Abstract
The presence of hepatitis C virus (HCV) RNA-containing particles in the low-density fractions of plasma has been associated with high infectivity. However, the nature of circulating HCV particles and their association with immunoglobulins or lipoproteins as well as the characterization of cell entry have all been subject to conflicting reports. For a better analysis of HCV RNA-containing particles, we quantified HCV RNA in the low-density fractions of plasma corresponding to the very-low-density lipoprotein (VLDL), intermediate-density lipoprotein, and low-density lipoprotein (LDL) fractions from untreated chronically HCV-infected patients. HCV RNA was always found in at least one of these fractions and represented 8 to 95% of the total plasma HCV RNA. Surprisingly, immunoglobulins G and M were also found in the low-density fractions and could be used to purify the HCV RNA-containing particles (lipo-viro-particles [LVP]). Purified LVP were rich in triglycerides; contained at least apolipoprotein B, HCV RNA, and core protein; and appeared as large spherical particles with a diameter of more than 100 nm and with internal structures. Delipidation of these particles resulted in capsid-like structures recognized by anti-HCV core protein antibody. Purified LVP efficiently bind and enter hepatocyte cell lines, while serum or whole-density fractions do not. Binding of these particles was competed out by VLDL and LDL from noninfected donors and was blocked by anti-apolipoprotein B and E antibodies, whereas upregulation of the LDL receptor increased their internalization. These results suggest that the infectivity of LVP is mediated by endogenous proteins rather than by viral components providing a mechanism of escape from the humoral immune response.
Although hepatitis C virus (HCV) infection is a major cause of chronic liver disease worldwide, the virus has not yet been cultured in vitro and little is known about its biological and physicochemical properties (16). A recent accumulation of data revealed the density heterogeneity of HCV RNA-containing particles (7, 19, 27). By use of nonquantitative PCR to detect HCV RNA in gradient fractions of infected human serum, HCV RNA-containing particles were found at densities of between 1.03 and 1.25 g/ml, with considerable variations from serum sample to serum sample (41, 42). Titration of infectivity in chimpanzees revealed a relationship between the density of particles and infectivity; the highest infectivity of plasma was associated with the majority of HCV RNA being found in the low-density fraction (density of <1.06 g/ml), while HCV RNA found in higher-density fractions seemed to be poorly infectious (5, 15).
The unusually low density of some HCV RNA-containing particles suggested an association of the virus with plasma lipoproteins (41, 42). The main function of lipoproteins is to transport and deliver lipids and lipid-soluble materials throughout the body. Lipoproteins are 20- to 80-nm particles which consist of a hydrophobic core of neutral lipid surrounded by a monolayer of amphipathic phospholipids and free cholesterol in which apolipoproteins reside. Synthesis and secretion of these particles occur in hepatocytes in the form of very-low-density lipoprotein (VLDL) (density of <1.0063 g/ml) with apolipoproteins B and E (ApoB and ApoE, respectively). Transformation of VLDL in the circulation gives rise to particles of a smaller size, with intermediate to low density (intermediate-density lipoprotein [IDL] and low-density lipoprotein [LDL]), enriched in cholesteryl esters, depleted of triglycerides, and containing only ApoB (14, 39). The interaction of HCV with plasma lipoproteins was first confirmed when Thomssen et al. (41) found that low-density materials can be precipitated with anti-ApoB antisera (41, 42). Several investigators have since extended this observation and shown that HCV envelope proteins bind to human lipoproteins of various densities (28). It is not known, however, whether HCV simply binds to circulating lipoproteins or whether an interaction occurs during lipoprotein synthesis by infected hepatocytes to form a hybrid virus-like particle.
Identification of the HCV receptor is a major challenge for the development of both cell culture systems and therapy. Two cell surface receptors, the LDL receptor and CD81, interact with HCV and HCV envelope protein E2, respectively, leading to the hypothesis that they both act as viral receptors (28, 30, 31, 40). Although several reports have shown a highly specific interaction between HCV E2 and cellular CD81 (31), most recent studies have indicated that putative lipoprotein-associated HCV particles may infect cells via the LDL receptor (1, 44). Characterization of this pathway has become challenging, especially since it appears to be preferentially active on hepatocytes.
The apparent heterogeneity of data concerning the various putative forms of HCV RNA-containing particles may be due to the lack of systematic and comparative analyses. Using a quantitative approach (22), we conducted an analysis of chronically HCV-infected patients to determine the representativeness and the precise nature and infectivity of HCV particles in low-density plasma fractions corresponding to VLDL, IDL, and LDL.
MATERIALS AND METHODS
Samples.
Thirty-six volunteers attending the Liver Unit at Necker Hospital, Paris, France, were selected in accordance with hospital ethics committee statements; they were chronically HCV-infected patients with chronic active hepatitis and had not been given antiviral therapy for more than 6 months. Screening for hepatitis B virus or human immunodeficiency virus infection was negative. Blood was obtained by venous puncture. EDTA at a 1 mM final concentration was added to 40 ml of blood, and samples were sent to the laboratory at an ambient temperature. Plasma and serum were immediately processed for density fraction separation, and aliquots were stored at −80°C. For control and competition experiments, plasma from noninfected blood donors was provided by the Etablissement de Transfusion Sanguine in Lyon, France.
Preparation of low-density fractions.
Plasma from infected patients or from blood donors was separated by sequential ultracentrifugation to obtain three low-density fractions whose densities corresponded to those of VLDL, IDL, and LDL (25). The lowest-density fraction, with a density of <1.0063 g/ml, was obtained by centrifugation of plasma for 4 h at 4°C and 543,000 × g with a TLA100.4 rotor and a TL100 ultracentrifuge (Beckman Instruments S. A., Gagny, France). After collection of the first fraction, the density of the remaining plasma was adjusted to 1.025 g/ml with NaBr (Sigma). The second fraction, with a density of between 1.006 and 1.025 g/ml, was collected after centrifugation under the same conditions. A last run at a density of 1.055 g/ml (by the addition of NaBr) allowed the collection of the third fraction, with a density of between 1.025 and 1.055 g/ml. All fractions were then extensively dialyzed at 4°C against 150 mM NaCl-0.24 mM EDTA (pH 7.4) buffer, filtered through 0.22-μm-pore-size filters (Millipore S. A., Saint Quentin, France), and stored at 4°C in the dark.
Immunopurification.
A 10-μl quantity of protein A-coated magnetic beads (Miltenyi Biotec, Paris, France) was incubated at room temperature with 1 ml of the low-density fractions in phosphate-buffered saline (PBS) with gentle rocking for 30 min. The beads were then passed through a magnetic column (Miltenyi Biotec), washed with PBS, and collected in 500 μl of PBS or Dulbecco modified Eagle medium (DMEM)-0.2% bovine serum albumin (BSA) (Gibco/BRL, Life Technologies, Cergy Pontoise, France). Immunoprecipitated particles were designated purified lipo-viro-particles (LVP).
Electron microscopy.
Dilution of HCV-infected low-density fractions or purified LVP were made in PBS. For some experiments, purified LVP were delipidated by incubation for 30 min with gentle rocking in an 85% ether-15% butanol solution. Phases were separated by centrifugation, and the aqueous phase was recovered. Formvar-carbon support film-coated 200-mesh copper grids (Electron Microscopy Sciences, Fort Washington, Pa.) were floated on drops of samples for 3 min at room temperature and stained for 3 min by flotation on 4% (wt/vol) phosphotungstic acid buffered at pH 7.2 with NaOH. The grids were then dried and examined with a JEOL apparatus at the Centre Commun d'Imagerie de Laennec in Lyon, France.
