Abstract
Certain glycosaminoglycans (GAGs), including heparin, inhibit infection by murine leukemia virus (MLV). We now show that this is due to inhibition of virus attachment independent of the interaction between viral envelope proteins (Env) and their cellular receptors. Heparin blocked the binding of both Env-deficient and amphotropic MLV (MLV-A) particles to NIH 3T3 fibroblasts, CHO cells which lack the amphotropic retroviral receptor Pit-2, and CHO cells transfected with Pit-2 (CHO-Pit-2). Heparin also inhibited the transduction of NIH 3T3 cells by MLV-A over a similar concentration range. This effect was observed within 15 min of exposure to retrovirus. Preloading target cells with heparin had no effect on transduction and both MLV-A and Env-deficient retrovirus bound efficiently to heparin-coated agarose beads, suggesting that heparin interacts with the virus rather than the target cell. This requires both a strong negative charge and a specific structure since GAGs with different charge and carbohydrate composition inhibited virus infection variably. The specificity of GAG-virus interaction also depends on the producer cells, since virus packaged by murine GP+EnvAM12 cells was 1,000-fold more sensitive to inhibition by chondroitin sulfate A than was virus packaged by human FLYA13 packaging cells. No evidence for an interaction between MLV and cell surface proteoglycans was found, however, since the attachment of MLV-A and envelope-defective virus to proteoglycan-deficient CHOpgsA-745 cells was similar to that seen with both wild-type and CHO-Pit-2 cells. Although the molecular mechanism is unclear, this study presents evidence that Env receptor-independent attachment is an important step in MLV infection.
The attachment of a number of viruses to the cell surface is known to involve proteoglycans, which are widely distributed molecules in which a membrane-linked protein core is posttranslationally modified by the addition of variably charged sulfated glycosaminoglycans (GAGs) (8). Examples include enveloped viruses such as the herpesviruses (22, 29, 32), respiratory syncytial virus (12), and human immunodeficiency virus (21, 24), as well as nonenveloped viruses such as adenovirus (5), adeno-associated virus (30), and foot-and-mouth disease virus (9). In most cases, additional receptors are involved in virus entry so that the absence of surface proteoglycans or competition by soluble GAGs inhibits rather than abolishes infectivity. Cell surface proteoglycans may thus serve to concentrate virus at the cell surface to increase the efficiency of interaction with secondary receptors in the same way that they localize other ligands such as basic fibroblast growth factor (11).
Infection by murine leukemia viruses (MLV) is mediated by interaction between the viral envelope protein (Env) and specific cellular receptors such as the phosphate channel Pit-2, the receptor for amphotropic MLV (MLV-A) (19, 31). In some cells, the level of expression of Pit-2 correlates with transduction efficiency, and this may be an important limiting factor for human hematopoietic stem cells which express very low levels of this molecule (13, 16). Although membrane fusion and virus entry certainly appear to require an interaction between Env and its cognate cellular receptor, recent evidence suggests that initial virus binding is Env independent (25). By using a system in which cell-associated virus was detected by confocal immunofluorescence microscopy for viral capsid, it was demonstrated that both Env-deficient and ecotropic MLV bound to cells regardless of whether the cells expressed the ecotropic receptor. The contribution of such Env-independent attachment to infectivity was not totally clear from this work, although MLV-A poorly infected certain human suspension cells which adsorbed virus particles less efficiently than adherent cell lines. Since soluble GAGs have previously been shown to inhibit transduction by MLV (1, 2, 14, 15) and since GAG-mediated interactions are involved in the initial binding of other viruses to the cell surface, we investigated whether a similar mechanism is responsible for Env-independent binding of MLV.
MATERIALS AND METHODS
Cells and materials.
Wild-type CHO cells (CHO-WT [CHO-K1]) were obtained from European Collection of Cell Cultures (Porton Down, Wiltshire, United Kingdom) and CHOpgsA-745 cells (6), and NIH 3T3 fibroblasts were from the American Type Culture Collection (Bethesda, Md.). Surface proteoglycan expression was assessed by flow cytometry by using fluorescein isothiocyanate (FITC)-labeled anti-heparan sulfate (CN Biosciences, Nottingham, United Kingdom) and gating on live cells by propidium iodide exclusion. The (amphotropic) packaging cell line GP+EnvAM12 and Env-deficient packager GP 101 were obtained from A. Bank (Columbia University, New York, N.Y.) (17, 18). The HT1080-based FLYA13 amphotropic packaging cells and their Env-deficient counterpart have been previously described (4). CHO-WT cells were transfected with the amphotropic retroviral receptor Pit-2 as previously reported (16). All cells were maintained at 37°C under 5% CO2 in complete medium (CM) comprising Dulbecco modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), penicillin G (100 U/ml), and streptomycin (10 mg/ml; all from Gibco-BRL, Paisley, United Kingdom). LacZ pseudotypes were generated by transfecting GP+EnvAM12 and FLYA13 with MFGnlsLacZ, an MLV-based vector containing the β-galactosidase gene linked to a nuclear localization signal. To collect retroviral supernatant, producer cells were grown to 90% confluence in 175-cm2 tissue culture flasks, the medium was removed, and 20 ml of CM added, followed by incubation overnight. The supernatant was removed, centrifuged at 800 × g for 5 min, filtered through a 0.45-μm-pore-size filter (Millipore, Watford, United Kingdom), and stored at −80°C.
Clinical-grade preservative-free heparin (Monoparin; 150 U/mg, 9 to 18 kDa) was obtained from CP Pharmaceuticals (Wrexham, United Kingdom.), and the chemically modified heparins—i.e., (i) completely desulfated N-acetylated (CDSNAc), (ii) completely desulfated N-resulfated (CDSNS), and (iii) N-desulfated N-acetylated (NDSNAc) heparin—were obtained from Seikagaku, Inc. (AMS Biotechnology Suppliers, Abingdon, United Kingdom). All other soluble GAGs were supplied by Sigma (Poole, United Kingdom).
Virus-binding assays.
