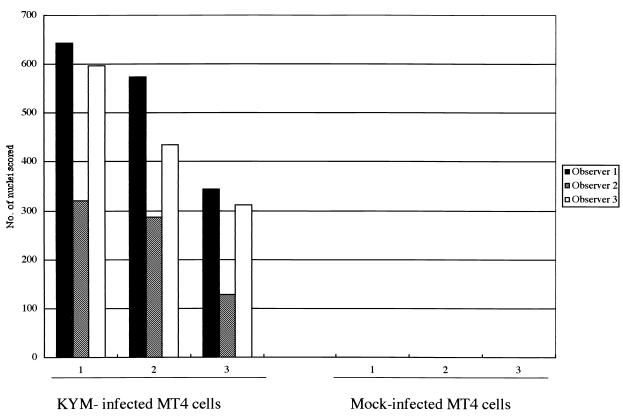
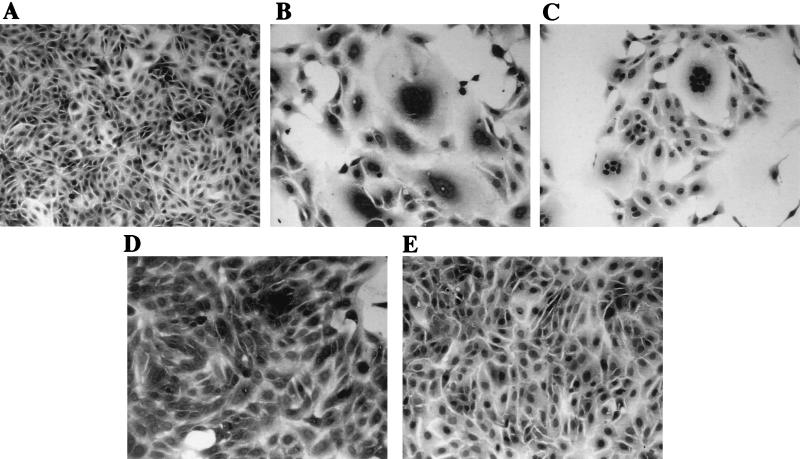
Although strain HST of HHV-6B did not induce polykaryocytes in several cell types in which they were induced by U1102, HST might induce fusion in susceptible cells. To examine whether HST induced polykaryocytes in MT4 cells, in which the virus is known to be able to replicate, MT4 cells and HSB-2 cells were infected with cell-free HST and U1102 (MOI, 0.01). After 24 h, the cells were fixed and stained with HE. HST formed multinucleated cells only in the MT4 cells (Fig. 5F), not in the HSB-2 cells (Fig. 5C), whereas U1102 formed multinucleated giant cells in both lines (Fig. 5B and E). To examine whether HHV-6B could cause FFWO in MT4 cells, cell-cell fusion in MT4 cells infected with HST (MOI, 0.01) in the presence of inhibitor of viral DNA polymerase PFA (300 μg/ml) was examined. Polykaryocyte formation was found in MT4 cells infected with HST in the presence of PFA as well as in its absence (data not shown). As well, polykaryocytes were observed in MT4 cells inoculated with UV-inactivated HST (data not shown). Finally, we investigated whether polykaryocytes were induced by HHV-6B in MT4 cells in the presence of CHX (50 μg/ml). Indeed, HHV-6B strains HST and KYM both induced polykaryocytes in MT4 cells in the presence of CHX (MOI, 0.1) (Fig. 6). However, the numbers of nuclei in syncytia in HHV-6B-infected MT4 cells were relatively less than those in HHV-6A-infected Sup-T1 and MT4 cells (data not shown).
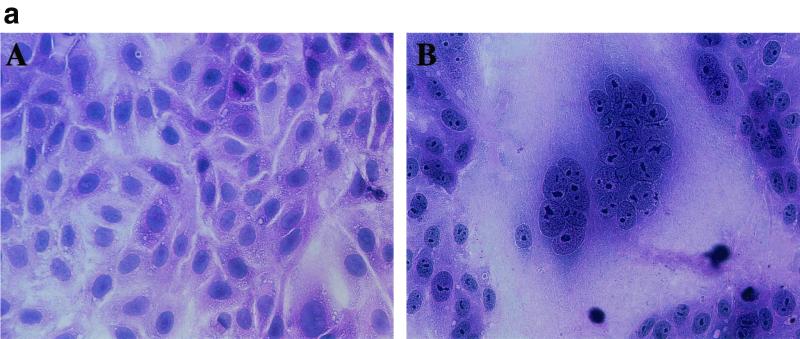
FIG. 5.
HHV-6B-induced polykaryocytes in MT4 cells. HSB-2 cells were infected with mock virus (A), U1102 (B), or HST (C). MT4 cells were mock infected (D) or infected with U1102 (E) or HST (F). HST induced polykaryocytes only in MT4 cells, not in HSB-2 cells. The results shown are from one of three experiments.
FIG. 6.
HHV-6B-induced FFWO in MT4 cells. MT4 cells were mock infected or infected with clinical isolate HHV-6B KYM and cultured with CHX (50 μg/ml) for 2 h. The cells were spun down, fixed, and stained with HE. Fusion was quantified by counting the nuclei in polykaryocytes with more than five nuclei. Variations in fusion scored in three experiments are shown.
Human CD46 is essential for polykaryocyte induction by HHV-6A.