For immunoelectron microscopy, Formvar-carbon support film-coated 200-mesh nickel grids were floated on LVP that had been delipidated in 0.5% Tween 80-PBS for 30 min at room temperature with gentle rocking and were incubated for 1 h at room temperature by flotation on 0.05 M Tris-HCl (pH 7.4) containing 1% BSA and 0.1% cold fish gelatin (Sigma). The grids were then floated on a drop of monoclonal antibody 19D9D6 (18) or isotypic control immunoglobulin G1 (IgG1) (5 μg/ml) in Tris-HCl (pH 7.4) overnight in a moist chamber at 4°C. The grids were successively washed by flotation on drops of Tris-HCl (pH 7.4, pH 8.2, and pH 8.2) with 1% BSA. The second antibody was incubated by floating grids on a 1:40 dilution of goat anti-mouse IgG-colloidal gold particles (10-nm diameter; BioCell Research Laboratories, Cardiff, United Kingdom) in Tris-HCl (pH 8.2)-1% BSA for 1 h at room temperature. Washings were first performed with Tris-HCl at pH 8.2 and then at pH 7.4 and finally with water. The grids were negatively stained by flotation on 3% uranyl acetate.
Protein, ApoB, and lipid quantitation.
Protein concentrations were determined according to the Lowry method as modified by Markwell (Sigma). Protein concentrations were calculated from a calibration curve by using BSA as a standard. ApoB concentrations in fractions and sera were determined by using an immunochemical kit according to the manufacturer's protocol (ApoB kit; bioMérieux S. A., Marcy l'Etoile, France). The concentrations were determined from a calibration curve established with the ApoB kit standard. Total cholesterol, phospholipid, and triglyceride concentrations were calculated with Cholesterol RTU, Phospholipides Enzymatique PAP 150, and Triglycerides Enzymatic PAP 150 kits (bioMérieux) according to the manufacturer's recommendations but with the inclusion of standard curves to calculate the concentrations.
ApoB concentrations in purified LVP were determined by an enzyme-linked immunosorbent assay. Ninety-six-well flat-bottom enzyme-linked immunosorbent assay plates (Maxisorb; Nunc) were coated overnight at 4°C with 100 μl of monoclonal anti-human ApoB antibody (5 μg/ml; clone 1609; Biodesign, Saco, Maine) in PBS and then blocked with 2% BSA for 1 h. Samples were first incubated for 30 min at room temperature in PBS-0.2% BSA supplemented with 10 μg of human IgG/ml before being distributed on the plates at 100 μl/well. After 2 h of incubation at 37°C and washing with PBS-0.05% Tween 20, peroxidase-conjugated goat anti-human ApoB antibodies (1.6 μg/ml; Biodesign) in PBS-0.2% BSA were incubated at 100 μl/well for 90 min at 37°C. The plates were washed, and ortho-phenylenediamine substrate (Sigma) was added at 150 μl/well. The reaction was developed for 10 min, and the plates were read at 490 nm. Standard curves were established with LDL dilutions ranging from 2 to 100 ng of ApoB/ml. Controls included human IgG-saturated protein A-coated magnetic beads prepared under the same conditions.
HCV RNA quantitation.
RNA was extracted from 50 μl of serum, 50 μl of low-density fraction, or 10 μl of purified LVP with a QIAamp viral RNA kit (Qiagen S. A., Courtaboeuf, France); the RNA was eluted in 50 μl of water and stored at −80°C. Samples with a high lipid content were first diluted with 2 volumes of normal human serum to inactivate PCR inhibitors. HCV RNA quantitation was performed by real-time PCR of the 5′ HCV noncoding region as previously described but with minor modifications (22). Briefly, RNA (4 μl) was reverse transcribed by using a Thermoscript reverse transcriptase kit (Gibco/BRL) with the RC21 primer (4). Real-time PCR were carried out with 2 μl of cDNA and the RC1 and RC21 primers by using an LC FastStart DNA Master SYBR Green I kit and a LightCycler apparatus (Roche Diagnostics, Meylan, France).
Direct calculation of the proportion of HCV RNA associated with low-density fractions overestimated the association because of incomplete RNA recovery from the remaining plasma and pellet after each centrifugation. Therefore, an index of the association of HCV RNA with low-density fractions was determined with ApoB included as an internal standard for the lipoprotein compartment. The index of the association of HCV RNA with low-density fractions was calculated as follows: (RNA copy number per milligram of ApoB in low-density fractions/RNA copy number per milligram of ApoB in serum) × 100.
5′ HCV noncoding region sequencing and genotyping.
PCR amplification products synthesized during the HCV RNA quantitation process with the LightCycler were directly sequenced in both directions by using PRISM Ready Reaction AmplitaqFS BigDye Terminator cycle sequencing kit (PE/Applied Biosystems, Roissy, France) and Applied Biosystems 377 and 373A automated DNA sequencers. The HCV genotype was determined by comparison of the nucleotide sequence with the HCVDB Hepatitis Sequence Database (http://hepatitis.ibcp.fr).
Western blotting.
Five-microgram quantities of low-density fraction proteins were resolved by sodium dodecyl sulfate (SDS)-10% polyacrylamide gel electrophoresis (PAGE) and transferred to polyvinylidene difluoride (PVDF) membranes (Millipore). The membranes were incubated in blocking solution (20 mM Tris-HCl [pH 7.4], 150 mM NaCl, 0.05% Tween 20, 5% skim milk) and revealed with goat serum anti-human IgG or IgM conjugated to horseradish peroxidase (Jackson ImmunoResearch, West Grove, Pa.). Labeled antibodies were visualized by enhanced chemiluminescence detection (Amersham Pharmacia Biotech Europe, Freiburg, Germany).
Purified LVP were delipidated by ether-butanol extraction as described above. Proteins were separated by SDS-14% PAGE and blotted onto PVDF membranes. Membranes were blocked as described above and incubated with anti-HCV core protein mouse monoclonal antibody 19D9D6 (18). Bound antibodies were detected with goat anti-mouse antibody conjugated to horseradish peroxidase (Promega, Lyon, France) and enhanced chemiluminescence.
Cells.
Human hepatoma cell lines PLC/PFR/5 (PLC) and HepG2 were cultured in DMEM (Gibco/BRL) supplemented with 10% fetal calf serum (FCS) (Biowhittaker, Emerainville, France) or 10% lipoprotein-deficient FCS (LPDS) (Sigma), 2 mM HEPES (Gibco/BRL), 1% nonessential amino acids (Gibco/BRL), and 50 IU of penicillin-streptomycin (Gibco/BRL)/ml at 37°C in a 5% CO2 atmosphere; cultures were split twice a week. HepG2 clone N3 stably expressing the HCV core protein was from T. Miyamura (34). Normal human fibroblast N1 and familial hypercholesterolemia-affected patient fibroblast FH (GM00488C; Human Genetic Cell Repository, Coriell Institute for Medical Research, Camden, N.J.) lines were maintained in DMEM supplemented with 10 and 20% FCS, respectively, 2 mM HEPES, 1% nonessential amino acids, and 50 IU of penicillin-streptomycin/ml.
Cell association, uptake, and kinetic assays.
For the HCV RNA cell association assay, 5 × 104 PLC cells per well were cultured in 96-well plates for 24 h with culture medium. PLC cells were washed three times with PBS and incubated for 3 h with serum, whole-density fractions, or purified LVP and with or without competitors in FCS-free medium containing 0.2% (wt/vol) BSA. After washing, cells were harvested in 350 μl of RNeasy kit lysis buffer (Qiagen), and RNA was extracted. HCV RNA was quantified as described above. For inhibition experiments, the monoclonal antibodies against the receptor-binding site of ApoB were 4G3 and 5E11. The receptor-binding site of ApoE was blocked with monoclonal antibody 1D7 (Ottawa Heart Institute Research Corporation, Ottawa, Ontario, Canada).