Cells were seeded onto 5-cm plastic tissue culture dishes at a concentration of 106 cells/dish. After overnight incubation, 2 ml of neat viral suspension with or without heparin was added for 1 h at 37°C. To assess the effect of temperature on virus attachment, incubation was in some cases performed at 4°C. Cells were then washed five times with phosphate-buffered saline (PBS), fixed with 4% paraformaldehyde for 15 min at room temperature, and permeabilized with 0.2% Triton X-100 for 15 min at room temperature. Samples were then washed with PBS and incubated with anti-RLV P30 antibody (Quality Biotech, Inc., Camden, N.J.) diluted 1/3,000 for 45 min at room temperature. Samples were washed three times with PBS, incubated with a 1/200 dilution of FITC-labeled anti-goat secondary antibody (Jackson ImmunoResearch, Inc., Luton, United Kingdom) for 45 min at room temperature, and extensively washed with PBS. After a final wash with distilled water, samples were mounted with immunofluorescence mounting medium (Dako, Ely, United Kingdom) and analyzed by confocal microscopy (an MRC 1024 microscope equipped with a krypton-argon laser; Bio-Rad, Hemel Hempstead, United Kingdom). Parallel sections perpendicular to the z axis were acquired every 0.5 μm. All pictures were obtained by using Kalman filtration and analyzed with Lasersharp software (Bio-Rad). The images shown were obtained by superimposing multiple perpendicular sections of the cells. A semiquantitative assessment of virion binding was derived by assessing the number of particles per cell. The results represent the mean ± the standard error of the mean from at least three cells.
The binding of Env-SU to CHO-WT cells and CHO-Pit-2 transfectants was assessed by flow cytometry as previously described (16). Briefly, cells were detached from the plastic by using EDTA, exposed to retroviral preparations for 1 h at 37°C, and incubated with anti-Env-SU (83A25; L. Evans, Rocky Mountain Laboratory, Hamilton, Mont.) after they were washed to remove excess virus. Cell-associated virus and soluble Env-SU were detected by using a 1:100 dilution of goat anti-rat F(ab)2 fragment conjugated to phycoerythrin (Immunotech, High Wycombe, United Kingdom). The possibility that autofluorescence of bound retrovirus was responsible for any of the signal obtained in these studies was excluded by preliminary experiments with ecotropic retrovirus which gave a signal similar to that of medium alone (data not shown); this was therefore used as a convenient and reproducible negative control. Env binding was measured by flow cytometry (EPICS Elite; Coulter Electronics, Luton, United Kingdom), excluding nonviable cells by TOPRO-5 uptake (Molecular Probes). Listmode data was analyzed by using FlowJo software (Tree Star, Inc., Stanford, Calif.)
Effect of heparin on retroviral transduction.
To measure the percent transduction by LacZ pseudotypes, NIH 3T3 cells were seeded at 5 × 103 per well in 48-well plates and incubated overnight to allow adherence. Medium was removed, and the cells were incubated with retroviral supernatant containing preservative-free clinical-grade heparin or other soluble GAGs for 72 h prior to staining with X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside). After the celles were washed and fixed with PBS containing 0.5% glutaraldehyde, X-Gal stain (X-Gal, 1 mg/ml; sodium deoxycholate, 0.01%; NP-40, 0.02%; MgCl2, 2 mM; potassium ferricyanide, 5 mM; potassium ferrocyanide, 5 mM [Sigma]) was added for 4 h at 37°C and then replaced with PBS containing 1 μg of propidium iodide/ml. The total cell number per high-power field was estimated on a fluorescent microscope, and the percent transduction was assessed by counting the number of blue cells under visible light. The effect of delayed addition of heparin and other GAGs was assessed in the same way.
The impact of heparin on viral titer was measured by endpoint dilution (26). NIH 3T3 cells were seeded at 2.5 × 103 per well in 96-well plates and incubated overnight. Heparin was added at doses of 100 to 0 U/ml to retroviral supernatants which were serially diluted in CM to give a range of concentrations from neat virus to 1:10−8. Dilutions of virus were added to the target cells, and the plates were incubated for 72 h and then stained for β-galactosidase expression as described above. The percentage of uninfected wells was measured, and the numbers of infectious units/milliliter were calculated with a Microsoft Excel spreadsheet (23). In some experiments cells were preloaded with heparin prior to measuring the viral titer by incubating them with 100 U of heparin or diluent control/ml for 1 h at 37°C, followed by washing with PBS.
Effect of heparin on cell proliferation.
In case heparin-mediated inhibition of transduction was due to an effect on the cell cycle, we measured the incorporation of [3H]thymidine into NIH 3T3 cells in the presence of various concentrations of heparin. NIH 3T3 cells were seeded at 2 × 103 per well in a 96-well plate. After 24 h of incubation, heparin or other soluble GAGs were added at various concentrations, [3H]thymidine (1 μCi/well) was added after a further 48 h, and the cells were harvested and counted in a liquid scintillation counter (Wallac, Cambridge, United Kingdom) 18 h later.
Binding of retrovirus to heparin-coated agarose beads.
Heparin-coated agarose beads (approximate diameter, 10 μm; Sigma) were washed twice in PBS and once in DMEM. Washed beads (0.5 ml) were then added to 10 ml of MLV-A or Env-defective MLV retroviral supernatant made in CM with 2% FCS and incubated with mixing for 12 h at 4°C in the presence or absence of 100 U of soluble heparin/ml. The agarose beads were washed twice in plain DMEM and once in PBS and then resuspended in an equal volume of 2× sample buffer (4% [wt/vol] sodium dodecyl sulfate [SDS], 0.15 M dithiothreitol, 20% [vol/vol] glycerol, 0.1% [wt/vol] bromophenol blue). The samples were boiled for 5 min and stored at −70°C prior to analysis by SDS-polyacrylamide gel electrophoresis. After semidry electrotransfer to a nitrocellulose membrane (Hybond C; Amersham), the samples were probed with a goat polyclonal antibody to the viral capsid protein (anti-RLV P30; Quality Biotech) and a mouse anti-goat second layer conjugated to horseradish peroxidase (Dako). Proteins were detected by using enhanced chemoluminescence (Amersham) and autoradiography.
