Abstract
Respiratory syncytial virus (RSV) is the most important virus mediating lower respiratory tract illness in infants and young children. RSV infection is associated with pulmonary inflammation and increased levels of substance P (SP), making the airways and leukocytes that express SP receptors susceptible to the proinflammatory effects of this peptide. This study examines combining neutralizing anti-F glycoprotein and anti-SP antibody treatment of RSV-infected BALB/c mice to inhibit RSV replication and inflammation associated with infection. BALB/c mice were prophylactically treated with antibody prior to RSV infection or were therapeutically treated at day 2 or 6 post-RSV infection. Prophylactic or therapeutic treatment with anti-SP antibodies promptly reduced pulmonary inflammatory cell infiltration and decreased the number of cells expressing proinflammatory cytokines, while anti-F antibody treatment reduced virus titers. The results suggest that combined anti-viral and anti-SP antibody treatment may be effective in treating RSV disease.
Respiratory syncytial virus (RSV) is the leading cause of lower respiratory tract disease in infants and young children worldwide. A member of the Paramyxoviridae family, RSV is an enveloped virus containing a negative-sense single-stranded RNA genome. The protective immune response to RSV infection is primarily directed against the two major surface viral glycoproteins, i.e., the G (attachment) and F (fusion) glycoproteins. The F glycoprotein appears to be most important for induction of protective immunity and is associated with a high serum neutralizing antibody response (6, 37) and activation of CD14 and Toll-like receptor-4 (21). Some monoclonal antibodies against the F glycoprotein provide passive protection against RSV disease (8, 13, 18, 42); therefore, the F glycoprotein has been the focus for therapeutic intervention in RSV disease.
At present, there is no RSV vaccine available, and the only options to address disease are prophylactic administration of enriched anti-RSV human immune globulin (Respigam) or anti-F glycoprotein monoclonal antibodies (palivizumab [Synagis]), both of which are recommended only for young children at high risk for RSV disease. In addition, ribavirin (Virazole), the only specific antiviral agent approved for RSV infection, has limited efficacy (10, 19, 41; M. I. Marks and J. McBride, abstract from Ribavirin Therapy for Respiratory Syncytial Virus Infections: a Scientific Workshop, Sept., 1989, Pediatr. Infect. Dis. J. 9:S84, 1990), and its use is limited for treatment of RSV infection in immune-compromised patients (10, 43).
Treatment with anti-RSV human immune globulin or anti-F glycoprotein neutralizing antibodies is effective in decreasing the titer of virus but does not appear to ameliorate the disease process, suggesting that a substantial portion of disease is associated with the host response to infection (27). The importance of the host response to infection is also suggested by the prominence of obstructed-airway disease and wheezing during RSV infection (reminiscent of asthma), the fact that serious disease can occur with repeated infections, and the occurrence of enhanced disease in younger children vaccinated with formalin-inactivated vaccine during subsequent RSV infection.
One inflammatory mediator associated with inflammation is the tachykinin neuropeptide substance P (SP) (40). SP is produced by afferent neurons and a variety of immune cells, including eosinophils, monocytes, macrophages (17, 30), lymphocytes (9), and dendritic cells (23). Numerous studies have directly associated SP with exacerbated inflammation (22, 25, 26, 29, 31, 32, 44). SP has been shown to affect inflammation by mediating vasodilation, thereby enhancing cell trafficking, as well as by affecting the cellular events involved in proliferation and cytokine and growth factor synthesis (3-5, 7, 11, 24, 28, 36). A recent study from our laboratory showed that RSV infection of BALB/c mice increases pulmonary SP levels, and these increased levels of SP exacerbated pulmonary inflammation (40). In that study, treatment of RSV-infected mice with anti-SP antibody decreased pulmonary inflammatory cells and proinflammatory cytokine expression (40). Similarly, RSV-infected rats have been shown to upregulate SP receptors in the lungs, an effect that was associated with increased pulmonary inflammation (20, 33). These findings suggest that SP might be important for RSV pathogenesis, and inhibiting SP might reduce RSV-associated inflammation.
In this study, we examine the effectiveness of combining antiviral treatment with a neutralizing anti-F glycoprotein monoclonal antibody with anti-SP antibody. The results show that prophylactic or therapeutic treatment with anti-SP markedly reduces pulmonary inflammation, suggesting that anti-SP antibodies should be considered as an adjunct to antiviral treatment to reduce RSV disease.
MATERIALS AND METHODS
Animals.
Six- to 8-week-old, specific-pathogen-free female BALB/c mice (Jackson Laboratory, Bar Harbor, Maine) were used in all experiments. The mice were housed in microisolator cages and were fed sterilized water and food ad libitum. All studies were performed in accordance with the guidelines of the Institutional Animal Care and Use Committee.
Virus and infection.
The A2 strain of RSV was used in all experiments and propagated in Vero cells (ATCC CCL 881) as previously described (39). Mice were anesthetized by intraperitoneal administration of avertin (2,2,2-tribromoethanol; 0.2 ml/g of body weight; Sigma-Aldrich, St. Louis, Mo.), and intranasally challenged with 106 PFU of RSV in Dulbecco's PBS (GIBCO Laboratories, Grand Island, N.Y.). No fewer than three mice per treatment were examined per time point.
Antibodies and treatment.
On day −1 prior to infection or day 2 or 6 postinfection (p.i.), mice were intraperitoneally treated with 150 μg of anti-F glycoprotein monoclonal antibody (anti-F)/mouse (1), rabbit anti-SP F(ab)2 antibody (Accurate Chemical and Scientific Corp., Westbury, N.Y.), or both anti-SP F(ab)2 and anti-F glycoprotein antibodies (anti-SP/F; 150 μg of each antibody/mouse) as previously described (40). Control mice were treated with 150 μg of normal rabbit F(ab)2 immunoglobulin (nIg) (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA)/mouse, purified mouse IgG1 isotype antibody (S1-68.1) (Pharmingen, San Diego, Calif.), or a combination of both control antibodies. The anti-F antibody (clone 143-6C) used in these studies is similar to palivizumab, i.e., it recognizes the same region on the F glycoprotein and both neutralize and inhibit RSV infection (1).
Quantitation of SP.
