Abstract
Pseudorabies virus (PRV), a swine alphaherpesvirus, is capable of causing viremia in vaccinated animals. Two mechanisms that may help PRV avoid recognition by the host immune system during this viremia are direct cell-to-cell spread in tissue and antibody-induced internalization of viral cell surface glycoproteins in PRV-infected blood monocytes, the carrier cells of the virus in the blood. PRV glycoprotein B (gB) is crucial during both processes. Here we show that mutating a tyrosine residue located in a YXXΦ motif in the gB cytoplasmic tail results in decreased efficiency of cell-to-cell spread and a strong reduction in antibody-induced internalization of viral cell surface glycoproteins. Mutating the dileucine motif in the gB tail led to an increased cell-to-cell spread of the virus and the formation of large syncytia.
Pseudorabies virus (PRV) is a swine alphaherpesvirus which, like most alphaherpesviruses, has evolved in several ways to subvert the immune system of its host. One noteworthy example of PRV immune modulation is the ability to replicate in the respiratory tracts of vaccinated animals. A viremia often results, giving rise to striking PRV symptoms, including abortion (24, 39). This viremia in vaccinated pigs requires cell-to-cell spread of PRV in tissue and transport of virus via infected monocytes in the blood (23, 24, 25, 39).
Mechanisms used by PRV, as well as by the prototypical alphaherpesvirus herpes simplex virus (HSV), to avoid recognition and destruction by the immune system include strategies to downregulate major histocompatibility complex class I-dependent antigen presentation in infected cells (3, 33; for a review, see reference 40), direct cell-to-cell spread of the virus, binding of complement factors via viral glycoprotein C (gC), Fc receptor activity of viral glycoprotein complex gE-gI, and, for PRV, the recently described antibody-induced internalization of viral cell surface proteins in PRV-infected blood monocytes, the natural carrier cells of the virus in vaccinated animals (9, 10, 14, 15, 17, 18).
For PRV, two of these mechanisms are mediated by viral glycoprotein gB: (i) the antibody-induced internalization of viral cell surface glycoproteins (a rapid and massive internalization of the majority of plasma membrane-anchored viral proteins upon aggregation of these proteins caused by the addition of PRV-specific antibodies, a process that likely results in inefficient antibody-dependent lysis of PRV-infected monocytes) and (ii) the direct cell-to-cell spread of the virus (10, 29, 31, 38).
PRV gB is a type I membrane glycoprotein of 913 amino acids (aa), consisting of an extracellular domain, a transmembrane region, and a 93-aa cytoplasmic C-terminal tail. At least three putative endocytosis motifs located within the cytoplasmic tail of gB are conserved throughout the alphaherpesvirus family. Two are tyrosine-based YXXΦ sequences (where Y stands for tyrosine, X stands for any amino acid, and Φ represents a bulky, hydrophobic group) and one is a dileucine (LL) motif. YXXΦ and LL motifs in the cytoplasmic tail of cellular receptors have been shown to be crucial for their endocytosis following ligand binding. Adaptor protein (AP) complexes such as AP-2 associate with these motifs and link the receptors to clathrin as a first step in the formation of clathrin-coated endocytosis vesicles (20). Such AP-2 binding to the putative endocytosis motifs in the PRV gB tail could explain how gB mediates the antibody-induced internalization of viral cell surface proteins.
Furthermore, these YXXΦ and LL motifs in the gB tail could also be of significance in gB-mediated cell-to-cell spread of PRV, since similar motifs in several cellular proteins direct basolateral targeting of these proteins by interacting with another subset of AP molecules (AP-1B) in polarized epithelial cells (12). Basolateral, unlike apical, expression brings viral proteins in close contact with neighboring cells, which may facilitate subsequent direct cell-to-cell spread. Recently, the C-terminal domain of PRV gB has been shown to modulate direct cell-to-cell spread of the virus (27). Furthermore, research has shown that HSV gE, another viral membrane protein involved in direct cell-to-cell spread, is specifically targeted to cell junctions on the lateral membranes. This sorting, which is important for direct spread of HSV from cell to cell, involves the cytoplasmic domain of gE as well as AP-1 molecules (19, 22).
To test the roles of the YXXΦ and LL motifs in the PRV gB tail in promoting efficient antibody-induced internalization of viral cell surface proteins and in direct cell-to-cell spread, we constructed viral mutants containing amino acid substitutions in the tyrosine residues, the LL motif, or both.
PRV mutants were constructed using the self-recombining bacterial artificial chromosome (BAC) containing the 142-kb PRV genome (PRV BAC) (32). First, a screening PRV BAC was constructed, in which 80% of the gB open reading frame (ORF) was replaced by a kanamycin resistance (Kanr) cassette (pHF22) as follows. A plasmid containing the PRV strain Becker (PRV Be) gB gene plus flanking sequences (pALM81) was partially digested by SphI to release the 4.6-kb fragment containing the PRV Be gB gene plus 700-bp upstream and 900-bp downstream flanking sequences. This fragment was cloned into an SphI-cut vector, creating pHF3. Approximately 80% of the gB ORF was excised from pHF3 by NotI-SalI digestion of pHF3, blunt ended using Klenow (New England Biolabs Inc., Beverly, Mass.), and replaced by a Kanr cassette (a SalI-digested, Klenow-blunt ended, 1.2-kb fragment of pUC4K [Promega, Madison, Wis.]), creating pHF7. The partially SphI-digested 4.6-kb fragment of pHF7 was cloned into the SphI-cut pGS284 plasmid (32), creating pHF9, which was used as a delivery vector for allelic exchange with the PRV BAC pGS469 (32) to create pHF22.