For kinetic and uptake experiments, HCV RNA-containing particles bound to cell surface receptors after the incubation period and three washings with PBS-0.2% BSA were removed by incubation with 10 mM suramin (Sigma) in PBS at 4°C for 1 h. Cells were washed three times in PBS and harvested in 350 μl of RNeasy kit lysis buffer. Internalized HCV RNA was quantified as described above.
The cell protein content in the RNeasy lysis buffer was directly measured after RNA binding to the RNeasy mini spin column. Nonspecific binding to plastic wells was controlled by incubating HCV samples under identical conditions in wells containing no cells.
RESULTS
Analysis of very-low-, intermediate-, and low-density plasma fractions.
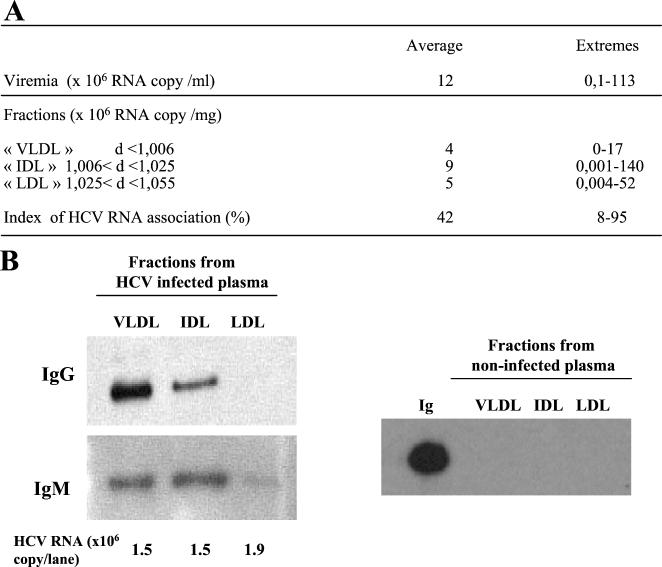
Plasma from 27 chronically infected patients was fractionated into three fractions corresponding to the floating densities of VLDL (<1.0063 g/ml), IDL (1.0063 to 1.025 g/ml), and LDL (1.025 to 1.055 g/ml). The amount of HCV RNA in each fraction was measured by real-time PCR as previously described (22). Figure 1A shows that all patients had HCV RNA in at least one of the three fractions irrespective of the amount of HCV RNA in the serum or the HCV genotype. The index of the association of HCV RNA with the density fractions varied from 8 to 95%, depending on the patient, with a mean of 42%.
FIG. 1.
Characterization of HCV RNA-containing particles in very-low- to low-density plasma fractions. (A) Serum and three plasma fractions corresponding to VLDL, IDL, and LDL were prepared from 27 chronically HCV-infected patients. The HCV genotype distribution was as follows: 1 genotype 1a, 15 genotype 1b, 1 genotype 2b, 5 genotype 3a, 5 genotype 4, and 3 ambiguous genotypes. Viremia was calculated as the number of HCV RNA copies per milliliter of serum. Viral loads in fractions were calculated as the number of HCV RNA copies per milligram of protein. The association of HCV RNA with low-density fractions is expressed as an index as described in Materials and Methods. d, density of fraction. (B) Fractions from infected and noninfected plasma samples were analyzed for the presence of immunoglobulins (Ig). Five-microgram quantities of fractions corresponding to VLDL, IDL, and LDL were separated by SDS-10% PAGE and transferred to PVDF membranes for IgG and IgM detection. A positive control experiment was performed by running 200 ng of purified IgG in the gel.
Some HCV particles with a high density have been reported to be associated with immunoglobulins (8, 15, 20, 42). To search for immunoglobulins, we subjected the three fractions to SDS-PAGE and Western blotting with anti-immunoglobulin antibodies (Fig. 1B). IgG and IgM were found in fractions from all patients but not noninfected donors, suggesting that HCV RNA-containing particles can be the target of a humoral response, with more immunoglobulins being found in the very-low- and intermediate-density fractions. We found no correlation between the amount of immunoglobulins and the amount of HCV RNA.
Purification and characterization of HCV RNA-containing particles.
To verify whether immunoglobulins were directed to HCV RNA-containing particles, immunoglobulins from various fractions were purified with protein A-coated magnetic beads, and the quantities of HCV RNA in the purified and nonpurified materials were determined. HCV RNA always copurified with immunoglobulins. Table 1 shows four representative examples of copurification indicating that the association of HCV RNA-containing particles with immunoglobulins ranged from 11 to 85% irrespective of the density of the fraction. The association of HCV RNA-containing particles with immunoglobulins therefore depends on individual variations rather than on density.
TABLE 1.
Quantitation of low-density HCV RNA-containing particles associated with immunoglobulins (Ig)
| Patient | Fraction density | HCV RNA copy no. (106) in the following fractiona:
|
Yield (%)b | % HCV RNA associated with Igc | ||
|---|---|---|---|---|---|---|
| Whole | Ig+ LVP− | Ig− | ||||
| 1 | <1.0063 | 9 | 1 | 8 | 99 | 11 |
| 2 | <1.0063 | 19.3 | 13.2 | 2.65 | 82 | 68 |
| 3 | 1.025-1.055 | 4.32 | 0.73 | 2.8 | 81 | 17 |
| 4 | 1.025-1.055 | 1.04 | 0.89 | <0.006 | 85 | 85 |
Ig+ LVP−, Ig positive LVP depleted; Ig−, Ig negative.
Expressed as the percentage of HCV RNA copies in the Ig+ and Ig− fractions/HCV RNA copies in the whole fraction.
Expressed as the percentage of HCV RNA copies in the Ig+ LVP− fraction/HCV RNA copies in the whole fraction.
The presence of immunoglobulins on HCV RNA-containing particles was then used for an additional step of purification with protein A-coated magnetic beads. This step allowed the separation of immunoglobulin-positive HCV RNA-containing particles from normal lipoproteins and from particles not covered by immunoglobulins. Such immunopurified material was referred to as purified LVP. Triglycerides, total cholesterol (free cholesterol and cholesteryl esters), and ApoB in the density fractions and in the corresponding purified LVP were quantified (Table 2). As expected, the triglyceride/cholesterol ratios of the density fractions before LVP purification were 3.4 ± 1.7 and 0.42 ± 0.2 for the very-low- and low-density fractions, respectively. Conversely, the triglyceride/cholesterol ratios of purified LVP from the very-low- and low-density fractions did not vary (3.1 ± 1.6 and 2.8 ± 1.9, respectively). Although the triglyceride/cholesterol ratios of VLDL and of LVP purified from the very-low-density fraction appeared in the same range, the lipid contents of LDL and of LVP purified from the low-density fraction differed significantly (0.42 ± 0.2 and 2.8 ± 1.9, respectively; P ≤ 0.01). In addition, LVP purified from the very-low-density fraction contained more triglyceride per ApoB molecule than LVP purified from the low-density fraction, explaining the difference in density. LVP purified from both very-low- and low-density fractions contained more triglyceride per ApoB molecule than lipoproteins from the same fractions.
TABLE 2.
Biochemical characterization of the whole fraction and corresponding purified LVP
| Fraction density | Ratio of the indicated lipidsa in:
|
|||
|---|---|---|---|---|
| Whole fraction
|
Purified LVP
|
|||
| TG/Chol | TG/ApoB | TG/Chol | TG/ApoB | |
| <1.006 | 3.4 ± 1.7 | 15 ± 1.3b | 3.1 ± 1.6 | 160 ± 47b |
| 1.025-1.055 | 0.42 ± 0.2c | 0.8 ± 0.2b | 2.8 ± 1.9c | 26 ± 20b |
TG, triglycerides; Chol, cholesterol. Cholesterol data were obtained from six samples, and ApoB data were obtained from four samples.
