Abstract
Cytomegalovirus (CMV) is the most significant infectious cause of brain disorders in humans involving the developing brain. It is hypothesized that the brain disorders occur after recurrent reactivation of the latent infection in some kinds of cells in the brains. In order to test this hypothesis, we examined the reactivation of latent murine CMV (MCMV) infection in the mouse brain by transfer to brain slice culture. We infected neonatal and young adult mice intracerebrally with recombinant MCMV in which the lacZ gene was inserted into a late gene. The brains were removed 6 months after infection and used to prepare brain slices that were then cultured for up to 4 weeks. Reactivation of latent infection in the brains was detected by β-galactosidase (β-Gal) staining to assess β-galactosidase expression. Viral replication was also confirmed by the plaque assay. Reactivation was observed in about 75% of the mice infected during the neonatal period 6 months after infection. Unexpectedly, reactivation was also observed in 75% of mice infected as young adults, although the infection ratio in the brain slices was significantly lower than that in neonatally infected mice. β-Gal-positive cells were observed in marginal regions of the brains or immature neural cells in the ventricular walls. Immunohistochemical staining showed that the β-Gal-positive reactivated cells were neural stem or progenitor cells. These results suggest that brain disorders may occur long after infection by reactivation of latent infection in the immature neural cells in the brain.
Human cytomegalovirus (HCMV) is the most significant infectious cause of congenital anomalies of the central nervous system (CNS) caused by intrauterine infection in humans (13, 59), with an average incidence of approximately 1% of all live births (10, 51). It is estimated that approximately 5 to 10% of infected infants have severe brain damage (2, 4, 7, 13). Another nearly 10% of infected infants have subclinical congenital infection and will subsequently have brain disorders, including mental retardation, seizures, and epilepsy (9, 33). Since studies of human subjects have obvious limitations, we have developed model systems for brain abnormalities induced by murine cytomegalovirus (MCMV) in fetal and neonatal mice (17, 24, 55-57).
MCMV infection has been used extensively as a model for CMV latency. Latent infection has been demonstrated in the spleen (15, 28, 38, 39), kidney (14, 18), salivary gland (6, 48), and lung (3). In addition, human hematopoietic cells, including bone marrow cells, have been found to contain latent HCMV (12, 30). Latent CMV DNA is present in diverse organs in different copy numbers, and the existence of multiple organ sites of CMV latency implies the possibility of independent events of recurrence at these sites (5, 8, 41).
Although the nervous system is one of the principal target organs in congenital HCMV infection and HCMV-infected AIDS patients (7), little attention has been focused on latent CMV infection and the recurrence of CMV in the brain. Regarding the susceptibility of brain cells to HCMV, various human brain-derived cultured cells, including glial (32), microglial (40), neuronal (37), or endothelial (36) cells, have been reported to support HCMV replication, However, the infectious dynamics of human brain cells in vivo are unknown, aside from speculation based on autopsy cases (4, 35). We have reported the infectious dynamics of neuronal and glial cells in acute and subacute phases of infection in the developing mouse brain (48, 57). However, there is little information available about the rate of latency and sites and cell types involved in latent CMV infection in the brain.
It is possible that subclinical infections or brain disorders that occur during the prolonged period after congenital infection are based on persistent infection or intermittent reactivation of latent infection in the brain. In order to examine the hypothesis that latent CMV infection occurs in the brain, we studied the occurrence and sites of reactivation of latent MCMV in mouse brain by transferring brain tissues to brain slice cultures.
MATERIALS AND METHODS
Virus.
Recombinant MCMV (RM461) in which the γ-0.85 gene was inserted with the Escherichia coli lacZ gene and which was constructed to express β-galactosidase (β-Gal) under the control of the HCMV immediate-early (IE) ie1/ie2 promoter or enhancer was provided by E. S. Mocarski (Stanford University, Stanford, Calif.) (52). RM461 has a disruption of a viral CC chemokine homolog gene (43, 44). The virus was quantified by the plaque assay method of Wentworth and French (60) with mouse embryonic fibroblasts of the BALB/c strain as reported previously (57).
Virus infection and establishment of MCMV latency.
For neonatal infection, BALB/c mice were injected intracerebrally with RM461 (5 × 102 PFU/2 μl) within 24 h after birth with a Hamilton syringe. For young adult infection, BALB/c mice (6 weeks old) were injected intracerebrally with RM461 (5 × 104 PFU/50 μl) by using a disposable 1-ml syringe. After infection, these two groups of mice were maintained for more than 180 days (6 months). For the uninfected control groups, neonatal and young adult mice were injected with minimum essential medium (MEM) and maintained for the same period. Body weight was measured once a week during the course of the experiments.
Organotypic brain slice culture.
The brain slice culture was performed basically according to the method of Stoppini et al. (53) with some modification (49). The whole brain, dissected from each mouse with complete removal of the meninx and kept in cold Hanks' balanced solution (HBSS; Gibco BRL) on ice, fixed to a stage with instant glue, and cut coronally at a 500-nm thickness with a microslicer (Dohan EM, Kyoto, Japan). Slices were transferred to porous transparent membranes (Millicell-CM, 0.4 mm diameter; Millipore, Bedford, Mass.), which were floated on culture medium in six-well plates (Becton Dickinson, Franklin Lakes, N.J.). Slices were maintained at 37°C in a humidified atmosphere of 95% air and 5% CO2 with a change of medium once a week. For labeling with 5-bromo-2-deoxyuridine (BrdU), brain slices were cultured in the presence of 10 μM BrdU (Boehringer Mannheim, Mannheim, Germany) for the last 24 h before harvesting.
β-Gal staining.
After incubation for 3 to 4 weeks, brain slices on membranes were fixed in 4% paraformaldehyde for 30 min and washed with phosphate-buffered saline at 4°C. The staining for β-Gal activity was performed by the method described by Reynolds et al. (42) with the substrate 5-bromo-4-chloro-3-indolyl-β-galactoside (X-Gal).
Quantitative analysis of virus-infected area in brain slices.
