Abstract
In the thymus, epithelial cells comprise a heterogeneous population required for the generation of functional T lymphocytes, suggesting that thymic epithelium disruption by viruses may compromise T-cell lymphopoiesis in this organ. In a previous report, we demonstrated that in vitro, measles virus induced differentiation of cortical thymic epithelial cells as characterized by (i) cell growth arrest, (ii) morphological and phenotypic changes, and (iii) apoptotis as a final step of this process. In the present report, we have analyzed the mechanisms involved. First, measles virus-induced differentiation of thymic epithelial cells is shown to be strictly dependent on beta interferon (IFN-β) secretion. In addition, transfection with double-stranded RNA, a common intermediate of replication for a broad spectrum of viruses, is reported to similarly mediate thymic epithelial cell differentiation through IFN-β induction. Finally, we demonstrated that recombinant IFN-α, IFN-β, or IFN-γ was sufficient to induce differentiation and apoptosis of uninfected thymic epithelial cells. These observations suggested that interferon secretion by either infected cells or activated leukocytes, such as plasmacytoid dendritic cells or lymphocytes, may induce thymic epithelium disruption in a pathological context. Thus, we have identified a new mechanism that may contribute to thymic atrophy and altered T-cell lymphopoiesis associated with many infections.
The thymus is a primary lymphoid organ that ensures T-lymphocyte differentiation from bone marrow-derived progenitors (for reviews, see references 3 and 21). Thymic microenvironments are composed of a network of thymic epithelial cells (TEC), present in both the cortex and the medulla, and hematopoietic cells such as macrophages and dendritic cells. Thymocytes migrate, while differentiating, through distinct microenvironments in the subcapsular cortex, the deep cortex, the cortico-medullary junction, and the medulla, where they are finally released, as mature T cells, into the bloodstream. Thymic microenvironments sustain thymocyte development, providing survival, proliferation, and differentiation signals that allow T-cell receptor gene rearrangements. Moreover, interaction of immature T cells with thymic microenvironments ensures the production of major histocompatibility complex-restricted, self-tolerant T cells. Within the cortex, positive selection of immature T cells expressing functional T-cell receptor is mediated mainly by cortical TEC, whereas in the medulla, negative selection of developing autoreactive T cells is ensured by antigen-presenting cells of hematopoietic origin and, to a lesser extent, the TEC subset. Thus, thymic microenvironments support thymocyte survival and differentiation into mature T cells.
Among the factors that profoundly affect T-cell lymphopoiesis is infection by measles virus (MV), a single-stranded RNA (ssRNA) virus from the Paramyxoviridae family that induces a severe lymphopenia and immunosuppression in humans (16). MV initially replicates in the respiratory tract and then spreads to local lymphoid tissue, where virus replication can occur in macrophages and/or dendritic cells. This secondary viremia allows the spreading of the virus to other lymphoid organs, including the thymus (11, 27, 38). Autopsies of patients with fatal measles infections demonstrated degenerative and/or necrotic lesions in the thymus associated with a rapid and predominant loss of the thymic cortex (38). A marked involution of the thymic medulla was also observed, and MV antigens were found in Hassal's corpuscules (11, 38). We previously demonstrated that, in vitro, MV replication profoundly affects human cortical TEC biology (37). MV-infected TEC undergo major changes in morphology, associated with cell growth arrest, decreased cell-cell contact, and acquisition of stellate shapes and a squamous phenotype. Moreover, MV was shown to promote a shift from low- to high-molecular-weight cytokeratins, the latter being characteristic of highly differentiated epithelial cells such as keratinocytes or medullar TEC (20, 30). Finally, terminally differentiated TEC underwent (i) loss of adhesion to plastic, (ii) accumulation in the culture supernatant as floating cells, and (iii) death by apoptosis. In the SCID-hu mouse model, TEC have also been described as a major target for MV after direct intrathymic injection of the virus, and massive apoptosis of mainly uninfected thymocytes was observed (4). Together, these results suggest that disruption of thymic microenvironments by MV could profoundly affect T-cell lymphopoiesis in vivo and contribute to MV-induced lymphopenia and immunosuppression.
Interestingly, other viruses have also been reported to affect the thymic microenvironment, leading to a severe atrophy of this organ. Infection with human immunodeficiency virus (HIV), feline immunodeficiency virus, and simian immunodeficiency virus is followed by thymic regression in their respective hosts, accompanied by alteration of the epithelial cells of the cortex and massive apoptosis of thymocytes (1, 8, 28, 32, 34, 39). HIV type 1 (HIV-1) infection of the human thymus was reported to result in thymocyte depletion by at least two different mechanisms (34). First, T-cell lymphopoiesis is interrupted by direct infection and destruction of intrathymic progenitor cells at the CD3− CD4+ CD8− differentiation stage. Second, uninfected thymocytes within multiple stages of differentiation are induced to die of apoptosis through a yet-undetermined pathway. These two mechanisms are differentially induced depending on the HIV-1 isolate tested. Interestingly, TEC death was also clearly reported in a SCID-hu model of intrathymic infection by HIV, suggesting a potential link with uninfected thymocyte apoptosis (32). Murine hepatitis virus is an RNA virus that replicates in a small number of TEC but induces drastic involution of the thymus and deletion of uninfected double-positive thymocytes (14). Thus, viruses that spread to the thymus mediate thymocyte deletion through two distinct mechanisms. Some viruses replicate in thymocytes directly, leading to apoptosis; other viruses preferentially affect thymic architecture and physiology, leading to the disruption of microenvironments required for thymocyte differentiation and survival (4, 14). In addition, coxsackievirus (25) and Sindbis virus (35), two RNA viruses, were reported to induce thymic atrophy in the absence of any detectable viral antigens in this organ, suggesting that a systemic stress factor induced by the infection could be responsible for the disruption of thymic microenvironments.