The P value for a comparison of TG/ApoB ratios between whole fraction and purified LVP was ≤0.04.
The P value for a comparison of TG/Chol ratios between whole fraction and purified LVP was ≤0.01.
The lipid compositions of lipoproteins and purified LVP suggested that LVP are unusual particles and not just aggregates of lipoproteins and HCV virions. We found no correlation between the amount of ApoB and the amount of HCV RNA in purified LVP (data not shown).
Electron microscopy of purified LVP.
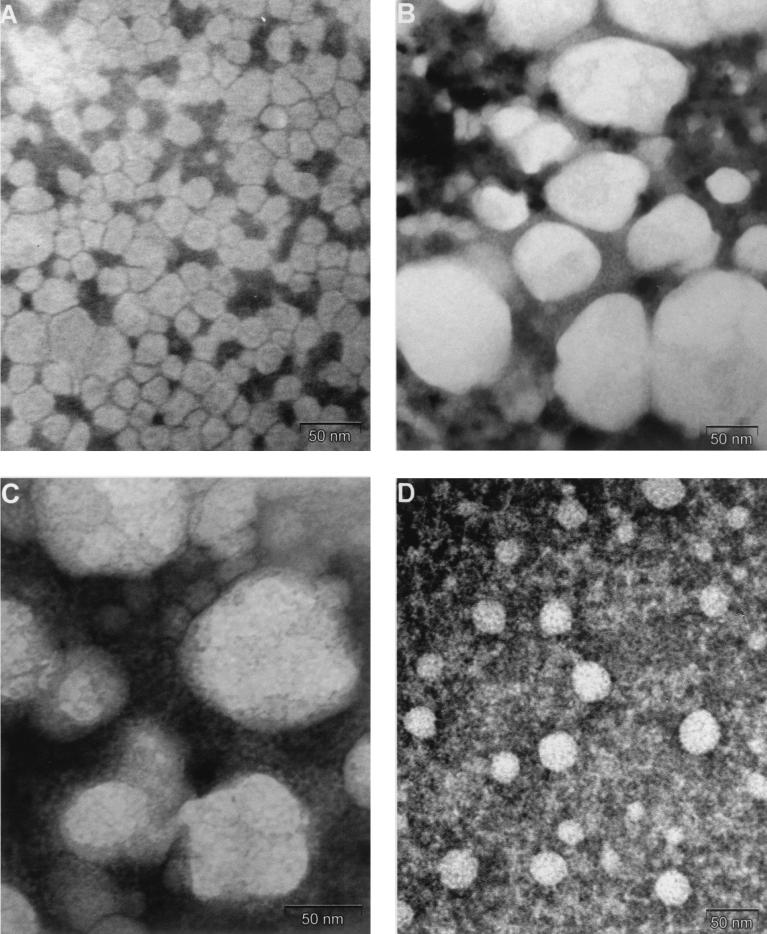
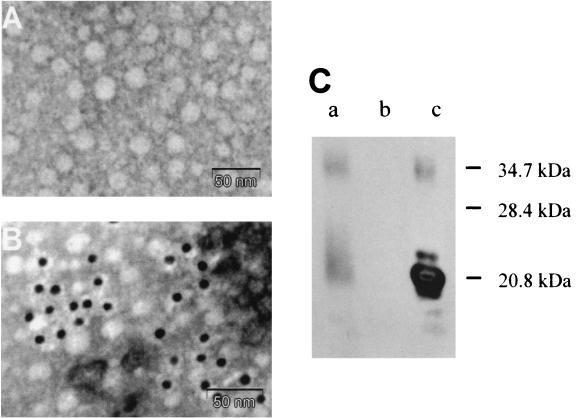
Electron microscopy was performed with purified LVP obtained from the plasma fraction corresponding to LDL and with the same fraction depleted of LVP. The LDL fraction from infected plasma after LVP depletion displayed a homogeneous spherical structure with an average diameter of 25 nm, similar to that of normal LDL (Fig. 2A). In contrast, purified LVP (Fig. 2B) were unusually large spherical structures with an average diameter of 100 nm. Internal structures began to be visible when purified LVP were observed at the highest magnification (Fig. 2C). These structures could be better observed after delipidation of purified LVP with ether-butanol and appeared as capsid-like particles with some heterogeneity. The largest structures were 30 to 35 nm in diameter, and smaller particles might have represented defective or incomplete capsids (Fig. 2D). These structures were poorly recognized by immunoelectron microscopy with a monoclonal antibody to the basic and hydrophilic N-terminal region of the HCV core protein (18) (data not shown). However, after more drastic treatment with Tween 80, particles appeared smaller and partially disrupted, with irregular borders (Fig. 3A), and could be identified by immunoelectron microscopy with the same monoclonal antibody (Fig. 3B). The presence of HCV core protein in purified LVP after delipidation was further demonstrated by Western blotting with the same anti-HCV core protein monoclonal antibody (Fig. 3C). Under our experimental conditions, both monomers and dimers of HCV core protein were detected in LVP and in a core protein-expressing cell line. In contrast, we failed to detect any HCV envelope proteins in purified LVP by Western blotting despite the use of monoclonal antibodies recognizing different conformational and linear HCV envelope epitopes (provided by J. Dubuisson, Institut Pasteur, Lille, France) (31).
FIG. 2.
Electron microscopy of fractions corresponding to LDL from infected and noninfected patients. (A) LDL fraction depleted of immunoglobulin-positive LVP from an infected donor. (B) LVP purified from the LDL fraction of an infected donor. Protein A-coated magnetic beads are seen as dark granules 10 to 20 nm in diameter. Purified LVP appears as spherical particles whose average diameter is 100 nm (extremes, 50 to 150 nm). (C) Same fraction as in panel B but at a higher magnification (×250,000), showing the internal structures. (D) Ether-butanol-treated purified LVP adsorbed on grids and stained with phosphotungstic acid. Capsid-like particles 25 to 35 nm in diameter can be seen.
FIG. 3.
Immunodetection of HCV core protein in purified and delipidated LVP. (A) Control immunoelectron microscopy with an irrelevant primary monoclonal antibody and a gold-labeled secondary antibody. No significant labeled antibody binding occurred. (B) Immunoelectron microscopy of Tween 80-treated LVP with anti-HCV core protein monoclonal antibody 19D9D6 and a 10-nm-gold-labeled secondary antibody. The binding of core protein-specific antibody on the particles can be seen. (C) Western blot of purified and delipidated LVP. Ether-butanol-treated LVP corresponding to 3 × 106 HCV RNA copies (lane a), HepG2 cells (negative control) (lane b), and HepG2 cells stably transfected with HCV core cDNA (positive control) (lane c) are shown. Detection of HCV core protein was performed with monoclonal antibody 19D9D6.
Cellular binding and uptake of purified LVP.
Purification of LVP combined with a previously described method for measuring HCV RNA allowed a precise analysis of the binding, association, and internalization of purified particles tested with hepatocyte cell lines.
