Abstract
Producing a prophylactic vaccine for human immunodeficiency virus (HIV) has proven to be a challenge. Most biological isolates of HIV are difficult to neutralize, so that conventional subunit-based antibody-inducing vaccines are unlikely to be very effective. In the rhesus macaque model, some protection was afforded by DNA/recombinant viral vector vaccines. However, these studies used as the challenge virus SHIV-89.6P, which is neutralizable, making it difficult to determine whether the observed protection was due to cellular immunity, humoral immunity, or a combination of both. In this study, we used a DNA prime/modified vaccinia virus Ankara boost regimen to immunize rhesus macaques against nearly all simian immunodeficiency virus (SIV) proteins. These animals were challenged intrarectally with pathogenic molecularly cloned SIVmac239, which is resistant to neutralization. The immunization regimen resulted in the induction of virus-specific CD8+ and CD4+ responses in all vaccinees. Although anamnestic neutralizing antibody responses against laboratory-adapted SIVmac251 developed after the challenge, no neutralizing antibodies against SIVmac239 were detectable. Vaccinated animals had significantly reduced peak viremia compared with controls (P < 0.01). However, despite the induction of virus-specific cellular immune responses and reduced peak viral loads, most animals still suffered from gradual CD4 depletion and progressed to disease.
It is estimated that in excess of 35 million individuals are infected with human immunodeficiency virus (HIV), and the epidemic does not appear to be slowing (AIDS Epidemic Update, 2000, http://www.unaidsorg/epidemic_update/report_dec00/index_dec.html). The catastrophic implications of such a worldwide pandemic have placed enormous pressure on the scientific community to produce a prophylactic or therapeutic vaccine as quickly as possible.
The infection of rhesus macaques with simian immunodeficiency virus (SIV) is used routinely as a model for AIDS (43, 95). In this model, the greatest degree of protection has been achieved with attenuated viral vaccines (7, 26, 62, 70, 102, 103). However, attenuated viruses have inherent risks (27, 28, 35, 85, 101) and, furthermore, have been shown to cause disease in infants (11, 12, 22). Therefore, the use of attenuated retroviruses as an AIDS vaccine is controversial (66, 84). So far, the mechanism of protection induced by attenuated viruses is still unknown (6, 8, 69, 94, 98). The degree of protection achieved with attenuated viruses is variable and depends on the degree of attenuation of the vaccine virus (44), the time point of challenge (25), and the challenge virus chosen (102); all of these factors make determining the mechanism of protection very difficult. Depending on the challenge virus used, some studies have suggested a role for neutralizing antibodies (21, 103), cytotoxic T lymphocytes (CTL) (44), or perhaps both (21) as protective mechanisms.
Due to the present inability to generate neutralizing antibodies against a wide variety of HIV strains (19, 55, 61), it is believed that current HIV vaccine candidates will have to rely on virus-specific T-cell-mediated responses. T-cell-mediated responses have been associated with the control of viral replication after infection (17, 28, 42, 47, 57, 71, 82, 87, 106). Virus-specific cellular immune responses can be induced in a variety of ways by using DNA vaccines (13, 15, 20, 29, 36, 46, 99), recombinant viral vector vaccines (14, 16, 64, 67, 72, 91), or a combination of both (5, 9, 40, 45, 80). However, the degree of protection achieved against infection with pathogenic immunodeficiency viruses by these approaches has been variable. This result may be explained in part by the differences in the virulence of the challenge viruses used in these studies and/or the different routes used to challenge the animals, since both variables can influence the outcome of a challenge (16, 44, 60, 102).
In this study, we immunized rhesus macaques with nearly all the proteins of SIV to maximize the breadth of immune response induction against the virus. We used a DNA prime/modified vaccinia virus Ankara (MVA) boost regimen to maximize the induction of CD4+- and CD8+-T-cell responses. We measured the virus-specific cellular immune responses induced by the vaccine against the entire peptide sequence of SIV and then mucosally challenged animals with SIVmac239 to evaluate the potential role of these responses in the control of viral replication. This molecular clone is difficult to neutralize and is, in this respect, similar to most field strains of HIV. Despite the elicitation of both CD8+ and CD4+ virus-specific responses and a reduction in peak viral loads, most animals suffered from gradual CD4+-T-cell loss and progressed to disease.
MATERIALS AND METHODS
Animals.
Rhesus macaques (Macaca mulatta) were maintained in accordance with the Guide for the Care and Use of Laboratory Animals (67a) and under the approval of the University of Wisconsin Research Animal Resource Center review committee.
Peptides.
Overlapping peptides (20-mer and 15-mer peptides) were synthesized at Chiron (Raleigh, N.C.) or the Natural and Medical Science Institute (University of Tübingen, Tübingen, Germany) on the basis of SIVmac239 protein sequences, with the exception of Pol peptides, which corresponded to SIVmac251 sequences. Lyophilized peptides were resuspended in phosphate-buffered saline (PBS) with 10% dimethyl sulfoxide (Sigma Chemical Co., St. Louis, Mo.). Consecutive 20-mer and 15-mer peptides overlapped by 10 and 11 amino acids, respectively. Pools of peptides contained 10 peptides each at a final concentration of 1 mg/ml.
Generation of the SIVmac17E-Fr gag-pol-env DNA vaccine.
The macrophage-tropic clone SIVmac17E-Fred (SIVmac17E-Fr) is closely related to SIVmac239 (10, 92). Molecularly cloned SIVmac17E-Fr gag coding sequences were isolated by using StuI and BamHI sites and cloned into pCMV-BGHpA/AMP, a vector containing the pUC19 origin of replication, the ampicillin resistance gene, the cytomegalovirus immediate-early promoter, and the polyadenylation signal from the bovine growth hormone gene. This cloning yielded intermediate plasmid p185-31. The cytomegalovirus-gag-bovine growth hormone sequences from p185-31 were then subcloned into a plasmid containing the pBR322 origin of replication, resulting in intermediate plasmid pSIVgag-int. pol-env sequences that included vif, vpx, vpr, tat, and rev were isolated from SIVmac17E-Fr and ligated into pSIVgag-int by using BsiEI and DraIII sites to generate vaccine plasmid pSIV17E-Fr gag-pol-env. The final plasmid thus encodes SIVmac17E-Fr gag-pol-env, including vif, vpx, vpr, tat, and rev, except that the 5′ LTR is deleted and the 3′ LTR is truncated by 360 bp. SIV nef was truncated at the sequence for amino acid 93 by insertion of a stop codon.
Generation of the SIVmac17E-Fr nef and SIVmac239 rev DNA vaccines.
The template DNA used for PCR of the SIV nef gene was pSIV17E-Fr gag-pol-env (see above), which expresses most of the SIV proteins, except for full-length Nef. Two PCRs were performed to create a full-length Nef coding fragment. Template pSIV17E-Fr gag-pol-env was PCR amplified with primers A (5′-GGA GCT AGC ATG GGT GGA GCT ATT TCC ATG-3′) and B (5′-C TAC CAA GTC ATC ATC CTC ATC TAT ATC-3′) and with primers C (5′-GAT ATA GAT GAG GAT GAT GAC TTG GTA G-3′) and D (5′-GGA TCC TCA GCG AGT TTC CTT CTT GTC AGC-3′) under standard PCR conditions (PCR core buffer with 15 mM MgCl2 [Promega], 0.400 μM each sense and antisense primers, 200 μM each deoxynucleoside triphosphate, 2.5 U of Taq polymerase [Promega], 1.0 ng of template DNA, water to 100 μl, mineral oil overlay). Thermocycler conditions were an initial denaturation step at 95°C for 4 min followed by 30 cycles of 1 min of denaturation at 95°C, 1 min 15 s of annealing at 55°C, and 1 min of extension at 72°C. A final 10-min extension step at 72°C followed before reaction mixtures were stored at 4°C.
PCR products AB and CD were electrophoresed on a 1% agarose gel, stained, cut out of the gel, and soaked for 30 min at 65°C in 100 μl of water to elute the PCR fragments. A second PCR was performed under standard conditions with primers A and D and with 1 μl of eluates from products AB and CD as templates in the reaction. The PCR fragment was phenol-chloroform extracted, ethanol precipitated, and resuspended in water. An aliquot was cut with NheI and BamHI (New England Biolabs) to prepare an insert fragment. A vector fragment was prepared by removing the hepatitis B core antigen from plasmid pHBcAg (49) by cutting with NheI and BamHI. The two fragments were ligated, resulting in pSIVNef-TPA. The Nef insert in pSIVNef-TPA was sequenced, and one change (isoleucine to asparagine) at position 140 was discovered. The pSIVNef-TPA vector has the leader sequence of the human tissue of plasminogen activator protein (TPA) in front of the nef gene, allowing for secretion of the product. A second, very similar vector for nef was constructed by using a vector fragment of a TPA leader-less version of plasmid pHBcAg, resulting in plasmid pSIVNef, which promotes the retention of the antigen and may enhance major histocompatibility complex (MHC) class I presentation.
The SIVmac239 rev vector (pSIVrev) was constructed in a similar fashion as described previously (31).
DNA vaccinations.
Animals received DNA immunizations at 4- to 9-week intervals. A particle delivery device was used to deliver DNA directly into cells of the epidermis as described previously (24, 105). A 10th DNA immunization was administered following a 23-week rest period (Fig. 1). Four vaccine plasmids encoding SIV Gag, Pol, Env, Vif, Vpr, Vpx, Tat, and Rev (pSIV17E-Fr gag-pol-env), SIV Nef (pSIVNef-TPA and pSIVNef), and SIV Rev (pSIVrev) were coadministered and included all the proteins of SIV. All protein sequences were derived from the macrophage-tropic clone SIVmac17E-Fr, except for that of Rev, which was derived from SIVmac239 (see above) (10, 76, 92). The macrophage-tropic clone SIVmac17E-Fr is closely related to SIVmac239 (10, 92). Plasmid DNA was precipitated onto 1 to 3 μM gold particles (Degussa, Plainfield, N.J.) in the presence of 0.1 M spermidine (Sigma) and 2.5 M CaCl2 (Fujisawa, Inc., Melrose Park, Ill.) at a rate of 4.4 μg of DNA per mg of gold. The four plasmids were coprecipitated onto gold beads at a rate of 2.0 μg of pSIV17E-Fr gag-pol-env, 1.0 μg of pSIVNef-TPA, 1.0 μg of pSIVNef, and 0.4 μg of pSIVrev DNA per mg of gold. Abdominal fur was clipped from rhesus macaques, the skin was swabbed with 70% alcohol, and DNA-coated gold particles were introduced into the abdominal epidermis by use of a particle delivery device at a constant helium pressure of 500 lb/in2. For the primary immunization, a total dose of 8.8 μg of DNA was administered by delivering 0.25 mg of gold/site into each of eight sites. For all subsequent immunizations, a total dose of 35.2 μg of DNA per immunization was administered by delivering 1 mg of gold/site into each of eight sites.
FIG. 1.
Immunization and challenge schedule. rMVA, recombinant MVA; i.d., intradermally; i.r., intrarectally.
Generation and inoculation of MVA vector vaccines.
MVA recombinant viruses used in this study separately express the env, gag-pol, tat, rev, or nef coding sequences of SIVmac251 32H clone pJ5 (83) under the transcriptional control of vaccinia virus-specific early and late promoters P7.5 (gag-pol, tat, rev, and nef) and sP (env). To generate vaccine preparations, MVA recombinant and nonrecombinant viruses were amplified on chicken embryo fibroblast (CEF) cells derived from embryonated eggs of a specific-pathogen-free stock. CEF cells were grown in minimal essential medium (Biochrom, Berlin, Germany) supplemented with 10% fetal bovine serum (Biochrom) and were maintained in a humidified air-5% CO2 atmosphere at 37°C. Viruses were purified by ultracentrifugation through a cushion of 36% (wt/vol) sucrose in 10 mM Tris-Cl (pH 8.0) and reconstituted in PBS, and titers were determined by immunostaining of virus-infected cell foci on CEF cell monolayers by using a vaccinia virus-specific rabbit polyclonal antibody (Biogenesis Ltd., Poole, United Kingdom). Virus preparations were divided into aliquots to contain 5 × 108 infectious units/ml and stored at −70°C. The vector vaccine preparations were tested in vitro for their capacity to synthesize SIVmac target antigens by monitoring for Gag, Env, Rev, and Nef proteins by Western blot analyses and assaying the trancriptional activation of HIV LTR-controlled luciferase reporter gene expression to confirm Tat production (data not shown).
