Abstract
Background
Parents of six children are facing a trial on charges of aggravated manslaughter in the care a 5 1/2 month old infant who died suddenly and neglect of their four older children for causing them to be malnourished by feeding them all an exclusively raw foods vegan diet. Both parents declined plea bargains and plan to defend themselves in court.
Case presentation
The fifth child born to a married couple was breast-fed until 2 1/2 months. Subsequently, the parents fed the baby an exclusively raw foods diet prepared in a blender at home. The four older children, ages 18 months – 6 1/2 years also ate an exclusively raw foods vegan diet. None of the four older children had significant previous injuries or serious illnesses. At autopsy, the infant weighed 3180 mg (6.99 pounds) and appeared emaciated. The thymus gland was absent and parathyroid glands were not located. The lungs were "congested." DiGeorge anomaly cannot be ruled out from these findings. Although, the coroner ruled that "malnutrition" was the sole cause of death, malnutrition, according to the World Health Organization definition, cannot be diagnosed in this infant. Compared with standard growth charts, the older children fell 2.1–4.1 standard deviations below the mean for North American children in height and weight. Labs were normal except for a low cholesterol level in all and a low prealbumin in one of three children tested. Therefore, malnutrition cannot be diagnosed in these children. The pediatrician diagnosed rickets in the four-year-old. However, chest x-rays were normal in all and long bone x-rays showed minimal changes in one child – no sign of rickets. The clinical diagnosis of rickets was not confirmed by the Center for Disease Control's criteria. A psychologist diagnosed the 18-month-old as developmentally delayed to the level of a 15-month-old, but this diagnosis is questionable.
Conclusion
The raw foods vegan diet and possibly inherited small stature from the father's side account for their relatively low heights and weights. Catch-up growth will probably occur on the standard American diet but would have also been expected if they had remained on a vegan diet.
Background
The American Dietetic Association (ADA) and Dietitians of Canada position paper on vegetarian diets officially recognizes that well-planned vegan diets are appropriate for infancy and childhood.[1] The American Academy of Pediatrics concurs.[2,3] However, the ADA position paper also concludes that raw foods diets impair growth and therefore cannot be recommended for infants and children. The single reference for this statement is a book by Mark J. Messina and Virginia L. Messina titled, The Dietitian's Guide to Vegetarian Diet: Issues and Applications.[4] This book has no references to studies supporting this conclusion and the references were not available from the authors or the American Dietetic Association.
No reference is cited in the ADA position paper or the Pediatric Nutrition Handbook of any specific case of an infant or child on a raw foods diet having any diet related nutritional deficiency or deficiency related adverse health problem.
Case report
A girl was the fifth child born to a married couple of African American ancestry by way of Jamaica and Puerto Rico. The father delivered the baby at home, which the parents did not weight at birth. The mother breast-fed the baby until nipple bleeding forced her to stop at 2 1/2 months. Subsequently, they fed the baby about nine ounces, five to six times each day of an exclusively raw foods diet prepared in a blender at home (see Table 1). The only support for this part of the history was the observations by Department of Children's Services investigators on three occasions that there was plenty of food in the house. Shortly after the infant died, police investigators took the homemade formula for analysis but checked it only for drugs and not for nutrient content. The couple also fed their older children, ages 18 months – 6 1/2 years a raw foods diet (Table 1). The mother did not estimate the amount of food consumed by the older children other than to say that they ate as much as they wanted.
Table 1.