The heparin-agarose binding experiments were also performed with a lysate of retrovirus. A total of 10 ml of MLV-A and Env-deficient MLV suspension containing 2% FCS was pelleted in an ultracentrifuge by using a Beckman SW41Ti rotor at 100,000 × g for 90 min at 4°C. Supernatant was removed, and the virus pellet was resuspended in 100 μl of lysis buffer containing antiproteolytic agents (50 mM HEPES buffer [pH 7.5], 100 mM NaCl, 1% [vol/vol] Triton X-100, 1 mM concentrations each of EDTA and EGTA, 1 mM Pefabloc [Roche, Lewes, United Kingdom], and 10 μg of aprotinin, pepstatin, and leupeptin/ml [all from Sigma]). A 10-μl aliquot of the lysate was retained and added to an equal volume of 2× sample buffer and then boiled and stored as previously described. The remainder was made up to 10 ml in PBS and added to 0.5 ml of washed heparin-agarose beads with protease inhibitors and processed in the presence or absence of heparin as described above.
RESULTS
The effect of soluble GAGs on transduction by MLV-A is packaging cell dependent.
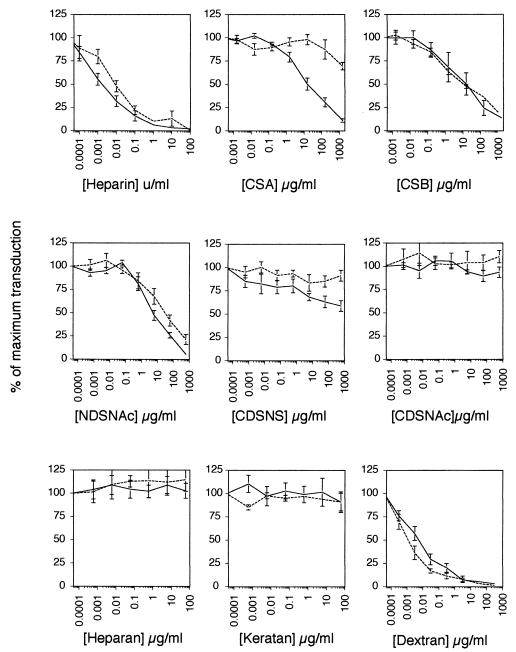
In keeping with previous reports (1, 2, 14, 15), soluble heparin potently inhibited the transduction of NIH 3T3 cells by MLV-A (Fig. 1). Other GAGs had a variable effect; the completely uncharged GAG CDSNAc heparin had no effect on transduction, whereas the partially charged NDSNAc heparin had a reduced effect. Heparan and keratan sulfates, which are both sulfated and negatively charged, were, however, completely without effect, indicating that the inhibitory effect of GAGs on transduction is mediated by both electrostatic charge and the sequence of the carbohydrate backbone.
FIG. 1.
Effect of heparin and other GAGs on transduction of NIH 3T3 cells with MLV vector MFGnlsLacZ produced in either murine GP+EnvAM12 (——) or human FLYA13 (- - -) amphotropic packaging cells.
When the effect of GAGs on virus packaged by different cell types was compared, marked differences were evident. For example, virus packaged by the murine AM12 cells was 1,000 times more sensitive to chondroitin sulfate A than virus packaged by human FLY cells. Significant differences were also seen in the sensitivity to NDSNAc heparin and CDSNS heparin, with AM12 virus being more sensitive to inhibition than FLY-derived retrovirus. Thus, the factor(s) responsible for GAG-mediated inhibition of transduction derives at least in part from the packaging cell. In contrast, dextran sulfate was more efficient on FLY-derived virus, suggesting that there is a qualitative rather than a quantitative difference between the GAG interacting factors from the two cell types.
To determine whether soluble GAGs inhibit transduction through an interaction with the virus or the target cell, we compared the infectivity of NIH 3T3 cells that had been preincubated with heparin with those that had been incubated with diluent alone. No difference in titer was observed for three separate preparations of retrovirus (Table 1), suggesting that GAGs exert their effect on transduction through an interaction with the virus and not the target cell surface.
TABLE 1.
Effect on retroviral titer of target cell pretreatment with heparina
| Pretreatment (U/ml) | Mean IU/ml (SEM) | n | P |
|---|---|---|---|
| Heparin (100) | 4.33 × 107 (1.37 × 107) | 3 | 0.44 |
| Mock | 2.29 × 107 (0.82 × 107) | 3 |
IU, infectious units; n, number of experiments.
Soluble GAGs inhibit an early event in transduction by MLV-A.
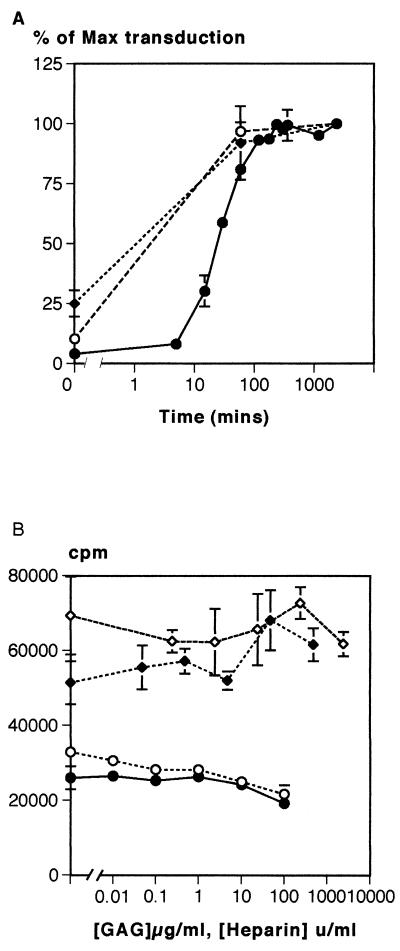
To further elucidate the mechanism by which GAGs inhibit transduction, we performed time course experiments in which the addition of GAGs to the virus supernatant and target cells was delayed (Fig. 2A). These experiments revealed that the inhibitory effect of heparin was confined to the first hour after the addition of virus to the cells. Similar findings were observed with dextran sulfate, and the effect was not cell type specific since the transduction of CHO-Pit-2 cells was inhibited in a similar fashion. Preincubation of retrovirus with GAGs prior to addition to target cells did not alter the effect on transduction (data not shown). Since cell proliferation is required for the integration of MLV genome (20, 27) and GAGs have been reported to inhibit proliferation (8), we investigated their effect on [3H]thymidine incorporation in the cells used in these experiments. Although at the very highest doses some reduction of [3H]thymidine incorporation was observed, over the range in which transduction was inhibited, there was no effect (Fig. 2B).