The formation of multinuclear cells led us to investigate whether there was a requirement for an interaction between viral envelope glycoproteins and one or more specific cell surface receptors. Human CD46 has been shown to serve as an entry receptor for HHV-6A and -6B (50). CHO cells are highly resistant to infection by HHV-6 and to cell-cell fusion induced by HHV-6A, as shown above. Therefore, CHO cells stably expressing human CD46 (CHO-CD46 cells) (25) were inoculated with U1102 and HST at an MOI of 0.01. CHO cells, which lack human CD46, failed to form polykaryocytes after infection with either U1102 or HST (data not shown); however, as shown in Fig. 7, U1102, but not HST, induced polykaryocytes in CHO-CD46 cells. Thus, the induction of polykaryocytes in target cells by U1102 requires an HHV-6 entry receptor, human CD46. In other words, human CD46 is essential for polykaryocyte formation induced by HHV-6A. We examined U1102-infected CHO-CD46 cells for evidence of the immediate-early and early expression of HHV-6A proteins by IFA. However, we could not detect their expression (data not shown).
FIG. 7.
U1102-induced syncytium formation required human CD46. CHO cells expressing CD46 (CHO-CD46) were mock infected (A) or inoculated with U1102 (B). After 10 h, the cells were fixed and stained with HE. Photographs of representative cell monolayers are shown. The results shown are from one of three experiments performed.
SCR2, SCR3, and SCR4 domains of CD46 are required for HHV-6A-mediated cell-cell fusion.
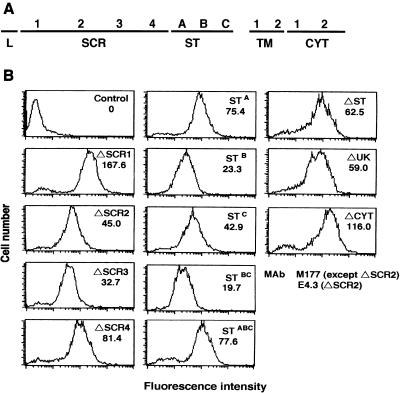
We next determined which domains of CD46 were important for syncytium formation induced by HHV-6A. Human CD46 is composed of four SCRs, a Ser/Thr (ST)-rich domain, a 13-amino-acid sequence of unknown significance (UK), a transmembrane domain, and a cytoplasmic tail (CYT) (31) (Fig. 8A). To determine which CD46 domains were important, human CD46 deletion mutants were stably expressed in CHO cells. The levels at which CD46 mutants ΔSCR1, ΔSCR2, ΔSCR3, ΔSCR4, ΔST, ΔUK, and ΔCYT (25) were expressed were examined by flow cytometry. All the deletion mutants were translated into proteins and expressed on the CHO cells (Fig. 8B). The levels of CD46 mutant expression for all the clones were similar. CHO cells expressing the CD46 deletion mutants were overlaid with U1102-infected Sup-T1 cells. The syncytia were observed under a light microscope. Syncytium formation was not found in the CHO cells expressing ΔSCR2, ΔSCR3, and ΔSCR4, although it was found in cells expressing ΔSCR1, ΔST, ΔUK, and ΔCYT (Table 1).
FIG. 8.
(A) Schematic drawing of the structure of human CD46. Four SCRs, an ST-rich domain, a 13-amino-acid region of unknown significance (UK), a transmembrane (TM) domain, and the cytoplasmic tail (CYT) are shown. (B) Flow-cytometric profiles of CHO transfectants expressing CD46 deletion mutants and ST CD46 variants. Stable transfectants were incubated with anti-CD46 MAb M177 or E4.3 for ΔSCR2, followed by an FITC-labeled secondary Ab, and then analyzed by flow cytometry. Control, CHO cells.
TABLE 1.
Summary of syncytium formation by CD46 deletion mutants and isoformsa
| Mutant | Syncytium formation |
|---|---|
| CD46 deletion mutants | |
| ΔSCR1 | + |
| ΔSCR2 | − |
| ΔSCR3 | − |
| ΔSCR4 | − |
| ΔCYT | + |
| ΔUK | + |
| ΔST | + |
| CD46 isoforms | |
| STBC | + |
| STABC | + |
| STA | + |
| STB | + |
| STC | + |
CHO cells expressing the indicated CD46 deletion mutants and isoforms were overlaid with strain U1102-infected Sup-T1 cells. After incubation at 37°C for 10 h, syncytium formation was observed under an inverted microscope.
Analysis of the CD46 genomic DNA revealed three similarly sized exons that each encode an ST domain of 14 or 15 amino acids, STA, STB, and STC (31, 43). PCR and nucleotide sequencing analyses have suggested that variable quantities of the three primary isoforms, STABC, STBC, and STC, are expressed on human blood cells and tissues; these isoforms are produced by alternative splicing (26, 44, 47). Here, we assessed the role of the ST region as an HHV-6 receptor using CHO cells expressing the following CD46 isoforms: STABC, STBC, STA, STB, and STC (24). The expression of all the isoforms was examined by flow cytometry (Fig. 8B). CHO cells expressing the CD46 ST isoforms were overlaid with U1102-infected Sup-T1 cells. Syncytium formation was found in the cells expressing all of the ST isoforms: STABC, STBC, STA, STB, and STC (Table 1). These results indicate that the SCR2, -3, and -4 domains are essential for HHV-6A-mediated syncytium formation.
To examine if the domains of CD46 that were essential for fusion were the same as or different from those needed for its function as an HHV-6 entry receptor, inhibition studies using anti-CD46 MAbs were performed (Table 2). The SCR2-recognizing MAb, M75, blocked GS-induced polykaryocyte formation and the expression of HHV-6 IE1 in Sup-T1 cells. The SCR-3-recognizing MAb, M160, completely blocked the GS-induced polykaryocytes in Sup-T1 cells but did not completely block the expression of IE1.