Approximately 1 year after the last DNA vaccination, all animals in the vaccine group were inoculated twice with recombinant MVA vaccines within a 13-week interval (Fig. 1). The animals received 108 infectious units of each MVA vector vaccine, containing DNA encoding SIVmacJ5 (83) Gag-Pol, Env, Nef, Rev, and Tat (no MVA was available expressing Vif, Vpr, and Vpx), delivered intradermally as well as intrarectally. Control animals received equal amounts of nonrecombinant MVA. No side effects or lesions were found associated with the inoculations.
Challenge with molecularly cloned SIVmac239/nef-open.
All animals were challenged intrarectally with a molecularly cloned virus, SIVmac239/nef-open (76), by using a dose of 6.31 × 104 50% intravenous rhesus monkey infectious doses (virus containing 8.5 ng of p27; 3.16 × 103 50% tissue culture infectious doses). The preparation, titration, and use of this challenge stock have been described previously (50). Based on in vivo titration experiments recently performed, this dose corresponded to approximately 10 intrarectal 50% rhesus monkey infectious doses (67). After the animals were tranquilized with ketamine (15 mg/kg of body weight intramuscularly), the anal area was gently wiped clean with soap and rinsed well with water. An inoculum was loaded into a 1-ml tuberculin syringe. The pelvic area of the animal was raised to a 45o angle with the head tilted forward. The syringe was carefully inserted to avoid trauma, and the material was inoculated while avoiding drainage from the anal area. Upon completion of the procedure, animals were kept in a slightly inverted position for 5 to 10 min.
Viral loads.
Viral RNA from SIV was quantitated by real-time PCR with a TaqMan assay kit (Perkin-Elmer Applied Biosystems, Foster City, Calif.). The forward primer was SIV-61F (5′-CCACCTACCATTAAGCCCGA-3′), the reverse primer was SIV-143R (5′-CTGGCACTACTTCTGCTCCAAA-3′), and the probe was SIV-84T (FAM reporter, TAMRA quencher) [5′-CATTAAATGCCTGGGTAAAATTGATAGAGGA(G/A)AAGAA-3′]. The reaction mixture contained TaqMan EZ buffer, 3 mM manganese acetate, 1.2 mM each deoxynucleoside triphosphate, 100 nM probe SIV-84T, 400 nM (final concentration) forward primer, 800 nM (final concentration) reverse primer, 2.5 U of recombinant Thermus thermophilus (rTth) DNA polymerase, and 2 μl of RNA sample or RNA standard. Cycling conditions were as follows: 50°C for 2 min, 60°C for 30 min, 95°C for 5 min, and 40 cycles at 95°C for 15 s, 60°C for 1 min, and 25°C for 2 min. Data were collected during the extension phase only.
Neutralizing antibody assays.
Neutralization of a T-cell-line-adapted stock of SIVmac251 or molecularly cloned SIVmac239/nef-open was measured with CEMx174 cells as described previously (59). Titers of neutralizing antibodies in this assay are reported as the reciprocal plasma dilution at which 50% of cells are protected from virus-induced killing, as measured by neutral red uptake. The assay stock of SIVmac251 was generated in H9 cells and was highly sensitive to neutralization. Neutralization of molecularly cloned SIVmac239/nef-open was also measured in human peripheral blood mononuclear cells (PBMC) as a reduction in p27 Gag antigen synthesis (59). Briefly, 500 50% tissue culture infectious doses of virus were incubated with a 1:5 dilution of plasma for 1 h at 37°C. Fifteen microliters of this virus-plasma mixture was then transferred to 150 μl of PBMC (500,000 cells; stimulated overnight with 6 μg of phytohemagglutinin/ml) in 96-well U-bottom culture plates. The final dilution of anticoagulant in plasma after the addition to cells was adequate to avoid cellular toxicity. The cells were washed three times after overnight incubation and then cultured in fresh interleukin 2-containing growth medium. SIV p27 Gag antigen synthesis was quantified 6 days after the addition of cells, at which time antigen synthesis was in an early exponential phase of increase in control wells (no-plasma sample). The percent reduction in p27 synthesis was calculated relative to the average amount of p27 synthesized in the presence of preimmunization plasma samples. Only samples showing a >80% reduction in p27 synthesis were considered positive in this assay. The assay stock of SIVmac239/nef-open was generated in human PBMC and was derived from the animal challenge stock.
Intracellular cytokine staining (ICS) with fresh PBMC.
PBMC were separated from whole heparinized blood by Ficoll-diatrizoate (Histopaque; Sigma) density gradient centrifugation. PBMC (106) were incubated with either staphylococcal enterotoxin B (10 μg/ml; Sigma) as a positive control or pools of 10 15-mer and 20-mer peptides (1 μg of each peptide) together with 0.5 μg of anti-CD28 (clone L293) and 0.5 μg of anti-CD49d (clone 9F10) (both from BD Pharmingen, San Diego, Calif.) in a total volume of 200 μl of RPMI 1640 supplemented with 10% fetal calf serum (FCS), 2 mM l-glutamine, 25 mM HEPES buffer, 25 μM 2-mercaptoethanol, 50 μg of streptomycin/ml, and 50 U of penicillin/ml. Anti-CD28 and anti-CD49d antibodies were added to provide optimal costimulation (74). After 1.5 h at 37°C, 10 μg of brefeldin A/ml was added, and the cells were further incubated for 5 h at 37°C. Brefeldin A inhibits the export of proteins from the endoplasmic reticulum and, therefore, results in the intracellular accumulation of cytokines, which would otherwise be secreted. The cells were washed twice with 1 ml of FACS buffer (PBS-2% FCS) and then stained with 6 μl of peridininchlorophyll a protein (PerCP)-conjugated anti-human CD8α (clone SK1; Becton Dickinson) and 4 μl of allophycocyanin (APC)-conjugated anti-human CD4 (clone SK3; Becton Dickinson) in 100 μl of FACS buffer for 40 min. After two washes with 1 ml of FACS buffer, the cells were fixed with 2% paraformaldehyde (PFA)-PBS solution overnight at 4°C. The cells were then washed once with FACS buffer, treated with permeabilization buffer (0.1% saponin in FACS buffer) for 10 min at room temperature, washed once more with 0.1% saponin buffer, and resuspended in 100 μl of 0.1% saponin buffer. Then, 1 μl of an anti-human gamma interferon (IFN-γ)-fluorescein isothiocyanate (FITC) monoclonal antibody (MAb) (clone 4S.B3; Pharmingen) and either 6 μl of an anti-CD69-phycoerythrin (PE) MAb (clone L78; Becton Dickinson) or 1 μl of an anti-human tumor necrosis factor alpha-PE MAb (clone MAb11; Pharmingen) were added. After 50 min of incubation at room temperature, the cells were washed two times with 0.1% saponin buffer, with a 10-min incubation before the last spin, and then fixed with 2% PFA-PBS. Samples were stored in the dark at 4°C, and the acquisition of 100,000 to 200,000 lymphocyte-gated events was performed with a FACSCalibur flow cytometer (Becton Dickinson) and analyzed with FlowJo software (Treestar). The background level of IFN-γ staining in PBMC (induced by a control influenza virus peptide, SNEGSYFFG) varied from animal to animal but was typically below 0.05% in the CD8+ lymphocytes and below 0.02% in the CD4+ lymphocytes. Only samples in which IFN-γ staining was at least twice that of the background or in which there was a distinct population of IFN-γ (bright)-positive cells (also positive for CD69 or tumor necrosis factor alpha) were considered positive. On the basis of these criteria, PBMC samples taken from all control animals 1 week after the injection of empty MVA did not produce any positive responses after stimulation with pools of peptides. All values are reported after the subtraction of background staining.
Lymphocyte subset determination.
PBMC (5 × 105) were stained with 10 μl of anti-human CD8-phycoerythrin-Cy5 (PECy5) (clone B9.11; Beckman Coulter), 10 μl of anti-human CD4-APC (clone SK3; Becton Dickinson), 5 μl of anti-human CD3ɛ-FITC (clone SP34; Pharmingen), and 5 μl of anti-human CD20-PE (clone L27; Becton Dickinson) in 200 μl of RPMI 1640 medium containing 10% FCS. Cells stained with immunoglobulins of matching isotypes were used as negative controls. Samples were incubated in the presence of antibodies for 45 min at an ambient temperature, washed, and fixed with 1% PFA-PBS solution for at least 30 min before the sample data were acquired with a FACSCalibur flow cytometer. The data were analyzed by using CellQuest software (Becton Dickinson). Absolute numbers of major lymphocyte subsets were determined by multiplying the percentage for each lymphocyte subset (percentages of CD3+ CD4+, CD3+ CD8+, or CD20+ lymphocytes) by the total lymphocyte count per microliter of blood, as obtained from a complete blood count. The complete blood count was determined by using an AcT10 Coulter Counter (Beckman Coulter).
Tetramer staining.
Fresh unstimulated PBMC (106) were washed two times with FACS buffer. In a 100-μl volume, cells were stained in the dark for 40 min at room temperature with Mamu-A*01/CM9 or Mamu-A*01/SL8 tetramer labeled with either PE or APC (5 μg/ml), anti-human CD3ɛ-FITC (SP34), and anti-CD8α-PerCP (clone SK1). The cells were then washed two times with 1 ml of FACS buffer and fixed with 2% PFA-PBS solution. Sample data were acquired with a FACSCalibur instrument and analyzed by using CellQuest software. Background tetramer staining of fresh unstimulated PBMC from naive Mamu-A*01-positive animals was routinely less than 0.08%.
Statistical analysis.
Differences in the levels of plasma SIV RNAs between groups were tested for statistical significance by using t tests after log transformation of the data to improve normality and homoscedasticity. In addition, the Levene test for homoscedasticity was conducted; if the results were found to be significant, the Welch correction for unequal variances was used. Finally, to further examine the robustness of the results, a nonparametric test, the Mann-Whitney U test, was performed. The P values for the nonparametric tests were calculated by exact methods. Associations between T-cell responses and viral loads were tested by Pearson correlation analysis and the Spearman correlation test, a nonparametric test, after log transformation of the data. All P values reported are two tailed. Survival analysis was conducted via Cox regression.
RESULTS
Cellular immune responses induced by vaccination and elicited after viral challenge.