Diets of the children
| Infant's diet | Children's diet |
| 24 ounces coconut water | Raw fruits, vegetables, and nuts |
| 8–9 ounces of blended sunflower, pecans, walnuts, brazil nuts, sesame seed, pumpkin seed, hazel nut and or almond/flax/coconut water and burdock root. | Green juice/veggie juice consisting of cucumber, celery, carrot, parsley, dandelion, kale, lemon, garlic, ginger, and other vegetables |
| Carrot juice, cucumber, celery, spinach, romaine lettuce, cilantro, kale, radishes, broccoli, tomato – 4–5 of these were included in one puree, blended with 1/2 avocado. Occasionally, a sliver of garlic or ginger was added. This puree would be placed in a baby bottle with the nipple cut a little larger to allow it to flow. | Nut or curry pate (almonds or sunnies, or brazil, or walnut, or other nuts with onion, garlic, basil, lime juice, dulse, carrots, celery, black pepper) on sticks or slices or chunks of celery, carrot, broccoli, cauliflower, or tomato |
| Papaya, strawberry, apple, banana, pear, watermelon, cantaloupe, figs, dates, raisins, and other fruits | |
| Nut milk several times per week | |
| Occasional raw pie | |
| 1/2 ounce of wheat grass with coconut water – 3 times a week | Coconut water and the coco jelly; three times a week, 1 ounce wheat grass |
| 8–10 ounces of fruit juice consisting of mango, cantaloupe, papaya, berries, oranges, banana, etc. blended up with a bit of coconut water | Big green salad with lettuce, cucumbers, tomatoes, arugula, collards, kale, sprouts, carrots, avocado and pate added |
| Banana date/strawberry/almond/macadamia ice cream made in food processor or juicer |
Figure 1 compares the infant's nutrient intake (approximately 650 Calories based on 50 ounces × 13 Calories/ounce) with the USDA Nutrient Database recommended daily amounts (RDA) of nutrients.[5] For comparison, Figure 2 graphically demonstrates a 650-Calorie diet from commercial vegan formulas and Figure 3 shows a 650-Calorie diet from breast milk.
Figure 1.
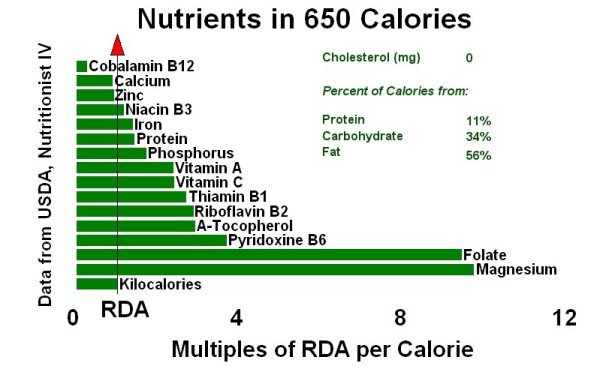
Infant's estimated diet.
Figure 2.
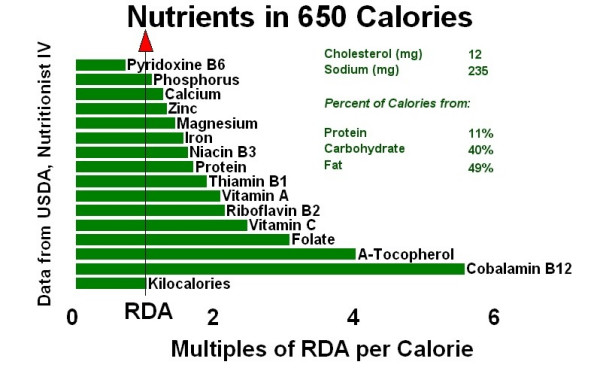
Four infant formulas.
Figure 3.
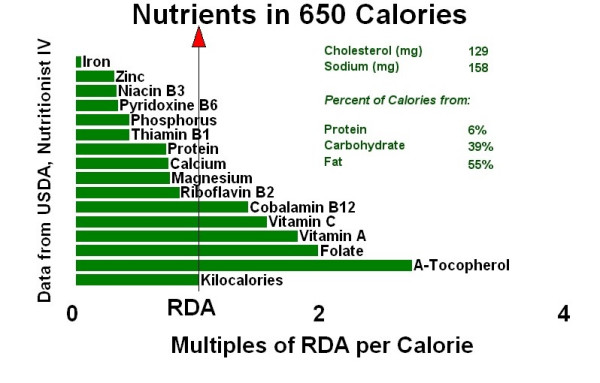
Human milk.
When the second youngest child was four months old, a child protective services worker visited the family at the request of a neighbor concerned about the small sizes of the children and their raw foods diets. This child protective services worker found no indicators of maltreatment and made no recommendation for physician referral. About 13 months later when the new baby was three months old, another neighbor's complaint that the children appeared underfed led to another visit by a representative of the Department of Children and Families. Again the assessment was that no maltreatment or reason for physician referral existed. Three days before the death of the infant, a third complaint led to a child protective services visit. Only the four older children were seen because the infant was out of the house with the father. This time the child protective services worker advised taking the children to a pediatrician but agreed to the mother's preference for a "natural doctor." The infant, who was out with the father during the visit, was scheduled to be seen subsequently, but she died before the visit. At age 5 1/2 months after three days of cold-like symptoms, the infant developed difficulty breathing. The parents called paramedics but attempts to resuscitate the infant were unsuccessful.