To construct the different PRV gB mutants, the 1.2-kb SalI-HindIII fragment of pHF3 was cloned, creating pHF6. The 200-bp fragment of pHF6 which resulted from digestion by EcoRI and partial digestion by XhoI was then subcloned to create pHF5. Subsequently, oligonucleotide mutagenesis of pHF5 was performed with the Altered Sites II kit (Promega). The oligonucleotide used to replace Y864 by an alanine (A)(5′-GCCCGGGACATGATCAGGGCCATGTCCATCGTGTCG-3′) introduced a BclI site, the oligonucleotide used to replace LL887 by two arginines (RR) (5′-GAACAGCGGGCCCGCGAGGAGGGCTAGCCGCGTCGGGGCG-3′) introduced an NheI site, and the oligonucleotide used to replace Y902 by A (5′-CACGCGCCGCCGACACGCCCAGCGCCTCGA-3′) removed an NgoMIV site, which facilitated the screening of mutated clones and the confirmation of recombinant viruses. All mutagenized plasmids were sequenced to confirm the mutagenesis, and the mutated DNA fragments were cloned back into pHF3. The 4.6-kb fragment resulting from partial digestion of the mutated pHF3 plasmids with SphI was then cloned into the SphI-cut pGS284, and these plasmids were used as delivery vectors for allelic exchange with pHF22 to create gB-mutated PRV BACs. A rescued virus was constructed in an identical manner using wild-type DNA. The PRV BACs with gB mutations were transferred into PK15 cells by using the CellPhect transfection kit (Amersham Pharmacia Biotech, Little Chalfont, England). When a total cytopathic effect was observed, infected cells and medium were collected.
All mutant viruses (PRV Y864A, PRV Y902A, PRV Y864A/Y902A, PRV LL887RR, PRV Y864A/Y902A/LL887RR, and PRV Rescue) contained the desired gB mutation as determined by PCR amplification of the 300 terminal bases of the 3′ end of the gB ORF and restriction enzyme analysis (using BclI, NheI, and NgoMIV) of this 300-bp fragment.
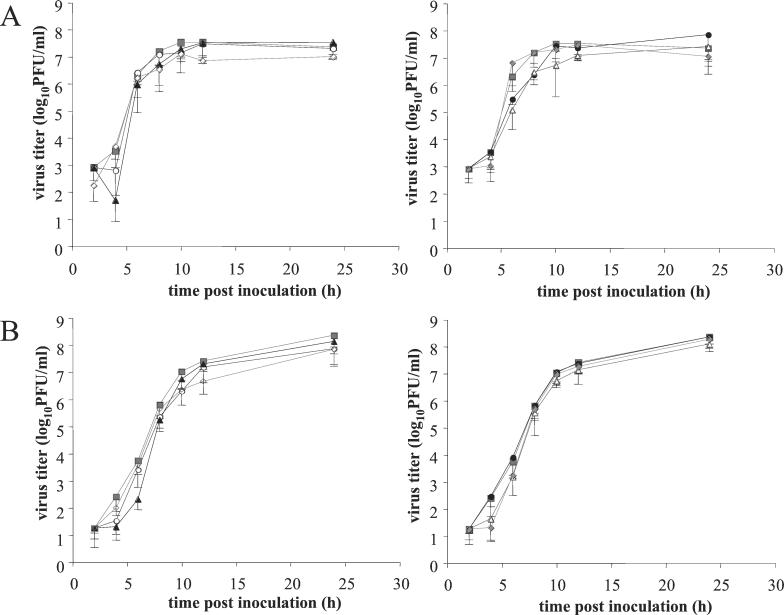
Single-step growth analyses in swine kidney (SK) cells (Fig. 1) showed that all mutant viruses grew as well as the wild-type virus and produced equivalent numbers of intracellular and extracellular infectious virus. The PRV gB tail contains two predicted α-helical domains. Previous studies have shown that the predicted α-helical domain II, encompassing both LL887 and Y902, is dispensable for virus growth (27), which is consistent with our present results. Similarly, the α-helical domain II of HSV type 1 (HSV-1) is not required for normal replication in Vero cells (5, 13). However, mutations disrupting α-helical domain I, which contains the Y864-based YM(S/A)I sequence, result in drastically reduced infectivity for both PRV and HSV-1 (5, 13, 27). For PRV, it has been shown that this reduced infectivity is accompanied by an inefficient incorporation of gB into the virion envelope (27). Since replacing the tyrosine in the YMSI sequence at position 864 by an alanine (Y864A) had no effect on virus growth or on gB incorporation into virions (data not shown), we believe that this mutation does not alter the structure of α-helical domain I.
FIG. 1.