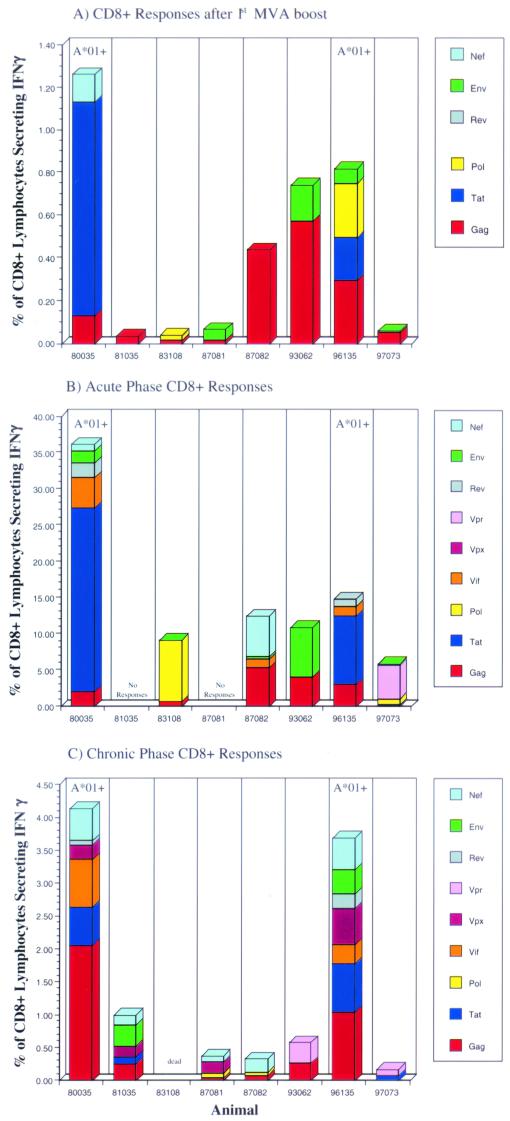
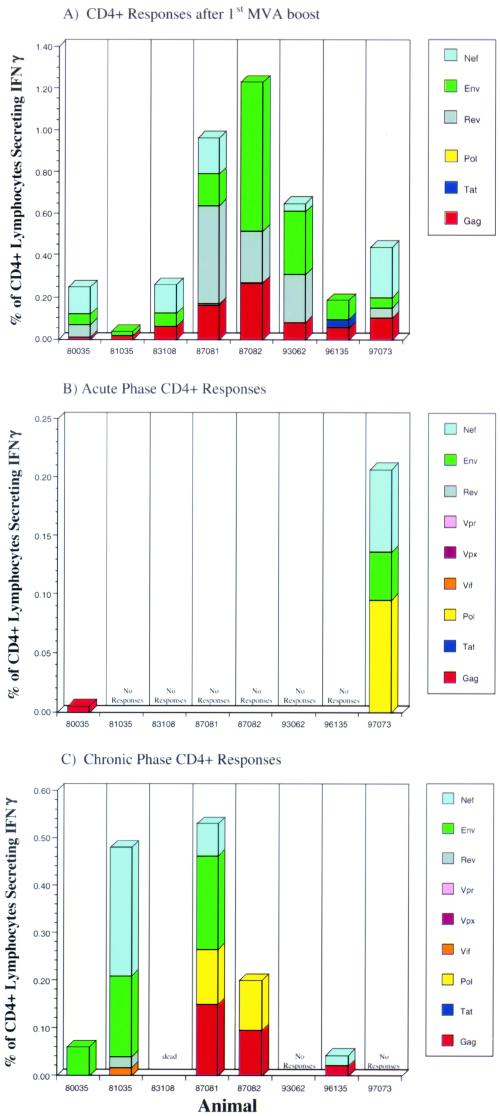
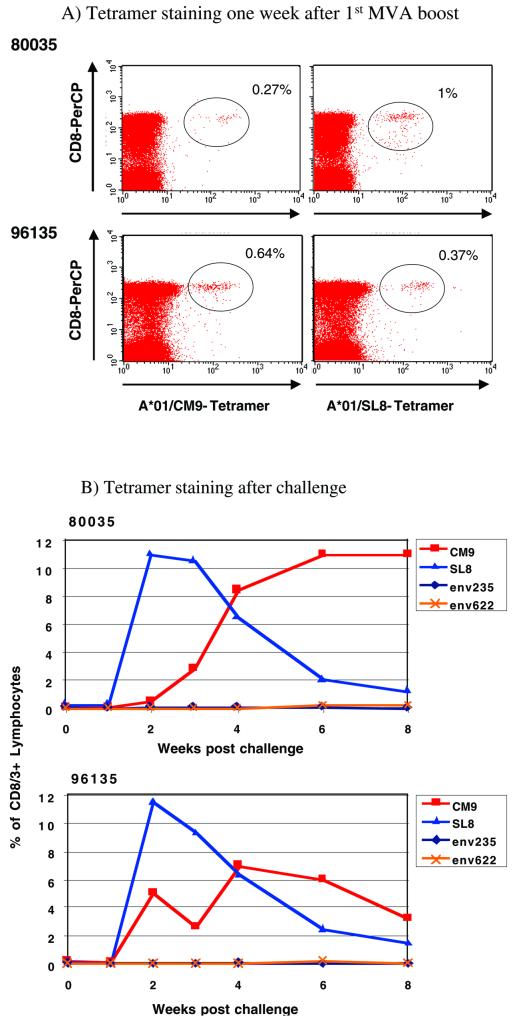
Eight rhesus monkeys were immunized 10 times with vaccines containing DNA encoding all the proteins from SIVmac. When tetramer staining was used, no CTL against the well-documented immunodominant Mamu-A*01-restricted epitopes Gag181-189CM9 (4) and Tat28-35SL8 (3) were detectable after the last DNA boost in fresh PBMC from Mamu-A*01-positive animals 80035 and 96135. However, CTL against these epitopes were detectable after in vitro stimulation (data not shown). To boost the cellular immune responses (5, 37, 38, 89), these animals were then immunized twice with recombinant MVA expressing Gag-Pol, Env, Nef, Rev, and Tat but not Vif, Vpr, and Vpx (Fig. 1). The cellular immune responses elicited following each MVA boost were determined by ICS. One week after the first MVA boost, freshly isolated PBMC were stimulated with pools of peptides (10 overlapping 15-mer and 20-mer peptides per pool) spanning the proteins Gag, Pol, Env, Nef, Tat, and Rev and were analyzed for the presence of virus-specific lymphocytes that produce cytokines. All animals showed both virus-specific CD8+ and virus-specific CD4+ responses after the first immunization with recombinant MVA (Fig. 2A and 3A), although the numbers of different proteins recognized and the frequencies of responding T cells differed greatly from animal to animal. The total frequency of SIV-specific CD8+ lymphocytes ranged from 0.05% to 1.26% of all CD8+ lymphocytes (Fig. 2A), with an average of 0.5%. The total frequency of SIV-specific CD4+ lymphocytes ranged from 0.04% to 1.23% of all CD4+ lymphocytes (Fig. 3A), with an average of 0.51%. Most animals mounted Gag-specific CD8+ (Fig. 2A) and CD4+ (Fig. 3A) responses. In particular, the two Mamu-A*01-positive animals (80035 and 96135) exhibited CD8+-T-cell responses against the well-documented immunodominant Gag181-189CM9 (4) and Tat28-35SL8 (3) epitopes, which were also detected by tetramer staining (Fig. 4A). Both tetramer staining and ICS revealed similar frequencies of CD8+ lymphocytes specific for these two Mamu-A*01-restricted epitopes, confirming that ICS is as sensitive as tetramer staining (5, 97). Four animals (81035, 83108, 87081, and 97073) showed only low-frequency CD8+ responses against a few peptide pools after the first immunization with recombinant MVA (Fig. 2A), but two of these four animals, 87081 and 97073, exhibited strong CD4+ responses (Fig. 3A).
FIG. 2.
ICS data for virus-specific CD8+-lymphocyte responses in animals vaccinated with DNA/MVA. The frequency of responding CD8+ cells against each protein is shown. These frequencies were obtained by adding the frequencies detected with peptide pools containing overlapping peptides from a single protein (after subtraction of the background). CD8+ responses after the first MVA boost (prior to infection), 2 weeks postinfection (acute phase of infection), and 12 weeks postinfection (chronic phase of infection) are shown.
FIG. 3.
ICS data for virus-specific CD4+-lymphocyte responses in animals vaccinated with DNA/MVA. The frequency of responding CD4+ cells against each protein is shown. These frequencies were obtained as described in the legend to Fig. 2. CD4+ responses after the first MVA boost (prior to infection), 2 weeks postinfection (acute phase of infection), and 12 weeks postinfection (chronic phase of infection) are shown.
FIG. 4.
Tetramer staining results for Mamu-A*01-positive animals, 80035 and 96135, after the first MVA boost and throughout infection. Percentages shown are for tetramer-specific CD8+ T cells. In addition to the tetramers against the Mamu-A*01-restricted epitopes Gag181-189CM9 and Tat28-35SL8, tetramers against two more Mamu-A*01-restricted epitopes, Env235-243CL9 and Env622-630TL9 (2), were tested postchallenge.
All animals were boosted a second time with the same recombinant MVA via the same routes. However, an elevation in the overall SIV-specific immune response was not observed. Moreover, not all of the responses previously detected in the peripheral blood following the first boost were detectable after the second boost (data not shown). The low frequencies of CD8+ responses against the two Mamu-A*01-restricted epitopes, Gag181-189CM9 and Tat28-35SL8, in the two Mamu-A*01-positive animals were also confirmed by tetramer staining (data not shown). Nevertheless, at 9 weeks after the last MVA boost (the day of challenge), both Mamu-A*01-positive animals still showed detectable tetramer-positive CD8+ responses against the two Mamu-A*01-restricted epitopes, Gag181-189CM9 (80035, 0.1% of CD3/8+ lymphocytes; 96135, 0.19% of CD3+ CD8+ lymphocytes) and Tat28-35SL8 (80035, 0.3% of CD3/8+ lymphocytes; 96135, 0.14% of CD3/8+ lymphocytes), as detected by tetramer staining. Only one other animal, 93062, showed a detectable CD8+ response against Gag (0.8% of all CD8+ lymphocytes) at this time point, as detected by ICS (data not shown).
Nine weeks after the last MVA boost, all vaccinated animals and 5 naive control animals were challenged intrarectally with molecularly cloned SIVmac239/nef-open. All animals became infected, as determined by positive detection of plasma SIV RNA by real-time PCR (Fig. 5) (see below). After challenge, cellular immune responses were measured by ICS with peptide pools spanning all SIV proteins, including Vpr, Vpx, and Vif, which were included in the DNA vaccine but not in the MVA boost. With the exception of two animals (81035 and 87081), in which no CD8+ responses were detectable 2 weeks postchallenge (acute phase), SIV-specific CD8+ lymphocytes increased in all animals to total frequencies ranging from 5.8% to 36.1% of all CD8+ lymphocytes (Fig. 2B), with an average of 16.6%. In contrast, CD4+ responses were low to undetectable 2 weeks postchallenge in all eight animals. Animal 80035 showed a borderline response against Gag, and animal 97073 mounted low but significant CD4+ responses against Pol, Env, and Nef (Fig. 3B). CD4+ responses were below the limits of detection in the remaining six animals. In the two Mamu-A*01-positive animals, the majority of the CD8+ responses were directed against the two Mamu-A*01-restricted epitopes, Gag181-189CM9 and Tat28-35SL8, as confirmed by tetramer staining (Fig. 4B). The frequency of Gag181-189CM9-specific CTL increased more slowly but remained high (7 to 11% of all CD3+ CD8+ lymphocytes) longer than that of Tat28-35SL8-specific CTL. Tat28-35SL8-specific CTL reached a high frequency at week 2 (11 to 12% of all CD3/8+ lymphocytes) but declined afterward (Fig. 4B). A similar rapid decline of Tat28-35SL8-specific CTL in Mamu-A*01-positive animals was previously shown to be due to the rapid selection of escape variants (3), which also occurred in the two Mamu-A*01-positive animals tested here (data not shown). Tetramer staining for two additional Mamu-A*01-restricted epitopes (Env235-243CL9 and Env622-630TL9) (2) revealed only very low frequencies of CD8+ T cells against these epitopes (Fig. 4B). CD8+ responses against these two epitopes were undetectable by ICS.
FIG. 5.
Viral load data for all animals up to 28 weeks postinfection, as determined by real-time PCR. (A) Control animals 90069, 90131, 92050, 92080, and 97086 were given empty MVA boosts only before intrarectal infection with SIVmac239. (B) Animals 80035, 81035, 83108, 87081, 87082, 93062, 96135, and 97073 were vaccinated 10 times with DNA and twice with recombinant MVA before intrarectal infection with SIVmac239. (C) Average viral loads of control animals versus vaccinees. A dagger indicates euthanasia of the animal.
By 12 weeks of infection (chronic phase), the frequencies of virus-specific CD8+ T cells in the vaccinees declined but persisted at significant levels, 0.17 to 4.1% of all CD8+ lymphocytes (Fig. 2C), with an average of 1.68%. Interestingly, significant CD8+ responses were detected at 12 weeks postinfection in animals 81035 and 87081, which did not show any detectable CD8+ responses at week 2 (Fig. 2C). Moreover, in contrast to the absence of CD4+ responses in most animals at week 2, five of the seven vaccinees showed detectable CD4+ responses by week 12, with frequencies ranging from 0.04% to 0.53% of all CD4+ lymphocytes (Fig. 3C), with an average of 0.18%.