TABLE 2.
Effects of anti-CD46 antibodies on GS-mediated syncytium formation and entryb
| MAb | Epitope | Block of HHV-6 functiona of:
|
|
|---|---|---|---|
| Syncytium formation | Expression of IE1 | ||
| J4-48 | SCR1 | − | − |
| M75 | SCR2 | +++ | +++ |
| M160 | SCR3 | +++ | + |
| OHV-2 | − | − | |
Block of CD46 function in syncytium formation and the expression of IE1. +++, syncytium formation and expression of IE1 were almost 90 to 100% inhibited by each antibody; +, almost 5 to 10% inhibited; −, not inhibited.
Sup-T1 cells were incubated at 37°C for 60 min with each anti-CD46 MAb or OHV-2 (10 μg/ml) in RPMI 1640 with 10% FCS and then centrifuged with GS at an 0.1 MOI of at 37°C for 60 min. The cells were washed three times and cultured at 37°C for 12 h. They were fixed and stained with HE or fixed with acetone and stained with rabbit antiserum specific for HHV-6 IE1. The blocking effects were evaluated by microscopy.
Syncytium formation induced by HHV-6A requires gB and gH.
Given that CHO cells that lack CD46 are unable to fuse, it is clear that one of the essential requirements for HHV-6-induced polykaryocyte formation is an interaction between HHV-6 glycoproteins and human CD46. To examine which glycoproteins contribute to polykaryocyte formation, a virus neutralization assay using MAbs against gH and gB was performed.
For the FFWO virus neutralization assay, the viral inoculum was preincubated for 1 h at room temperature with serial dilutions of mouse ascites fluid containing MAb 2E4 (anti-HHV-6A gH) or MAb 87-y-13 (anti-HHV-6A gB) or containing MAb REP A1 (anti-HHV-6B REP/U94) as a negative control. Vero cells (106) were then inoculated with treated or untreated viral stocks at 103 TCID50 by centrifugation, the virus was removed, and the cells were seeded in six-well plates. Syncytium formation was monitored by light-microscopic examination, and the cells were stained with HE.
The MAb against HHV-6 gB, which has been implicated in virus infectivity, and the MAb against gH, which has been implicated in virus entry and syncytium formation, caused the inhibition of U1102-induced polykaryocyte formation (Fig. 9D and E). MAbs against HHV-6 U94/REP had no effect (Fig. 9C). These results indicate that gH (2) and gB of HHV-6A are necessary to mediate polykaryocyte formation of the target cells.
FIG. 9.
Anti-gB and anti-gH MAbs neutralize U1102-mediated syncytium formation. An infectious, cell-free stock of U1102 was generated and preincubated for 60 min at room temperature with a 50-fold-diluted aliquot of ascites fluid containing the MAbs. Vero cells (106) were then infected with the treated virus preparation (103 TCID50), and the cells were fixed and stained with HE at 24 h p.i. (A) Negative control (uninfected Vero cells); (B) positive control (Vero cells infected with U1102 in the absence of any MAb); (C, D, and E) Vero cells infected with U1102 preincubated with anti-REP, anti-gB, and anti-gH MAbs, respectively. Note the presence of extensive syncytia in panels B and C but not in the panels in which U1102 was treated with anti-gB and anti-gH (D and E).
DISCUSSION
We showed here that HHV-6A, but not HHV-6B, induced syncytium formation in a variety of cells, including U373, 293T, HEL, Vero, Sup-T1, MT4, Jurkat, Molt-3, and HSB-2 cells, without viral replication. Syncytium formation was not induced in BALB/c 3T3, OMK, and CHO cells. Furthermore, we demonstrated that HHV-6A can induce FFWO—that is, HHV-6A mediates cell-cell fusion without viral protein synthesis—in cells that express human CD46. Human CD46 is reported to be a cellular receptor of HHV-6A and HHV-6B (50). It has also been identified as a human measles virus (MV) receptor (13, 36). CD46 is a ubiquitous type 1 glycoprotein expressed on the surfaces of all nucleated human cells examined to date. It was originally purified as a complement (C) regulatory protein; it binds C3b and C4b on host cells to allow factor I-mediated inactivation of these C fragments, and it plays an important role in protecting host cells from autologous C (51, 52). Therefore, CD46 is a designated membrane cofactor protein.
Our findings show that the failure of FFWO in BALB/c 3T3, which is a murine cell line, and OMK cells, which are derived from New World monkeys, and that the success in the Vero cell line, which is derived from Old World monkeys, may reflect the expression of different CD46 domains. The Vero cell CD46 is 86% identical to human CD46 in the ectodomain region and shows an MV receptor activity similar to that of human CD46 (35). In contrast, the amino acid sequence of the CD46 of B95a cells, an Epstein-Barr virus-transformed marmoset B-cell line (which is a simian cell line), is 76% identical to that of human CD46 (34). Considering these data, one possible reason for the failure to form HHV-6A-induced syncytia in OMK cells is that HHV-6 might not have been able to bind the CD46 of New World monkeys, although it could bind to the CD46 of Old World monkeys. In fact, Vero CD46 acts as a cofactor for human factor I, inactivates human C3b, and functions as a major receptor for MV (35), indicating that Vero CD46 is not only a structural but also a functional homologue of human CD46.