In the autopsy, (Table 2), the coroner found no evidence of dehydration on the ocular chemistries and ruled that "malnutrition" was the immediate and underlying cause of death.
Table 2.
Autopsy findings
| Gross and microscopic | Laboratory |
| Weight: 3180 mg (6.99 pounds) | Toxicology studies all negative |
| Length: 57 cm (22 1/2 inches) | Homemade infant formula: no drugs found; not tested for nutrients. |
| Head circumference: 38 cm (15 inches) | Ocular fluid: Calcium = 6.0 |
| Emaciated appearance | Ocular fluid: Chloride = 108 mmol/l |
| The complete absence of the thymus gland | Ocular fluid: Creatinine = 0.2 mg/dl |
| "Inconspicuous" parathyroid glands | Ocular fluid: Glucose = < 5.0 mg/dl (considered due to post mortem glucose metabolism) |
| Congested pulmonary parenchyma | |
| Lungs: postmortem changes and atelectasis | Ocular fluid: Potassium = 10.3 mmol/l |
| Liver: passive congestion, no fatty changes | Ocular fluid: BUN = 13 mg/dl |
| Pancreas: normal histology | Blood/CSF/lung post mortem cultures all showed heavy gram-negative rods (Klebsiella pneumoniae, citrobacter freundii, and enterococcus faecium). |
| Adrenals: spent | |
| Mediastinal soft tissue: thymus not present | |
| Parathyroid histology: no tissue submitted | |
| Spleen: "Immunochemistry for CD3 demonstrates the presence of T-lymphocytes towards the periphery of the Malpighian corpuscles and scattered throughout the red pulp. NOTE: The presence of T-lymphocytes in the spleen excludes the possibility of DiGeorge syndrome." | Ova, parasites, and viral inclusions not found in stool specimen |
| Brain: no abnormalities |
The older children were examined by a nurse practitioner supervised by a pediatrician (Table 3). Laboratory results (Table 4) showed low cholesterols in all children, mildly low prealbumin in the 18-month-old, slightly high INRs in two children, and alkaline phosphatases consistent with childhood.
Table 3.
History and physical examinations of the four older children
| 18-month-old girl | 39-month-old boy | 52-month-old boy | 78-month-old boy | |
| Past medical history | Unknown (ed note: no illnesses by the parents history) All born at home with assistance of a midwife or the husband. None were premature. All played outdoors in sunny Southern Florida more than 6 hours per week | |||
| Exclusive breast feeding period | 3 months | 3 months | 8 months | 12 months |
| Medications | Unknown (ed note: no medications by the parents history) | |||
| Family history | Unknown (ed note: mother 169 cm [5 feet 6 1/2 inches] and 52 1/4 kg [115 pounds], father 168 cm [5 feet 6 inches] and 70.5 kg [155 pounds]. The father weighted 104.5 kg [230 pounds] on the standard American diet about 7 years previously. The paternal grandmother was 152 cm tall [5 feet 0 inches] and the paternal grandfather was 170 cm tall [5 feet 7 inches]). | |||
| Immunizations | None | None | None | None |
| Diet | See Table 2 | |||
| Physical exam | Relevant positive and negative findings | |||
| General | Small thin female/male, alert | |||
| Length | 68-1/2 cm (27 inches: 3.68 SD below the mean height/age) | 83-1/3 cm (34 inches: 2.9 SD below the mean height/age) | 94 cm (37 inches: 2.82 SD below the mean height/age) | 104 cm (41 inches: 2.93 SD below the mean height/age) |
| Weight | 7.5 kg (16 pounds 8 ounces): 4.1 SD below the mean weight/age) | 11 kg (24 pounds 8 ounces: 2.06 SD below the mean weight/age) | 13 2/3 kg (30 pounds: 2.48 SD below the mean weight/age) | 15 2/3 kg (34 1/2 pounds: 2.98 SD below the mean weight/age) |
| Head circumference | 44 cm (17 1/3 inches) | 50 cm (19-2/3 inches with two | 52-1/2 cm (20-2/3 inches) | 54 cm (21 1/4 inches) |
| Mouth | Dental caries | Dental caries | Dental caries | Dental caries |
| Thorax | Ribcage prominent | Ribcage prominent | Bilateral beading of the ribs (Rachitic rosary)a | Ribcage prominent |
| Abdomen | Soft, protruding, no masses. Girth: 42 cm. (16 1/2 inches) | Soft, protruding, no masses. Girth: 49 cm (19 1/3 inches) | Soft, protruding, no masses. Girth 49-1/2 cm (19.5 inches) | Soft, protruding, no masses. Girth: 53 cm (21 inches) |
| Neuro | No deficits | No deficits | No deficits | No deficits |
| Skin | No rashes | No rashes | No rashes | No rashes |
| Labs | See Table 4 | |||
| Impression | Children with signs of severe malnutrition, including decreased subcutaneous fat tissue. These cases represent appropriate food deprivation, severe physical neglect, and failure to thrive.bc | |||
aPhotograph of child's chest: Beading of ribs was not seen by the pediatrician or other observers.