Kinetics of intracellular (A) and extracellular (B) virus titers for the different PRV mutants in SK cells inoculated at an MOI of 10 with PRV Be (▪), PRV Y864A (○), PRV Y902A (▴), PRV Y864A/Y902A (◊), PRV LL887RR (•), PRV Y864A/Y902A/LL887RR (▵), or PRV Rescue (⧫). Data represent means − standard deviations of triplicate assays.
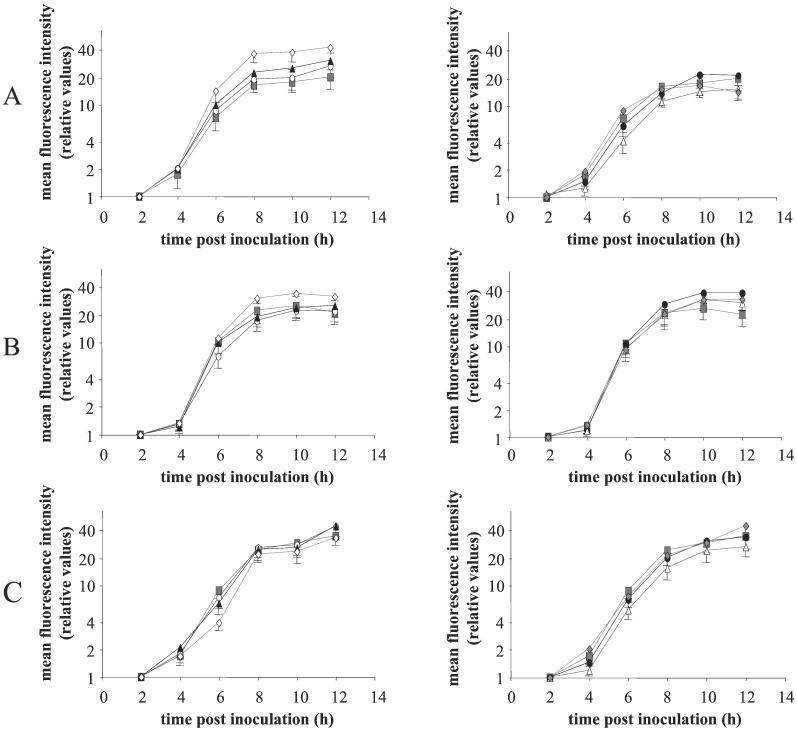
The kinetics of viral glycoprotein expression on the plasma membrane of infected SK cells were determined as described before (9). Briefly, cells were inoculated with the different mutant viruses at a multiplicity of infection (MOI) of 10, incubated in suspension on a rocking platform, and fixed in 1% paraformaldehyde at different time points postinoculation. Different viral cell surface glycoproteins (gB, gC, and gD) were stained using monoclonal antibodies (26) and fluorescein isothiocyanate (FITC)-labeled secondary antibodies (Molecular Probes, Eugene, Oreg.). Flow cytometric analysis (Fig. 2) (FACSCalibur; Becton Dickinson, San Jose, Calif.) showed that there were no notable differences in cell surface expression of the viral glycoproteins for the different mutants except for a small, significant increase (P < 0.05; two-way analysis of variance) in gB expression for the Y864A/Y902A mutant. This increase can possibly be explained by reduced spontaneous endocytosis of Y864A/Y902A-mutated gB during early stages of infection (34), resulting in higher levels of gB at the plasma membrane, as will be discussed below.
FIG. 2.
Kinetics of expression of viral glycoproteins gB (A), gC (B), and gD (C) on the surfaces of SK cells inoculated with PRV Be (▪), PRV Y864A (○), PRV Y902A (▴), PRV Y864A/Y902A (◊), PRV LL887RR (•), PRV Y864A/Y902A/LL887RR (Δ), or PRV Rescue (⧫). SK cells were fixed using 1% paraformaldehyde, and different viral cell surface glycoproteins were stained by incubating the cells with mouse anti-gB (A), anti-gC (B), or anti-gD (C) antibodies and subsequently with FITC-labeled goat anti-mouse antibodies. Fluorescence intensities of the cells were measured using flow cytometry. Data represent means − standard deviations of triplicate assays.