The expression of immediate-early or early proteins was not found in CHO-CD46 cells inoculated with U1102 but was found in Vero, HEL, U373, and Sup-T1 cells inoculated with U1102 (data not shown). Moreover, the observed syncytia gradually became larger in the Vero, HEL, U373, and Sup-T1 cells within 48 h. When the cells were infected with a lower number of infectious units, it was difficult to observe the syncytia in the cells immediately after infection, but on day 1 or 2 p.i., they could gradually be seen, indicating that cell-cell fusion also occurred after virus replication. The syncytia also became larger in CHO-CD46 cells but stopped growing within 10 to 18 h following infection. The attached viral glycoproteins might have been able to move about the cell surface to induce cell-cell fusion within 10 to 18 h. These data suggest that, although HHV-6A could induce syncytium formation in CHO-CD46 cells, the virus may not have been able to enter the cells or, if it did enter, to complete its replication cycle in them. Other human cellular factors may be required for virus replication in CHO cells, as indicated by Santoro et al. (50), and new viral protein synthesis may be required for the syncytia caused by HHV-6A to increase.
Moreover, we studied which CD46 domains contributed to the HHV-6A-induced cell-cell fusion using deletion mutants. We studied four parts of the CD46 protein: the four SCRs and the ST, UK, and CYT regions. Our results showed that the SCR2, -3, and -4 regions of the CD46 ectodomain were mainly involved in HHV-6A-mediated cell-cell fusion. However, the effects of the deletions may not only have been due to the loss of specific domains, but may have also resulted from steric hindrance or a conformational change of the receptor. Furthermore, we showed that, although one MAb, M75, which recognizes SCR2, blocked GS-induced polykaryocytes and the expression of HHV-6 IE1 in Sup-T1 cells, another MAb, M160, which recognizes SCR3, completely blocked GS-induced polykaryocytes in Sup-T1 cells but did not completely block the expression of IE1. In other words, MAb M75 could inhibit both virus-induced syncytium formation and viral entry, but MAb M160 could inhibit only syncytium formation, indicating that there might be CD46 domains that are essential for both cell-cell fusion and entry and some that are involved in only one of these functions and that cell-cell fusion induced by HHV-6A may also be induced by another mechanism that is different from the virion-cell fusion required for entry. Therefore, in this study, we have developed an assay for syncytium formation rather than an assay that identifies the end point of a virus entry experiment.
SCR1 and -2 have been reported to be mainly involved in MV binding and infection; SCR2, -3, and -4 sustain C3b inactivation by factor I; and SCR2 and -3 are essential for factor I-mediated inactivation of C4b (25). Interestingly, our results indicate that the ligands of CD46, C4b, MV, and HHV-6A, bind to different sets of CD46 SCRs and confer distinct functions on CD46; however, C3b binds to SCR2, -3, and -4, as does HHV-6A. The observation that SCR2, -3, and -4 are responsible for HHV-6A-mediated syncytium formation may indicate the involvement of C3b in the HHV-6-CD46 interaction.
The ST domain of CD46 is a critical factor for controlling the permissiveness of cells to MV infection (24). Therefore, we assessed the role of the ST region in HHV-6A-mediated syncytium formation. Cells expressing CD46 variants with a small ST domain (STC) and STBC were more effective as receptors for syncytium formation than cells expressing other ST domain variants (STA, STB, and ST ABC; data not shown). Hence, the ST domain modulated HHV-6 infectivity in addition to C inhibitory activity and MV infectivity. In this study, the STC/CYT2 CD46 variant was stably expressed in CHO cells. Based on evidence that the STC variant is the most susceptible to HHV-6A of the ST isoforms we tested, we propose that the predominant expression of STC in the brain is related to the high sensitivity of the brain to HHV-6-mediated encephalitis. In fact, fatal encephalitis cases that were caused by HHV-6A have been reported (28, 42).
We showed that HHV-6 could induce FFWO at an MOI of 0.01 to 0.1. In general, a high MOI is required for FFWO. Unfortunately, even in the most permissive systems (peripheral blood mononuclear cells, CBMCs, and T-cell lines), HHV-6 yields are very low (8). Cultures of CBMCs, the most productive cell type, do not yield more than 104 infectious units per ml, whereas the titer of an HSV-1 stock is generally as high as 109 to 1010 PFU per ml (8). Therefore, in this study, we inoculated the cells with viruses by centrifugation to obtain high infectivity. The technique of centrifugal inoculation of cell monolayers to improve infectivity has been described for viral agents (38). In studies with HHV-6, an approximately 100-fold enhancement of infectivity was obtained with a centrifugal force of 2,500 × g for 60 min (41). The mechanisms involved in this phenomenon have not been fully elucidated (20). For HHV-6, centrifugal infection might enhance the infectivity of extracellular particles for cellular debris contained in the virus stock, because a large number of extracellular particles of HHV-6 have been observed on the surfaces of the cells infected with virus (37). Analysis of varicella-zoster virus (VZV), for which it is also difficult to obtain high titers, showed a particle-to-infectivity ratio of 106:1 (53). We observed concentrated virus samples by electron microscopy, and these samples appeared to contain incomplete particles (data not shown). Another possibility is that, as with VZV, there are incomplete extracellular particles in the supernatant obtained from the cells infected with HHV-6, and the particle-to-infectivity ratio may be similar to that seen for VZV. Thus, even a low number of infectious units of HHV-6 might be able to induce cell-cell fusion.