SP levels in cell-free bronchoalveolar lavage fluid were analyzed using a competitive enzyme-linked immunoassay kit (Cayman Chemical, Ann Arbor, Mich.) in accordance with the manufacturer's instructions as described previously (40). The assay is based on the competition between free SP and an SP tracer for a limited number of SP-specific binding sites. The percent sample bound per maximum bound was calculated, and the SP concentration of each sample was determined based on the percent standard bound per maximum bound versus the standard SP concentration. The intra- and interassay coefficients of variation were ≤10%. The rabbit anti-SP F(ab2) antibody used in vivo does not interfere with the antibodies used to detect SP in the competitive enzyme-linked immunoassay.
Cell collection and analysis.
Mice were anesthetized with Avertin and exsanguinated by severing the right caudal artery. Bronchoalveolar leukocytes (BAL) were harvested by lavaging the lungs with PBS. The procedure used for extracellular staining of BAL was modified for microculture staining as described previously (39). Briefly, BAL were washed in Dulbecco's PBS (GIBCO) containing 1% bovine serum albumin and then stained (4°C; 30 min) with an appropriate dilution of the fluorescein isothiocyanate-conjugated or phycoerythrin-conjugated antibodies anti-CD3ɛ (145-2C11), anti-CD45R/B220 (RA3-6B2), anti-CD8 (Ly-2), anti-neutrophil (polymorphonuclear leukocyte [PMN]) (RB6-8C5), and anti-CD11b (M1/70) and isotype antibody controls (Pharmingen). Intracellular cytokine staining was modified for microculture staining as described previously (39). Briefly, BAL were incubated in PBS containing Golgi Stop (Pharmingen) for 3 h at 37°C to allow accumulation of intracellular cytokines. The cells were washed in PBS and stained (4°C; 30 min) with an appropriate dilution of anti-CD3 antibody, fixed, and permeabilized in Cytofix/Cytoperm (Pharmingen). The cells were washed in Cytofix/Cytoperm buffer and stained (4°C; 30 min) with appropriate dilutions of anti-interleukin 2 (IL-2) (JES6-5H4), anti-IL-4 (BVD4-1D11), anti-IL-5 (TRFK5), anti-IL-10 (JES3-16E3), anti-gamma interferon (IFN-γ) (XMG1.2), or anti-tumor necrosis factor alpha (TNF-α; MP6-XT22) antibody (Pharmingen) diluted in PBS containing Cytofix/Cytoperm as described previously (39). Extra- and intracellular staining was analyzed using a FACScan and Cell Quest software (Becton Dickinson, San Diego, Calif.). The total number of CD3+ BAL expressing a particular cytokine was determined by multiplying the total number of BAL by the percent CD3+ cells expressing that particular cytokine.
Virus titers.
Virus titers in the lungs of RSV-infected mice were determined as previously described (39). Briefly, lungs were aseptically removed from three to five mice per group on days 3, 5, 7, and 9 p.i. and stored at −70°C until they were assayed. Identical weights (0.1 g) of individual lung samples were homogenized in 1 ml of Dulbecco's PBS, and 10-fold serial dilutions of the lung homogenates were added to confluent Vero cell monolayers. Following adsorption (2 h; 37°C), the cell monolayers were overlaid with Dulbecco's modified Eagle's medium (GIBCO) containing 10% fetal bovine serum (HyClone, Logan, Utah) and incubated at 37°C for 3 to 4 days. Plaques were enumerated by immunostaining them with monoclonal antibodies against the G and F glycoproteins (130-2G and 131-2A, respectively).
Statistical analysis.
Statistical significance was determined using Student's t test, where a P value of <0.05 was considered statistically significant.
RESULTS
Prophylactic treatment of RSV.
Prophylactic treatment 1 day prior to RSV infection with anti-SP or anti-SP/F antibody was associated with a substantial decrease in pulmonary inflammatory cells compared to nIg-treated mice (Fig. 1). For example, treatment with anti-SP or anti-SP/F antibody decreased the total number of BAL in the lung by ∼50% on day 3 p.i. and by ∼75% on day 5 p.i. (the peak of virus replication [see Fig. 5]) compared to nIg antibody treatment (Fig. 1A). By day 7 p.i., the numbers of cells were similar for all treatments. For the leukocyte subsets, the decrease was not always evident on day 3 p.i. but was most evident on day 5 p.i. Anti-SP/F antibody treatment reduced the total number of CD8+ (P ≤ 0.05 [Fig. 1B]), CD4+ (Fig. 1C), B220+ (P ≤ 0.05 [Fig. 1D]), and CD11b+ (P ≤ 0.05 [Fig. 1E]) cells and PMN (P ≤ 0.05 [Fig. 1F]) 6- to 10-fold by day 5 p.i. compared to nIg-treated mice. In contrast, treatment with anti-F antibody alone was associated with an increase in CD8+ and CD11b+ cells on day 3 p.i. and had minimal effect on the leukocyte subsets at later time points (Fig. 1).
FIG. 1.
Prophylactic treatment. Shown are flow cytometry results following prophylactic treatment (day −1 prior to infection) of mice with anti-SP and anti-F antibodies. (A) Total pulmonary leukocyte trafficking. (B to F) BAL were stained with antibodies against CD8+ (B), CD4+ (C), B220+ (D), and CD11b+ (E) cells and PMN (RB6-8C5+) (F). The data are expressed as the mean number (103) of BAL/lung (± SEM) on days 3, 5, and 7 p.i. from three independent experiments. Asterisks indicate a significant difference (P < 0.05) between nIg-treated and antibody-treated mice.
FIG. 5.
SP levels in BAL. Mice were treated on day −1 prior to infection (A) or day 2 (B) or day 6 (C) p.i. with either nIg, anti-SP, anti-F, or anti-SP/F antibody. Cell-free BAL lavage fluid was harvested on days 3, 5, 7, and 9 p.i. and examined for levels of SP by enzyme-linked immunosorbent assay. The dashed lines represent the mean baseline SP concentration in the BAL from naïve mice. The data are expressed as the SP concentration (in picograms per milliliter) (+ SEM).
Therapeutic treatment of RSV infection.
