Abstract
Mice infected with the murine coronavirus, mouse hepatitis virus, strain JHM (MHV) develop an immune-mediated demyelinating encephalomyelitis. Adoptive transfer of MHV-immune splenocytes depleted of either CD4 or CD8 T cells to infected mice deficient in recombination activation gene 1 resulted in demyelination. We showed previously that the process of CD8 T-cell-mediated demyelination was strongly dependent on the expression of gamma interferon (IFN-γ) by donor cells. In this report, we show, in contrast, that demyelination and lymphocyte infiltration were increased in recipients of IFN-γ−/− CD4 T cells when compared to levels in mice receiving C57BL/6 CD4 T cells.
Mice infected with the neurotropic JHM strain of mouse hepatitis virus (MHV) develop acute encephalitis or acute and chronic demyelinating diseases that serve as useful models for the human disease multiple sclerosis (11). Demyelination in this model is immune mediated, since mice that are sublethally irradiated, mice with severe combined immunodeficiency, or mice deficient in recombination activation gene 1 (RAG1−/− mice) do not develop demyelination after infection with MHV but develop signs of acute encephalitis (hunching, ruffled fur, and lethargy) at approximately 2 weeks postinoculation (p.i.). However, adoptive transfer of splenocytes from immunocompetent donors results in demyelination within 7 days posttransfer (p.t.) (2, 14, 17). It was observed that demyelination developed reproducibly in MHV-infected RAG1−/− mice if splenocytes were transferred from C57BL/6 (B6) mice previously immunized with MHV (17). Transfer of cells from naïve mice did not reproducibly cause demyelination (17). Subsequently, it was shown that transfer of spleen populations depleted of both CD4 and CD8 T cells did not result in demyelination, although demyelination did occur if only one subset was depleted (16).
Recent studies have partly elucidated the inflammatory factors critical for demyelination in MHV-infected mice. Direct infection of mice deficient in different immune effector molecules, including gamma interferon (IFN-γ), tumor necrosis factor alpha (TNF-α), interleukin-10 (IL-10), or inducible nitric oxide synthase (NOS2) resulted in levels of demyelination equivalent to those detected in infected B6 mice (5, 8, 12, 18). Recently, it was shown, using MHV-infected RAG1−/− mice, that adoptive transfer of CD8 T-cell-enriched splenocytes from IFN-γ−/− but not from perforin−/− or TNF-α−/− mice resulted in an approximately 80% reduction in demyelination when compared to that in mice receiving similar populations from B6 mice (9). This result revealed the importance of analyzing individual T-cell subsets for their ability to cause demyelination, since the role of IFN-γ in CD8 T-cell-mediated demyelination was obscured when both CD4 and CD8 T cells were present.
IFN-γ is a key proinflammatory molecule and is also critical for lymphocyte homeostasis. In IFN-γ−/− or IFN-γ receptor−/− (IFN-γR−/−) mice with CD4 T-cell-mediated experimental autoimmune encephalomyelitis (EAE), disease is much severer than in wild-type counterparts. The number of lymphocytes in the mice's central nervous sytems (CNS) was greater in the absence of IFN-γ, and expression of chemokines, such as RANTES and macrophage chemoattractant protein (MCP)-1, was greatly decreased. Most striking, the number of neutrophils and the levels of macrophage-inducible protein (MIP)-2 and CCL1/T-cell activation gene 3 in the CNS, both chemoattractants for neutrophils, were increased. This increase in neutrophil numbers was postulated to contribute to the severer disease observed in these animals (1, 13, 15).
Collectively, these results suggested that, in the absence of IFN-γ, CD4 T-cell-mediated demyelination in MHV-infected mice would occur and would perhaps even be more extensive. To investigate this possibility, we measured the amount of demyelination that resulted from transfer of IFN-γ−/− CD4 T-cell-enriched populations to MHV-infected RAG1−/− mice.
Equivalent amount of demyelination after transfer of undepleted populations of B6 or IFN-γ−/− splenocytes from MHV-immune donors.