In contrast to the strong virus-specific CD8+ responses detected in most vaccinees, analysis by ICS showed that most control animals exhibited very weak virus-specific CD8+ responses at 3 weeks postchallenge, with frequencies of all CD8+ lymphocytes ranging between 0.28 and 0.6% in four of five control animals. Only control animal 92050 demonstrated significant SIV-specific CD8+ responses at this time point, with 2.8% of all CD8+ lymphocytes being specific for SIV sequences (overlapping peptides), as measured by ICS (data not shown) (65). At 3 weeks postchallenge, control animals also showed low to undetectable SIV-specific CD4+ responses (total frequencies of between 0 and 0.2%; average, 0.06%) (data not shown).
Consistent with the results seen in the acute phase, the control animals also showed weaker cellular immune responses than the vaccinees by 12 weeks postchallenge (chronic phase). Control animals 90131 and 92050, which died at weeks 16 and 38, respectively, showed no detectable SIV-specific CD8+ responses (data not shown), and the remaining three control animals showed only very weak CD8+ responses at this time point (total frequencies of between 0.1 and 0.48%; average, 0.34%) (data not shown). At this time point, only the control animal which was able to control its virus replication, animal 92080, showed detectable CD4+ responses (total frequency of 0.14%) (data not shown).
Neutralizing antibodies.
Even though SIVmac239 is extremely difficult to neutralize (48, 59), we assessed neutralizing antibody levels in all animals on the day of challenge and at 3, 6, and 10 weeks postchallenge, since the vaccine was capable of inducing an antibody response (data not shown). None of the vaccinated animals or control animals had SIVmac239-specific neutralizing antibody activity against a PBMC-grown stock of SIVmac239, as measured with CEMx174 target cells, at any of the time points tested (Tables 1 and 2). In addition, all plasma samples were negative for neutralizing activity against this PBMC-grown stock of SIVmac239, as measured with human PBMC (<80% reduction in p27 Gag antigen synthesis), even at low plasma dilutions (1:5) (data not shown). However, all the vaccinees demonstrated a low-level neutralizing antibody response against the laboratory-adapted virus strain, SIVmac251, on the day of challenge, as assessed with CEMx174 cells (Table 1). This response increased rapidly after infection (Table 1). The neutralizing antibody titers against SIVmac251 in control animals increased much more slowly or remained undetectable; when detected, titers observed in control animals were lower than those observed in the vaccinees (Tables 1 and 2).
TABLE 1.
Neutralizing antibody data from vaccinated animals at various weeks after challenge with SIVmac239
| Animal | wk postchallenge | Neutralizing antibody titera to:
|
|
|---|---|---|---|
| SIVmac251 | SIVmac239 | ||
| 80035 | 0 | 94 | <20 |
| 3 | 10,419 | <20 | |
| 6 | 23,097 | <20 | |
| 10 | 11,600 | <20 | |
| 81035 | 0 | 36 | <20 |
| 4 | 9,795 | <20 | |
| 6 | 6,996 | <20 | |
| 10 | 12,947 | <20 | |
| 83108 | 0 | 37 | <20 |
| 3 | 2,466 | <20 | |
| 87081 | 0 | 34 | <20 |
| 3 | 6,297 | <20 | |
| 6 | 1,369 | <20 | |
| 10 | 2,412 | <20 | |
| 87082 | 0 | 52 | <20 |
| 3 | 6,941 | <20 | |
| 6 | 2,024 | <20 | |
| 10 | 1,822 | <20 | |
| 93062 | 0 | 282 | <20 |
| 3 | 12,917 | <20 | |
| 6 | 5,630 | <20 | |
| 10 | 4,703 | <20 | |
| 96135 | 0 | 82 | <20 |
| 3 | 8,436 | <20 | |
| 6 | 8,333 | <20 | |
| 10 | 2,366 | <20 | |
| 97073 | 0 | 211 | <20 |
| 3 | 11,135 | <20 | |
| 6 | 7,274 | <20 | |
| 10 | 13,967 | <20 | |
Reciprocal serum dilution at which 50% of CEMx174 cells were protected from virus-induced killing, as measured by neutral red uptake. SIVmac251 was laboratory adapted.
TABLE 2.
Neutralizing antibody data from control animals at various weeks after challenge with SIVmac239
| Animal | wk postchallenge | Neutralizing antibody titera to:
|
|
|---|---|---|---|
| SIVmac251 | SIVmac239 | ||
| 90069 | 0 | <20 | <20 |
| 3 | <20 | <20 | |
| 6 | 856 | <20 | |
| 10 | 1,755 | <20 | |
| 90131 | 0 | <20 | <20 |
| 3 | <20 | <20 | |
| 6 | <20 | <20 | |
| 10 | <20 | <20 | |
| 92050 | 0 | <20 | <20 |
| 3 | 42 | <20 | |
| 6 | 919 | <20 | |
| 10 | 651 | <20 | |
| 92080 | 0 | 20 | <20 |
| 3 | 341 | <20 | |
| 6 | 2,139 | <20 | |
| 10 | 1,641 | <20 | |
| 97086 | 0 | 25 | <20 |
| 3 | 419 | <20 | |
| 6 | 8,010 | <20 | |
| 10 | 9,952 | <20 | |
Reciprocal serum dilution at which 50% of CEMx174 cells were protected from virus-induced killing, as measured by neutral red uptake. SIVmac251 was laboratory adapted.
Viral loads.
Plasma viral RNA levels after challenge were measured by real-time PCR. The control monkeys had 1.0 × 107 to 1.0 × 108 copies of virus per ml of plasma at the time of peak viremia at 2 weeks postchallenge (Fig. 5A). In the vaccinated animals, peak viremia was measured at 4.5 × 105 to 4.5 × 106 copies/ml (Fig. 5B). One vaccinated animal, 83108, died 3 weeks postinfection due to a non-SIV-related illness (see below) and was excluded from the analysis. On average, the vaccinees had a 1.5-log-lower viral load during peak viremia than the controls (Fig. 5C). This difference was significant (P < 0.001 [t test]; P = 0.003 [nonparametric test]). However, by 12 weeks postinfection, the viral loads in all vaccinees increased to levels comparable to those in the controls, and the significance of the difference in viral loads between the two groups was lost by week 6 or 8, depending on which statistical test was used.
Clinical disease progression.
Postchallenge CD4+-T-lymphocyte counts were determined over time in all animals as an indicator of disease progression (Fig. 6). With the exception of two vaccinees (96135 and 97073), all vaccinated animals demonstrated progressive declining CD4+-T-cell counts. Two vaccinees, 81035 and 87082, were euthanized with an AIDS-like disease at 28 and 32 weeks postinfection, respectively (Table 3 and Fig. 6B). Vaccinee 83108 was euthanized at 3 weeks postinfection due to diabetes (Table 3) and was therefore excluded from all analyses. Four of the five control animals demonstrated various degrees of postinfection CD4+-T-cell decline (Fig. 6A). The fifth control animal, 92080, showed virus replication to low levels (<10,000 copies per ml) for 24 weeks (Fig. 5A) and maintained CD4+-T-cell counts (Fig. 6A) for up to 36 weeks postinfection. However, at 32 weeks postchallenge, the viral load in this animal began to progressively increase; by 44 weeks postchallenge, the viral load was >400,000 copies/ml (data not shown). Control animal 90131, which showed the highest viral load (Fig. 5A), also demonstrated the most dramatic decline in CD4+-T-cell counts (Fig. 6A) and had to be euthanized due to an AIDS-related disease at week 16 (Table 3). Another control animal, 92050, was also euthanized at week 38 due to an AIDS-related disease (Table 3). There was no statistical difference (P > 0.05 [Cox regression]) between the survival rates of vaccinees and control animals (Fig. 7).
FIG. 6.
Postchallenge CD4+-T-lymphocyte counts in control and vaccinated animals. A dagger indicates euthanasia of the animal.
TABLE 3.
Significant clinical events attributable to SIVmac239 infection or subsequent immunodeficiency in control and vaccinated animals through 38 weeks postinfection
| Monkey | Disease-attributable clinical event(s) |
|---|---|
| Controls | |
| 90069 | None |
| 90131 | Moderate anorexia, moderate diarrhea, opportunistic infections (Staphylococcus aureus, Giardia spp., Cryptosporidium spp.); euthanasia at wk 14 |
| 92050 | Severe anemia, moderate thrombocytopenia; euthanasia at wk 38 |
| 92080 | None |
| 97086 | Moderate diarrhea |
| Vaccinees | |
| 80035 | Mild weight loss |
| 81035 | Mild anorexia, peritonitis; euthanasia at wks 28 |
| 83108 | Euthanasia due to clinical diabetes |
| 87081 | Mild gingivitis |
| 87082 | Moderate weight loss, moderate anorexia, suppurative lymphadenitis; euthanasia at wk 32 |
| 93062 | None |
| 96135 | None |
| 97073 | None |
FIG. 7.
Postchallenge mortality in vaccinated and control animals through 38 weeks postinfection.
Analysis of correlation between cell-mediated immunity and viral load.
Associations between total CD8 and CD4 responses detected after vaccination (preinfection), during the acute phase of infection or the chronic phase of infection, with either the viral load peak (week 2 or 3) or the set point (average of the viral loads between weeks 16 and 28) were tested by both Pearson and Spearman correlation analyses. Interestingly, a significant inverse correlation was found only between the total virus-specific CD8+ responses detected after vaccination and the viral load set point by Spearman analysis (P = 0.003) but not by Pearson analysis (P = 0.065). No correlation was found between the virus-specific CD4+ responses and viral load.
There was also no correlation between either the strength or the breadth of the virus-specific CD8+ responses detected in each animal and its ability to maintain CD4 counts. In fact, one vaccinee, 97073, maintained its CD4 counts throughout the study (Fig. 6B) but demonstrated only low virus-specific CD8+ responses throughout the study (Fig. 2). In contrast, animal 80035 demonstrated the strongest virus-specific CD8+ responses throughout the study (Fig. 2) but failed to maintain its CD4 counts throughout the study (Fig. 6B).
The magnitude of the virus-specific CD4+ responses did not appear to correlate with an ability to mount strong CD8+ cellular immune responses. Animal 87081 demonstrated strong virus-specific CD4+ responses prechallenge and during the chronic phase of infection (Fig. 3A and C) but showed very low to undetectable CD8+ responses throughout the study (Fig. 2). This animal also suffered a loss of its CD4+ T cells (Fig. 6B). In one case, the presence of a strong virus-specific CD4+ response during the chronic phase of infection also did not predict a better outcome, since animal 81035, which had demonstrated the strongest virus-specific CD4+ responses throughout the study (Fig. 3C), still progressed to AIDS.
DISCUSSION
We have demonstrated here that a DNA prime/recombinant MVA boost regimen induced strong SIV-specific CD8+ and CD4+ cellular immune responses and resulted in a significant reduction in viral replication during the acute phase after intrarectal challenge with SIVmac239. Nevertheless, the immunization did not protect the animals against the pathogenic consequences of infection with this virus. Most animals demonstrated a progressive loss of CD4+ cells, and two of seven vaccinees had to be euthanized by weeks 28 and 32 postchallenge due to progression to simian AIDS. These results are somewhat disappointing, since recent studies with similar DNA prime/viral vector boost approaches protected rhesus macaques from the pathogenic consequences of infection with SHIV-89.6P (9, 80). Even a cytokine-augmented DNA vaccination alone (15) and a vaccination with recombinant MVA alone (14) protected animals from disease progression after challenge with SHIV-89.6P. These results suggest that the discrepancy between our results with SIVmac239 and the results of previous studies, where similar regimens have afforded significant protection against SHIV-89.6P, may lie in critical differences between the challenge viruses used. It was previously documented that the virulence of a challenge virus can influence the outcome of a challenge (60, 102). SIVmac239 and SHIV-89.6P are both highly pathogenic viruses (50, 53, 76-79). However, each has some distinguishing characteristics. SHIV-89.6P induces a rapid CD4+-T-cell decline within 2 to 3 weeks after infection, depending on the route of challenge (52, 77-79), an outcome that is not seen with field strains of HIV. In contrast, infection with SIVmac239 results in slow, inexorable CD4 destruction (50, 53), an outcome that is similar to that seen with HIV infection (86). More importantly, SIVmac239 is very difficult to neutralize with antibodies (48, 59), much like many field strains of HIV (19, 55, 61).