The results for anti-SP antibody prophylaxis suggested that similar treatment might be therapeutically effective during RSV infection. Early anti-SP or anti-SP/F antibody treatment on day 2 p.i., prior to the period of maximal viral replication (day 5 p.i. [see Fig. 4]), led to a prompt and marked decrease of total pulmonary cells compared to nIg antibody-treated mice (Fig. 2A). One day after antibody treatment, the numbers for all cell subsets were decreased (P < 0.05) compared to anti-F or nIg antibody-treated mice. Treatment with anti-F antibody, relative to nIg, was associated with some reduction of CD8+, B220+, and CD11b+ cells and PMN, but not CD4+ cells, on day 3 p.i. (Fig. 2B and D to F). By day 5 p.i., anti-F antibody treatment alone was associated with a pronounced decrease in CD8+ cells and a modest decrease in the other leukocyte subsets.
FIG. 4.
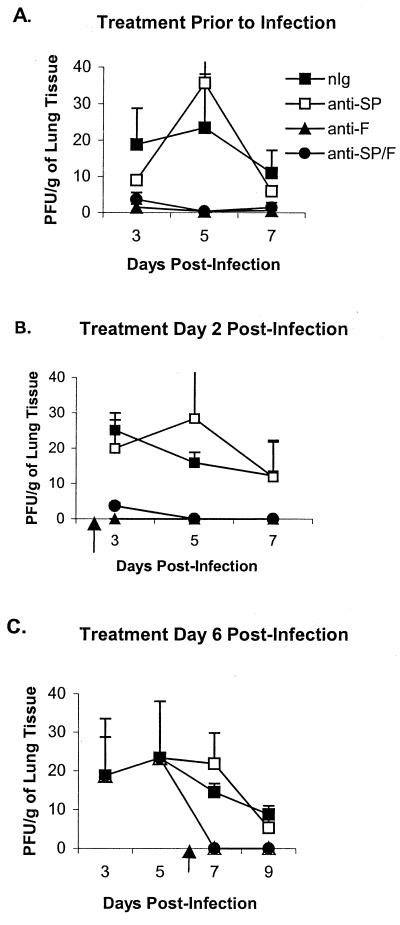
RSV lung titers following antibody treatment. The lungs of antibody treated-mice were harvested on days 3, 5, 7, and 9 post-RSV infection. (A) Prophylactic antibody treatment 1 day prior to infection. (B) Early therapeutic antibody treatment on day 2 p.i. (C) Late therapeutic antibody treatment on day 6 p.i. The results are expressed as 103 PFU/g (+ SEM).
FIG. 2.
Early therapeutic treatment. Shown are flow cytometry results following early (day 2 p.i.) therapeutic treatment of mice with anti-SP and anti-F antibodies. (A) Total pulmonary leukocyte trafficking. (B to F) BAL were stained with antibodies against CD8+ (B), CD4+ (C), B220+ (D), and CD11b+ (E) cells and PMN (RB6-8C5+) (F). The data are expressed as the number (103) of BAL/lung (± SEM) on days 3, 5, and 7 p.i. A representative experiment from two independent experiments is shown. Asterisks indicate a significant difference (P < 0.05) between nIg-treated and antibody-treated mice.
Late anti-SP antibody treatment in RSV infection was also effective (Fig. 3). Administration of anti-SP or anti-SP/F antibodies on day 6 p.i. reduced the total number of cells infiltrating the lung 1 day after treatment (Fig. 3A). Antibody treatment decreased the numbers of all leukocyte subsets examined on day 7 p.i. compared to both nIg and anti-F antibody-treated mice (Fig. 3B to F).
FIG. 3.
Late therapeutic treatment. Shown are flow cytometry results following late (day 6 p.i.) therapeutic treatment of mice with anti-SP and anti-F antibodies. (A) Total pulmonary leukocyte trafficking. (B to F) BAL were stained with antibodies against CD8+ (B), CD4+ (C), B220+ (D), and CD11b+ (E) cells and PMN (RB6-8C5+) (F). The data are expressed as the number (103) of BAL/lung (± SEM) on days 3, 5, 7, and 9 p.i. A representative experiment from two independent experiments is shown. Asterisks indicate a significant difference (P < 0.05) between nIg-treated and antibody-treated mice.
Cytokine expression after anti-SP and anti-F antibody treatments.
Similar to cell numbers, the levels of intracellular cytokine expression were markedly decreased by anti-SP or anti-SP/F antibody treatment (Tables 1, 2, and 3). This decrease is most easily seen by examining the percent decrease of CD3+ BAL for the different treatments. In mice treated prophylactically with anti-SP or anti-SP/F antibody, cytokine expression on days 3 and 5 p.i. was inhibited 56 to 87% (Table 1). By day 7 p.i., this inhibition was less distinct and ranged from 16 to 62%. Anti-F antibody treatment also reduced cytokine expression, but the effect was less than that observed with anti-SP/F antibody treatment (Table 1). For mice treated therapeutically on day 2 p.i. with anti-SP or anti-SP/F antibody, there was not a significant decrease in cytokine expression on day 3 p.i., except for IFN-γ and TNF-α in the anti-SP-treated mice; however, all treatment groups showed a decrease compared to nIg treatment on day 5 p.i. The decrease on day 5 p.i. was greater when anti-SP antibodies were included in the treatment (57 to 83%) than when they were not (anti-F treatment, 22 to 55%) (Table 2). On day 7 p.i., the greatest decrease in cytokine expression was again most evident with treatments that included anti-SP antibodies, and the decrease following all treatments was greatest for IL-4 and IFN-γ expression. Intracellular cytokine expression also decreased in mice treated with anti-SP or anti-SP/F antibody on day 6 p.i. (Table 3) and was greatest when anti-SP antibody was included in the treatment. Although treatment with anti-SP antibody reduced cytokine expression on day 7 p.i. (26 to 72%), the greatest reduction in cytokine expression was observed on day 9 p.i. (51 to 70%) for mice treated with anti-SP antibodies (Table 3).
TABLE 1.