In HSV, the fusion of the viral envelope with the plasma membrane requires gD, gB, gH, and gL (5). In addition, the fusion event is dependent on the expression of a gD receptor on the target cell membrane (5, 40, 57, 59). gB and the formation of heterodimer gH-gL are highly conserved, and homologues to these glycoproteins are encoded by all of the known herpesviruses, although gD is present in only some members of the Alphaherpesvirinae subfamily. The multinucleated giant cells caused by HSV have been suggested to be a hallmark of HSV-1 lesions in vivo (40). Spear et al. (54, 55) described evidence showing that, although the interaction of gD with a receptor is required for membrane fusion, gD is probably not the viral fusogen and perhaps not even an essential component of the actual fusion machinery. The basic fusion machinery has probably been conserved, although the means of triggering fusion activity might differ among the viruses. Under certain conditions, the expression of VZV gH-gL alone (14) or of pseudorabies virus gB plus gH-gL (27) can induce cell fusion, provided the cells are susceptible to the virus and therefore express some kind of receptor for membrane fusion. Thus, gB or gH-gL or both are candidates for the actual fusogenic glycoproteins.
Here, we used a neutralization assay to show that gB and gH were required to mediate syncytium formation for HHV-6A as well as other herpesviruses in target cells. Considering the previous reports and our results, it seems likely that the process of syncytium formation for HHV-6A and HSV is roughly as follows. First, the HHV-6 envelope glycoprotein(s) binds to human CD46, thereby triggering fusogenic activity, and then other viral envelope glycoproteins, probably gB and the gH-gL heterodimer (29, 30), act to form multinucleated cells. Alternatively, gB plus gH-gL might be sufficient to induce cell fusion, provided the cells express human CD46. Thus, our data agree with those of others and indicate that gB, gH-gL, or both are candidates for the actual fusogenic glycoproteins.
Several viral genes are required for the expression of the Syn phenotype for HSV. gE, gI, and gM have all been implicated in syncytium formation (9). gK is thought to play a central role in regulating membrane fusion, because mutations in gK can give rise to the Syn phenotype (11, 21, 22). On the other hand, amino acid substitutions in the cytoplasmic tail of HSV-1 gB have been reported to induce syncytium formation (6, 15, 17). In another report, a recombinant virus carrying a gD mutation that significantly reduces viral entry via HveA had a markedly reduced ability to induce syncytia in CHO-HVEM cells (58). These reports lead us to speculate why HHV-6A, but not HHV-6B, induced syncytia. There might be amino acid substitutions in envelope glycoproteins that are responsible for the different abilities of HHV-6A and HHV-6B to induce syncytium formation. Such amino acid substitutions might affect the interaction with human CD46. This might explain why a variety of cultured cells were highly susceptible to syncytium induction by HHV-6A but were resistant to HHV-6B-induced syncytium formation, even though HHV-6B mediated polykaryocyte formation in MT4 cells and CBMCs. Another possibility is that cellular factors somehow differentiate between HHV-6A and HHV-6B. One or more additional factors in the target cells might be required for the cell-cell fusion induced by HHV-6B, given that multinucleated giant cells could be found in the MT4 cells and CBMCs, which are susceptible to HHV-6 replication.
HHV-6A has been reported to cause gastroduodenitis and pancreatitis after heart transplantation in immunosuppressed individuals (45). In these cases, the presence of multinucleate giant cells and enveloped virions with a prominent tegument in human biopsy tissue was used as a morphological criterion to suggest the possibility of HHV-6 infection. The epithelium showed enlarged nuclei, prominent nuclei, and multinucleated-giant-cell formation. Mononuclear cells contained intranuclear and intracytoplasmic enveloped viral capsids with a thick tegumental layer, consistent with infection by HHV-6. Viral particles were not found in the multinucleate epithelial cells. The authors detected HHV-6A DNA, but not that of HHV-6B, in the specimens by PCR. The results of our study might indicate that, in such cases, virus need not be transferred from the circulating blood components to the tissues; rather, only the attachment of the virus may be required, given that our data show that HHV-6A can mediate the formation of multinucleated giant cells without viral replication in human tissues. The phenomenon we show here in vitro closely mimics the interactive cellular events occurring in vivo. Thus, our study shows that disease could be caused solely by the attachment of the virus, carried by blood components, through the epithelium or endothelium lining the blood vessels to the other tissues throughout the body.
In summary, our study demonstrates an important finding with implications for pathogenicity and the spread of the virus, which is that the transfer of HHV-6 to a variety of tissues might lead to syncytia without viral replication. This may help explain the observation of widespread viral effects on tissues in immunocompromised patients.
Acknowledgments
This study was supported in part by a grant-in-aid from the Ministry of Education, Science and Culture of Japan and was also supported in part by a Japan-China Sasakawa medical fellowship.
We thank G. Campadelli-Fiume for providing MAbs, and we thank M. Taniguchi and M. Matsumoto (Osaka Medical Center) for providing reagents and cells relative to CD46.