A previous study demonstrated that longer survival after infection with SHIV-89.6P was associated with the production of antiviral antibodies (52), indicating that antibodies against this virus may play a significant role in protection against SHIV-89.6P. Indeed, this protective capacity of neutralizing antibodies against infection with SHIV-89.6P or disease development after infection has been demonstrated by passive immunization (54, 56). It appears, therefore, that if animals can be protected against the acute CD4 depletion caused by SHIV-89.6P, then the development of effective antibody responses may ameliorate the disease course. Even animals without any detectable virus-specific immune responses after vaccination were still protected against the pathogenic consequences of a challenge with SHIV-89.6P (81), indicating that it may be much easier to protect against a challenge with this virus than it is to protect against SIVmac239. Because SIVmac239 is so difficult to neutralize and no neutralizing antibodies against this virus were detectable in our animals (Tables 1 and 2), it is unlikely that neutralizing antibodies played any role in the reduced viral loads observed during the acute phase of infection in all of our vaccinees, even though the neutralizing antibody titers against laboratory-adapted SIVmac251 increased (Tables 1 and 2). However, no correlation between vaccine-induced immune responses and reduced peak viral loads was detectable either. Interestingly, we detected a trend toward an inverse correlation between the total frequency of SIV-specific CD8+ responses detected after vaccination and the viral load set point, as determined by the average of the viral loads between weeks 16 and 28. Vaccinated animals demonstrated stronger and more sustained CD8+-T-cell responses postinfection than control animals. However, the viral load set point in the vaccinated animals was not significantly lower than that in the control animals. No correlation was observed between the strength and/or the breadth of the virus-specific CD8+ responses and the ability to maintain CD4 counts.
Although early viral replication dynamics have been correlated with disease progression (41, 72, 93), reduced peak viral replication alone is apparently not sufficient to protect animals from disease progression after infection with certain viruses, such as SIVmac 251 (51, 93) or SIVmac239 (this study). In addition to the reduced peak level of viral RNA in plasma, all vaccinees demonstrated some transient suppression of viral replication at weeks 6 to 12 postchallenge compared with nonvaccinated controls. It is unclear why the plasma virus concentrations started to rise again after this point. However, a previous study demonstrated a functional defect in SIV-specific CTL after 16 weeks of infection with SIVmac239 (97). Other groups have also described such a functionally impaired cellular immune system in SIVmac-infected animals (68, 104, 107), which could result in inefficient control of the virus by the cellular immune system in the absence of effective antibodies. Another explanation for the increase in viral replication could be escape of the virus from the cellular immune response through mutation of MHC class I-restricted epitopes, as has been indicated for HIV (18, 34, 75). Several studies have indicated that SIV can also accumulate mutations in MHC class I-restricted epitopes, abrogating recognition by CTL (3, 30, 63). We are currently exploring whether this property could explain the lack of control of viral replication after 12 weeks in these animals by sequencing regions of the virus against which cellular immune responses were directed at several time points after challenge.
The significant reduction in acute viral loads in the vaccinated animals and the inverse correlation between viral loads and CD8+ responses following vaccination reported here suggest that vaccines that elicit strong virus-specific CTL may be capable of controlling the replication of even highly pathogenic viruses. However, the failure of this vaccine strategy to achieve long-term protection from CD4+-T-cell decline following challenge with SIVmac239 is consistent with previous studies demonstrating that it is very difficult to protect against SIVmac239 (1, 26, 33, 58, 67, 99). Indeed, although several vaccines have been tested for the ability to protect against SIVmac239 (1, 26, 33, 58, 67, 99), only a few studies have reported similar vaccine-induced control of this virus postchallenge (67, 99). Although Mamu-A*01 had been associated with a better outcome after challenge with SIVmac251 (73), the two Mamu-A*01-positive animals selected for this study did not stand out with regard to their viral loads or disease progression after challenge with SIVmac239 in comparison to the non-Mamu-A*01 vaccinees. Therefore, the inclusion of the two Mamu-A*01-positive animals as vaccinees did not affect the outcome of this study.
Unfortunately, it is not possible to compare the levels of immune responses induced by our vaccine regimen to those that provided protection from disease progression after challenge with SIVmac239 (67, 99) or SHIV-89.6P (9, 14, 15) because of the different assay systems used. With regard to tetramer staining, however, the frequencies of Gag181-189CM9-specific CTL in our two Mamu-A*01-positive animals after the first MVA boost were comparable to those reported after DNA vaccination alone (15) and were approximately half the frequencies obtained after cytokine-augmented DNA vaccination, which resulted in the prevention of clinical AIDS after challenge with SHIV-89.6P (15). Nevertheless, the levels of Gag181-189CM9-specific CD8+ T cells that we obtained were similar to the levels obtained with recombinant MVA vaccination alone, which also prevented the development of disease after challenge with SHIV-89.6P (14). Much higher frequencies of Mamu-A*01/Gag181-189CM9-specific responses have been observed after DNA/MVA vaccination (5; T. U. Vogel et al., unpublished data); however, most of those animals were much younger than the ones used in this study. It is possible that immune responses in older animals may not be as strong as those in younger animals (32, 88, 96). However, with the limitations of having a small, diverse group of animals of different ages in this study, our data do not directly indicate such an influence of age on cellular immune responses.
The failure of our immunization strategy could be due to several other factors. The expression of certain proteins from immunodeficiency viruses might have had negative effects on the induction of immune responses. For example, Nef has been documented to downregulate the surface expression of MHC class I molecules (90) and, therefore, may result in less efficient antigen presentation by the MHC class I pathway (23). However, a study by Robinson et al. also included Nef as an immunogen and, nevertheless, protection against SHIV challenge was achieved (80). In addition, Env has been reported to be immunosuppressive (100). However, Env was also included as an immunogen in multiple studies, and it protected monkeys against the pathogenic consequences of a SHIV-89.6P challenge (9, 14, 15, 80, 81). Nevertheless, by preventing the immunosuppressive potential of some of our immunogens, we may be able to induce more effective immune responses.
In summary, we have demonstrated that the DNA prime/MVA boost regimen, which has proven to be safe and capable of inducing strong cellular immune responses against SIV in rhesus monkeys (5, 9, 38-40), suppressed viral replication during the acute phase of infection with SIVmac239. However, the vaccine regimen was unable to induce protection against the pathogenic consequences of an infection with SIVmac239. This failure may be related in part to the neutralization resistance and highly pathogenic potential of SIVmac239, against which protection has proven to be very difficult (1, 26, 33, 58, 67, 99).
Acknowledgments
Helen Horton and Thorsten U. Vogel contributed equally to this work.
We thank Sarah Fuenger, Max Liebl, and Edward Dunphy for assistance in performing ICS and data collection; Marion Ohlmann for expert MVA vaccine preparation; Dagna Sheerar and Eva Rakasz for assistance in determining lymphocyte subsets by flow cytometry; and Jason Reed and Jason Weinfurter for assistance in tissue processing from necropsies. We also thank Jody Helgeland for assistance with blood processing, Jacque Mitchen for coordinating all animal procedures, Carol L. Emerson for performing all animal procedures, and Amy L. Usborne for help in evaluating all clinical signs and necropsy reports.
This work was supported by NIH grants RR1537, AI46366, AI45461, and RR00167 (awarded to David I. Watkins) and AI85343 (awarded to David C. Montefiori) and by the European Community (Programme EVA, grant EU QLK2-2000-1040 awarded to Volker Erfle and Gerd Sutter). David I. Watkins is a recipient of an Elizabeth Glaser scientist award.
REFERENCES
- 1.Ahmad, S., B. Lohman, M. Marthas, L. Giavedoni, Z. el-Amad, N. L. Haigwood, C. J. Scandella, M. B. Gardner, P. A. Luciw, and T. Yilma. 1994. Reduced virus load in rhesus macaques immunized with recombinant gp160 and challenged with simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 10:195-204. [DOI] [PubMed] [Google Scholar]
- 2.Allen, T. M., B. R. Mothe, J. Sidney, P. Jing, J. L. Dzuris, M. E. Liebl, T. U. Vogel, D. H. O'Connor, X. Wang, M. C. Wussow, J. A. Thomson, J. D. Altman, D. I. Watkins, and A. Sette. 2001. CD8+ lymphocytes from simian immunodeficiency virus-infected rhesus macaques recognize 14 different epitopes bound by the major histocompatibility complex class I molecule mamu-A*01: implications for vaccine design and testing. J. Virol. 75:738-749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allen, T. M., D. H. O'Connor, J. Peicheng, J. L. Dzuris, B. R. Mothé, T. U. Vogel, E. Dunphy, M. E. Liebl, C. Emerson, N. Wilson, K. J. Kunstman, X. Wang, D. B. Allison, A. L. Hughes, R. C. Desrosiers, J. D. Altman, S. M. Wolinsky, A. Sette, and D. I. Watkins. 2000. Tat-specific cytotoxic T lymphocytes select for SIV escape variants during resolution of primary viremia. Nature 407:386-390. [DOI] [PubMed] [Google Scholar]
- 4.Allen, T. M., J. Sidney, M. F. del Guercio, R. L. Glickman, G. L. Lensmeyer, D. A. Wiebe, R. DeMars, C. D. Pauza, R. P. Johnson, A. Sette, and D. I. Watkins. 1998. Characterization of the peptide binding motif of a rhesus MHC class I molecule (Mamu-A*01) that binds an immunodominant CTL epitope from simian immunodeficiency virus. J. Immunol. 160:6062-6071. [PubMed] [Google Scholar]