FIG. 2.
(A) Effect of delayed addition of heparin on retroviral transduction. Heparin-mediated inhibition of transduction mainly occurred during the 30 min after initial exposure to the viral supernatant. There was no observable effect when the addition of heparin was delayed by more than 100 min. Symbols: •, NIH 3T3 + heparin; ♦, NIH 3T3 + dextran sulfate; ○, CHO-Pit-2 plus heparin. (B) Effect of heparin on the proliferation of NIH 3T3 fibroblasts as assessed by measuring the incorporation of [3H]thymidine. An effect on proliferation was only observed at heparin concentrations of ≥100 U/ml, far above the levels that inhibited transduction. Symbols: ○, CHO-Pit-2 plus heparin; •, NIH 3T3 + heparin; ♦, NIH 3T3 + dextran sulfate; ⋄, NIH 3T3 plus chondroitin sulfate.
Env-independent attachment of MLV is strongly inhibited by soluble heparin.
Since heparin and other soluble GAGs inhibit an early event in the transduction process, we investigated whether this was due to interference with virus binding to the cell surface. This was assessed by two methods; a flow cytometric assay that uses a monoclonal antibody to the SU component of the Env protein (16) and confocal immunofluorescence microscopy with an antibody to the viral capsid (CA) (25). The former detects the binding of both virus-associated and soluble SU to the MLV receptor and thus cannot measure attachment of Env-deficient virus, whereas the latter allows any intact cell-associated virions to be visualized.
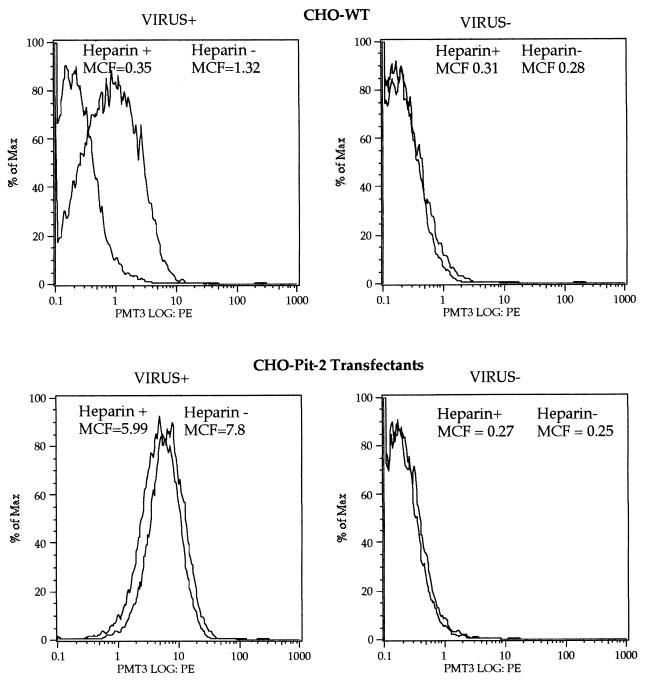
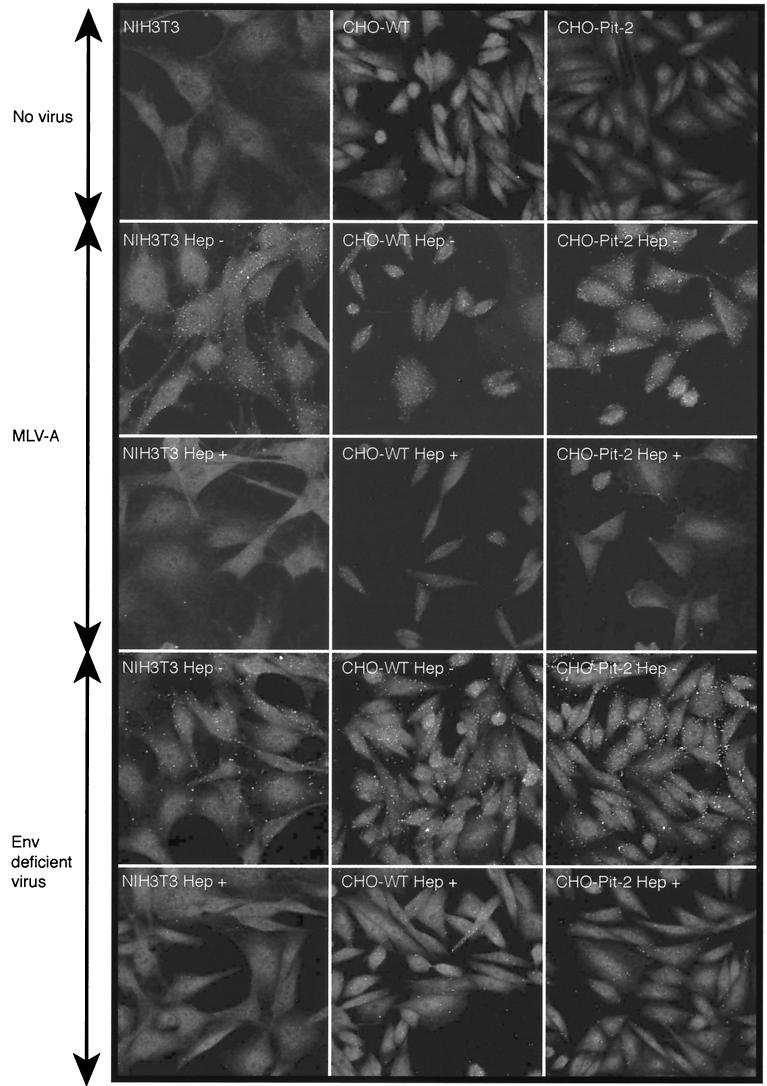
Flow cytometric analyses of Env-SU binding showed dependence on PiT-2 expression since there was a large shift in the CHO-Pit-2 cells but not in the CHO-WT cells (Fig. 3). This is consistent with previous data demonstrating that when “virus” binding is measured in this way, the signal is largely due to soluble rather than to virus-associated Env-SU (25). The small shift in CHO-WT cells, which is probably due to the binding of MLV-A particles, was abolished in the presence of heparin. Only a slight reduction in the Env-SU signal was observed in CHO-Pit-2 cells, suggesting that heparin inhibits the binding of virus particles but not free Env-SU. Accordingly, we hypothesized that heparin might be interfering with the attachment of virus particles and investigated this possibility by confocal microscopy. This revealed that soluble heparin inhibits the binding of both MLV-A and Env-deficient virus to NIH 3T3 fibroblasts, receptor-deficient CHO-WT cells and the CHO-Pit-2 transfectant (Fig. 4). These observations confirm previous studies (25) showing that attachment of MLV-A is independent of the presence of both Env and its cognate receptor Pit-2 and further demonstrate that such Env-independent attachment is inhibited by soluble GAGs such as heparin.