bThe 18-month-old girl was also diagnosed with developmental delay.
cThe 52-month-old boy was also diagnosed with clinical rickets
Table 4.
Laboratory data of the four older children
| Labs | 18-month-old girl | 39-month-old boy | 52 month-old boy | 78 month-old boy | Normal range |
| PT | 12.9 | 13.4 | 12.3 | 10.6 | 11.0–13.0 seconds |
| INR | 1.06 | 1.4 | 1.3 | 1.1 | 0.9 – 1.1 |
| PTT | 28.1 | 27 | 32 | 25 | < 35 seconds |
| Vit D 25-hydroxy | 27.5 | - | 32 | - | 10.0 – 60.0 IU |
| Glucose | 76 | - | - | - | 70–110 mg/dl |
| BUN | 10 | - | - | - | 8.0 – 23.0 mg/dl |
| Creatinine | 0.5 | - | - | 0.7 – 1.4 mg/dl | |
| Alk phosphatase | 351 | 232 | - | 351 | 39 – 117 IU |
| Calcium | 9.4 | 10.4 | 10.1 | 9.4 | 8.5 – 10.4 mg/dl |
| Magnesium | - | 1.8 | 2.0 | 2.4 | 1.5 – 2.5 mg/dl |
| Phosphate | 4.0 | 3.0 – 6.0 mg/dl | |||
| Bicarbonate | 29 | - | - | - | 22.0 – 29.0 mg/dl |
| Sodium | 139.3 | - | - | - | 133.0 – 145.0 mg/dl |
| Chloride | 101.0 | - | - | - | 96.0–108.0 mg/dl |
| Potassium | 4.4 | - | - | - | 3.3 – 5.1 mg/dl |
| Triglycerides | 128 | 48 | - | 67 | < 200 mg/dl |
| Cholesterol | 144 | 129 | 120 | 124 | < 200 mg/dl |
| VLDL | 25.6 | - | - | - | 5 – 55 mg/dl |
| LDL | 73 | 82 | 63 | 73 | |
| HDL | 65 | 37 | 43 | 68 | |
| Prealbumin | 15 | 11 | 14 | 14–30 mg/dl | |
| Chest X-ray | Normal | Normal | Normal | Normal | |
| Long bone X-ray | - | Normal | Normal | Minimal changes* |
* Minimal metaphyseal banding of proximal femora possibly associated with some interruption of growth on a transient basis of the proximal femora
Based on a "Denver Developmental" screen,[6] the pediatrician referred the 18-month-old girl to a psychologist for a formal assessment. The consulting psychologist used the Battelle Inventory Screening Test[7] to evaluate the development of the girl. On adaptive fine motor skills, overall motor skills, expressive language, and communication she scored on average at the level of a 15-month-old. On the cognitive assessment, she scored at about her age. According to the pediatrician, the psychology board does not allow psychologists to release the raw data of their examinations to anyone other than another psychologist. Consequently, the psychologist did not reveal the data supporting the conclusion that the 18-month-old was developmentally delayed to a 15-month-old level.
After an investigation, including the infant's autopsy, the parents were charged with aggravated manslaughter in the care of the infant and neglect of all their four older children for feeding them an exclusively raw foods vegan diet. The court incarcerated the parents and placed the older children in foster care. Realizing that all four children were small yet appeared quite healthy; the court initially ordered the raw foods vegan diet to be continued in foster care. After about two weeks, the court reversed itself and ordered an omnivore diet for the four older children because of the testimony of the pediatrician who diagnosed severe malnutrition in all four, rickets in the four-year-old based entirely on his clinical finding of a rachitic rosary on the ribs, and developmental delay in the 18-month-old based on the psychologist's report.