- 5.Allen, T. M., T. U. Vogel, D. H. Fuller, B. R. Mothe, S. Steffen, J. E. Boyson, T. Shipley, J. Fuller, T. Hanke, A. Sette, J. D. Altman, B. Moss, A. J. McMichael, and D. I. Watkins. 2000. Induction of AIDS virus-specific CTL activity in fresh, unstimulated peripheral blood lymphocytes from rhesus macaques vaccinated with a DNA prime/modified vaccinia virus Ankara boost regimen. J. Immunol. 164:4968-4978. [DOI] [PubMed] [Google Scholar]
- 6.Almond, N., T. Corcoran, R. Hull, B. Walker, J. Rose, R. Sangster, K. Silvera, P. Silvera, M. Cranage, E. Rud, and E. J. Stott. 1997. Mechanisms of protection induced by attenuated simian immunodeficiency virus. IV. Protection against challenge with virus grown in autologous simian cells. J. Med. Primatol. 26:34-43. [DOI] [PubMed] [Google Scholar]
- 7.Almond, N., K. Kent, M. Cranage, E. Rud, B. Clarke, and E. J. Stott. 1995. Protection by attenuated simian immunodeficiency virus in macaques against challenge with virus-infected cells. Lancet 345:1342-1344. [DOI] [PubMed] [Google Scholar]
- 8.Almond, N., J. Rose, R. Sangster, P. Silvera, R. Stebbings, B. Walker, and E. J. Stott. 1997. Mechanisms of protection induced by attenuated simian immunodeficiency virus. I. Protection cannot be transferred with immune serum. J. Gen. Virol. 78:1919-1922. [DOI] [PubMed] [Google Scholar]
- 9.Amara, R. R., F. Villinger, J. D. Altman, S. L. Lydy, S. P. O'Neil, S. I. Staprans, D. C. Montefiori, Y. Xu, J. G. Herndon, L. S. Wyatt, M. A. Candido, N. L. Kozyr, P. L. Earl, J. M. Smith, H. L. Ma, B. D. Grimm, M. L. Hulsey, J. Miller, H. M. McClure, J. M. McNicholl, B. Moss, and H. L. Robinson. 2001. Control of a mucosal challenge and prevention of AIDS by a multiprotein DNA/MVA vaccine. Science 292:69-74. [DOI] [PubMed] [Google Scholar]
- 10.Anderson, M. G., D. Hauer, D. P. Sharma, S. V. Joag, O. Narayan, M. C. Zink, and J. E. Clements. 1993. Analysis of envelope changes acquired by SIVmac239 during neuroadaption in rhesus macaques. Virology 195:616-626. [DOI] [PubMed] [Google Scholar]
- 11.Baba, T. W., Y. S. Jeong, D. Pennick, R. Bronson, M. F. Greene, and R. M. Ruprecht. 1995. Pathogenicity of live, attenuated SIV after mucosal infection of neonatal macaques. Science 267:1820-1825. [DOI] [PubMed] [Google Scholar]
- 12.Baba, T. W., V. Liska, A. H. Khimani, N. B. Ray, P. J. Dailey, D. Penninck, R. Bronson, M. F. Greene, H. M. McClure, L. N. Martin, and R. M. Ruprecht. 1999. Live attenuated, multiply deleted simian immunodeficiency virus causes AIDS in infant and adult macaques. Nat. Med. 5:194-203. [DOI] [PubMed] [Google Scholar]
- 13.Barouch, D. H., A. Craiu, S. Santra, M. A. Egan, J. E. Schmitz, M. J. Kuroda, T. M. Fu, J. H. Nam, L. S. Wyatt, M. A. Lifton, G. R. Krivulka, C. E. Nickerson, C. I. Lord, B. Moss, M. G. Lewis, V. M. Hirsch, J. W. Shiver, and N. L. Letvin. 2001. Elicitation of high-frequency cytotoxic T-lymphocyte responses against both dominant and subdominant simian-human immunodeficiency virus epitopes by DNA vaccination of rhesus monkeys. J. Virol. 75:2462-2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barouch, D. H., S. Santra, M. J. Kuroda, J. E. Schmitz, R. Plishka, A. Buckler-White, A. E. Gaitan, R. Zin, J. H. Nam, L. S. Wyatt, M. A. Lifton, C. E. Nickerson, B. Moss, D. C. Montefiori, V. M. Hirsch, and N. L. Letvin. 2001. Reduction of simian-human immunodeficiency virus 89.6P viremia in rhesus monkeys by recombinant modified vaccinia virus Ankara vaccination. J. Virol. 75:5151-5158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barouch, D. H., S. Santra, J. E. Schmitz, M. J. Kuroda, T. M. Fu, W. Wagner, M. Bilska, A. Craiu, X. X. Zheng, G. R. Krivulka, K. Beaudry, M. A. Lifton, C. E. Nickerson, W. L. Trigona, K. Punt, D. C. Freed, L. Guan, S. Dubey, D. Casimiro, A. Simon, M. E. Davies, M. Chastain, T. B. Strom, R. S. Gelman, D. C. Montefiori, and M. G. Lewis. 2000. Control of viremia and prevention of clinical AIDS in rhesus monkeys by cytokine-augmented DNA vaccination. Science 290:486-492. [DOI] [PubMed] [Google Scholar]
- 16.Benson, J., C. Chougnet, M. Robert-Guroff, D. Montefiori, P. Markham, G. Shearer, R. C. Gallo, M. Cranage, E. Paoletti, K. Limbach, D. Venzon, J. Tartaglia, and G. Franchini. 1998. Recombinant vaccine-induced protection against the highly pathogenic simian immunodeficiency virus SIV(mac251): dependence on route of challenge exposure. J. Virol. 72:4170-4182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Borrow, P., H. Lewicki, B. H. Hahn, G. M. Shaw, and M. B. A. Oldstone. 1994. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J. Virol. 68:6103-6110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Borrow, P., H. Lewicki, X. Wei, M. S. Horwitz, N. Peffer, H. Meyers, J. A. Nelson, J. E. Gairin, B. H. Hahn, M. B. Oldstone, and G. M. Shaw. 1997. Antiviral pressure exerted by HIV-1-specific cytotoxic T lymphocytes (CTLs) during primary infection demonstrated by rapid selection of CTL escape virus. Nat. Med. 3:205-211. [DOI] [PubMed] [Google Scholar]
- 19.Bures, R., A. Gaitan, T. Zhu, C. Graziosi, K. M. McGrath, J. Tartaglia, P. Caudrelier, R. El Habib, M. Klein, A. Lazzarin, D. M. Stablein, M. Deers, L. Corey, M. L. Greenberg, D. H. Schwartz, and D. C. Montefiori. 2000. Immunization with recombinant canarypox vectors expressing membrane-anchored glycoprotein 120 followed by glycoprotein 160 boosting fails to generate antibodies that neutralize R5 primary isolates of human immunodeficiency virus type 1. AIDS Res. Hum. Retrovir. 16:2019-2035. [DOI] [PubMed] [Google Scholar]
- 20.Cafaro, A., F. Titti, C. Fracasso, M. T. Maggiorella, S. Baroncelli, A. Caputo, D. Goletti, A. Borsetti, M. Pace, E. Fanales-Belasio, B. Ridolfi, D. R. Negri, L. Sernicola, R. Belli, F. Corrias, I. Macchia, P. Leone, Z. Michelini, P. ten Haaft, S. Butto, P. Verani, and B. Ensoli. 2001. Vaccination with DNA containing tat coding sequences and unmethylated CpG motifs protects cynomolgus monkeys upon infection with simian/human immunodeficiency virus (SHIV89.6P). Vaccine 19:2862-2877. [DOI] [PubMed] [Google Scholar]
- 21.Clements, J. E., R. C. Montelaro, M. C. Zink, A. M. Amedee, S. Miller, A. M. Trichel, B. Jagerski, D. Hauer, L. N. Martin, and R. P. Bohm. 1995. Cross-protective immune responses induced in rhesus macaques by immunization with attenuated macrophage-tropic simian immunodeficiency virus. J. Virol. 69:2737-2744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cohen, J. 1997. Weakened SIV vaccine still kills. Science 278:24-25. [DOI] [PubMed] [Google Scholar]
- 23.Collins, K. L., B. K. Chen, S. A. Kalams, B. D. Walker, and D. Baltimore. 1998. HIV-1 Nef protein protects infected primary cells against killing by cytotoxic T lymphocytes. Nature 391:397-401. [DOI] [PubMed] [Google Scholar]
- 24.Condon, C., S. C. Watkins, C. M. Celluzzi, K. Thompson, and L. D. Falo, Jr. 1996. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2:1122-1128. [DOI] [PubMed] [Google Scholar]
- 25.Connor, R. I., D. C. Montefiori, J. M. Binley, J. P. Moore, S. Bonhoeffer, A. Gettie, E. A. Fenamore, K. E. Sheridan, D. D. Ho, P. J. Dailey, and P. A. Marx. 1998. Temporal analyses of virus replication, immune responses, and efficacy in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J. Virol. 72:7501-7509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daniel, M. D., F. Kirchhoff, S. C. Czajak, P. K. Sehgal, and R. C. Desrosiers. 1992. Protective effects of a live attenuated SIV vaccine with a deletion in the nef gene. Science 258:1938-1941. [DOI] [PubMed] [Google Scholar]
- 27.Desrosiers, R. C. 1994. Safety issues facing development of a live-attenuated, multiply deleted HIV-1 vaccine. AIDS Res. Hum. Retrovir. 10:331-332. [DOI] [PubMed] [Google Scholar]
- 28.Dittmer, U., T. Nisslein, W. Bodemer, H. Petry, U. Sauermann, C. Stahl-Hennig, and G. Hunsmann. 1995. Cellular immune response of rhesus monkeys infected with a partially attenuated nef deletion mutant of the simian immunodeficiency virus. Virology 212:392-397. [DOI] [PubMed] [Google Scholar]
- 29.Egan, M. A., W. A. Charini, M. J. Kuroda, J. E. Schmitz, P. Racz, K. Tenner-Racz, K. Manson, M. Wyand, M. A. Lifton, C. E. Nickerson, T. Fu, J. W. Shiver, and N. L. Letvin. 2000. Simian immunodeficiency virus (SIV) gag DNA-vaccinated rhesus monkeys develop secondary cytotoxic T-lymphocyte responses and control viral replication after pathogenic SIV infection. J. Virol. 74:7485-7495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Evans, D. T., D. H. O'Connor, P. Jing, J. L. Dzuris, J. Sidney, J. da Silva, T. M. Allen, H. Horton, J. E. Venham, R. A. Rudersdorf, T. Vogel, C. D. Pauza, R. E. Bontrop, R. DeMars, A. Sette, A. L. Hughes, and D. I. Watkins. 1999. Virus-specific cytotoxic T-lymphocyte responses select for amino-acid variation in simian immunodeficiency virus Env and Nef. Nat. Med. 5:1270-1276. [DOI] [PubMed] [Google Scholar]
- 31.Fuller, D. H., L. Simpson, K. S. Cole, J. E. Clements, D. L. Panicali, R. C. Montelaro, M. Murphey-Corb, and J. R. Haynes. 1997. Gene gun-based nucleic acid immunization alone or in combination with recombinant vaccinia vectors suppresses virus burden in rhesus macaques challenged with a heterologous SIV. Immunol. Cell Biol. 75:389-396. [DOI] [PubMed] [Google Scholar]
- 32.Gardner, E. M., E. D. Bernstein, M. Dorfman, E. Abrutyn, and D. M. Murasko. 1997. The age-associated decline in immune function of healthy individuals is not related to changes in plasma concentrations of beta-carotene, retinol, alpha-tocopherol or zinc. Mech. Ageing Dev. 94:55-69. [DOI] [PubMed] [Google Scholar]
- 33.Giorgi, J. V., L. E. Hultin, and R. C. Desrosiers. 1996. The immunopathogenesis of retroviral diseases: no immunophenotypic alterations in T, B, and NK cell subsets in SIVmac239-challenged rhesus macaques protected by SIV delta nef vaccination. J. Med. Primatol. 25:186-191. [DOI] [PubMed] [Google Scholar]
- 34.Goulder, P. J., R. E. Phillips, R. A. Colbert, S. McAdam, G. Ogg, M. A. Nowak, P. Giangrande, G. Luzzi, B. Morgan, A. Edwards, A. J. McMichael, and S. Rowland-Jones. 1997. Late escape from an immunodominant cytotoxic T-lymphocyte response associated with progression to AIDS. Nat. Med. 3:212-217. [DOI] [PubMed] [Google Scholar]
- 35.Gundlach, B. R., M. G. Lewis, S. Sopper, T. Schnell, J. Sodroski, C. Stahl-Hennig, and K. Uberla. 2000. Evidence for recombination of live, attenuated immunodeficiency virus vaccine with challenge virus to a more virulent strain. J. Virol. 74:3537-3542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Haigwood, N. L., C. C. Pierce, M. N. Robertson, A. J. Watson, D. C. Montefiori, M. Rabin, J. B. Lynch, L. Kuller, J. Thompson, W. R. Morton, R. E. Benveniste, S. L. Hu, P. Greenberg, and S. P. Mossman. 1999. Protection from pathogenic SIV challenge using multigenic DNA vaccines. Immunol. Lett. 66:183-188. [DOI] [PubMed] [Google Scholar]
- 37.Hanke, T., T. J. Blanchard, J. Schneider, C. M. Hannan, M. Becker, S. C. Gilbert, A. V. Hill, G. L. Smith, and A. McMichael. 1998. Enhancement of MHC class I-restricted peptide-specific T cell induction by a DNA prime/MVA boost vaccination regime. Vaccine 16:439-445. [DOI] [PubMed] [Google Scholar]