FIG. 3.
Effect of heparin (100 U/ml) on the binding of MLV-A to CHO-WT cells and CHO-Pit-2 transfectants as measured by staining for SU and flow cytometry. A total of 10,000 viable cells were analyzed. % of Max, percentage of the maximum event number. Some virus binding to CHO-WT cells was observed with a mean channel fluorescence (MCF) of 1.32 compared to an MCF of 0.28 in the absence of virus. The binding of MLV-A to CHO-Pit-2 transfectants was much more efficient, with an MCF of 7.8 compared to and MCF of 0.25 in the absence of virus. Heparin reduced the binding of MLV-A to both CHO-WT and CHO-Pit-2 cells by a similar amount (the MCF was reduced in the presence of heparin by 0.97 for CHO-WT and by 1.81 for CHO-Pit-2), a result compatible with an effect on Env-independent virus binding.
FIG. 4.
Binding of MLV-A and Env-deficient retrovirus to NIH 3T3 fibroblasts, CHO-WT cells, and CHO-Pit-2 transfectants as measured by confocal immunofluorescence microscopy. The virus binds to all cell types regardless of the presence of Env or the level of expression of its cognate receptor, Pit-2. In each case, marked inhibition of attachment by soluble heparin (100 U/ml) is seen.
Since it could be argued that the binding of virus for an hour at 37°C might allow significant internalization to occur, virus attachment to NIH 3T3 fibroblasts was measured at 4 and 37°C. In keeping with previous results (25), the binding of virus particles was similar at both temperatures for NIH 3T3, CHO-WT, and CHO-Pit-2 cells, indicating that the assay predominantly detects cell surface rather than internalized virus (Table 2). The fact that the attachment of Env-deficient virus, which cannot enter the cell by membrane fusion, was broadly similar to MLV-A further supports the view that the assay mainly detects virions at the cell surface.
TABLE 2.
Effect of temperature on the binding of MLV-A
| Cell type | Heparina | Virions/cell at:
|
|||
|---|---|---|---|---|---|
| 4°C
|
37°C
|
||||
| Mean | SEM | Mean | SEM | ||
| CHO-WT | − | 75 | 6.75 | 73.3 | 8.84 |
| CHO-WT | + | 9 | 4.42 | 7.7 | 2.9 |
| CHO-Pit-2 | − | 68.7 | 10.8 | 70 | 10.3 |
| CHO-Pit-2 | + | 8.3 | 1.1 | ||
| NIH 3T3 | − | 70 | 1 | 82.3 | 19.6 |
| NIH 3T3 | + | 11.3 | 3.9 | 8.3 | 2.9 |
With (+) or without (−) 100 U of heparin/ml.
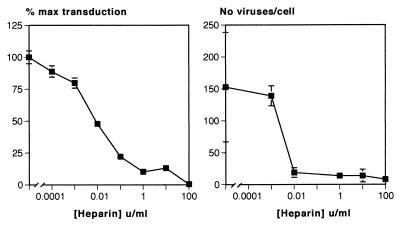
The effect of heparin on attachment was extremely potent with a similar dose-response relationship to the inhibition of transduction (Fig. 5). If we assume equilibrium conditions, an average molecular mass for heparin of 12.5 kDa, and an activity of 150 U/mg, the 50% inhibitory dose for both the inhibition of transduction of 0.01 to 0.001 U/ml equates to a Kd for the interaction between heparin and the virus of 5.3 to 0.53 μM. There was also a correlation between the potency of the effect of other GAGs on transduction and attachment. At a dose of 0.6 μg/ml, CDSNS heparin reduced transduction to 93% ± 3.7% and binding to 80% ± 4%, whereas the more potent GAG dextran sulfate reduced transduction and attachment to 11.4% ± 3.4% and 17% ± 11%, respectively, at the same concentration.
FIG. 5.
Dose-response relationship for heparin-mediated inhibition of retroviral attachment to NIH 3T3 cells compared to the effect on transduction. An effect of heparin on attachment was seen at concentrations similar to those required for the inhibition of transduction, with a 50% inhibitory concentration of between 0.001 and 0.01 U/ml.
Env-independent binding of MLV to heparin.