These observations led us to search for a factor responsible for MV-induced differentiation and apoptosis of TEC, looking for an endogenous signal that (i) is inducible by a broad spectrum of viruses and (ii) mediates thymic epithelium disruption. In the present report, we demonstrate that MV and double-stranded RNA (dsRNA), a common intermediate of viral replication, both induce terminal TEC differentiation through beta interferon (IFN-β) synthesis. Moreover, recombinant human IFN-β (rhIFN-β) but also rhIFN-α and rhIFN-γ are shown to trigger similar processes. Thus, IFNs are shown to induce cortical TEC differentiation leading to apoptosis of thymic epithelium in vitro, suggesting their involvement in thymic epithelium atrophy.
MATERIALS AND METHODS
Reagents.
The monoclonal antibodies (MAbs) used were MAb CAM5.2 (immunoglobulin G2b [IgG2b] isotype) (Becton Dickinson, Mountain View, Calif.) and MAb AE3 (IgG1 isotype) (Diagnostic Biosystems, Fremont, Calif.), which specifically recognize low-molecular-weight cytokeratins (K8 and K18) and high-molecular-weight cytokeratins (K1, K2, and K5), respectively. MAb K20 (CD29, β1-integrin) was kindly provided by A. Bernard (Nice, France). Clone 55 (anti-H antibody) was provided by T. F. Wild (Lyon, France). Mouse IgG1 and IgG2 isotypic controls (Immunotech, Marseille, France) were used for negative controls of immunofluorescence. Sheep anti-human IFN-α or IFN-β polyclonal antibodies (BioSource International, Camarillo, Calif.) were also used. rhIFNs (rhIFN-α, rhIFN-β, and rhIFN-γ) were purchased from Calbiochem (San Diego, Calif.). Synthetic ssRNA [poly(C)] and dsRNA [poly(I · C)] were from Amersham Pharmacia Biotech Europe (Orsay, France).
Cell lines.
The P1.4D6 cortical TEC clone from human postnatal thymus was kindly provided by M. L. Toribio (13). Human epithelial cell lines MDA-MB-231 (breast adenocarcinoma), HT-29 (colon adenocarcinoma), and HeLa (cervix carcinoma) were from the American Type Culture Collection (Manassas, Va.). Cells were cultured in RPMI 1640 (Gibco Life Technologies, Inc., Grand Island, N.Y.) supplemented with 2 mM l-Glutamine (Gibco Life Technologies), 10 mM HEPES (Gibco Life Technologies), 40 μg of gentamicin (Gibco Life Technologies) per ml, and 10% fetal calf serum (Gibco Life Technologies). TEC were cultivated as previously described (37).
MV infection and viral titration.
For MV infection experiments, TEC were seeded at 5 × 103 cells/cm2 for 24 h and then infected at 1 PFU/cell with MV (Hallé strain). After 2 h of incubation at 37°C, cells were extensively washed three times. As controls, TEC were pulsed with MV inactivated by 254-nm UV rays for 30 min (UV-MV) or with a mock preparation. The mock preparation corresponds to uninfected Vero cell supernatant. After infection, cells were treated with or without neutralizing anti-IFN-α or anti-IFN-β at 2,000 or 500 IU/ml, respectively. Three days later, cells were treated again with anti-IFNs. Production of infectious MV particles from cell-free supernatants was measured at the indicated times, and the median tissue culture infective dose was calculated as previously described (12). Briefly, cell-free supernatants were diluted fivefold and incubated with Vero cell monolayers for 7 days.
TEC transfection with ssRNA or dsRNA.
TEC were plated at 5 × 104 cells/cm2 for 24 h and then transfected with 5 μg of ssRNA [poly(C)] or dsRNA [poly(I · C)] per ml with Fugene (Boehringer Mannheim, Meylan, France). After transfection, cells were treated or not with neutralizing anti-IFN-α or anti-IFN-β at 2,000 and 500 IU/ml, respectively. One day later, cells were treated again with anti-IFNs.
TEC treatment with rhIFN-α, rhIFN-β, or rhIFN-γ.
For rhIFN-β treatment, TEC were plated at 2.5 × 104 cells/cm2, cultured for 24 h, and then treated with 5,000 IU of rhIFN-β per ml for 7 days. For rhIFN-α and rhIFN-γ treatments, TEC were seeded at 5 × 103 cells/cm2, cultured for 24 h, and then treated for 7 days with 5,000 IU of rhIFN-α or 250 IU of rhIFN-γ per ml.
RNase protection assay.
RNA was extracted from 4 × 106 cells by using RNA NOW-TC reagent (Biogentex, Seabrook, Tex.). The RNase protection assay was performed using 4 μg of RNA with the RiboQuant multiprobe system from Pharmingen (San Diego, Calif.), following the manufacturer's specifications. In brief, RNA was hybridized overnight with the in vitro-translated 32P-labeled probe (hCK-3; Pharmingen). Following hybridization, samples were treated with RNase A plus RNase T1 and proteinase K, phenol-chloroform extracted, and ethanol precipitated. The protected fragments were resolved by electrophoresis on a 5% acrylamide-urea gel and exposed on a phosphor screen (Molecular Dynamics Inc., Sunnyvale, Calif.) for 12 h to quantify the intensities of the bands.
Phenotypic analysis.
Expression of cell surface and intracellular antigens was determined as previously described (37). After labeling, cells were analyzed by using a FACScalibur flow cytometer (Becton Dickinson Cellquest software). Integrated fluorescence was measured, and data were collected from at least 10,000 events.
Cell viability and apoptosis analysis.
The viability and apoptosis of TEC cultures were assayed using DIOC6 (3) (Molecular Probe, Inc., Eugene, Oreg.) as previously described (37). Briefly, cells were incubated for 15 min at 37°C with 40 nM DIOC6 (3) in culture medium to evaluate transmembrane potential (ΔΨm). As DIOC6 (3) stains living cells, the early decrease of DIOC6 (3) fluorescence is a measure of apoptosis. Integrated fluorescence was then measured by flow cytometry, and data were collected from at least 1 min for the evaluation of the cell number. Cell apoptosis was confirmed by using a fluorescein isothiocyanate (FITC) conjugate of the cell-permeable caspase inhibitor VAD-FMK (Promega, Madison, Wis.). This structure allows delivery of the inhibitor into the cells, where it irreversibly binds to activated caspases, serving as a specific in situ marker for apoptosis. Cells were incubated for 20 min at room temperature in the presence of FITC-VAD-FMK at 5 μM. Cells were then washed twice, and FITC-VAD-FMK labeling was determined by flow cytometry. Under our differentiation conditions, more than 95% of attached TEC were alive, and conversely, more than 95% of floating cells retrieved from the culture supernatant were apoptotic.
