Abstract
Nectin-1, a cell adhesion molecule belonging to the immunoglobulin superfamily, can bind to virion glycoprotein D (gD) to mediate entry of herpes simplex viruses (HSV) and pseudorabies virus (PRV). Nectin-1 colocalizes with E-cadherin at adherens junctions in epithelial cells. The disruption of cell junctions can result in the redistribution of nectin-1. To determine whether disruption of junctions by calcium depletion influenced the susceptibility of epithelial cells to viral entry, Madin-Darby canine kidney cells expressing endogenous nectin-1 or transfected human nectin-1 were tested for the ability to bind soluble forms of viral gD and to be infected by HSV and PRV, before and after calcium depletion. Confocal microscopy revealed that binding of HSV and PRV gD was localized to adherens junctions in cells maintained in normal medium but was distributed, along with nectin-1, over the entire cell surface after calcium depletion. Both the binding of gD and the fraction of cells that could be infected by HSV-1 and PRV were enhanced by calcium depletion. Taken together, these results provide evidence that nectin-1 confined to adherens junctions in epithelial cells is not very accessible to virus, whereas dissociation of cell junctions releases nectin-1 to serve more efficiently as an entry receptor.
Human herpes simplex virus types 1 and 2 (HSV-1 and HSV-2), porcine pseudorabies virus (PRV), and bovine herpesvirus 1 are members of the alphaherpesvirus subfamily of herpesviruses. At the time of primary infection, these viruses replicate in mucosal epithelia or skin and then enter nerve endings of sensory neurons to establish latent infections. Upon reactivation from latency, viruses are produced in neuronal cells and transported back to the epithelia to cause recurrent lesions. Virus can also move through neuronal connections to the central nervous system to cause encephalitis.
Human and animal representatives of the alphaherpesviruses have a broad host range and similar requirements for entry into cells (30). The first step is usually binding of virion envelope glycoprotein C (gC) and/or gB to cell surface glycosaminoglycans, preferentially heparan sulfate. The subsequent interaction of virion gD with one of its receptors triggers the penetration of virus, which occurs through fusion of the viral envelope with the cell membrane and requires four virion glycoproteins, gB, gD, gH, and gL.
Three classes of cell surface molecules can serve as gD receptors for the entry of HSV-1 or HSV-2 (30). These include a member of the tumor necrosis factor receptor family (22), two members of the immunoglobulin (Ig) superfamily (6, 12, 18, 33) and specific sites in heparan sulfate generated by the action of certain isoforms of 3-O-sulfotransferase (28). The Ig superfamily members were recently named nectin-1 and nectin-2 (26, 32) and were previously called Prr1 and Prr2 (9, 19), respectively, or HveC and HveB (12, 33), respectively. Human nectin-1 and nectin-2 can serve as entry receptors for PRV as well as HSV. Many human cell types, such as epithelial cells, express multiple HSV entry receptors, including herpesvirus entry mediator (HVEM), nectin-1, and nectin-2 (12, 33).
Nectin-1 and nectin-2 are related to nectin-3 and nectin-4 as well as to the poliovirus receptor (9, 19, 24, 26). All five proteins, each encoded by a different gene, have three extracellular Ig-like domains, an N-terminal V-like domain, and two C2-like domains and are homologous with respect to amino acid sequence. The nectins are Ca2+-independent cell adhesion molecules that can engage in homophilic or heterophilic interactions. In some instances, HSV-1 gD can block these interactions (17, 25). Differentially spliced transcripts from the nectin genes can be translated to yield multiple isoforms with identical ectodomains. Most of the membrane-bound isoforms can bind through their cytoplasmic tails to the PDZ domain in l-afadin. The nectins and l-afadin colocalize with E-cadherin and catenins in adherens junctions of epithelial cells (31, 32). Depletion of extracellular Ca2+ can cause rapid disruption of adherens junctions with redistribution of nectin and turnover of E-cadherin (1, 15).
If nectin-1 or nectin-2 is localized to adherens junctions in epithelial cells, are these gD receptors accessible for binding to virus? In this study, we used Madin-Darby canine kidney (MDCK) cells to examine the effects of junctional disruption on cell surface localization of nectin-1, binding to the cells of HSV-1 gD and PRV gD, and susceptibility of the cells to viral entry. MDCK cells expressing transfected human nectin-1 or an endogenous entry receptor were used because, in either case, the only receptors available for HSV and PRV entry were human nectin-1 and/or a molecule antigenically related to human nectin-1, probably dog nectin-1. Thus, use of the MDCK cells facilitated investigation of the relationship between cell surface distribution of a single type of entry receptor and susceptibility of the cells to HSV and PRV entry.
MATERIALS AND METHODS
Cells and viruses.
MDCK cells (34-CCL, American Type Culture Collection) were grown in Dulbecco’s modified Eagle’s medium (DME) with 10% fetal calf serum (FCS). MDCK cells stably expressing human Flag-nectin 1α (32), designated here MDCK-nectin-1 cells, were provided by Y. Takai (Osaka University Medical School, Osaka, Japan) and grown in DME with 10% FCS and G418 at 300 μg/ml (Gibco-BRL). The epitope tag in Flag-nectin-1α is on the natural N terminus resulting from signal peptidase cleavage. Control experiments done, as described previously (12), to compare the entry activities of nectin-1α and Flag-nectin-1α revealed that the epitope tag did not prevent HSV-1 or PRV entry, although entry was not as efficient as that observed with the untagged form of receptor (data not shown).
The β-galactosidase reporter viruses have been described previously (2, 12, 33). HSV-1(KOS)tk12 was propagated and titers were determined on Vero cells, and PRV(Kaplan)gH− (a gift from T. Mettenleiter, Federal Research Center for Virus Diseases of Animals, Insel Riems, Germany) was propagated and titers were determined on gH−expressing Vero-SW78 cells.
Ca2+ switch protocol.
Cells were grown in DME containing 2 mM Ca2+ with 10% FCS (NC medium). To deplete the cells of Ca2+ as described previously (4), the cells were washed with phosphate-buffered saline and transferred for 1 or 2 h to Ca2+-free DME with 1 mM EDTA and 10% FCS that had been depleted of Ca2+ by passage over Chelex-100 (Bio-Rad) (LC medium, ∼2 μM Ca2+).
gD:Fc binding assay.
The production and quantitation of herpesvirus gD:Fc hybrid proteins and assay for binding of the gD:Fcs to cells were described previously (10, 11). HSV-1 gD:Fc has the first 345 amino acids (first 320 amino acids after signal peptide cleavage) of HSV-1(KOS) gD fused through a linker of 6 amino acids (YRARIH) to 231 amino acids at the C terminus of rabbit IgG heavy chain. The PRV gD:Fc is similar except that the first 408 amino acids (first 391 amino acids after signal peptide cleavage) of PRV(Kaplan) gD is fused through a 3-amino-acid linker (RIH) to the rabbit sequence. MDCK cells grown in 96-well plates were incubated with serial dilutions of HSV-1 gD:Fc or PRV gD:Fc for 1 h at 37°C. Cells were washed, fixed with 2% formaldehyde and 0.2% glutaraldehyde, and sequentially incubated with biotinylated anti-rabbit IgG (Sigma), Amdex streptavidin-conjugated horseradish peroxidase (HRP; Amersham), and HRP substrate (BioFx Lab). Binding was monitored at 370 nm in a Spectra Max 250 enzyme-linked immunosorbent assay reader. Alternatively, cells grown on coverslips were incubated with gD:Fc, and binding was visualized with Alexa 488-conjugated goat anti-rabbit IgG (Molecular Probes). To assess the ability of antibodies to block gD:Fc binding, serial dilutions of monoclonal antibodies recognizing nectin-1 (R1.302, Immunotech) (6) or nectin-2 (R2.477, Immunotech) (18) were added to cells 1 h prior to the addition of a constant amount of gD:Fc.
Viral entry assay.
The assay for viral entry was described previously (22). Serial dilutions of β-galactosidase-expressing viruses were added to cells grown in 96-well plates and incubated for 6 h. Cells were washed, permeabilized, and incubated with β-galactosidase substrate, O-nitrophenyl-β-d-galactopyranoside (ONPG; Sigma). The reaction was monitored at 410 nm to quantitate viral entry. Alternatively, infected cells were permeabilized and incubated with the β-galactosidase substrate, X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside; Gibco-BRL), which yields an insoluble blue reaction product. The stained cells were photographed with an Olympus microscope (model CK2), equipped with a 35-mm camera, controlled by the Olympus automatic exposure photographic system (PM-10AK3).
Immunofluorescence and microscopy.
Indirect immunofluorescence was carried out as described previously (32). The primary antibodies used were mouse monoclonal anti-nectin-1 antibodies, R1.302 (6) and CK6 and CK8 (16), mouse anti-Flag (M1, Sigma), and rat anti-E-cadherin (ECCD2; Zymed) antibodies. The secondary antibodies were Alexa 488-conjugated goat anti-mouse IgG and Alexa 568-conjugated goat anti-rat IgG. Immunofluorescence observations were made with a Zeiss LSM510 confocal microscope equipped with a 100×, 1.4 NA oil immersion objective. Orthogonal sections were made in a z stack of 30 images (1-μm interval) according to the LSM510 operating manual.
RESULTS AND DISCUSSION
Distribution of nectin-1 and E-cadherin before and after disruption of adherens junctions.
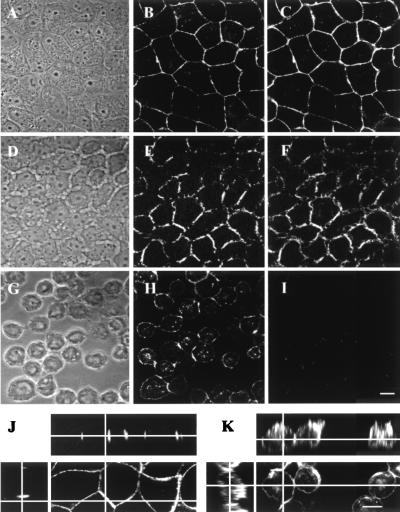
MDCK cells and MDCK cells stably transfected to express Flag-tagged human nectin-1α (referred to here as MDCK-nectin-1 cells) were grown to confluence in medium containing standard levels of Ca2+ (2 mM; NC medium) and then either maintained in this medium or switched to medium with greatly reduced levels of Ca2+ (∼2 μM; LC medium). As shown previously (32), Flag-tagged nectin-1 and E-cadherin colocalized at cell junctions in MDCK-nectin-1 cells maintained in NC medium (Fig. 1A to C). Following depletion of Ca2+ from the culture medium, dissociation of cell-cell junctions was in initial stages by 1 h (Fig. 1D to F) and was nearly complete by 2 h (Fig. 1G to I). E-cadherin was no longer detectable after 2 h (Fig. 1G and I), as previously shown (15). Under the same conditions, nectin-1 remained detectable on the cell surfaces (Fig. 1H) as previously described (1). Orthogonal sectional views demonstrated that nectin-1 was localized only at cell-cell junctions in NC medium and redistributed over large areas of the cell surface after Ca2+depletion (Fig. 1J and K).
FIG. 1.
Localization of Flag-tagged nectin-1 and E-cadherin in MDCK-nectin-1 cells before and after a switch to medium depleted of Ca2+. Cells were maintained in NC medium (A to C) or switched to LC medium for 1 h (D to F) or 2 h (G to I) and then double stained with mouse anti-Flag (B, E, H, J, and K) and rat anti-E-cadherin (C, F, and I) antibodies. (A, D, and G) Phase-contrast images. (J and K) Orthogonal sections of anti-Flag-stained MDCK-nectin-1 cells maintained in NC medium (J) or in LC medium for 2 h (K). Bar, 10 μm.
The antibody used to detect nectin-1 in Fig. 1 was specific for the Flag tag on the human nectin-1 expressed in the MDCK-nectin-1 cells. Similar results were obtained with anti-nectin-1 antibodies (CK6, CK8, and R1.302). These antibodies also bound to an endogenous dog antigen in the MDCK cells maintained in NC medium (data not shown), resulting in a weak fluorescence pattern indistinguishable from that observed in Fig. 1. Evidence is presented below that MDCK cells express a viral entry receptor related to human nectin-1, probably dog nectin-1. Although dog nectin-1 has not been sequenced, human, monkey, pig, cow, mouse, and hamster forms of nectin-1 were shown to exhibit more than 92% amino acid sequence identity within the ectodomain for all pairwise comparisons (21, 27). Mammalian nectin-1 is much more highly conserved in sequence than are nectin-2 (9, 23, 29) and HVEM (14, 22).
Enhanced binding of gD to epithelial cells depleted of Ca2+.
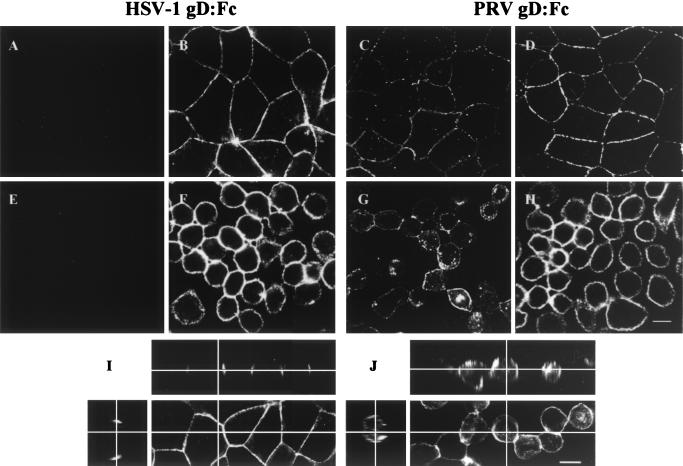
To assess the presence and distribution of gD receptors, MDCK cells and MDCK-nectin-1 cells were maintained in NC medium or switched to LC medium for 2 h and then were incubated with soluble forms of HSV-1 gD and PRV gD (gD:Fc hybrid molecules), followed by fixation and addition of labeled anti-Fc antibodies. Figure 2 shows that both the HSV-1 and PRV gD:Fcs bound to the MDCK-nectin-1 cells. Also, PRV, but not HSV-1, gD:Fc bound to the untransfected MDCK cells. PRV gD is known to bind to human nectin-1 with higher affinity than HSV-1 gD (8). One explanation for the results in Fig. 2A, C, E, and G is that PRV gD also binds with higher affinity to the endogenous dog receptor than does HSV-1 gD and that the latter interaction is too weak to be detected by the use of soluble gD:Fc. MDCK cells must express an HSV-1 receptor, because MDCK cells can be infected by HSV-1.
FIG. 2.
Binding of HSV-1 gD:Fc and PRV-gD:Fc to MDCK cells (A, C, E, and G) and MDCK-nectin-1 cells (B, D, F, and H). The cells were maintained in NC medium (A to D) or switched to LC medium for 2 h (E to H) and then incubated with either HSV-1 gD:Fc (A, B, E, F, I, and J) or PRV gD:Fc (C, D, G, and H), followed by fixation and incubation with Alexa 488-conjugated goat anti-rabbit IgG. (I and J) Orthogonal sections of HSV-1 gD:Fc-stained MDCK-nectin-1 cells maintained in NC medium (I) or LC medium for 2 h (J). Bar, 10 μm.
The binding of both gD:Fcs was localized to the junctions of cells maintained in NC medium (Fig. 2B, C, D, and I) and was distributed over the cell surface after the switch to LC medium for 2 h (Fig. 2F, G, H, and J). This localization and subsequent redistribution are indistinguishable from those observed for human nectin-1 (compare with Fig. 1).
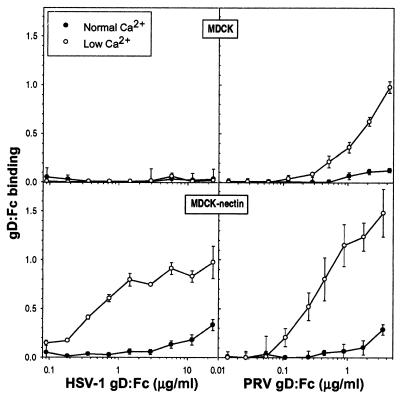
To investigate whether depletion of Ca2+ and the redistribution of gD receptors were accompanied by altered amounts of gD:Fc bound to cells, a quantitative binding assay was performed on live MDCK cells or MDCK-nectin-1 cells maintained in NC or LC medium. There was no stable binding of HSV-1 gD:Fc to MDCK cells, regardless of the conditions (Fig. 3), consistent with the immunofluorescence observations in Fig. 2. Greater amounts of HSV-1 gD:Fc bound to MDCK-nectin-1 cells incubated for 2 h in LC medium than to cells maintained in NC medium. There was also enhanced binding of PRV gD:Fc to both MDCK cells and MDCK-nectin-1 cells in response to Ca2+ depletion (Fig. 3).
FIG. 3.
Enhanced binding of HSV-1 gD:Fc and PRV-gD:Fc to MDCK cells and MDCK-nectin-1 cells after a switch to LC medium. Cells grown in 96-well plates were maintained in NC medium or switched to LC medium for 2 h and incubated with either HSV-1 gD:Fc or PRV gD:Fc for 1 h. Cells were then washed, fixed, and sequentially incubated with biotinylated goat anti-rabbit IgG, streptavidin-conjugated HRP, and HRP substrate. Values are optical densities at 370 nm; means of triplicate determinations with standard deviations for one representative experiment of five replicates are shown.
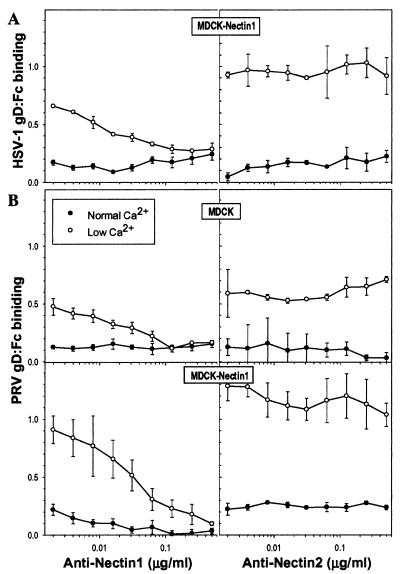
To confirm that the enhanced gD binding in response to Ca2+ depletion depended on direct interaction with nectin-1, binding of gD:Fc was quantitated in the absence or presence of serial dilutions of anti-nectin-1 or anti-nectin-2 monoclonal antibodies. The anti-nectin-1 antibody used (R1.302) was previously shown to compete with soluble forms of HSV-1 and PRV gD for binding to human and porcine forms of nectin-1 (5, 16, 21). This antibody completely inhibited the enhanced binding of HSV-1 gD:Fc to MDCK-nectin-1 cells depleted for Ca2+ whereas the anti-nectin-2 antibody was without effect (Fig. 4A). Anti-nectin-1, but not anti-nectin-2, also inhibited the enhanced binding of PRV gD:Fc to both the MDCK cells and the MDCK-nectin-1 cells depleted of Ca2+ (Fig. 4B). Thus, the enhanced binding of HSV-1 and PRV gD:Fcs after Ca2+ depletion was due to increased availability of human nectin-1 or an endogenous gD receptor that is antigenically related to human nectin-1, probably dog nectin-1.
FIG. 4.
Anti-nectin-1 antibody blocks the enhanced binding of HSV-1 gD:Fc and PRV gD:Fc to MDCK cells and MDCK-nectin-1 cells. Cells in 96-well plates were maintained in NC medium or switched to LC medium for 2 h and incubated with serial dilutions of monoclonal antibodies against nectin-1 (R1.302.12) or nectin-2 (R2.477.1) for 1 h prior to addition of a single dose of HSV-1 gD:Fc (24 μg/ml) (A) or PRV gD:Fc (4 μg/ml) (B). After 1 h of incubation, cells were washed, fixed, and incubated with biotinylated secondary antibody, streptavidin-conjugated HRP, and HRP substrate. Values are optical densities at 370 nm; means of triplicate determinations with standard deviations for one representative experiment of five replicates are shown.
Enhanced entry of HSV-1 and PRV into epithelial cells depleted of Ca2+.
Studies done previously with MDCK cells showed that disruption of cell junctions by Ca2+ depletion resulted in increased ability of HSV-1 to infect, replicate, or spread from cell to cell, as assessed by infectious-center assays on HeLa cell monolayers (13). However, it was not determined whether the increase in number of infectious centers was due to enhanced entry of virus into the cells or enhanced replication and spread of virus within the MDCK monolayer. To resolve this question, entry activities of HSV-1 and PRV were compared before and after the disruption of adherens junctions in MDCK and MDCK-nectin-1 cells.
Viral entry assays were performed using cells maintained in NC medium or switched for 2 h to LC medium prior to incubation with serial dilutions of HSV-1(KOS)tk12 or PRV-gH−. These viruses express β-galactosidase from a lacZ cassette inserted within their genome. At 6 h after addition of virus, cells were lysed to quantitate β-galactosidase activity using either ONPG or X-Gal as the substrate. Expression of β-galactosidase signals the entry of virus into cells and expression of viral and reporter genes.
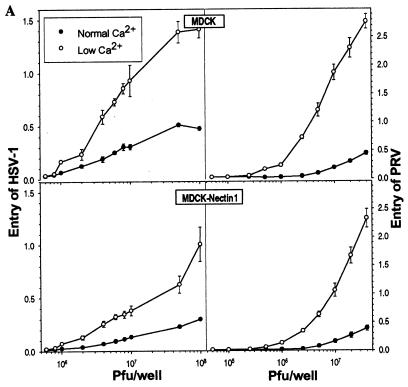
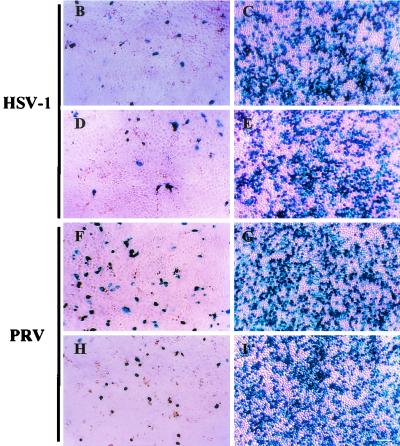
Depletion of Ca2+ from the culture medium significantly enhanced the entry of both HSV-1 and PRV into MDCK cells and MDCK-nectin-1 cells. Quantitative results presented in Fig. 5A show that viral entry was more efficient for the cells depleted of Ca2+ than for cells maintained in NC medium at each of the input doses of virus tested. The levels of β-galactosidase activity were related to the numbers of cells infected at each virus dose, as shown by the X-Gal-stained cells in Fig. 5B to I. Thus, the disruption of cell junctions and release of junctional nectin-1 to larger areas of the cell surface correlated with enhanced susceptibility of the cells to viral entry. We estimate that this enhancement was at least 10-fold, based on the amounts of virus required to achieve equivalent numbers of infected cells under the two conditions.
FIG. 5.
Enhanced entry of HSV-1 and PRV after a switch to LC medium. Cells in 96-well plates (2 × 104 to 4 × 104 cells per well) were maintained in NC medium or switched to LC medium for 2 h and incubated with serial dilutions of β-galactosidase-expressing HSV-1 or PRV. After 6 h, cells were processed for incubation with the β-galactosidase substrate ONPG (A) or X-Gal (B to I). (A) β-Galactosidase activity was quantitated as a measure of viral entry. Values are optical densities at 410 nm; means of triplicate determinations with standard deviations for one representative experiment of five replicates are shown. (B to I) X-Gal staining at a single virus dose of 107 PFU per well in MDCK cells (B, C, F, and G) and MDCK-nectin-1 cells (D, E, H, and I), cultured in either NC medium (B, D, F, and H) or LC medium (C, E, G, and I) prior to infection. Bar, 100 μm.
Even though HSV-1 infection of the untransfected MDCK cells was enhanced by Ca2+ depletion, we were not able to detect a gD receptor using HSV-1 gD:Fc (Fig. 2 and 3). It seems likely that HSV-1 and PRV used the same endogenous MDCK receptor for entry. Nectin-1 is the most highly conserved of all the alphaherpesvirus entry receptors and the only one that, in human, mouse, and pig forms, has been shown to serve as an entry receptor for both HSV-1 and PRV (6, 12, 20, 21, 29). The MDCK cells clearly express a PRV receptor related to human nectin-1, based on the ability of the antibody specific for human nectin-1 (R1.302) to inhibit the binding of PRV gD:Fc to the cells. Concentrations of this antibody that effectively blocked the binding of gD:Fc to the MDCK cells and MDCK-nectin-1 cells (Fig. 4), up to 2.5 μg/ml, preincubated with the cells at 37°C for 30 min, failed to inhibit infection of the cells by either HSV-1 or PRV before or after Ca2+ depletion (data not shown). The same antibody was shown to prevent HSV infection of cells that expressed human (6) or porcine (21) nectin-1 when higher concentrations of antibody were preincubated with the cells at 4°C for more than 90 min. In this study, preincubation at 4°C was not done, so as to maintain normal cell architecture. Both the availability of an endogenous dog receptor and the particular conditions used here for treating cells with the R1.302 antibody may explain the failure to protect MDCK cells from infection.
Insights into viral entry of epithelial cells.
Recently, two cell surface glycoproteins, the coxsackievirus and adenovirus receptor (CAR) and the junction adhesion molecule, were identified as transmembrane components of the tight junction in epithelial cells as well as entry receptors for coxsackie virus and adenovirus and for reovirus, respectively (3, 7). Disruption of tight junctions by Ca2+ depletion enhanced CAR-mediated coxsackievirus and adenovirus infection in polarized T-84 epithelial cells (7). Sequestration of CAR in tight junctions apparently limits viral infection, similar to our findings that the localization of nectin-1 to adherens junctions actually impairs its ability to mediate HSV-1 and PRV entry. Although soluble forms of gD could bind to nectin-1 even when it was localized to cell junctions and presumably engaged in homophilic trans interactions, the junctions probably do not allow access of virions to the nectin-1 so engaged. Thus, if nectin-1 is the principal receptor for viral entry into epithelial cells at the portal of entry into the natural host, then some damage to the epithelium may be required for efficient viral entry. Alternatively, other gD receptors, including forms of nectin-1 that do not bind to l-afadin (nectin-1β), could mediate entry of virus into the first cells of an intact epithelium to be infected.
Acknowledgments
We thank Y. Takai for the MDCK cells expressing Flag-tagged human nectin-1, R. Eisenberg and G. Cohen for anti-nectin-1 antibodies (CK6 and CK8), R. Goldman for use of his confocal microscope, and N. Susmarski and A. Fridberg for technical assistance.
This work was supported by National Institutes of Health grant R37 AI36293.
REFERENCES
- 1.Asakura, T., H. Nakanishi, T. Sakisaka, K. Takahashi, K. Mandai, M. Nishimura, T. Sasaki, and Y. Takai. 1999. Similar and differential behaviour between the nectin-afadin-ponsin and cadherin-catenin systems during the formation and disruption of the polarized junctional alignment in epithelial cells. Genes Cells 4:573-581. [DOI] [PubMed] [Google Scholar]
- 2.Babic, N., B. G. Klupp, B. Makoschey, A. Karger, A. Flamand, and T. C. Mettenleiter. 1996. Glycoprotein gH of pseudorabies virus is essential for penetration and propagation in cell culture and in the nervous system of mice. J. Gen. Virol. 77:2277-2285. [DOI] [PubMed] [Google Scholar]
- 3.Barton, E. S., J. C. Forrest, J. L. Connolly, J. D. Chappell, Y. Liu, F. J. Schnell, A. Nusrat, C. A. Parkos, and T. S. Dermody. 2001. Junction adhesion molecule is a receptor for reovirus. Cell 104:441-451. [DOI] [PubMed] [Google Scholar]
- 4.Clement, S., A. V. Trejo-Skalli, L. Gu, P. T. Velasco, L. Lorand, and R. D. Goldman. 1997. A transglutaminase-related antigen associates with keratin filaments in some mouse epidermal cells. J. Investig. Dermatol. 109:778-782. [DOI] [PubMed] [Google Scholar]
- 5.Cocchi, F., M. Lopez, L. Menotti, M. Aoubala, P. Dubreuil, and G. Campadelli-Fiume. 1998. The V domain of herpesvirus Ig-like receptor (HIgR) contains a major functional region in herpes simplex virus-1 entry into cells and interacts physically with the viral glycoprotein D. Proc. Natl. Acad. Sci. USA 95:15700-15705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cocchi, F., L. Menotti, P. Mirandola, M. Lopez, and G. Campadelli-Fiume. 1998. The ectodomain of a novel member of the immunoglobulin subfamily related to the poliovirus receptor has the attributes of a bona fide receptor for herpes simplex virus types 1 and 2 in human cells. J. Virol. 72:9992-10002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cohen, C. J., J. T. C. Shieh, R. J. Pickles, T. Okegawa, J.-T. Hsieh, and J. M. Bergelson. 2001. The coxsackievirus and adenovirus receptor is a transmembrane component of the tight junction. Proc. Natl. Acad. Sci. USA 98:15191-15196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Connolly, S. A., J. C. Whitbeck, A. H. Rux, C. Krummenacher, S. Van Drunen Littel-van den Hurk, G. H. Cohen, and R. J. Eisenberg. 2001. Glycoprotein D homologs in herpes simplex virus type 1, pseudorabies virus, and bovine herpes virus type 1 bind directly to human HveC (nectin-1) with different affinities. Virology 280:7-18. [DOI] [PubMed] [Google Scholar]
- 9.Eberlé, F., P. Dubreuil, M. G. Mattei, E. Devilard, and M. Lopez. 1995. The human PRR2 gene, related to the human poliovirus receptor gene (PVR), is the true homolog of the murine MPH gene. Gene 159:267-272. [DOI] [PubMed] [Google Scholar]
- 10.Geraghty, R. J., A. Fridberg, C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 2001. Use of chimeric nectin-1 (HveC)-related receptors to demonstrate that ability to bind alphaherpesvirus gD is not necessarily sufficient for viral entry. Virology 285:366-375. [DOI] [PubMed] [Google Scholar]
- 11.Geraghty, R. J., C. R. Jogger, and P. G. Spear. 2000. Cellular expression of alphaherpesvirus gD interferes with entry of homologous and heterologous alphaherpesviruses by blocking access to a shared gD receptor. Virology 268:147-158. [DOI] [PubMed] [Google Scholar]
- 12.Geraghty, R. J., C. Krummenacher, G. H. Cohen, R. J. Eisenberg, and P. G. Spear. 1998. Entry of alphaherpesviruses mediated by poliovirus receptor-related protein 1 and poliovirus receptor. Science 280:1618-1620. [DOI] [PubMed] [Google Scholar]
- 13.Hayashi, K. 1995. Role of tight junctions of polarized epithelial MDCK cells in the replication of herpes simplex virus type 1. J. Med. Virol. 47:323-329. [DOI] [PubMed] [Google Scholar]
- 14.Hsu, H., I. Solovyev, A. Colombero, R. Elliott, M. Kelley, and W. J. Boyle. 1997. ATAR, a novel tumor necrosis factor receptor family member, signals through TRAF2 and TRAF5*. J. Biol. Chem. 272:13471-13474. [DOI] [PubMed] [Google Scholar]
- 15.Kartenbeck, J., M. Schmelz, W. W. Franke, and B. Geiger. 1991. Endocytosis of junctional cadherins in bovine kidney epithelial (MDBK) cells cultured in low Ca2+ ion medium. J. Cell Biol. 113:881-892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Krummenacher, C., I. Baribaud, M. Ponce de Leon, J. C. Whitbeck, H. Lou, G. H. Cohen, and R. J. Eisenberg. 2000. Localization of a binding site for herpes simplex virus glycoprotein D on herpesvirus entry mediator C by using antireceptor monoclonal antibodies. J. Virol. 74:10863-10872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krummenacher, C., I. Baribaud, J. F. Sanzo, G. H. Cohen, and R. J. Eisenberg. 2002. Effects of herpes simplex virus on structure and function of nectin-1/HveC. J. Virol. 76:2424-2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lopez, M., F. Cocchi, L. Menotti, E. Avitabile, P. Dubreuil, and G. Campadelli-Fiume. 2000. Nectin2α (PRR2α or HveB) and nectin2δ are low-efficiency mediators for entry of herpes simplex virus mutants carrying the Leu25Pro substitution in glycoprotein D. J. Virol. 74:1267-1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez, M., F. Eberlé, M. G. Mattei, J. Gabert, F. Birg, F. Bardin, C. Maroc, and P. Dubreuil. 1995. Complementary DNA characterization and chromosomal localization of a human gene related to the poliovirus receptor-encoding gene. Gene 155:261-265. [DOI] [PubMed] [Google Scholar]
- 20.Menotti, L., M. Lopez, E. Avitabile, A. Stefan, F. Cocchi, J. Adelaide, E. Lecocq, P. Dubreuil, and G. Campadelli-Fiume. 2000. The murine homolog of human nectin1δ serves as a species nonspecific mediator for entry of human and animal αherpesviruses in a pathway independent of a detectable binding to gD. Proc. Nat. Acad. Sci. USA 97:4867-4872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Milne, R. S. B., S. A. Connolly, C. Krummenacher, R. J. Eisenberg, and G. H. Cohen. 2001. Porcine HveC, a member of the highly conserved HveC/nectin 1 family, is a functional alphaherpesvirus receptor. Virology 281:315-328. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery, R. I., M. S. Warner, B. J. Lum, and P. G. Spear. 1996. Herpes simplex virus-1 entry into cells mediated by a novel member of the TNF/NGF receptor family. Cell 87:427-436. [DOI] [PubMed] [Google Scholar]
- 23.Morrison, M. E., and V. R. Racaniello. 1992. Molecular cloning and expression of a murine homolog of the human poliovirus receptor gene. J. Virol. 66:2807-2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reymond, N., S. Fabre, E. Lecocq, J. Adelaide, P. Dubreuil, and M. Lopez. 2001. Nectin4/PRR4, a new afadin-associated member of the nectin family that trans-interacts with nectin1/PRR1 through V domain interaction. J. Biol. Chem. 276:43205-43215. [DOI] [PubMed] [Google Scholar]
- 25.Sakisaka, T., T. Taniguchi, H. Nakanishi, K. Takahashi, M. Miyahara, W. Ikeda, S. Yokoyama, Y. F. Peng, K. Yamanishi, and Y. Takai. 2001. Requirement of interaction of nectin-1 alpha/HveC with afadin for efficient cell-cell spread of herpes simplex virus type 1. J. Virol. 75:4734-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Satoh-Horikawa, K., H. Nakanishi, K. Takahashi, M. Miyahara, M. Nishimura, K. Tachibana, A. Mizoguchi, and Y. Takai. 2000. Nectin-3, a new member of immunoglobulin-like cell adhesion molecules that shows homophilic and heterophilic cell-cell adhesion activities. J. Biol. Chem. 275:10291-10299. [DOI] [PubMed] [Google Scholar]
- 27.Shukla, D., M. Dal Canto, C. L. Rowe, and P. G. Spear. 2000. Striking similarity of murine nectin-1α to human nectin-1α (HveC) in sequence and activity as a gD receptor for alphaherpesvirus entry. J. Virol. 74:11773-11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shukla, D., J. Liu, P. Blaiklock, N. W. Shworak, X. Bai, J. D. Esko, G. H. Cohen, R. J. Eisenberg, R. D. Rosenberg, and P. G. Spear. 1999. A novel role for 3-O-sulfated heparan sulfate in herpes simplex virus 1 entry. Cell 99:13-22. [DOI] [PubMed] [Google Scholar]
- 29.Shukla, D., C. L. Rowe, Y. Dong, V. R. Racaniello, and P. G. Spear. 1999. The murine homolog (Mph) of human herpesvirus entry protein B (HveB) mediates entry of pseudorabies virus but not herpes simplex virus types 1 and 2. J. Virol. 73:4493-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spear, P. G., R. J. Eisenberg, and G. H. Cohen. 2000. Three classes of cell surface receptors for alphaherpesvirus entry. Virology 275:1-8. [DOI] [PubMed] [Google Scholar]
- 31.Tachibana, K., H. Nakanishi, K. Mandai, K. Ozaki, W. Ikeda, Y. Yamamoto, A. Nagafuchi, S. Tsukita, and Y. Takai. 2000. Two cell adhesion molecules, nectin and cadherin, interact through their cytoplasmic domain-associated proteins. J. Cell Biol. 150:1161-1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takahashi, K., H. Nakanishi, M. Miyahara, K. Mandai, K. Satoh, A. Satoh, H. Nishioka, J. Aoki, A. Nomoto, A. Mizoguchi, and Y. Takai. 1999. Nectin/PRR: an immunoglobulin-like cell adhesion molecule recruited to cadherin-based adherens junctions through interaction with afadin, a PDZ domain-containing protein. J. Cell Biol. 145:539-549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Warner, M. S., R. J. Geraghty, W. M. Martinez, R. I. Montgomery, J. C. Whitbeck, R. Xu, R. J. Eisenberg, G. H. Cohen, and P. G. Spear. 1998. A cell surface protein with herpesvirus entry activity (HveB) confers susceptibility to infection by mutants of herpes simplex virus type 1, herpes simplex virus type 2 and pseudorabies virus. Virology 246:179-189. [DOI] [PubMed] [Google Scholar]