Intracellular cytokine expression by CD3+ T cells following anti-SP and anti-F prophylactic antibody treatment prior to RSV infectiona
| Day | Cytokine | Total cytokine-expressing CD3+ BAL (103) ± SEMb
|
||||||
|---|---|---|---|---|---|---|---|---|
| nIg | anti-SP | % Reductionc | anti-F | % Reduction | anti-SP/F | % Reduction | ||
| 3 | IL-2 | 13.7 ± 4.2 | 3.8 ± 0.5 | 72 | 9.0 ± 1.5 | 34 | 4.8 ± 1.7 | 65 |
| IL-4 | 13.0 ± 4.0 | 3.8 ± 0.5 | 70 | 8.2 ± 1.3 | 37 | 4.5 ± 1.6 | 65 | |
| IL-5 | 12.7 ± 3.9 | 4.2 ± 0.6 | 67 | 9.5 ± 1.6 | 25 | 5.3 ± 1.8 | 58 | |
| IL-10 | 8.8 ± 2.7 | 2.2 ± 0.3 | 75 | 5.5 ± 0.9 | 38 | 2.5 ± 0.9 | 72 | |
| IFN-γ | 16.2 ± 5.0 | 5.1 ± 0.7 | 69 | 11.4 ± 1.9 | 30 | 6.4 ± 2.2 | 60 | |
| TNF-α | 19.3 ± 5.9 | 6.3 ± 0.9 | 67 | 15.6 ± 2.6 | 19 | 8.5 ± 3.0 | 56 | |
| 5 | IL-2 | 17.3 ± 5.5 | 2.2 ± 0.4 | 87 | 11.6 ± 1.4 | 33 | 4.9 ± 1.5 | 72 |
| IL-4 | 16.9 ± 5.4 | 2.5 ± 0.5 | 85 | 11.1 ± 1.3 | 34 | 5.5 ± 1.7 | 67 | |
| IL-5 | 18.2 ± 5.8 | 2.8 ± 0.5 | 85 | 12.3 ± 1.4 | 32 | 6.6 ± 2.0 | 64 | |
| IL-10 | 12.7 ± 4.1 | 1.8 ± 0.3 | 86 | 9.1 ± 1.1 | 28 | 4.0 ± 1.2 | 69 | |
| IFN-γ | 19.3 ± 6.1 | 3.1 ± 0.6 | 84 | 15.1 ± 1.8 | 22 | 6.4 ± 2.0 | 67 | |
| TNF-α | 27.8 ± 8.9 | 5.0 ± 0.9 | 82 | 20.2 ± 2.4 | 27 | 8.6 ± 2.6 | 69 | |
| 7 | IL-2 | 12.0 ± 3.0 | 4.8 ± 1.0 | 60 | 6.1 ± 0.6 | 49 | 6.7 ± 2.7 | 44 |
| IL-4 | 10.6 ± 2.7 | 4.0 ± 0.9 | 62 | 5.5 ± 0.5 | 48 | 6.4 ± 2.6 | 40 | |
| IL-5 | 8.9 ± 2.3 | 4.6 ± 1.0 | 48 | 7.0 ± 0.7 | 21 | 6.6 ± 2.7 | 26 | |
| IL-10 | 4.5 ± 1.1 | 2.5 ± 0.6 | 44 | 4.0 ± 0.4 | 11 | 3.8 ± 1.5 | 16 | |
| IFN-γ | 10.3 ± 2.6 | 5.4 ± 1.2 | 48 | 7.6 ± 0.7 | 26 | 7.6 ± 3.0 | 26 | |
| TNF-α | 21.2 ± 5.4 | 9.7 ± 2.1 | 54 | 3.8 ± 1.3 | 82 | 12.8 ± 5.2 | 40 | |
BALB/c mice were treated with control nIg, anti-SP, anti-F, or anti-SP/F antibodies 24 h prior to RSV infection. BAL samples from three mice per group were examined on days 3, 5, and 7 p.i. The results of three independent experiments are shown.
Data are represented as total CD3+ BAL expressing IL-2, -4, -5, or -10, IFN-γ, or TNF-α per lung ± SEM. The total number of CD3+ BAL expressing a particular cytokine was determined by multiplying the total number of BAL by the percent CD3+ cells expressing that cytokine.
Percent reduction is the change in total cytokine-expressing CD3+ cells after anti-SP, anti-F, or anti-SP/F treatment relative to total cytokine-expressing CD3+ cells after nIg treatment.
TABLE 2.
Intracellular cytokine expression by CD3+ T cells following anti-SP and anti-F therapeutic antibody treatment on day 2 Post-RSV infectiona
| Day | Cytokine | Total cytokine-expressing CD3+ BAL (103) ± SEMb
|
||||||
|---|---|---|---|---|---|---|---|---|
| nIg | anti-SP | % Reductionc | anti-F | % Reduction | anti-SP/F | % Reduction | ||
| 3 | IL-2 | 13.6 ± 3.1 | 15.3 ± 3.5 | 0 | 15.7 ± 1.8 | 0 | 12.5 ± 1.6 | 8 |
| IL-4 | 15.0 ± 3.5 | 14.3 ± 3.3 | 5 | 15.8 ± 1.8 | 0 | 11.8 ± 1.5 | 21 | |
| IL-5 | 13.5 ± 3.2 | 14.3 ± 3.3 | 6 | 14.0 ± 1.6 | 0 | 10.1 ± 1.3 | 25 | |
| IL-10 | 10.2 ± 2.4 | 11.4 ± 2.6 | 12 | 12.5 ± 1.4 | 0 | 8.4 ± 1.1 | 18 | |
| IFN-γ | 16.7 ± 3.9 | 7.6 ± 1.8 | 54 | 16.7 ± 1.9 | 0 | 13.9 ± 1.8 | 17 | |
| TNF-α | 16.1 ± 3.7 | 7.3 ± 1.7 | 55 | 16.2 ± 1.9 | 0 | 12.8 ± 3.2 | 21 | |
| 5 | IL-2 | 10.2 ± 0 | 4.4 ± 0.9 | 57 | 5.3 ± 0.6 | 48 | 2.7 ± 0.5 | 74 |
| IL-4 | 17.3 ± 0 | 3.3 ± 0.6 | 81 | 9.1 ± 1.1 | 53 | 4.3 ± 0.8 | 75 | |
| IL-5 | 14.3 ± 0 | 2.6 ± 0.5 | 82 | 8.0 ± 0.9 | 44 | 3.5 ± 0.7 | 75 | |
| IL-10 | 11.3 ± 0 | 1.9 ± 0.4 | 83 | 5.1 ± 0.6 | 55 | 2.3 ± 0.4 | 80 | |
| IFN-γ | 16.9 ± 0 | 4.2 ± 0.8 | 75 | 13.2 ± 1.5 | 22 | 5.4 ± 1.0 | 68 | |
| TNF-α | 25.0 ± 0 | 5.8 ± 1.1 | 77 | 13.8 ± 1.6 | 45 | 5.8 ± 1.1 | 77 | |