By using Adobe Photoshop version 5.5 software (Adobe Systems, Inc.), the ratio of the X-Gal-positive area to the whole brain slice area was calculated. Photos were taken of each brain slice stained with X-Gal and converted into digital information. The intensities of the entire brain slices and X-Gal-stained areas were converted into pixel counts. The infection ratio was expressed by dividing the pixel count of the X-Gal-stained area by the pixel count of the entire brain slice area.
Quantitative analysis of viral growth in brain slices after latent infection.
The brains of the mice 180 days after neonatal infection were sliced into six pieces and cultured as described above. Each slice was taken before and 1, 2, 3, and 4 weeks after culture, kept at −80°C, and then minced in 500 μl of MEM. Freeze-thaw supernatants were assayed for virus by plaque assay as described above.
Immunohistochemistry.
After X-Gal staining, brain slices were embedded in paraffin and serial sectioned (5 μm in thickness). After deparaffinization, sections were pretreated with 0.3% hydrogen peroxide and incubated with goat serum blocking solution for 10 min. In the serial brain sections, sections adjacent to those reacted with X-Gal were reacted with the primary antibodies rat monoclonal antibody (MAb) specific to mouse Musashi-1 (Msi1; 1:1,000) (16), rabbit antibody to glial fibrillary acidic protein (GFAP; Dako Corp., Carpinteria, Calif.), rabbit antibody to nestin (a gift from H. Kitani, National Institute of Animal Health, Tsukuba, Japan) (54) for undifferentiated neural precursor cells (22), or mouse MAb to proliferating cell nuclear antigen (PCNA; 1:100; Sigma) and then incubated with biotinylated secondary antibodies and with horseradish peroxidase-conjugated streptavidin and colored with 3-amino-9-ethylcarbazole (AEC) or colored with 3,3′-diaminobenzidine (DAB) (48).
RESULTS
Growth curve after infection.
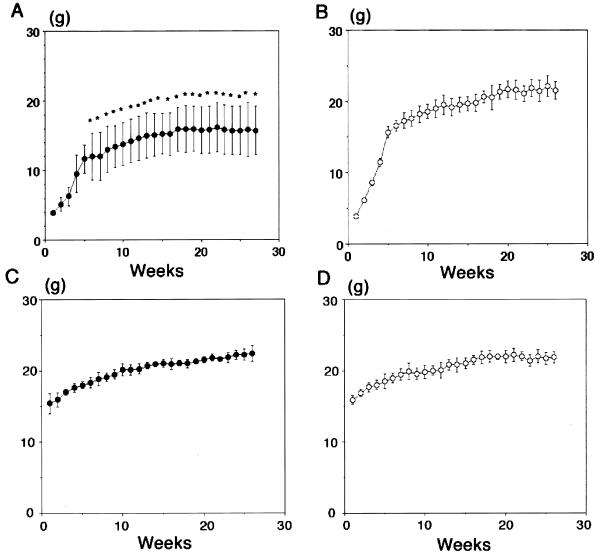
After cerebral infection with the mutant MCMV (RM461), which had a disruption of a viral CC chemokine homolog gene (43, 44), mice were maintained for 180 to 210 days before use for preparing brain slice cultures. Representative growth curves are shown in Fig. 1. The growth curve of the neonatal infection group was significantly different in terms of body weight (Fig. 1A) from that of MEM-injected mice (Fig. 1B). In contrast, the change in body weight of the mice infected as young adults during the experimental period was small (Fig. 1C), as was that of their uninfected counterparts (Fig. 1D).
FIG. 1.
Comparison of growth curves between uninfected mice and mice infected either during neonatal period (A and B) or as young adults (C and D). BALB/c mice were injected intracerebrally with 5 × 102 PFU of mutant MCMV (RM461), which contains a disruption of CC chemokine homolog gene (43, 44) (A), or 2 μl of MEM (B) and maintained for more than 180 days. ∗, P < 0.05 versus MEM-injected control mice (Student's t test). Young adult mice (6 weeks old) were also injected intracerebrally with 5 × 105 PFU of mutant MCMV (RM461) (C) or injected with 50 μl of MEM (D). Each group consisted of five mice that were maintained for more than 180 days after injection. Body weight was measured every week. Each group consists of more than six animals. Bars indicate standard deviation.
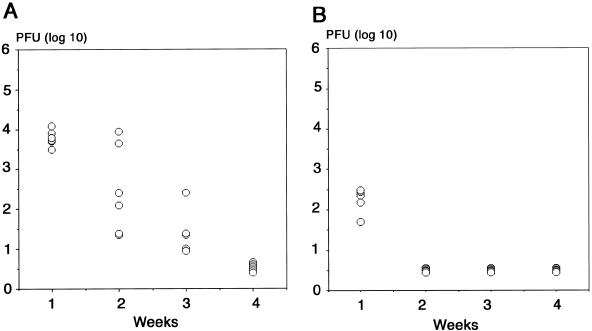
The viral titers of the infected brains were measured by plaque assay. The viral titer had decreased below the limit of detection of virus by 4 weeks after infection in both the neonatal (Fig. 2A) and young adult groups (Fig. 2B). Virus-infected cells detected by X-Gal staining just after brain slices were prepared were observed in the periventricular region 7 (Fig. 3A) to 14 (Fig. 3B) days after neonatal infection and disappeared 21 days after the infection (Fig. 3C).
FIG. 2.
Viral titers of brains from neonatal (A) and young adult mice (B) after intracerebral infection with 5 × 102 and 5 × 105 PFU of RM461, respectively. Half a gram of brain tissue was minced in 0.5 ml of MEM and centrifuged at 4°C. The viral titer in the supernatant was quantified by the plaque assay method (60). The area below the horizontal line indicates the limit of detection of virus.
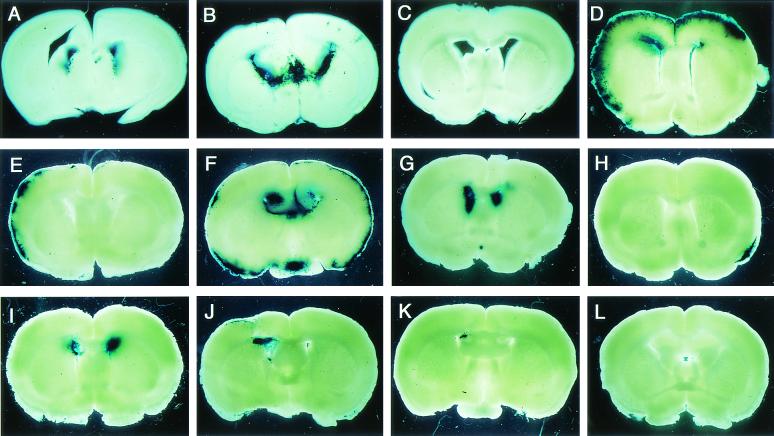
FIG. 3.
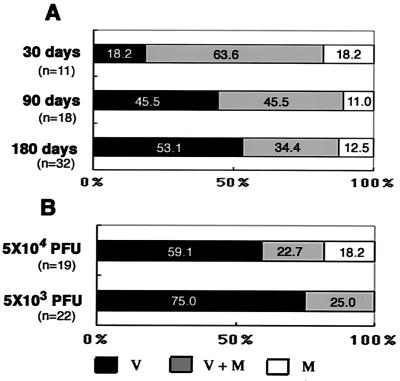
β-Gal expression in brain slices from mice infected during the neonatal period or as young adults. (A to C) Uncultured brain slices from mice infected with 5 × 102 PFU of RM461 within 24 h after birth (neonatal infection) and maintained for 7 (A), 14 (B), or 28 (C) days. (D) Brain slices cultured for 4 weeks from mice with neonatal infection and fed 90 days (D). (E to G) Brain slices from mice infected neonatally and maintained for 180 days. (H and I) Brain slices cultured for 4 weeks from mice infected at 6 weeks of age with 5 × 104 PFU (young adult infection) and maintained for 180 days. (J and K) Brain slices cultured for 4 weeks from mice infected as young adults, but with 5 × 103 PFU, and maintained for 180 days. (L) Brain slices cultured for 4 weeks from mice injected with MEM within 24 h after birth and maintained for 180 days.
Reactivation of latent infection in subventricular zone and cerebral marginal region by brain slice culturing.
Brain slices from the mice 30 and 90 days after the neonatal infection were cultured for 4 weeks and stained by X-Gal. X-Gal-positive areas, which consisted of infected cells, appeared massively in the cerebral marginal region and subventricular region (Fig. 3D). In some of the brain slices cultured for around 90 days, a streak-like positive reaction penetrated from the cerebral marginal region into the cerebral cortex (Fig. 3D). In the brain slices from the mice maintained for 180 days after neonatal infection, there were three types of distribution of X-Gal-positive area: cerebral marginal region only (M) (Fig. 3E), both cerebral marginal region and subventricular region (M+V) (Fig. 3F), and subventricular region only (V) (Fig. 3G). X-Gal-positive cells were also observed in brain slices from the mice infected as young adults with either 5 × 104 PFU (Fig. 3H and I) or 5 × 103 PFU (Fig. 3J and K). No X-Gal-positive area was detected in the brain slices of mice injected with MEM and maintained for 180 days (Fig. 3L).
Viral replication in the cultured brain slices after the latent infection.
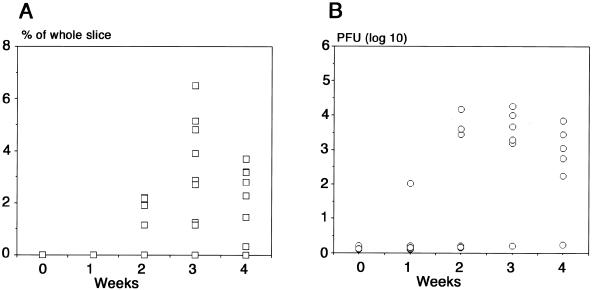
Brain slices were obtained from the six mice 180 days after neonatal infection. Five slices were taken from each mouse before and 1, 2, 3, and 4 weeks after culture. Viral titers were quantified by the plaque assay (Fig. 4B). Brain slices were taken in the same manner from nine mice 180 days after neonatal infection and stained with X-Gal, and the β-Gal-positive area per whole brain slice area was quantified as described in Materials and Methods (Fig. 4A). Viral replication in the brain slice was detected around 2 weeks and peaked at 3 weeks after culture (Fig. 4B). This tendency was almost paralleled by the ratio of X-Gal-stained area (Fig. 4).
FIG. 4.
Quantitative measurements of reactivation of latent infection after transfer to brain slice cultures either by measuring the X-Gal-positive area (A) or by plaque assay (B). (A) Brain slices were cultured from nine mice 180 days after intracerebral neonatal infection (5 × 102 PFU). Five brain slices from each mouse before and 1, 2, 3, and 4 weeks after cultures were stained by X-Gal. The infection ratio was expressed by dividing the pixel counts of the X-Gal-stained area by those of the whole brain slice area with Adobe Photoshop software. (B) Brain slices were cultured from six mice 180 days after neonatal infection in the same manner. Brain slices before and after cultures were frozen at −80°C, and the virus titers in the brain slices were measured by plaque assay.
High rate of reactivation of latent infection in the brains.
Reactivation of latent infection in the brains was determined by examination of three brain slices from each brain. The rate of reactivation in the brain was about 75% in mice maintained for 180 days after neonatal infection. Surprisingly, the rate of reactivation in the brain was also 75% in mice infected as young adults (Table 1). However, the rate of reactivation was decreased to 42% when young adult mice were infected with a 10-fold-lower virus titer (5 × 103 PFU). No reactivation was observed in the brain slices from the brains of mice maintained for 180 days after injection of MEM as a control. Furthermore, no virus was detected by plaque assay in the brains of latently infected mice just before transfer of brain tissue to the slice cultures.
TABLE 1.
Reactivation of latent infection of MCMV in mouse brains after transfer to brain slice culturea
| Infection group | Virus titer (PFU) | No. (%) of reactivated mice/virus-injected mouse
|
||
|---|---|---|---|---|
| 30 days | 90 days | 180 days | ||
| Neonatal | 5 × 102 | 18/18 (100) | 10/14 (71.4) | 39/52 (75.0) |
| Uninfectedb | 0 (MEM) | 0/8 (0) | ||
| Young adultsc | 5 × 104 | NDd | 7/9 (77.7) | 21/28 (75.0) |
| 5 × 103 | ND | ND | 8/19 (42.1) | |
| Uninfected | 0 (MEM) | 0/5 (0) | ||
BALB/c mice were infected by intracerebral injection with mutant MCMV (RM461). The mice were maintained for the times given and sacrificed at appropriate times. Brains were sliced, cultured for 4 weeks, and stained with X-Gal. Reactivation was determined in three slices from each brain.
Virus was injected within 24 h after birth.
Virus was injected at 6 weeks after birth.
ND, not determined.
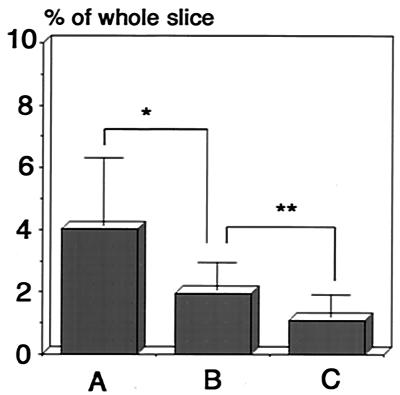
Virus-infected cells stained with X-Gal were preferentially observed in the periventricular regions compared to the cerebral marginal regions because infected mice were maintained for longer times (Fig. 5A). This tendency was most prominent in the brains of mice infected as young adults, especially in the mice infected with the smaller amount of virus (Fig. 5B). The number of virus-infected cells was significantly lower in the brain slices from the mice infected as young adults than in those from neonatally infected mice when the ratios of X-Gal-stained area per brain slice area were calculated (Fig. 6A and B). The infection ratio was further decreased in brain slices from the mice infected with a low virus titer as young adults (Fig. 6C).
FIG. 5.
Distribution of β-Gal-expressing region in cultured brain slices. (A) Brain slices from mice infected with 5 × 102 PFU 24 h after birth (neonatal infection) maintained for 30, 90, or 180 days. (B) Brain slices from mice infected at 6 weeks of age (young adults) with 5 × 104 or 5 × 103 PFU and maintained for 180 days. All of the brain slices were cultured for 4 weeks, fixed, and stained with X-Gal. V, X-Gal-positive area only in the ventricular region; M+V, X-Gal-positive area in both regions; M, X-Gal-positive area only in the cerebral marginal region. The ratio was expressed as percentages of numbers of mice with brains of each group. Three brain slices were examined per brain.
FIG. 6.
Comparison of the ratios of infected area to whole brain area in brain slices from mice infected neonatally to those of mice infected as young adults. The infection ratio was expressed by dividing the pixel count of the X-Gal-stained area by the pixel count of the whole brain slice area by using Adobe Photoshop software. (A) Brain slices from mice infected with 5 × 102 PFU 24 h after birth (neonatal infection). (B) Brain slices from mice infected with 5 × 104 at 6 weeks of age (young adult). (C) Brain slices from mice infected with 5 × 103 PFU as young adults. All mice were maintained for more than 180 days. ∗, P < 0.01; ∗∗, P < 0.05 (Student's t test).
Preferential reactivation in the neural progenitor cells in latently infected brains.
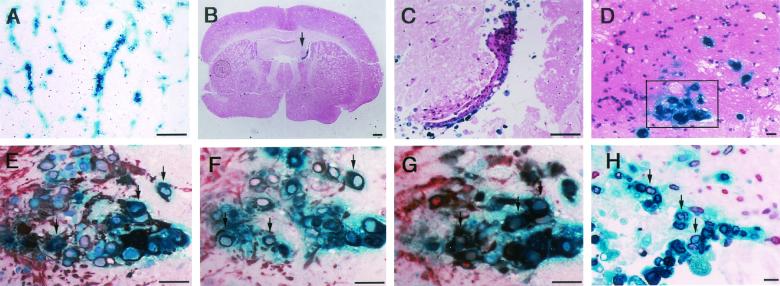
Paraffin-embedded sections of cultured brain slices were histologically examined by either staining with hematoxylin and eosin or immunostaining. The streak-like X-Gal-positive area observed in the cerebral cortex from the marginal region (Fig. 3D) was extended along the endothelial cells (Fig. 7A). X-Gal-positive cells were preferentially observed in the ventricular wall, where cells tended to proliferate as clusters (Fig. 7B and C). In order to identify features of the reactivated cells, immunostaining of X-Gal-positive cells in sections from brain slices cultured for 2 weeks (Fig. 7D), which were thought to be close to the beginning of reactivation, was performed with specific antibodies to neural markers. X-Gal-positive cells were double stained with antibodies to GFAP (Fig. 7E), nestin (Fig. 7F), and Musashi-1 (Fig. 7G), which are thought to be specific for neural stem or progenitor cells. X-Gal-positive cells were not double stained with antibody to MAP2, a neuronal cell marker (data not shown). X-Gal-positive cells in the marginal parts of the brain were also stained with antibodies specific to nestin and Musashi-1 (data not shown). X-Gal-stained cells were also preferentially labeled with BrdU, which was added to the medium of brain slice cultures for the last 24 h before harvesting (Fig. 7H).
FIG. 7.
Histochemical and immunohistochemical examination of β-Gal-expressing cells. After X-Gal staining, brain slices were embedded in paraffin and serial sectioned for histological and immunohistological analysis. Vascular distribution of X-Gal-positive cells in 4-week-cultured brain slices from mice infected during the neonatal period and maintained for 90 days (A). X-Gal-positive cells are seen in the ventricular region (arrow) in the 4-week-cultured brain slices from mice infected as young adults and maintained for 180 days (B and C). In order to identify features of the reactivated cells (D [inset]), GFAP (E), X-Gal-positive cells in sections from brain slices cultured for 2 weeks were immunostained with antibodies specific to nestin (F), and Musashi-1 (G). Brain slices were labeled with BrdU for the last 24 h before analysis and stained with the antibody specific to BrdU (H). Size bars: panels A to C, 300 μm; panels D to H, 50 μm. In panels E to H, arrows indicate representative double-stained cells.
DISCUSSION
In the present study, we first showed that latent infection with CMV occurs in the mouse brain and defined the localization and cell type of the latent infection in the brains by detecting reactivation sites by the method of brain slice culturing after latent infection. Although latent MCMV DNA has been detected in diverse organs of mice (3, 8), little attention has been paid to the brain as a latent infection site. However, brain infection by CMV is an important problem as a cause of brain disorders in fetuses (4, 13, 35, 51) or immunocompromised adults (7, 31, 61).
Among the present results, it is worth noting that the latent infection was reactivated in about 75% of brains by their transfer to brain slice culture. Unexpectedly, the same high rate of reactivation occurred in the brains of mice infected as young adults, although the degree of reactivation was lower than in the mice infected during the neonatal period in terms of the amount of reactivated cells. From this result, it is possible that latent infection and reactivation may occur even in patients infected with HCMV in adulthood. Schmader et al. (47) reported that young, blood-transfused mice reactivated MCMV in submaxillary salivary glands significantly more often than did old transfused mice after a latency of 6 months. Reddehase et al. (41) reported that recurrence was detected in the lungs and spleens after whole-body irradiation in more than 30% of mice infected in the neonatal period, but recurrence was observed in only 6.7% of the lungs of mice infected in adulthood, although this recurrence was observed with a different reactivation method 1 year after infection.
Although the mechanism of reactivation in the brain slice culture is not known, the basic mechanism may be similar to explant reactivation, in which latent cells are reactivated by cocultivation of cell suspensions with susceptible fibroblasts (15, 52, 62). The advantages of using brain slice cultures include detection of latent sites of the brain and the cell type of the reactivation. During the course of latent infection, reactivation was observed in three parts of the brains: endothelial cells, cerebral marginal regions, and the subventricular regions. Reactivation of the cerebral endothlial cells was prominent about 90 days after infection in neonatal mice, but was not prominently observed 180 days after infection. Koffron et al. (19) reported that endothelial cells are a major site of MCMV DNA latency in infected animals in which several organs were examined by PCR-in situ hybridization, as reported in patients with active HCMV infection (11, 34). It was suggested that there is significant heterogeneity in endothelial cell type with regard to organ type and vessel type (63). In the case of mice infected in young adulthood, reactivation in endothelial cells was rarely observed during the course of infection.
In the latently infected mice maintained for more than 180 days after infection, reactivation was observed in both the submeningeal region of the cerebrum and the subventricular zone. However, the degree of reactivation in the subventricular region was higher than that in the cerebral marginal region. In the mice infected during young adulthood, this tendency was prominent, especially in mice infected with a reduced amount of virus. From these results, it can be postulated that the subventricular region is the most preferential site of latent infection in the brain (Fig. 3 and 5).
The subventricular zone has been focused on as the site of the neural progenitor cells, including the neural stem cells (26, 45, 46, 58). The present results show that the reactivated cells expressed nestin, Musashi-1, and GFAP, and most of the cells were also labeled with BrdU, suggesting that they are neural stem or progenitor cells (27, 45, 46). The stem cells have the potential to self-renew and to differentiate into neurons, astrocytes, and oligodendrocytes. The progenitor cells are cells with a more restricted potential, such as only a neuronal or astroglial lineage, than stem cells (27). However, it is difficult to distinguish neural progenitor cells from neural stem cells by assessing neural markers. According to the results of our recent studies, the neural progenitor cells in the subventricular regions in the mouse brain are specifically susceptible to MCMV infection in vivo (23), as are cultured neural stem or progenitor cells (21). In human autopsy samples, periventricular regions are susceptible to HCMV, showing necrosis and calcification (4, 13, 35). Activation of the MCMV IE promoter was also observed in glial progenitor cells in the ventricular zone in transgenic mice (23). Some submeningeal cells, in which reactivation was also observed, expressed nestin, Musashi-1, and GFAP, similar to the neural progenitor cells. It is reported that radial glial cells might have the same potential as neural progenitor cells and are not necessarily located in the subventricular region (1). Recently it was suggested that immature cells capable of neural differentiation also exist in the subpial layer in the adult brain (29).
Although there is close similarity, it is not known whether there is a correlation between the susceptibility of neural progenitor cells to CMV infection and their tendency to be a preferential site of CMV latency. At this moment, it is difficult to distinguish neural stem cells from progenitor cells by using the neural markers. However, it is possible that the neural progenitor cells are transient cells in the process of differentiating from neural stem cells to mature cells. It is not possible that these transient cells might be sites of latency. We hypothesize that latent CMV infection may occur in neural stem cells. Once latently infected stem cells are committed to differentiate to progenitor cells by some kind of stimulation, latent virus is reactivated in these cells, in which a lot of factors for viral gene expression are induced, eventually leading to permissive infection. Recently we showed that MCMV IE promoter was not expressed in neural stem cells, but was expressed extensively in the glial neural progenitor cells (23). It is possible that neural stem cells might have strong suppressors for expression of the IE promoter (25) or might be deficient of some necessary factors for activation of the promoter. At present, it is difficult to demonstrate latent virus genome directly in neural stem cells, presumably because of the rareness of these cells and the difficulty in isolating such cells without induction of reactivation. Therefore, we can only speculate about the existence of latency in neural stem cells based on detection of reactivated neural progenitor cells in brain slice culture. Interestingly, our hypothesis that neural stem or progenitor cells may be the site of latent infection in the brain may be related to the observation that hematopoietic progenitor cells are similarly a site of latent infection (12, 20). It was reported that reactivation occurred when these cells were stimulated to differentiate by certain factors, such as allogeneic stimulation (50).
Finally, it is suggested that brain disorders that occur long after birth in individuals with congenital CMV infection or in immunocompromised adults may occur by intermittent reactivation of latent infection in the brains. The most preferential site of latent CMV infection may be neural stem or progenitor cells in the subventricular regions, which are pivotal sites for brain development. Since neural stem or progenitor cells have potential therapeutic use to treat brain disorders, it may be important to avoid the use of neural stem or progenitor cells latently infected with HCMV as donor cells.
Acknowledgments
We thank Hiromi Suzuki and Masaaki Kaneta for excellent technical assistance. We also thank E. S. Mocarski, Department of Microbiology and Immunology, Stanford University School of Medicine, for providing recombinant MCMV (RM461).
This work was supported in part by grant 2470054 from Ministry of Education, Culture, Science, and Technology, Japan.
REFERENCES
- 1.Alvarez-Buylla, A., J. M. Gricia-Verdugo, and A. D. Tramontin. 2001. A unified hypothesis on lineage of neural stem cells. Nature Rev. 2:287-293. [DOI] [PubMed] [Google Scholar]
- 2.Bale, J. F., Jr. 1984. Human cytomegalovirus infection and disorders of the nervous system. Arch. Neurol. 41:310-320. [DOI] [PubMed] [Google Scholar]
- 3.Balthesen, M., L. Dreher, P. Lucin, and M. J. Reddehase. 1994. The establishment of cytomegalovirus latency in organs is not linked to local virus production during primary infection. J. Gen. Virol. 75:2329-2336. [DOI] [PubMed] [Google Scholar]
- 4.Becroft, D. M. O. 1981. Prenatal cytomegalovirus infection: epidemiology, pathology, pathogenesis, p. 203-241. In H. S. Rosenberg and J. Bernstein (ed.), Perspective in pediatric pathology. Masson, New York, N.Y. [PubMed]
- 5.Bruggeman, C. A. 1993. Cytomegalovirus and latency: an overview. Virchows Arch. B Cell Pathol. 64:325-333. [DOI] [PubMed] [Google Scholar]
- 6.Cheung, K.-S., and D. J. Lang. 1977. Detection of latent cytomegalovirus in murine salivary and prostate explant cultures and cells. Infect. Immun. 15:568-575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cinque, P., R. Marenzi, and D. Ceresa. 1997. Cytomegalovirus infections of the nervous system. Intervirology 40:85-97. [DOI] [PubMed] [Google Scholar]
- 8.Collins, T., C. Pomeroy, and C. Jordan. 1993. Detection of latent cytomegalovirus DNA in diverse organs of mice. J. Infect. Dis. 168:725-729. [DOI] [PubMed] [Google Scholar]
- 9.Conboy, T. J., R. F. Pass, S. Stagno, W. T. Britt, C. A. Alford, C. E. McFarland, and T. J. Boll. 1986. Intellectual development in school-aged children with asymptomatic congenital cytomegalovirus infection. Pediatrics 77:801-806. [PubMed] [Google Scholar]
- 10.Demmler, G. J. 1991. Infectious Disease Society of American and Centers of Disease Control: summary of a workshop on surveillance for congenital cytomegalovirus disease. Rev. Infect. Dis. 13:315-329. [DOI] [PubMed] [Google Scholar]
- 11.Grefte, A., M. van der Giessen, W. van Son, and T. H. The. 1993. Circulating cytomegalovirus (CMV)-infected endothelial cells in patients with an active CMV infection. J. Infect. Dis. 167:270-277. [DOI] [PubMed] [Google Scholar]
- 12.Hahn, G., R. Jores, and E. S. Mocarski. 1998. Cytomegalovirus remains latent in a common precursor of dendritic and myeloid cells. Proc. Natl. Acad. Sci. USA 95:3937-3942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ho, M. 1991. Cytomegalovirus: biology and infection, p. 205-277. Plenum, New York, N.Y.
- 14.Hummel, M., Z. Zhang, S. Yan, I. Deplaen, P. Golia, T. Varghese, G. Thomas, and M. I. Abecassis. 2001. Allogeneic transplantation induces expression of cytomegalovirus immediate-early genes in vivo: a model for reactivation from latency. J. Virol. 75:4814-4822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan, M. C., and V. L. Mar. 1982. Spontaneous activation of latent cytomegalovirus from murine spleen explant. Role of lymphocytes and macrophages in release and replication of virus. J. Clin. Investig. 70:762-768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaneko, Y., S. Sakakibara, T. Imai, A. Suzuki, Y. Nakamura, K. Sawamoto, Y. Ogawa, Y. Toyama, T. Miyata, and H. Okano. 2000. Musashi1: an evolutionally conserved marker for CNS progenitor cells including neural stem cells. Dev. Neurosci. 22:138-152. [DOI] [PubMed] [Google Scholar]
- 17.Kashiwai, A., N. Kawamura, C. Kadota, and Y. Tsutsui. 1992. Susceptibility of mouse embryo to murine cytomegalovirus infection in early and mid-gestation satages. Arch. Virol. 127:37-48. [DOI] [PubMed] [Google Scholar]
- 18.Klotman, M. E., D. Starnes, and J. D. Hamilton. 1985. The source of murine CMV in mice receiving kidney allograft. J. Infect. Dis. 153:1192-1195. [DOI] [PubMed] [Google Scholar]
- 19.Koffron, A. J., M. Hummel, B. K. Patterson, S. Yan, D. B. Kaufman, J. P. Fryer, F. P. Stuart, and M. I. Abecassis. 1998. Cellular localization of latent murine cytomegalovirus. J. Virol. 72:95-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kondo, K., H. Kaneshima, and E. S. Mocarski. 1994. Human cytomegalovirus latent infection of granulocyte-macrophage progenitors. Proc. Natl. Acad. Sci. USA 91:11879-11883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kosugi, I., Y. Shinmura, H. Kawasaki, Y. Arai, R.-Y. Li, S. Baba, and Y. Tsutsui. 2000. Cytomegalovirus infection of the CNS stem cells from mouse embryo: a model for developmental brain disorders induced by cytomegalovirus. Lab. Investig. 80:1373-1383. [DOI] [PubMed] [Google Scholar]
- 22.Lendahl, U., L. B. Zimmerman, and R. D. G. McKay. 1990. CNS stem cells express a new class of intermediate filament protein. Cell 60:585-595. [DOI] [PubMed] [Google Scholar]
- 23.Li, R.-Y., S. Baba, I. Kosugi, Y. Arai, H. Kawasaki, Y. Shinmura, S. Sakakibara, H. Okano, and Y. Tsutsui. 2001. Activation of murine cytomegalovirus immediate-early promoter in cerebral ventricular zone and glial progenitor cells in transgenic mice. Glia 35:41-52. [DOI] [PubMed] [Google Scholar]
- 24.Li, R.-Y., and Y. Tsutsui. 2000. Growth retardation and microcephaly induced in mice by placental infection with murine cytomegalovirus. Teratology 62:79-85. [DOI] [PubMed] [Google Scholar]
- 25.Liu, R., J. Baillie, J. G. P. Sissons, and J. H. Sinclair. 1994. The transcription factor YY1 binds to negative regulatory elements in the human cytomegalovirus major immediate early enhancer/promoter and mediates repression in non-permissive cells. Nucleic Acids Res. 22:2453-2459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Luskin, M. B., J. G. Parnavelas, and J. A. Barfield. 1993. Neurons, astrocytes and oligodendrocytes of the rat cerebral cortex originate from separate progenitor cells: an ultrastructural analysis of clonally related cells. J. Neurosci. 13:1730-1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKay, R. 1997. Stem cells in the central nervous system. Science 276:66-71. [DOI] [PubMed] [Google Scholar]
- 28.Mercer, J. A., C. A. Wiley, and D. H. Spector. 1988. Pathogenesis of murine cytomegalovirus infection: identification of infected cells in the spleen during acute and latent infections. J. Virol. 62:987-997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mercier, F., and G. I. Hatton. 2001. Connexin 26 and basic fibroblast growth factor are expressed primarily in the subpial and subependymal layers in adult brain parenchyma: roles in stem cell proliferation and morphological plasticity? J. Comp. Neurol. 431:88-104. [DOI] [PubMed] [Google Scholar]
- 30.Minton, E. J., C. Tysoe, J. H. Sinclair, and J. G. P. Sissons. 1994. Human cytomegalovirus infection of the monocyte/macrophage lineage in bone marrow. J. Virol. 68:4017-4021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morgello, S., E. S. Cho, S. Nielsen, O. Devinsky, and C. K. Petito. 1987. Cytomegalovirus encephalitis in patients with acquired immunodeficiency syndrome: an autopsy study of 30 cases and a review of literature. Hum. Pathol. 18:289-297. [DOI] [PubMed] [Google Scholar]
- 32.Ogura, T., J. Tanaka, S. Kamiya, H. Sato, H. Ogura, and M. Hatano. 1986. Human cytomegalovirus persistent infection in a human central nervous system cell line: production of a variant virus with different growth characteristics. J. Gen. Virol. 67:2605-2616. [DOI] [PubMed] [Google Scholar]
- 33.Pass, R. F., S. Stagno, G. J. Myers, and C. A. Alford. 1980. Outcome of symptomatic congenital cytomegalovirus infection: results of long-term longitudinal follow-up. Pediatrics 66:758-762. [PubMed] [Google Scholar]
- 34.Percivalle, E., M. G. Revello, L. Vago, F. Morini, and G. Gerna. 1993. Circulating endothelial giant cells permissive for human cytomegalovirus (HCMV) are detected in disseminated HCMV infections with organ involvement. J. Clin. Investig. 92:663-670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Perlman, J. M., and C. Argyle. 1992. Lethal cytomegalovirus infection in premature infants: clinical, radiological, and neuropathological findings. Ann. Neurol. 13:64-68. [DOI] [PubMed] [Google Scholar]
- 36.Poland, S. D., P. Costello, G. A. Dekaban, and G. P. A. Rice. 1990. Cytomegalovirus in the brain: in vitro infection of human brain-derived cells. J. Infect. Dis. 162:1252-1262. [DOI] [PubMed] [Google Scholar]
- 37.Poland, S. D., L. Bambbrick, G. A. Dekaban, and G. P. A. Rice. 1994. The extent of human cytomegalovirus replication in primary neurons is dependent on host cell differentiation. J. Infect. Dis. 170:1267-1271. [DOI] [PubMed] [Google Scholar]
- 38.Pollock, J. L., and H. W. Virgin IV. 1995. Latency, without persistence, of murine cytomegalovirus in the spleen and kidney. J. Virol. 69:1762-1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pomeroy, C., P. J. Hilleren, and M. C. Jordan. 1991. Latent murine cytomegalovirus DNA in splenic stromal cells of mice. J. Virol. 65:3330-3334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pulliam, L. 1991. Cytomegalovirus preferentially infects monocyte derived macrophage/microglial cells in human brain cultures: neuropathology differs between strains. J. Neuropathol. Exp. Neurol. 50:432-440. [DOI] [PubMed] [Google Scholar]
- 41.Reddehase, M. J., M. Balthesen, M. Rapp, S. Jonjic, I. Pavic, and U. H. Koszinowski. 1994. The conditions of primary infection define the load of latent viral genome in organs and the risk of recurrent cytomegalovirus disease. J. Exp. Med. 179:185-193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Reynolds, K., E. Mezey, and A. Zimmer. 1991. Activity of the β-retinoic acid receptor promoter in transgenic mice. Mech. Dev. 36:15-29. [DOI] [PubMed] [Google Scholar]
- 43.Saederup, N., Y. C. Lin, D. J. Dairaghi, T. J. Schall, and E. S. Mocarski. 1999. Cytomegalovirus-encoded β chemokine promoters monocyte-associated viremia in the host. Proc. Natl. Acad. Sci. USA 96:10881-10886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Saederup, N., S. A. Aguirre, T. E. Sparer, D. M. Bouley, and E. S. Mocarski. 2001. Murine cytomegalovirus CC chemokine homolog MCK-2 (m131-129) is a determinant of dissemination that increases inflammation at initial sites of infection. J. Virol. 75:9966-9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sakakibara, S., T. Imai, J. Aruga, K. Nakajima, D. Yasutomi, T. Nagata, Y. Kurihara, S. Uesugi, T. Miyata, M. Ogawa, K. Mikoshiba, and H. Okano. 1996. Mouse-Musashi-1, a neural RNA-binding protein highly enriched in the mammalian CNS stem cell. Dev. Biol. 176:230-242. [DOI] [PubMed] [Google Scholar]
- 46.Sakakibara, S., and H. Okano. 1997. Expression of neural RNA-binding proteins in the postnatal CNS: implication of their roles in neurol and glial cell development. J. Neurosci. 17:8300-8312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmader, K. E., R. Rahija, K. R. Porter, G. Daley, and J. D. Hamilton. 1992. Aging and reactivation of latent murine cytomegalovirus. J. Infect. Dis. 166:1403-1407. [DOI] [PubMed] [Google Scholar]
- 48.Shinmura, Y., S. Aiba-Masago, I. Kosugi, R.-Y. Li, S. Baba, and Y. Tsutsui. 1997. Differential expression of the immediate-early and early antigens in neuronal and glial cells of developing mouse brains infected with murine cytomegalovirus. Am. J. Pathol. 151:1331-1340. [PMC free article] [PubMed] [Google Scholar]
- 49.Shinmura, Y., I. Kosugi, M. Kaneta, and Y. Tsutsui. 1999. Migration of virus-infected neuronal cells in cerebral slice cultures of developing mouse brains after in vitro infection with murine cytomegalovirus. Acta Neuropathol. 98:590-596. [DOI] [PubMed] [Google Scholar]
- 50.Soderberg-Naucher, C., K. N. Fish, and J. A. Nelson. 1997. Reactivation of latent human cytomegalovirus by allogeneic stimulation of blood cells from healthy donors. Cell 91:119-126. [DOI] [PubMed] [Google Scholar]
- 51.Stagno, S., R. F. Pass, G. Cloud, W. T. Britt, R. E. Henderson, P. O. Walton, D. A. Veren, F. Page, and C. A. Alford. 1986. Primary cytomegalovirus infection in pregnancy: incidence, transmission to fetus, and clinical course. JAMA 256:1904-1908. [PubMed] [Google Scholar]
- 52.Stoddart, C. A., R. D. Cardin, J. M. Boname, W. C. Manning, G. B. Abenes, and E. S. Mocarski. 1994. Peripheral blood mononuclear phagocytes mediate dissemination of murine cytomegalovirus. J. Virol. 68:6243-6253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stoppini, L., P. A. Buchs, and D. Muller. 1991. A simple method for organotypic culture of nervous tissue. J. Neurosci. Methods 37:173-182. [DOI] [PubMed] [Google Scholar]
- 54.Tomooka, Y., H. Kitani, N. Jing, M. Matsushima, and T. Sakakura. 1993. Reconstruction of neural tube-like structure in vitro from primary neural precursor cells. Proc. Natl. Acad. Sci. USA 90:9683-9687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tsutsui, Y. 1995. Developmental disorders of the mouse brain induced by murine cytomegalovirus: animal models for congenital cytomegalovirus infection. Pathol. Int. 45:91-102. [DOI] [PubMed] [Google Scholar]
- 56.Tsutsui, Y., A. Kashiwai, N. Kawamura, and C. Kadota. 1993. Microphthalmia and cerebral atrophy induced in mouse embryos by infection with murine cytomegalovirus in midgestation. Am. J. Pathol. 143:804-812. [PMC free article] [PubMed] [Google Scholar]
- 57.Tsutsui, Y., A. Kashiwai, N. Kawamura, S. Aiba-Masago, and I. Kosugi. 1995. Prolonged infection of mouse brain neurons with murine cytomegalovirus after pre- and perinatal infection. Arch. Virol. 140:1725-1736. [DOI] [PubMed] [Google Scholar]
- 58.Turner, D., and C. Cepko. 1987. A common progenitor for neurons and glia persist in rat retina late in development. Nature 328:131-136. [DOI] [PubMed] [Google Scholar]
- 59.Weller, T. H. 1971. The cytomegalovirus: ubiquitous agents with protean clinical manifestation. N. Engl. J. Med. 258:203-214. [DOI] [PubMed] [Google Scholar]
- 60.Wentworth, B. B., and L. French. 1970. Plaque assay of cytomegalovirus strains of human origin. Proc. Soc. Exp. Biol. Med. 135:253-258. [DOI] [PubMed] [Google Scholar]
- 61.Wiley, C. A., and J. A. Nelson. 1988. Role of human immunodeficiency virus and cytomegalovirus in AIDS encephalitis. Am. J. Pathol. 133:73-81. [PMC free article] [PubMed] [Google Scholar]
- 62.Wise, T. G., J. E. Manischewitz, G. V. Quinnan, G. S. Aukakh, and E. A. Ennis. 1979. Latent cytomegalovirus infectionof BALB/c mouse spleen detected by an explant culture technique. J. Gen. Virol. 114:551-556. [DOI] [PubMed] [Google Scholar]
- 63.Zetter, B. R. 1988. Endothelial cell heterogeneity: influence of vessel size, organ localization, and species specificity on the properties of cultured endothelial cells, p. 63-79. In U. Ryan (ed.), Endothelial cells, vol. II. CRC Press, Inc., Boca Raton, Fla. [Google Scholar]