IFN-α/β detection assay.
At different times, supernatants were collected for IFN-α/β assay. For MV cultures, supernatants were UV inactivated (30 min at 254 nm). The supernatants were serially diluted twofold in a 96-microwell plate and added to confluent Vero cell monolayers. After incubation for 24 h at 37°C, the cells were infected with vesicular stomatitis virus at 0.1 PFU/cell. Cytopathic effects were scored 24 h later. Titration end points represented dilutions that gave destruction of 50% of the cells. IFN titers are expressed as international units per milliliter with reference to a standard IFN curve (Sigma, Saint Quentin Fallavier, France). IFN-α levels in the TEC supernatants were determined by enzyme-linked immunosorbent assay according to the specifications of the manufacturer (R&D Systems).
RESULTS
MV induces terminal TEC differentiation via an IFN-β loop.
In our previous report, MV replication was shown to induce terminal differentiation of human cortical TEC (37). In order to identify the factor responsible for TEC differentiation, cytokine induction by MV was investigated by RNase protection assays at day 3 postinfection. Transforming growth factor β (TGF-β) family factors and IFN-β were tested first, since they were previously reported to inhibit TEC or epithelial cell proliferation (17, 26). As shown in Fig. 1A, TGF-β1 and TGF-β2 mRNAs were constitutively expressed in TEC but were not modulated by either MV or UV-MV. Tumor necrosis factor beta (TNF-β), lymphotoxin beta, TNF-α, IFN-γ, IFN-β, and TGF-β3 mRNAs were not detected in TEC treated with either mock supernatant or UV-MV. However, IFN-β mRNA expression was specifically induced in MV-infected TEC. The supernatants from MV-infected TEC were tested for IFN-α/β secretion. TEC produced significant amounts of IFN-α/β upon MV replication, while mock supernatant or UV-MV had no effects (Fig. 1B). Up to 160 IU/ml was detected in the culture supernatants, but this could be underestimated if IFN-α/β were engaged in TEC stimulation. As shown in Fig. 2B, IFN-α could not be detected in the supernatant of MV-infected TEC on day 7, suggesting that IFN-β is mainly produced. This led us to investigate the contribution of IFN-β in the differentiation process of TEC.
FIG. 1.
Contribution of IFN-β to MV-induced TEC differentiation. TEC were seeded at 5 × 103 cells/cm2 for 24 h and then infected with MV at 0.1 infectious particle per cell or treated with UV-MV or mock supernatant (Mo). (A) On day 3, cells were harvested and expression of cytokine mRNAs was determined by RNase protection assay. L32 mRNA, encoding a constitutively expressed ribosomal protein, was used as a control probe. Lt-β, lymphotoxin beta. (B) On days 3, 5, and 7, supernatants were collected and tested for IFN-α/β with a biological quantification assay. Results correspond to the means from three independent experiments (standard deviations were less than 10%). (C) On day 7, cells were stained with May-Grunwald Giemsa stain and differentiation stages were determined under a microscope (magnification, ×170). As indicated, anti-IFN-α (2,000 U/ml) or anti-IFN-β (500 U/ml) was added just after the infection and 3 days later. For each culture condition, production of infectious MV particles from cell-free supernatants collected on day 5 is indicated. Results are from one representative experiment of three. TCID50, median tissue culture infective dose.
FIG. 2.
IFN-α/β synthesis by dsRNA-transfected TEC. TEC were seeded at 5 × 104 cells/cm2 for 24 h and then transfected with 5 μg of either ssRNA [poly(C)] or dsRNA [poly(I · C)] per ml. (A) Supernatants were collected 8, 24, 48, and 72 h after transfection. IFN-α/β were tested by using a biological quantification assay. Results correspond to the means from three independent experiments (standard deviations were less than 10%). (B) Supernatants from ssRNA- or dsRNA-transfected TEC were collected on day 3, and IFN-α synthesis was determined by enzyme-linked immunosorbent assay. Supernatants from MV-infected TEC and activated T lymphocytes were also tested at days 7 and 3 postinfection, respectively. rhIFN-α and rhIFN-β (5,000 IU/ml) were included in the assay as positive and negative controls, respectively. Results correspond to one representative experiment of three.
Cells were either treated with mock supernatant, treated with UV-MV, or infected with MV and cultured in the presence of either neutralizing anti-IFN-α or anti-IFN-β polyclonal antibodies. Because IFN-α/β were originally reported to control viral replication in infected cells, addition of anti-IFN-β antibodies was expected to boost MV replication. However, neutralizing antibodies had no significant effects on the production of infectious MV particles by TEC (Fig. 1C). Therefore, these results demonstrated that MV replication is not under IFN-α/β control in infected TEC. This is in agreement with a previous report from Naniche et al. in which replication of the Edmonston MV strain was shown to be poorly controlled by IFN-α/β, suggesting that MV bypasses the inhibitory effects of these cytokines (29). On day 7, cells were stained with May-Grunwald Giemsa stain and TEC differentiation was examined under the microscope. TEC treated with UV-MV (Fig. 1C) or mock supernatant (data not shown) formed monolayers of highly packed and regular clones, characteristic of undifferentiated epithelial cells in culture. In contrast, MV-infected TEC stopped growing, lost the classical epithelium-like morphology, decreased cell-cell contacts, and acquired the squamous aspect of highly differentiated TEC with fusiform to stellate shapes of dispersed single cells. The TEC differentiation process was completely blocked by anti-IFN-β treatment but not anti-IFN-α antibodies (Fig. 1C). These results demonstrated that IFN-β synthesis by MV-infected TEC mediated their differentiation.
dsRNA induces TEC differentiation via an IFN-β loop.