REFERENCES
- 1.Ablashi, D. V., N. Balachandran, S. F. Josephs, C. L. Hung, G. R. Krueger, B. Kramarsky, S. Z. Salahuddin, and R. C. Gallo. 1991. Genomic polymorphism, growth properties, and immunologic variations in human herpesvirus-6 isolates. Virology 184:545-552. [DOI] [PubMed] [Google Scholar]
- 2.Anderson, R. A., and U. A. Gompels. 1999. N- and C-terminal external domains of human herpesvirus-6 glycoprotein H affect a fusion-associated conformation mediated by glycoprotein L binding the N terminus. J. Gen. Virol 80:1485-1494. [DOI] [PubMed] [Google Scholar]
- 3.Aubin, J. T., H. Collandre, D. Candotti, D. Ingrand, C. Rouzioux, M. Burgard, S. Richard, J. M. Huraux, and H. Agut. 1991. Several groups among human herpesvirus 6 strains can be distinguished by Southern blotting and polymerase chain reaction. J. Clin. Microbiol. 29:367-372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bratt, M. A., and W. R. Gallaher. 1969. Preliminary analysis of the requirements for fusion from within and fusion from without by Newcastle disease virus. Proc. Natl. Acad. Sci. USA 64:536-543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Browne, H., B. Bruun, and T. Minson. 2001. Plasma membrane requirements for cell fusion induced by herpes simplex virus type 1 glycoproteins gB, gD, gH and gL. J. Gen. Virol 82:1419-1422. [DOI] [PubMed] [Google Scholar]
- 6.Bzik, D. J., B. A. Fox, N. A. DeLuca, and S. Person. 1984. Nucleotide sequence of a region of the herpes simplex virus type 1 gB glycoprotein gene: mutations affecting rate of virus entry and cell fusion. Virology 137:185-190. [DOI] [PubMed] [Google Scholar]
- 7.Campadelli-Fiume, G., S. Guerrini, X. Liu, and L. Foa-Tomasi. 1993. Monoclonal antibodies to glycoprotein B differentiate human herpesvirus 6 into two clusters, variants A and B. J. Gen Virol 74:2257-2262. [DOI] [PubMed] [Google Scholar]
- 8.Campadelli-Fiume, G., P. Mirandola, and L. Menotti. 1999. Human herpesvirus 6: an emerging pathogen. Emerg. Infect. Dis. 5:353-366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis-Poynter, N., S. Bell, T. Minson, and H. Browne. 1994. Analysis of the contributions of herpes simplex virus type 1 membrane proteins to the induction of cell-cell fusion. J. Virol. 68:7586-7590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dhepakson, P., Y. Mori, Y. B. Jiang, H. L. Huang, P. Akkapaiboon, T. Okuno, and K. Yamanishi. 2002. Human herpesvirus-6 rep/U94 gene product has single-stranded DNA-binding activity. J. Gen. Virol 83:847-854. [DOI] [PubMed] [Google Scholar]
- 11.Dolter, K. E., R. Ramaswamy, and T. C. Holland. 1994. Syncytial mutations in the herpes simplex virus type 1 gK (UL53) gene occur in two distinct domains. J. Virol. 68:8277-8281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominguez, G., T. R. Dambaugh, F. R. Stamey, S. Dewhurst, N. Inoue, and P. E. Pellett. 1999. Human herpesvirus 6B genome sequence: coding content and comparison with human herpesvirus 6A. J. Virol. 73:8040-8052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dorig, R. E., A. Marcil, A. Chopra, and C. D. Richardson. 1993. The human CD46 molecule is a receptor for measles virus (Edmonston strain). Cell 75:295-305. [DOI] [PubMed] [Google Scholar]
- 14.Duus, K. M., C. Hatfield, and C. Grose. 1995. Cell surface expression and fusion by the varicella-zoster virus gH:gL glycoprotein complex: analysis by laser scanning confocal microscopy. Virology 210:429-440. [DOI] [PubMed] [Google Scholar]
- 15.Engel, J. P., E. P. Boyer, and J. L. Goodman. 1993. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology 192:112-120. [DOI] [PubMed] [Google Scholar]
- 16.Foa-Tomasi, L., A. Boscaro, S. di Gaeta, and G. Campadelli-Fiume. 1991. Monoclonal antibodies to gp100 inhibit penetration of human herpesvirus 6 and polykaryocyte formation in susceptible cells. J. Virol. 65:4124-4129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gage, P. J., M. Levine, and J. C. Glorioso. 1993. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 67:2191-2201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gompels, U. A., J. Nicholas, G. Lawrence, M. Jones, B. J. Thomson, M. E. Martin, S. Efstathiou, M. Craxton, and H. A. Macaulay. 1995. The DNA sequence of human herpesvirus-6: structure, coding content, and genome evolution. Virology 209:29-51. [DOI] [PubMed] [Google Scholar]
- 19.He, J., M. McCarthy, Y. Zhou, B. Chandran, and C. Wood. 1996. Infection of primary human fetal astrocytes by human herpesvirus 6. J. Virol. 70:1296-1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hudson, J. B., V. Misra, and T. R. Mosmann. 1976. Cytomegalovirus infectivity: analysis of the phenomenon of centrifugal enhancement of infectivity. Virology 72:235-243. [DOI] [PubMed] [Google Scholar]