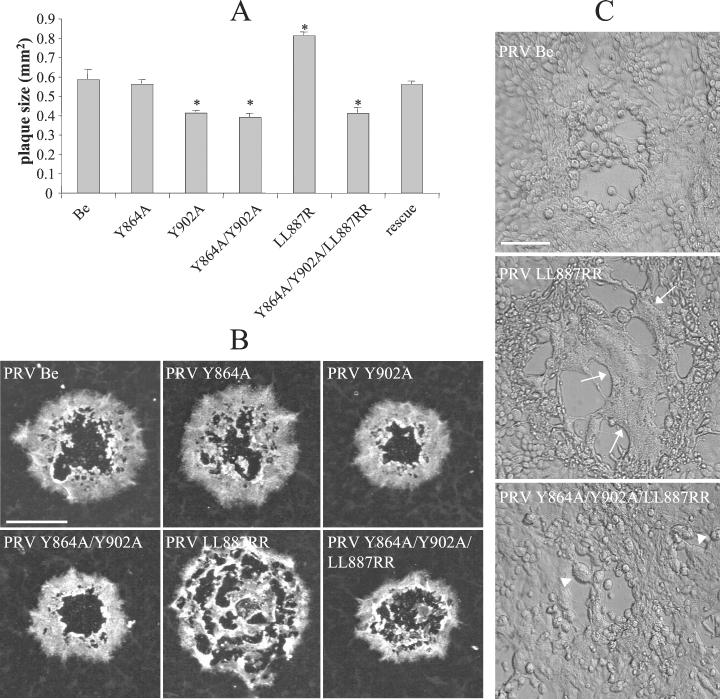
PRV plaque size reflects both virus yield per cell and the ability of the infection to spread from cell to cell. Plaque formation was assayed and measurement of plaque diameters was performed 48 h postinoculation (p.i.) on epithelial swine testicle (ST) cells (36) overlaid with 1% carboxymethylcellulose as described before (27, 35). Plaque diameters were then used to calculate the average surface areas of the plaques. Since all gB mutants grew to the same titers and produced equivalent amounts of extracellular infectious virus in SK cells (Fig. 1), the plaque sizes of these mutants represented a means to estimate the efficiency of cell-to-cell spread. The data shown in Fig. 3A and B indicate that replacing the tyrosine at position 864 by an alanine (Y864A) had no effect on plaque size. However, replacing tyrosine 902 with an alanine (Y902A) resulted in significantly smaller plaques (34% of wild-type plaque size; P < 0.01). The double-mutant PRV Y864A/Y902A formed small plaques indistinguishable in size from those of mutant Y902A. By contrast, replacing the LL motif at position 887 by two arginines (LL887RR) resulted in larger plaques (42% increase in plaque size; P < 0.01), an effect which was accompanied by the formation of large syncytia (fused cells; Fig. 3C). The triple-mutant PRV Y864A/Y902A/LL887RR again formed small plaques of the same size as those of the Y902A mutant, accompanied by small syncytia. Finally, the plaque size of the rescue virus was identical to that of the wild-type parental virus.
FIG. 3.
(A and B) Plaque sizes for the different PRV mutants on ST cells. Monolayers of ST cells were inoculated at an MOI of 0.001 with the different mutants. At 1 h p.i., the medium was replaced with 1% carboxymethylcellulose. At 48 h p.i., cells were fixed in 100% methanol and viral antigens were stained using FITC-labeled PRV-specific porcine antibodies. Plaques were visualized by fluorescence microscopy, and plaque diameters were measured and the values obtained were used to calculate the relative plaque surface areas. Data represent means ± standard deviations of triplicate assays. Asterisks indicate significant differences (P < 0.01). Bar, 0.5 mm. (C) Differential interference contrast images of large syncytia induced by the LL887RR mutant virus (arrows) and small syncytia induced by the Y864A/Y902A/LL887RR triple mutant (arrowheads) on ST cells 24 h p.i. at an MOI of 0.001. Bar, 70 μm.
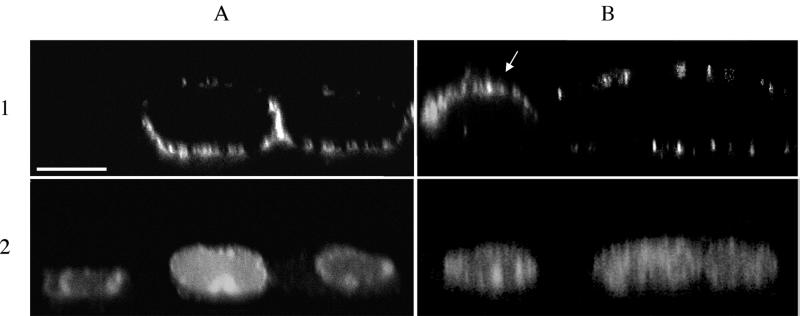
Thus, viruses harboring the Y902A mutation form small plaques on ST cells. This phenotype might reflect improper localization of the gB protein in infected cells. Accordingly, we visualized gB localization at 8 h after infection on the apical and basolateral cell surfaces of a monolayer of polarized epithelial ST cells (36), grown on collagen-coated permeable filter supports (0.4 μm pore size, Millicell CM; Millipore, Bedford, Mass.) essentially as described before (4, 7). Five-day-old monolayers of cells were inoculated with the different PRV mutants at an MOI of 10 from both the apical and basolateral sides. At 8 h p.i., cells were paraformaldehyde fixed and gB on the cell surface was stained by incubating the apical and basal sides of the filter support with gB-specific mouse monoclonal antibodies (26) followed by FITC-labeled goat anti-mouse antibodies (Molecular Probes). Nuclei were counterstained using propidium iodide. Afterwards, filters were cut out and mounted on a microscope slide and localization of gB on the basolateral and apical cell surfaces was analyzed by confocal microscopy (Leica TCS SP 2 confocal microscope; Leica Microsystems, Heidelberg, Germany) as described before (7). Figure 4 shows vertical sections through the z axis of ST cells infected with wild-type or Y902A PRV. In PRV wild-type-infected cells, gB was predominantly expressed on the U-shaped basolateral surfaces. Most Y902A-infected cells did not show basolateral targeting of gB but showed either apical (Fig. 4B) or random cell surface expression of gB. Several cellular proteins targeted to the basolateral surface in epithelial cells also depend on the presence of YXXΦ motifs in their cytoplasmic tail. Sorting is mediated by interaction with a specific AP complex (AP-1B) (12). These YXXΦ motifs are identical to endocytosis motifs, and whether they function as determinants for basolateral targeting (interaction with AP-1B), endocytosis (interaction with AP-2), or both depends on the amino acids in the vicinity of these motifs (16, 21). We suggest that predominant sorting of gB to the basolateral cell surface is important for efficient functioning of gB during cell-to-cell spread of PRV, as has been reported for the HSV gE protein. HSV gE is sorted to cell junctions at the lateral cell surface, and this sorting is necessary for gE-promoted cell-to-cell spread of the virus (8, 22) and requires the gE cytoplasmic tail and AP-1 proteins (19, 22). Interestingly, for PRV gE, mutation of the two YXXΦ motifs in the cytoplasmic tail results in a modest 15% decrease in plaque size (35). Whether this reduced plaque size is caused by inefficient lateral sorting of gE is not known.