Both parents declined plea bargains offering probation, because it did not include the return of their surviving children. The trial in October 2005 resulted in an acquittal on the manslaughter charge regarding the infant and a conviction of neglect of the four older children by feeding them the exclusively raw foods diet. Sentencing is scheduled for December 15, 2005.
Discussion
Some 12 million infants and young children die each year in developing countries from complications of marasmus (protein-calorie deficiency) and kwashiorkor (severe protein deficiency).[8] Diarrhea, dehydration, and infection are generally the immediate causes of death in malnourished children. Kwashiorkor manifests clinically with stunted growth, mental apathy, edema, a desquamating patchy rash, and pigment changes in the hair and skin. Children with marasmus typically retain mental alertness and do not have edema or rash. Autopsies of children dying of kwashiorkor and marasmus show pancreatic atrophy or fibrosis[9,10] and fatty changes or fibrosis in the liver.[10] The infant under discussion did not have the typical autopsy findings of either kwashiorkor or marasmus. She had no rash, edema, or pancreatic or hepatic histological changes.
Laboratory findings in severely malnourished children include low albumin, protein, prealbumin, BUN, cholesterol, transferrin, ferritin, B12, folate, and lymphocyte count and severe anemia. Notably, the pediatrician ordered only the BUN in one child, cholesterols in all four, and prealbumins in three children (Table 4).
Although the four older siblings were 2.1–4.1 SD below the mean on the USA growth charts, they had none of the clinical or laboratory findings associated with kwashiorkor and marasmus. According to the World Health Organization, a child that is significantly below the mean on growth charts cannot be definitively diagnosed as malnourished without confirmation by other clinical or laboratory evidence.[11]
Regarding the issue of the risk of post neonatal death (aged 1–12 months) from the complications of protein-energy malnutrition, we can refer to vital statistics data from the Center for Disease Control/National Center for Health Statistics for 2001 and 2002. In those 2 years, there were about 10,000 post neonatal infants who were more than 3 standard deviations below the mean on the CDC growth charts (8 million × .0013 [fraction of any normally distributed population below 3SD]). Out of approximately 18,000 post neonatal deaths in 2001 and 2002, none were caused by marasmus or kwashiorkor. Only 4 had the underlying cause of "severe malnutrition, not otherwise specified."[12,13] Growth chart evidence alone does not mean malnutrition. Vegan children tend to have catch up growth by age 10.[14]
Total cholesterol levels of the four children (144, 129, 120, 154 mg/dl) were all below 160 mg/dl, which suggests possible malnutrition in laboratory assessment panels. However, no published article documents any health risks of children or adults having total cholesterols <160 mg/dl due to a vegan diet. Reducing risk factors for atherosclerosis is a prime health reason for becoming a vegan and indicates a problem with indiscriminately applying laboratory malnutrition assessment indices to vegans.
Of the prealbumin tests obtained on the three older children (i.e., 11, 14, and 15 mg/dl), one was slightly below the "normal" range (14–30 mg/dl). According to the Family Practice Notebook, prealbumin < 5 indicates severe protein malnutrition and predicts a poor prognosis.[15]
The nutrient analysis of this infant's diet (Figure 1) shows that it meets the USDA certified recommended daily requirements (RDA) for all nutrients except vitamin B12, calcium, and zinc. Unlike folate for which we have stores for only days or a few weeks, vitamin B12 stores in the liver may be gradually used over months and years. Breast milk of vegan mothers generally has less vitamin B12 than that of omnivore mothers. We do not have the serum vitamin B12 amount of this mother during breast-feeding. It may have been low although she had no symptoms of anemia. During the 2 1/2 months of breast feeding, the infant accumulated an unknown amount of vitamin B12. The infant and older children were all at risk for B12 deficiency, however, B12 deficiency was unlikely to have triggered the acute event that caused the infant's death.
Rickets diagnosed in the 4-year-old
The Center for Disease Control (CDC) defines vitamin D deficient rickets (International Classification of Diseases, Ninth Revision, Clinical Modification [ICD-9-CM] [1] codes of 268.0 [active rickets], 268.9 [unspecified vitamin D deficiency], or 268.2 [unspecified osteomalacia]) as having a low serum 25-hydroxy-vitamin-D level (below laboratory reference range) combined with one or more of the following radiographic changes: osteopenia, widening of growth plates, fraying and cupping of the metaphysis, or craniomalacia.[16] Low phosphorus, normal or low calcium, markedly high alkaline phosphatase, and high parathyroid hormone levels are also typically seen but not part of the CDC's criteria for the diagnosis.[17] None of the children had any of these findings.