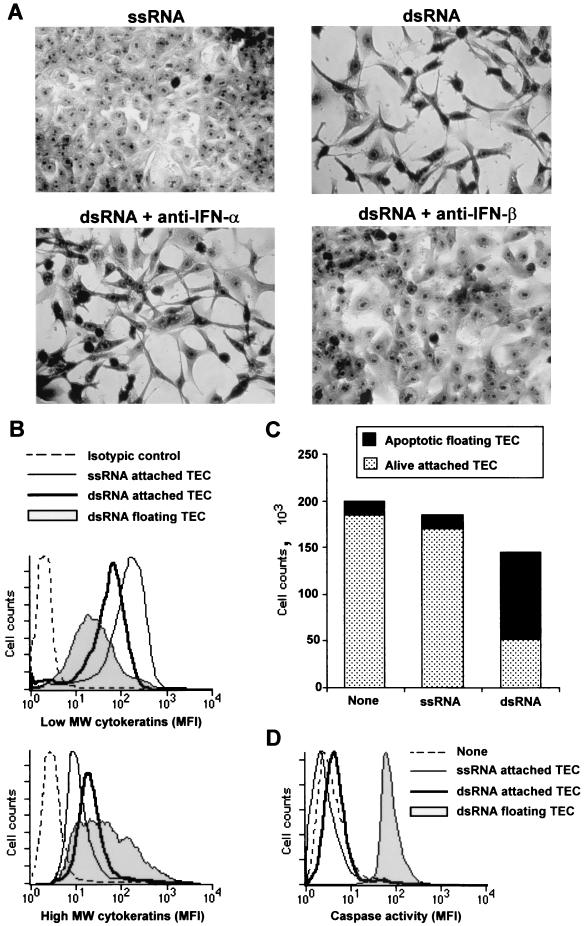
To demonstrate that terminal TEC differentiation is potentially inducible by a broad spectrum of viruses, cells were transfected with optimal concentrations of dsRNA, a common intermediate of viral replication cycle that activates antiviral response in many cell types (18), or with ssRNA as a negative control. As shown in Fig. 2A, significant amounts of IFN-α/β are produced by dsRNA- but not ssRNA-transfected TEC. IFN-α/β production was detected as soon as 8 h after the transfection and reached a maximum on day 2. IFN-α could not be detected in the supernatants of MV-infected or dsRNA-transfected TEC on day 3, suggesting that mainly IFN-β is produced (Fig. 2B). TEC differentiation was analyzed on days 3 and 5 posttransfection (Fig. 3). ssRNA-transfected TEC formed monolayers of highly packed and regular clones, characteristic of undifferentiated epithelial cells in culture (Fig. 3A). In contrast, dsRNA-transfected cells stopped growing and acquired the squamous morphology of highly differentiated TEC with fusiform to stellate shapes of dispersed single cells (Fig. 3A).
FIG. 3.
IFN-β-mediated differentiation of dsRNA-transfected TEC. TEC were seeded at 5 × 104 cells/cm2 for 24 h and then transfected with 5 μg of either ssRNA [poly(C)] or dsRNA [poly(I · C)] per ml. Anti-IFN-α (2,000 U/ml) or anti-IFN-β (500 U/ml) was added just after the transfection and 1 day later. (A) Cells were stained with May-Grunwald Giemsa stain, and differentiation stages were determined under a microscope on day 5 (magnification, ×170). (B) High-molecular-weight (MW) and low-molecular-weight cytokeratin expression was determined by flow cytometry on day 3 posttransfection. Attached ssRNA-transfected TEC, attached dsRNA-transfected TEC, or floating cells were harvested. Cells were permeabilized and stained with either MAb CAM 5.2 (specific for low-molecular-weight cytokeratins), MAb AE3 (specific for high-molecular-weight cytokeratins), or an isotypic control. Results are one representative experiment of three. MFI, mean fluorescence intensity. (C) Attached and floating cells were recovered from the culture wells on day 3. Both cell counts and viability, determined by DIOC6 (3) incorporation as a measure of mitochondrial transmembrane potential (ΔΨm), were analyzed by flow cytometry. Results correspond to the means from three independent experiments (standard deviations were less than 10%). (D) Caspase activity was determined by using FITC-VAD-FMK as a specific marker of activated caspases. Labeling was analyzed by flow cytometry. Results are from one representative experiment of three.
Epithelial cell differentiation is also characterized by a shift from low- to high-molecular-weight cytokeratin expression (6, 36, 40). This previously allowed us to demonstrate that floating cells, retrieved from the supernatant of MV-infected cultures, corresponded to the final step of TEC differentiation process (37). When transfected with dsRNA but not ssRNA, numerous floating TEC were similarly retrieved from the culture supernatant (Fig. 3C). Intracellular immunostaining was performed on day 3 with either MAb CAM5.2 (specific for low-molecular-weight cytokeratins) or MAb AE3 (specific for high-molecular-weight cytokeratins) to determine the TEC differentiation stage. When untreated (data not shown) or transfected with ssRNA, TEC expressed high levels of low-molecular-weight cytokeratins (Fig. 3B, upper panel). In contrast, dsRNA transfection led to the progressive loss of low-molecular-weight cytokeratins, with intermediate levels of expression in attached cells and low levels of expression in floating cells (Fig. 3B, upper panel). Conversely, high-molecular-weight cytokeratin expression was up-regulated as a function of differentiation stage when TEC were transfected with dsRNA but not ssRNA (Fig. 3B, lower panel). This process was accompanied by CD29/β1-integrin down-regulation (data not shown), which may account for progressive loss of TEC adhesion to the plastic surface during differentiation. As previously reported for MV-induced differentiation, terminally differentiated cells retrieved from the culture supernatants finally died by apoptosis, as assessed by the loss of mitochondrial transmembrane potential (ΔΨm) and caspase activation (Fig. 3C and D).