| 7 | IL-2 | 4.0 ± 0.3 | 3.0 ± 0.7d | 25 | 4.8 ± 1.2 | 0 | 2.4 ± 0.3e | 40 |
| IL-4 | 7.5 ± 0.6 | 1.6 ± 0.3f | 79 | 4.2 ± 1.1g | 44 | 2.6 ± 0.3h | 65 | |
| IL-5 | 5.0 ± 0.4 | 3.5 ± 0.8i | 30 | 4.5 ± 1.1 | 10 | 2.2 ± 0.3j | 56 | |
| IL-10 | 2.5 ± 0.2 | 1.6 ± 0.3k | 36 | 2.7 ± 0.7 | 0 | 1.6 ± 0.2l | 35 | |
| IFN-γ | 11.2 ± 0.9 | 5.5 ± 1.2m | 51 | 7.6 ± 2.0n | 32 | 4.0 ± 0.5o | 64 | |
| TNF-α | 10.4 ± 0.8 | 6.6 ± 1.4p | 37 | 8.7 ± 2.2q | 16 | 4.2 ± 0.5r | 60 | |
BALB/c mice were infected with RSV. Two days p.i., the animals were i.p. treated with 150 μg of nIg, anti-SP, anti-F, or anti-SP/F antibodies/mouse. BAL samples from three mice per group were examined on days 3, 5, and 7 p.i. The results of a representative experiment are shown.
Data are represented as the total CD3+ BAL expressing IL-2, -4, -5, or -10; IFN-γ, or TNF-α per lung ± SEM. The total number of CD3+ BAL expressing a particular cytokine was determined by multiplying the total number of BAL by the percent CD3+ cells expressing that cytokine. Boldface values indicate a significant difference.
Percent reduction is the change in total cytokine-expressing CD3+ cells after anti-SP, anti-F, or anti-SP/F treatment relative to total cytokine-expressing CD3+ cells after nIg treatment.
P value comparing nIg control to anti-SP; P = 0.150.
P value comparing nIg control to anti-SP/F; P = 0.017.
P value comparing nIg control to anti-SP; P = 0.009.
P value comparing nIg control to anti-F; P = 0.054.
P value comparing nIg control to anti-SP/F; P = 0.002.
P value comparing nIg control to anti-SP; P = 0.077.
P value comparing nIg control to anti-SP/F; P = 0.003.
P value comparing nIg control to anti-SP; P = 0.017.
P value comparing nIg control to anti-SP/F; P = 0.031.
P value comparing nIg control to anti-SP; P = 0.018.
P value comparing nIg control to anti-F; P = 0.117.
P value comparing nIg control to anti-SP/F; P = 0.002.
P value comparing nIg control to anti-SP; P = 0.084.
P value comparing nIg control to anti-F; P = 0.110.
P value comparing nIg control to anti-SP/F; P = 0.003.
TABLE 3.
Intracellular cytokine expression by CD3+ T cells following anti-SP and anti-F therapeutic antibody treatment on day 6 post-RSV infectiona
| Day | Cytokine | Total cytokine-expressing CD3+ BAL (103) ± SEMb
|
||||||
|---|---|---|---|---|---|---|---|---|
| nIg | anti-SP | % Reductionc | anti-F | % Reduction | anti-SP/F | % Reduction | ||
| 7 | IL-2 | 9.7 ± 2.5 | 6.8 ± 1.1 | 30 | 8.4 ± 2.1 | 13 | 7.2 ± 2.2 | 26 |
| IL-4 | 11.1 ± 2.8 | 7.3 ± 1.2 | 34 | 7.7 ± 1.9 | 31 | 6.2 ± 1.9 | 44 | |
| IL-5 | 12.3 ± 3.1 | 6.6 ± 1.1 | 46 | 5.3 ± 1.3 | 57 | 5.2 ± 1.6 | 58 | |
| IL-10 | 6.7 ± 1.7 | 4.8 ± 0.8 | 28 | 13.7 ± 3.4 | 0 | 2.3 ± 0.7 | 66 | |
| IFN-γ | 16.0 ± 4.0 | 8.7 ± 1.4 | 46 | 13.8 ± 3.5 | 14 | 8.0 ± 2.5 | 50 | |
| TNF-α | 31.0 ± 7.9 | 10.0 ± 1.7 | 68 | 13.5 ± 3.4 | 56 | 8.8 ± 2.7 | 72 | |
| 9 | IL-2 | 11.7 ± 2.7 | 3.5 ± 0.7d | 70 | 7.1 ± 2.8 | 39 | 4.5 ± 1.3e | 62 |
| IL-4 | 10.3 ± 2.4 | 4.2 ± 0.8 | 59 | 6.0 ± 2.3 | 41 | 4.6 ± 1.3 | 55 | |
| IL-5 | 10.0 ± 2.4 | 4.2 ± 0.8 | 58 | 6.5 ± 2.5 | 35 | 4.9 ± 1.4 | 51 | |
| IL-10 | 7.8 ± 1.8 | 3.1 ± 0.6 | 60 | 4.5 ± 1.7 | 42 | 3.1 ± 0.9 | 60 | |
| IFN-γ | 13.9 ± 3.3 | 5.7 ± 1.1 | 59 | 8.8 ± 3.4 | 37 | 6.4 ± 1.9 | 54 | |
| TNF-α | 29.2 ± 6.9 | 10.5 ± 2.0 | 64 | 18.4 ± 7.1 | 37 | 12.4 ± 3.6 | 58 | |
BALB/c mice were infected with RSV. Six days p.i., the animals were i.p. treated with nIg, anti-SP, anti-F, or anti-SP/F antibodies. BAL samples from three mice per group were examined on days 3, 5, 7, and 9 p.i. The results of a representative experiment are shown.