- 38.Hanke, T., and A. McMichael. 1999. Pre-clinical development of a multi-CTL epitope-based DNA prime MVA boost vaccine for AIDS. Immunol. Lett. 66:177-181. [DOI] [PubMed] [Google Scholar]
- 39.Hanke, T., V. C. Neumann, T. J. Blanchard, P. Sweeney, A. V. Hill, G. L. Smith, and A. McMichael. 1999. Effective induction of HIV-specific CTL by multi-epitope using gene gun in a combined vaccination regime. Vaccine 17:589-596. [DOI] [PubMed] [Google Scholar]
- 40.Hanke, T., R. V. Samuel, T. J. Blanchard, V. C. Neumann, T. M. Allen, J. E. Boyson, S. A. Sharpe, N. Cook, G. L. Smith, D. I. Watkins, M. P. Cranage, and A. J. McMichael. 1999. Effective induction of simian immunodeficiency virus-specific cytotoxic T lymphocytes in macaques by using a multiepitope gene and DNA prime-modified vaccinia virus Ankara boost vaccination regimen. J. Virol. 73:7524-7532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hirsch, V. M., T. R. Fuerst, G. Sutter, M. W. Carroll, L. C. Yang, S. Goldstein, M. Piatak, Jr., W. R. Elkins, W. G. Alvord, D. C. Montefiori, B. Moss, and J. D. Lifson. 1996. Patterns of viral replication correlate with outcome in simian immunodeficiency virus (SIV)-infected macaques: effect of prior immunization with a trivalent SIV vaccine in modified vaccinia virus Ankara. J. Virol. 70:3741-3752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin, X., D. E. Bauer, S. E. Tuttleton, S. Lewin, A. Gettie, J. Blanchard, C. E. Irwin, J. T. Safrit, J. Mittler, L. Weinberger, L. G. Kostrikis, L. Zhang, A. S. Perelson, and D. D. Ho. 1999. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected macaques. J. Exp. Med. 189:991-998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson, R. P. 1996. Macaque models for AIDS vaccine development. Curr. Opin. Immunol. 8:554-560. [DOI] [PubMed] [Google Scholar]
- 44.Johnson, R. P., J. D. Lifson, S. C. Czajak, K. S. Cole, K. H. Manson, R. Glickman, J. Yang, D. C. Montefiori, R. Montelaro, M. S. Wyand, and R. C. Desrosiers. 1999. Highly attenuated vaccine strains of simian immunodeficiency virus protect against vaginal challenge: inverse relationship of degree of protection with level of attenuation. J. Virol. 73:4952-4961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kent, S. J., A. Zhao, S. J. Best, J. D. Chandler, D. B. Boyle, and I. A. Ramshaw. 1998. Enhanced T-cell immunogenicity and protective efficacy of a human immunodeficiency virus type 1 vaccine regimen consisting of consecutive priming with DNA and boosting with recombinant fowlpox virus. J. Virol. 72:10180-10188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kim, J. J., J. S. Yang, L. K. Nottingham, D. J. Lee, M. Lee, K. H. Manson, M. S. Wyand, J. D. Boyer, K. E. Ugen, and D. B. Weiner. 2001. Protection from immunodeficiency virus challenges in rhesus macaques by multicomponent DNA immunization. Virology 285:204-217. [DOI] [PubMed] [Google Scholar]
- 47.Koup, R. A., J. T. Safrit, Y. Cao, C. A. Andrews, G. McLeod, W. Borkowsky, C. Farthing, and D. D. Ho. 1994. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J. Virol. 68:4650-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Langlois, A. J., R. C. Desrosiers, M. G. Lewis, V. N. KewalRamani, D. R. Littman, J. Y. Zhou, K. Manson, M. S. Wyand, D. P. Bolognesi, and D. C. Montefiori. 1998. Neutralizing antibodies in sera from macaques immunized with attenuated simian immunodeficiency virus. J. Virol. 72:6950-6955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lesinski, G. B., S. L. Smithson, N. Srivastava, D. Chen, G. Widera, and M. A. Westerink. 2001. A DNA vaccine encoding a peptide mimic of Streptococcus pneumoniae serotype 4 capsular polysaccharide induces specific anti-carbohydrate antibodies in Balb/c mice. Vaccine 19:1717-1726. [DOI] [PubMed] [Google Scholar]
- 50.Lewis, M. G., S. Bellah, K. McKinnon, J. Yalley-Ogunro, P. M. Zack, W. R. Elkins, R. C. Desrosiers, and G. A. Eddy. 1994. Titration and characterization of two rhesus-derived SIVmac challenge stocks. AIDS Res. Hum. Retrovir. 10:213-220. [DOI] [PubMed] [Google Scholar]
- 51.Lu, S., J. Arthos, D. C. Montefiori, Y. Yasutomi, K. Manson, F. Mustafa, E. Johnson, J. C. Santoro, J. Wissink, J. I. Mullins, J. R. Haynes, N. L. Letvin, M. Wyand, and H. L. Robinson. 1996. Simian immunodeficiency virus DNA vaccine trial in macaques. J. Virol. 70:3978-3991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lu, Y., C. D. Pauza, X. Lu, D. C. Montefiori, and C. J. Miller. 1998. Rhesus macaques that become systemically infected with pathogenic SHIV 89.6-PD after intravenous, rectal, or vaginal inoculation and fail to make an antiviral antibody response rapidly develop AIDS. J. Acquir. Immune Defic. Syndr. Hum. Retrovirol. 19:6-18. [DOI] [PubMed] [Google Scholar]
- 53.Luciw, P. A., K. E. Shaw, R. E. Unger, V. Planelles, M. W. Stout, J. E. Lackner, E. Pratt-Lowe, N. J. Leung, B. Banapour, and M. L. Marthas. 1992. Genetic and biological comparisons of pathogenic and nonpathogenic molecular clones of simian immunodeficiency virus (SIVmac). AIDS Res. Hum. Retrovir. 8:395-402. [DOI] [PubMed] [Google Scholar]
- 54.Mascola, J. R., M. G. Lewis, G. Stiegler, D. Harris, T. C. VanCott, D. Hayes, M. K. Louder, C. R. Brown, C. V. Sapan, S. S. Frankel, Y. Lu, M. L. Robb, H. Katinger, and D. L. Birx. 1999. Protection of macaques against pathogenic simian/human immunodeficiency virus 89.6PD by passive transfer of neutralizing antibodies. J. Virol. 73:4009-4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mascola, J. R., S. W. Snyder, O. S. Weislow, S. M. Belay, R. B. Belshe, D. H. Schwartz, M. L. Clements, R. Dolin, B. S. Graham, G. J. Gorse, M. C. Keefer, M. J. McElrath, M. C. Walker, K. F. Wagner, J. G. McNeil, F. E. McCutchan, D. S. Burke, et al. 1996. Immunization with envelope subunit vaccine products elicits neutralizing antibodies against laboratory-adapted but not primary isolates of human immunodeficiency virus type 1. J. Infect. Dis. 173:340-348. [DOI] [PubMed] [Google Scholar]
- 56.Mascola, J. R., G. Stiegler, T. C. VanCott, H. Katinger, C. B. Carpenter, C. E. Hanson, H. Beary, D. Hayes, S. S. Frankel, D. L. Birx, and M. G. Lewis. 2000. Protection of macaques against vaginal transmission of a pathogenic HIV-1/SIV chimeric virus by passive infusion of neutralizing antibodies. Nat. Med. 6:207-210. [DOI] [PubMed] [Google Scholar]
- 57.Matano, T., R. Shibata, C. Siemon, M. Connors, H. C. Lane, and M. A. Martin. 1998. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J. Virol. 72:164-169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Miller, C. J., M. B. McChesney, X. Lu, P. J. Dailey, C. Chutkowski, D. Lu, P. Brosio, B. Roberts, and Y. Lu. 1997. Rhesus macaques previously infected with simian/human immunodeficiency virus are protected from vaginal challenge with pathogenic SIVmac239. J. Virol. 71:1911-1921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Montefiori, D. C., T. W. Baba, A. Li, M. Bilska, and R. M. Ruprecht. 1996. Neutralizing and infection-enhancing antibody responses do not correlate with the differential pathogenicity of SIVmac239delta3 in adult and infant rhesus monkeys. J. Immunol. 157:5528-5535. [PubMed] [Google Scholar]
- 60.Mooij, P., W. M. Bogers, H. Oostermeijer, W. Koornstra, P. J. Ten Haaft, B. E. Verstrepen, G. Van Der Auwera, and J. L. Heeney. 2000. Evidence for viral virulence as a predominant factor limiting human immunodeficiency virus vaccine efficacy. J. Virol. 74:4017-4027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Moore, J. P., P. W. Parren, and D. R. Burton. 2001. Genetic subtypes, humoral immunity, and human immunodeficiency virus type 1 vaccine development. J. Virol. 75:5721-5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Mori, K., Y. Yasutomi, S. Ohgimoto, T. Nakasone, S. Takamura, T. Shioda, and Y. Nagai. 2001. Quintuple deglycosylation mutant of simian immunodeficiency virus SIVmac239 in rhesus macaques: robust primary replication, tightly contained chronic infection, and elicitation of potent immunity against the parental wild-type strain. J. Virol. 75:4023-4028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mortara, L., F. Letourneur, H. Gras-Masse, A. Venet, J. G. Guillet, and I. Bourgault-Villada. 1998. Selection of virus variants and emergence of virus escape mutants after immunization with an epitope vaccine. J. Virol. 72:1403-1410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mossman, S. P., F. Bex, P. Berglund, J. Arthos, S. P. O'Neil, D. Riley, D. H. Maul, C. Bruck, P. Momin, A. Burny, P. N. Fultz, J. I. Mullins, P. Liljestrom, and E. A. Hoover. 1996. Protection against lethal simian immunodeficiency virus SIVsmmPBj14 disease by a recombinant Semliki Forest virus gp160 vaccine and by a gp120 subunit vaccine. J. Virol. 70:1953-1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mothe, B. R., H. Horton, D. K. Carter, T. M. Allen, M. E. Liebl, P. Skinner, T. U. Vogel, S. Fuenger, K. Vielhuber, W. Rehrauer, N. Wilson, G. Franchini, J. D. Altman, A. Haase, L. J. Picker, D. B. Allison, and D. I. Watkins. 2002. Dominance of CD8 responses specific for epitopes bound by a single major histocompatibility complex class I molecule during the acute phase of viral infection. J. Virol. 76:875-884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murphey-Corb, M. 1997. Live-attenuated HIV vaccines: how safe is safe enough? Nat. Med. 3:17-18. [DOI] [PubMed] [Google Scholar]
- 67.Murphy, C. G., W. T. Lucas, R. E. Means, S. Czajak, C. L. Hale, J. D. Lifson, A. Kaur, R. P. Johnson, D. M. Knipe, and R. C. Desrosiers. 2000. Vaccine protection against simian immunodeficiency virus by recombinant strains of herpes simplex virus. J. Virol. 74:7745-7754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67a.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 68.Neben, K., M. Heidbreder, J. Muller, A. Marxer, H. Petry, A. Didier, A. Schimpl, T. Hunig, and T. Kerkau. 1999. Impaired thymopoietic potential of immature CD3(−)CD4(+)CD8(−) T cell precursors from SIV-infected rhesus monkeys. Int. Immunol. 11:1509-1518. [DOI] [PubMed] [Google Scholar]
- 69.Nixon, D. F., S. M. Donahoe, W. M. Kakimoto, R. V. Samuel, K. J. Metzner, A. Gettie, T. Hanke, P. A. Marx, and R. I. Connor. 2000. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and protection against challenge in rhesus macaques immunized with a live attenuated simian immunodeficiency virus vaccine. Virology 266:203-210. [DOI] [PubMed] [Google Scholar]
- 70.Norley, S., B. Beer, D. Binninger-Schinzel, C. Cosma, and R. Kurth. 1996. Protection from pathogenic SIVmac challenge following short-term infection with a nef-deficient attenuated virus. Virology 219:195-205. [DOI] [PubMed] [Google Scholar]