Developmental delay in the 18-month-old girl
A licensed psychologist, using the Battelle Developmental Inventory Screening Test (BDIST), made the diagnosis of developmental delay in the 18-month-old girl. An analysis of the BDIST in 104 children 7 to 83-months-old compared with a battery of other psychological tests showed both poor sensitivity (failing to detect 25% of the children with developmental problems, such as mental retardation, borderline intelligence, language delays, and learning disabilities) and poor specificity (27% of the non developmentally delayed children failed the BDIST).[18]
Accuracy of the BDIST in diagnosing developmental delay in this 18-month-old toddler may have also been compromised by the trauma due to the recent separation from her parents.
DiGeorge anomaly in the infant
The differential diagnosis of an infant with no thymus gland and "inconspicuous parathyroid glands" includes only one thing: DiGeorge syndrome. Normally, an infant's thymus should be about 8 times the size of the thyroid gland. Involution of the thymus gland is frequently found in infants and children with malnutrition.[8] However, no case of the complete absence of a thymus gland due to malnutrition alone has ever been reported in the medical literature.
DiGeorge syndrome is a misnomer and should be called "DiGeorge anomaly" because the constellation of defects results from a failure of an embryological field (third and fourth pharyngeal pouches) to develop normally rather than a single cause.[19]
Patients with DiGeorge anomaly may have velocardiofacial syndrome ([VCFS] or Shprintzen syndrome), conotruncal anomaly face syndrome, Caylor syndrome, Opitz-GBBB syndrome, or CHARGE (coloboma, heart anomalies, atresia of choanae, retardation [mental and somatic], genital hypoplasia, and ear anomalies) syndrome. Absence or hypoplasia of thymus and parathyroid glands is consistently found. Circulating T-cells tend to be decreased but not absent and functionally deficient with poor response to mitogens.[20] Consequently, the presence of CD3+ T-cells in the spleen at post mortem examination does not rule out DiGeorge anomaly. T-cell function with mitogens or skin tests cannot be measured in post mortem specimens.
In 2002, the last year for which vital statistics are available, 41 death certificates mentioned DiGeorge syndrome. Of those, 31 died before age 12 months. Only one survived childhood.[12] If DiGeorge patients survive problems with congenital heart disease and hypocalcemia typically presenting in the first month of life, they generally succumb to infection due to the T-cell immunodeficiency.[21] In an autopsy series of 24 DiGeorge patients from a large specialty hospital, most of the children surviving the first month of life had failure to thrive and developmental delay.[19]
Conclusion
In the infant who died, DiGeorge anomaly cannot be ruled out and malnutrition, according to WHO criteria, cannot be diagnosed. The 18-month-old's recent separation from her parents and the cultural bias and poor sensitivity and specificity of the Battelle Developmental Inventory Screening Test make the diagnosis of developmental delay highly questionable. The older children were not malnourished by the WHO's definition. The clinical diagnosis of rickets in the 4-year-old was not confirmed by the CDC's criteria. The raw foods vegan diet and possibly inherited small stature from the father's side account for their relatively low heights and weights. Catch-up growth will probably occur on the standard American diet but would have also been expected if they had remained on a vegan diet.
Abbreviations
American Dietetic Association: ADA
Battelle Developmental Inventory Screening Test: BDIST
Blood urea nitrogen: BUN
Center for Disease Control: CDC
Cyanocobalamin: Vitamin B12 or B12
Standard deviation: SD
United States Department of Agriculture: USDA
World Health Organization: WHO
Competing interests
The author(s) declare that they have no competing interest.
Authors' contributions
DKC conceived of drafting the case report, researched the appropriate medical records, and wrote the text. WH created figures 1, 2, 3, using the nutritional intake data supplied by the mother in the case. Both authors read and approved the final manuscript.
Acknowledgments
Acknowledgements
None.
Contributor Information
David K Cundiff, Email: dkcundiff3@verizon.net.
William Harris, Email: HARRISMDW001@hawaii.rr.com.