Data are represented as total CD3+ BAL expressing IL-2, -4, -5, or -10, IFN-γ, or TNF-α per lung ± SEM. The total number of CD3+ BAL expressing a particular cytokine was determined by multiplying the total number of BAL by the percent CD3+ cells expressing that cytokine. Boldface values indicate a significant difference.
Percent reduction is the change in total cytokine-expressing CD3+ cells after anti-SP, anti-F, or anti-SP/F treatment relative to total cytokine-expressing CD3+ cells after nIg treatment.
P value comparing nIg control to anti-SP; P = 0.042.
P value comparing nIg control to anti-SP; P = 0.073.
Virus replication and SP levels.
As expected, administration of anti-F antibodies alone markedly decreased the titer of virus in the lungs. Treatment with anti-SP antibodies did not alter the titer of the virus recovered (Fig. 4), i.e., the titer of virus in mice treated with anti-SP antibodies was not significantly different from the titer of nIg-treated mice, and the titer of virus in mice treated with anti-SP/F antibody was not significantly different from that in mice treated with anti-F antibody alone. As previously shown, SP levels increased with RSV infection from a baseline level in naïve mice ranging from 3 to 5 (Fig. 5) to 70 pg/ml on day 5 p.i. before decreasing to 45 pg/ml on day 7 p.i. (Fig. 5A). Administration of anti-SP antibodies reduced detectable SP levels 53 to 88% compared to nIg-treated mice. Of note, treatment with anti-F antibodies on days 1 (Fig. 5A) and 2 (Fig. 5B) p.i. also decreased levels of SP, presumably by decreasing virus replication. Despite the decrease in SP levels after treatment with anti-F antibodies, there was only a marginal decrease in pulmonary cell infiltration, suggesting that other factors may also contribute to the inflammatory response associated with RSV infection.
DISCUSSION
In the absence of a safe and effective RSV vaccine, an effective RSV treatment could be of substantial benefit to patients of any age with serious RSV disease. In the immunologically normal patient, it is likely that anti-inflammatory agents will be needed in conjunction with an anti-viral drug, immune globulin, or monoclonal antibody treatment to decrease RSV disease. Immunosuppressive medications, particularly steroids, have a significant role in the management of many pediatric illnesses. Several steroid-based agents have been examined for treating inflammation associated with respiratory virus infections; however, all suppressed the immune response and negatively affected virus clearance. In one animal study, topical treatment of RSV-infected cotton rats with triamcinolone, a synthetic glucosteroid, reduced pulmonary pathology but resulted in delayed virus clearance (34). In another study by this group, triamcinolone treatment of parainfluenza virus-3-infected cotton rats also reduced pulmonary pathology but resulted in a 10-fold increase in virus titers (35). Similarly, hydrocortisone treatment of pneumovirus-infected BALB/c mice had a deleterious effect of enhancing viral replication and accelerating mortality (12). Prolonged virus shedding has also been shown in clinical studies of RSV-infected children with compromised immune function due to steroid therapy (16).
The concept of combining nonsteroidal anti-inflammatory treatment with antiviral agents has been examined for nonrespiratory viruses, including rhinovirus-induced rhinitis (15) and herpes simplex virus keratitis (14). In one study, treatment of rhinovirus infections with the anti-viral agent IFN-α and two anti-inflammatory agents (ipratropium and naproxen) reduced the overall symptoms and disease severity in treated individuals (15). Likewise, combined treatment of herpetic stromal keratitis with acyclovir and cyclosporin A reduced stromal infiltration and the severity of stromal disease (14).
An alternative target for reducing inflammation associated with respiratory virus infection may be SP. SP has been shown to be an important contributor to airway inflammation and disease. Recent data from animal studies show that RSV upregulates the SP receptor (20, 33), and elevated levels of SP have been detected in patients with asthma and obstructive airway disease (38). Previously, we observed elevated pulmonary SP levels associated with RSV infection in BALB/c mice and showed that treatment with anti-SP antibodies was effective in reducing the number of inflammatory cells and proinflammatory cytokine expression (40). In this study, we demonstrate the effectiveness of combining anti-SP antibody with a neutralizing anti-F antibody in controlling both virus replication and RSV-associated inflammation. The anti-F monoclonal IgG antibody used in this study (143-6C), recognizes the same antigenic site (site A) as the anti-F monoclonal antibody used for prophylaxis in humans (palivizumab) (2, 18). As expected, this neutralizing monoclonal antibody inhibited virus replication when given as prophylactic or therapeutic treatment, and as expected from previous studies, anti-F antibody treatment was inadequate at reducing pulmonary inflammation. In contrast, both prophylactic and therapeutic treatment with anti-SP antibodies promptly reduced the number of inflammatory cells, and unlike steroid therapy, it did not prolong virus replication. In addition, treatment with anti-SP antibodies decreased the number of cells expressing proinflammatory cytokines.
In summary, this study shows that the anti-SP antibodies can effectively reduce inflammation and suggests that anti-SP treatment may complement the antiviral effects of neutralizing anti-F antibody treatment or antiviral drugs to provide an effective treatment for RSV disease.
Acknowledgments
This research was supported in part by an appointment to the Research Participation Program at the Centers for Disease Control and Prevention, National Center for Infectious Diseases, Division of Viral and Rickettsial Diseases, administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Centers for Disease Control and Prevention.
We thank Les Jones (Centers for Disease Control and Prevention, Atlanta, Ga.) for technical assistance.