FIG. 4.
Apical-basolateral cell surface expression of PRV gB. Polarized monolayers of ST cells, grown on permeable filter supports, were inoculated with PRV Be (A) or PRV Y902A (B) at an MOI of 10. At 8 h p.i., cells were washed, paraformaldehyde fixed, washed again, and incubated subsequently from both the apical and basolateral sides with mouse anti-gB antibodies and FITC-labeled goat anti-mouse antibodies. Nuclei were counterstained using propidium iodide. Vertical sections through the z axis of the cells are shown with viral glycoproteins in panels 1 and nuclei in panels 2. The arrow indicates a PRV Y902A-infected cell with apical expression of gB. Bar, 10 μm.
Replacing the two leucines at position 887 to 888 in the gB tail with two arginines (LL887RR) resulted in a significant increase in plaque size (42%; P < 0.01) (Fig. 4). Although LL motifs can affect basolateral targeting of proteins (21), the mutated PRV gB LL motif had no obvious effect on basolateral sorting (data not shown). Therefore, and since the LL887RR mutation not only increases plaque size but also induces the formation of large syncytia (Fig. 3C), we suggest that this mutation enhances the fusogenic activity of gB rather than altering its sorting. The LL motif is located at the N-terminal start of the predicted α-helical domain II in the PRV gB tail. It has already been shown for HSV that all mutations disrupting the α-helical domain II of gB cause extensive cell fusion in Vero cells (5, 13). Recently, Nixdorf et al. demonstrated that the truncation of the last 29 C-terminal amino acids of the PRV gB tail, which encompass the entire α-helical domain II, caused a twofold increase in plaque size without affecting virion entry rate (27). Perhaps the enhanced fusogenic activity observed for the LL887RR mutant reflects a conformational change which disrupts α-helical domain II. How changes in the structure of α-helical domain II in the carboxy-terminal tail of gB can alter the fusogenic activity of gB remains puzzling. One possibility could be that α-helical domain II defines an interaction domain of a protein(s) involved in gB fusion regulation. Such putative control proteins could be transmembrane or cytosolic proteins (viral or cellular) that modulate the fusogenic activity of gB, for example, by anchoring gB to the cellular cytoskeleton. Abolishing this interaction could lead to enhanced fusogenic activity of gB. That the Y902A mutation (small plaques) is epistatic to the LL887RR mutation (large plaques) supports our idea that Y902 is important for localization of gB while LL887 is important for regulation of gB function when it is properly localized.
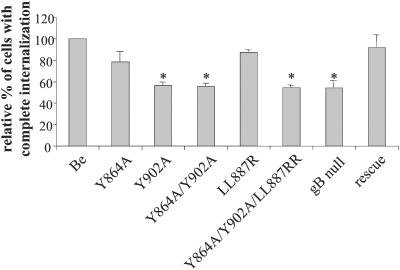
Finally, we tested whether the different mutations had an effect on the efficiency of antibody-induced internalization of viral cell surface glycoproteins in porcine monocytes. The internalization assay was performed as described before (38). Briefly, isolated porcine blood monocytes were inoculated with the different mutants in vitro (the PRV gB null mutant was kindly provided by T. C. Mettenleiter [31]). At 13 h p.i., cells were washed and incubated with FITC-labeled, PRV-specific porcine polyclonal immunoglobulin G for 1 h at 37°C to induce internalization of the viral cell surface proteins. Afterwards, cells were paraformaldehyde fixed and the percentage of cells with internalized viral cell surface proteins was determined by fluorescence microscopy analysis as described before (38). The Y864A mutation had no significant effect on the efficiency of antibody-induced internalization (Fig. 5). By contrast, the Y902A mutation markedly reduced antibody-induced internalization (44%; P < 0.01). This reduction is identical to that observed after infection by a PRV gB null mutant. The effect of the PRV Y864A/Y902A double mutation was indistinguishable from that of the Y902 mutation. The LL887RR mutation had no effect on the efficiency of antibody-induced internalization, whereas the triple mutation (Y864A/Y902A/LL887RR) again gave a reduction in antibody-induced internalization comparable to the reduction observed when the single-mutant PRV Y902A strain was used. The rescue strain gave results similar to those with the wild-type infection.