RAG1−/− mice (obtained from the Jackson Laboratory [Bar Harbor, Maine] and bred at the University of Iowa) were infected by intracranial injection with 103 PFU of the neuroattenuated J2.2-V-1 strain of MHV (generously provided by J. Fleming [University of Wisconsin, Madison]). These mice develop acute encephalitis at 14 to 18 days p.i. without evidence of demyelination (17). Adoptive transfer of MHV-immune splenocytes from either B6 (National Cancer Institute, Bethesda, Md.) or IFN-γ−/− (Jackson Laboratory) mice resulted in clinical signs consistent with a demyelinating encephalomyelitis (wobbly gait, hind limb paresis, and mild lethargy) at 7 to 9 days p.i. In these experiments, B6 or IFN-γ−/− mice were immunized intraperitoneally with MHV 7 days prior to transfer of 5 × 106 splenocytes to each infected RAG1−/− recipient (17). Similar numbers of MHV-specific CD4 and CD8 T cells were detected in the CNS of recipients of B6 or IFN-γ−/− cells at the time of harvest at 7 to 9 days p.i. (data not shown).
We quantified demyelination using 8-μm spinal cord sections stained with Luxol Fast Blue (LFB) as described previously (19). Briefly, images of stained sections were digitalized using an Optiphot charge-coupled camera attached to a Leitz diaplan light microscope. An image analysis program, Vtrace (Image Analysis Facility, University of Iowa), was used to delineate myelinated and demyelinated areas. The total demyelinated area per spinal cord was determined and divided by the sum of the demyelinated and myelinated areas. Similar amounts of demyelination were present in spinal cords of mice receiving MHV-immune spleen cells from either B6 or IFN-γ−/− mice (Table 1). In agreement with a previous report (8), virus clearance was significantly diminished in recipients of IFN-γ−/− cells (Table 1). Thus, these results show that IFN-γ was required for optimal virus clearance but not for MHV-induced demyelination when both CD4 and CD8 T cells were present.
TABLE 1.
Demyelination and virus titers in adoptive transfer recipients
| Enrichment group | Time | % Demyelination (no.) | No. of samples with detectable virus/total no. | Titer (log10 PFU/g of tissue ± SE) |
|---|---|---|---|---|
| Virus only | Days 10 to 13 p.i. | N/Ad | 6/6 | 6.00 ± 0.13a |
| Undepleted cells | Days 11 to 13 p.i.; day 7 to 9 p.t. | |||
| B6 | 15.6 ± 2.0 (9) | 3/5 | 3.04 ± 0.44b | |
| IFN-γ−/− | 17.7 ± 2.1 (7) | 4/4 | 4.77 ± 0.08 | |
| CD4 T cells | Day 10 or 11 p.i.; day 6 or 7 p.t. | |||
| B6 | 8.0 ± 1.9 (4)c | 3/3 | 4.96 ± 0.23 | |
| IFN-γ−/− | 20.0 ± 3.0 (7) | 8/8 | 4.93 ± 0.12 |
Virus titers were significantly higher in the absence of adoptive transfer (P < 0.005).
Virus titers were significantly lower in recipients of undepleted B6 splenocytes than in members of all other groups (P < 0.02).
A significant difference was observed in amounts of demyelination between recipients of CD4 T-cell-enriched B6 and IFN-γ−/− populations (P < 0.05) as measured by Student's t test.
N/A, not available.
Transfer of CD4 T-cell-enriched splenocytes from immunized IFN-γ−/− mice resulted in increased demyelination.
CD4 T-cell-enriched splenocytes were prepared by two rounds of complement lysis, as previously described (16). At the time of harvest from recipient mice at 6 or 7 days p.t., MHV-specific CD8 T cells were depleted by at least 99% when assayed by intracellular cytokine staining for TNF-α (data not shown). Transfer of IFN-γ−/− CD4 T-cell-enriched splenocytes resulted in a clinical disease similar to that observed in recipients of B6 CD4 T-cell-enriched populations (16), with predominant signs of severe encephalitis, including lethargy, ruffled fur, and hunching.