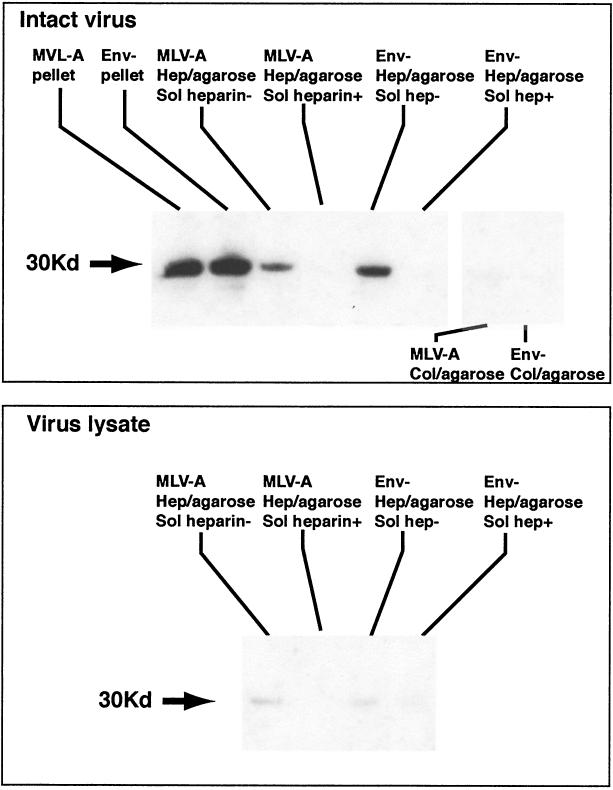
To further characterize the interaction between virus and GAGs, the binding of retrovirus to heparin was directly studied by using agarose beads coated with heparin. Beads were used to capture and precipitate virus particles, which were then analyzed by Western blotting with anti-RLV p30 antibody. Heparin-coated beads captured both MLV-A and Env-deficient viral particles with similar efficiency (Fig. 6). No virus was captured when excess soluble heparin was added to the virus suspension or when control beads coated with collagen were used. Only intact virions were capable of binding to heparin since no capsid was detected when heparin-coated beads were exposed to a lysate of virus particles. These results prove that MLV virus particles can directly interact with heparin by an Env-independent mechanism.
FIG. 6.
Binding of MLV-A and Env-deficient retrovirus to heparin-agarose and collagen-agarose beads. Supernatants of MLV-A and Env-deficient retrovirus was incubated with heparin- or collagen-coated beads in the presence or absence of excess soluble heparin, and agarose-bound virus was detected by SDS-polyacrylamide gel electrophoresis and Western blotting for the capsid protein. Specific binding of Env-deficient and amphotropic MLV to heparin but not to collagen was observed. There was no binding of virus lysate (bottom panel), confirming that the heparin-binding activity is present in the virus membrane. MLV-A, supernatant of FLYA13 packaging cells transfected with MFGnlsLacZ genome; Env−, supernatant of Env-deficient counterpart of same producer cell line; Hep/agarose, virus bound to heparin-agarose beads; Col/agarose, virus bound to collagen-agarose beads.
Attachment of MLV to proteoglycan-deficient cells.
Since a number of other viruses have been shown to interact with cell surface proteoglycans, we studied the attachment of MLV to proteoglycan-deficient CHOpgsA-745 cells (6), which completely lack these molecules because of a deficiency of xylose transferase, the enzyme responsible for initiating their biosynthesis. Proteoglycan deficiency was confirmed by staining with an FITC-conjugated antibody to heparan sulfate (data not shown). Contrary to expectations, the attachment of both MLV-A and Env-deficient virus to CHO-WT and CHOpgsA-745 cells was similar, and both were inhibited in the presence of excess soluble heparin (Fig. 7B). This result suggests that although GAG binding sites are present on the virus surface, cell surface proteoglycans are not involved in Env-independent attachment of MLV to CHO cells.
FIG. 7.
Effect of heparin on the attachment of amphotropic or Env-deficient MLV to CHO-WT or to proteoglycan-deficient CHOpgsA-745 cells. Virus binding to wild-type and proteoglycan-deficient CHO cells was equivalent in the absence of heparin (Hep−) and was similarly inhibited in the presence of 100 U of heparin/ml (Hep+).
DISCUSSION
Retroviral infection is initiated by adsorption of the virus particle to the cell surface and fusion of the viral lipid bilayer membrane with that of the target cell. Membrane fusion depends on the presence of the viral envelope protein (Env), a trimeric molecule with a surface (SU) and a transmembrane component, and a specific cellular receptor (7). Although virus entry and infection clearly require the presence of Env and its receptor, we have recently shown that Env-deficient viral particles attach to cells with the same efficiency as virus bearing the amphotropic envelope (MLV-A) (25). The results presented here show that heparin and other soluble GAGs inhibit both transduction and Env-independent attachment to the cell surface. Several lines of evidence suggest that the effects on transduction are a consequence of the inhibition of attachment. Transduction experiments in which the addition of heparin was delayed showed that the inhibitory effects are confined to the first hour of the infection process. A striking correlation between the potency with which GAGs inhibit transduction and their effect on attachment was also noted. Heparin inhibits transduction and attachment over a similar concentration range with a 50% effective concentration for both effects of between 0.01 and 0.001 U/ml. The same is true for other soluble GAGs; dextran sulfate, which strongly inhibits transduction, is also a potent inhibitor of attachment, whereas CDSNS heparin shows weak activity in both respects. Finally, the inhibition of both transduction and attachment involve an interaction between soluble GAGs and packaging-cell-derived factor(s) that are distinct from the viral Env protein. Direct binding of virus to GAGs was confirmed by experiments showing Env-independent binding of MLV to heparin-coated agarose beads. Taken together, these findings suggest that the mechanism by which GAGs inhibit attachment and transduction is through competition for packaging-cell-derived GAG binding factors on the virus surface. Unlike other viruses, however, the ligand for these molecules does not appear to be cell surface proteoglycan, since Env-independent attachment of MLV to proteoglycan-deficient CHOpgsA-745 and CHO-WT cells was similar.