FIG. 5.
Efficiency of antibody-induced internalization of viral cell surface proteins. Isolated porcine blood monocytes were inoculated with the different PRV mutants at an MOI of 10. At 13 h p.i., monocytes were incubated with FITC-labeled porcine polyclonal PRV-specific antibodies for 1 h at 37°C. Afterwards, the percentage of infected monocytes with internalized viral cell surface proteins was determined using fluorescence microscopy. Percentages shown are relative to that of PRV Be-infected monocytes. Data represent means ± standard deviations of triplicate assays. Asterisks indicate significant differences (P < 0.01).
Hence, Y902 in the PRV gB tail is essential for efficient functioning of gB during the antibody-induced internalization of viral cell surface proteins in PRV-infected monocytes. Our hypothesis is that AP-2 AP complexes, docked at the plasma membrane, bind to the YQRL motif at positions 902 to 905 in the PRV gB tail and thereby recruit the antibody-induced patches of viral proteins in the coated pits as a first step in the formation of clathrin-coated endocytosis vesicles. A previous report showed that the antibody-induced internalization is clathrin mediated (38), and we are presently investigating whether gB and AP-2 molecules can physically interact with each other.
A question that remains is why deletion of gB or changing Y902 to alanine in the gB tail does not completely abolish the antibody-triggered internalization. Since deleting gD also results in a ±50% reduction in the efficiency of antibody-induced internalization (38), and since gD also contains a tyrosine-based putative endocytosis motif in its cytoplasmic tail, it is possible that gB and gD have overlapping or redundant tasks in antibody-induced internalization of viral membrane proteins. To investigate this idea, we plan to construct PRV mutants with missense mutations in the PRV gD tail.
When analyzing the kinetics of plasma membrane expression of gB on SK cells infected with the different mutants, we observed a small but significant (P < 0.05) increase in gB expression after inoculation with the PRV Y864A/Y902A double mutant. The increased cell surface expression of gB when the PRV Y864A/Y902A mutant was used can possibly be explained by the spontaneous (i.e., non-antibody-dependent) endocytosis of gB from the cell surface during early stages of infection (<6 h after infection) (34). Such spontaneous endocytosis of some, but not all, viral proteins from the cell surfaces of infected cells has been described for several herpesviruses, including PRV, HSV, varicella-zoster virus, and human cytomegalovirus (1, 2, 11, 28, 30, 37, 41). For PRV, spontaneous endocytosis has been demonstrated for gB, gE, and Us9 (but not gC or gI) during the first 6 h of infection (6, 34). PRV gE, like gB, contains two YXXΦ domains in its cytoplasmic tail, and one of these has been shown to be crucial for its spontaneous endocytosis (35). Similarly, the YXXΦ motifs in the gB tail could be important for its spontaneous endocytosis. Indeed, deleting α-helical domain II from the cytoplasmic tail of PRV gB, which contains the Y902-based YXXΦ motif, has been shown to strongly reduce spontaneous endocytosis of gB (27). Hence, the mutant YXXΦ motifs in the gB tail may decrease spontaneous gB endocytosis during early stages of infection, resulting in increased levels of gB on the plasma membrane. The PRV Y864A/Y902A/LL887RR triple mutant had no effect on the levels of expression of gB on the cell surface. It is unclear if this result reflects an additional effect of the LL887RR mutation on the spontaneous endocytosis process or an effect on the intracellular trafficking pathway of gB.
In conclusion, we have found that the Y902 residue in the PRV gB cytoplasmic tail is crucial for the correct functioning of gB during antibody-induced internalization of viral cell surface proteins in PRV-infected monocytes. Moreover, the same Y902 residue is important for efficient cell-to-cell spread of the virus. We believe that the latter phenotype reflects the role of Y902 in the targeting of gB to the basolateral cell surface. Furthermore, we found that mutating an LL motif at position 887-888 has no effect on antibody-triggered endocytosis but, rather, promotes an increased efficiency of cell-to-cell spread of the virus and increased formation of syncytia. We speculate that the area surrounding the LL motif defines an interaction domain for proteins that regulate the fusogenic activity of gB.
Acknowledgments
We thank Christoph Hengartner and Greg Smith for invaluable help and ideas with the construction of the mutants, Gerlinde Van de Walle for excellent help with the internalization assays, Chantal Vanmaercke for excellent technical assistance, and T. C. Mettenleiter for the kind gift of the gB-null PRV strain as well as the gB-complementing cell line.
H.W.F. was supported with postdoctoral fellowships from the Belgian American Educational Foundation and the Fulbright organization. This research was supported by a cooperative research action fund of the Research Council of Ghent University.