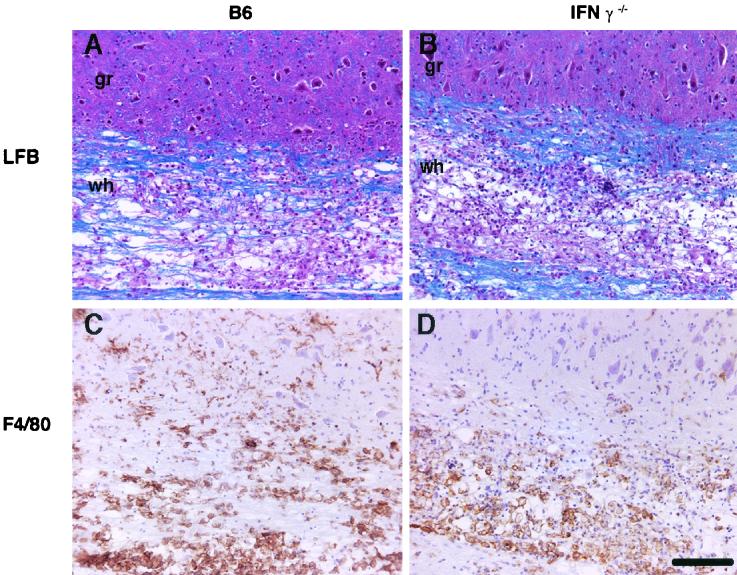
In agreement with our previous results (16), approximately 8% of the spinal cord was demyelinated in recipients of B6 CD4 T-cell-enriched splenocytes (Table 1). However, we detected significantly more demyelination in the spinal cords of recipients of IFN-γ−/− CD4 T cells (20.0% ± 3.0%, P < 0.05). This effect was due neither to increased levels of virus in recipients of IFN-γ−/− cells (Table 1) nor to differences in the distribution of virus antigen in the spinal cords of the two types of recipients (data not shown). Large numbers of macrophages/microglia (detected with anti-F4/80 monoclonal antibody [CI:A3-1; Serotec, Oxford, England]) were observed in the spinal cords of recipients of either B6 or IFN-γ−/− cells (Fig. 1C and D). Of note, while similar numbers of macrophages/microglia were detected in the white matter, greater numbers of these cells were identified in the gray matter of recipients of B6 splenocytes than in that of recipients of IFN-γ−/− cells.
FIG. 1.
Demyelination and cellular infiltration in recipients of B6 and IFN-γ−/− splenocytes. MHV-infected RAG1−/− mice received CD4 T-cell-enriched splenocytes from immune B6 (A and C) or IFN-γ−/− (B and D) donors at 4 days p.i. Mice were sacrificed at 7 days p.t. (A and B) Representative longitudinal sections of spinal cord were stained with LFB to detect myelin. Although demyelination was detected in both types of recipients, larger amounts were detected in recipients of IFN-γ−/− cells (summarized in Table 1) (C and D) Sections were stained for macrophages/microglia. Equivalent numbers of macrophages/microglia were present in the white (wh) matter of both groups, but the number of these cells in the gray (gr) matter of recipients of B6 cells was significantly greater than in the gray matter of those receiving IFN-γ−/− cells (summarized in Table 3). Scale bar, 200 μm.
Quantification of MHV-specific CD4 T cells and macrophages/microglia in the infected CNS.
To extend these results, the numbers of lymphocytes and macrophages/microglia in the infected brain and spinal cord were quantified. We measured the number of MHV-specific CD4 T cells in the CNS of recipients using intracellular staining for TNF-α after stimulation with peptide corresponding to an immunodominant CD4 T-cell epitope (residues 133 to 147 of the transmembrane [M] protein [epitope M133], I-Ab restricted) and fluorescence-activated cell sorter (FACS) analysis as previously described (9, 16). The absolute number of MHV-specific cells was calculated by multiplying the fraction of MHV-specific cells by the fraction of CD4 T lymphocytes by the total number of cells per brain. Approximately twofold more epitope M133-specific CD4 T cells were detected in the CNS of recipients of IFN-γ−/− CD4 T-cell-enriched splenocytes (P = 0.10 by Student's t test) (Table 2).