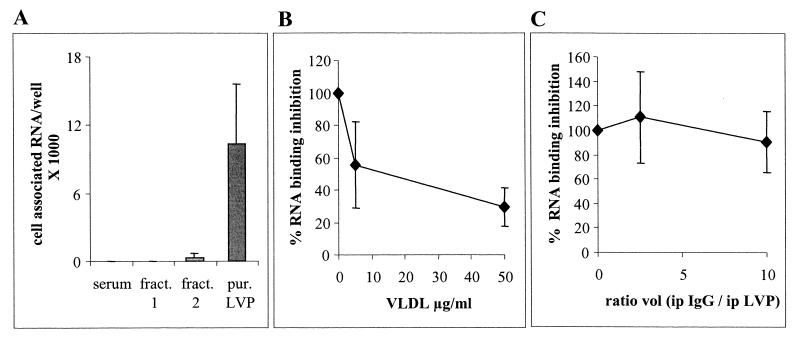
In the first experiment, samples containing 50,000 copies of HCV RNA were incubated for 3 h with PLC cells at 37°C. Cells were then washed, and RNA was extracted. Cell-associated HCV RNA was quantified and represented the amount of bound or internalized virus during this time period. We used four sources of HCV RNA from the same patient: serum, whole-density fraction corresponding to VLDL, the same fraction depleted of LVP (and therefore containing immunoglobulin-negative HCV RNA-containing particles and normal lipoproteins), and purified LVP. Maximum association was obtained with purified LVP (Fig. 4A). No association or an extremely weak association was detected when serum or fractions were used as a viral source.
FIG. 4.
Cell association and uptake of purified LVP. (A) A total of 50,000 copies of HCV RNA from serum, fraction (fract.) 1 (whole fraction containing lipoproteins and LVP), fraction 2 (whole fraction depleted of immunoglobulin-positive LVP but containing lipoproteins and remaining immunoglobulin-negative LVP), and purified (pur.) LVP were incubated with PLC cells (50,000 cells/well). Cell association was quantified after 3 h of incubation and extensive washing and expressed as HCV RNA copy number per well. Data are representative of three independent experiments. (B and C) Purified LVP (50,000 HCV RNA copies) were coincubated with increasing amount of VLDL from a noninfected donor (B) or with increasing ratios of protein A-coated magnetic beads saturated with purified human IgG to purified LVP (C). Quantitation of cell-associated HCV RNA was performed after 3 h of incubation. ip, immunopurified LVP. Error bars indicated standard deviations.
As nonpurified LVP contained in fractions could not associate with cells, it is likely that these fractions contained inhibitors of LVP binding. Lipoproteins were thus tested for their ability to inhibit the cell association of purified LVP. Competition experiments revealed that the cell association of purified LVP could be blocked in a dose-dependent manner by VLDL from healthy donors, reaching more than 75% inhibition with 50 μg of VLDL/ml (Fig. 4B). LDL inhibited the cell association of purified LVP in a similar fashion (data not shown). In contrast, no inhibition was observed with an excess of IgG (Fig. 4C).
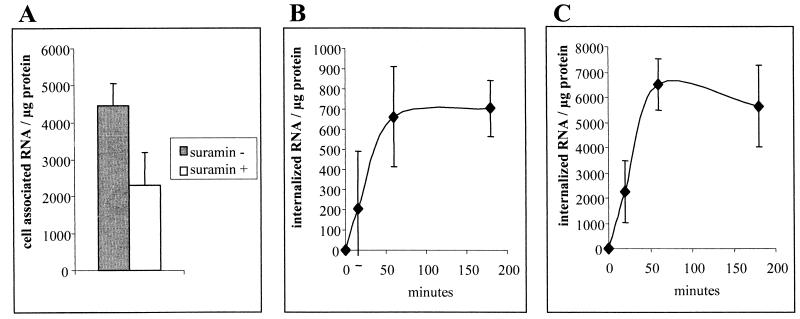
Suramin is a polysulfonylnaphthylurea that has been extensively used to study receptor kinetics and specificity. In particular, it has been shown to separate lipoproteins from their cell surface receptors (37) and to inhibit HCV infection of HepG2 cells as well as the binding of human immunodeficiency virus to CD4 and galactosylceramide (12, 45). Suramin treatment was thus applied to distinguish surface binding of purified LVP from internalization. Quantitation of HCV RNA remaining associated with cells after suramin treatment provides an estimation of what has been internalized during incubation with LVP. When purified LVP were incubated at 37°C for 3 h, half of the HCV RNA was removed by subsequent suramin treatment (Fig. 5A). This result indicated that 50% of the purified LVP bound to hepatocarcinoma PLC cells were internalized during the 3-h incubation period.
FIG. 5.
Kinetics of internalization of immunoglobulin-positive purified LPV. (A) After 3 h of incubation at 37°C with purified LVP and extensive washing, PLC cells were subjected to suramin treatment at 4°C. The number of HCV RNA copies after suramin treatment is representative of internalized purified LVP. As uptake does not occur at 4°C, the HCV RNA copy number remaining associated with cells following incubation at 4°C and subsequent suramin treatment is indicative of the efficiency of suramin. The efficiency of suramin treatment was greater than 95% (data not shown). Data are expressed as HCV RNA copy number per microgram of protein. (B and C) Purified LVP were incubated with PLC cells for various periods of time before suramin treatment and quantitation of internalized HCV RNA. (B) Incubation with 50,000 copies of HCV RNA from purified LVP. (C) Incubation with 200,000 copies of HCV RNA from purified LVP. Data are representative of three independent experiments. Error bars indicated standard deviations.
Figure 5B and C show the amounts of purified LVP internalized by PLC cells as a function of time. Two examples are shown to illustrate representative data obtained with different amounts of purified LVP. For both viral preparations, the process of viral uptake was rapid and achieved saturation in 1 h. The rates of uptake did not differ but reached a higher plateau when more HCV RNA copies were provided during the incubation period.
Cell entry pathway for purified LVP.
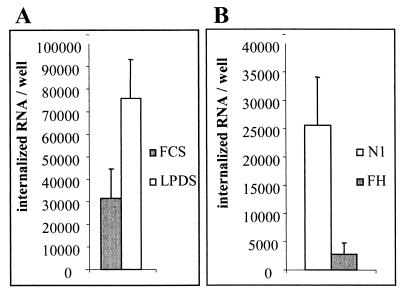
The activity of the LDL receptor is regulated by cholesterol. As a consequence, LDL receptor activity is upregulated when cells are grown in LPDS instead of FCS (37). To determine whether the internalization pathway for HCV is regulated by cholesterol, cells were grown in 10% LPDS for 24 h before incubation with purified LVP. Figure 6A shows a twofold increase in the uptake of purified LVP when cells were grown in LPDS. This result is in agreement with the twofold increase in LDL receptor expression and activity usually obtained under these experimental conditions (data not shown).
FIG. 6.
Endocytosis pathway for purified LVP. (A) A total of 200,000 copies of HCV RNA from purified LVP were incubated for 3 h with 50,000 HepG2 cells grown for 24 h in LPDS or FCS. Internalized HCV RNA was calculated after suramin treatment. (B) A total of 500,000 copies of HCV RNA from purified LVP were incubated with 20,000 N1 or FH fibroblasts for 3 h before suramin treatment and quantitation of internalized HCV RNA. Error bars indicated standard deviations.
N1 normal human fibroblasts were used to study the internalization of LVP in nonhepatocarcinoma cells. As shown in Fig. 6B, N1 fibroblasts expressing the LDL receptor could internalize substantial amounts of HCV RNA. However, the inoculum had to be more concentrated for N1 fibroblasts than for HepG2 cells to internalize similar amounts of purified LVP in 3 h. The uptake of purified LVP was also analyzed by using LDL receptor-negative fibroblasts (FH) derived from a patient with familial hypercholesterolemia (13). Purified LVP could still be internalized by FH fibroblasts, but the internalization was 10 times less efficient than that seen with fibroblasts expressing the LDL receptor.