REFERENCES
- 1.Alconada, A., U. Bauer, and B. Hoflack. 1996. A tyrosine-based motif and a casein kinase II phosphorylation site regulate the intracellular trafficking of the varicella-zoster virus glycoprotein I, a protein localized in the trans-Golgi network. EMBO J. 15:6096-6110. [PMC free article] [PubMed] [Google Scholar]
- 2.Alconada, A., U. Bauer, B. Sodeik, and B. Hoflack. 1999. Intracellular traffic of herpes simplex virus glycoprotein gE: characterization of the sorting signals required for its trans-Golgi network localization. J. Virol. 73:377-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ambagala, A. P., S. Hinkley, and S. Srikumaran. 2000. An early pseudorabies virus protein down-regulates porcine MHC class I expression by inhibition of transporter associated with antigen processing (TAP). J. Immunol. 164:93-99. [DOI] [PubMed] [Google Scholar]
- 4.Bacallao, R., C. Antony, C. Dotti, E. Karsenti, E. H. Stelzer, and K. Simons. 1989. The subcellular organization of Madin-Darby canine kidney cells during the formation of a polarized epithelium. J. Cell Biol. 109:2817-2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baghian, A., L. Huang, S. Newman, S. Jayachandra, and K. G. Kousoulas. 1993. Truncation of the carboxy-terminal 28 amino acids of glycoprotein B specified by herpes simplex virus type 1 mutant amb1511-7 causes extensive cell fusion. J. Virol. 67:2396-2401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brideau, A. D., T. del Rio, E. J. Wolffe, and L. W. Enquist. 1999. Intracellular trafficking and localization of the pseudorabies virus Us9 type II envelope protein to host and viral membranes. J. Virol. 73:4372-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chodosh, J., Y. Gan, V. P. Holder, and J. W. Sixbey. 2000. Patterned entry and egress by Epstein-Barr virus in polarized CR2-positive epithelial cells. Virology 266:387-396. [DOI] [PubMed] [Google Scholar]
- 8.Dingwell, K. S., and D. C. Johnson. 1998. The herpes simplex virus gE-gI complex facilitates cell-to-cell spread and binds to components of cell junctions. J. Virol. 72:8933-8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Favoreel, H. W., H. J. Nauwynck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1997. Antibody-induced and cytoskeleton-mediated redistribution and shedding of viral glycoproteins, expressed on pseudorabies virus-infected cells. J. Virol. 71:8254-8261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Favoreel, H. W., H. J. Nauwynck, H. M. Halewyck, P. Van Oostveldt, T. C. Mettenleiter, and M. B. Pensaert. 1999. Antibody-induced endocytosis of viral glycoproteins and major histocompatibility complex class I on pseudorabies virus-infected monocytes. J. Gen. Virol. 80:1283-1291. [DOI] [PubMed] [Google Scholar]
- 11.Fish, K. N., C. Soderberg-Naucler, and J. A. Nelson. 1998. Steady-state plasma membrane expression of human cytomegalovirus gB is determined by the phosphorylation state of Ser900. J. Virol. 72:6657-6664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fölsch, H., H. Ohno, J. S. Bonifacino, and I. Mellman. 1999. A novel clathrin adaptor complex mediates basolateral targeting in polarized epithelial cells. Cell 99:189-198. [DOI] [PubMed] [Google Scholar]
- 13.Foster, T. P., J. M. Melancon, and K. G. Kousoulas. 2001. An α-helical domain within the carboxyl terminus of herpes simplex virus type 1 (HSV-1) glycoprotein B (gB) is associated with cell fusion and resistance to heparin inhibition of cell fusion. Virology 287:18-29. [DOI] [PubMed] [Google Scholar]
- 14.Frank, I., and H. M. Friedman. 1989. A novel function for the herpes simplex virus type I Fc receptor: participation in bipolar bridging of antiviral immunoglobulin G. J. Virol. 63:4479-4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Friedman, H. M., G. H. Cohen, R. J. Eisenberg, C. A. Seidel, and D. B. Cines. 1984. Glycoprotein C of herpes simplex virus 1 acts as a receptor for the C3b complement component on infected cells. Nature 309:633-635. [DOI] [PubMed] [Google Scholar]
- 16.Gut, A., F. Kappeler, N. Hyka, M. S. Balda, H. P. Hauri, and K. Matter. 1998. Carbohydrate-mediated Golgi to cell surface transport and apical targeting of membrane proteins. EMBO J. 17:1919-1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huemer, H. P., C. Larcher, and N. E. Coe. 1992. Pseudorabies virus glycoprotein III derived from virions and infected cells binds to the third component of complement. Virus Res. 23:271-280. [DOI] [PubMed] [Google Scholar]
- 18.Johnson, D. C., M. C. Frame, M. W. Ligas, A. M. Cross, and N. D. Stow. 1988. Herpes simplex virus immunoglobulin G Fc receptor activity depends on a complex of two viral glycoproteins, gE and gI. J. Virol. 62:1347-1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Johnson, D. C., M. Webb, T. W. Wisner, and C. Brunetti. 2001. Herpes simplex virus gE/gI sorts nascent virions to epithelial cell junctions, promoting virus spread. J. Virol. 75:821-833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchhausen, T., J. S. Bonifacino, and H. Riezman. 1997. Linking cargo to vesicle formation: receptor tail interactions with coat proteins. Curr. Opin. Cell Biol. 9:488-495. [DOI] [PubMed] [Google Scholar]