TABLE 2.
Lymphocyte and neutrophil infiltration into the CNS of recipients of CD4 T-cell-enriched splenocytes
| Donor cella type | No. of CD4 cells | No. of M133b cells | No. of Mac-1/ GR1 cells |
|---|---|---|---|
| B6 | (1.8 ± 0.3) × 105 | (3.3 ± 0.5) × 104 | (8.1 ± 2.4) × 104 |
| IFN-γ−/− | (2.6 ± 0.6) × 105 | (6.2 ± 1.4) × 104 | (9.3 ± 3.9) × 104 |
Each group consisted of three individual mice (six to nine per group) that received CD4 T-cell-enriched splenocytes from B6 or IFN-γ−/− mice.
Measured by intracellular staining for TNF-α. Values are shown after subtraction of background staining. The absolute number (± standard error) of virus-specific cells was calculated as described in the text.
Since the histological analysis shown in Fig. 1 suggested that there were greater numbers of macrophages/microglia in the gray matter of B6 recipients, we counted the number of these cells in the gray and white matter of the spinal cord. We counted all of the F4/80+ cells in 0.63-mm-wide cross-sections at eight levels within spinal cords harvested from three recipients of B6 cells and three recipients of IFN-γ−/− cells. There were approximately twofold more macrophages/microglia in spinal cords harvested from recipients of B6 splenocytes than in those harvested from mice receiving cells from IFN-γ−/− donors (Table 3). In agreement with the results shown in Fig. 1, we detected an increase in the number of macrophages/microglia in the gray matter of recipients of B6 cells. We also identified similar numbers of macrophages/microglia in the white matter of recipients of both types of cells (Table 3). These results were unexpected, because in MHV-infected RAG1−/− recipients of B6 or IFN-γ−/− CD8 T cells, the number of macrophages/microglia in the white matter correlated with the extent of demyelination (9).
TABLE 3.
Macrophages microglia in gray and white matter of spinal cords
| Donor cella | No. in
|
||
|---|---|---|---|
| total spinal cord | gray matter | white matter | |
| B6 | 422 ± 60b | 209 ± 30b | 213 ± 38 |
| IFN-γ−/− | 213 ± 23 | 56 ± 8 | 158 ± 20 |
Each group consisted of three individual animals that received CD4 T-cell-enriched splenocytes from B6 or IFN-γ−/− mice. F4/80+ cells in 0.63-mm-wide cross-sections at eight levels within the spinal cords were counted.
A significant difference was observed in the numbers of F4/80+ cells in the total spinal cord and in gray matter between recipients of CD4 T-cell-enriched B6 and IFN-γ−/− populations (P < 0.002) as measured by Student's t test.
Decreased levels of proinflammatory chemokine mRNA in the CNS of recipients of IFN-γ−/− CD4 T cells.
As described above, disease in IFN-γ−/− or IFN-γR−/− mice with EAE is severer than in wild-type mice and is associated with increased neutrophil infiltration and altered chemokine expression (7). To determine if the increased demyelination observed in recipients of IFN-γ−/− CD4 T-cell-enriched populations reflected a change in chemokine or cytokine profile or in neutrophil infiltration into the CNS, we measured levels of chemokine/cytokine RNA in the infected CNS by RNase protection assay and determined the number of neutrophils by FACS analysis. Chemokines such as CXCL9/monocyte induced by IFN-γ (Mig), CXCL10/IFN-inducible protein (IP)-10, CCL5/RANTES, CCL4/MIP-1β, CCL2/MCP-1, and CCL7/MCP-3 are critical for lymphocyte and macrophage infiltration into sites of inflammation (6, 20) and are up regulated in the CNS of mice infected with MHV (3, 4).