- 71.Ogg, G. S., X. Jin, S. Bonhoeffer, P. R. Dunbar, M. A. Nowak, S. Monard, J. P. Segal, Y. Cao, S. L. Rowland-Jones, V. Cerundolo, A. Hurley, M. Markowitz, D. D. Ho, D. F. Nixon, and A. J. McMichael. 1998. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science 279:2103-2106. [DOI] [PubMed] [Google Scholar]
- 72.Ourmanov, I., C. R. Brown, B. Moss, M. Carroll, L. Wyatt, L. Pletneva, S. Goldstein, D. Venzon, and V. M. Hirsch. 2000. Comparative efficacy of recombinant modified vaccinia virus Ankara expressing simian immunodeficiency virus (SIV) Gag-Pol and/or Env in macaques challenged with pathogenic SIV. J. Virol. 74:2740-2751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Pal, R., D. Venzon, N. L. Letvin, S. Santra, D. C. Montefiori, N. R. Miller, E. Tryniszewska, M. G. Lewis, T. C. VanCott, V. Hirsch, R. Woodward, A. Gibson, M. Grace, E. Dobratz, P. D. Markham, Z. Hel, J. Nacsa, M. Klein, J. Tartaglia, and G. Franchini. 2002. ALVAC-SIV-gag-pol-env-based vaccination and macaque major histocompatibility complex class I (A*01) delay simian immunodeficiency virus SIVmac-induced immunodeficiency. J. Virol. 76:292-302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Picker, L. J., M. K. Singh, Z. Zdraveski, J. R. Treer, S. L. Waldrop, P. R. Bergstresser, and V. C. Maino. 1995. Direct demonstration of cytokine synthesis heterogeneity among human memory/effector T cells by flow cytometry. Blood 86:1408-1419. [PubMed] [Google Scholar]
- 75.Price, D. A., P. J. Goulder, P. Klenerman, A. K. Sewell, P. J. Easterbrook, M. Troop, C. R. Bangham, and R. E. Phillips. 1997. Positive selection of HIV-1 cytotoxic T lymphocyte escape variants during primary infection. Proc. Natl. Acad. Sci. USA 94:1890-1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Regier, D. A., and R. C. Desrosiers. 1990. The complete nucleotide sequence of a pathogenic molecular clone of simian immunodeficiency virus. AIDS Res. Hum. Retrovir. 6:1221-1231. [DOI] [PubMed] [Google Scholar]
- 77.Reimann, K. A., J. T. Li, R. Veazey, M. Halloran, I. W. Park, G. B. Karlsson, J. Sodroski, and N. L. Letvin. 1996. A chimeric simian/human immunodeficiency virus expressing a primary patient human immunodeficiency virus type 1 isolate env causes an AIDS-like disease after in vivo passage in rhesus monkeys. J. Virol. 70:6922-6928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Reimann, K. A., J. T. Li, G. Voss, C. Lekutis, K. Tenner-Racz, P. Racz, W. Lin, D. C. Montefiori, D. E. Lee-Parritz, Y. Lu, R. G. Collman, J. Sodroski, and N. L. Letvin. 1996. An env gene derived from a primary human immunodeficiency virus type 1 isolate confers high in vivo replicative capacity to a chimeric simian/human immunodeficiency virus in rhesus monkeys. J. Virol. 70:3198-3206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Reimann, K. A., A. Watson, P. J. Dailey, W. Lin, C. I. Lord, T. D. Steenbeke, R. A. Parker, M. K. Axthelm, and G. B. Karlsson. 1999. Viral burden and disease progression in rhesus monkeys infected with chimeric simian-human immunodeficiency viruses. Virology 256:15-21. [DOI] [PubMed] [Google Scholar]
- 80.Robinson, H. L., D. C. Montefiori, R. P. Johnson, K. H. Manson, M. L. Kalish, J. D. Lifson, T. A. Rizvi, S. Lu, S. L. Hu, G. P. Mazzara, D. L. Panicali, J. G. Herndon, R. Glickman, M. A. Candido, S. L. Lydy, M. S. Wyand, and H. M. McClure. 1999. Neutralizing antibody-independent containment of immunodeficiency virus challenges by DNA priming and recombinant pox virus booster immunizations. Nat. Med. 5:526-534. [DOI] [PubMed] [Google Scholar]
- 81.Rose, N. F., P. A. Marx, A. Luckay, D. F. Nixon, W. J. Moretto, S. M. Donahoe, D. Montefiori, A. Roberts, L. Buonocore, and J. K. Rose. 2001. An effective AIDS vaccine based on live attenuated vesicular stomatitis virus recombinants. Cell 106:539-549. [DOI] [PubMed] [Google Scholar]
- 82.Rosenberg, E. S., J. M. Billingsley, A. M. Caliendo, S. L. Boswell, P. E. Sax, S. A. Kalams, and B. D. Walker. 1997. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science 278:1447-1450. [DOI] [PubMed] [Google Scholar]
- 83.Rud, E. W., M. Cranage, J. Yon, J. Quirk, L. Ogilvie, N. Cook, S. Webster, M. Dennis, and B. E. Clarke. 1994. Molecular and biological characterization of simian immunodeficiency virus macaque strain 32H proviral clones containing nef size variants. J. Gen. Virol. 75:529-543. [DOI] [PubMed] [Google Scholar]
- 84.Ruprecht, R. M., T. W. Baba, and V. Liska. 1996. Attenuated HIV vaccine: caveats. Science 271:1790-1792. [PubMed] [Google Scholar]
- 85.Sawai, E. T., M. S. Hamza, M. Ye, K. E. Shaw, and P. A. Luciw. 2000. Pathogenic conversion of live attenuated simian immunodeficiency virus vaccines is associated with expression of truncated Nef. J. Virol. 74:2038-2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schellekens, P. T., M. Tersmette, M. T. Roos, R. P. Keet, F. de Wolf, R. A. Coutinho, and F. Miedema. 1992. Biphasic rate of CD4+ cell count decline during progression to AIDS correlates with HIV-1 phenotype. AIDS 6:665-669. [DOI] [PubMed] [Google Scholar]
- 87.Schmitz, J. E., M. J. Kuroda, S. Santra, V. G. Sasseville, M. A. Simon, M. A. Lifton, P. Racz, K. Tenner-Racz, M. Dalesandro, B. J. Scallon, J. Ghrayeb, M. A. Forman, D. C. Montefiori, E. P. Rieber, N. L. Letvin, and K. A. Reimann. 1999. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science 283:857-860. [DOI] [PubMed] [Google Scholar]
- 88.Schmucker, D. L., K. Thoreux, and R. L. Owen. 2001. Aging impairs intestinal immunity. Mech. Ageing Dev. 122:1397-1411. [DOI] [PubMed] [Google Scholar]
- 89.Schneider, J., S. C. Gilbert, T. J. Blanchard, T. Hanke, K. J. Robson, C. M. Hannan, M. Becker, R. Sinden, G. L. Smith, and A. V. Hill. 1998. Enhanced immunogenicity for CD8+ T cell induction and complete protective efficacy of malaria DNA vaccination by boosting with modified vaccinia virus Ankara. Nat. Med. 4:397-402. [DOI] [PubMed] [Google Scholar]
- 90.Schwartz, O., V. Marechal, G. S. Le, F. Lemonnier, and J. M. Heard. 1996. Endocytosis of major histocompatibility complex class I molecules is induced by the HIV-1 Nef protein. Nat. Med. 2:338-342. [DOI] [PubMed] [Google Scholar]
- 91.Seth, A., I. Ourmanov, J. E. Schmitz, M. J. Kuroda, M. A. Lifton, C. E. Nickerson, L. Wyatt, M. Carroll, B. Moss, D. Venzon, N. L. Letvin, and V. M. Hirsch. 2000. Immunization with a modified vaccinia virus expressing simian immunodeficiency virus (SIV) Gag-Pol primes for an anamnestic Gag-specific cytotoxic T-lymphocyte response and is associated with reduction of viremia after SIV challenge. J. Virol. 74:2502-2509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sharma, D. P., M. C. Zink, M. Anderson, R. Adams, J. E. Clements, S. V. Joag, and O. Narayan. 1992. Derivation of neurotropic simian immunodeficiency virus from exclusively lymphocytetropic parental virus: pathogenesis of infection in macaques. J. Virol. 66:3550-3556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Staprans, S. I., P. J. Dailey, A. Rosenthal, C. Horton, R. M. Grant, N. Lerche, and M. B. Feinberg. 1999. Simian immunodeficiency virus disease course is predicted by the extent of virus replication during primary infection. J. Virol. 73:4829-4839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Stebbings, R., J. Stott, N. Almond, R. Hull, J. Lines, P. Silvera, R. Sangster, T. Corcoran, J. Rose, S. Cobbold, F. Gotch, A. McMichael, and B. Walker. 1998. Mechanisms of protection induced by attenuated simian immunodeficiency virus. II. Lymphocyte depletion does not abrogate protection. AIDS Res. Hum. Retrovir. 14:1187-1198. [DOI] [PubMed] [Google Scholar]
- 95.Stott, J., and N. Almond. 1995. Assessing animal models of AIDS. Nat. Med. 1:295-297. [DOI] [PubMed] [Google Scholar]
- 96.Taylor, L. D., C. K. Daniels, and D. L. Schmucker. 1992. Ageing compromises gastrointestinal mucosal immune response in the rhesus monkey. Immunology 75:614-618. [PMC free article] [PubMed] [Google Scholar]
- 97.Vogel, T. U., T. M. Allen, J. D. Altman, and D. I. Watkins. 2001. Functional impairment of simian immunodeficiency virus-specific CD8+ T cells during the chronic phase of infection. J. Virol. 75:2458-2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Vogel, T. U., J. Fournier, A. Sherring, D. Ko, M. Parenteau, D. Bogdanovic, J. Mihowich, and E. W. Rud. 1998. Presence of circulating CTL induced by infection with wild-type or attenuated SIV and their correlation with protection from pathogenic SHIV challenge. J. Med. Primatol. 27:65-72. [DOI] [PubMed] [Google Scholar]
- 99.Wang, S. W., P. A. Kozlowski, G. Schmelz, K. Manson, M. S. Wyand, R. Glickman, D. Montefiori, J. D. Lifson, R. P. Johnson, M. R. Neutra, and A. Aldovini. 2000. Effective induction of simian immunodeficiency virus-specific systemic and mucosal immune responses in primates by vaccination with proviral DNA producing intact but noninfectious virions. J. Virol. 74:10514-10522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Weinhold, K. J., H. K. Lyerly, S. D. Stanley, A. A. Austin, T. J. Matthews, and D. P. Bolognesi. 1989. HIV-1 GP120-mediated immune suppression and lymphocyte destruction in the absence of viral infection. J. Immunol. 142:3091-3097. [PubMed] [Google Scholar]
- 101.Whatmore, A. M., N. Cook, G. A. Hall, S. Sharpe, E. W. Rud, and M. P. Cranage. 1995. Repair and evolution of nef in vivo modulates simian immunodeficiency virus virulence. J. Virol. 69:5117-5123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wyand, M. S., K. Manson, D. C. Montefiori, J. D. Lifson, R. P. Johnson, and R. C. Desrosiers. 1999. Protection by live, attenuated simian immunodeficiency virus against heterologous challenge. J. Virol. 73:8356-8363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wyand, M. S., K. H. Manson, M. M. Garcia, D. Montefiori, and R. C. Desrosiers. 1996. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J. Virol. 70:3724-3733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Xiong, Y., M. A. Luscher, J. D. Altman, M. Hulsey, H. L. Robinson, M. Ostrowski, B. H. Barber, and K. S. MacDonald. 2001. Simian immunodeficiency virus (SIV) infection of a rhesus macaque induces SIV-specific CD8+ T cells with a defect in effector function that is reversible on extended interleukin-2 incubation. J. Virol. 75:3028-3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Yang, N. S., J. Burkholder, B. Roberts, B. Martinell, and D. McCabe. 1990. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc. Natl. Acad. Sci. USA 87:9568-9572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Yasutomi, Y., K. A. Reimann, C. I. Lord, M. D. Miller, and N. L. Letvin. 1993. Simian immunodeficiency virus-specific CD8+ lymphocyte response in acutely infected rhesus monkeys. J. Virol. 67:1707-1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zeitz, M., R. Ullrich, T. Schneider, S. Kewenig, and E. Riecken. 1998. Mucosal immunodeficiency in HIV/SIV infection. Pathobiology 66:151-157. [DOI] [PubMed] [Google Scholar]