It could be argued that the effects of GAGs in the attachment and transduction assays are nonspecific, since these molecules have a strong negative charge and might interfere with these processes through purely electrostatic effects. There is evidence that charge is a factor in the interaction between the virus and GAGs since there was a broad correlation between the degree of sulfation and inhibition of transduction. The sequence of the carbohydrate backbone of the GAG also influenced the interaction, however, with some charged molecules such as heparan and keratan sulfate having little or no effect on transduction. The different patterns of sensitivity to GAG-mediated inhibition of virus derived from human and murine packaging cells presumably reflect the incorporation of different GAG-binding molecules into virus derived from different species, and this provides further evidence for specificity in the interaction. Finally, the concentration of heparin that inhibits both attachment and transduction at between 0.01 and 0.001 U/ml equates to a Kd in the micromolar range, a result similar to that observed for other specific interactions involving GAGs, such as that between heparin and antithrombin-3 (28).
As previously noted (25), there was a marked disparity in the results of virus-binding assays with antibodies to Env and viral capsid. Only a small proportion of virus binding was inhibited by heparin when anti-SU was used to detect bound virus, whereas confocal immunofluorescence detection of viral capsid revealed a much more marked effect. The Env signal was strongly influenced by the overexpression of Pit-2 (16), whereas the confocal assay detected similar binding regardless of the level of expression of Pit-2 by the target cell or the presence of Env on the virus. The most likely explanation for these findings is that the assay with anti-SU predominantly detects soluble SU, with only a small heparin-sensitive proportion attributable to virus-associated SU. Conversely, the confocal capsid assay detects only intact virus bound to the cell by Env-independent mechanisms that are almost completely inhibited by excess soluble heparin.
These results shed new light on the mechanism of infection by oncoretroviruses and have a number of implications for the design of gene therapy vectors and protocols. The finding that the inhibition of transduction is a consequence of the inhibition of attachment strongly suggests that Env-independent interactions of the virus with the cell surface are an important factor in virus entry. Interestingly, even at the highest concentration of heparin, inhibition of transduction was not complete with low level but consistent infection in all cell lines studied. This implies that the role of GAG-mediated interactions is to increase the efficiency of infection, possibly by concentrating virus at the cell surface, where binding of Env to its receptor would be more likely. This view is in keeping with other systems, such as that of basic fibroblast growth factor (11), in which GAGs serve to localize ligand at the cell surface, facilitating subsequent binding to the primary receptor.
The observation that there are differences in the interaction between GAGs and virus from different packaging cells suggests that virus tropism might be influenced by cellular factors rather than molecules encoded by the virus itself. Studies of a neuropathogenic variant of Friend MLV have recently shown that central nervous system tropism can be conferred by heparin-binding structures (10). In this case, heparin-binding activity was mapped to a glutamine-to-lysine mutation in the receptor-binding domain of the envelope protein. Interestingly, infectivity was enhanced in the presence of low concentrations but was inhibited by high levels of heparin, suggesting that heparin serves as a molecular bridge between GAG-binding domains on the virus and cell surface. Precedents for such a mechanism include Chlamydia trachomatis (33) and Leishmania donovani (3) promastigotes, both of which attach to the cell surface through such a GAG bridge. The possibility is further supported by previous work showing that careful titration of the concentrations of Polybrene and soluble GAGs can enhance infection by MLV-A by up to 72% (15). The same mechanism might also explain our observation that Env-independent attachment to CHO-WT and the proteoglycan-deficient mutant CHOpgsA-745 are similar. Thus, soluble GAGs secreted by packaging or target cells might form a bridge between packaging-cell-derived GAG-binding factors on the virus and similar molecules on the target cell membrane. This model predicts that at the low concentrations similar to those produced by most cells, soluble GAGs should enhance virus attachment and transduction, whereas high levels would be inhibitory by blocking binding sites on the virus and cell surfaces.
In summary, we have shown that heparin-sensitive interactions mediate Env-independent attachment of MLV and that this process influences the efficiency of infection. Although the molecules responsible for these events have not been identified in here, viral particles possess a heparin-binding activity that derives from the host cell membrane.
Acknowledgments
S.J.W. was supported by a grant from the European Union Biotech 4 program. The research at the Wohl Virion Centre was supported by the UK Medical Research Council.
REFERENCES
- 1.Arai, T., K. Matsumoto, K. Saitoh, M. Ui, T. Ito, M. Murakami, Y. Kanegae, I. Saito, F. L. Cosset, Y. Takeuchi, and H. Iba. 1998. A new system for stringent, high-titer vesicular stomatitis virus G protein-pseudotyped retrovirus vector induction by introduction of Cre recombinase into stable prepackaging cell lines. J. Virol. 72:1115-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Batra, R. K., J. C. Olsen, D. K. Hoganson, B. Caterson, and R. C. Boucher. 1997. Retroviral gene transfer is inhibited by chondroitin sulfate proteoglycans/glycosaminoglycans in malignant pleural effusions. J. Biol. Chem. 272:11736-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butcher, B. A., L. A. Sklar, L. C. Seamer, and R. H. Glew. 1992. Heparin enhances the interaction of infective Leishmania donovani promastigotes with mouse peritoneal macrophages: a fluorescence flow cytometric analysis. J. Immunol. 148:2879-2886. [PubMed] [Google Scholar]