Levels of all of these proinflammatory chemokine RNAs were increased in recipients of B6 cells compared to those in mice that did not receive splenocytes (Fig. 2). In contrast, levels of IP-10 and RANTES did not increase after adoptive transfer of IFN-γ−/− CD4 T cells (Fig. 2B), although there were increases in MIP-1β, MCP-1, MCP-3, and MIP-2 mRNA, similar to the changes detected in recipients of B6 cells (Fig. 2C). Consistent with the equivalent increases in MIP-2 RNA, the number of neutrophils was the same in recipients of either B6 or IFN-γ−/− CD4 T-cell-enriched populations as measured by FACS analysis (Table 2). Neutrophils were detected using anti-CD11b and anti-GR1/Ly6G (RB6-8C5; PharMingen) monoclonal antibodies. We also detected no significant differences in levels of TNF-α, IL-1β, and IL-6 between recipients of B6 and IFN-γ−/− cells by RNase protection assay (data not shown). Thus, our experiments did not identify a cytokine or chemokine that might be critical for the increased demyelination observed in recipients of IFN-γ−/− CD4 T cells.
FIG. 2.
Kinetics of chemokine and cytokine mRNA expression in the spinal cords of MHV-infected mice before and after transfer of CD4 T-cell-enriched cells from MHV-immune B6 or IFN-γ−/− donors. (A) Five micrograms of total RNA obtained from the spinal cords of mice 10 days p.i. (7 days p.t.) was analyzed by RNase protection assays. RNA was isolated from spinal cords using Tri Reagent (Molecular Research Center, Cincinnati, Ohio). Samples were hybridized with a probe set designed to detect six chemokines and an internal L32 control (provided by Iain Campbell, Scripps Research Institute). Donor strains are indicated above the lanes. Each lane contains a sample from an individual mouse. (B and C) Quantification of chemokine mRNA levels. Gels were exposed to a phosphorimaging screen and were analyzed using NIH Image 1.61 software. Levels are normalized to L32 and allow comparison of chemokine mRNA levels. RNA from seven recipients of B6 cells, three recipients of IFN-γ−/− cells, and five mice that received no cells were used in these analyses. Note the difference in scale between panels B and C. Levels of IP-10 and RANTES were significantly decreased in recipients of IFN-γ−/− CD4 T-cell-enriched populations (P < 0.05) compared to levels in B6 controls.
Previous studies have shown that CD4 T cells are sufficient for MHV-induced demyelination (2, 4). In this report, we show that CD4 T-cell-mediated demyelination in MHV-infected mice is increased in the absence of IFN-γ. These results parallel results obtained in analyses of mice with EAE. Thus, in both MHV-infected mice and in mice with EAE, the absence of IFN-γ diminishes demyelination mediated by CD8 T cells and enhances that mediated by CD4 T cells (7, 10). Perhaps most relevant to understanding the increase in demyelination was the observed twofold increase in the number of MHV-specific CD4 T cells in the CNS of recipients of IFN-γ−/− splenocytes (Table 2). Whether these cells contribute to demyelination by directly lysing virus-infected oligodendrocytes or other cells in the CNS or by another mechanism remains to be determined.
Acknowledgments
We thank J. Harty for critical review of the manuscript.
This research was supported in part by grants from the National Institutes of Health (NS40438-01) and the National Multiple Sclerosis Society (RG 2864-B-3).