REFERENCES
- 1.Anderson, L. J., P. Bingham, and J. C. Hierholzer. 1988. Neutralization of respiratory syncytial virus by individual and mixtures of F and G protein monoclonal antibodies. J. Virol. 62:4232-4238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beeler, J. A., and K. van Wyke Coelingh. 1989. Neutralization epitopes of the F glycoprotein of respiratory syncytial virus: effect of mutation upon fusion function. J. Virol. 63:2941-2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berczi, I., I. M. Chalmers, E. Nagy, and R. J. Warrington. 1996. The immune effects of neuropeptides. Baillieres Clin. Rheumatol. 10:227-257. [DOI] [PubMed] [Google Scholar]
- 4.Berman, A. S., C. Chancellor-Freeland, G. Zhu, and P. H. Black. 1996. Substance P primes murine peritoneal macrophages for an augmented proinflammatory cytokine response to lipopolysaccharide. Neuroimmunomodulation 3:141-149. [DOI] [PubMed] [Google Scholar]
- 5.Calvo, N., J. Reiriz, E. Perez-Navarro, and J. Alberch. 1996. Tachykinins protect cholinergic neurons from quinolinic acid excitotoxicity in striatal cultures. Brain Res. 740:323-328. [DOI] [PubMed] [Google Scholar]
- 6.Connors, M., P. L. Collins, C. Y. Firestone, and B. R. Murphy. 1991. Respiratory syncytial virus (RSV) F, G, M2 (22K), and N proteins each induce resistance to RSV challenge, but resistance induced by M2 and N proteins is relatively short-lived. J. Virol. 65:1634-1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Covas, M. J., L. A. Pinto, and R. M. Victorino. 1994. Disturbed immunoregulatory properties of the neuropeptide substance P on lymphocyte proliferation in HIV infection. Clin. Exp. Immunol. 96:384-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Crowe, J. E., Jr., B. R. Murphy, R. M. Chanock, R. A. Williamson, C. F. Barbas III, and D. R. Burton. 1994. Recombinant human respiratory syncytial virus (RSV) monoclonal antibody Fab is effective therapeutically when introduced directly into the lungs of RSV-infected mice. Proc. Natl. Acad. Sci. USA 91:1386-1390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Giorgio, R., P. L. Tazzari, G. Barbara, V. Stanghellini, and R. Corinaldesi. 1998. Detection of substance P immunoreactivity in human peripheral leukocytes. J. Neuroimmunol. 82:175-181. [DOI] [PubMed] [Google Scholar]
- 10.De Vincenzo, J. P., D. Leombruno, R. J. Soiffer, and G. R. Siber. 1996. Immunotherapy of respiratory syncytial virus pneumonia following bone marrow transplantation. Bone Marrow Transplant. 17:1051-1056. [PubMed] [Google Scholar]
- 11.Dickerson, C., B. Undem, B. Bullock, and R. A. Winchurch. 1998. Neuropeptide regulation of proinflammatory cytokine responses. J. Leukoc. Biol. 63:602-605. [DOI] [PubMed] [Google Scholar]
- 12.Domachowske, J. B., C. A. Bonville, D. Ali-Ahmad, K. D. Dyer, A. J. Easton, and H. F. Rosenberg. 2001. Glucocorticoid administration accelerates mortality of pneumovirus-infected mice. J. Infect. Dis. 184:1518-1523. [DOI] [PubMed] [Google Scholar]
- 13.Fisher, R. G., J. E. Crowe, Jr., T. R. Johnson, Y. W. Tang, and B. S. Graham. 1999. Passive IgA monoclonal antibody is no more effective than IgG at protecting mice from mucosal challenge with respiratory syncytial virus. J. Infect. Dis. 180:1324-1327. [DOI] [PubMed] [Google Scholar]
- 14.Gunduz, K., and O. Ozdemir. 1997. Topical cyclosporin as an adjunct to topical acyclovir treatment in herpetic stromal keratitis. Ophthalmol. Res. 29:405-408. [DOI] [PubMed] [Google Scholar]
- 15.Gwaltney, J. M., Jr. 1992. Combined antiviral and antimediator treatment of rhinovirus colds. J. Infect. Dis. 166:776-782. [DOI] [PubMed] [Google Scholar]
- 16.Hall, C. B., K. R. Powell, N. E. MacDonald, C. L. Gala, M. E. Menegus, S. C. Suffin, and H. J. Cohen. 1986. Respiratory syncytial viral infection in children with compromised immune function. N. Engl. J. Med. 315:77-81. [DOI] [PubMed] [Google Scholar]
- 17.Ho, W. Z., J. P. Lai, X. H. Zhu, M. Uvaydova, and S. D. Douglas. 1997. Human monocytes and macrophages express substance P and neurokinin-1 receptor. J. Immunol. 159:5654-5660. [PubMed] [Google Scholar]
- 18.Johnson, S., C. Oliver, G. A. Prince, V. G. Hemming, D. S. Pfarr, S. C. Wang, M. Dormitzer, J. O'Grady, S. Koenig, J. K. Tamura, R. Woods, G. Bansal, D. Couchenour, E. Tsao, W. C. Hall, and J. F. Young. 1997. Development of a humanized monoclonal antibody (MEDI-493) with potent in vitro and in vivo activity against respiratory syncytial virus. J. Infect. Dis. 176:1215-1224. [DOI] [PubMed] [Google Scholar]
- 19.Khoshoo, V., and D. Edell. 2000. Ribavarin in ventilated respiratory syncytial virus bronchiolitis: a randomized, placebo-controlled trial. Am. J. Respir. Crit. Care Med. 162:333-334. [DOI] [PubMed] [Google Scholar]
- 20.King, K. A., C. Hu, M. M. Rodriguez, R. Romaguera, X. Jiang, and G. Piedimonte. 2001. Exaggerated neurogenic inflammation and substance P receptor upregulation in RSV-infected weanling rats. Am. J. Respir. Cell Mol. Biol. 24:101-107. [DOI] [PubMed] [Google Scholar]
- 21.Kurt-Jones, E. A., L. Popova, L. Kwinn, L. M. Haynes, L. P. Jones, R. A. Tripp, E. E. Walsh, M. W. Freeman, D. T. Golenbock, L. J. Anderson, and R. W. Finberg. 2000. Pattern recognition receptors TLR4 and CD14 mediate response to respiratory syncytial virus. Nat. Immunol. 1:398-401. [DOI] [PubMed] [Google Scholar]