These results demonstrated that dsRNA induced terminal differentiation of TEC, as assessed by combined morphological and phenotypic changes, with progressive loss of adhesion and apoptosis as an end point of this process. Importantly, the changes reported here were not by-products of the apoptotic cascade, since staurosporine-treated or serum-deprived TEC were undergoing apoptosis but did not acquire, at any stage of the apoptotic process, the morphology of differentiated epithelial cells (data not shown). To test whether IFN-α/β participate in dsRNA-induced differentiation of TEC, transfected cells were treated with either neutralizing anti-IFN-α or anti-IFN-β polyclonal antibodies. The TEC differentiation process induced by dsRNA transfection was completely blocked by anti-IFN-β treatment, while anti-IFN-α had no effects (Fig. 3A). Consequently, dsRNA, but not ssRNA, induces terminal TEC differentiation via an autocrine/paracrine IFN-β loop.
rhIFNs induce terminal differentiation of TEC.
In order to demonstrate that IFN-β signaling is sufficient to induce TEC differentiation, cells were treated with optimal concentration of rhIFN-β (5,000 IU/ml) and TEC differentiation was analyzed on day 7 (Fig. 4). Untreated TEC formed characteristic monolayers of undifferentiated epithelial cells (Fig. 4A). In contrast, rhIFN-β-treated TEC stopped growing and acquired the typical morphology of highly differentiated cells (Fig. 4A). Furthermore, rhIFN-β treatment led to the progressive loss of low-molecular-weight cytokeratins and, conversely, up-regulated high-molecular-weight cytokeratin expression (data not shown). This differentiation process was also accompanied by CD29/β1-integrin down-regulation in rhIFN-β-treated TEC (data not shown). rhIFN-β-induced differentiation led to apoptosis of terminally differentiated floating cells as assessed by DIOC6 (3) staining and caspase activity measure (Fig. 4B and C). This process was initiated when 500 IU/ml was applied, but 5,000 IU/ml was required to induce full differentiation of TEC (data not shown).
FIG. 4.
Recombinant IFN-β induces terminal differentiation of TEC. TEC were seeded at 2.5 × 104 cells/cm2 for 24 h then treated or not with rhIFN-β at 5,000 IU/ml. TEC differentiation was analyzed on day 7. (A) Cells were stained with May-Grunwald Giemsa stain, and differentiation stages were determined under a microscope (magnification, ×170). (B) Attached and floating cells were recovered from the culture flasks. Both cell counts and viability, determined by DIOC6 (3) incorporation as a measure of mitochondrial transmembrane potential (ΔΨm), were analyzed by flow cytometry. Results correspond to the means from three independent experiments (standard deviations were less than 10%). (C) Caspase activity was determined by using FITC-VAD-FMK as a specific marker of activated caspases. Labeling was analyzed by flow cytometry. Results are from one representative experiment of three.
To demonstrate that TEC differentiation is not induced exclusively by IFN-β, TEC were finally treated with optimal concentrations of either rhIFN-α (5,000 IU/ml) or rhIFN-γ (250 IU/ml). On day 7, TEC morphology was evaluated under the microscope. Both rhIFN-α and rhIFN-γ treatments stopped TEC proliferation and induced differentiation as assessed by acquisition of a squamous morphology with fusiform to stellate shapes of single-dispersed cells and loss of cell-cell contacts (Fig. 5A). As reported for rhIFN-β, both rhIFN-α and rhIFN-γ promoted a shift from low- to high-molecular-weight cytokeratins (data not shown). Moreover, terminally differentiated floating cells retrieved from the culture supernatants were apoptotic as assessed by caspase activity (Fig. 5B) and DIOC6 staining (data not shown). Thus, both IFN-α/β and IFN-γ induce terminal TEC differentiation that leads to apoptosis.
FIG. 5.
Differentiation of either IFN-α- or IFN-γ-treated TEC. TEC were seeded at 5 × 103 cells/cm2 for 24 h and then treated with either rhIFN-α at 5,000 IU/ml or rhIFN-γ at 250 IU/ml. (A) TEC differentiation was analyzed on day 7. Cells were stained with May-Grunwald Giemsa stain, and differentiation stages were determined under a microscope (magnification, ×170). Results are from one representative experiment of three. (B) Caspase activity in attached and floating cells recovered from the culture was determined by using FITC-VAD-FMK as a specific marker of activated caspases. Labeling was analyzed by flow cytometry. Results are from one representative experiment of three.
Differentiation induced by MV and rhIFN-α is cell type specific.
In addition to TEC, epithelial cell lines originating from other tissues were tested for their ability to differentiate when infected with MV or treated with rhIFN-α. MDA-MB-231 (breast adenocarcinoma), HT-29 (colon adenocarcinoma), and HeLa (cervix carcinoma) cells were infected with MV, and replication was determined on the basis of MV H viral protein expression. MV efficiently replicated in all cell lines tested and induced IFN-α/β production (Fig. 6A). Both MV and rhIFN-α induced growth arrest and morphological differentiation of the breast adenocarcinoma cell line MDA-MB-231, as assessed by their stellate shapes (Fig. 6B). Cells finally died by apoptosis as an end point of this differentiation process (data not shown). In contrast, MV and rhIFN-α induced growth arrest of HT-29 and HeLa cells with no significant modification of their morphology (Fig. 6A). These results demonstrated that MV- and IFN-α-induced differentiation of epithelial cells is not unique to thymic epithelium but appears to be highly tissue specific and cell type dependent.
FIG. 6.
Differentiation induced by MV and rhIFN-α is cell type specific. Cells were seeded at 5 × 103 cells/cm2 for 24 h and then infected with MV at 0.1 infectious particle per cell or treated with rhIFN-α at 5,000 IU/ml. Cells and supernatants were collected on day 7. (A) MV H hemagglutinin expression was determined by immunostaining and flow cytometry analysis. IFN-α/β in the culture supernatants were quantified by using a biological assay. Cells were stained with May-Grunwald Giemsa stain, and morphological differentiation was determined under a microscope (magnification, ×200). Results are from one representative experiment of three. MFI, mean fluorescence intensity; ND, not determined. (B) Representative morphology of untreated, MV-infected, or rhIFN-α-treated MDA-MB-231 breast adenocarcinoma cells stained with May-Grunwald Giemsa stain (magnification, ×170).