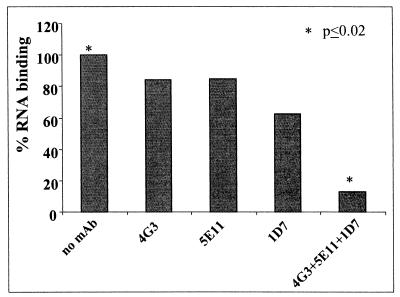
These data suggest that the LDL receptor pathway is the main route of entry for purified LVP. The LDL receptor recognizes lipoproteins through the direct binding of ApoB on LDL and ApoB and ApoE on VLDL (14, 39). We therefore tested whether the binding of purified LVP to the LDL receptor could be blocked by antibodies directed to the receptor-binding sites of ApoB and ApoE (26, 43) (Fig. 7). Two anti-ApoB monoclonal antibodies recognizing different epitopes within the ApoB receptor-binding site and one anti-ApoE monoclonal antibody directed to the receptor-binding site of ApoE only slightly inhibited the cell association of purified LVP when used independently. In contrast, a strong blockage of purified LVP cell association (>85%; P < 0.02) was obtained when all three antiapolipoprotein antibodies were used together. The participation of the anti-ApoE monoclonal antibody in the blockage of LVP binding also indicated that LVP contained ApoE.
FIG. 7.
Characterization of LVP binding to the LDL receptor. Purified LVP (6.6 × 106 HCV RNA copies) were incubated for 1 h with 200 μg of purified human IgG/ml. A total of 300,000 HCV RNA copies were diluted in FCS-free medium supplemented with 0.2% BSA and 50 μg of each anti-ApoB or anti-ApoE monoclonal antibody/ml alone or together. The samples were allowed to stand at room temperature for 1 h with rocking. The samples were then incubated for 45 min on 40,000 HepG2 cells that had been grown for 24 h in medium supplemented with LPDS. Quantitation of cell-associated HCV RNA was then performed. Monoclonal antibodies were 4G3 (anti-ApoB), 5E11 (anti-ApoB), and 1D7 (anti-ApoE). The P value for a comparison of results obtained with the control and the pool of antibodies was <0.02.
DISCUSSION
HCV RNA is detected over a large range of densities in gradient fractions of infected human plasma, with variations from serum sample to serum sample (7, 15, 27, 42). The presence of low-density fractions containing HCV RNA and the possibility of immunoprecipitation of HCV RNA with anti-ApoB antibodies suggested an association of viral components with lipoproteins (32, 41, 42). To clarify this association, we performed sequential centrifugations to prepare fractions containing VLDL, IDL, and LDL from infected plasma (25). Blood samples were processed within a few hours after collection without freezing or storage at low temperature. Real-time PCR allowed accurate quantitation of HCV RNA in plasma. However, HCV RNA amplification from low-density fractions was impeded by inhibitors, most likely of lipid origin. These inhibitors were inactivated by the addition of normal human serum (data not shown). With this optimized procedure, we confirmed the presence of HCV RNA in low-density fractions and showed that low-density HCV RNA-containing particles are a constant feature of chronic HCV infection.
It has been suggested that high-density HCV RNA-containing particles are immune complexes whereas particles of lower density are free of immunoglobulins (8, 15, 21, 42). Surprisingly, IgG and IgM were detected in the low-density fractions from all chronically HCV-infected patients but not from controls. This result indicated that low-density HCV RNA-containing particles should contain large amounts of very-low-density material to allow flotation during centrifugation despite the presence of immunoglobulins. These particles could then be purified with protein A-coated magnetic beads and further characterized. In addition to HCV RNA and immunoglobulins, these purified particles contained the viral core protein and ApoB. They were very rich in triglycerides, explaining their low density. Variations in the quantities of triglycerides might also explain why we found these particles at densities of between 1.006 and 1.055 g/ml. Importantly, the differences in lipid compositions between LVP and their lipoprotein counterparts strongly suggested that LVP are not just classical flavivirus-like virions attached to normal lipoproteins by HCV envelope glycoproteins, as previously suggested (28, 41, 42). This suggestion was confirmed by electron microscopy of purified LVP, which appeared as spherical particles with a diameter of more than 100 nm and not as HCV virions bound to lipoproteins. LVP also contained internal structures only visible at a high magnification. After delipidation, these structures were 30 to 35 nm in diameter, the size of a flavivirus capsid (33). The presence of capsids after delipidation of purified LVP is in agreement with reports showing naked capsids by electron microscopy after treatment of serum or low-density fractions with detergent (17, 24, 32). A monoclonal antibody directed to the N-terminal region of the HCV core protein clearly identified these particles as HCV capsids by immunoelectron microscopy. The presence of HCV core protein in delipidated LVP was further confirmed by Western blotting. Interestingly, the absence of a correlation between the amount of ApoB and the amount of HCV RNA suggests that some of the particles may be defective or empty particles.
We did not detect viral envelope glycoproteins in purified LVP. Anti-HCV envelope antibodies raised against recombinant proteins may not bind to native envelope proteins (9, 29), or epitopes may be altered during sample preparation. Alternatively, HCV envelope glycoproteins may be lost during high-speed centrifugation. The presence of immunoglobulins on LVP strongly suggests that these immunoglobulins are directed to surface viral components, especially envelope glycoproteins. However, because of the small amount of material available, we have not yet determined the specificities of these antibodies. For the same reason, we do not know whether IgM antibodies present in these complexes are directed to viral structures or are monoclonal or polyclonal IgM anti-IgG antibodies; the latter would provide an explanation for the high frequency of cryoglobulinemia observed in chronically infected patients (1).
Recent reports indicated that HCV may use the LDL receptor for binding and entry (2, 28, 44). Here we provide evidence that LVP behave as ligands of the LDL receptor: (i) purified LVP binding was inhibited by low concentrations of VLDL and LDL; (ii) the kinetics of purified LVP internalization were rapid, reached saturation, and were compatible with LDL receptor-mediated endocytosis (6); (iii) the upregulation of LDL receptor expression significantly increased purified LVP internalization; (iv) purified LVP internalization by LDL receptor-deficient fibroblasts was considerably reduced compared to that by normal fibroblasts (6, 13); and (v) monoclonal antibodies to ApoB and ApoE together blocked purified LVP cell association. These data indicate that purified LVP binding relies on apolipoproteins and their receptors and explain why this binding is efficiently inhibited by normal VLDL or LDL. Furthermore, these data also suggest that HCV envelope glycoproteins which were not demonstrated to bind to the LDL receptor may not have a major role in this pathway (44). This scenario may also provide a mechanism for LVP to evade the humoral immune response. Nevertheless, antibodies against envelope glycoproteins on LVP, particularly against hypervariable region 1 of E2, may facilitate the clearance of LVP immune complexes and participate in protection against infection (11).
A long-standing observation has been that molecules from the host cell membrane can be incorporated into viral particles (23). ApoB is an endoplasmic reticulum (ER) membrane-associated protein which initiates VLDL assembly, triglyceride loading in the presence of microsomal triglyceride transfer protein, and VLDL budding into the ER lumen. Nascent VLDL (one ApoB molecule per particle) is packaged in secretory vesicles and delivered into the hepatic extracellular space by exocytosis. Within the bloodstream, VLDL acquires ApoE, and three enzymes modify its content; it becomes enriched in cholesteryl esters and depleted in triglycerides. VLDL is thus converted to lipoproteins with higher densities, IDL and LDL (35, 38). Most of the flavivirus replication steps occur in association with the ER. Some regions of the polyprotein, including the envelope protein, are translocated into the lumen of the ER, whereas the core protein and most of the nonstructural proteins remain localized on the cytoplasmic side. The orientations of the core and envelope proteins with respect to the ER membrane suggest that nucleocapsids acquire an envelope when budding into the ER lumen. Later stages in virion maturation include the modification of envelope glycans, implying that virions move by vesicular transport through the cellular secretory pathway and are released by exocytosis (33). The lack of an efficient system for cell culture replication has so far hampered the understanding of HCV particle assembly. However, the absence of complex glycans, the localization of transfected HCV glycoproteins in the ER, and the absence of these proteins on the cell surface suggest that initial virion morphogenesis may occur by budding into intracellular vesicles (10). During virion and VLDL budding into the ER lumen, both vesicles may interfere, resulting in ApoB-bearing vesicles which are enriched in triglycerides by microsomal triglyceride transfer protein and form very-low-density lipoproteins containing RNA-filled HCV capsids. Binding of the HCV core protein to intracellular lipid droplets and to apolipoprotein AII further supports the hypothesis of an interaction between lipid metabolism and HCV (3, 36).