- 21.Hutchinson, L., K. Goldsmith, D. Snoddy, H. Ghosh, F. L. Graham, and D. C. Johnson. 1992. Identification and characterization of a novel herpes simplex virus glycoprotein, gK, involved in cell fusion. J. Virol. 66:5603-5609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hutchinson, L., C. Roop-Beauchamp, and D. C. Johnson. 1995. Herpes simplex virus glycoprotein K is known to influence fusion of infected cells, yet is not on the cell surface. J. Virol. 69:4556-4563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Isegawa, Y., T. Mukai, K. Nakano, M. Kagawa, J. Chen, Y. Mori, T. Sunagawa, K. Kawanishi, J. Sashihara, A. Hata, P. Zou, H. Kosuge, and K. Yamanishi. 1999. Comparison of the complete DNA sequences of human herpesvirus 6 variants A and B. J. Virol. 73:8053-8063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Iwata, K., T. Seya, S. Ueda, H. Ariga, and S. Nagasawa. 1994. Modulation of complement regulatory function and measles virus receptor function by the serine-threonine-rich domains of membrane cofactor protein (CD46). Biochem. J. 304:169-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwata, K., T. Seya, Y. Yanagi, J. M. Pesando, P. M. Johnson, M. Okabe, S. Ueda, H. Ariga, and S. Nagasawa. 1995. Diversity of sites for measles virus binding and for inactivation of complement C3b and C4b on membrane cofactor protein CD46. J. Biol. Chem. 270:15148-15152. [DOI] [PubMed] [Google Scholar]
- 26.Johnstone, R. W., S. M. Russell, B. E. Loveland, and I. F. McKenzie. 1993. Polymorphic expression of CD46 protein isoforms due to tissue-specific RNA splicing. Mol. Immunol. 30:1231-1241. [DOI] [PubMed] [Google Scholar]
- 27.Klupp, B. G., R. Nixdorf, and T. C. Mettenleiter. 2000. Pseudorabies virus glycoprotein M inhibits membrane fusion. J. Virol. 74:6760-6768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Knox, K. K., D. P. Harrington, and D. R. Carrigan. 1995. Fulminant human herpesvirus six encephalitis in a human immunodeficiency virus-infected infant. J. Med. Virol. 45:288-292. [DOI] [PubMed] [Google Scholar]
- 29.Liu, D. X., U. A. Gompels, L. Foa-Tomasi, and G. Campadelli-Fiume. 1993. Human herpesvirus-6 glycoprotein H and L homologs are components of the gp100 complex and the gH external domain is the target for neutralizing monoclonal antibodies. Virology 197:12-22. [DOI] [PubMed] [Google Scholar]
- 30.Liu, D. X., U. A. Gompels, J. Nicholas, and C. Lelliott. 1993. Identification and expression of the human herpesvirus 6 glycoprotein H and interaction with an accessory 40K glycoprotein. J. Gen. Virol. 74:1847-1857. [DOI] [PubMed] [Google Scholar]
- 31.Lublin, D. M., M. K. Liszewski, T. W. Post, M. A. Arce, M. M. Le Beau, M. B. Rebentisch, L. S. Lemons, T. Seya, and J. P. Atkinson. 1988. Molecular cloning and chromosomal localization of human membrane cofactor protein (MCP). Evidence for inclusion in the multigene family of complement-regulatory proteins. J. Exp. Med. 168:181-194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mori, Y., P. Dhepakson, T. Shimamoto, K. Ueda, Y. Gomi, H. Tani, Y. Matsuura, and K. Yamanishi. 2000. Expression of human herpesvirus 6B rep within infected cells and binding of its gene product to the TATA-binding protein in vitro and in vivo. J. Virol. 74:6096-6104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mori, Y., H. Yagi, T. Shimamoto, Y. Isegawa, T. Sunagawa, R. Inagi, K. Kondo, Y. Tano, and K. Yamanishi. 1998. Analysis of human herpesvirus 6 U3 gene, which is a positional homolog of human cytomegalovirus UL 24 gene. Virology 249:129-139. [DOI] [PubMed] [Google Scholar]
- 34.Murakami, Y., T. Seya, M. Kurita, A. Fukui, S. Ueda, and S. Nagasawa. 1998. Molecular cloning of membrane cofactor protein (MCP; CD46) on B95a cell, an Epstein-Barr virus-transformed marmoset B cell line: B95a-MCP is susceptible to infection by the CAM, but not the Nagahata strain of the measles virus. Biochem. J. 330:1351-1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Murakami, Y., T. Seya, M. Kurita, and S. Nagasawa. 1996. Molecular cloning of a complementary DNA for a membrane cofactor protein (MCP, CD46)/measles virus receptor on Vero cells and its functional characterization. Biol. Pharm. Bull. 19:1541-1545. [DOI] [PubMed] [Google Scholar]
- 36.Naniche, D., G. Varior-Krishnan, F. Cervoni, T. F. Wild, B. Rossi, C. Rabourdin-Combe, and D. Gerlier. 1993. Human membrane cofactor protein (CD46) acts as a cellular receptor for measles virus. J. Virol. 67:6025-6032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nii, S., M. Yoshida, F. Uno, T. Kurata, K. Ikuta, and K. Yamanishi. 1990. Replication of human herpesvirus 6 (HHV-6): morphological aspects. Adv. Exp. Med. Biol. 278:19-28. [DOI] [PubMed] [Google Scholar]
- 38.Osborn, J. E., and D. L. Walker. 1968. Enhancement of infectivity of murine cytomegalovirus in vitro by centrifugal inoculation. J. Virol. 2:853-858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pellett, P. E., J. B. Black, and M. Yamamoto. 1992. Human herpesvirus 6: the virus and the search for its role as a human pathogen. Adv. Virus Res. 41:1-52. [DOI] [PubMed] [Google Scholar]
- 40.Pertel, P. E., A. Fridberg, M. L. Parish, and P. G. Spear. 2001. Cell fusion induced by herpes simplex virus glycoproteins gB, gD, and gH-gL requires a gD receptor but not necessarily heparan sulfate. Virology 279:313-324. [DOI] [PubMed] [Google Scholar]