DISCUSSION
Combined mechanisms could account for thymic involution associated with MV infection. In the SCID-hu model, TEC have been described as a major target of MV in the thymus, leading to apoptosis of thymocytes (4). It has been recently demonstrated that MV-H hemagglutinin and MV-F fusion glycoproteins inhibit Akt kinase activity in mature T cells, leading to the disruption of interleukin-2 receptor signaling and inhibition of their proliferation (5). In addition, spontaneous apoptosis of thymocytes in the thymuses of Akt1−/− mice was reported (10). Therefore, it can be assumed that H and F expression on the cell surfaces of MV-infected TEC could disrupt the Akt pathway in thymocytes, leading to apoptosis. As an additional pathway, MV replication was shown to induce terminal differentiation and apoptosis of TEC in vitro (37). The latter process could mediate thymic epithelium disruption, leading to apoptosis of uninfected thymocytes through survival signal deprivation.
In the present study, we demonstrated that MV-induced differentiation and apoptosis of TEC were dependent on IFN-β production. IFN-α/β were originally described for their antiviral activity, and their induction corresponds to the engagement of an innate response against viruses. However, in our culture system, where IFN-α/β failed to inhibit MV replication, the induction of a terminal TEC differentiation revealed an alternative and deleterious effect of these cytokines. Furthermore, dsRNA was also reported to induce TEC differentiation through IFN-β synthesis. Finally, we demonstrated that viral infection of TEC is not required to induce their terminal differentiation and apoptosis, since either recombinant IFN-α, -β, or -γ is sufficient for the induction of this process.
While dsRNA is a common structural signature for viral replication, the sources differ widely among RNA and DNA viruses (18). For viruses with dsRNA genomes, the obvious source of dsRNA is the genome itself. The RNA genomes of retroviruses contain large domains of secondary structure with dsRNA. For ssRNA viruses, genome replication leads to the synthesis of a dsRNA intermediate. When DNA viruses replicate, dsRNA accumulates, since complementary mRNAs are produced from viral genes transcribed in opposing directions and hybridize. In infected cells, dsRNA coated with viral proteins prevents the exposure of naked molecules. However, very small amounts of incorrectly coated dsRNA might be present in infected cells and activate the known dsRNA-dependent enzymes that induce IFN-α/β expression (18). Indeed, minute quantities of dsRNA, as little as one molecule per cell, were reported to trigger IFN-α/β synthesis and antiviral responses (24). Importantly, TEC differentiation was dependent on dsRNA expression inside the cells, since extracellular dsRNA had no effects and transfection was required (data not shown). This observation suggested that intracellular receptors, such as the dsRNA-dependent kinase PKR, but not recently described receptors for extracellular dsRNA, such as TLR3, are involved in IFN-α/β induction in TEC (2). On the basis of these results, we propose that virtually all viruses that are capable of infecting TEC and accumulating dsRNA could affect thymic epithelium architecture, function, and maintenance through the induction of IFN-α/β.
We also reported that IFN-α or IFN-β was efficient in inducing differentiation of noninfected TEC. IFN-α and -β had similar effects on TEC, because a common receptor composed of two chains, IFNAR1 and IFNAR2, is shared by those two cytokines (22). The recently described thymic plasmacytoid dendritic cells (pDCs), also known as IFN-α/β-producing cells, were characterized as a major resident population in the human thymus (7, 9, 31). Thymic pDCs were shown to be a major source of IFN-α/β when stimulated with either influenza virus or HIV-1 (7, 19). Thus, pDCs could produce IFN-α/β doses required to mediate terminal differentiation of uninfected TEC when viruses spread to the thymus, leading to thymic epithelium disruption and atrophy. Furthermore, coxsackievirus (25) and Sindbis virus (35) were reported to induce thymic atrophy in the absence of any detectable viral antigens in this organ, suggesting that a systemic stress factor induced by the infection is responsible for the disruption of thymic microenvironments. This systemic stress factor could be IFN-α/β, since daily injections of IFN-α (4 × 105 IU for 6 days) were also reported to induce severe thymic atrophy and impaired thymocyte development in mice (23). Thymic atrophy induced by IFN-α/β was originally attributed to the antiproliferative activity of these cytokines. Indeed, IFN-α/β were shown to inhibit interleukin-7-driven survival and proliferation of thymocytes at the early CD4− CD8− CD44+ CD25+ differentiation stage (33). Our present report sustains the additional hypothesis that systemic IFN-α/β induction could also mediate thymic involution through their deleterious effects on thymic epithelium, leading to impaired T-cell lymphopoiesis.
In addition, we demonstrated that IFN-γ could also mediate terminal TEC differentiation in vitro. IFN-γ interacts with a receptor composed of two chains, IFNGR1 and IFNGR2, which activate Jak1 and Jak2 kinases, leading to STAT1 activation (22). Interestingly, the receptor for IFN-α/β activates Jak1 and Tyk2, leading to the recruitment of STAT1 and STAT2. Thus, IFN-α/β and IFN-γ activate a common signaling cascade leading to STAT1 activation. This transcription factor, shared by IFN-α/β and IFN-γ signaling pathways, could therefore participate in the induction of the TEC differentiation program. In the case of viral infection, large amounts of IFN-γ are produced by activated T lymphocytes. Thus, entry of antivirally active T lymphocytes into the thymuses of virus-infected mice could also mediate thymic epithelium involution through the expression of IFN-γ (15).
When viruses spread to the thymus, pDCs, infected TEC, or antivirally active T cells could produce large amounts of IFN-α/β or IFN-γ. On the basis of our present results, those IFNs could disrupt thymic epithelium function and survival, leading to thymic atrophy. Together, these results support the idea that thymic atrophy associated with viral infections could be due not only to the blockade of thymocyte proliferation by IFNs but also to the disruption of thymic epithelium function and survival by these cytokines.