The quantitative analysis of LVP binding and internalization showed that the entry of the viral genome into target cells via LVP relies mainly on host molecules, apolipoproteins and lipoprotein receptors, providing an efficient mechanism of escape from neutralizing antibodies directed to envelope proteins. As LDL receptor expression is ubiquitous, such an entry mechanism may not restrict HCV infection to hepatocytes and may lead to extrahepatic compartments of viral replication.
Acknowledgments
This work was supported by INSERM, Agence Nationale de Recherche Contre le SIDA, and bioMérieux.
We thank Fabienne Chambrion, Hôpital Necker, Paris, France, for excellent work in collecting blood samples; Jean Dubuisson, Institut Pasteur, Lille, France, for providing anti-envelope protein antibodies; Simone Peyrol, Faculté de Médecine RTH Laennec, for help with electron microscopy; and Geneviève Sibai, bioMérieux, for the preparation of human immunoglobulins.
REFERENCES
- 1.Agnello, V. 1997. Hepatitis C virus infection and type II cryoglobulinemia: an immunological perspective. Hepatology 26:1375-1379. [DOI] [PubMed] [Google Scholar]
- 2.Agnello, V., G. Abel, M. Elfahal, G. B. Knight, and Q. X. Zhang. 1999. Hepatitis C virus and other flaviviridae viruses enter cells via low density lipoprotein receptor. Proc. Natl. Acad. Sci. USA 96:12766-12771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barba, G., F. Harper, T. Harada, M. Kohara, S. Goulinet, Y. Matsuura, G. Eder, Z. Schaff, M. J. Chapman, T. Miyamura, and C. Brechot. 1997. Hepatitis C virus core protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc. Natl. Acad. Sci. USA 94:1200-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Besnard, N. C., and P. M. Andre. 1994. Automated quantitative determination of hepatitis C virus viremia by reverse transcription-PCR. J. Clin. Microbiol. 32:1887-1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bradley, D., K. McCaustland, K. Krawczynski, J. Spelbring, C. Humphrey, and E. H. Cook. 1991. Hepatitis C virus: buoyant density of the factor VIII-derived isolate in sucrose. J. Med. Virol. 34:206-208. [DOI] [PubMed] [Google Scholar]
- 6.Brown, M. S., R. G. Anderson, and J. L. Goldstein. 1983. Recycling receptors: the round-trip itinerary of migrant membrane proteins. Cell 32:663-667. [DOI] [PubMed] [Google Scholar]
- 7.Carrick, R. J., G. G. Schlauder, D. A. Peterson, and I. K. Mushahwar. 1992. Examination of the buoyant density of hepatitis C virus by the polymerase chain reaction. J. Virol. Methods 39:279-289. [DOI] [PubMed] [Google Scholar]
- 8.Choo, S. H., H. S. So, J. M. Cho, and W. S. Ryu. 1995. Association of hepatitis C virus particles with immunoglobulin: a mechanism for persistent infection. J. Gen. Virol. 76:2337-2341. [DOI] [PubMed] [Google Scholar]
- 9.Cocquerel, L., J. C. Meunier, A. Pillez, C. Wychowski, and J. Dubuisson. 1998. A retention signal necessary and sufficient for endoplasmic reticulum localization maps to the transmembrane domain of hepatitis C virus glycoprotein E2. J. Virol. 72:2183-2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duvet, S., L. Cocquerel, A. Pillez, R. Cacan, A. Verbert, D. Moradpour, C. Wychowski, and J. Dubuisson. 1998. Hepatitis C virus glycoprotein complex localization in the endoplasmic reticulum involves a determinant for retention and not retrieval. J. Biol. Chem. 273:32088-32095. [DOI] [PubMed] [Google Scholar]
- 11.Farci, P., A. Shimoda, D. Wong, T. Cabezon, D. De Gioannis, A. Strazzera, Y. Shimizu, M. Shapiro, H. J. Alter, and R. H. Purcell. 1996. Prevention of hepatitis C virus infection in chimpanzees by hyperimmune serum against the hypervariable region 1 of the envelope 2 protein. Proc. Natl. Acad. Sci. USA 93:15394-15399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Garson, J. A., D. Lubach, J. Passas, K. Whitby, and P. R. Grant. 1999. Suramin blocks hepatitis C binding to human hepatoma cells in vitro. J. Med. Virol. 57:238-242. [PubMed] [Google Scholar]
- 13.Goldstein, J. L., M. K. Sobhani, J. R. Faust, and M. S. Brown. 1976. Heterozygous familial hypercholesterolemia: failure of normal allele to compensate for mutant allele at a regulated genetic locus. Cell 9:195-203. [DOI] [PubMed] [Google Scholar]
- 14.Havel, R. J., and J. P. Kane. 1995. Introduction: structure and metabolism of plasma lipoproteins, p. 18416-18451. In C. R. Scriver, A. L. Beaudet, W. S. Sly, and D. Walle (ed.), The metabolic and molecular bases of inherited diseases, 7th ed. McGraw-Hill Book Co., New York, N.Y.
- 15.Hijikata, M., Y. K. Shimizu, H. Kato, A. Iwamoto, J. W. Shih, H. J. Alter, R. H. Purcell, and H. Yoshikura. 1993. Equilibrium centrifugation studies of hepatitis C virus: evidence for circulating immune complexes. J. Virol. 67:1953-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoofnagle, J. H. 1997. Hepatitis C: the clinical spectrum of disease. Hepatology 26:15S-20S. [DOI] [PubMed] [Google Scholar]
- 17.Ishida, S., M. Kaito, M. Kohara, K. Tsukiyama-Kohora, N. Fujita, J. Ikoma, Y. Adachi, and S. Watanabe. 2001. Hepatitis C virus core particle detected by immunoelectron microscopy and optical rotation technique. Hepatol. Res. 20:335-347. [DOI] [PubMed] [Google Scholar]
- 18.Jolivet-Reynaud, C., P. Dalbon, F. Viola, S. Yvon, G. Paranhos-Baccala, N. Piga, L. Bridon, M. A. Trabaud, N. Battail, G. Sibai, and M. Jolivet. 1998. HCV core immunodominant region analysis using mouse monoclonal antibodies and human sera: characterization of major epitopes useful for antigen detection. J. Med. Virol. 56:300-309. [DOI] [PubMed] [Google Scholar]
- 19.Kanto, T., N. Hayashi, T. Takehara, H. Hagiwara, E. Mita, M. Naito, A. Kasahara, H. Fusamoto, and T. Kamada. 1994. Buoyant density of hepatitis C virus recovered from infected hosts: two different features in sucrose equilibrium density-gradient centrifugation related to degree of liver inflammation. Hepatology 19:296-302. [PubMed] [Google Scholar]
- 20.Kanto, T., N. Hayashi, T. Takehara, H. Hagiwara, E. Mita, M. Naito, A. Kasahara, H. Fusamoto, and T. Kamada. 1995. Density analysis of hepatitis C virus particle population in the circulation of infected hosts: implications for virus neutralization or persistence. J. Hepatol. 22:440-448. [DOI] [PubMed] [Google Scholar]
- 21.Kanto, T., N. Hayashi, T. Takehara, H. Hagiwara, E. Mita, M. Oshita, K. Katayama, A. Kasahara, H. Fusamoto, and T. Kamada. 1995. Serial density analysis of hepatitis C virus particle populations in chronic hepatitis C patients treated with interferon-alpha. J. Med. Virol. 46:230-237. [DOI] [PubMed] [Google Scholar]
- 22.Komurian-Pradel, F., G. Paranhos-Baccala, M. Sodoyer, P. Chevallier, B. Mandrand, V. Lotteau, and P. Andre. 2001. Quantitation of HCV RNA using real-time PCR and fluorimetry. J. Virol. Methods 95:111-119. [DOI] [PubMed] [Google Scholar]
- 23.Lodish, H. F., and M. Porter. 1980. Specific incorporation of host cell surface proteins into budding vesicular stomatitis virus particles. Cell 19:161-169. [DOI] [PubMed] [Google Scholar]
- 24.Maillard, P., K. Krawczynski, J. Nitkiewicz, C. Bronnert, M. Sidorkiewicz, P. Gounon, J. Dubuisson, G. Faure, R. Crainic, and A. Budkowska. 2001. Nonenveloped nucleocapsids of hepatitis C virus in the serum of infected patients. J. Virol. 75:8240-8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mills, G. L., P. A. Lane, and P. K. Weech. 1984. A guidebook to lipoprotein technique. Elsevier, Amsterdam, The Netherlands.
- 26.Milne, R., R. Theolis, Jr., R. Maurice, R. J. Pease, P. K. Weech, E. Rassart, J. C. Fruchart, J. Scott, and Y. L. Marcel. 1989. The use of monoclonal antibodies to localize the low density lipoprotein receptor-binding domain of apolipoprotein B. J. Biol. Chem. 264:19754-19760. [PubMed] [Google Scholar]
- 27.Miyamoto, H., H. Okamoto, K. Sato, T. Tanaka, and S. Mishiro. 1992. Extraordinarily low density of hepatitis C virus estimated by sucrose density gradient centrifugation and the polymerase chain reaction. J. Gen. Virol. 73:715-718. [DOI] [PubMed] [Google Scholar]
- 28.Monazahian, M., S. Kippenberger, A. Muller, H. Seitz, I. Bohme, S. Grethe, and R. Thomssen. 2000. Binding of human lipoproteins (low, very low, high density lipoproteins) to recombinant envelope proteins of hepatitis C virus. Med. Microbiol. Immunol. (Berlin) 188:177-184. [DOI] [PubMed] [Google Scholar]
- 29.Patel, A. H., J. Wood, F. Penin, J. Dubuisson, and J. A. McKeating. 2000. Construction and characterization of chimeric hepatitis C virus E2 glycoproteins: analysis of regions critical for glycoprotein aggregation and CD81 binding. J. Gen. Virol. 81:2873-2883. [DOI] [PubMed] [Google Scholar]
- 30.Petracca, R., F. Falugi, G. Galli, N. Norais, D. Rosa, S. Campagnoli, V. Burgio, E. Di Stasio, B. Giardina, M. Houghton, S. Abrignani, and G. Grandi. 2000. Structure-function analysis of hepatitis C virus envelope-CD81 binding. J. Virol. 74:4824-4830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pileri, P., Y. Uematsu, S. Campagnoli, G. Galli, F. Falugi, R. Petracca, A. J. Weiner, M. Houghton, D. Rosa, G. Grandi, and S. Abrignani. 1998. Binding of hepatitis C virus to CD81. Science 282:938-941. [DOI] [PubMed] [Google Scholar]
- 32.Prince, A. M., T. Huima-Byron, T. S. Parker, and D. M. Levine. 1996. Visualization of hepatitis C virions and putative defective interfering particles isolated from low-density lipoproteins. J. Viral Hepatol. 3:11-17. [DOI] [PubMed] [Google Scholar]
- 33.Rice, C. M. 1996. Flaviviridae: the viruses and their replication, p. 931-959. In B. N. Fields, D. M. Knipe, P. M. Howley, R. M. Chanock, J. L. Melnick, T. P. Monach, B. Roizman, and S. E. Straus (ed.), Fields virology, 3rd ed., vol. 1. Lippincott-Raven Publishers, Philadelphia, Pa. [Google Scholar]
- 34.Ruggieri, A., T. Harada, Y. Matsuura, and T. Miyamura. 1997. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology 229:68-76. [DOI] [PubMed] [Google Scholar]
- 35.Rustaeus, S., K. Lindberg, P. Stillemark, C. Claesson, L. Asp, T. Larsson, J. Boren, and S. O. Olofsson. 1999. Assembly of very low density lipoprotein: a two-step process of apolipoprotein B core lipidation. J. Nutr. 129:463S-466S. [DOI] [PubMed] [Google Scholar]
- 36.Sabile, A., G. Perlemuter, F. Bono, K. Kohara, F. Demaugre, M. Kohara, Y. Matsuura, T. Miyamura, C. Brechot, and G. Barba. 1999. Hepatitis C virus core protein binds to apolipoprotein AII and its secretion is modulated by fibrates. Hepatology 30:1064-1076. [DOI] [PubMed] [Google Scholar]
- 37.Schneider, W. J., U. Beisiegel, J. L. Goldstein, and M. S. Brown. 1982. Purification of the low density lipoprotein receptor, an acidic glycoprotein of 164,000 molecular weight. J. Biol. Chem. 257:2664-2673. [PubMed] [Google Scholar]
- 38.Shelness, G. S., M. F. Ingram, X. F. Huang, and J. A. DeLozier. 1999. Apolipoprotein B in the rough endoplasmic reticulum: translation, translocation and the initiation of lipoprotein assembly. J. Nutr. 129:456S-462S. [DOI] [PubMed] [Google Scholar]
- 39.Steinberg, D. 1988. Metabolism of lipoproteins and their role in the pathogenesis of atherosclerosis, vol. 18. Raven Press, Ltd., New York, N.Y.
- 40.Takikawa, S., K. Ishii, H. Aizaki, T. Suzuki, H. Asakura, Y. Matsuura, and T. Miyamura. 2000. Cell fusion activity of hepatitis C virus envelope proteins. J. Virol. 74:5066-5074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thomssen, R., S. Bonk, C. Propfe, K. H. Heermann, H. G. Kochel, and A. Uy. 1992. Association of hepatitis C virus in human sera with beta-lipoprotein. Med. Microbiol. Immunol. 181:293-300. [DOI] [PubMed] [Google Scholar]
- 42.Thomssen, R., S. Bonk, and A. Thiele. 1993. Density heterogeneities of hepatitis C virus in human sera due to the binding of beta-lipoproteins and immunoglobulins. Med. Microbiol. Immunol. (Berlin) 182:329-334. [DOI] [PubMed] [Google Scholar]
- 43.Weisgraber, K. H., T. L. Innerarity, K. J. Harder, R. W. Mahley, R. W. Milne, Y. L. Marcel, and J. T. Sparrow. 1983. The receptor-binding domain of human apolipoprotein E. Monoclonal antibody inhibition of binding. J. Biol. Chem. 258:12348-12354. [PubMed] [Google Scholar]
- 44.Wunschmann, S., J. D. Medh, D. Klinzmann, W. N. Schmidt, and J. T. Stapleton. 2000. Characterization of hepatitis C virus (HCV) and HCV E2 interactions with CD81 and the low-density lipoprotein receptor. J. Virol. 74:10055-10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yao, X. J., M. A. Wainberg, M. Richard, and M. Pollak. 1991. The ability of suramin to block CD4-gp120 binding is reversed in the presence of albumin. Antimicrob. Agents Chemother. 35:2636-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]