- 22.Lambert, N., P. L. Lescoulie, B. Yassine-Diab, G. Enault, B. Mazieres, C. De Preval, and A. Cantagrel. 1998. Substance P enhances cytokine-induced vascular cell adhesion molecule-1 (VCAM-1) expression on cultured rheumatoid fibroblast-like synoviocytes. Clin. Exp. Immunol. 113:269-275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambrecht, B. N., P. R. Germonpre, E. G. Everaert, I. Carro-Muino, M. De Veerman, C. de Felipe, S. P. Hunt, K. Thielemans, G. F. Joos, and R. A. Pauwels. 1999. Endogenously produced substance P contributes to lymphocyte proliferation induced by dendritic cells and direct TCR ligation. Eur. J. Immunol. 29:3815-3825. [DOI] [PubMed] [Google Scholar]
- 24.Lee, H. R., W. Z. Ho, and S. D. Douglas. 1994. Substance P augments tumor necrosis factor release in human monocyte-derived macrophages. Clin. Diagn. Lab. Immunol. 1:419-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maggi, C. A. 1997. The effects of tachykinins on inflammatory and immune cells. Regul. Pept. 70:75-90. [DOI] [PubMed] [Google Scholar]
- 26.Maggi, C. A. 1995. The mammalian tachykinin receptors. Gen. Pharmacol. 26:911-944. [DOI] [PubMed] [Google Scholar]
- 27.Malley, R., J. DeVincenzo, O. Ramilo, P. H. Dennehy, H. C. Meissner, W. C. Gruber, P. J. Sanchez, H. Jafri, J. Balsley, D. Carlin, S. Buckingham, L. Vernacchio, and D. M. Ambrosino. 1998. Reduction of respiratory syncytial virus (RSV) in tracheal aspirates in intubated infants by use of humanized monoclonal antibody to RSV F protein. J. Infect. Dis. 178:1555-1561. [DOI] [PubMed] [Google Scholar]
- 28.Manske, J. M., E. L. Sullivan, and S. M. Andersen. 1995. Substance P mediated stimulation of cytokine levels in cultured murine bone marrow stromal cells. Adv. Exp. Med. Biol. 383:53-64. [DOI] [PubMed] [Google Scholar]
- 29.Nakagawa, N., H. Sano, and I. Iwamoto. 1995. Substance P induces the expression of intercellular adhesion molecule-1 on vascular endothelial cells and enhances neutrophil transendothelial migration. Peptides 16:721-725. [DOI] [PubMed] [Google Scholar]
- 30.Pascual, D. W., and K. L. Bost. 1990. Substance P production by P388D1 macrophages: a possible autocrine function for this neuropeptide. Immunology 71:52-56. [PMC free article] [PubMed] [Google Scholar]
- 31.Payan, D. G. 1989. Neuropeptides and inflammation: the role of substance P. Annu. Rev. Med. 40:341-352. [DOI] [PubMed] [Google Scholar]
- 32.Pernow, B. 1985. Role of tachykinins in neurogenic inflammation. J. Immunol. 135:812S-815S. [PubMed] [Google Scholar]
- 33.Piedimonte, G., M. M. Rodriguez, K. A. King, S. McLean, and X. Jiang. 1999. Respiratory syncytial virus upregulates expression of the substance P receptor in rat lungs. Am. J. Physiol. 277:L831-L840. [DOI] [PubMed] [Google Scholar]
- 34.Prince, G. A., A. Mathews, S. J. Curtis, and D. D. Porter. 2000. Treatment of respiratory syncytial virus bronchiolitis and pneumonia in a cotton rat model with systemically administered monoclonal antibody (Palivizumab) and glucocorticosteroid. J. Infect. Dis. 182:1326-1330. [Google Scholar]
- 35.Prince, G. A., and D. D. Porter. 1996. Treatment of parainfluenza virus type 3 bronchiolitis and pneumonia in a cotton rat model using topical antibody and glucocorticosteroid. J. Infect. Dis. 173:598-608. [DOI] [PubMed] [Google Scholar]
- 36.Rameshwar, P., and P. Gascon. 1995. Substance P (SP) mediates production of stem cell factor and interleukin-1 in bone marrow stroma: potential autoregulatory role for these cytokines in SP receptor expression and induction. Blood 86:482-490. [PubMed] [Google Scholar]
- 37.Stott, E. J., G. Taylor, L. A. Ball, K. Anderson, K. K. Young, A. M. King, and G. W. Wertz. 1987. Immune and histopathological responses in animals vaccinated with recombinant vaccinia viruses that express individual genes of human respiratory syncytial virus. J. Virol. 61:3855-3861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomaki, M., M. Ichinose, M. Miura, Y. Hirayama, H. Yamauchi, N. Nakajima, and K. Shirato. 1995. Elevated substance P content in induced sputum from patients with asthma and patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 151:613-617. [DOI] [PubMed] [Google Scholar]
- 39.Tripp, R. A., D. Moore, L. Jones, W. Sullender, J. Winter, and L. J. Anderson. 1999. Respiratory syncytial virus G and/or SH protein alters Th1 cytokines, natural killer cells, and neutrophils responding to pulmonary infection in BALB/c mice. J. Virol. 73:7099-7107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tripp, R. A., D. Moore, J. Winter, and L. J. Anderson. 2000. Respiratory syncytial virus infection and G and/or SH protein expression contribute to substance P, which mediates inflammation and enhanced pulmonary disease in BALB/c mice. J. Virol. 74:1614-1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Walker, T. A., S. Khurana, and S. J. Tilden. 1994. Viral respiratory infections. Pediatr. Clin. N. Am. 41:1365-1381. [DOI] [PubMed] [Google Scholar]
- 42.Weltzin, R., S. A. Hsu, E. S. Mittler, K. Georgakopoulos, and T. P. Monath. 1994. Intranasal monoclonal immunoglobulin A against respiratory syncytial virus protects against upper and lower respiratory tract infections in mice. Antimicrob. Agents Chemother. 38:2785-2791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Win, N., D. Mitchell, S. Pugh, and N. H. Russell. 1992. Successful therapy with ribavirin of late onset respiratory syncytial virus pneumonitis complicating allogeneic bone transplantation. Clin. Lab. Haematol. 14:29-32. [PubMed] [Google Scholar]
- 44.Yonehara, N., Y. Imai, T. Shibutani, and R. Inoki. 1989. Participation of substance P in inflammatory responses. Adv. Exp. Med. Biol. 247B:529-534. [DOI] [PubMed] [Google Scholar]