References
- Anon Position of the American Dietetic Association and Dietitians of Canada: Vegetarian diets. Journal of the American Dietetic Association. 2003;103:748–765. doi: 10.1053/jada.2003.50142. [DOI] [PubMed] [Google Scholar]
- Pediatric Nutrition Handbook. American Academy Of Pediatrics. http://www.aap.org/bst/showdetl.cfm?&DID=15&Product_ID=760&CatID=132 Accessed May 19, 2005.
- Mangels AR, Messina V. Considerations in planning vegan diets: infants. J Am Diet Assoc. 2001;101:670–677. doi: 10.1016/S0002-8223(01)00169-9. [DOI] [PubMed] [Google Scholar]
- Messina M, Messina V. The Dietitian's Guide to Vegetarian Diet: Issues and Applications. Second. Gathersburg, MD: Jones and Bartlett; 2004. [Google Scholar]
- USDA Nutrient Database for Standard Reference, Release 18. Nutrient Data Laboratory Home Page. US Department of Agriculture Agricultural Research Service. 2006. http://www.ars.usda.gov/ba/bhnrc/ndl Accessed January 10, 2006.
- Frankenburg WK, Dodds J, Archer P, Shapiro H, Bresnick B. The Denver II: A major revision and restandardization of the Denver Developmental Screening Test. Pediatrics. 1992;89:91–97. [PubMed] [Google Scholar]
- Berls AT, McEwen IR. Battelle developmental inventory. Phys Ther. 1999;79:776–783. [PubMed] [Google Scholar]
- Chevalier P, Sevilla R, Zalles L, et al. Immuno-nutritional recovery of children with severe malnutrition. Sante. 1996;6:201–208. [PubMed] [Google Scholar]
- Brooks SE, Golden MH. The exocrine pancreas in kwashiorkor and marasmus. Light and electron microscopy. West Indian Med J. 1992;41:56–60. [PubMed] [Google Scholar]
- Blackburn WR, Vinijchaikul K. The pancreas in kwashiorkor. An electron microscopic study. Lab Invest. 1969;20:305–318. [PubMed] [Google Scholar]
- The WHO Family of International Classifications. World Health Organization. http://www3.who.int/icd/vol1htm2003/fr-icd.htm Accessed January 17, 2005.
- National Vital Statistics System, Mortality, 2001–2002. Hyattsville, MD: Center for Disease Control/National Center for Health Statistics; [Google Scholar]
- Deaths: final data for 2002 NVSR. Vol. 53. Hyattville, MD 20782: National Center for Health Statistics. Center for Disease Control. National Institutes of Health. Public Health Service 2005-1120; October 12, 2004. [Google Scholar]
- O'Connell JM, Dibley MJ, Sierra J, Wallace B, Marks JS, Yip R. Growth of vegetarian children: The Farm Study. Pediatrics. 1989;84:475–481. [PubMed] [Google Scholar]
- Moses S. Serum prealbumin. Family Practice Notebook. http://www.fpnotebook.com/PHA55.htm March 5, 2005. Accessed May 17, 2005.
- Anon Severe malnutrition among young children in Georgia, January 1997 – June 1999. MMWR. pp. 224–7.http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5012a3.htm March 30, 2001. [PubMed]
- Carvalho NF, Kenney RD, Carrington PH, Hall DE. Severe nutritional deficiencies in toddlers resulting from health food milk alternatives. Pediatrics. 107:e46. doi: 10.1542/peds.107.4.e46. April 1, 2001. [DOI] [PubMed] [Google Scholar]
- Glascoe FP, Byrne KE. The usefulness of the Battelle Developmental Inventory Screening Test. Clin Pediatr. 1993;32:273–280. doi: 10.1177/000992289303200504. [DOI] [PubMed] [Google Scholar]
- Conley ME, Beckwith JB, Mancer JF, Tenckhoff L. The spectrum of the DiGeorge syndrome. J Pediatr. 1979;94:883–890. doi: 10.1016/s0022-3476(79)80207-3. [DOI] [PubMed] [Google Scholar]
- Markert ML, Hummell DS, Rosenblatt HM, et al. Complete DiGeorge syndrome: persistence of profound immunodeficiency. J Pediatr. 1998;132:15–21. doi: 10.1016/S0022-3476(98)70478-0. [DOI] [PubMed] [Google Scholar]
- Guduri S, Hussain I. DiGeorge syndrome. eMedicine. http://www.emedicine.com/med/topic567.htm#section~introduction. May 28, 2002:


