Abstract
Herpes simplex virus (HSV) exhibits altered gene regulation in neuronal compared to nonneuronal tissues. It has been hypothesized that initiation of DNA synthesis at the viral origins of replication (oriS and oriL) is a critical step in the upregulation of transcriptional activity of flanking divergent promoters, thereby increasing productive gene expression in neurons. Notably, oriS is flanked by the immediate-early (IE) ICP4 and ICP22/47 promoters, and oriL is flanked by the early (E) UL29 and UL30 promoters. To test this hypothesis further, a series of constructs were generated in which these promoters were placed upstream of luciferase genes. In addition, DNA replication origins were deleted in the context of these promoter constructs. All cassettes were recombined into the viral genome of HSV type 1 strain KOS at a site distal to its native origins. Recombinant reporter expression was monitored in vitro and in vivo to determine the role of viral origins of DNA replication in the regulation of their flanking promoters. Reporter gene expression was unaffected by the presence or absence of oriS or oriL, with the exception of a twofold increase in ICP22/47 promoter activity in the absence of oriS. DNA synthesis inhibitors resulted in a decrease of both IE- and E-promoter activity in primary cells but not continuous cell cultures. Reporter activity was readily assayed in vivo during acute infection and reactivation from latency and was also sensitive to DNA synthesis inhibitors. In all assays, reporter gene expression was unaffected by the presence or absence of either oriS or oriL. These data support the requirement of DNA synthesis for full viral gene expression in vivo but suggest that the origin elements play no role in the regulation of their flanking promoters.
Herpes simplex virus (HSV) gene expression during the course of in vitro infection has been well characterized (5, 6, 20, 26). Shortly after viral entry, a complex consisting of the viral tegument protein virion protein 16 (VP16) and the cellular factors Oct-1 and host cell factor transactivate immediate early (IE) promoters regulating ICP0, -4, -22, -27, and -47. These gene products, in turn, upregulate the expression of early (E) proteins involved in viral DNA replication and nucleotide metabolism. Concomitant with the initiation of viral DNA synthesis, late transcripts coding for structural and tegument proteins are expressed, allowing viral assembly and subsequent egress from the infected cell. Relatively little, however, is known about the molecular events regulating viral gene expression during infection in vivo. Specifically, the pattern of gene regulation during the establishment of and reactivation from viral latency remains, largely, a mystery (16). Previous reports have suggested that viral DNA synthesis is required for productive gene expression within the nervous system. Null mutants lacking thymidine kinase, an E gene, are unable to replicate in neuronal tissues and demonstrate a paradoxical decrease in both viral IE and E transcription in murine trigeminal ganglia during establishment of latency (10, 12). Treatment of latently infected explanted ganglia with DNA synthesis inhibitors also results in reduced IE and E transcription, suggesting that viral DNA synthesis is necessary for optimal gene expression during reactivation (11). Reduced IE and E gene expression has also been observed upon infection of cultured superior cervical ganglion neurons in the presence of the viral DNA synthesis inhibitor acyclovir (ACV) (14). In the same study, infection of neurons with an origin binding protein (UL9)-null virus resulted in even further reduced levels of E gene expression, which was refractory to inhibition by ACV. In Vero cells, however, reporter expression was unaffected by ACV treatment or UL9 deletion. These data suggest that initiation of viral DNA synthesis at the origins of replication, rather than genome amplification, is a critical step in productive gene expression in neurons.
HSV contains three origins of replication: two copies of oriS located in the IRS/TRS regions and one copy of oriL located within the unique long region. All are highly homologous and contain UL9 binding sites flanking AT-rich spacers. Deletions of oriL or both copies of oriS have little effect on viral replication in vitro, suggesting a role other than DNA replication for origin conservation (7, 15). Intriguingly, all of the origins are flanked by promoters regulating gene products critical for the life cycle of the virus. The IE promoters driving ICP4 and ICP22/47 expression flank oriS, whereas the E promoters regulating UL29 and UL30 flank oriL (17, 28). These flanking promoters are required for efficient origin function, but the converse role of the origins in the regulation of flanking gene expression is unknown (29). Such studies of origin function are complicated by the fact that origin disruption has multiple effects on both gene expression and viral DNA synthesis. In this study, the functional significance of the origins of replication, with regard to their role in the regulation of flanking genes, was addressed by using reporter cassettes inserted into the HSV type 1 (HSV-1) genome at a site distal to its native origins. This approach leaves the native origins intact, allowing specific study of the contribution of origins on the regulation of flanking promoters in an ectopic reporter cassette. Results demonstrate that the origins of replication have no significant role in the regulation of flanking promoters in vitro or in vivo.
MATERIALS AND METHODS
Cells and virus.
All viruses were propagated on Vero cells as previously described (18) and were constructed in the context of the KOS strain of HSV-1. Dissociated trigeminal ganglion (dTG) cultures were generated as described previously (9) to obtain a single-cell suspension. Cells were plated on collagen-coated 24-well plates at approximately 0.5 ganglion per well. CD-1 mouse embryonic fibroblasts (MEFs) were harvested as previously described (8). Both Vero cells and MEFs were plated at 105 cells/well 18 to 20 h before infection for all culture experiments. Cycloheximide reversal (CHR) experiments were performed as described previously (23). DNA synthesis inhibition experiments were performed similarly to CHR experiments. Briefly, monolayers were pretreated with 400 μg of phosphonoacetic acid (PAA; Sigma, St. Louis, Mo.) per ml or 50 μM ACV (Sigma) for 1 h followed by infection at a multiplicity of infection (MOI) of 5 in the presence of drug. Eight hours postinfection, cells were harvested in 100 μl of passive lysis buffer (Promega, Madison, Wis.), and luciferase activity was measured.
Plasmids and generation of recombinant viruses.
The luciferase reporter plasmid pDlux and the subsequent pDlux/oriS clone have been described (23). Site-directed mutagenesis was performed to specifically delete the minimum oriS sequence to yield pDlux/ΔoriS (Fig. 1). Plasmid 1502 (kindly supplied by Sandra Weller), containing oriL and flanking regulatory regions, was propagated in Sure 2 cells (Stratagene, La Jolla, Calif.). A 435-bp SmaI/BamHI fragment from plasmid 1502 encoding oriL and flanking regulatory regions was isolated and blunt-end ligated into the NheI site of pDlux to yield pDlux/oriL (Fig. 1). This clone was transformed and propagated in Sure 2 cells. A previously described 144-bp deletion of oriL (27) was obtained by passage of pDlux/oriL through DH5α to yield pDlux/ΔoriL. All pDlux cassettes were cloned into the BglII site at position 106750 of plasmid pUIC. Origin-dependent plasmid amplification assays were performed as follows: subconfluent Vero monolayers in 35-mm culture plates were transfected with 1 μg of plasmid using Lipofectamine (GibcoBRL, Carlsbad, Calif.) per the manufacturer's protocol. At 24 h posttransfection, cells were infected with KOS at an MOI of 5 in the presence or absence of 400 μg of PAA per ml or 50 μM ACV. At 24 h postinfection, cells were lysed, DNA was extracted, and equal amounts were digested with PstI or PstI/DpnI. Digested plasmid was separated by gel electrophoresis, blotted, probed, and visualized with a Storm phosphorimager (Molecular Dynamics, Sunnyvale, Calif.). Transient transfections of reporter constructs were performed as described for plasmid amplification assays. At 24 h posttransfection, monolayers were treated with replication inhibitors as described above and harvested 8 h posttreatment for luciferase expression. All clones were sequenced to confirm appropriate deletions, PstI linearized, and cotransfected with infectious KOS DNA to allow recombination. Putative recombinant viruses were plaque purified three times, and genotype was confirmed via Southern blot hybridization (data not shown).
FIG. 1.
Maps of reporter gene plasmids and viruses used in this study. (A) Prototypical arrangement of the HSV-1 genome, showing the unique long (UL) and unique short (US) segments flanked by internal (a′, b′, and c′) and terminal (a, b, and c) repeats. The location of reporter cassette insertions within the viral genome is shown. The BglII site is at nucleotide position 106750, in the C terminus of ICP49.5. (B) KOS/Dlux/oriS has been described (23). The oriS-containing fragment is an 823-bp BamHI-NruI fragment comprising HSV-1 nucleotide positions 131399 to 132221. The center of oriS maps to 131999 with the ICP4 transcript starting at position 131429 and ICP22/47 starting at 132126. Regions within the reporter cassettes that encode origin elements and UL9 binding sites (boxes I, II, and III) are enlarged. Origin deletions are in italics, with the limits of the deletion in bold. (C) KOS/Dlux/ΔoriS was generated in the same manner as KOS/Dlux/oriS, but the reporter cassette harbors a 62-bp deletion of the oriS region. (D) KOS/Dlux/oriL was constructed by recombination with a cassette encoding the UL29/30 and oriL region regulating luciferases. The oriL-containing fragment is a 436-bp BamHI-SmaI fragment comprising HSV-1 nucleotide positions 62238 to 62673. The center of oriL maps to 62475, with the UL29 transcript starting at position 62331 and UL30 starting at 62617. Origin deletions are in italics, with the limits of the deletion in bold. (E) KOS/Dlux/ΔoriL was generated in the same manner as KOS/Dlux/oriL but with a 144-bp deletion in the oriL region.
Animal procedures.
Outbred CD-1 female mice (weight, 21 to 25 g; Charles River Breeding Laboratories, Inc., Kingston, N.Y.) were infected, harvested, and assayed for viral growth and reporter gene activity as previously described (23). Periocular disease was scored in a masked fashion on a semiquantitative scale as previously described (23).
Establishment of latency and reactivation assays.
Trigeminal ganglia from latently infected animals were harvested 28 days postinfection and pooled. To assess establishment of latency, DNA was isolated from individual ganglia with DNeasy columns following manufacturer's protocols (Qiagen, Valencia, Calif.). Real-time PCR was performed on an iCycler (Bio-Rad, Richmond, Calif.) with the following thermocycle parameters: 95°C for 20 s, 62°C for 20 s, and 72°C for 20 s. Reaction mixtures (50 μl) contained 10× buffer, 1.5 mM MgCl2, 50 μM deoxynucleoside triphosphates, 1.25 U of Taq (Promega), 0.5 μl of a 1:500 dilution of SYBR green (Molecular Probes, Eugene, Oreg.), and 10 pmol of each primer specific for the HSV-1 UL41 gene (5′-GGCGGATACGAAGACGACG-3′ and 5′-GCCACATAACTGCGGTGCTC-3′). A standard curve of viral infectious DNA in the background of mouse DNA was included in each set of reactions. All reactions were run in triplicate, and copy number was determined by comparison to infectious DNA standards using iCycler software. To assess reactivation, individual trigeminal ganglia were dissociated (9) and plated on collagen-coated 12-well plates. Supernatants were assayed every 12 h for progeny virus from 1 to 5 days postplating.
RESULTS
Plasmid and recombinant virus construction.
The previously characterized bidirectional reporter construct, pDlux, was used to generate cassettes encoding both oriS and oriL regulatory regions yielding pDlux/oriS and pDlux/oriL. Site-directed mutagenesis was performed in order to delete a 62-bp region of pDlux/oriS encompassing the oriS genetic element including all UL9 binding sites and the AT-rich region to yield pDlux/ΔoriS. pDlux/oriL was transformed and propagated in DH5α in order to generate a previously characterized spontaneous deletion of the oriL genetic element termed pDlux/ΔoriL (Fig. 1). Cassettes encompassing the regulatory regions and luciferase genes were isolated and ligated into pUIC to allow recombination into HSV-1 strain KOS (Fig. 1). The appropriate sequences were confirmed by DNA sequencing prior to virus generation (data not shown).
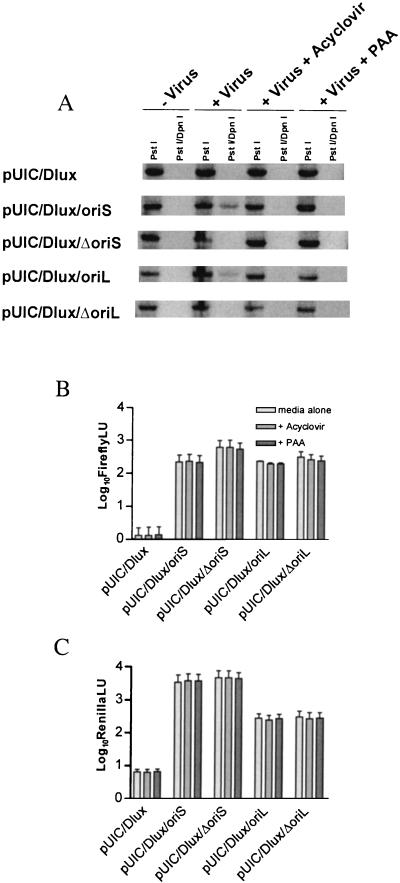
Origin-dependent DNA replication activity was determined by using origin-dependent plasmid amplification assays (Fig. 2A). As expected, origin-containing plasmids (pUICDlux/oriS and pUICDlux/oriL) replicated after superinfection with KOS, whereas origin deletion plasmids (pUICDlux/ΔoriS and pUICDlux/ΔoriL) did not. In addition, the DNA replication inhibitors PAA at 400 μg/ml or ACV at 50 μM completely abolished plasmid amplification, validating their use at the given concentrations in subsequent assays. To determine the effect of the origin deletions on basal-level promoter regulation, luciferase expression was assessed in transiently transfected Vero monolayers (Fig. 2B). Luciferase activity was normalized to plasmid DNA extracted from transfected cell lysate. The 62-bp deletion of oriS resulted in a two- to threefold increase in (firefly) luciferase regulated by the ICP22/47 promoter; however, ICP4, UL29, and UL30 promoter regulation appeared to be unaffected by either oriS or oriL deletions. In addition, the DNA synthesis inhibitors PAA and ACV had no effect on basal level reporter expression in transfection assays. Taken together, these data suggest that oriS and oriL have little if any effect on basal promoter function in continuous cell culture. In addition, these data show that PAA and ACV are inhibitory to DNA replication but not promoter activity at the concentrations used. All subsequent experiments in this study were performed with the recombinant viruses generated from these plasmids (Fig. 1). Southern blotting following three rounds of plaque purification showed band sizes in complete concordance with expected values and confirmed that recombination events with native origins had not occurred to repair the deleted origins in KOS/Dlux/ΔoriS and KOS/Dlux/ΔoriL (data not shown).
FIG. 2.
Origin-dependent amplification of, and luciferase expression from, reporter plasmids. (A) Total DNA harvested from Vero cells transfected with reporter plasmids and mock infected or infected with KOS in the presence or absence of DNA replication inhibitors. DNA was linearized, blotted, and probed. Amplified plasmid was differentiated from input plasmid by DpnI digestion. (B) Monolayers transfected with reporter plasmids were harvested after 32 h and assayed for firefly luciferase expression in the presence or absence of DNA replication inhibitors, which were added at 24 h. (C) Monolayers transfected with reporter plasmids were harvested after 32 h and assayed for Renilla luciferase expression in the presence of absence of DNA replication inhibitors, which were added at 24 h. All data are means and standard errors of the means for at least two independent experiments in duplicate. LU, light units.
Replication and reporter gene expression in vitro.
The growth of all viruses was examined in African green monkey kidney cells (Vero cells), CD-1 MEFs, and CD-1 dTG. At various times postinfection, infectious-virus titers in supernatants were determined and monolayers were harvested to measure luciferase activity. The kinetics of viral growth and egress were equivalent among all recombinant viruses tested (Fig. 3A). Viral egress was detectable in all cultures at approximately 12 h postinfection in contrast with previous reports of slower growth kinetics in cells of neuronal origin, although this may be explained in part by the relatively lower percentage of neurons in our dTG cultures (14). A slight growth decrease of all recombinants at 24 h, relative to KOS, was observed in dTG and MEFs, suggesting that insertion of the luciferase cassettes into the BglII site (position 106750 [23]) has a slightly detrimental effect on viral replication in primary cell cultures.
FIG. 3.
Growth kinetics and reporter activity of recombinant viruses in vitro. Vero, MEF, and dTG cultures were infected at an MOI of 5 with either KOS, KOS/Dlux/oriS, KOS/Dlux/ΔoriS, KOS/Dlux/oriL, or KOS/Dlux/ΔoriL. (A) Plaque assays of supernatants in which infectious particles titers were determined on Vero cells. The limit of detection was 10 PFU/ml. (B) Kinetics of firefly luciferase expression for the four recombinant viruses in each of the three cell types. (C) Kinetics of Renilla luciferase expression for the four recombinant viruses in each of the three cell types. Data are means and standard errors of the means for at least two independent experiments in duplicate. LU, light units.
Reporter gene activity was detectable early in infection and rose concomitant with the detection of viral progeny in all cell cultures tested. Reporter activity was unaltered by the presence or absence of the origins of DNA replication and was generated comparably by all promoters (Fig. 3B and C). The only exception was a two- to threefold increase in luciferase regulated by ICP22/47 in the absence of oriS. This result agrees with transient-transfection data showing a slight effect of the oriS deletion on basal expression of the ICP22/47 promoter, possibly reflecting promoter synergy as a result of this deletion. In order to validate the appropriate kinetics of luciferase expression from all recombinant viruses, a CHR procedure was used, which allows assignment of transcripts to specific kinetic classes (2, 5, 6, 20). Vero cell cultures were infected in the presence or absence of cycloheximide, and reporter activity was measured after removal of cycloheximide in the presence of actinomycin D (Fig. 4). If luciferases were being regulated appropriately, we would expect to see only IE-regulated reporter activity (ICP4 or ICP22/47) in a CHR experiment. Infections of Vero cells under CHR conditions resulted in detectable luciferase expression from the ICP22/47 promoter, although it was significantly reduced relative to untreated controls, and overexpression of luciferase from the ICP4 promoter (Fig. 4A to D). In contrast, luciferase activity directed by the UL29 or UL30 promoter was essentially abolished by CHR (Fig. 4E to H). These results are in strong agreement with previously published data and demonstrate that luciferases expressed from KOS/Dlux/oriS and KOS/Dlux//ΔoriS are regulated as IE genes whereas luciferases encoded by KOS/Dlux/oriL and KOS/Dlux//ΔoriL are regulated as E genes in Vero cell culture (4, 26). The presence or absence of either origin had no effect on reporter expression regardless of treatment.
FIG. 4.
Luciferase reporter activity for recombinant viruses under CHR or control conditions. Promoter activity measured, with or without origin is indicated above each graph (A to H). Vero, MEF, and dTG cultures were pretreated for 1 h and infected at an MOI of 5 under control (C) or CHR (R) conditions. Data show activity as a percentage of that in untreated cultures and are means and standard errors of the means for at least two independent experiments in duplicate. Asterisks indicate a statistical difference as determined by Student's t test (P < 0.05).
Reporter gene regulation in cells of primary origin was compared with regulation in Vero cells (Fig. 4). Many similarities were observed, but there were some notable differences under CHR conditions. In particular, ICP22/47 directed expression of luciferase was completely abolished in dTG cultures (Fig. 4A and B). In addition, luciferase expression regulated by ICP4 was significantly reduced in MEFs and dTG in contrast to the overexpression observed in Vero cells (Fig. 4C and D). Patterns of expression were independent of the presence or absence of either origin of DNA replication. These results suggests a partial dependence on de novo protein synthesis for appropriate IE gene expression in primary cells.
Previous studies have reported productive viral gene expression requires DNA synthesis in neuronal but not nonneuronal tissues and cells (10, 11, 14). A role for viral components, specifically UL9, contacting oriS or oriL has been suggested to explain, in part, this requirement. To correlate these earlier studies with our own, as well as to determine whether the origins have an effect on the regulation of flanking genes, cultures were infected in the presence and absence of PAA and assayed for reporter expression. To ensure that viral egress and reinfection were not confounding our results, reporter activity was assayed 8 h postinfection. Viral DNA synthesis inhibition by PAA was confirmed by slot blot analysis (data not shown) as well as origin-dependent plasmid amplification assays (Fig. 2A). Treatment with PAA resulted in a significant decrease in expression from all promoters in primary cell cultures (Fig. 5). Similar results were obtained in experiments using cultured rat superior cervical ganglion neurons and using ACV and PAA (data not shown). This effect was observed regardless of the presence or absence of either origin. Assuming that PAA is not acting nonspecifically to downregulate promoters in these assays, these results are consistent with previous studies suggesting that DNA replication is critical for maximal IE gene expression in neurons (14). These data additionally suggest that there may be a requirement for DNA synthesis for optimum IE gene expression in primary cells, other than those of neuronal origin.
FIG. 5.
Luciferase reporter activity for recombinant viruses under viral DNA synthesis inhibition or control conditions. Promoter activity in the presence or absence of either origin is indicated above each graph (A to H). Vero, MEF, and dTG cultures were left untreated (C) or pretreated for 1 h with PAA (P) and infected at an MOI of 5 for 8 h prior to assay for luciferase activity. Data show activity as a percentage of that in untreated cultures and are means and standard errors of the means for at least two independent experiments in duplicate. Asterisks indicate a statistical difference as determined by Student's t test (P < 0.05).
Growth kinetics and gene expression in vivo. (i) Acute replication.
Previous studies observed a tissue- and cell-type-specific alteration in the viral gene expression cascade during in vivo infection (10-12). Specifically, viruses which are unable to replicate in the nervous system demonstrate reduced IE and E gene expression relative to wild-type virus during acute infection. Reporter viruses were used to monitor viral IE and E gene expression patterns and determine the effect that origin elements have on these patterns during acute infection in various tissues. Mice were infected via scarified cornea with all recombinant viruses. Whole eyes, trigeminal ganglia, and periocular skin were harvested at various times postinfection, and progeny virus titers were determined. Reporter gene expression was measured in the same lysates to correlate the presence of virus with luciferase activity. Recombinants were compared to wild-type strain KOS for viral growth as well as the ability to cause periocular disease. Viral replication and disease scores were comparable for all of the recombinant viruses, with slight decreases in ganglion replication and disease scores relative to KOS (Fig. 6). This is in agreement with a slight growth decrease of recombinants in primary cell cultures of all recombinants, as well as the phenotype of other recombinants harboring insertions within this locus. The profile of luciferase activity and titer for all viruses peaked in the eyes at 24 h, in the trigeminal ganglia at 60 h, and in the periocular tissue at 96 h, in agreement with previously published observations (23) (Fig. 6, 7, and 8). Detection of viral progeny and reporter activity in the trigeminal ganglia at 24 to 36 h was also in strong agreement with previous detection of viral genome and transcription in this tissue using PCR methods (12). Luciferase expression varied slightly in the presence or absence of either origin; however, no consistently significant difference was observed regardless of tissue tested, promoter monitored, or time point taken (Fig. 7 and 8). These data are consistent with our in vitro data showing that DNA replication, rather than initiation at the origin, is critical for viral gene expression in vivo.
FIG. 6.
Growth and pathogenesis of KOS and recombinant viruses after ocular infection. Mice were infected via corneal scarification and inoculation of 2 × 107 PFU per eye. At various times postinfection, eyes (A), trigeminal ganglia (B), and periocular tissue (C) were harvested and assayed for infectious virus. Data points represent eight tissues from four independent experiments. (D) Periocular disease development was monitored after ocular infection over a 30-day time course. Data are means and standard errors of the means for at least 30 scores.
FIG. 7.
Firefly luciferase expression in the presence or absence of either oriS or oriL genetic elements in vivo. Mice were infected via corneal scarification and inoculation of 2 × 107 PFU per eye. At various times postinfection, eyes (A and B), trigeminal ganglia (C and D), and periocular tissues (E and F) were harvested and assayed for firefly activity regulated by either the ICP22/47 or UL29 promoter. Data are means and standard errors of the means for eight tissues from four independent experiments. LU, light units.
FIG. 8.
Renilla luciferase expression in the presence or absence of either oriS or oriL genetic elements in vivo. Mice were infected via corneal scarification and inoculation of 2 × 107 PFU per eye. At various times postinfection, eyes (A and B), trigeminal ganglia (C and D), and periocular tissues (E and F) were harvested and assayed for Renilla luciferase activity regulated by either the ICP4 or UL30 promoter. Data are means of and standard errors of the means for eight tissues from four independent experiments. LU, light units.
(ii) Reactivation from latency.
To ensure that all recombinants retained the ability to establish latency, real-time quantitative PCR was performed to determine viral genome loads in latently infected trigeminal ganglia. Total DNA from individual trigeminal ganglia was harvested from mice 28 days postinfection. PCR using primers specific for the HSV-1 UL41 gene was performed in triplicate, and results were compared to a standard curve of infectious viral DNA in the background of mouse DNA. The data suggest that despite a slight growth defect in the trigeminal ganglia, all recombinants establish latent infections with genome loads equivalent to that of KOS (Table 1). In addition, our results are in agreement with previously published measurements of viral genome loads during latency (21). To ensure the ability of all recombinants to reactivate, latently infected trigeminal ganglia were dissociated and plated, and supernatant was monitored at various times for viral egress. Sporadic viral egress from dissociated cultures was detectable by 36 h postexplantation. All recombinants retained the ability to reactivate from latency equivalently to wild-type strain KOS, validating their use in monitoring promoter regulation during reactivation from latency (Table 1).
TABLE 1.
Establishment and reactivation from latency of KOS and recombinant viruses
| Virus | Log10 genomes/TGa | Reactivationb (no. of ganglia with virions) |
|---|---|---|
| KOS | 4.525 ± 0.111 (12) | 12 |
| KOS/Dlux/oriS | 4.408 ± 0.106 (13) | 11 |
| KOS/Dlux/ΔoriS | 4.561 ± 0.102 (14) | 11 |
| KOS/Dlux/oriL | 4.600 ± 0.142 (14) | 12 |
| KOS/Dlux/ΔoriL | 4.614 ± 0.154 (12) | 10 |
Establishment of latency was determined by real-time quantitative PCR for viral genome. Copy numbers were determined by using a standard dose curve of infectious viral DNA in a background of mouse DNA. Numbers of ganglia analyzed are shown in parentheses; data are means ± standard errors of the means. TG, trigeminal gangion.
dTG were harvested from mice 28 days postinfection. Supernatants were harvested at various times postexplantation, and titers were determined to monitor viral egress. A total of 16 ganglia were tested in each case.
Previous studies have reported viral gene expression during reactivation from latency as measured by in situ hybridization (11). These studies compared viral gene expression between PAA-treated and untreated latently infected ganglia at 48 h postexplantation and demonstrated that DNA synthesis inhibition led to a reduction in IE and E gene expression. To confirm this effect with our reporter virus system, as well as to explore whether origins can influence viral gene expression during initial steps in reactivation, latently infected trigeminal ganglia were explanted in the presence or absence of ACV and PAA. From 32 to 36 h postexplantation, ganglia were harvested and assayed for luciferase expression. This time frame was chosen so as not to be significantly impacted by reactivating virus, which may acutely reinfect cells. The results suggest a sporadic pattern of gene expression from individual trigeminal ganglia in untreated controls in agreement with the asynchronous nature of viral reactivation (Fig. 9). Little or no luciferase activity was observed in PAA-treated ganglia, in strong agreement with published data demonstrating that this DNA synthesis inhibitor has an inhibitory effect on IE and E viral gene expression during reactivation (11). Quantitative assessment of the effects of origins on gene expression was impossible due to the sporadic and low levels of luciferase expression observed, but overall the results observed were consistent with the previous observations in this study that the presence of a functional origin does not significantly affect the regulation of flanking promoters.
FIG. 9.
Luciferase activity from latently infected trigeminal ganglia following explantation in the presence or absence of PAA. Reporter gene expression in the presence or the absence of oriS or oriL as regulated by ICP22/47, ICP4, UL29, and UL30 is shown (A to H). Trigeminal ganglia were harvested from mice 28 days postinfection and explanted into medium alone or medium containing PAA. At 32 to 36 h postexplantation, ganglia were mechanically disrupted and assayed for luciferase expression.
DISCUSSION
We have previously described recombinant viruses with reporter cassettes inserted into the UL49.5 open reading frame of strain KOS (23). This insertion site, although not completely neutral, results in little or no alteration in in vitro or in vivo growth, with the exception of the trigeminal ganglia, where a reproducible 5- to 10-fold decrease in replication is observed. This decrease in recombinant viral replication, relative to KOS, is also seen in growth curves in dTG and MEFs as well as primary superior cervical ganglion cultures, suggesting that insertions at this site are slightly detrimental to growth in primary tissues. Despite this, these recombinants replicate, cause disease, and establish and reactivate from latency comparably to wild-type virus. As such, they are useful tools for monitoring IE and E gene expression throughout all stages of infection in a number of different in vitro and in vivo systems.
A number of studies have demonstrated that full, productive viral gene expression requires DNA synthesis in neuronal cells (10, 11, 13). In addition, components of the replication complex, specifically UL9, have been implicated in appropriate gene expression in neurons, leading to the hypothesis that initiation of viral DNA synthesis, in the absence of genome amplification, may contribute to gene regulation (14). This hypothesis is provocative, considering that each origin of replication is directly flanked by promoters regulating IE and E gene products. In this study we constructed and utilized a series of reporter viruses to determine the role of the viral origins of DNA synthesis in flanking promoter regulation. Insertion of HSV-derived promoters into ectopic loci in the HSV genome does not disrupt their regulation, providing a useful tool for their study (1, 19). One caveat is that the system used here assumes that the additional origins function with an efficiency similar to that of the native origins in the context of viral infection. In agreement with previously published data, however, we find that full productive gene expression does depend on viral DNA synthesis in cells of neuronal tissue origin in vitro and in vivo during reactivation. This effect was observed regardless of the presence or absence of either origin of replication in vitro or in vivo, suggesting that the elements, or components binding to them, do not play a role in the regulation of flanking promoters. This argument is further supported by recent studies utilizing adenoviral vectors to assess the ability of different HSV proteins to cause reactivation from latency (3). Those authors demonstrated that infection of latently infected trigeminal ganglia cultures with a VP16-expressing adenoviral vector resulted in efficient reactivation from latency whereas infection with UL9-expressing adenovirus had no effect. This suggested that although UL9 may be necessary, it is not sufficient to induce reactivation. With respect to oriL and its potential role in regulation of flanking promoters, some previous studies have made predictions of regulatory sequences based on alignments with the tk promoter (22, 30). Based on these studies, the major transcriptional initiation sites and putative TATA boxes for UL29 and UL30 and the “first distal signal” for UL29 are all intact in the oriL deletion virus. The “second distal signal” for UL29 and the Sp1 site for UL30 were deleted. Given the very small changes in luciferase activity seen in any cell type or tissue resulting from the deletion of oriL, we can conclude that these elements are largely dispensable for UL29/30 regulation in cell culture and in vivo.
Inhibition of DNA synthesis has been reported to lead to decreased IE and E viral gene expression in neuronal tissues in vivo and in cultured neuronal cells but showed minimal alterations in Vero cells (11, 14). One interpretation of these studies is that the inhibition of DNA replication leading to reduced IE gene expression is a neuron-specific phenomenon. The present study shows a similar effect in MEFs, suggesting that inhibition of IE and E viral gene expression by PAA and ACV may extend to other primary cell types.
We also report here that CHR conditions yield different patterns of ICP4 and ICP22/47 promoter activities depending upon the cell type infected. Our data obtained with Vero cells are in agreement with previous observations with hyperactivity of the ICP4 promoter and reduced activity of ICP22/47 (26). In primary cells in contrast, ICP4 promoter activity was reduced in the presence of cycloheximide, suggesting that maximal ICP4 expression requires protein synthesis in these cells. In dTG, there was a complete block of ICP22/47 promoter activity in the absence of protein synthesis. One likely hypothesis to explain these results is that tegument-derived VP16 can only weakly transactivate these IE promoters in primary cells. This result may have important implications during infection of the sensory nervous system, as both ICP4 and ICP22 are required for viral growth in vivo. Inefficient VP16 transactivation has been proposed to explain the inability of the virus to maintain a lytic program in the neurons (24, 25). Interestingly, VP16 or ICP4 delivered by an adenoviral vector results in reactivation from latently infected trigeminal ganglia cultures, suggesting that under the appropriate conditions or dosage, these proteins can direct lytic gene expression in neuronal cells (3). The role of VP16 in regulating IE promoters in neuronal tissue is an active area of research, and we propose that the recombinant viruses and cultures reported in this study offer a relevant system for studying VP16's role in gene expression during neuronal infection.
The mechanism through which IE and E promoters show sensitivity to DNA replication inhibitors in primary cells is unknown. Our data are in strong agreement with previous studies suggesting that genome amplification is required for productive viral gene expression and may be a critical deciding factor between a lytic or latent infection (9). Cells of primary origin may require DNA amplification for reasons such as repressor protein titration and template alteration as previously proposed. In addition, the consequences of DNA replication, such as L gene expression, may play a role in maintaining productive gene expression in vivo. In this environment, L gene products such as VP16 may be critical to achieve a full productive cascade of viral transcription. This is consistent with previous reports that VP16 transactivation mutants grow poorly or not at all in primary and neuronal tissue (20, 21). This study, however, strongly argues against a direct role of origin function in the regulation of flanking promoters.
Acknowledgments
We thank Skip Virgin, Sam Speck, Lynda Morrison, and members of their laboratories for helpful discussions and Sandy Weller for generous provision of reagents.
This study was supported by NIH grants RO1 EY09083 to David A. Leib and P30-EY02687 to the Department of Ophthalmology and Visual Sciences. Support from Research to Prevent Blindness to the department and a Robert E. McCormick Scholarship to David A. Leib are gratefully acknowledged.
REFERENCES
- 1.Drolet, B. S., G. C. Perng, R. J. Villosis, S. M. Slanina, A. B. Nesburn, and S. L. Wechsler. 1999. Expression of the first 811 nucleotides of the herpes simplex virus type 1 latency-associated transcript (LAT) partially restores wild-type spontaneous reactivation to a LAT-null mutant. Virology 253:96-106. [DOI] [PubMed] [Google Scholar]
- 2.Gelman, I. H., and S. Silverstein. 1987. Dissection of immediate-early gene promoters from herpes simplex virus: sequences that respond to the virus transcriptional activators. J. Virol. 61:3167-3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Halford, W. P., C. D. Kemp, J. A. Isler, D. J. Davido, and P. A. Schaffer. 2001. ICP0, ICP4, or VP16 expressed from adenovirus vectors induces reactivation of latent herpes simplex virus type 1 in primary cultures of latently infected trigeminal ganglion cells. J. Virol. 75:6143-6153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harris-Hamilton, E., and S. L. Bachenheimer. 1985. Accumulation of herpes simplex virus type 1 RNAs of different kinetic classes in the cytoplasm of infected cells. J. Virol. 53:144-151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Honess, R. W., and B. Roizman. 1974. Regulation of herpesvirus macromolecular synthesis. I. Cascade regulation of the synthesis of three groups of viral proteins. J. Virol. 14:8-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Honess, R. W., and B. Roizman. 1975. Regulation of herpesvirus macromolecular synthesis: sequential transition of polypeptide synthesis requires functional viral polypeptides. Proc. Natl. Acad. Sci. USA 72:1276-1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Igarashi, K., R. Fawl, R. J. Roller, and B. Roizman. 1993. Construction and properties of a recombinant herpes simplex virus 1 lacking both S-component origins of DNA synthesis. J. Virol. 67:2123-2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jacobson, J. G., D. A. Leib, D. J. Goldstein, C. L. Bogard, P. A. Schaffer, S. K. Weller, and D. M. Coen. 1989. A herpes simplex virus ribonucleotide reductase deletion mutant is defective for productive acute and reactivatable latent infections of mice and for replication in mouse cells. Virology 173:276-283. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy, P. G., R. P. Lisak, and M. C. Raff. 1980. Cell type-specific markers for human glial and neuronal cells in culture. Lab. Investig. 43:342-351. [PubMed] [Google Scholar]
- 10.Kosz-Vnenchak, M., D. M. Coen, and D. M. Knipe. 1990. Restricted expression of herpes simplex virus lytic genes during establishment of latent infection by thymidine kinase-negative mutant viruses. J. Virol. 64:5396-5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kosz-Vnenchak, M., J. Jacobson, D. M. Coen, and D. M. Knipe. 1993. Evidence for a novel regulatory pathway for herpes simplex virus gene expression in trigeminal ganglion neurons. J. Virol. 67:5383-5393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kramer, M. F., S. H. Chen, D. M. Knipe, and D. M. Coen. 1998. Accumulation of viral transcripts and DNA during establishment of latency by herpes simplex virus. J. Virol. 72:1177-1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kramer, M. F., and D. M. Coen. 1995. Quantification of transcripts from the ICP4 and thymidine kinase genes in mouse ganglia latently infected with herpes simplex virus. J. Virol. 69:1389-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nichol, P. F., J. Y. Chang, E. M. Johnson, Jr., and P. D. Olivo. 1996. Herpes simplex virus gene expression in neurons: viral DNA synthesis is a critical regulatory event in the branch point between the lytic and latent pathways. J. Virol. 70:5476-5486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Polvino-Bodnar, M., P. K. Orberg, and P. A. Schaffer. 1987. Herpes simplex virus type 1 oriL is not required for virus replication or for the establishment and reactivation of latent infection in mice. J. Virol. 61:3528-3535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Preston, C. M. 2000. Repression of viral transcription during herpes simplex virus latency. J. Gen. Virol. 81:1-19. [DOI] [PubMed] [Google Scholar]
- 17.Quinn, J. P., and D. J. McGeoch. 1985. DNA sequence of the region in the genome of herpes simplex virus type 1 containing the genes for DNA polymerase and the major DNA binding protein. Nucleic Acids Res. 13:8143-8163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rader, K. A., C. E. Ackland-Berglund, J. K. Miller, J. S. Pepose, and D. A. Leib. 1993. In vivo characterization of site-directed mutations in the promoter of the herpes simplex virus type 1 latency-associated transcripts. J. Gen. Virol. 74:1859-1869. [DOI] [PubMed] [Google Scholar]
- 19.Roemer, K., P. A. Johnson, and T. Friedmann. 1991. Activity of the simian virus 40 early promoter-enhancer in herpes simplex virus type 1 vectors is dependent on its position, the infected cell type, and the presence of Vmw175. J. Virol. 65:6900-6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roizman, B., M. Kozak, R. W. Honess, and G. Hayward. 1975. Regulation of herpesvirus macromolecular synthesis: evidence for multilevel regulation of herpes simplex 1 RNA and protein synthesis. Cold Spring Harbor Symp. Quant. Biol. 39:687-701. [DOI] [PubMed] [Google Scholar]
- 21.Strelow, L. I., and D. A. Leib. 1995. Role of the virion host shutoff (vhs) of herpes simplex virus type 1 in latency and pathogenesis. J. Virol. 69:6779-6786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Su, L., and D. M. Knipe. 1987. Mapping of the transcriptional initiation site of the herpes simplex virus type 1 ICP8 gene in infected and transfected cells. J. Virol. 61:615-620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Summers, B. C., T. P. Margolis, and D. A. Leib. 2001. Herpes simplex virus type 1 corneal infection results in periocular disease by zosteriform spread. J. Virol. 75:5069-5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tal-Singer, R., R. Pichyangkura, E. Chung, T. M. Lasner, B. P. Randazzo, J. Q. Trojanowski, N. W. Fraser, and S. J. Triezenberg. 1999. The transcriptional activation domain of VP16 is required for efficient infection and establishment of latency by HSV-1 in the murine peripheral and central nervous systems. Virology 259:20-33. [DOI] [PubMed] [Google Scholar]
- 25.Valyi-Nagy, T., S. L. Deshmane, J. G. Spivack, I. Steiner, C. I. Ace, C. M. Preston, and N. W. Fraser. 1991. Investigation of herpes simplex virus type 1 (HSV-1) gene expression and DNA synthesis during the establishment of latent infection by an HSV-1 mutant, in1814, that does not replicate in mouse trigeminal ganglia. J. Gen. Virol. 72:641-649. [DOI] [PubMed] [Google Scholar]
- 26.Weinheimer, S. P., and S. L. McKnight. 1987. Transcriptional and post-transcriptional controls establish the cascade of herpes simplex virus protein synthesis. J. Mol. Biol. 195:819-833. [DOI] [PubMed] [Google Scholar]
- 27.Weller, S. K., A. Spadaro, J. E. Schaffer, A. W. Murray, A. M. Maxam, and P. A. Schaffer. 1985. Cloning, sequencing, and functional analysis of oriL, a herpes simplex virus type 1 origin of DNA synthesis. Mol. Cell. Biol. 5:930-942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Whitton, J. L., and J. B. Clements. 1984. Replication origins and a sequence involved in coordinate induction of the immediate-early gene family are conserved in an intergenic region of herpes simplex virus. Nucleic Acids Res. 12:2061-2079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong, S. W., and P. A. Schaffer. 1991. Elements in the transcriptional regulatory region flanking herpes simplex virus type 1 oriS stimulate origin function. J. Virol. 65:2601-2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yager, D. R., and D. M. Coen. 1988. Analysis of the transcript of the herpes simplex virus DNA polymerase gene provides evidence that polymerase expression is inefficient at the level of translation. J. Virol. 62:2007-2015. [DOI] [PMC free article] [PubMed] [Google Scholar]