- 41.Pietroboni, G. R., G. B. Harnett, and M. R. Bucens. 1989. Centrifugal enhancement of human immunodeficiency virus (HIV) and human herpesvirus type 6 (HHV-6) infection in vitro. J. Virol. Methods 24:85-90. [DOI] [PubMed] [Google Scholar]
- 42.Portolani, M., M. Pecorari, M. G. Tamassia, W. Gennari, F. Beretti, and G. Guaraldi. 2001. Case of fatal encephalitis by HHV-6 variant A. J. Med. Virol. 65:133-137. [PubMed] [Google Scholar]
- 43.Post, T. W., M. K. Liszewski, E. M. Adams, I. Tedja, E. A. Miller, and J. P. Atkinson. 1991. Membrane cofactor protein of the complement system: alternative splicing of serine/threonine/proline-rich exons and cytoplasmic tails produces multiple isoforms that correlate with protein phenotype. J. Exp. Med. 174:93-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Purcell, D. F., S. M. Russell, N. J. Deacon, M. A. Brown, D. J. Hooker, and I. F. McKenzie. 1991. Alternatively spliced RNAs encode several isoforms of CD46 (MCP), a regulator of complement activation. Immunogenetics 33:335-344. [DOI] [PubMed] [Google Scholar]
- 45.Randhawa, P. S., F. J. Jenkins, M. A. Nalesnik, J. Martens, P. A. Williams, A. Ries, S. Pham, and A. J. Demetris. 1997. Herpesvirus 6 variant A infection after heart transplantation with giant cell transformation in bile ductular and gastroduodenal epithelium. Am. J. Surg. Pathol. 21:847-853. [DOI] [PubMed] [Google Scholar]
- 46.Rotola, A., D. Di Luca, E. Cassai, D. Ricotta, A. Giulio, A. Turano, A. Caruso, and C. Muneretto. 2000. Human herpesvirus 6 infects and replicates in aortic endothelium. J. Clin. Microbiol. 38:3135-3136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell, S. M., R. L. Sparrow, I. F. McKenzie, and D. F. Purcell. 1992. Tissue-specific and allelic expression of the complement regulator CD46 is controlled by alternative splicing. Eur. J. Immunol. 22:1513-1518. [DOI] [PubMed] [Google Scholar]
- 48.Saharkhiz-Langroodi, A., and T. C. Holland. 1997. Identification of the fusion-from-without determinants of herpes simplex virus type 1 glycoprotein B. Virology 227:153-159. [DOI] [PubMed] [Google Scholar]
- 49.Salahuddin, S. Z., D. V. Ablashi, P. D. Markham, S. F. Josephs, S. Sturzenegger, M. Kaplan, G. Halligan, P. Biberfeld, F. Wong-Staal, B. Kramarsky, et al. 1986. Isolation of a new virus, HBLV, in patients with lymphoproliferative disorders. Science 234:596-601. [DOI] [PubMed] [Google Scholar]
- 50.Santoro, F., P. E. Kennedy, G. Locatelli, M. S. Malnati, E. A. Berger, and P. Lusso. 1999. CD46 is a cellular receptor for human herpesvirus 6. Cell 99:817-827. [DOI] [PubMed] [Google Scholar]
- 51.Seya, T., T. Hara, M. Matsumoto, Y. Sugita, and H. Akedo. 1990. Complement-mediated tumor cell damage induced by antibodies against membrane cofactor protein (MCP, CD46). J. Exp. Med. 172:1673-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Seya, T., J. R. Turner, and J. P. Atkinson. 1986. Purification and characterization of a membrane protein (gp45-70) that is a cofactor for cleavage of C3b and C4b. J. Exp. Med. 163:837-855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shiraki, K., and M. Takahashi. 1982. Virus particles and glycoprotein excreted from cultured cells infected with varicella-zoster virus (VZV). J. Gen. Virol. 61:271-275. [DOI] [PubMed] [Google Scholar]
- 54.Spear, P. G. 2001. A first step toward understanding membrane fusion induced by herpes simplex virus. Mol. Cell 8:2-4. [DOI] [PubMed] [Google Scholar]
- 55.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 56.Takeda, K., M. Haque, T. Sunagawa, T. Okuno, Y. Isegawa, and K. Yamanishi. 1997. Identification of a variant B-specific neutralizing epitope on glycoprotein H of human herpesvirus-6. J. Gen. Virol. 78:2171-2178. [DOI] [PubMed] [Google Scholar]
- 57.Terry-Allison, T., R. I. Montgomery, M. S. Warner, R. J. Geraghty, and P. G. Spear. 2001. Contributions of gD receptors and glycosaminoglycan sulfation to cell fusion mediated by herpes simplex virus 1. Virus Res. 74:39-45. [DOI] [PubMed] [Google Scholar]
- 58.Terry-Allison, T., R. I. Montgomery, J. C. Whitbeck, R. Xu, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. HveA (herpesvirus entry mediator A), a coreceptor for herpes simplex virus entry, also participates in virus-induced cell fusion. J. Virol. 72:5802-5810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Turner, A., B. Bruun, T. Minson, and H. Browne. 1998. Glycoproteins gB, gD, and gHgL of herpes simplex virus type 1 are necessary and sufficient to mediate membrane fusion in a Cos cell transfection system. J. Virol. 72:873-875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu, C. A., and J. D. Shanley. 1998. Chronic infection of human umbilical vein endothelial cells by human herpesvirus-6. J. Gen. Virol. 79:1247-1256. [DOI] [PubMed] [Google Scholar]
- 61.Wyatt, L. S., N. Balachandran, and N. Frenkel. 1990. Variations in the replication and antigenic properties of human herpesvirus 6 strains. J. Infect. Dis. 162:852-857. [DOI] [PubMed] [Google Scholar]
- 62.Yamanishi, K., T. Okuno, K. Shiraki, M. Takahashi, T. Kondo, Y. Asano, and T. Kurata. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065-1067. [DOI] [PubMed] [Google Scholar]