Acknowledgments
This work was supported in part by institutional grants from the Ministère de l'Education National, de l'Enseignement Supérieur et de la Recherche, and l'Institut National de la Santé et de la Recherche Médicale and by additional support from the Association pour la Recherche sur le Cancer (CRC 5753), the Agence National de Recherche sur le SIDA (FF049G), and the Programme de Recherche de la Région Rhône-Alpes (UR503-2A7-HHC02F).
We are grateful to J. P. Revillard and D. Gerlier for reading the manuscript and for helpful suggestions. We thank A. Lefeuvre, who kindly gave us supernatants from MV-infected lymphocytes. We also thank M. L. Toribio, who gave us the P1.4D6 TEC clone, and we thank D. Kaiserlian for providing the HT-29 cell line. The expert technical assistance of M. Belviso and A. Anginot is acknowledged. The useful comments of A. Astier, M. C. Trescol-Biémont, A. Evlashev, and L. Genestier are greatly appreciated.
REFERENCES
- 1.Aldrovandi, G. M., G. Feuer, L. Gao, B. Jamieson, M. Kristeva, I. S. Chen, and J. A. Zack. 1993. The SCID-hu mouse as a model for HIV-1 infection. Nature 363:732-736. [DOI] [PubMed] [Google Scholar]
- 2.Alexopoulou, L., A. C. Holt, R. Medzhitov, and R. A. Flavell. 2001. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature 413:732-738. [DOI] [PubMed] [Google Scholar]
- 3.Anderson, G., N. C. Moore, J. J. Owen, and E. J. Jenkinson. 1996. Cellular interactions in thymocyte development. Annu. Rev. Immunol. 14:73-99. [DOI] [PubMed] [Google Scholar]
- 4.Auwaerter, P. G., H. Kaneshima, J. M. McCune, G. Wiegand, and D. E. Griffin. 1996. Measles virus infection of thymic epithelium in the SCID-hu mouse leads to thymocyte apoptosis. J. Virol. 70:3734-3740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avota, E., A. Avots, S. Niewiesk, L. P. Kane, U. Bommhardt, V. ter Meulen, and S. Schneider-Schaulies. 2001. Disruption of Akt kinase activation is important for immunosuppression induced by measles virus. Nat. Med. 7:725-731. [DOI] [PubMed] [Google Scholar]
- 6.Banks-Schlegel, S. P. 1982. Keratin alterations during embryonic epidermal differentiation: a presage of adult epidermal maturation. J. Cell Biol. 93:551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bendriss-Vermare, N., C. Barthelemy, I. Durand, C. Bruand, C. Dezutter-Dambuyant, N. Moulian, S. Berrih-Aknin, C. Caux, G. Trinchieri, and F. Briere. 2001. Human thymus contains IFN-alpha-producing CD11c(−), myeloid CD11c(+), and mature interdigitating dendritic cells. J. Clin. Invest. 107:835-844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bonyhadi, M. L., L. Rabin, S. Salimi, D. A. Brown, J. Kosek, J. M. McCune, and H. Kaneshima. 1993. HIV induces thymus depletion in vivo. Nature 363:728-732. [DOI] [PubMed] [Google Scholar]
- 9.Cella, M., D. Jarrossay, F. Facchetti, O. Alebardi, H. Nakajima, A. Lanzavecchia, and M. Colonna. 1999. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat. Med. 5:919-923. [DOI] [PubMed] [Google Scholar]
- 10.Chen, W. S., P. Z. Xu, K. Gottlob, M. L. Chen, K. Sokol, T. Shiyanova, I. Roninson, W. Weng, R. Suzuki, K. Tobe, T. Kadowaki, and N. Hay. 2001. Growth retardation and increased apoptosis in mice with homozygous disruption of the Akt1 gene. Genes Dev. 15:2203-2208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chino, F., H. Kodama, T. Ohkawa, and Y. Egashira. 1979. Alterations of the thymus and peripheral lymphoid tissues in fatal measles. A review of 14 autopsy cases. Acta Pathol. Jpn. 29:493-507. [DOI] [PubMed] [Google Scholar]
- 12.Evlashev, A., E. Moyse, H. Valentin, O. Azocar, M. C. Trescol-Biemont, J. C. Marie, C. Rabourdin-Combe, and B. Horvat. 2000. Productive measles virus brain infection and apoptosis in CD46 transgenic mice. J. Virol. 74:1373-1382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez, E., A. Vicente, A. Zapata, B. Brera, J. J. Lozano, C. Martinez, and M. L. Toribio. 1994. Establishment and characterization of cloned human thymic epithelial cell lines. Analysis of adhesion molecule expression and cytokine production. Blood 83:3245-3254. [PubMed] [Google Scholar]
- 14.Godfraind, C., K. V. Holmes, and J. P. Coutelier. 1995. Thymus involution induced by mouse hepatitis virus A59 in BALB/c mice. J. Virol. 69:6541-6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gossmann, J., J. Lohler, and F. Lehmann-Grube. 1991. Entry of antivirally active T lymphocytes into the thymus of virus-infected mice. J. Immunol. 146:293-297. [PubMed] [Google Scholar]
- 16.Griffin, D. E. 1995. Immune responses during measles virus infection. Curr. Top. Microbiol. Immunol. 191:117-134. [DOI] [PubMed] [Google Scholar]
- 17.Hong, Y. K., D. S. Chung, Y. A. Joe, Y. J. Yang, K. M. Kim, Y. S. Park, W. K. Yung, and J. K. Kang. 2000. Efficient inhibition of in vivo human malignant glioma growth and angiogenesis by interferon-beta treatment at early stage of tumor development. Clin. Cancer Res. 6:3354-3360. [PubMed] [Google Scholar]
- 18.Jacobs, B. L., and J. O. Langland. 1996. When two strands are better than one: the mediators and modulators of the cellular responses to double-stranded RNA. Virology 219:339-349. [DOI] [PubMed] [Google Scholar]
- 19.Keir, M. E., C. A. Stoddart, V. Linquist-Stepps, M. E. Moreno, and J. M. McCune. 2002. IFN-alpha secretion by type 2 predendritic cells up-regulates MHC class I in the HIV-1-infected thymus. J. Immunol. 168:325-331. [DOI] [PubMed] [Google Scholar]
- 20.Laster, A. J., T. Itoh, T. J. Palker, and B. F. Haynes. 1986. The human thymic microenvironment: thymic epithelium contains specific keratins associated with early and late stages of epidermal keratinocyte maturation. Differentiation 31:67-77. [DOI] [PubMed] [Google Scholar]
- 21.Laufer, T. M., L. H. Glimcher, and D. Lo. 1999. Using thymus anatomy to dissect T cell repertoire selection. Semin. Immunol. 11:65-70. [DOI] [PubMed] [Google Scholar]
- 22.Levy, D. E., and A. Garcia-Sastre. 2001. The virus battles: IFN induction of the antiviral state and mechanisms of viral evasion. Cytokine Growth Factor Rev. 12:143-156. [DOI] [PubMed] [Google Scholar]
- 23.Lin, Q., C. Dong, and M. D. Cooper. 1998. Impairment of T and B cell development by treatment with a type I interferon. J. Exp. Med. 187:79-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Marcus, P. I., and M. J. Sekellick. 1977. Defective interfering particles with covalently linked [+/−]RNA induce interferon. Nature 266:815-819. [DOI] [PubMed] [Google Scholar]
- 25.Matteucci, D., A. Toniolo, P. G. Conaldi, F. Basolo, Z. Gori, and M. Bendinelli. 1985. Systemic lymphoid atrophy in coxsackievirus B3-infected mice: effects of virus and immunopotentiating agents. J. Infect. Dis. 151:1100-1108. [DOI] [PubMed] [Google Scholar]
- 26.Meilin, A., J. Shoham, L. Schreiber, and Y. Sharabi. 1995. The role of thymocytes in regulating thymic epithelial cell growth and function. Scand. J. Immunol. 42:185-190. [DOI] [PubMed] [Google Scholar]
- 27.Moench, T. R., D. E. Griffin, C. R. Obriecht, A. J. Vaisberg, and R. T. Johnson. 1988. Acute measles in patients with and without neurological involvement: distribution of measles virus antigen and RNA. J. Infect. Dis. 158:433-442. [DOI] [PubMed] [Google Scholar]
- 28.Muller, J. G., V. Krenn, S. Czub, C. Stahl-Hennig, C. Coulibaly, G. Hunsmann, and H. K. Muller-Hermelink. 1993. The thymic epithelial reticulum and interdigitating cells in SIV-induced thymus atrophy and its comparison with other forms of thymus involution. Res. Virol. 144:93-98. [DOI] [PubMed] [Google Scholar]
- 29.Naniche, D., A. Yeh, D. Eto, M. Manchester, R. M. Friedman, and M. B. Oldstone. 2000. Evasion of host defenses by measles virus: wild-type measles virus infection interferes with induction of alpha/beta interferon production. J. Virol. 74:7478-7484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nicolas, J. F., A. Reano, D. Kaiserlian, and J. Thivolet. 1989. Epithelial cell heterogeneity in mammalian thymus: monoclonal antibody to high molecular weight keratins exclusively binds to Hassall's corpuscles. Histochem. J. 21:357-364. [DOI] [PubMed] [Google Scholar]
- 31.Siegal, F. P., N. Kadowaki, M. Shodell, P. A. Fitzgerald-Bocarsly, K. Shah, S. Ho, S. Antonenko, and Y. J. Liu. 1999. The nature of the principal type 1 interferon-producing cells in human blood. Science 284:1835-1837. [DOI] [PubMed] [Google Scholar]
- 32.Stanley, S. K., J. M. McCune, H. Kaneshima, J. S. Justement, M. Sullivan, E. Boone, M. Baseler, J. Adelsberger, M. Bonyhadi, J. Orenstein, et al. 1993. Human immunodeficiency virus infection of the human thymus and disruption of the thymic microenvironment in the SCID-hu mouse. J. Exp. Med. 178:1151-1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su, D. M., J. Wang, Q. Lin, M. D. Cooper, and T. Watanabe. 1997. Interferons alpha/beta inhibit IL-7-induced proliferation of CD4− CD8− CD3− CD44+ CD25+ thymocytes, but do not inhibit that of CD4− CD8− CD3− CD44− CD25− thymocytes. Immunology 90:543-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Su, L., H. Kaneshima, M. Bonyhadi, S. Salimi, D. Kraft, L. Rabin, and J. M. McCune. 1995. HIV-1-induced thymocyte depletion is associated with indirect cytopathogenicity and infection of progenitor cells in vivo. Immunity 2:25-36. [DOI] [PubMed] [Google Scholar]
- 35.Trgovcich, J., J. F. Aronson, and R. E. Johnston. 1996. Fatal Sindbis virus infection of neonatal mice in the absence of encephalitis. Virology 224:73-83. [DOI] [PubMed] [Google Scholar]
- 36.Tseng, S. C., M. J. Jarvinen, W. G. Nelson, J. W. Huang, J. Woodcock-Mitchell, and T. T. Sun. 1982. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell 30:361-372. [DOI] [PubMed] [Google Scholar]
- 37.Valentin, H., O. Azocar, B. Horvat, R. Williems, R. Garrone, A. Evlashev, M. L. Toribio, and C. Rabourdin-Combe. 1999. Measles virus infection induces terminal differentiation of human thymic epithelial cells. J. Virol. 73:2212-2221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.White, R. G., and J. F. Boyd. 1973. The effect of measles on the thymus and other lymphoid tissues. Clin. Exp. Immunol. 13:343-357. [PMC free article] [PubMed] [Google Scholar]
- 39.Woo, J. C., G. A. Dean, N. C. Pedersen, and P. F. Moore. 1997. Immunopathologic changes in the thymus during the acute stage of experimentally induced feline immunodeficiency virus infection in juvenile cats. J. Virol. 71:8632-8641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Woodcock-Mitchell, J., R. Eichner, W. G. Nelson, and T. T. Sun. 1982. Immunolocalization of keratin polypeptides in human epidermis using monoclonal antibodies. J. Cell Biol. 95:580-588. [DOI] [PMC free article] [PubMed] [Google Scholar]