- 4.Cosset, F. L., Y. Takeuchi, J. L. Battini, R. A. Weiss, and M. K. L. Collins. 1995. High-titer packaging cells producing recombinant retroviruses resistant to human serum. J. Virol. 69:7430-7436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dechecchi, M. C., A. Tamanini, A. Bonizzato, and G. Cabrini. 2000. Heparan sulfate glycosaminoglycans are involved in adenovirus type 5- and 2-host cell interactions. Virology 268:382-390. [DOI] [PubMed] [Google Scholar]
- 6.Esko, J. D., T. E. Stewart, and W. H. Taylor. 1985. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 82:3197-3201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hunter, E. 1997. Viral entry and receptors, p. 71-119. In J. M. Coffin, S. H. Hughes, and H. E. Varmus (ed.), Retroviruses. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [PubMed]
- 8.Jackson, R. L., S. J. Busch, and A. D. Cardin. 1991. Glycosaminoglycans: molecular properties, protein interactions, and role in physiological processes. Physiol. Rev. 71:481-539. [DOI] [PubMed] [Google Scholar]
- 9.Jackson, T., F. M. Ellard, R. A. Ghazaleh, S. M. Brookes, W. E. Blakemore, A. H. Corteyn, D. I. Stuart, J. W. Newman, and A. M. King. 1996. Efficient infection of cells in culture by type O foot-and-mouth disease virus requires binding to cell surface heparan sulfate. J. Virol. 70:5282-5287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jinno-Oue, A., M. Oue, and S. K. Ruscetti. 2001. A unique heparin-binding domain in the envelope protein of the neuropathogenic PVC-211 murine leukemia virus may contribute to its brain capillary endothelial cell tropism. J. Virol. 75:12439-12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kjellen, L., and U. Lindahl. 1991. Proteoglycans: structures and interactions. Annu. Rev. Biochem. 60:443-475. [DOI] [PubMed] [Google Scholar]
- 12.Krusat, T., and H. J. Streckert. 1997. Heparin-dependent attachment of respiratory syncytial virus (RSV) to host cells. Arch. Virol. 142:1247-1254. [DOI] [PubMed] [Google Scholar]
- 13.Kurre, P., H. P. Kiem, J. Morris, S. Heyward, J. L. Battini, and A. D. Miller. 1999. Efficient transduction by an amphotropic retrovirus vector is dependent on high-level expression of the cell surface virus receptor. J. Virol. 73:495-500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Le Doux, J. M., J. R. Morgan, R. G. Snow, and M. L. Yarmush. 1996. Proteoglycans secreted by packaging cell lines inhibit retrovirus infection. J. Virol. 70:6468-6473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Le Doux, J. M., J. R. Morgan, and M. L. Yarmush. 1999. Differential inhibition of retrovirus transduction by proteoglycans and free glycosaminoglycans. Biotechnol. Prog. 15:397-406. [DOI] [PubMed] [Google Scholar]
- 16.Macdonald, C., S. J. Walker, M. J. Watts, S. J. Ings, D. C. Linch, and S. Devereux. 2000. Effect of changes in expression of the amphotropic retroviral receptor Pit-2 on transduction efficiency and viral titre: implications for gene therapy. Hum. Gene Ther. 11:587-595. [DOI] [PubMed] [Google Scholar]
- 17.Markowitz, D., S. Goff, and A. Bank. 1988. Construction and use of a safe and efficient amphotropic packaging cell line. Virology 167:400-406. [PubMed] [Google Scholar]
- 18.Markowitz, D., S. Goff, and A. Bank. 1988. A safe packaging line for gene-transfer: separating viral genes on two different plasmids. J. Virol. 62:1120-1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Miller, D., R. Edwards, and A. Miller. 1994. Cloning of the cellular receptor for amphotropic murine retroviruses reveals homology to that for gibbon ape leukaemia virus. Proc. Natl. Acad. Sci. USA 91:78-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller, D. G., M. A. Adam, and A. D. Miller. 1990. Gene transfer by retrovirus vectors occurs only in cells that are actively replicating at the time of infection. Mol. Cell. Biol. 10:4239-4242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mondor, I., S. Ugolini, and Q. J. Sattentau. 1998. Human immunodeficiency virus type 1 attachment to HeLa CD4 cells is CD4 independent and gp120 dependent and requires cell surface heparans. J. Virol. 72:3623-3634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Neyts, J., R. Snoeck, D. Schols, J. Balzarini, J. D. Esko, A. Van Schepdael, and E. De Clercq. 1992. Sulfated polymers inhibit the interaction of human cytomegalovirus with cell surface heparan sulfate. Virology 189:48-58. [DOI] [PubMed] [Google Scholar]
- 23.O'Reilly, D., L. Miller, and V. Lucknow. 1994. Excel spreadsheet for TCID50 calculation, p. 304-305. In Baculovirus expression vectors: a laboratory manual, 1st ed. Oxford University Press, Oxford, England.
- 24.Patel, M., M. Yanagishita, G. Roderiquez, D. C. Bou-Habib, T. Oravecz, V. C. Hascall, and M. A. Norcross. 1993. Cell-surface heparan sulfate proteoglycan mediates HIV-1 infection of T-cell lines. AIDS Res. Hum. Retrovir. 9:167-174. [DOI] [PubMed] [Google Scholar]
- 25.Pizzato, M., S. A. Marlow, E. D. Blair, and Y. Takeuchi. 1999. Initial binding of murine leukemia virus particles to cells does not require specific Env-receptor interaction. J. Virol. 73:8599-8611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reed, L., and H. Muench. 1938. A simple method for estimating 50% endpoints. Am. J. Hyg. 27:439-497. [Google Scholar]
- 27.Roe, T., T. C. Reynolds, G. Yu, and P. O. Brown. 1993. Integration of murine leukemia virus DNA depends on mitosis. EMBO J. 12:2099-2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg, R. D. 1985. Role of heparin and heparinlike molecules in thrombosis and atherosclerosis. Fed. Proc. 44:404-409. [PubMed] [Google Scholar]
- 29.Secchiero, P., D. Sun, A. L. De Vico, R. W. Crowley, M. S. Reitz, Jr., G. Zauli, P. Lusso, and R. C. Gallo. 1997. Role of the extracellular domain of human herpesvirus 7 glycoprotein B in virus binding to cell surface heparan sulfate proteoglycans. J. Virol. 71:4571-4580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Summerford, C., and R. J. Samulski. 1998. Membrane-associated heparan sulfate proteoglycan is a receptor for adeno-associated virus type 2 virions. J. Virol. 72:1438-1445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van Zeijl, M., S. Johann, E. Closs, J. Cunningham, R. Eddy, T. Shows, and B. O'Hara. 1994. A human amphotropic retrovirus receptor is a second member of the gibbon ape leukaemia virus receptor family. Proc. Natl. Acad. Sci. USA 91:1168-1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.WuDunn, D., and P. G. Spear. 1989. Initial interaction of herpes simplex virus with cells is binding to heparan sulfate. J. Virol. 63:52-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang, J. P., and R. S. Stephens. 1992. Mechanism of C. trachomatis attachment to eukaryotic host cells. Cell 69:861-869. [DOI] [PubMed] [Google Scholar]