- 21.Matter, K., E. M. Yamamoto, and I. Mellman. 1994. Structural requirements and sequence motifs for polarized sorting and endocytosis of LDL and Fc receptors in MDCK cells. J. Cell Biol. 126:991-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McMillan, T. N., and D. C. Johnson. 2001. Cytoplasmic domain of herpes simplex virus gE causes accumulation in the trans-Golgi network, a site of virus envelopment and sorting of virions to cell junctions. J. Virol. 75:1928-1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nauwynck, H. J. 1997. Functional aspects of Aujeszky's disease (pseudorabies) viral proteins with relation to invasion, virulence and immunogenicity. Vet. Microbiol. 55:3-11. [DOI] [PubMed] [Google Scholar]
- 24.Nauwynck, H. J., and M. B. Pensaert. 1992. Abortion induced by cell-associated pseudorabies virus in vaccinated sows. Am. J. Vet. Res. 53:489-493. [PubMed] [Google Scholar]
- 25.Nauwynck, H. J., and M. B. Pensaert. 1995. Cell-free and cell-associated viremia in pigs after oronasal infection with Aujeszky's disease virus. Vet. Microbiol. 43:307-314. [DOI] [PubMed] [Google Scholar]
- 26.Nauwynck, H. J., and M. B. Pensaert. 1995. Effect of specific antibodies on the cell-associated spread of pseudorabies virus in monolayers of different cell types. Arch. Virol. 140:1137-1146. [DOI] [PubMed] [Google Scholar]
- 27.Nixdorf, R., B. G. Klupp, A. Karger, and T. C. Mettenleiter. 2000. Effects of truncation of the carboxy terminus of pseudorabies virus glycoprotein B on infectivity. J. Virol. 74:7137-7145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Olson, J. K., and C. Grose. 1997. Endocytosis and recycling of varicella-zoster virus Fc receptor glycoprotein E: internalization mediated by a YXXL motif in the cytoplasmic tail. J. Virol. 71:4042-4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Peeters, B., N. de Wind, M. Hooisma, F. Wagenaar, A. Gielkens, and R. Moormann. 1992. Pseudorabies virus envelope glycoproteins gp50 and gII are essential for virus penetration, but only gII is involved in membrane fusion. J. Virol. 66:894-905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Radsak, K., M. Eickmann, T. Mockenhaupt, E. Bogner, H. Kern, A. Eis-Hübinger, and M. Reschke. 1996. Retrieval of human cytomegalovirus glycoprotein B from infected cell surface for virus envelopment. Arch. Virol. 141:557-572. [DOI] [PubMed] [Google Scholar]
- 31.Rauh, I., and T. C. Mettenleiter. 1991. Pseudorabies virus glycoproteins gII and gp50 are essential for virus penetration. J. Virol. 65:5348-5356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith, G. A., and L. W. Enquist. 2000. A self-recombining bacterial artificial chromosome and its application for analysis of herpesvirus pathogenesis. Proc. Natl. Acad. Sci. USA 97:4873-4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sparks-Thissen, R. L., and L. W. Enquist. 1999. Differential regulation of Dk and Kk major histocompatibility complex class I proteins on the cell surface after infection of murine cells by pseudorabies virus. J. Virol. 73:5748-5756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tirabassi, R. S., and L. W. Enquist. 1998. Role of envelope protein gE endocytosis in the pseudorabies virus life cycle. J. Virol. 72:4571-4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tirabassi, R. S., and L. W. Enquist. 1999. Mutation of the YXXL endocytosis motif in the cytoplasmic tail of pseudorabies virus gE. J. Virol. 73:2717-2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres, J. M., C. Alonso, A. Ortega, S. Mittal, F. Graham, and L. Enjuanes. 1996. Tropism of human adenovirus type 5-based vectors in swine and their ability to protect against transmissible gastroenteritis coronavirus. J. Virol. 70:3770-3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tugizov, S., E. Maidji, J. Xiao, and L. Pereira. 1999. An acidic cluster in the cytosolic domain of human cytomegalovirus glycoprotein B is a signal for endocytosis from the plasma membrane. J. Virol. 73:8677-8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van de Walle, G. R., H. W. Favoreel, H. J. Nauwynck, P. Van Oostveldt, and M. B. Pensaert. 2001. Involvement of cellular cytoskeleton proteins in antibody-induced internalization of viral glycoproteins in pseudorabies virus-infected monocytes. Virology 288:129-138. [DOI] [PubMed] [Google Scholar]
- 39.Wittmann, G., J. Jakubik, and R. Ahl. 1980. Multiplication and distribution of Aujeszky's disease (pseudorabies) virus in vaccinated and non-vaccinated pigs after intranasal infection. Arch. Virol. 66:227-240. [DOI] [PubMed] [Google Scholar]
- 40.York, I. A. 1996. Immune evasion strategies of the herpesviruses. Chem. Biol. 3:331-335. [DOI] [PubMed] [Google Scholar]
- 41.Zhu, Z., Y. Hao, M. D. Gershon, R. T. Ambron, and A. A. Gershon. 1996. Targeting of glycoprotein I (gE) of varicella-zoster virus to the trans-Golgi network by an AYRV sequence and an acidic amino acid-rich patch in the cytosolic domain of the molecule. J. Virol. 70:6563-6575. [DOI] [PMC free article] [PubMed] [Google Scholar]