REFERENCES
- 1.Chu, C. Q., S. Wittmer, and D. K. Dalton. 2000. Failure to suppress the expansion of the activated CD4 T cell population in interferon γ-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J. Exp Med. 192:123-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Houtman, J. J., and J. O. Fleming. 1996. Dissociation of demyelination and viral clearance in congenitally immunodeficient mice infected with murine coronavirus JHM. J. Neurovirol. 2:101-110. [DOI] [PubMed] [Google Scholar]
- 3.Lane, T. E., V. Asensio, N. Yu, A. D. Paoletti, I. Campbell, and M. J. Buchmeier. 1998. Dynamic regulation of α- and β-chemokine expression in the central nervous system during mouse hepatitis virus-induced demyelinating disease. J. Immunol. 160:970-978. [PubMed] [Google Scholar]
- 4.Lane, T. E., M. T. Liu, B. P. Chen, V. C. Asensio, R. M. Samawi, A. D. Paoletti, I. L. Campbell, S. L. Kunkel, H. S. Fox, and M. J. Buchmeier. 2000. A central role for CD4+ T cells and RANTES in virus-induced central nervous system inflammation and demyelination. J. Virol. 74:1415-1424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin, M. T., D. Hinton, B. Parra, S. Stohlman, and R. van der Veen. 1998. The role of IL-10 in mouse hepatitis virus-induced demyelinating encephalomyelitis. Virology 245:270-280. [DOI] [PubMed] [Google Scholar]
- 6.Luster, A. 1998. Chemokines—chemotactic cytokines that mediate inflammation. N. Engl. J. Med. 338:436-445. [DOI] [PubMed] [Google Scholar]
- 7.Owens, T., H. Wekerle, and J. Antel. 2001. Genetic models for CNS inflammation. Nat. Med. 7:161-164. [DOI] [PubMed] [Google Scholar]
- 8.Parra, B., D. Hinton, N. Marten, C. Bergmann, M. T. Lin, C. S. Yang, and S. A. Stohlman. 1999. IFN-γ is required for viral clearance from central nervous system oligodendroglia. J. Immunol. 162:1641-1647. [PubMed] [Google Scholar]
- 9.Pewe, L. L., and S. Perlman. 2002. CD8 T cell-mediated demyelination is IFN-γ dependent in mice infected with a neurotropic coronavirus. J. Immunol. 168:1547-1551. [DOI] [PubMed] [Google Scholar]
- 10.Steinman, L. 2001. Myelin-specific CD8 T cells in the pathogenesis of experimental allergic encephalitis and multiple sclerosis. J. Exp Med. 194:F27-F30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stohlman, S. A., C. C. Bergmann, and S. Perlman. 1998. Mouse hepatitis virus, p. 537-557. In R. Ahmed and I. Chen (ed.), Persistent viral infections. John Wiley & Sons, Ltd., New York, N.Y.
- 12.Stohlman, S. A., D. R. Hinton, D. Cua, E. Dimacali, J. Sensintaffar, F. M. Hofman, S. M. Tahara, and Q. Yao. 1995. Tumor necrosis factor expression during mouse hepatitis virus-induced demyelinating encephalomyelitis. J. Virol. 69:5898-5903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tran, E. H., E. N. Prince, and T. Owens. 2000. IFN-gamma shapes immune invasion of the central nervous system via regulation of chemokines. J. Immunol. 164:2759-2768. [DOI] [PubMed] [Google Scholar]
- 14.Wang, F., S. A. Stohlman, and J. O. Fleming. 1990. Demyelination induced by murine hepatitis virus JHM strain (MHV-4) is immunologically mediated. J. Neuroimmunol. 30:31-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willenborg, D. O., S. Fordham, C. C. Bernard, W. B. Cowden, and I. A. Ramshaw. 1996. IFN-gamma plays a critical down-regulatory role in the induction and effector phase of myelin oligodendrocyte glycoprotein-induced autoimmune encephalomyelitis. J. Immunol. 157:3223-3227. [PubMed] [Google Scholar]
- 16.Wu, G. F., A. A. Dandekar, L. Pewe, and S. Perlman. 2000. CD4 and CD8 T cells have redundant but not identical roles in virus-induced demyelination. J. Immunol. 165:2278-2286. [DOI] [PubMed] [Google Scholar]
- 17.Wu, G. F., and S. Perlman. 1999. Macrophage infiltration, but not apoptosis, is correlated with immune-mediated demyelination following murine infection with a neurotropic coronavirus. J. Virol. 73:8771-8780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu, G. F., L. Pewe, and S. Perlman. 2000. Coronavirus-induced demyelination occurs in the absence of inducible nitric oxide synthase. J. Virol. 74:7683-7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xue, S., N. Sun, N. van Rooijen, and S. Perlman. 1999. Depletion of blood-borne macrophages does not reduce demyelination in mice infected with a neurotropic coronavirus. J. Virol. 73:6327-6334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zlotnik, A., and O. Yoshie. 2000. Chemokines: a new classification system and their role in immunity. Immunity 12:121-127. [DOI] [PubMed] [Google Scholar]